Abstract
Numerous studies have established a pivotal role for Aβ42 in Alzheimer's disease (AD) pathogenesis. In contrast, although Aβ40 is the predominant form of amyloid β (Aβ) produced and accumulates to a variable degree in the human AD brain, its role in AD pathogenesis has not been established. It has generally been assumed that an increase in Aβ40 would accelerate amyloid plaque formation in vivo. We have crossed BRI-Aβ40 mice that selectively express high levels of Aβ40 with both Tg2576 (APPswe, K670N+M671L) mice and BRI-Aβ42A mice expressing Aβ42 selectively and analyzed parenchymal and cerebrovascular Aβ deposition in the bitransgenic mice compared with their singly transgenic littermates. In the bitransgenic mice, the increased steady-state levels of Aβ40 decreased Aβ deposition by 60–90%. These results demonstrate that Aβ42 and Aβ40 have opposing effects on amyloid deposition: Aβ42 promotes amyloid deposition but Aβ40 inhibits it. In addition, increasing Aβ40 levels protected BRI-Aβ40/Tg2576 mice from the premature-death phenotype observed in Tg2576 mice. The protective properties of Aβ40 with respect to amyloid deposition suggest that strategies that preferentially target Aβ40 may actually worsen the disease course and that selective increases in Aβ40 levels may actually reduce the risk for development of AD.
Keywords: Alzheimer's disease, amyloid β, aggregation, premature death, transgenic mice, cerebral amyloid angiopathy
Introduction
Accumulation of amyloid β (Aβ) is hypothesized to initiate a pathogenic cascade that eventually results in Alzheimer's disease (AD) (Hardy and Selkoe, 2002). Sequential amyloid β precursor protein (APP) processing by β-secretase and γ-secretase produces a major Aβ species, Aβ1-40, and a number of minor species, including Aβ1-42 (Steiner and Haass, 2000). Studies of AD-causing mutations in APP, presenilin 1 (PSEN1), and presenilin 2 (PSEN2) genes demonstrate that the vast majority of these mutations alter APP processing in a manner that either increases the absolute or relative levels of Aβ42 (Price et al., 1998). In vitro, Aβ42 aggregates into amyloid much more rapidly than Aβ40 (Caughey and Lansbury, 2003). In vivo, Aβ42 is the predominant form of Aβ that accumulates in the AD brain and is essential for seeding Aβ deposition (Younkin, 1998; Fryer and Holtzman, 2005).
Previously, we have described transgenic mice that selectively express either Aβ1-40 or Aβ1-42 in the secretory pathway without human APP overexpression by fusing Aβ40 or Aβ42 peptide sequences to the C-terminal end of the BRI protein (McGowan et al., 2005). BRI-Aβ40 mice expressing high levels of Aβ40 had no pathology at any age. In contrast, BRI-Aβ42A mice expressing ∼10-fold lower levels of Aβ42 developed amyloid deposits in the cerebellum as early as 3 months (McGowan et al., 2005). These data suggest that Aβ42, but not Aβ40, is sufficient to drive amyloid deposition in vivo. Such studies demonstrate a key role for Aβ42 in initiating AD pathology but do not provide a great deal of insight into the role that Aβ40 plays in AD pathogenesis.
Aβ40 does accumulate in the AD brain, but the extent of Aβ40 accumulation relative to Aβ42 is highly variable and is usually attributed to accumulation of Aβ40 in cerebral vessels (Gravina et al., 1995). Given that Aβ40 is the predominant form of Aβ produced and that therapeutic strategies targeting Aβ typically do not selectively target any single Aβ species, we have conducted experiments to directly examine the contribution of Aβ40 to amyloid deposition in vivo. We bred BRI-Aβ40 mice with both Tg2576 mice and BRI-Aβ42A mice. The bitransgenic mice from crossbreedings had increased steady-state soluble Aβ levels but significantly less parenchymal and vascular amyloid deposition compared with their respective single transgenic Tg2576 or BRI-Aβ42A littermates. These results demonstrate Aβ40 has anti-amyloidogenic effect in vivo.
Materials and Methods
Generation of mice.
Transgenic mice expressing BRI-Aβ40 and BRI-Aβ42 under the control of mouse prion promoter were generated as described previously (McGowan et al., 2005). Hemizygous BRI-Aβ40 mice or BRI-Aβ42A mice were crossed with hemizygous Tg2576 (APPswe) mice (Hsiao et al., 1996). To generate the bitransgenic BRI-Aβ40/BRI-Aβ42 mice, hemizygous BRI-Aβ40 mice were mated with hemizygous BRI-Aβ42A or BRI-Aβ42B mice. BRI-Aβ mice were maintained on a B6/C3 hybrid background, and Tg2576 mice were maintained on a B6/SJL background. All animal procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee.
Quantification of parenchymal amyloid deposition.
Hemibrains were immersion fixed in 10% formalin and processed for paraffin embedding. Brain tissue sections (5 μm) were immunostained with anti-total Aβ antibody (Ab) (33.1.1, 1:1000; a gift from T. Golde, Mayo Clinic) on a Dako (Glostrup, Denmark) autostainer. Sections were counterstained with hematoxylin. Six sections per brain through the hippocampus, piriform cortex (bregma, −1.70 to −2.80 mm), or cerebellum (paraflocculus, crus ansiform, and simple lobules; bregma, −5.40 to −6.36 mm) were used for quantification (n = 5–7 mice per genotype at each age group). The Aβ plaque burden was determined using MetaMorph software (Molecular Devices, Palo Alto, CA). For quantification of cored plaques, serial sections of those analyzed for Aβ burden were stained with thioflavine S (ThioS), and the number of ThioS-positive plaques in the hippocampus, entorhinal/piriform cortex, or the cerebellum was counted. All of the above analyses were performed in a blinded manner.
Quantification of vascular amyloid deposition.
For quantification of cerebral amyloid angiopathy (CAA), 5 μm paraffin-embedded sections at 30 μm intervals through the parietal or cerebellar cortex leptomeninges were immunostained with biotinylated-Ab9 antibody (anti-Aβ1-16, 1:500; a gift from T. Golde) overnight at 4°C (n = 5–7 mice per genotype at each age group, n = 6 sections per mouse). Positively stained blood vessels were visually assessed using modified Vonsattel's scoring system as described previously (Greenberg and Vonsattel, 1997). The CAA severity score was calculated by multiplying the number of CAA vessels with the CAA severity grade.
