Abstract
Aberrant phosphorylation of tau is associated with a number of neurodegenerative diseases, including Alzheimer's disease (AD). The molecular mechanisms by which tau phosphorylation is regulated under normal and disease conditions are not well understood. Microtubule affinity regulating kinase (MARK) and PAR-1 have been identified as physiological tau kinases, and aberrant phosphorylation of MARK/PAR-1 target sites in tau has been observed in AD patients and animal models. Here we show that phosphorylation of PAR-1 by the tumor suppressor protein LKB1 is required for PAR-1 activation, which in turn promotes tau phosphorylation in Drosophila. Diverse stress stimuli, such as high osmolarity and overexpression of the human β-amyloid precursor protein, can promote PAR-1 activation and tau phosphorylation in an LKB1-dependent manner. These results reveal a new function for the tumor suppressor protein LKB1 in a signaling cascade through which the phosphorylation and function of tau is regulated by diverse signals under physiological and pathological conditions.
Keywords: Alzheimer's disease, APP, Drosophila, LKB1, MARK/PAR-1, neurodegeneration, tau phosphorylation, stress
Introduction
Tau is a soluble microtubule (MT)-binding protein normally found in axons. It becomes abnormally phosphorylated and insoluble and forms neurofibrillary tangles (NFTs) in neurodegenerative tauopathies (Grundke-Iqbal et al., 1986; Greenberg and Davies, 1990; Lee et al., 1991). The association of mutations in tau with frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17) supports that tau abnormalities can cause neurodegeneration (Foster et al., 1997; Hutton et al., 1998; Poorkaj et al., 1998; Spillantini et al., 1998). In Alzheimer's disease (AD), NFTs often surround amyloid plaques composed of amyloid β (Aβ) peptides. Overexpression of wild-type or mutant human tau (h-tau) in mice could result in limited tangle pathology and neuronal loss, without accompanying plaque pathology (Ishihara et al., 1999; Lewis et al., 2000; Probst et al., 2000). Induction of amyloid pathology in these animals could promote NFT formation (Gotz et al., 2001; Lewis et al., 2001; Oddo et al., 2003). Additional studies demonstrated that blocking Aβ production could remove amyloid pathology as well as early-stage tau lesions (Oddo et al., 2004). These results suggest that NFT pathology is a downstream event resulting from amyloid-induced changes as proposed by the amyloid cascade hypothesis (Hardy and Selkoe, 2002).
Several kinases and phosphatases have been shown to regulate tau phosphorylation in vitro (Lee et al., 2001). Overexpression studies using transgenic mice have implicated cyclin-dependent kinase 5 (Cdk5) and glycogen synthase kinase-3β (GSK-3β) in tau phosphorylation, aggregation, and tangle formation (Lucas et al., 2001; Cruz et al., 2003; Noble et al., 2003). Recent studies in Drosophila have implicated partitioning defective-1 (PAR-1) as a physiological tau kinase in regulating tau phosphorylation and toxicity. Microtubule affinity regulating kinase (MARK), the mammalian homolog of PAR-1, could phosphorylate tau in vitro and was found to associate with NFT in AD brains (Drewes et al., 1997; Chin et al., 2000; Biernat et al., 2002). Overexpression of PAR-1 in Drosophila leads to elevated phosphorylation and enhanced toxicity of h-tau, whereas loss of PAR-1 function or mutating PAR-1-directed phosphorylation sites (12E8 sites) in tau attenuated h-tau toxicity (Nishimura et al., 2004). Phosphorylation of tau at PAR-1-directed sites is increased in AD and amyloid precursor protein (APP) transgenic mice (Augustinack et al., 2002; Perez et al., 2005), underscoring the potential importance of this phosphorylation event in disease pathogenesis. Mammalian cell culture studies have implicated atypical protein kinase C (aPKC) in negatively regulating MARK kinase (MARKK) activity (Hurov et al., 2004), whereas MARKK and LKB1 positively regulate MARK (Timm et al., 2003; Brajenovic et al., 2004; Lizcano et al., 2004).
Here we show that PAR-1 is regulated by phosphorylation of a conserved Thr in the activation loop of the kinase domain. We have identified LKB1 as the kinase responsible for this phosphorylation event. We also found that this phosphorylation event responds to various stress stimuli such as high osmolarity and APP accumulation. Our results identify LKB1 as an activating kinase of PAR-1 under normal physiological conditions and raise the possibility that aberrant activation of PAR-1 may provide one of the molecular links in the pathogenic cascade of tauopathies.
Materials and Methods
Drosophila genetics.
Fly culture and crosses were performed according to standard procedures. All general fly stocks were obtained from the Bloomington Drosophila Stock Center (Bloomington, IN). The UAS-APP and UAS-h-tau transgenic lines were provided by Dr. Larry Goldstein (University of California San Diego, La Jolla, CA) and Dr. Mel Feany (Harvard University, Cambridge, MA), respectively. To generate UAS-LKB1 RNA interference (RNAi) transgenics, genomic DNA/cDNA hybrid constructs were generated as described previously (Kalidas and Smith, 2002). To make UAS-LKB1 transgenics, a full-length LKB1 cDNA was cloned into a pUAST vector. To generate PAR-1(T408A) and PAR-1(T408E) transgenics, the corresponding point mutations were introduced into a myc-tagged PAR-1α cDNA (Sun et al., 2001), using PCR-based site-directed mutagenesis. After DNA sequencing to verify sequence accuracy, the cDNA inserts were cloned into the pUAST vector. Standard procedures were followed for embryo injection and recovery of transgenic lines.
Western blotting.
