Abstract
Insect flight is one of the fastest, most intense and most energy-demanding motor behaviors. It is modulated on multiple levels by the biogenic amine octopamine. Within the CNS, octopamine acts directly on the flight central pattern generator, and it affects motivational states. In the periphery, octopamine sensitizes sensory receptors, alters muscle contraction kinetics, and enhances flight muscle glycolysis. This study addresses the roles for octopamine and its precursor tyramine in flight behavior by genetic and pharmacological manipulation in Drosophila. Octopamine is not the natural signal for flight initiation because flies lacking octopamine [tyramine-β-hydroxylase (TβH) null mutants] can fly. However, they show profound differences with respect to flight initiation and flight maintenance compared with wild-type controls. The morphology, kinematics, and development of the flight machinery are not impaired in TβH mutants because wing-beat frequencies and amplitudes, flight muscle structure, and overall dendritic structure of flight motoneurons are unaffected in TβH mutants. Accordingly, the flight behavior phenotypes can be rescued acutely in adult flies. Flight deficits are rescued by substituting octopamine but also by blocking the receptors for tyramine, which is enriched in TβH mutants. Conversely, ablating all neurons containing octopamine or tyramine phenocopies TβH mutants. Therefore, both octopamine and tyramine systems are simultaneously involved in regulating flight initiation and maintenance. Different sets of rescue experiments indicate different sites of action for both amines. These findings are consistent with a complex system of multiple amines orchestrating the control of motor behaviors on multiple levels rather than single amines eliciting single behaviors.
Keywords: octopamine, Drosophila, tyramine, motor behavior, modulation, invertebrate
Introduction
How are rhythmical motor behaviors initiated, maintained, and terminated? For many years, neuroscientists have debated whether motor behaviors were produced by chains of reflexes or by intrinsically oscillating central networks. Pioneering work on locust flight set the stage for today's well accepted concept of central pattern generation by demonstrating that rhythmic motor output could be induced by nonrhythmical stimulation of the nerve cord without sensory feedback (Wilson, 1961, 1966; Wilson and Wyman, 1965; Edwards, 2006). The underlying networks are central pattern generators (CPGs), which are found at the heart of motor networks in all animals (Kiehn and Kullander, 2004; Grillner et al., 2005; Marder et al., 2005).
Neuromodulators play a major role in activating and modifying CPG activity (Marder and Bucher, 2001). The central release of specific neuromodulators or mixtures of different modulators can initiate distinct motor patterns (Nusbaum et al., 2001). Pioneering studies in locusts have demonstrated that microinjection of the biogenic amine octopamine (OA) into distinct neuropil regions elicits either walking or flight motor patterns in isolated ventral nerve cords (Sombati and Hoyle, 1984). This has led to the “orchestration hypothesis” (Hoyle, 1985) assuming that neuromodulator release into specific neuropils configures distinct neural assemblies to produce coordinated network activity. Monoamines have also been assigned to aggression, motivation, and mood in vertebrates and invertebrates (Baier et al., 2002; Kravitz and Huber, 2003; Stevenson et al., 2005; Popova, 2006). Furthermore, specific cognitive functions have been assigned to monoamine codes, such as that in flies OA mediates appetitive learning but dopamine mediates aversive learning (Schwaerzel et al., 2003; Riemensperger et al., 2005). In mammals, dysfunctions in monoamine neurotransmission are implicated in neurological disorders, including Parkinson's disease, schizophrenia, anxiety, and depression (Kobayashi, 2001; Taylor et al., 2005).
