Abstract
Rett syndrome (RTT) is caused by loss-of-function mutations in the gene encoding methyl-CpG-binding protein 2 (MeCP2). Although MeCP2 is thought to act as a transcriptional repressor of brain-derived neurotrophic factor (BDNF), Mecp2 null mice, which develop an RTT-like phenotype, exhibit progressive deficits in BDNF expression. These deficits are particularly significant in the brainstem and nodose cranial sensory ganglia (NGs), structures critical for cardiorespiratory homeostasis, and may be linked to the severe respiratory abnormalities characteristic of RTT. Therefore, the present study used Mecp2 null mice to further define the role of MeCP2 in regulation of BDNF expression and neural function, focusing on NG neurons and respiratory control. We find that mutant neurons express significantly lower levels of BDNF than wild-type cells in vitro, as in vivo, under both depolarizing and nondepolarizing conditions. However, BDNF levels in mutant NG cells can be increased by chronic depolarization in vitro or by treatment of Mecp2 null mice with CX546, an ampakine drug that facilitates activation of glutamatergic AMPA receptors. Ampakine-treated Mecp2 null mice also exhibit marked functional improvement, characterized by restoration of normal breathing frequency and minute volume. These data demonstrate that BDNF expression remains plastic in Mecp2 null mice and raise the possibility that ampakine compounds could be of therapeutic value in the treatment of RTT.
Keywords: Mecp2 null mice, respiratory frequency, minute volume, nodose ganglion, neurotrophin expression, AMPA receptors modulator
Introduction
Rett syndrome (RTT) is an X-linked neurodevelopmental disorder caused by mutations in the methyl-CpG-binding protein 2 gene (MECP2) (Amir et al., 1999). Six to 18 months after birth, RTT patients begin a neurological decline characterized by regression of acquired skills, behavioral disturbances with autistic features (Hagberg et al., 1983), motor stereotypies, seizures, autonomic dysfunction, and severely disordered breathing (Shahbazian and Zoghbi, 2002). Respiratory abnormalities in RTT include alternating periods of hyperventilation and breath holds and forced and apneustic breathing (Weese-Mayer et al., 2006, and references therein) and may contribute to up to 26% of deaths in RTT (Kerr et al., 1997). The primary cause of these breathing alterations is unknown, and current hypotheses include cortical dysfunction (Elian and Rudolf, 1991; Marcus et al., 1994), brainstem immaturity (Julu et al., 2001), decreased noradrenergic transmission in ponto-medullary respiratory networks (Viemari et al., 2005), and hyperexcitability in pontine and vagal afferent pathways (Stettner et al., 2007). There is no treatment currently available for respiratory dysfunction in RTT.
Recent studies suggest that alterations in brain-derived neurotrophic factor (BDNF) signaling contribute to RTT pathophysiology. For example, Mecp2 null mice exhibit progressive deficits in BDNF levels after birth (Chang et al., 2006; Wang et al., 2006), and genetic restoration of BDNF in the forebrain improves somatomotor function and extends lifespan (Chang et al., 2006). Moreover, neural structures important for cardiorespiratory control, including the nodose cranial sensory ganglia (NGs) and brainstem, exhibit the earliest and most significant known deficits in BDNF expression in the Mecp2 null mouse brain (Wang et al., 2006). Because BDNF is required for the development of NG and brainstem respiratory neurons, as well as breathing (Katz, 2005), we hypothesize that BDNF deficits contribute to the RTT-like respiratory phenotype of Mecp2 null mice.
The fact that Mecp2 null mice exhibit decreased BDNF expression contrasts with the prevailing view that Mecp2 is a transcriptional repressor of Bdnf (Chen et al., 2003). One model proposed to explain this apparent discrepancy is that decreased neuronal activity in Mecp2 null mutants (Dani et al., 2005) reduces activity-dependent BDNF expression, thereby masking any effect of derepression (Chang et al., 2006). To test this hypothesis, we examined BDNF expression in NG neurons cultured under depolarizing and nondepolarizing conditions. Because the NG comprises a single neuronal cell type (sensory neurons) and exhibits the Mecp2 null BDNF phenotype in vitro as in vivo (Wang et al., 2006), it provides a simple model for exploring mechanisms that underlie BDNF regulation by MeCP2. Our data indicate that Mecp2 null cells exhibit significantly lower levels of BDNF expression than wild type, under both depolarizing and nondepolarizing conditions. However, BDNF levels in mutant cells can be elevated to wild-type resting levels by depolarizing stimuli in vitro. Similarly, we find that treatment of Mecp2 null mice with the ampakine drug 1-(1,4-benzodioxan-6-yl-carbonyl)piperidine (CX546), which enhances activation of glutamatergic AMPA receptors (Nagarajan et al., 2001), elevates NG BDNF levels in vivo. Moreover, ampakine treatment significantly improves respiratory function in Mecp2 null mice, suggesting that this class of compounds may be of therapeutic value in RTT.
Materials and Methods
Animals.