To generate the p-T408 antibody, a synthetic peptide GSKLDpTFCGSP was used to elicit immune response in rabbits. Purification of the phosphospecific antibody was performed by Open Biosystems (Huntsville, AL). Purified p-T408 antibody was used at 1:2000 dilutions in Western blot analysis. To detect phosphorylation of endogenous PAR-1 at T408, protein extracts prepared from dissected larval brain complexes from wild-type and par-1 mutants were probed by Western blot analysis. To detect LKB1 protein in control and LKB1 RNAi animals, an affinity-purified rabbit anti-LKB1 antibody (a gift from Dr. Helen McNeil, Samuel Lunenfeld Research Institute, Toronto, Ontario, Canada) was used. A mouse monoclonal anti-myc antibody (4A6; Millipore, Bedford, MA) was used to detect transgenic PAR-1 protein. Anti-actin and anti-β-tubulin antibodies (Sigma, St. Louis, MO) were used as loading controls.
In vitro kinase assay.
Glutathione S-transferase (GST) fusion proteins of the kinase domain of wild-type PAR-1 or T408A mutant PAR-1 were expressed in bacteria and purified using glutathione agarose beads. These GST–PAR-1 proteins were used as substrates in in vitro kinase assays, in which the purified mammalian LKB1/STRAD/MO25 complex (Millipore) was used as the source of kinase. Phosphorylation events were detected by Western blotting with p-T408 antibody. To control for the specificity of the kinase reaction, FLAG-tagged wild-type LKB1 or kinase-dead LKB1 (LKB1K194A) were cotransfected with His-tagged STRAD in HEK293 cells. The LKB1 complex was purified from cell lysates by immunoprecipitation, as described previously (Lizcano et al., 2004; Shaw et al., 2004), and was used in kinase assays in which the kinase domains of wild-type PAR-1 or PAR-1(T408A) were used as substrates. The in vitro kinase assay conditions were essentially as described previously (Nishimura et al., 2004).
Osmotic stress treatment.
Newly eclosed adult flies or third instar larvae were placed in instant fly food hydrated with 1.5 m NaCl. After treatment for 5 h of the larvae or for 12–24 h of the adult flies, whole larvae or adult fly heads were homogenized to prepare protein extracts. Proteins were separated on SDS-PAGE and probed with p-T408, myc, tubulin, or tau antibodies.
Scanning electron microscopy and photoreceptor staining.
For the analysis of neurodegeneration in the fly eye, scanning electron microscopic analysis of eye morphology and toluidine blue staining of photoreceptor neurons were performed essentially as described previously (Nishimura et al., 2004). For each genotype, at least five samples were analyzed.
Results
Phosphorylation of T408 residue is critical for PAR-1 activity
PAR-1/MARK kinases belong to the AMP-activated protein kinase (AMPK) family. Previous in vitro biochemical studies suggest that phosphorylation of a conserved Thr residue in the activation loop of the kinase domain is critical for the activation of AMPK-related kinase (Lizcano et al., 2004). To test whether this residue is critical for PAR-1 activity in vivo, we mutated it into Ala (T408A) to block phosphorylation or Glu (T408E) to mimic phosphorylation. Transgenic flies expressing these mutant proteins were generated using the UAS-Gal4 system. As reported previously (Nishimura et al., 2004), overexpression of wild-type PAR-1 can cause eye degeneration (Fig. 1A). This is mediated in part by hyperphosphorylation of endogenous fly tau, which is expressed in the fly retina as well as other tissues (Heidary and Fortini, 2001; Nishimura et al., 2004). Furthermore, although coexpression of PAR-1 and wild-type h-tau synergistically enhanced neurodegeneration, coexpression of h-tau(S2A), in which the PAR-1 phosphorylation sites are rendered nonphosphorylatable, blocked the enhancing effect of PAR-1 (Nishimura et al., 2004). These results suggest that tau is a major target through which aberrantly activated PAR-1 causes neurotoxicity. However, because high levels of PAR-1 overexpression can cause a stronger phenotype than tau overexpression, it is possible that when over activated, PAR-1 may also act on other substrates.
Figure 1.
In vivo phosphorylation of PAR-1 at T408 and its importance in regulating PAR-1 activity. A–D, Scanning electron microscopic images of transgenic fly eyes overexpressing wild-type (WT) PAR-1 (A), PAR-1(T408A) (B), PAR-1(T408E) (C), or wild-type control (D) fly eyes. E, F, Western blot analysis showing that whereas PAR-1 overexpression caused an approximate fourfold increase in wild-type h-tau (E) or h-tau(R406W) (F) phosphorylation at 12E8 sites, PAR-1(T408A) or PAR-1(T408E) had little effects. Tubulin served as a loading control. G, Detection of T408 phosphorylation of wild-type PAR-1 but not PAR-1(T408A) or PAR-1(T408E) produced from transgenes (bottom). Exogenous PAR-1 proteins expressed from the transgenes were tagged with a myc tag and detected with an anti-myc antibody. H, Detection of p-T408-positive endogenous PAR-1 protein from adult fly head extracts prepared from wild-type and hypomorphic PAR-1 (PAR-1W3/PAR-119A) mutant animals. ←, PAR-1-specific bands; *, nonspecific bands.
We found that expression of equivalent levels of PAR-1(T408A) or PAR-1(T408E) as wild-type PAR-1 had no effect on eye morphology (Fig. 1B,C), suggesting that T408 is critical for PAR-1 kinase activity and that a mere negative charge at this position cannot substitute for phosphorylation. This latter result is consistent with previous in vitro studies of similar mutations in mammalian MARK family kinases, which caused either no or subtle activation of kinase activity in some family members (Timm et al., 2003; Lizcano et al., 2004). To test whether the T408A and T408E mutations affected PAR-1 activity in other processes, we overexpressed the mutant PAR-1 proteins using a ubiquitous promoter. Although ubiquitous overexpression of wild-type PAR-1 was toxic and led to embryonic lethality, PAR-1(T408A) or PAR-1(T408E) overexpression had no effect on animal viability. Furthermore, unlike wild-type PAR-1, neither PAR-1(T408A) nor PAR-1(T408E) was capable of promoting tau phosphorylation (Fig. 1E,F). The T408 residue is therefore important for PAR-1 activity in various in vivo settings.