Aβ sandwich ELISA.
For brain Aβ ELISAs, forebrain and hindbrain Aβ levels were determined independently, and the olfactory bulb was excluded from analysis. For plasma Aβ analysis, blood was collected in EDTA-coated tubes after cardiac puncture. Blood samples were centrifuged at 3000 rpm for 10 min at 4°C, and the plasma was aliquoted and stored at −80°C until used. Aβ levels were determined by end-specific sandwich ELISAs using Ab9 (anti-Aβ1-16 Ab) as the capture Ab for Aβ40, 13.1.1–HRP (anti-Aβ35-40 Ab) as the detection Ab for Aβ40, 2.1.3 (anti-Aβ35-42 Ab) as the capture Ab for Aβ42, and Ab9–HRP as the detection Ab for Aβ42, as described previously (Kawarabayashi et al., 2001) (n = 5–7 mice per genotype at each age group). Aβ levels were normalized to our previous results using the same sets of mice as internal controls to minimize potential ELISA variability.
Survival analysis.
Survival rates were analyzed using Kaplan–Meier methods. Holm–Sidak methods (post hoc) were used for all pairwise multiple comparison tests (SigmaStat 3.0; Systat Software, San Jose, CA). The extraneous deaths were censored. All comparisons were made between littermates to limit any potentially confounding effects from background strain differences.
Western blotting.
Snap-frozen forebrain samples were homogenized in radioimmunoprecipitation assay (RIPA) buffer (Boston BioProducts, Worcester, MA) with 1% protease inhibitor mixture (Roche, Indianapolis, IN). The homogenate was centrifuged at 100,000 × g for 1 h at 4°C. Protein concentration in supernatants was determined using the BCA protein assay (Pierce, Woburn, MA). Protein samples (20 μg) were run on Bis-Tris 12% XT gels or Bis-Tris 4–12% XT gels (Bio-Rad, Hercules, CA) and transferred to 0.2 μm nitrocellose membranes. Blots were microwaved for 2 min in 0.1 m PBS twice and probed with Ab 82E1 (anti-Aβ1-16, 1:1000; IBL, Gunma, Japan) and CT20 (anti-APP C-terminal 20 amino acids, 1:1000; a gift from T. Golde). Blots were stripped and reprobed with anti β-actin (1:1000; Sigma, St. Louis, MO) as a loading control. Relative band intensity was measured using ImageJ software.
In vitro Aβ aggregation assay.
Synthetic Aβ40 or Aβ42 peptides (Bachem, Torrance, CA) were dissolved in DMSO and diluted in TBS at various molar ratios as indicated. Aβ mixtures were either directly used for analysis or incubated for 2 h at 37°C without shaking. Mixtures were run on 4–20% Tris-HCl gels under nondenaturing conditions and transferred to a 0.4 μm polyvinylidene difluoride membrane as described previously (Klug et al., 2003). The blot was probed with 1:1000 Ab9.
Statistical analysis.
Aβ levels, amyloid plaque burden, and CAA severity were analyzed by using ANOVA with the post hoc Holm–Sidak multiple comparison test or two-tailed Student's t test (SigmaStat 3.0). If the data set did not meet the parametric test assumptions, either the Kruskal–Wallis test followed by the post hoc Dunn's multiple comparison or the Mann–Whitney rank sum test was performed (SigmaStat 3.0). To test whether the Aβ levels in the bitransgenic mice were consistent with an additive sum of Aβ levels in the single transgenic littermates, a multiple linear regression with no intercept test was used (StatsDirect 2.5.6). All comparisons were made between littermates. Variance was reported as SEM.
Results
Aβ40 inhibits amyloid deposition in bitransgenic BRI-Aβ40/Tg2576 mice
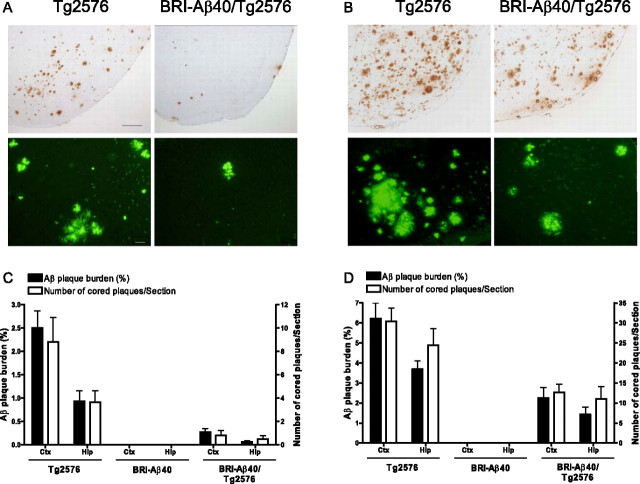
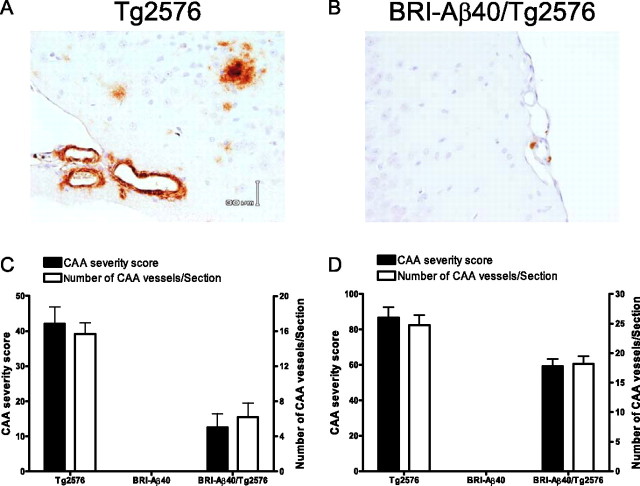
We crossed hemizygous BRI-Aβ40 mice that produce only Aβ40 with hemizygous APPswe (Tg2576) mice overexpressing a normal profile of human Aβ peptides (Hsiao et al., 1996), generating offspring with nontransgenic (non-Tg), BRI-Aβ40, Tg2576, and BRI-Aβ40/Tg2576 genotypes. The extent of parenchymal and vascular Aβ deposition in an aging series of littermates (8–20 months of age) was analyzed by biochemical and immunohistochemical methods. The forebrain and hindbrain were analyzed independently because of regional differences in Aβ production between BRI-Aβ mice and Tg2576 mice (McGowan et al., 2005). As noted previously, BRI-Aβ40 mice did not develop amyloid pathology at any age (Fig. 1 C,D) (McGowan et al., 2005). Surprisingly, BRI-Aβ40/Tg2576 mice had dramatic (60–90%) reductions in both immunohistochemical Aβ loads and ThioS-positive plaques compared with age-matched Tg2576 littermates (Fig. 1). Likewise, biochemical analyses of Aβ levels showed 60–80% reductions in RIPA-insoluble, formic acid (FA)-extractable Aβ40 and Aβ42 levels in the forebrain and hindbrain of the bitransgenic mice (Fig. 2), although steady-state soluble Aβ (Aβ40 plus Aβ42) levels were increased by approximately twofold to fourfold in bitransgenic mice compared with Tg2576 littermates (see Fig. 5 A,B). Leptomeningeal CAA, with a typical concentric Aβ immunostaining pattern, was also reduced in BRI-Aβ40/Tg2576 mice compared with Tg2576 littermates (Fig. 3). The CAA severity score and the number of CAA-affected leptomeningeal vessels per section were decreased by ∼60 and ∼30% at 15 and 20 months of age, respectively (Fig. 3 C,D).