Mecp2tm1–1Jae mice (Chen et al., 2001), developed by Dr. R. Jaenisch (Whitehead Institute, Massachusetts Institute of Technology, Cambridge, MA) and obtained from the Mutant Mouse Regional Resource Center (University of California Davis, Davis, CA), were maintained on a mixed background (129Sv, C57BL/6, BALB/c). Male Mecp2 nulls (Mecp2 −/y) were generated by crossing heterozygous Mecp2tm1–1Jae knock-out females with Mecp2tm1–1Jae wild-type males (Mecp2+/y). All experimental procedures were approved by the Institutional Animal Care and Use Committee at Case Western Reserve University.
Cell cultures.
Wild-type and Mecp2 null mice were killed with CO2 on postnatal day 35 (P35). The NGs were removed, digested in 0.1% collagenase (Sigma, St. Louis, MO) in Earle's balanced salt solution (Invitrogen, San Diego, CA) for 70 min at 37°C, triturated in culture medium (see below) containing 0.15% BSA, and plated at a density of one NG per well into 96-well flat-bottom ELISA plates coated with poly-d-lysine. Cultures were grown for 3 d in DMEM/F-12 medium supplemented with 5% fetal bovine serum (Invitrogen) and 1% penicillin–streptomycin–neomycin, with or without 40 mm potassium chloride (KCl) or 1.5 μm tetrodotoxin (TTX).
Ampakine treatment.
Beginning on P25, wild-type and Mecp2 null littermates were acclimatized to the injection protocol to reduce stress, first by handling for 10 min/d for 3 d, followed by saline injections (0.9% NaCl, i.p., b.i.d.) at 8:00 A.M. and 8:00 P.M. for an additional 3 d. Subsequently, mice were assigned either to drug treatment (40 mg/kg CX546 in 16.5% 2-hydroxypropyl-β-cyclodextrin, i.p., b.i.d.) or vehicle injections (cyclodextrin alone). On the day of their last injection, mice were trained in the plethysmograph recording chamber for 1 h. Eighteen to 24 h after their last injection, on P35, mice were returned to the chamber for recording of respiratory activity.
Plethysmography.
Breathing was recorded in unrestrained mice using a whole-body flow plethysmograph (Buxco II; Buxco Research Systems, Wilmington, NC) in which a constant bias flow supply connected to the animal recording chamber ensured continuous inflow of fresh air (1 L/min). Ambient temperature was maintained between 23 and 25°C. Breathing traces were analyzed using Biosystem XA software (Buxco Research Systems). After the recording sessions, mice were euthanized with CO2 and tissue was processed for BDNF immunoassay.
BDNF reverse transcription-PCR.
Total RNA was isolated from intact P35 NG using the RNeasy Mini kit (Qiagen, Valencia, CA). For each sample, 500 ng of total RNA was digested with DNase I (Invitrogen, Carlsbad, CA) and reverse transcribed by oligodT priming using SuperScriptIII (Invitrogen). The amount of each Bdnf transcript present in the sample was measured by quantitative real-time PCR (qRT-PCR) using SYBR Green detection (Applied Biosystems, Foster City, CA). Bdnf mRNA levels were normalized to β-tubulin III mRNA levels to adjust for small differences in input RNA. The following primers were used for qRT-PCR: Bdnf exon 8 (coding exon), forward (F) 5′-gatgccgcaaacatgtctatga-3′ and reverse (R) 5′-taatactgtcacacacgctcagctc-3′; Bdnf exon 1, F 5′-cactgagcaaagccgaacttctc-3′ and R 5′-tcacctggtggaacattgtggc-3′; Bdnf exon 2, F 5′-agcggtgtaggctggaatagactc-3′ and R 5′-ggtggaacttctttgcggcttac-3′; Bdnf exon 4, F 5′-cgccatgcaatttccactatcaataatttaac-3′ and R 5′-cgccttcatgcaaccgaagtatg-3′; Bdnf exon 5, F 5′-gatccgagagctttgtgtggac-3′ and R 5′-gccttcatgcaaccgaagtatg-3′; β-tubulin III, F 5′-cgacaatgaagccctctacgac-3′ and R 5′-atggtggcagacacaaggtggttg-3′.
BDNF immunoassay.
BDNF protein levels in intact NGs or in cultured NG cells were measured by ELISA using the BDNF Emax Immunoassay System (Promega, Madison, WI). Protein extracts from one intact NG or from an equivalent number of cultured cells were used for ELISA.
MeCP2 and β-tubulin III double staining.
Mice were killed with CO2 and perfused with 4% paraformaldehyde, and the head was sectioned at 10 μm with a cryostat. Sections were stained with rabbit polyclonal anti-MeCP2 (Upstate Biotechnology, Lake Placid, NY) and chicken polyclonal anti-β-tubulin III (Aves Labs, Ft. Lauderdale, FL).
Statistical analysis.
Differences between wild-type and mutant mice, and between vehicle-treated and CX546-treated mice, were tested using an unpaired t test or ANOVA I with Tukey's multiple comparison post hoc analysis. A p value <0.05 was considered statistically significant. Data are presented as mean ± SEM.