However, recent work from areas as diverse as Parkinson's disease (Scholtissen et al., 2006) and Drosophila larval motor behavior suggests that the chemical codes producing specific motor behavior outputs are bouquets of different amines rather than single ones (Saraswati et al., 2004; Fox et al., 2006). This study tests this hypothesis by genetic and pharmacological dissection of flight behavior in Drosophila. For >20 years, OA has been assigned as the sole modulator controlling insect flight. In contrast, we demonstrate that flight is controlled by the combined action of OA and tyramine (TA). OA and TA are decarboxylation products of the amino acid tyrosine, with TA as the biological precursor of OA. In insect flight systems, OA assumes a variety of physiological roles affecting central neuron excitability (Ramirez and Pearson, 1991), synaptic transmission (Evans and O'Shea, 1979; Leitch et al., 2003), sensory sensitivity (Matheson, 1997), hormone release (Orchard et al., 1993), and muscle metabolism (Mentel et al., 2003). Almost every organ is equipped with OA receptors (Roeder, 1999). TA receptors have been cloned recently in many insect species (Blenau and Baumann, 2003), and physiological functions for TA have been demonstrated (McClung and Hirsh, 1999; Nagaya et al., 2002). The multiple possible levels of OA and TA action on Drosophila flight behavior are discussed.
Materials and Methods
Animals
Drosophila melanogaster flies were kept in standard 68 ml vials with cotton stoppers on a yeast–syrup–cornmeal–agar diet at 25°C and 50–60% humidity with a 12 h light/dark regimen. Flies were used for experiments 3–5 d after eclosion. Various strains were used for the experiments (Table 1).
Table 1.
Genotypes and sources of flies
| Strains | Genotypes | Source |
|---|---|---|
| w+ | +;+;+;+ | Dr. H. Scholz, University of Wuerzburg, Wuerzburg, Germany |
| TβHnM18 | TβH nM18/FM7c;+;+;+ | Monastirioti et al., 1996 |
| TβHnM18 hsp–TβH | w-TβHnM18/FM6;+;P{hsp–TβH};+ | Schwaerzel et al., 2003 |
| dTdc2–Gal4 | w1118;P{Tdc2–Gal4};+;+ | Cole et al., 2005 |
| UAS–reaper | w1118;+;P{w+mc=UAS–reaper}/TM3 Sb;+ | Drosophila Stock Center, Indiana University, Bloomington, IN |
| w1118 | w1118;+;+;+ | |
| UAS–2xeGFP | w-;+; P{w+mC=UAS–2xEGFP};+ | Halfon et al., 2002 |
TβH-lines.
TβHnM18 flies have a null mutation at the tyramine-β-hydroxylase (TβH) locus. The phenotype includes an approximately eightfold increase in tyramine concentration and completely lacks OA (Monastirioti et al., 1996). The strain exhibits female sterility, caused by their inability to lay eggs. Otherwise, the flies appear normal, without dramatic effects on their behavior or lifespan. Because the original TβHM18 stock (Monastirioti et al., 1996) carries an additional mutation in the white (w) gene, the mutant and control stocks from Schwaerzel et al. (2003) were used, as mutations in the white gene might cause unspecific phenotypic effects. The octopamine mutants are recombinant flies with the w+ allele, and the corresponding nonrecombinant w+ lines serve as controls (Schwaerzel et al., 2003). Flies of the TβHnM18 hsp–TβH strain contain the TβH cDNA under control of the heat-shock protein 70 (HSP70) promoter in the TβH mutant background, making OA synthesis inducible by heat shock (HS) (Schwaerzel et al., 2003).
Gal4 driver lines.
The Drosophila tyrosine decarboxylase 2 (dTdc2)–galactosidase-4 (Gal4) driver is expressed in clusters of neurons throughout brain and nerve cord. The gene encoding the neuronal enzyme tyrosine decarboxylase (TDC) was identified recently, and the coding section of the yeast GAL4 gene was inserted into it, immediately before the coding start (Cole et al., 2005). We made use of this genetic tool, driving the apoptosis-inducing construct upstream activating sequence (UAS)–reaper and the construct for the enhanced green fluorescent protein (UAS–2xeGFP).
Reporter strains.