Figure 1.
Decreased amyloid deposition in BRI-Aβ40/Tg2576 mice. A, B, Representative entorhinal/piriform cortex sections from Tg2576 and BRI-Aβ40/Tg2576 mice at 15 (A) and 20 (B) months of age were immunostained with 33.1.1 (anti-Aβ1-16; top panels) or stained with ThioS (bottom panels). Scale bars: (in A) top panels, 200 μm; bottom panels, 50 μm. C, D, The amyloid plaque burden and number of ThioS-positive cored plaques in the entorhinal/piriform cortex (Ctx) and hippocampus (Hip) at 15 (C) and 20 (D) months were quantified. There was a significant decrease in both the Aβ plaque burden and number of ThioS-positive plaques in BRI-Aβ40/Tg2576 mice compared with Tg2576 littermates (p < 0.05). For statistical analysis, see Materials and Methods.
Figure 2.
Decreased Aβ accumulation in BRI-Aβ40/Tg2576 mice. A, RIPA-insoluble, FA-extractable Aβ40 and Aβ42 levels in the forebrain of BRI-Aβ40/Tg2576 were significantly reduced compared with Tg2576 littermates at all ages (Aβ40 levels at p = 0.01, p < 0.001, and p < 0.001 at 11, 15, and 20 months, respectively, and Aβ42 levels at p < 0.001 at all age). There was no evidence for accumulation of insoluble Aβ in the BRI-Aβ40 mice at any age. B, Because Tg2576 have only minimal accumulation of FA-extractable Aβ up to 15 months of age, comparisons of FA–Aβ levels in BRI-Aβ40 × Tg2576 progeny were determined at 20 months of age. There was an ∼80% reduction in FA–Aβ42 in the hindbrain of bitransgenic BRI-Aβ40/Tg2576 mice compared with single transgenic Tg2576 littermates (p = 0.022 by rank sum test). Decreased FA–Aβ40 levels were also detected but did not reach statistical significance (p = 0.101 by rank sum test). M, Months.
Figure 5.
Steady-state RIPA-soluble brain Aβ levels and plasma Aβ levels in BRI-Aβ40/Tg2576 and BRI-Aβ40/BRI-Aβ42A mice before amyloid deposition. To ensure that there was no change in transgene expression levels or alteration in production of Aβ levels in any of the bigenic mice, RIPA-soluble Aβ levels in forebrain, hindbrain, and plasma were analyzed by Aβ sandwich ELISAs. A–C, The levels of RIPA-soluble Aβ40 and Aβ42 in forebrain (A), hindbrain (B), and plasma (C) of BRI-Aβ40/Tg2576 mice were consistent with an additive sum of Aβ levels from their single transgenic littermates at 8 months of age (p > 0.1). D, E, Bitransgenic BRI-Aβ40/BRI-Aβ42A mice had comparable Aβ40 and Aβ42 levels in hindbrain (D) and plasma (E) compared with BRI-Aβ40 and BRI-Aβ42A single transgenic littermates at 2.5 months of age, respectively (p > 0.1). For statistical analysis, see Materials and Methods.
Figure 3.
Increased Aβ40 levels reduce congophilic amyloid angiopathy. A, B, CAA in cortical leptomeningeal vessels immunostained with a biotinylated-Ab9 antibody was shown in Tg2576 (A) and BRI-Aβ40/Tg2576 (B) mice at 15 months of age. C, D, At both 15 (C) and 20 (D) months of age, there was a decrease in both the CAA severity score and number of CAA-affected vessels in BRI-Aβ40/Tg2576 mice compared with Tg2576 age-matched littermates (p < 0.05; t test).
Aβ40 inhibits amyloid deposition in bitransgenic BRI-Aβ40/BRI-Aβ42A mice
Next we examined Aβ deposition in bitransgenic BRI-Aβ40/BRI-Aβ42A mice produced by crossing hemizygous BRI-Aβ40 mice with hemizygous BRI-Aβ42A mice. BRI-Aβ42A mice initially develop amyloid deposition in the cerebellum at ∼3 months of age, whereas forebrain pathology was consistently observed only after ∼12 months of age (McGowan et al., 2005). Extensive premature death observed in BRI-Aβ40/BRI-Aβ42A mice limited rigorous pathological analyses to an 8 month time point (see Fig. 7 A). At this age, Aβ plaque burden, the number of ThioS-positive cored plaques in the cerebellum, and FA-fraction Aβ42 levels were significantly decreased by ∼75% in BRI-Aβ40/BRI-Aβ42A mice (Fig. 4), although steady-state soluble brain Aβ (Aβ40 plus Aβ42) levels were increased by ∼10-fold in bitransgenic mice compared with BRI-Aβ42A littermates (Fig. 5 D). BRI-Aβ40/BRI-Aβ42A mice also had markedly less CAA than BRI-Aβ42A littermates (Fig. 4 D). The results from BRI-Aβ40/BRI-Aβ42A mice confirmed our findings from BRI-Aβ40/Tg2576 mice, indicating Aβ40 inhibited amyloid deposition in vivo.