Results
Bdnf gene expression is reduced in Mecp2 null cells in vivo
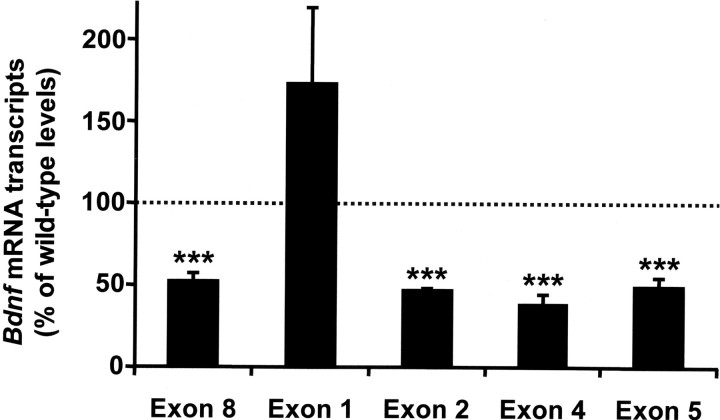
We previously found that BDNF protein content of peripheral and CNS tissues is markedly reduced in Mecp2 null mice by 5 weeks of age (Wang et al., 2006; see also Chang et al., 2006). To determine whether these deficits are reflective of decreased Bdnf gene expression, we compared Bdnf mRNA levels in wild-type and mutant animals, using the NG as a model. This analysis revealed that on P35, total Bdnf mRNA was reduced by 50% in the Mecp2 null NG compared with wild-type controls (Fig. 1), paralleling the deficit in BDNF protein (Wang et al., 2006). However, not all Bdnf transcripts were similarly affected. For example, although Bdnf splice variants containing exon 2, 4, or 5 were all decreased by ∼50% in mutant tissue compared with wild type, transcripts containing exon 1 were unchanged. These data indicate that MeCP2 function is required to maintain normal levels of BDNF expression by regulating specific isoforms of Bdnf mRNA.
Figure 1.
Mecp2 null mutation is associated with decreased expression of specific Bdnf transcripts in nodose neurons. Bdnf transcript levels in intact NG from wild-type and Mecp2 null mice were determined using qRT-PCR. The Bdnf gene has a complex structure in which multiple promoters drive the expression of different mRNA isoforms containing alternative noncoding 5′ exons spliced to a common downstream coding exon [exon 8; nomenclature of Liu et al. (2006)]. Total Bdnf mRNA levels (Exon 8), as well as transcripts containing exons 2, 4, and 5 were markedly decreased in mutant NG compared with wild type, whereas transcripts containing exon 1 were expressed at levels that were not significantly different from wild type. Results are the mean ± SEM (n = 4). ***p < 0.001, ANOVA I with post hoc Tukey's test.
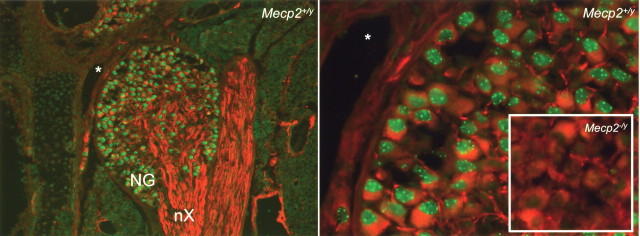
The marked deficit in BDNF content found in P35 Mecp2 null NG neurons in vivo is maintained in dissociate cell culture (Wang et al., 2006), suggesting that it may be a cell-autonomous effect of MeCP2 loss. However, MeCP2 expression in peripheral neurons has not previously been described. Therefore, initial studies examined the localization of MeCP2 immunoreactivity in the NG and found robust expression in all neurons at P0 through P35 (Fig. 2 and data not shown).
Figure 2.
MeCP2 protein is expressed in nodose neurons. Left, Double immunostaining for MeCP2 (green) and β-tubulin III (red) in the newborn wild-type (Mecp2 +/y) mouse NG. nX, Vagal nerve. Right, Higher magnification of the same section shown on the left, illustrating the concentration of MeCP2-immunoreactive protein in heterochromatin foci. The inset shows that the MeCP2 antibody used in these studies does not produce any specific staining in the NG from a Mecp2 null mouse (Mecp2 −/y). The asterisk represents an anatomical landmark shared by both panels.