The cell death gene reaper (White et al., 1994) acts dominantly to kill cells in which it is expressed. Because it has been incorporated into a UAS vector (Zhou et al., 1997), cell-specific ablation can be accomplished efficiently and accurately. The F1 transheterozygote offspring of the dTdc2–Gal4 × UAS–reaper cross served as the experimental strain. Parent dTdc2–Gal4 and UAS–reaper strains were used as controls. The white-eyed w1118 strain was also chosen as control line, because it is the original nonrecombinant line from which the dTdc2–Gal4 and the UAS–reaper strains have been created. dTdc2–Gal4 and UAS–reaper were backcrossed with white, and the progeny was used as heterozygous control. For visualization of octopaminergic and tyraminergic cells, dTdc2–Gal4 virgins were crossed with UAS–2xeGFP (two times enhanced green fluorescent protein) (Halfon et al., 2002) males.
Treatments for behavioral rescue experiments
Octopamine.
Flies were raised on OA-containing medium. To obtain an OA (O0250; Sigma, St. Louis, MO) concentration of 10 mg/ml, each vial containing 15 ml of freshly prepared standard food was supplemented with 150 mg of octopamine diluted in 900 μl of distilled water. The OA solution was added while the food was still liquid but at a temperature below 50°C. Distilled water without OA (also 900 μl) was added to control vials. Four-day-old flies were transferred to the vials for oviposition and removed after 24 h. The progeny was raised on the OA-supplemented food and used for experiments later.
Yohimbine.
To feed yohimbine (YH) (Y3125; Sigma), a 5% sucrose (S1888; Sigma) solution with or without yohimbine added (10 mg/ml) was pipetted onto five pieces of filter paper in cylindrical vials before transferring 10–20 mutants into the vials. After 1–2 h, the animals were singled out and prepared for testing.
Heat shock.
Flies (TβHnM18 hsp–TβH) were kept at 37°C for 45 min twice with a 6 h interval and were then allowed to recover for 12 h before experiments.
Behavioral testing
Three- to 5-d-old male flies were briefly immobilized by cold anesthesia and glued [clear glass adhesive (Duro; Pacer Technology, Rancho Cucamonga, CA)] with head and thorax to a triangle-shaped copper hook (0.02 mm diameter). Adhesion was achieved by exposure to UV light for 10 s. The animals are then kept individually in small chambers containing a few grains of sucrose until testing (1–5 h).
The fly, glued to the hook as described above, was attached to the experimental setup via a clamp to accomplish stationary flight. For observation, the fly was illuminated from behind and above (150 W, 15 V; Schott, Elmsford, NY) and fixed in front of a polystyrene panel. Additionally, it was shielded by another polystyrene panel from the experimenter. Tarsal contact with a bead of polystyrene prevented flight initiation before the experiment started. A digital high-speed camera (1000 pictures per second; Motion Scope; Redlake Imaging, Morgan Hill, CA) was positioned behind the test animal. To initiate flight, the polystyrene bead was removed, and the fly was gently aspirated. The time until the fly ceased flying was recorded (initial flight). The fly was aspirated as a stimulation to fly, each time it stopped flying. When no flight reaction was shown after three consecutive stimulations, the experiment was completed and the total flight time was recorded (extended flight). Every stimulus after the first one, to which the fly showed a response, was recorded. Each fly was filmed during the first few seconds of flight, and the recordings were saved on a personal computer for later analysis. The person scoring the flight time was unaware of the treatment group of the animal. All animals were included in the study, including those that did not show any flight behavior.
Neuroanatomical stainings
Immunocytochemistry.