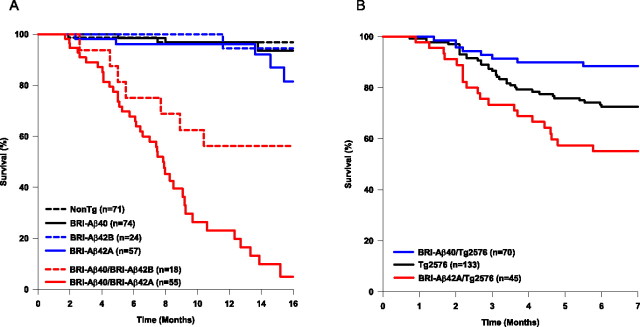
Figure 7.
Aβ modulates premature death. A, Survival rates for progeny of BRI-Aβ40 mice bred with BRI-Aβ42A mice (highest-expressing Aβ42 line) and BRI-Aβ42B mice (lower-expressing Aβ42 line) were calculated using Kaplan–Meier methods. Bitransgenic BRI-Aβ40/BRI-Aβ42A had an accelerated mortality, whereas non-Tg, single transgenic BRI-Aβ40, and BRI-Aβ42A mice did not show any premature death (p < 0.01). BRI-Aβ40/BRI-Aβ42B mice had a decreased premature death rate compared with BRI-Aβ40/BRI-Aβ42A mice (p < 0.01). B, Kaplan–Meier survival curves for progeny of BRI-Aβ40 and BRI-Aβ42A crossed with Tg2576 mice. BRI-Aβ42A/Tg2576 mice had a significantly increased premature death rate compared with Tg2576 littermates (p < 0.05). Although BRI-Aβ40/Tg2576 only had 10% premature death at 7 months of age, Tg2576 mice had ∼30% early death (p < 0.01). There was no difference in survival rates for Tg2576 progeny from breeding with either BRI-Aβ40 mice or BRI-Aβ42A mice (p = 0.884), thus Tg2576 data were pooled. However, only littermates from breeding experiments were used for multiple comparison tests. For statistical analysis, see Materials and Methods.
Figure 4.
Decreased amyloid deposition in BRI-Aβ40/BRI-Aβ42A mice. A, Serial cerebellar sections from 8-month-old mice were immunostained with 33.1.1 (anti-Aβ1-16; top panels) and stained with ThioS (bottom panels). Scale bar: top panels, 200 μm; bottom panels, 50 μm. B, Both the Aβ plaque burden (p = 0.007; t test) and the number of ThioS-positive plaques (p < 0.001; t test) were significantly reduced in BRI-Aβ40/BRI-Aβ42A mice compared with age-matched BRI-Aβ42A littermates. C, Similarly, RIPA-insoluble, FA-extractable Aβ42 levels in the cerebellum of BRI-Aβ40/BRI-Aβ42A mice were markedly lower compared with BRI-Aβ42A littermates (p = 0.01; t test). D, Both the severity of CAA and the number of CAA-affected vessels in cerebellar leptomeninges were reduced in BRI-Aβ40/BRI-Aβ42A mice compared with BRI-Aβ42A mice (p < 0.001; rank sum test). There was no amyloid pathology, CAA, or accumulation of RIPA-insoluble FA–Aβ in BRI-Aβ40 mice.
No alteration in steady-state soluble Aβ levels before amyloid deposition
Because reduced Aβ deposition in BRI-Aβ40/Tg2576 and BRI-Aβ40/BRI-Aβ42A mice might be attributable to effects of transgene expression and/or Aβ production and because these would be reflected by changes in steady-state Aβ levels, we measured RIPA-soluble brain Aβ levels and plasma Aβ levels before the significant accumulation of Aβ in the brain. RIPA-soluble Aβ levels in the brain and plasma of BRI-Aβ40/Tg2576 mice were consistent with an additive sum of soluble Aβ levels of their single transgenic littermates at 8 months of age (Fig. 5 A–C). Indeed, these data demonstrate that the large decrease in amyloid deposition is attributable to a doubling of Aβ40 levels in BRI-Aβ40/Tg2576 mice (Fig. 5 A). Similarly, BRI-Aβ40/BRI-Aβ42A mice had no significant differences in soluble Aβ40 and Aβ42 levels compared with BRI-Aβ40 and BRI-Aβ42A littermates, respectively (Fig. 5 D,E). These results confirm that the reduced amyloid deposition observed in BRI-Aβ40/Tg2576 mice and BRI-Aβ40/BRI-Aβ42A mice was not caused by decreased steady-state soluble Aβ levels.
No alteration in APP processing in bitransgenic BRI-Aβ40/Tg2576 mice
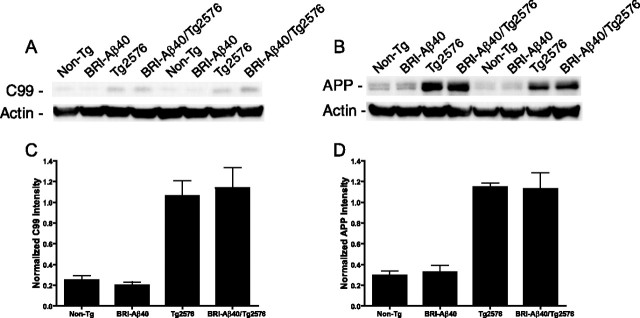
Recently, an interaction between BRI and wild-type APP (APPwt) was reported in vitro. The binding of BRI to APPwt resulted in decreased Aβ and increased C99 levels, with inconclusive effects of BRI on full-length APP and total secreted APP levels (Fotinopoulou et al., 2005; Matsuda et al., 2005). As noted above, we saw no evidence for altered production of Aβ in the crossed mice. To further investigate the possible inhibition of APP processing by BRI-Aβ40 protein in the transgenic mice, we examined C99 and APP protein levels by Western blot analysis (Fig. 6 A,B). Steady-state C99 and APP protein levels were unchanged between the Tg2576 mice and BRI-Aβ40/Tg2576 mice (Fig. 6 C,D). These results together with the data from the bitransgenic BRI-Aβ40/BRIAβ42 mice indicate that the reduced amyloid burden and decreased accumulation of FA-fraction Aβ are not attributable to interference in APP processing by the BRI transgene.
Figure 6.
No alteration in amyloidogenic APP processing in BRI-Aβ40/Tg2576 mice. RIPA-soluble forebrain extracts from 8-month-old BRI-Aβ40/Tg2576, Tg2576, BRI-Aβ40, and non-Tg littermates were analyzed by Western blotting. A, B, Blots were probed with 82E1 (anti-Aβ1-16) (A) or CT20 (anti-APP C-terminal 20 amino acids) (B), stripped, and reprobed with anti-β actin to assess loading. C, D, The relative levels of C99 (C) and APP (D) after normalization to β-actin were equivalent between Tg2576 mice and BRI-Aβ40/Tg2576 mice, indicating that there was no alteration in the processing of APP in the bitransgenic mice (p > 0.1). C99 and APP levels were equivalent between non-Tg and BRI-Aβ40 mice that express only endogenous mouse APP (p > 0.1). n = 5 mice per genotype. For statistical analysis, see Materials and Methods.