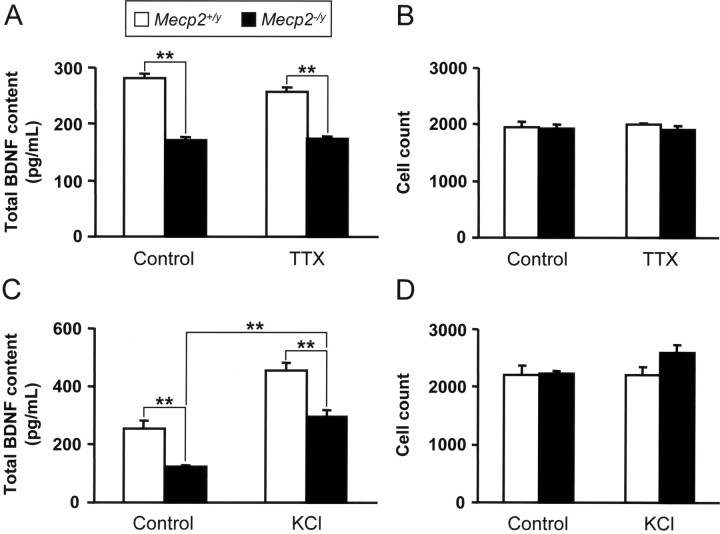
To test the hypothesis that differences in BDNF content between wild-type and Mecp2 null cells result from different levels of activity (Chang et al., 2006), BDNF levels were compared in P35 NG neurons from wild-type and Mecp2 null mice grown in dissociated culture for 3 d under control (nondepolarizing) and depolarizing (40 mm KCl) conditions. Under control conditions, NG neurons exhibit resting membrane potentials of approximately −70 mV and are not spontaneously active (Schild and Kunze, 1997; Brosenitsch et al., 1998). However, to eliminate any possible depolarizing influence of voltage-gated sodium channels, some cultures were grown in the presence of 1.5 μm TTX [NG neurons also express TTX-insensitive Na channels; however, these activate at substantially more positive membrane potentials (Schild and Kunze, 1997)]. In both control and TTX-treated cultures, Mecp2 null neurons exhibit 40–50% less BDNF than wild-type neurons (Fig. 3 A), as in vivo, without any change in cell survival (Fig. 3 B).
Figure 3.
BDNF levels are depressed in P35 Mecp2 −/y NG neurons under resting and depdarizing conditions. A, C, Summary data showing that BDNF content is decreased by 40–50% in NG cultures from Mecp2 null mutants, regardless of the activity state of the cells [i.e., electrically silent (A; treated with TTX) or chronic depolarization (C, treated with KCl)]. Results show that KCl treatment can increase the BDNF level in mutant cells as in wild-type controls. B, D, Neuron survival was unaffected by either TTX (B) or KCl (D). Results are the mean ± SEM (n = 6). **p < 0.01, ANOVA I with post hoc Tukey's test.
To further test the role of membrane depolarization in the BDNF phenotype of Mecp2 null neurons, NG cultures were grown in the absence and presence of a depolarizing concentration of KCl (40 mm). In both wild-type and mutant cultures, KCl depolarization resulted in a significant increase in BDNF protein compared with unstimulated controls (Fig. 3 C), with no change in cell survival (Fig. 3 D). However, even under depolarizing conditions, mutant cells expressed significantly lower levels of BDNF than wild-type cells. These data indicate that Mecp2 is required for normal levels of BDNF expression in NG neurons under both resting and depolarizing conditions. In addition, these experiments show that chronic depolarization of mutant neurons can stimulate BDNF protein expression to wild-type resting levels. This observation is consistent with previous observations showing increased expression of Bdnf exon 4 mRNA in cultured newborn Mecp2 null cortical cells after KCl treatment (Chen et al., 2003).
Ampakine stimulation of BDNF expression in vivo
The fact that depolarization of Mecp2 null NG neurons could increase BDNF expression in vitro raised the possibility that neuronal activation could rescue the BDNF deficit in vivo. To approach this issue, we examined the effect of an ampakine drug, CX546, on BDNF protein expression in the NG in intact P35 wild-type and Mecp2 null mice. Ampakines are fast-acting molecules that acutely lengthen the duration of AMPA receptor-mediated inward currents and thereby increase the activity of neurons that express AMPA receptors (Nagarajan et al., 2001). As a result, repeated ampakine treatment leads to an increase in activity-dependent expression of BDNF, in vivo and in vitro (Lauterborn et al., 2000, 2003; Rex et al., 2006).
P35 wild-type and Mecp2 null littermates were treated for 3 d with CX546 (40 mg/kg in cyclodextrin, i.p., b.i.d.) or vehicle. Twenty-four hours after the last injection, respiratory activity was measured (see below), the mice were killed, and the NG was removed for BDNF ELISA. NG BDNF content in vehicle-treated Mecp2 null mice was significantly reduced compared with vehicle-treated wild-type controls, as described previously in naive untreated animals (wild type, 170 ± 14 pg BDNF/ml vs mutant, 72 ± 3 pg BDNF/ml; n = 6; p < 0.001, ANOVA I). Treatment of wild-type mice with CX546 had no effect on NG BDNF content. However, treatment of Mecp2 null mice resulted in a significant 42% increase in BDNF protein content compared with vehicle-treated mutants (wild-type CX546, 167 ± 5 pg BDNF/ml vs mutant, CX546 114 ± 4 pg BDNF/ml; n = 6; p < 0.001, ANOVA I).
Ampakine treatment restores wild-type mean respiratory frequency and minute volume in Mecp2tm1–1Jae null mice
NG neurons secrete BDNF in an activity-dependent manner (Balkowiec and Katz, 2000), and BDNF acutely modulates glutamatergic transmission at second-order neurons in the nucleus tractus solitarius (nTS) (Balkowiec et al., 2000), the primary relay for peripheral afferent input to the brainstem respiratory rhythm generating network. Therefore, we hypothesize that BDNF deficits in NG neurons contribute to the pathogenesis of respiratory dysfunction in RTT by disrupting synaptic modulation in nTS.