To determine whether T408 residue is phosphorylated in vivo, we generated a phosphospecific antibody (p-T408 antibody). This antibody could recognize transgene-derived, wild-type PAR-1 (Fig. 1G) or endogenous PAR-1 protein (Fig. 1H). The specificity of this antibody for p-T408 was demonstrated by the fact that it did not recognize wild-type PAR-1, PAR-1(T408A), and PAR-1(T408E) proteins expressed in bacteria or PAR-1(T408A) and PAR-1(T408E) proteins produced in transgenic flies (Fig. 1G; see Fig. 3A).
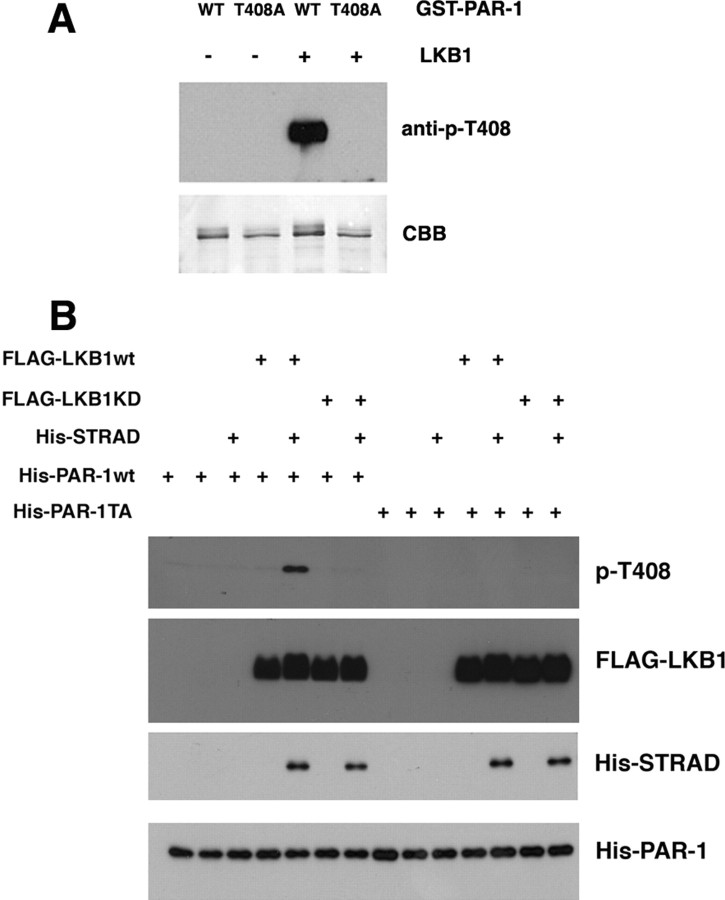
Figure 3.
Direct phosphorylation of PAR-1 T408 site by LKB1. A, In vitro kinase assay showing phosphorylation of wild-type (WT) PAR-1 but not PAR-1(T408A) recombinant proteins by mammalian LKB1/STRAD/MO25 complex. Top, PAR-1 T408 phosphorylation detected with the p-T408 antibody. Bottom, Coomassie blue staining of the recombinant substrate proteins to show equal loading. B, In vitro kinase assays using wild-type LKB1 and kinase-dead LKB1(LKB1KD), which contains a K194A mutation, to demonstrate the specificity of LKB1 phosphorylation of PAR-1. HEK293 cells were transfected with the indicated FLAG-LKB1 and His-STRAD constructs. Cell lysates were immunoprecipitated with anti-Flag agarose beads, and the immunocomplex was used in in vitro kinase assays using the indicated His-PAR-1 recombinant protein as the substrate. Phosphorylation of PAR-1 was detected with anti-p-T408 antibody. The amounts of immunoprecipitated LKB1, STRAD, and the input PAR-1 substrates were detected by Western blotting with the corresponding anti-epitope tag antibodies.
LKB1 phosphorylates PAR-1 T408 site in vitro and in vivo
We next sought to identify the kinase(s) responsible for phosphorylating PAR-1 T408. Two kinases, LKB1 and MARKK/TAO1, have been reported to phosphorylate mammalian MARK in vitro (Timm et al., 2003; Brajenovic et al., 2004; Lizcano et al., 2004). The exact relationship between LKB1 and MARK/PAR-1 is, however, unclear because data that suggest LKB1 acting upstream or downstream of MARK/PAR-1 have both been reported (Martin and St. Johnston, 2003; Spicer et al., 2003; Brajenovic et al., 2004; Lizcano et al., 2004). We investigated whether LKB1 is the kinase responsible for PAR-1 T408 phosphorylation in vivo. We first tested whether LKB1 is sufficient to promote PAR-1 T408 phosphorylation. Western blot analysis showed that coexpression of LKB1 strongly enhanced PAR-1 T408 phosphorylation (Fig. 2F), whereas coexpression of a control kinase AMPK has little effect (data not shown). Interestingly, LKB1 coexpression also led to an increase in PAR-1 protein level. This effect is likely phosphorylation dependent, because LKB1 coexpression had little effect on PAR-1(T408A) protein level (supplemental Fig. 1A, available at www.jneurosci.org as supplemental material). Coexpression of LKB1 and PAR-1 strongly enhanced PAR-1-induced eye degeneration (Fig. 2C), although overexpression of LKB1 alone had little effect (Fig. 2B). The lack of an obvious effect on eye morphology by LKB1 overexpression alone is probably because the level of activated endogenous PAR-1 is still below the threshold level needed for neurotoxicity, because the toxic effect of PAR-1 overexpression in the eye is strictly dose dependent (Nishimura et al., 2004). Indeed, Western blot analysis showed that the level of phosphorylated endogenous PAR-1 in LKB1 overexpression animals was much lower than that of exogenous PAR-1 phosphorylated by endogenous LKB1 in animals overexpressing PAR-1 (supplemental Fig. 1B, available at www.jneurosci.org as supplemental material). It is also possible that the PAR-1α isoform expressed from the transgenes used in this study has higher kinase activity than the other PAR-1 isoforms known to exist in vivo, resulting from the absence of a putative autoinhibitory domain at the C terminus of PAR-1α (Elbert et al., 2005).