Aβ modulates premature-death phenotype
In contrast to many lines of mutant APP mice that exhibit a premature-death phenotype (Moechars et al., 1999b; Leissring et al., 2003), BRI-Aβ40 or BRI-Aβ42 mice did not have accelerated mortality (Fig. 7 A). Surprisingly, BRI-Aβ40/BRI-Aβ42A mice had a progressive premature-death phenotype that approached 100% death by 16 months of age (p < 0.001; compared with singly transgenic and non-Tg littermates) (Fig. 7 A). When BRI-Aβ40 mice were crossed with a second line of BRI-Aβ42B mice that express lower levels of Aβ42 (∼50% less than BRI-Aβ42A line), bitransgenic BRI-Aβ40/BRI-Aβ42B mice still died prematurely, although at a slower rate than BRI-Aβ40/BRI-Aβ42A mice. BRI-Aβ42A/Tg2576 mice exhibited an enhanced premature-death phenotype (∼50%) relative to Tg2576 littermates (∼30%), whereas BRI-Aβ40/Tg2576 mice had a significantly reduced death rate (∼10%) compared with their Tg2576 littermates at 7 months of age (Fig. 7 B).
Aβ40 inhibits Aβ42 aggregation in vitro
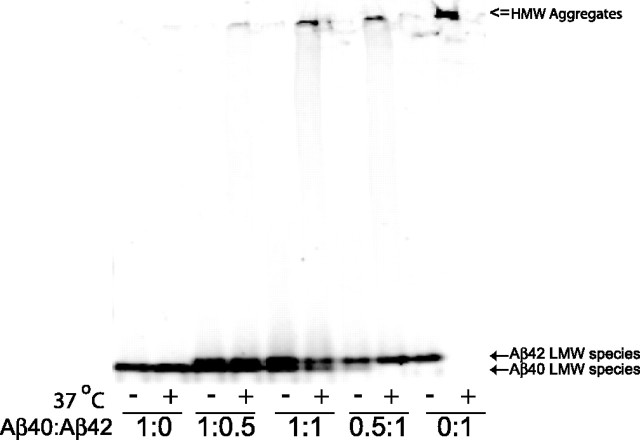
To understand the underlying mechanism by which Aβ40 reduced amyloid deposition in BRI-Aβ40/Tg2576 and BRI-Aβ40/BRI-Aβ42A mice, we determined whether Aβ40 could directly inhibit Aβ42 fibrillogenesis in vitro using an Aβ aggregation assay. When freshly prepared Aβ42 mixtures were incubated for 2 h, all Aβ42 aggregated as high molecular complexes (Fig. 8). However, addition of Aβ40 to Aβ42 preparation led to a reduction in high molecular complex formation, and most Aβ42 still remained as low molecular weight species (Fig. 8). These nondenaturing electrophoresis results indicate that Aβ40 directly inhibits the Aβ42 aggregation process in vitro.
Figure 8.
Aβ40 inhibits Aβ42 aggregation in vitro. Synthetic Aβ40 or Aβ42 peptides were mixed at various molar ratios (1:1.5 and 0.5:0.75 μm). Aβ mixtures were either directly used for analysis (indicated by “−”) or incubated for 2 h at 37°C (indicated by “=”) and electrophoresed on 4–20% Tris-HCl gels under nondenaturing conditions. The blot was probed with Ab9. After a 2 h incubation, the Aβ42-only preparation (Aβ40:Aβ42 ratio; 0:1) formed very high molecular weight (HMW) aggregates (indicated by “⇐” at the top of blot) without any low molecular weight LMW species, whereas the Aβ40 only preparation (Aβ40:Aβ42 ratio; 1:0) remained as a LMW species (indicated by ← at the bottom of blot). When Aβ40 was mixed with Aβ42 (Aβ40:Aβ42 ratio; 0.5:1 and 1:1), Aβ40 inhibited the formation of HMW Aβ42 aggregates, and the vast majority of Aβ42 remained as LMW species (indicated by ← at the bottom of the blot).
Discussion
Our surprising results unequivocally demonstrate that Aβ40 has a strong anti-amyloidogenic effect in vivo; increasing Aβ40 levels in the brain of Tg2576 or BRI-Aβ42A mice protected against amyloid pathology. Moreover, the magnitude of this effect is quite unexpected: approximately twofold increases of Aβ40 levels in the forebrain of the BRI-Aβ40/Tg2576 mice had a lifelong inhibitory effect on Aβ deposition ranging from ∼80% reduction at 11 months to ∼50% at 20 months, compared with Aβ deposition in Tg2576 littermates. By inference, decreasing Aβ40 levels should increase amyloid pathology. Several studies do support the notion that decreasing Aβ40 levels exacerbates the AD phenotype. Decreases in Aβ40, without an increase in Aβ42, have been associated with a subset of AD causing PSEN mutations and the APPV715M mutation (Ancolio et al., 1999; Bentahir et al., 2006; Kumar-Singh et al., 2006). In addition, several transgenic modeling studies support the notion that Aβ40 may be protective. Small reductions in brain Aβ40 levels, with no change in Aβ42 levels, resulting from expression of an artificial exon 10 deletion mutant PSEN1 transgene were also associated with exacerbated amyloid plaque pathology in Tg2576 mice (Deng et al., 2006). Finally, results from transgenic mice expressing wild-type and various mutant forms of APP also suggest that increased Aβ40 levels might reduce amyloid deposition (Mucke et al., 2000).
Because these studies rely on comparisons of Aβ deposition between wild-type and mutant APP transgenic mice or APP mice crossed with wild-type and artificial mutant PSEN transgenic mice, there are numerous confounds that prevent definitive assertions regarding the role of Aβ40. Mutations in APP and PSEN can alter production of not only Aβ42 but also other Aβ peptides (e.g., Aβ1-38) and both the levels of APP processing derivatives and the subcellular localization of processing. In addition, mutations in PSEN can have a variety of effects on protein trafficking, clearance, and intracellular signaling (Koo and Kopan, 2004; Zhang et al., 2006). These complicating factors are mostly avoided in our study by using the BRI-Aβ fusion system. Selective expression of Aβ1-40 decreased Aβ deposition in Tg2576 mice, whereas selective expression of Aβ1-42 in Tg2576 mice had the exact opposite effect on the pathology of plaques (McGowan et al., 2005).