For immunohistochemical stainings of Drosophila CNS with GFP antibody (Ab), fly CNS was removed in saline. After fixation for 1 h in 4% paraformaldehyde (PFA) (10 ml of PBS plus 0.4 g of PFA, pH 7.4), the CNS was treated with a mixture of enzymes (collagenase/dispase, 1 mg/ml each) for 1 min to ensure better penetration of antibodies (Abs) into the tissue and then washed in PBS (0.1 m) overnight at 4°C. Preparations were then washed six times for 30 min in 0.5% Triton X-100 in PBS (PBSTx), again to increase the penetration of Ab into the tissue. Subsequently, the CNS was placed for 2 d in a 1:200 dilution of the anti-GFP primary Ab mouse serum in 0.3% PBSTx at 4°C. They were then rinsed eight times for 15 min in PBS and then incubated at 4°C overnight in a 1:500 dilution of the secondary Ab serum that was coupled to a fluorescent dye [anti-mouse cyanine 2 (Cy2)] in PBS. After rinsing the preparations eight times for 15 min in PBS, they were dehydrated in an ascending ethanol series (50, 70, 90, and 100%, 10 min each) and then transferred to a microscope slide and cleared in methylsalicylate. For immunohistochemical stainings of Drosophila CNS for presynaptic active zones with bruchpilot antibody (Wagh et al., 2006) (gift from E. Buchner, University of Wuerzburg, Wuerzburg, Germany), the same protocol was followed with the exception that the primary Ab was diluted 1:100 in 0.3% PBSTx.
Phalloidin stainings.
Flies were opened via a dorsal longitudinal cut in saline and then fixed in 4% PFA. After 1 h, they were transferred into PBS, and flight muscles were removed and washed three times for 1 h in 0.5% PBSTx. After treatment with 2 μl/ml Oregon Green phalloidin, 0.3% PBSTx for 36 h, the muscles were washed six times for 15 min in PBS and finally embedded in glycerin on a microscope slide.
Confocal microscopy.
The preparations were viewed under a Leica (Bensheim, Germany) SP2 confocal laser-scanning microscope with 40× oil immersion objective. Stacks of optical sections (0.5 μm) were acquired. Both Cy2 and Oregon Green phalloidin were excited with an argon laser at 488 nm, and emitted light was detected between 500 and 530 nm.
Data analysis
Wing-beat amplitude.
For wing-beat amplitude measurements, Redlake Imaging MotionScope software (DEL Imaging Systems, Cheshire, CT) was used to capture the first 100 frames. After image inversion, the image stacks were imported into AMIRA software (TGS, San Diego, CA) for overlaying of all frames (projection view) and then measuring wing angles using the angle-measuring tool.
Wing-beat frequency.
To measure the wing-beat frequency, the number of frames per 10 wing beats was counted, starting from frame 1, 100 and 300 in each sequence, and subsequently the mean was calculated.
Sarcomere length.
For sarcomere-length survey, the images of phalloidin-stained muscles were imported into AMIRA software, and sarcomeres were measured with the line-measuring tool. For each animal, the lengths of 31–41 sarcomeres were measured.
Flight time per stimulation.
To calculate flight time per stimulation, the total flight time was divided by the number of stimulations, including the initial one.
Statistics.
The flight data approximately conformed to a Poisson distribution, and hence nonparametric tests were used. For comparison of more than two groups, a Kruskal–Wallis ANOVA was used to test the hypothesis that the samples were drawn from the same population. When differences between the samples occurred, Mann–Whitney U tests were performed for planned comparisons of two samples. Two groups were always compared with a Mann–Whitney U test. To display the measurements, box-and-whisker plots were chosen, and medians were used as central values. Boxes included the medial 25–75%, and, because the data show many extreme scores, the whiskers included 15–85% of the data values. Outliers were not shown. Significant differences were accepted at p < 0.05.
A full rescue is scored when the rescue group differs significantly from the mutant but not from the wild-type control. For a partial rescue, the rescue line must either differ significantly from both mutant and wild type or not differ from both. No rescue is achieved when no significant difference is obtained between the mutant flies and the rescue line and a significant difference remains for the wild-type controls.
Results
Flight initiation and maintenance deficits in flies lacking octopamine
There currently is only one viable strain lacking OA, a null mutant in the TβH gene, TβHnM18 (Monastirioti et al., 1996). Mutants lacking OA are able to fly, clearly demonstrating that OA is not required for flight initiation. However, TβHnM18 mutants show a drastic decrease in the initial flight duration (Fig. 1a), in all subsequent flight episodes [i.e., average flight duration per stimulation (Fig. 1b, Average flight duration)] and thus also in total flight duration (Fig. 1c, Total flight duration). Moreover, the mutants resume flight less often after stimulation compared with control animals (Fig. 1d, Flight initiations). Therefore, TβHnM18 mutants take off significantly less often in response to wind stimuli than wild-type controls (Fig. 1d), and, once airborne, they fly for significantly shorter durations (Fig. 1a–c).