Figure 2.
LKB1 acts upstream of PAR-1. A–E, Scanning electron microscopic images showing modification of PAR-1-induced eye degeneration by LKB1 overexpression or RNAi knockdown. The eyes of the following genotypes are shown: GMR-Gal4>UAS-PAR-1 (A), GMR-Gal4>UAS-LKB1 (B), GMR-Gal4>UAS-PAR-1/UAS-LKB1 (C), GMR-Gal4>UAS-LKB1 RNAi (D), GMR-Gal4>UAS-PAR-1/UAS-LKB1 RNAi (E). F, Western blot analysis showing increased PAR-1 T408 phosphorylation after LKB1 overexpression. Although LKB1 coexpression also caused an increase in PAR-1 protein, after normalization of total PAR-1 level, there is still a 2.5-fold increase in p-T408 signal. G, Western blot analysis showing decreased PAR-1 T408 phosphorylation after LKB1 knockdown by RNAi. H, Control experiment showing that coexpression of a W RNAi construct had no effect on PAR-1 T408 phosphorylation. I, Western blot analysis demonstrating RNAi knockdown of LKB1 protein expression. W RNAi serves as an RNAi control, and tubulin serves as a loading control.
We also tested whether LKB1 is necessary for PAR-1 T408 phosphorylation. Because LKB1 null mutants are embryonic lethal, we adopted the transgenic RNAi technique to knockdown LKB1 in a tissue-specific manner to circumvent the lethality problem. The LKB1 RNAi construct efficiently and specifically inhibited LKB1 protein expression (Fig. 2I). We observed a clear suppression of PAR-1-induced rough eye phenotype after LKB1 RNAi (Fig. 2E), which was correlated with a significant reduction in PAR-1 T408 phosphorylation (Fig. 2G). Quantification of Western blot data showed that LKB1 RNAi led to an ∼80% reduction in PAR-1 T408 phosphorylation (supplemental Fig. 1C, available at www.jneurosci.org as supplemental material). In contrast, coexpression of a control white RNAi construct (W RNAi) showed no effect on PAR-1 phosphorylation (Fig. 2H) or toxicity (data not shown), suggesting that the LKB1 RNAi effect was not simply as a result of double-stranded RNA expression. The residual PAR-1 T408 phosphorylation in the LKB1 RNAi background could be because of incomplete inhibition of LKB1 by RNAi or phosphorylation of PAR-1 by redundant kinases such as MARKK/TAO1. To begin to address the contribution of MARKK/TAO1 in phosphorylating PAR-1 T408, we coexpressed PAR-1 with fly MARKK/TAO1 or the mouse homolog. Surprisingly, coexpression of MARKK/TAO1 led to a reduction in PAR-1 T408 phosphorylation (supplemental Fig. 1D, available at www.jneurosci.org as supplemental material). Although the precise function and mechanism of action of MARKK/TAO1 in regulating PAR-1 T408 phosphorylation remain to be elucidated, this result suggests that LKB1 and MARKK/TAO1 may exert distinct effects on PAR-1 T408 phosphorylation in Drosophila.
To test whether LKB1 directly phosphorylates PAR-1 at T408, we performed in vitro kinase assays using recombinant GST-PAR-1 and GST-PAR-1(T408A) as substrates and a commercially available LKB1/STRAD/MO25 complex as the kinase. This LKB1 kinase complex was purified from transfected mammalian cell lines and is the kinase-active form (Hawley et al., 2003). The LKB1 complex efficiently phosphorylated wild-type PAR-1 but not PAR-1(T408A) (Fig. 3A), indicating that it directly phosphorylated PAR-1 at T408. In contrast, an LKB1 protein complex containing the kinase-dead form of LKB1 (K194A) was unable to phosphorylate the PAR-1 T408 site (Fig. 3B). These results demonstrate that LKB1 directly acts on the PAR-1 T408 site. It is possible that, in addition to being a phosphorylation site for LKB1, the T408 site may mediate the association of PAR-1 and LKB1 to form a putative PAR-1/LKB1 complex and that this complex may be the functional entity that is responsible for the toxic effects of PAR-1 and LKB1. This could explain why the T408A and T408E mutations both abolished PAR-1 toxicity, should these mutations disrupt PAR-1 and LKB1 interaction. We tested this possibility by coexpressing LKB1 and wild-type PAR-1 or PAR-1 T408A and PAR-1 T408E mutants. We could not detect interaction between LKB1 and wild-type PAR-1 or the mutant forms of PAR-1, although we could readily detect the interaction between LKB1 and its known binding partner STRAD (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Similarly, a previous proteomic analysis also did not detect stable association between LKB1 and MARK (Brajenovic et al., 2004). Thus, although we cannot rule out the possibility that a transient complex forms between PAR-1 and LKB1, it is unlikely that a stable association between LKB1 and PAR-1 is the predominant mechanism of action.