Although there is much debate regarding the role of amyloid in AD pathogenesis (Le et al., 2001; Caughey and Lansbury, 2003; D'Amore et al., 2003; Lombardo et al., 2003; Tsai et al., 2004), an increasing body of evidence suggests that soluble oligomeric and protofibrillar Aβ species, such as Aβ*56, may cause the synaptic dysfunction and memory deficits in mice (Klein et al., 2004; Cleary et al., 2005; Glabe, 2006; Lesne et al., 2006). Therefore, the effect of increasing Aβ40 levels on oligomer formation and behavioral abnormalities in Tg2576 mice requires additional investigation.
There are several in vitro studies demonstrating that Aβ40 directly interferes with Aβ42 aggregation by delaying the Aβ42-mediated nucleation step at an early stage in the fibrillogenesis process (Snyder et al., 1994; Hasegawa et al., 1999; Zou et al., 2003). Additional studies have shown that wild-type Aβ40 can stabilize aggregation of Arctic mutant Aβ40 (E22G) (Lashuel et al., 2003). Our results from an in vitro Aβ aggregation assay confirm these previous studies, indicating that a direct inhibitory effect of Aβ40 on Aβ42 aggregation into amyloid is the most likely mechanism that accounts for our in vivo findings.
Increased Aβ40 levels in BRI-Aβ40/Tg2576 and BRI-Aβ40/BRI-Aβ42A mice led to a reduction in CAA, demonstrating that Aβ40 has anti-amyloidogenic effects on not only parenchymal but also vascular amyloid deposition. This is a surprising result given that Aβ40 is reported to be the predominant Aβ species deposited in vessels (Gravina et al., 1995). Although previous studies suggested that a higher ratio of Aβ40 to Aβ42 might promote the formation of CAA over parenchymal deposition (Herzig et al., 2004; Fryer and Holtzman, 2005; Fryer et al., 2005), in other studies, selective increases in Aβ42 were associated with more CAA (Van Dorpe et al., 2000; Samura et al., 2006; Van Dooren et al., 2006). Our previous studies showed that high-level production of wild-type Aβ40 by itself is not sufficient to cause CAA (McGowan et al., 2005). In any case, additional studies will be needed to understand the factors that promote Aβ40 accumulation within vessels in AD.
Premature death has been observed in many mutant APP transgenic mice on multiple background strains, although no one has been able to determine the cause of death (Hsiao et al., 1995; Moechars et al., 1999b; Leissring et al., 2003). BRI-Aβ40/BRI-Aβ42A mice had a progressive and ongoing death rate, whereas mortality in BRI-Aβ40/Tg2576 and BRI-Aβ42A/Tg2576 mice stabilized after 6 months of age. Premature death occurs well before plaque deposition in BRI-Aβ40/BRI-Aβ42A mice, implying that early high mortality is not directly associated with plaque formation as reported previously (Moechars et al., 1999a,b; Leissring et al., 2003).
Bitransgenic BRI-Aβ40/Tg2576 mice had a significantly reduced premature death rate with a concomitant decrease in Aβ deposition compared with their Tg2576 littermates. Reduction in Aβ levels by increased α-secretase activity or by enhanced proteolysis of Aβ has been linked to prevention of high mortality in APP transgenic mice and Drosophila (Leissring et al., 2003; Etcheberrigaray et al., 2004; Finelli et al., 2004). Together, these studies provide indirect evidence that alterations in Aβ levels can modulate the premature-death phenotype, although one study suggests that this is not because of Aβ (Krezowski et al., 2004). Although it is difficult to completely exclude other transgene-related and genetic background effects, our data suggest that an interaction between Aβ40 and Aβ42 is required for the premature-death phenotype. However, the rate and extent of premature death are influenced by total levels of Aβ, the ratio of Aβ40 to Aβ42, and the genetic background.
The inhibition of amyloid deposition by Aβ40 may have critical implications for AD therapy. Our data support the strategy that selectively targeting Aβ42 by allosterically modulating γ-secretase may be preferential to nonselective inhibition of γ-secretase activity (Weggen et al., 2001; Eriksen et al., 2003; Lleo et al., 2004). Indeed, strategies that preferentially target Aβ40 production, as some γ-secretase inhibitors do, could exacerbate amyloid deposition. Notably, there are several other examples of anti-aggregation effects of homologous proteins. β-Synuclein inhibits α-synuclein aggregation in mice, and mouse tau may retard human tau aggregation (Rochet et al., 2000; Hashimoto et al., 2001; Andorfer et al., 2003). Thus, a common mechanism underlying many neurodegenerative diseases characterized by accumulation of misfolded proteins may be an imbalance between pro-amyloidogenic (i.e., Aβ42 and α-synuclein) and anti-amyloidogenic (i.e., Aβ40 and β-synuclein) proteins.
Footnotes
This work was supported by National Institute on Aging Grant RO1 AG022595 to E.M. Additional resources from the Mayo Foundation, provided by a gift from Robert and Clarice Smith, were used to support the Tg2576 mouse colony. We thank Dr. J. Crook and M. Heckman for advice on statistical analysis; Dr. T. Rosenberry for valuable discussion; F. Conkle and the Veterinary Medicine staff for animal maintenance; L. Rousseau, V. Phillips, and M. Castanedes-Casey for expert histology; and Drs. J. Eriksen and C. Zehr for Metamorph programming.