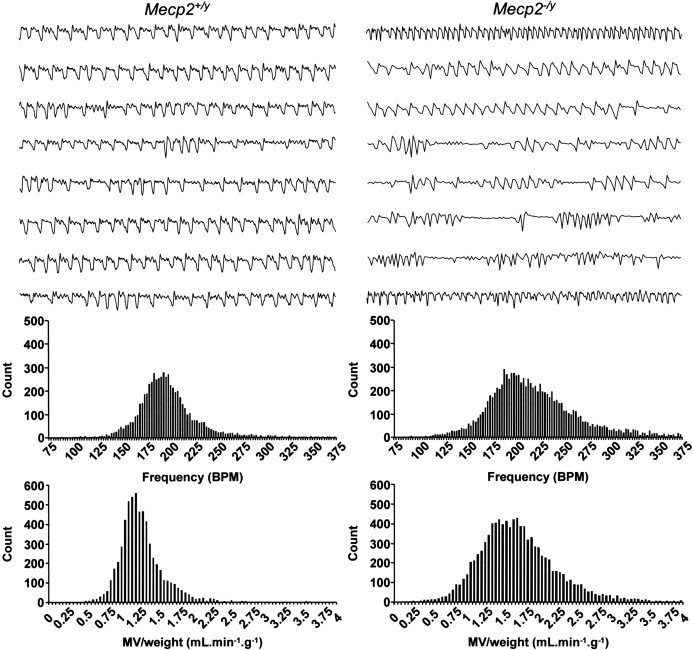
To examine whether or not ampakine enhancement of BDNF expression in Mecp2 null NG neurons is associated with recovery of neural function, we compared respiratory activity in wild-type and mutant mice after treatment with CX546 in vivo as described above. Respiratory function was monitored by whole-body plethysmography 18–24 h after the last drug injection as described in Materials and Methods. Analysis of naive untreated wild-type and mutant animals revealed a highly disordered breathing pattern in the mutants compared with wild-type controls (Fig. 4). The mutant breathing pattern is characterized by a highly variable frequency (coefficient of variation of breathing frequency: wild type, 18.8 ± 0.7% vs mutant, 22.0 ± 1.2%; n = 6 for wild type and n = 7 for mutants; p < 0.05, unpaired t test) and occasional long breathing pauses compared with wild types, similar to human RTT patients (Julu et al., 2001; Weese-Mayer et al., 2006) and other models of RTT (Mecp2tm1–1Bird null mice) (Viemari et al., 2005; Stettner et al., 2007). More detailed analysis of breathing parameters revealed that the phenotype observed in mutant mice is associated with repetitive episodes of very high breathing frequency (Fig. 4), resulting in a 23% increase in mean respiratory frequency compared with wild-type controls (p < 0.001, unpaired t test; n = 6 for wild type and n = 7 for mutants), similar to RTT patients (Weese-Mayer et al., 2006). Consequently, the mean value for minute volume/weight (tidal volume/weight × breathing frequency) is also increased in mutants (Fig. 4) (wild type, 0.97 ± 0.11 ml/min/g vs mutant, 1.38 ± 0.13 ml/min/g; n = 6 for wild-types and n = 7 for mutants; p < 0.05, unpaired t test). In contrast, there was no significant difference in tidal volume/weight alone between wild-type and mutant animals (wild type, 5.4 ± 0.6 μl/g vs mutant, 6.4 ± 0.5 μl/g; n = 6 for wild types and n = 7 for mutants).
Figure 4.
Mecp2 null mice exhibit a Rett-like respiratory phenotype at 5 weeks of age (P35). Representative plethysmographic recordings from wild-type (Mecp2 +/y) and Mecp2 null (Mecp2 −/y) mice are shown. Each trace is 10 s quiet breathing in room air. The bottom graphs are frequency histograms from control (compilation of 9776 breath cycles) and mutant (compilation of 6065 breath cycles) mice showing the higher incidence of fast breaths in mutant mice compared with controls, along with a shift to higher values of minute volume/weight. BPM, Breaths per minute; MV, minute volume.