LKB1 promotes tau phosphorylation at PAR-1-dependent sites and modulates tau toxicity
The results presented so far provide compelling in vivo evidence that LKB1 is an upstream activating kinase of PAR-1. PAR-1/MARK kinases are known to regulate MT dynamics by phosphorylating tau in the MT-binding domains. To test whether LKB1 is involved in this process, we first asked whether LKB1 and tau genetically interact. Overexpression of wild-type h-tau or FTDP-17-associated h-tau(R406W) in the fly eye is toxic (Wittmann et al., 2001; Jackson et al., 2002; Nishimura et al., 2004), as indicated by a mild photoreceptor loss (Fig. 4B). Coexpression of an LKB1 transgene strongly enhanced tau toxicity, resulting in a severe disorganization of the eye and drastic loss of photoreceptor neurons and surrounding cells (Fig. 4C), although overexpression of LKB1 alone had little effect (Fig. 4A), presumably because of the low level of endogenous phospho-PAR-1 shown previously. Thus, LKB1 can genetically modify tau toxicity. To test whether the effect of LKB1 on tau toxicity is mediated by PAR-1, we coexpressed LKB1 and tauS2A, in which the PAR-1 phosphorylation sites are rendered nonphosphorylatable. As shown in Figure 4E, tauS2A blocked the effect of LKB1 on tau toxicity, supporting the involvement of PAR-1 in mediating the effect of LKB1 on tau toxicity.
Figure 4.

Enhancement of h-tau phosphorylation and toxicity by LKB1. A–E, Toluidine blue staining of photoreceptor neurons in transgenic flies overexpressing LKB1 (A) or h-tau(R406W) (B), coexpressing LKB1 and h-tau(R406W) (C), overexpressing h-tau(R406W)S2A (D), or coexpressing LKB1 and h-tau(R406W)S2A (E). Arrows mark ommatidial clusters showing photoreceptor loss. Note that all of the photoreceptor neurons are preserved in A, D, and E; a small number of photoreceptors are lost in B; whereas the majority of the photoreceptor neurons are lost in C. F, Western blot analysis showing increased phosphorylation of h-tau(R406W) at 12E8 and AT8 sites, whereas the phosphorylation at AT180 site was minimally changed after LKB1 coexpression. The T14 antibody recognizes total h-tau. G, Western blot analysis showing no change in tau phosphorylation at the 12E8 site after AMPK coexpression.
We next tested whether LKB1 modulates tau toxicity by affecting its phosphorylation. We found that coexpression of LKB1 significantly increased tau phosphorylation at 12E8 sites, which are PAR-1 recognition sites (Fig. 4F). This effect of LKB1 on tau phosphorylation is likely indirect and mediated by PAR-1, because PAR-1 but not LKB1 could directly phosphorylate tau at 12E8 sites in kinase assays (Nishimura et al., 2004) (data not shown). As a kinase control, we coexpressed h-tau with AMPK, a kinase closely related to PAR-1 and a known substrate of LKB1. AMPK has no obvious effect on tau 12E8 site phosphorylation (Fig. 4G).
Phosphorylation of tau by PAR-1 was previously shown to initiate a multisite phosphorylation process that leads to the generation of a subset of disease-diagnostic phosphoepitopes such as AT8, without affecting other phosphorylation sites such as AT180 sites (Nishimura et al., 2004). We found that in LKB1 and tau coexpression flies, there was a significant increase in AT8 site phosphorylation, whereas the AT180 site was minimally affected (Fig. 4F). These data are consistent with LKB1 acting through PAR-1 to regulate tau phosphorylation. We also tested whether LKB1 may affect phosphorylation of endogenous tau. Overexpression of LKB1 increased whereas LKB1 RNAi decreased endogenous tau phosphorylation at 12E8 sites (supplemental Fig. 3, available at www.jneurosci.org as supplemental material).
Overexpression of APP promotes PAR-1 T408 phosphorylation and tau phosphorylation in an LKB1-dependent manner
We wanted to identify upstream signals that could regulate PAR-1 and tau phosphorylation. We first tested whether APP-related toxicity might play a role. Overexpression of full-length human APP protein has been shown to be toxic in Drosophila CNS neurons, presumably by disrupting axonal transport (Gunawardena and Goldstein, 2001). We found that co-overexpression of human APP with PAR-1 promoted PAR-1 T408 phosphorylation (Fig. 5I) and enhanced PAR-1-induced eye degeneration (Fig. 5C), although APP itself had no obvious effect in the eye (Fig. 5A). Coexpression of a control UAS-green fluorescent protein transgene did not affect PAR-1 T408 phosphorylation or toxicity. Enhancement of PAR-1 T408 phosphorylation was also observed when mutant APP proteins containing the London or Swedish mutations were coexpressed with PAR-1 (Fig. 5I). To test whether LKB1 is required for mediating the effect of APP on PAR-1 T408 phosphorylation, we used the LKB1 RNAi transgene. LKB1 RNAi reduced the enhancing effect of APP on PAR-1 T408 phosphorylation and toxicity (Fig. 4D,J), whereas a control W RNAi transgene had no such effect (supplemental Fig. 4A, available at www.jneurosci.org as supplemental material). These results suggest that LKB1 is required for the enhancement of PAR-1 phosphorylation and activity by APP.
Figure 5.