References
- Ancolio K, Dumanchin C, Barelli H, Warter JM, Brice A, Campion D, Frebourg T, Checler F. Unusual phenotypic alteration of beta amyloid precursor protein (betaAPP) maturation by a new Val-715 → Met betaAPP-770 mutation responsible for probable early-onset Alzheimer's disease. Proc Natl Acad Sci USA. 1999;96:4119–4124. doi: 10.1073/pnas.96.7.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86:582–590. doi: 10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- Bentahir M, Nyabi O, Verhamme J, Tolia A, Horre K, Wiltfang J, Esselmann H, De Strooper B. Presenilin clinical mutations can affect gamma-secretase activity by different mechanisms. J Neurochem. 2006;96:732–742. doi: 10.1111/j.1471-4159.2005.03578.x. [DOI] [PubMed] [Google Scholar]
- Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- D'Amore JD, Kajdasz ST, McLellan ME, Bacskai BJ, Stern EA, Hyman BT. In vivo multiphoton imaging of a transgenic mouse model of Alzheimer disease reveals marked thioflavine-S-associated alterations in neurite trajectories. J Neuropathol Exp Neurol. 2003;62:137–145. doi: 10.1093/jnen/62.2.137. [DOI] [PubMed] [Google Scholar]
- Deng Y, Tarassishin L, Kallhoff V, Peethumnongsin E, Wu L, Li Y, Zheng H. Delection of presenilin 1 hydrophilic loop sequence leads to impaired γ-secretase activity and exacerbated amyloid pathology. J Neurosci. 2006;26:3845–3854. doi: 10.1523/JNEUROSCI.5384-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen JL, Sagi SA, Smith TE, Weggen S, Das P, McLendon DC, Ozols VV, Jessing KW, Zavitz KH, Koo EH, Golde TE. NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo. J Clin Invest. 2003;112:440–449. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etcheberrigaray R, Tan M, Dewachter I, Kuiperi C, Van der Auwera I, Wera S, Qiao L, Bank B, Nelson TJ, Kozikowski AP, Van Leuven F, Alkon DL. Therapeutic effects of PKC activators in Alzheimer's disease transgenic mice. Proc Natl Acad Sci USA. 2004;101:11141–11146. doi: 10.1073/pnas.0403921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finelli A, Kelkar A, Song HJ, Yang H, Konsolaki M. A model for studying Alzheimer's Abeta42-induced toxicity in Drosophila melanogaster . Mol Cell Neurosci. 2004;26:365–375. doi: 10.1016/j.mcn.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Fotinopoulou A, Tsachaki M, Vlavaki M, Poulopoulos A, Rostagno A, Frangione B, Ghiso J, Efthimiopoulos S. BRI2 interacts with amyloid precursor protein (APP) and regulates amyloid beta (Abeta) production. J Biol Chem. 2005;280:30768–30772. doi: 10.1074/jbc.C500231200. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Holtzman DM. The bad seed in Alzheimer's disease. Neuron. 2005;47:167–168. doi: 10.1016/j.neuron.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Simmons K, Parsadanian M, Bales KR, Paul SM, Sullivan PM, Holtzman DM. Human apolipoprotein E4 alters the amyloid-β 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J Neurosci. 2005;25:2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabe CG. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol Aging. 2006;27:570–575. doi: 10.1016/j.neurobiolaging.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Gravina SA, Ho L, Eckman CB, Long KE, Otvos L, Jr, Younkin LH, Suzuki N, Younkin SG. Amyloid beta protein (A beta) in Alzheimer's disease brain. Biochemical and immunocytochemical analysis with antibodies specific for forms ending at A beta 40 or A beta 42(43) J Biol Chem. 1995;270:7013–7016. doi: 10.1074/jbc.270.13.7013. [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Vonsattel JP. Diagnosis of cerebral amyloid angiopathy. Sensitivity and specificity of cortical biopsy. Stroke. 1997;28:1418–1422. doi: 10.1161/01.str.28.7.1418. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Yamaguchi I, Omata S, Gejyo F, Naiki H. Interaction between A beta(1–42) and A beta(1–40) in Alzheimer's beta-amyloid fibril formation in vitro. Biochemistry. 1999;38:15514–15521. doi: 10.1021/bi991161m. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Rockenstein E, Mante M, Mallory M, Masliah E. beta-Synuclein inhibits alpha-synuclein aggregation: a possible role as an anti-parkinsonian factor. Neuron. 2001;32:213–223. doi: 10.1016/s0896-6273(01)00462-7. [DOI] [PubMed] [Google Scholar]
- Herzig MC, Winkler DT, Burgermeister P, Pfeifer M, Kohler E, Schmidt SD, Danner S, Abramowski D, Sturchler-Pierrat C, Burki K, Van Duinen SG, Maat-Schieman ML, Staufenbiel M, Mathews PM, Jucker M. Abeta is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat Neurosci. 2004;7:954–960. doi: 10.1038/nn1302. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–103. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Hsiao KK, Borchelt DR, Olson K, Johannsdottir R, Kitt C, Yunis W, Xu S, Eckman C, Younkin S, Price D, Iadecola C, Clark HB, Carlson G. Age-related CNS disorder and early death in transgenic FVB/N mice overexpressing Alzheimer amyloid precursor proteins. Neuron. 1995;15:1203–1218. doi: 10.1016/0896-6273(95)90107-8. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein WL, Stine WB, Jr, Teplow DB. Small assemblies of unmodified amyloid beta-protein are the proximate neurotoxin in Alzheimer's disease. Neurobiol Aging. 2004;25:569–580. doi: 10.1016/j.neurobiolaging.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Klug GM, Losic D, Subasinghe SS, Aguilar MI, Martin LL, Small DH. Beta-amyloid protein oligomers induced by metal ions and acid pH are distinct from those generated by slow spontaneous ageing at neutral pH. Eur J Biochem. 2003;270:4282–4293. doi: 10.1046/j.1432-1033.2003.03815.x. [DOI] [PubMed] [Google Scholar]
- Koo EH, Kopan R. Potential role of presenilin-regulated signaling pathways in sporadic neurodegeneration. Nat Med. 2004;10(Suppl):S26–S33. doi: 10.1038/nm1065. [DOI] [PubMed] [Google Scholar]
- Krezowski J, Knudson D, Ebeling C, Pitstick R, Giri RK, Schenk D, Westaway D, Younkin L, Younkin SG, Ashe KH, Carlson GA. Identification of loci determining susceptibility to the lethal effects of amyloid precursor protein transgene overexpression. Hum Mol Genet. 2004;13:1989–1997. doi: 10.1093/hmg/ddh210. [DOI] [PubMed] [Google Scholar]