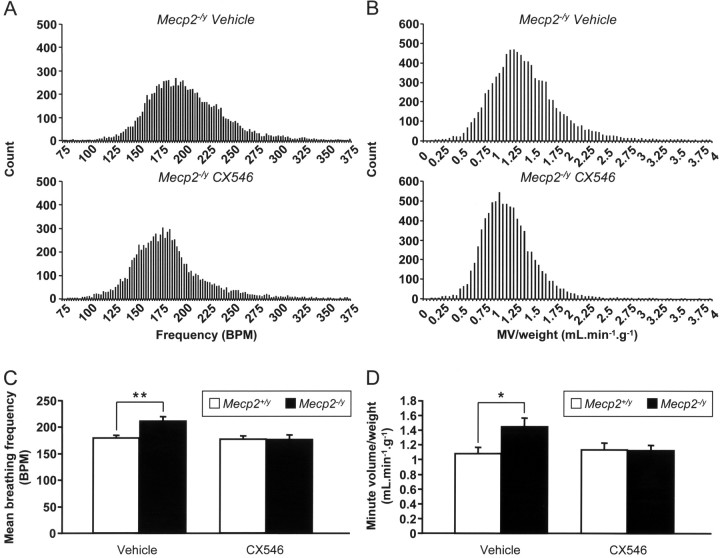
Three-day treatment with CX546 did not significantly affect breathing frequency, tidal volume/weight, and minute volume/weight in P35 Mecp2tm1–1Jae wild-type mice (vehicle vs CX546: frequency, 179 ± 3 vs 177 ± 6 breaths/min; tidal volume/weight, 6.1 ± 0.4 vs 6.4 ± 0.5 μl/g; minute volume/weight, 1.09 ± 0.07 ml/min/g vs 1.14 ± 0.09 ml/min/g; n = 8 for vehicle and n = 7 for CX546). In contrast, ampakine treatment of mutant animals sharply decreased the episodes of high breathing frequency, leading to restoration of wild-type mean breathing frequency (Fig. 5 A,C) (wild-type CX546, 177 ± 6 breaths/min vs mutant CX546, 176 ± 8 breaths/min; n = 7 for wild types and n = 9 for mutants) and minute volume/weight (Fig. 5 B,D) (wild-type CX546, 1.14 ± 0.09 ml/min/g vs mutant CX546, 1.13 ± 0.07 ml/min/g; n = 7 for wild types and n = 9 for mutants). However, ampakine treatment did not decrease the higher variability in breathing frequency characteristic of mutant animals (coefficient of variation of breathing frequency: wild-type CX546, 18.5 ± 1.2% vs mutant CX546, 23.4 ± 1.5%; n = 7 for wild types and n = 9 for mutants). Tidal volume/weight was not affected in mutants by ampakine treatment and was similar to wild type (wild-type CX546 vs mutant CX546, 6.4 ± 0.5 vs 6.5 ± 0.3 μl/g; n = 7 for wild types and n = 9 for mutants).
Figure 5.
Chronic treatment with CX546 restores normal breathing frequency and minute volume/weight in P35 Mecp2 null mice. A, B, Representative histograms of breathing frequency (A) and minute volume/weight (B) from two mutant mice, one treated with vehicle (9227 breath cycles) and one treated with CX546 (8393 breath cycles), showing that drug treatment (40 mg/kg, b.i.d for 3 d) decreases episodes of high breathing frequency and minute volume/weight. C, D, Summary data for breathing frequency (C) and minute volume/weight (D) for all animals. Ampakine treatment completely restores wild-type frequency and minute volume/weight in mutant animals and has no effect in wild types. Results are the mean ± SEM (n = 8 for vehicle-treated wild types; n = 7 for CX546-treated wild types; n = 8 for vehicle-treated mutants; n = 9 for CX546-treated mutants). *p < 0.05; **p < 0.01, ANOVA I with post hoc Tukey's test. BPM, Breaths per minute.
Discussion
Our results demonstrate that MeCP2 is required for normal levels of BDNF expression in nodose sensory neurons under both resting and depolarizing conditions in vitro. Moreover, chronic depolarization in vitro, or ampakine treatment in vivo, can elevate BDNF levels in Mecp2 null cells. Furthermore, ampakine treatment results in a restoration of wild-type breathing frequency and minute volume/weight in Mecp2 null mice.
Previous studies in cultured newborn cortical neurons indicated that MeCP2 represses Bdnf expression at rest (Chen et al., 2003) and that release from MeCP2-mediated repression is required for normal levels of activity-dependent expression of BDNF (Martinowich et al., 2003; Zhou et al., 2006). However, Mecp2 null mice exhibit deficits in BDNF protein (Chang et al., 2006; Wang et al., 2006) and mRNA (present study) in vivo. Moreover, reduced Bdnf gene expression has recently been reported in the frontal cortex of RTT patients (Deng et al., 2007). A proposed explanation for these discrepancies between in vivo and in vitro studies is that Mecp2 null cortical neurons are less active in vivo than wild-type cells (Dani et al., 2005), leading to a reduction in activity-dependent BDNF expression that masks any effects of BDNF derepression (Chen et al., 2003; Chang et al., 2006; Sun and Wu, 2006). However, our data indicate that, as in vivo, Mecp2 null NG neurons grown in dissociated cell culture express significantly less BDNF than wild-type cells and that this deficit persists under both nondepolarizing and depolarizing conditions in culture. These observations indicate that reduced activity alone may be insufficient to explain the BDNF deficit in Mecp2 null neurons. This apparent difference in BDNF regulation in Mecp2 null mouse cortical and NG neurons, respectively, may indicate a role for cell context, including cell type and age, in determining the interaction between these two genes. For example, it is possible that in P35 NG neurons, unlike newborn cortical neurons (Chen et al., 2003), MeCP2 indirectly regulates BDNF expression, perhaps by repressing a gene or genes that, in turn, repress BDNF. There are other differences between the present study and that of Chen et al. (2003) that may also be important, including the fact that Chen et al. (2003) only looked at regulation of the exon 4-containing Bdnf transcript and stimulated their cultures with KCl for 6 h, compared with 3 d in the present study.