LKB1 mediates the effect of APP and osmotic stress on PAR-1 and tau phosphorylation and toxicity. A–D, Scanning electron microscopic eye images of transgenic flies overexpressing APP695 (A) or PAR-1 (B), coexpressing APP695 and PAR-1(C), or coexpressing APP695, PAR-1, and LKB1 RNAi transgenes (D). E–H, Scanning electron microscopic eye images of transgenic flies overexpressing APP695 (E) or h-tau(R406W) (F), coexpressing APP695 and h-tau(R406W) (G), or coexpressing APP695, h-tau(R406W), and LKB1 RNAi transgenes (H). I, Western blot analysis showing enhancement of PAR-1 T408 phosphorylation by overexpression of wild-type APP or APP containing the London mutation. Wild-type APP and APP(London) caused an approximate twofold and threefold increase in PAR-1 p-T408 levels, respectively. J, Western blot analysis showing attenuation of the effects of APP overexpression on PAR-1 T408 phosphorylation and tau 12E8 site phosphorylation by LKB1 RNAi. Whereas APP caused an approximate threefold increase in PAR-1 p-T408 levels and a twofold increase in h-tau 12E8 signals, LKB1 RNAi attenuated this enhancing effect. K, L, Western blot analyses showing enhancement of PAR-1 T408 phosphorylation (K) or tau 12E8 phosphorylation (L) by NaCl treatment. NaCl treatment caused an approximate fourfold increase in PAR-1 p-T408 level and a twofold increase in h-tau 12E8 phosphorylation. These effects were blocked by LKB1 RNAi but not W RNAi. Tubulin served as a loading control in all panels.
We next tested whether APP can promote tau phosphorylation and whether LKB1 activity is also required for this effect. Coexpression of APP with the wild-type h-tau or h-tau(R406W) promoted tau phosphorylation at 12E8 sites (Fig. 5J) and enhanced tau toxicity (Fig. 5G). Enhanced 12E8 site phosphorylation of tau was also observed in previous studies of APP and tau double transgenic mice (Lewis et al., 2001; Perez et al., 2005), but the kinase responsible for this effect was not known. We found that LKB1 RNAi but not W RNAi attenuated the effect of APP on tau phosphorylation and toxicity (Fig. 5H,J) (supplemental Fig. 4B, available at www.jneurosci.org as supplemental material). This result suggests that PAR-1 activation and tau phosphorylation are downstream events in a putative signaling pathway initiated by APP accumulation.
Osmotic stress also regulates PAR-1 and tau phosphorylation in an LKB1-dependent manner
We next tested whether other stress stimuli may regulate PAR-1 and tau phosphorylation in an LKB1-dependent manner. For this purpose, we tested the effect of high osmolarity, because tau phosphorylation at 12E8 sites was known to be sensitive to high osmolarity in cultured mammalian cells (Jenkins et al., 2000). PAR-1 transgenic animals fed with food containing a high salt concentration (1.5 m NaCl) showed a significant increase in PAR-1 T408 phosphorylation (Fig. 5K). That high salt treatment leads to osmotic stress was supported by the fact that this treatment led to activation of p38 mitogen-activated protein kinase (MAPK) phosphorylation (supplemental Fig. 4C, available at www.jneurosci.org as supplemental material) and eventual animal mortality. To test whether this effect was dependent on LKB1, we performed the same treatment in an LKB1 RNAi background. LKB1 RNAi significantly attenuated the effect of osmotic stress on PAR-1 T408 phosphorylation, whereas W RNAi showed little effect (Fig. 5K). NaCl treatment of tau transgenic flies also enhanced tau phosphorylation at 12E8 sites, and the effect was significantly reduced by LKB1 RNAi but not W RNAi (Fig. 5L). Thus, LKB1 is required for PAR-1 activation and tau phosphorylation in response to both APP overexpression and osmotic stress.
Discussion
Aberrant phosphorylation of tau protein is associated with a number of neurodegenerative disorders. The molecular mechanism by which tau phosphorylation is regulated under physiological and pathological conditions is not understood. Here we show that phosphorylation of a conserved T408 residue in the activation loop of the kinase domain of PAR-1, an established tau kinase, is critical for its activity. Mutations that render this site unphosphorylatable abolished the biological activity of PAR-1 and its biochemical activity in phosphorylating tau. Using a combination of biochemical and genetic analyses, we found that the tumor suppressor protein LKB1 is an upstream activating kinase that acts on the T408 site of PAR-1. Consistent with LKB1 acting upstream of PAR-1, we found that LKB1 promotes tau phosphorylation at PAR-1-dependent sites and that tauS2A, which is nonphosphorylatable by PAR-1, blocked the effect of LKB1 on tau toxicity.
Our results also demonstrate that PAR-1 activation and tau phosphorylation respond to diverse cellular signals such as overexpressed APP and osmotic stress in an LKB1-dependent manner. We hypothesize that in disease conditions, accumulation of APP and possibly APP-derived peptides may trigger a stress signaling process that causes aberrant PAR-1 and tau phosphorylation. This signaling process may directly impinge on LKB1, but the detailed biochemical mechanism awaits additional investigation. Previous studies have shown that the cellular localization and activity of LKB1 is regulated through its interaction with a catalytically inactive protein kinase STRAD and an armadillo repeat-containing protein MO25. LKB1 activity can also be regulated by phosphorylation and by its interaction with molecular chaperone Hsp90 (Alessi et al., 2006). Overexpression of APP may act through one or a combination of these mechanisms to regulate LKB1 function. Overexpressed APP may also act through aPKC to regulate PAR-1 phosphorylation and activity. A recent study showed that the three components of the γ-secretase complex might influence tau hyperphosphorylation and neurodegeneration through γ-secretase-independent mechanisms. Specifically, it was shown that presenilin and nicastrin prevent tau toxicity by modulating the phosphatidylinositol 3′-kinase (PI3K)/Akt/GSK-3β phosphorylation pathway, whereas aph-1 regulates the aPKC/PAR-1 pathway (Doglio et al., 2006). It is possible that under APP overexpression conditions, sequestration of γ-secretase components by APP may affect the PI3K/Akt/GSK-3β and aPKC/PAR-1 pathways of tau phosphorylation, leading to tau hyperphosphorylation and neurodegeneration.
Osmotic stress is known to activate the MAPK stress-signaling pathway. Significantly, recent findings have provided strong evidence that the p38 MAPK signaling cascade is overactivated in AD (Zhu et al., 2001; Johnson and Bailey, 2003; Sun et al., 2003). These results underscore the disease relevance of our observation of the regulation of PAR-1 and tau phosphorylation by osmotic stress. Future studies are needed to address the molecular mechanisms by which osmotic stress may regulate PAR-1 activation and tau phosphorylation.