- Kumar-Singh S, Theuns J, Van Broeck B, Pirici D, Vennekens K, Corsmit E, Cruts M, Dermaut B, Wang R, Van Broeckhoven C. Mean age-of-onset of familial Alzheimer disease caused by presenilin mutations correlates with both increased Abeta42 and decreased Abeta40. Hum Mutat. 2006;27:686–695. doi: 10.1002/humu.20336. [DOI] [PubMed] [Google Scholar]
- Lashuel HA, Hartley DM, Petre BM, Wall JS, Simon MN, Walz T, Lansbury PT., Jr Mixtures of wild-type and a pathogenic (E22G) form of Abeta40 in vitro accumulate protofibrils, including amyloid pores. J Mol Biol. 2003;332:795–808. doi: 10.1016/s0022-2836(03)00927-6. [DOI] [PubMed] [Google Scholar]
- Le R, Cruz L, Urbanc B, Knowles RB, Hsiao-Ashe K, Duff K, Irizarry MC, Stanley HE, Hyman BT. Plaque-induced abnormalities in neurite geometry in transgenic models of Alzheimer disease: implications for neural system disruption. J Neuropathol Exp Neurol. 2001;60:753–758. doi: 10.1093/jnen/60.8.753. [DOI] [PubMed] [Google Scholar]
- Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40:1087–1093. doi: 10.1016/s0896-6273(03)00787-6. [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Lleo A, Berezovska O, Herl L, Raju S, Deng A, Bacskai BJ, Frosch MP, Irizarry M, Hyman BT. Nonsteroidal anti-inflammatory drugs lower Abeta(42) and change presenilin 1 conformation. Nat Med. 2004;10:1065–1066. doi: 10.1038/nm1112. [DOI] [PubMed] [Google Scholar]
- Lombardo JA, Stern EA, McLellan ME, Kajdasz ST, Hickey GA, Bacskai BJ, Hyman BT. Amyloid-β antibody treatment leads to rapid normalization of plaque-induced neuritic alterations. J Neurosci. 2003;23:10879–10883. doi: 10.1523/JNEUROSCI.23-34-10879.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Giliberto L, Matsuda Y, Davies P, McGowan E, Pickford F, Ghiso J, Frangione B, D'Adamio L. The familial dementia BRI2 gene binds the Alzheimer gene amyloid-beta precursor protein and inhibits amyloid-beta production. J Biol Chem. 2005;280:28912–28916. doi: 10.1074/jbc.C500217200. [DOI] [PubMed] [Google Scholar]
- McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, Jansen K, Delucia M, Lin WL, Dolios G, Wang R, Eckman CB, Dickson DW, Hutton M, Hardy J, Golde T. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moechars D, Lorent K, Van Leuven F. Premature death in transgenic mice that overexpress a mutant amyloid precursor protein is preceded by severe neurodegeneration and apoptosis. Neuroscience. 1999a;91:819–830. doi: 10.1016/s0306-4522(98)00599-5. [DOI] [PubMed] [Google Scholar]
- Moechars D, Dewachter I, Lorent K, Reverse D, Baekelandt V, Naidu A, Tesseur I, Spittaels K, Haute CV, Checler F, Godaux E, Cordell B, Van Leuven F. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem. 1999b;274:6483–6492. doi: 10.1074/jbc.274.10.6483. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of Aβ1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DL, Tanzi RE, Borchelt DR, Sisodia SS. Alzheimer's disease: genetic studies and transgenic models. Annu Rev Genet. 1998;32:461–493. doi: 10.1146/annurev.genet.32.1.461. [DOI] [PubMed] [Google Scholar]
- Rochet JC, Conway KA, Lansbury PT., Jr Inhibition of fibrillization and accumulation of prefibrillar oligomers in mixtures of human and mouse alpha-synuclein. Biochemistry. 2000;39:10619–10626. doi: 10.1021/bi001315u. [DOI] [PubMed] [Google Scholar]
- Samura E, Shoji M, Kawarabayashi T, Sasaki A, Matsubara E, Murakami T, Wuhua X, Tamura S, Ikeda M, Ishiguro K. Enhanced accumulation of tau in doubly transgenic mice expressing mutant [beta]APP and presenilin-1. Brain Res. 2006;1094:192–199. doi: 10.1016/j.brainres.2005.12.134. [DOI] [PubMed] [Google Scholar]
- Snyder SW, Ladror US, Wade WS, Wang GT, Barrett LW, Matayoshi ED, Huffaker HJ, Krafft GA, Holzman TF. Amyloid-beta aggregation: selective inhibition of aggregation in mixtures of amyloid with different chain lengths. Biophys J. 1994;67:1216–1228. doi: 10.1016/S0006-3495(94)80591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Haass C. Intramembrane proteolysis by presenilins. Nat Rev Mol Cell Biol. 2000;1:217–224. doi: 10.1038/35043065. [DOI] [PubMed] [Google Scholar]
- Tsai J, Grutzendler J, Duff K, Gan WB. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci. 2004;7:1181–1183. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- Van Dooren T, Muyllaert D, Borghgraef P, Cresens A, Devijver H, Van der Auwera I, Wera S, Dewachter I, Van Leuven F. Neuronal or glial expression of human apolipoprotein e4 affects parenchymal and vascular amyloid pathology differentially in different brain regions of double- and triple-transgenic mice. Am J Pathol. 2006;168:245–260. doi: 10.2353/ajpath.2006.050752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dorpe J, Smeijers L, Dewachter I, Nuyens D, Spittaels K, Van Den Haute C, Mercken M, Moechars D, Laenen I, Kuiperi C, Bruynseels K, Tesseur I, Loos R, Vanderstichele H, Checler F, Sciot R, Van Leuven F. Prominent cerebral amyloid angiopathy in transgenic mice overexpressing the London mutant of human APP in neurons. Am J Pathol. 2000;157:1283–1298. doi: 10.1016/S0002-9440(10)64644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Younkin SG. The role of A beta 42 in Alzheimer's disease. J Physiol (Paris) 1998;92:289–292. doi: 10.1016/s0928-4257(98)80035-1. [DOI] [PubMed] [Google Scholar]
- Zhang M, Haapasalo A, Kim DY, Ingano LA, Pettingell WH, Kovacs DM. Presenilin/gamma-secretase activity regulates protein clearance from the endocytic recycling compartment. FASEB J. 2006;20:1176–1178. doi: 10.1096/fj.05-5531fje. [DOI] [PubMed] [Google Scholar]
- Zou K, Kim D, Kakio A, Byun K, Gong JS, Kim J, Kim M, Sawamura N, Nishimoto S, Matsuzaki K, Lee B, Yanagisawa K, Michikawa M. Amyloid beta-protein (Abeta)1–40 protects neurons from damage induced by Abeta1–42 in culture and in rat brain. J Neurochem. 2003;87:609–619. doi: 10.1046/j.1471-4159.2003.02018.x. [DOI] [PubMed] [Google Scholar]