The fact that BDNF expression remains plastic in Mecp2 null NG neurons and can be increased by depolarizing stimuli in vitro led us to test whether or not BDNF levels could be increased in Mecp2 null mice in vivo by the ampakine drug CX546. Ampakines are a family of small molecules that trigger short-term increases in the duration of AMPA-mediated inward currents (Nagarajan et al., 2001). In addition, repeated treatment with ampakines can increase the efficiency of long-term potentiation in the hippocampus and facilitate memory processes (Ingvar et al., 1997; Rex et al., 2006; Wezenberg et al., 2006). These long-term effects of ampakine treatment result from their ability to increase Bdnf mRNA and protein expression (Lauterborn et al., 2000, 2003; Rex et al., 2006).
Our study reveals that chronic treatment with CX546 significantly improves respiratory behavior in adult symptomatic Mecp2 null mice. Indeed, drug treatment significantly decreased breathing frequency and minute volume/weight, two parameters that are markedly increased in RTT patients and may contribute to severe hypocapnic alkalemia and hypoxemia (Southall et al., 1988). The respiratory improvement was not an acute effect of ampakine treatment, because CX546 has an extremely short half-life (<1 h) (Hampson et al., 1998; Wezenberg et al., 2006) and breathing was analyzed 18–24 h after the last drug injection. Thus, improved breathing is attributable to long-term effects of the ampakine treatment. Although mechanisms that underlie improved respiration in ampakine-treated Mecp2 null mice remain to be defined, our data are consistent with a role for increased BDNF expression in the NG. NG neurons project centrally to the brainstem nTS, the primary site for afferent input to the brainstem respiratory rhythm generating network, where BDNF inhibits glutamatergic excitation of second-order vagal sensory relay neurons (Balkowiec et al., 2000). In Mecp2 null mice, BDNF is severely depleted in NG afferents and their projections to nTS (Wang et al., 2006), and activity of postsynaptic neurons is increased (D. D. Kline, personal communication) compared with wild-type controls. Thus, we suspect that elevated respiratory frequency in Mecp2 null mice may result, in part, from increased excitability in nTS and that ampakine treatment restores wild-type respiratory frequency by enhancing BDNF modulation of primary afferent transmission. This possibility is supported by recent findings that breathing dysfunction in Mecp2 null mice results from enhanced excitatory (or decreased inhibitory) neurotransmission affecting both vagal sensory and brainstem respiratory cell groups. In particular, Stettner et al. (2007) described hyperexcitability of pontine cell groups involved in the regulation of postinspiratory discharge (Kolliker-Fuse and lateral parabrachial nuclei) and a loss of desensitization in vagal afferent control of breathing in Mecp2 null mice. It is also possible that ampakine treatment has direct effects in the brainstem as well.
Neuropathological studies in RTT patients and Mecp2 null mice indicate relatively subtle structural abnormalities, such as decreased dendritic arbor complexity (Chen et al., 2001; Armstrong, 2002; Kishi and Macklis, 2004), that likely reflect disruptions in transynaptic signaling rather than overt neuronal degeneration, raising the possibility that functional deficits in RTT may be reversible. This possibility has recently been strengthened by the demonstration that postnatal re-expression of Mecp2 in severely symptomatic Mecp2 null mice is associated with symptom reversal (Guy et al., 2007). Our findings demonstrate that ampakine treatment of symptomatic Mecp2 null mice can significantly improve respiratory function, raising the possibility that this class of compounds may be of therapeutic value in the treatment of RTT patients.
Footnotes
This work was supported by grants from the National Heart, Lung, and Blood Institute; the Rett Syndrome Research Foundation; and the National Institutes of Health to D.M.K. and M.E.G. We gratefully acknowledge Dr. Diana Kunze for critical review of this manuscript and David T. Hellard for technical help.
References
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Armstrong DD. Neuropathology of Rett syndrome. Ment Retard Dev Disabil Res Rev. 2002;8:72–76. doi: 10.1002/mrdd.10027. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ . J Neurosci. 2000;20:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Kunze DL, Katz DM. Brain-derived neurotrophic factor acutely inhibits AMPA-mediated currents in developing sensory relay neurons. J Neurosci. 2000;20:1904–1911. doi: 10.1523/JNEUROSCI.20-05-01904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosenitsch TA, Salgado-Commissariat D, Kunze DL, Katz DM. A role for L-type calcium channels in developmental regulation of transmitter phenotype in primary sensory neurons. J Neurosci. 1998;18:1047–1055. doi: 10.1523/JNEUROSCI.18-03-01047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49:341–348. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2005;102:12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng V, Matagne V, Banine F, Frerking M, Ohliger P, Budden S, Pevsner J, Dissen GA, Sherman LS, Ojeda SR. FXYD1 is an MeCP2 target gene overexpressed in the brains of Rett syndrome patients and Mecp2-null mice. Hum Mol Genet. 2007;16:640–650. doi: 10.1093/hmg/ddm007. [DOI] [PubMed] [Google Scholar]