One of the advantages of studying disease mechanisms using the fly models is that the powerful genetic tools available in Drosophila can be applied. For example, the involvement of LKB1 and PAR-1 in tau phosphorylation is supported by both overexpression and loss-of-function studies. In our previous studies (Nishimura et al., 2004), we have shown that overexpression of PAR-1 promotes tau phosphorylation at 12E8 sites, whereas loss of PAR-1 function abolished tau phosphorylation at these sites. Here, we show that overexpression of LKB1 also promotes tau phosphorylation at the 12E8 sites, whereas loss of LKB1 function attenuated 12E8 site phosphorylation. These loss-of-function and overexpression studies thus strongly support the physiological roles of PAR-1 and LKB1 in tau phosphorylation. Because of the lack of appropriate vertebrate animal models of LKB1 or MARK-mediated neurodegeneration, it is not clear whether the signaling cascades described in this study are directly relevant to disease conditions in vertebrate animal models or human patients. Interestingly, there are reports of aberrant phosphorylation of tau at MARK/PAR-1 sites in APP and tau double transgenic models (Lewis et al., 2001; Perez et al., 2005), although no genetic test has been done to demonstrate that mammalian LKB1 or MARK are critically involved in this process. There are also reports of changes of activation states of stress-induced kinases and levels of tau phosphorylation in neurons surrounding amyloid plaques in APP transgenic mice (Ferrer et al., 2005). It would be interesting to determine whether the LKB1/PAR-1/tau phosphorylation cascade plays a role in linking the APP and tau pathology in this experimental setting. It would also be important to extend the findings reported here to human postmortem tissues.
The stress responsiveness of the LKB1/PAR-1 pathway was corroborated by observations in rodents that LKB1 and MARK were upregulated during focal cerebral ischemia and that MARK could be activated by electroconvulsive shock (Schneider et al., 2004; Jeon et al., 2005). It is possible that LKB1 may occupy a nodal position through which various physiological or pathological signals are integrated to regulate PAR-1/MARK and tau. The stress responsiveness of the LKB1/PAR-1/tau pathway suggests that it may serve an adaptive or even protective role under normal physiological conditions. Under pathological conditions, however, excessive or prolonged activation of this pathway becomes detrimental. This would be analogous to the opposing effects of transient versus prolonged activation of Cdk5 on synaptic plasticity and hippocampus-dependent memory (Fischer et al., 2005). Additional characterization of the signaling processes that regulate LKB1 and PAR-1 should enhance our understanding of the molecular events in the disease process and identify novel therapeutic targets.
Footnotes
This work was supported by the McKnight and Beckman Foundations and National Institutes of Health Grant NS043167 (B.L.). We thank Dr. Su Guo for reading this manuscript and members of the Lu laboratory for discussions. We are grateful to Dr. Zhinong Huang for the GST-PAR-1 cDNA constructs, Drs. Larry Goldstein and Mel Feany and the Bloomington Drosophila Stock Center for fly stocks, Dr. Helen McNeil for sharing the LKB1 antibody, Dr. Peter Seubert for the 12E8 antibody, Drs. Ruben Shaw and Dario Alessi for LKB1 and STRAD cDNA clones, and Drs. Stephan Gehrke and Jon Kosek for help with scanning electron microscopy.
References
- Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol (Berl) 2002;103:26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- Biernat J, Wu YZ, Timm T, Zheng-Fischhofer Q, Mandelkow E, Meijer L, Mandelkow EM. Protein kinase MARK/PAR-1 is required for neurite outgrowth and establishment of neuronal polarity. Mol Biol Cell. 2002;13:4013–4028. doi: 10.1091/mbc.02-03-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brajenovic M, Joberty G, Kuster B, Bouwmeester T, Drewes G. Comprehensive proteomic analysis of human Par protein complexes reveals an interconnected protein network. J Biol Chem. 2004;279:12804–12811. doi: 10.1074/jbc.M312171200. [DOI] [PubMed] [Google Scholar]
- Chin JY, Knowles RB, Schneider A, Drewes G, Mandelkow EM, Hyman BT. Microtubule-affinity regulating kinase (MARK) is tightly associated with neurofibrillary tangles in Alzheimer brain: a fluorescence resonance energy transfer study. J Neuropathol Exp Neurol. 2000;59:966–971. doi: 10.1093/jnen/59.11.966. [DOI] [PubMed] [Google Scholar]
- Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- Doglio LE, Kanwar R, Jackson GR, Perez M, Avila J, Dingwall C, Dotti CG, Fortini ME, Feiguin F. gamma-cleavage-independent functions of presenilin, nicastrin, and Aph-1 regulate cell-junction organization and prevent tau toxicity in vivo. Neuron. 2006;50:359–375. doi: 10.1016/j.neuron.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- Elbert M, Rossi G, Brennwald P. The yeast par-1 homologs kin1 and kin2 show genetic and physical interactions with components of the exocytic machinery. Mol Biol Cell. 2005;16:532–549. doi: 10.1091/mbc.E04-07-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Gomez-Isla T, Puig B, Freixes M, Ribe E, Dalfo E, Avila J. Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer's disease and tauopathies. Curr Alzheimer Res. 2005;2:3–18. doi: 10.2174/1567205052772713. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai LH. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48:825–838. doi: 10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Foster NL, Wilhelmsen K, Sima AA, Jones MZ, D'Amato CJ, Gilman S. Frontotemporal dementia and parkinsonism linked to chromosome 17: a consensus conference. Conference participants. Ann Neurol. 1997;41:706–715. doi: 10.1002/ana.410410606. [DOI] [PubMed] [Google Scholar]
- Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- Greenberg SG, Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci USA. 1990;87:5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena S, Goldstein LS. Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron. 2001;32:389–401. doi: 10.1016/s0896-6273(01)00496-2. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidary G, Fortini ME. Identification and characterization of the Drosophila tau homolog. Mech Dev. 2001;108:171–178. doi: 10.1016/s0925-4773(01)00487-7. [DOI] [PubMed] [Google Scholar]
- Hurov JB, Watkins JL, Piwnica-Worms H. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr Biol. 2004;14:736–741. doi: 10.1016/j.cub.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VM. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24:751–762. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- Jackson GR, Wiedau-Pazos M, Sang TK, Wagle N, Brown CA, Massachi S, Geschwind DH. Human wild-type tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron. 2002;34:509–519. doi: 10.1016/s0896-6273(02)00706-7. [DOI] [PubMed] [Google Scholar]
- Jenkins SM, Zinnerman M, Garner C, Johnson GV. Modulation of tau phosphorylation and intracellular localization by cellular stress. Biochem J. 2000;345:263–270. [PMC free article] [PubMed] [Google Scholar]
- Jeon S, Kim YS, Park J, Bae CD. Microtubule affinity-regulating kinase 1 (MARK1) is activated by electroconvulsive shock in the rat hippocampus. J Neurochem. 2005;95:1608–1618. doi: 10.1111/j.1471-4159.2005.03505.x. [DOI] [PubMed] [Google Scholar]
- Johnson GV, Bailey CD. The p38 MAP kinase signaling pathway in Alzheimer's disease. Exp Neurol. 2003;183:263–268. doi: 10.1016/s0014-4886(03)00268-1. [DOI] [PubMed] [Google Scholar]
- Kalidas S, Smith DP. Novel genomic cDNA hybrids produce effective RNA interference in adult Drosophila. Neuron. 2002;33:177–184. doi: 10.1016/s0896-6273(02)00560-3. [DOI] [PubMed] [Google Scholar]
- Lee VM, Balin BJ, Otvos L, Jr, Trojanowski JQ. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JJ, Hernandez F, Gomez-Ramos P, Moran MA, Hen R, Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SG, St Johnston D. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature. 2003;421:379–384. doi: 10.1038/nature01296. [DOI] [PubMed] [Google Scholar]
- Nishimura I, Yang Y, Lu B. PAR-1 kinase plays an initiator role in a temporally ordered phosphorylation process that confers tau toxicity in Drosophila. Cell. 2004;116:671–682. doi: 10.1016/s0092-8674(04)00170-9. [DOI] [PubMed] [Google Scholar]
- Noble W, Olm V, Takata K, Casey E, Mary O, Meyerson J, Gaynor K, LaFrancois J, Wang L, Kondo T, Davies P, Burns M, Veeranna, Nixon R, Dickson D, Matsuoka Y, Ahlijanian M, Lau LF, Duff K. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron. 2003;38:555–565. doi: 10.1016/s0896-6273(03)00259-9. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Perez M, Ribe E, Rubio A, Lim F, Moran MA, Ramos PG, Ferrer I, Isla MT, Avila J. Characterization of a double (amyloid precursor protein-tau) transgenic: tau phosphorylation and aggregation. Neuroscience. 2005;130:339–347. doi: 10.1016/j.neuroscience.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–825. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- Probst A, Gotz J, Wiederhold KH, Tolnay M, Mistl C, Jaton AL, Hong M, Ishihara T, Lee VM, Trojanowski JQ, Jakes R, Crowther RA, Spillantini MG, Burki K, Goedert M. Axonopathy and amyotrophy in mice transgenic for human four-repeat tau protein. Acta Neuropathol (Berl) 2000;99:469–481. doi: 10.1007/s004010051148. [DOI] [PubMed] [Google Scholar]
- Schneider A, Laage R, von Ahsen O, Fischer A, Rossner M, Scheek S, Grunewald S, Kuner R, Weber D, Kruger C, Klaussner B, Gotz B, Hiemisch H, Newrzella D, Martin-Villalba A, Bach A, Schwaninger M. Identification of regulated genes during permanent focal cerebral ischaemia: characterization of the protein kinase 9b5/MARKL1/MARK4. J Neurochem. 2004;88:1114–1126. doi: 10.1046/j.1471-4159.2003.02228.x. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer J, Rayter S, Young N, Elliott R, Ashworth A, Smith D. Regulation of the Wnt signalling component PAR1A by the Peutz-Jeghers syndrome kinase LKB1. Oncogene. 2003;22:4752–4756. doi: 10.1038/sj.onc.1206669. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A, Liu M, Nguyen XV, Bing G. P38 MAP kinase is activated at early stages in Alzheimer's disease brain. Exp Neurol. 2003;183:394–405. doi: 10.1016/s0014-4886(03)00180-8. [DOI] [PubMed] [Google Scholar]
- Sun TQ, Lu B, Feng JJ, Reinhard C, Jan YN, Fantl WJ, Williams LT. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nat Cell Biol. 2001;3:628–636. doi: 10.1038/35083016. [DOI] [PubMed] [Google Scholar]
- Timm T, Li XY, Biernat J, Jiao J, Mandelkow E, Vandekerckhove J, Mandelkow EM. MARKK, a Ste20-like kinase, activates the polarity-inducing kinase MARK/PAR-1. EMBO J. 2003;22:5090–5101. doi: 10.1093/emboj/cdg447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, Feany MB. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- Zhu X, Rottkamp CA, Hartzler A, Sun Z, Takeda A, Boux H, Shimohama S, Perry G, Smith MA. Activation of MKK6, an upstream activator of p38, in Alzheimer's disease. J Neurochem. 2001;79:311–318. doi: 10.1046/j.1471-4159.2001.00597.x. [DOI] [PubMed] [Google Scholar]