- Elian M, Rudolf ND. EEG and respiration in Rett syndrome. Acta Neurol Scand. 1991;83:123–128. doi: 10.1111/j.1600-0404.1991.tb04660.x. [DOI] [PubMed] [Google Scholar]
- Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome: report of 35 cases. Ann Neurol. 1983;14:471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Rogers G, Lynch G, Deadwyler SA. Facilitative effects of the ampakine CX516 on short-term memory in rats: enhancement of delayed-nonmatch-to-sample performance. J Neurosci. 1998;18:2740–2747. doi: 10.1523/JNEUROSCI.18-07-02740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvar M, Ambros-Ingerson J, Davis M, Granger R, Kessler M, Rogers GA, Schehr RS, Lynch G. Enhancement by an ampakine of memory encoding in humans. Exp Neurol. 1997;146:553–559. doi: 10.1006/exnr.1997.6581. [DOI] [PubMed] [Google Scholar]
- Julu PO, Kerr AM, Apartopoulos F, Al-Rawas S, Engerstrom IW, Engerstrom L, Jamal GA, Hansen S. Characterisation of breathing and associated central autonomic dysfunction in the Rett disorder. Arch Dis Child. 2001;85:29–37. doi: 10.1136/adc.85.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DM. Regulation of respiratory neuron development by neurotrophic and transcriptional signaling mechanisms. Respir Physiol Neurobiol. 2005;149:99–109. doi: 10.1016/j.resp.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Kerr AM, Armstrong DD, Prescott RJ, Doyle D, Kearney DL. Rett syndrome: analysis of deaths in the British survey. Eur Child Adolesc Psychiatry. 1997;6:71–74. [PubMed] [Google Scholar]
- Kishi N, Macklis JD. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci. 2004;27:306–321. doi: 10.1016/j.mcn.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC, Lynch G, Vanderklish P, Arai A, Gall CM. Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J Neurosci. 2000;20:8–21. doi: 10.1523/JNEUROSCI.20-01-00008.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Truong GS, Baudry M, Bi X, Lynch G, Gall CM. Chronic elevation of brain-derived neurotrophic factor by ampakines. J Pharmacol Exp Ther. 2003;307:297–305. doi: 10.1124/jpet.103.053694. [DOI] [PubMed] [Google Scholar]
- Liu QR, Lu L, Zhu XG, Gong JP, Shaham Y, Uhl GR. Rodent BDNF genes, novel promoters, novel splice variants, regulation by cocaine. Brain Res. 2006;1067:1–12. doi: 10.1016/j.brainres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Marcus CL, Carroll JL, McColley SA, Loughlin GM, Curtis S, Pyzik P, Naidu S. Polysomnographic characteristics of patients with Rett syndrome. J Pediatr. 1994;125:218–224. doi: 10.1016/s0022-3476(94)70196-2. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- Nagarajan N, Quast C, Boxall AR, Shahid M, Rosenmund C. Mechanism and impact of allosteric AMPA receptor modulation by the ampakine CX546. Neuropharmacology. 2001;41:650–663. doi: 10.1016/s0028-3908(01)00133-2. [DOI] [PubMed] [Google Scholar]
- Rex CS, Lauterborn JC, Lin CY, Kramar EA, Rogers GA, Gall CM, Lynch G. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol. 2006;96:677–685. doi: 10.1152/jn.00336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild JH, Kunze DL. Experimental and modeling study of Na+ current heterogeneity in rat nodose neurons and its impact on neuronal discharge. J Neurophysiol. 1997;78:3198–3209. doi: 10.1152/jn.1997.78.6.3198. [DOI] [PubMed] [Google Scholar]
- Shahbazian MD, Zoghbi HY. Rett syndrome and MeCP2: linking epigenetics and neuronal function. Am J Hum Genet. 2002;71:1259–1272. doi: 10.1086/345360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southall DP, Kerr AM, Tirosh E, Amos P, Lang MH, Stephenson JB. Hyperventilation in the awake state: potentially treatable component of Rett syndrome. Arch Dis Child. 1988;63:1039–1048. doi: 10.1136/adc.63.9.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettner GM, Huppke P, Brendel C, Richter DW, Gartner J, Dutschmann M. Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2−/y knockout mice. J Physiol (Lond) 2007;579:863–876. doi: 10.1113/jphysiol.2006.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YE, Wu H. The ups and downs of BDNF in Rett syndrome. Neuron. 2006;49:321–323. doi: 10.1016/j.neuron.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Pena F, Zanella S, Bevengut M, Barthelemy-Requin M, Herzing LB, Moncla A, Mancini J, Ramirez JM, Villard L, Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Chan SA, Ogier M, Hellard D, Wang Q, Smith C, Katz DM. Dysregulation of brain-derived neurotrophic factor expression and neurosecretory function in Mecp2 null mice. J Neurosci. 2006;26:10911–10915. doi: 10.1523/JNEUROSCI.1810-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Silvestri JM, Ramirez JM. Autonomic nervous system dysregulation: breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatr Res. 2006;60:443–449. doi: 10.1203/01.pdr.0000238302.84552.d0. [DOI] [PubMed] [Google Scholar]
- Wezenberg E, Jan Verkes R, Ruigt GS, Hulstijn W, Sabbe BG. Acute effects of the ampakine farampator on memory and information processing in healthy elderly volunteers. Neuropsychopharmacology. 2006;32:1272–1283. doi: 10.1038/sj.npp.1301257. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, Hu L, Steen JA, Weitz CJ, Greenberg ME. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]