Figure 1.
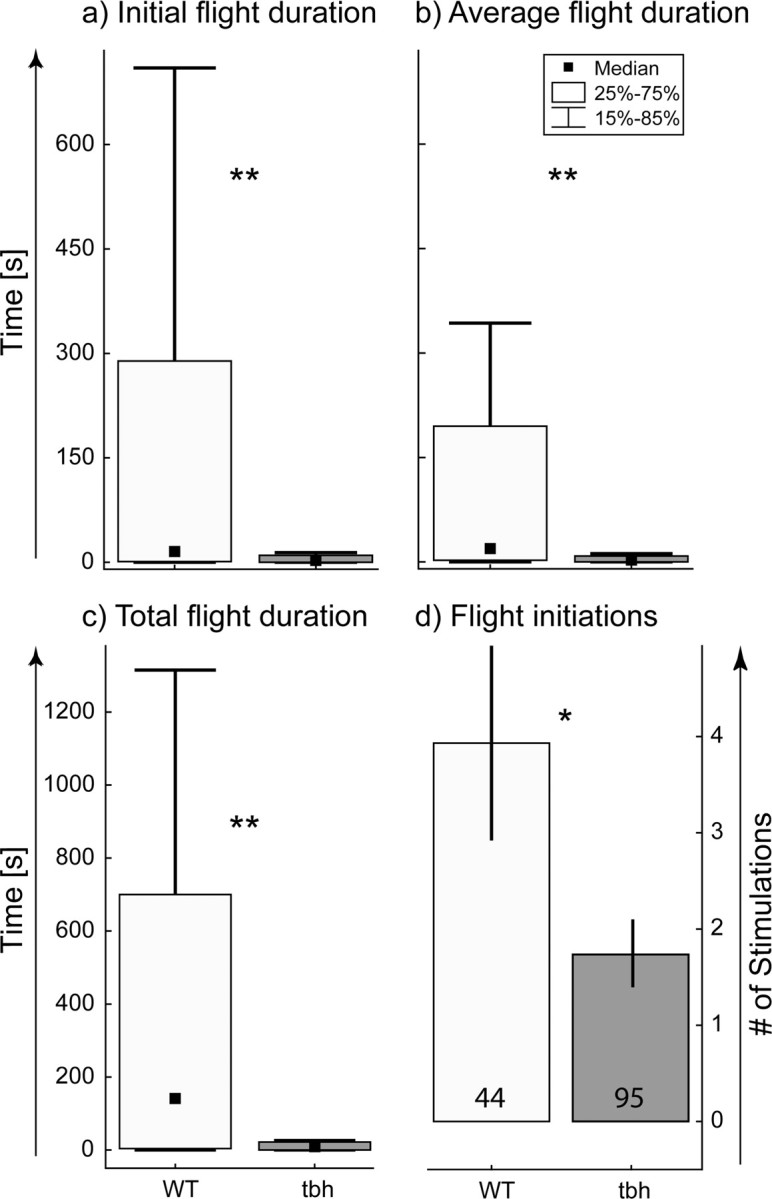
Comparison of flight initiation and maintenance between TβH mutant and wild-type flies. For a–c, the black squares indicate the median, the boxes signify the 25 and the 75 percentiles, and the error bars range from the 15 to the 85 percentiles. a shows the flight duration until the first stop for wild-type (WT; light gray bar) and TβH null mutant (tbh; dark gray bar) flies. b indicates the duration of all flight bouts for wild-type and TβH flies. c shows the total flight duration for wild-type and TβH flies. d shows the mean number of stimuli to which wild-type and TβH mutant flies responded with flight bouts before they did not respond to three consecutive stimuli (error bars are SEMs). The number of animals per group is indicated in the bars. *p < 0.05, **p < 0.01, Mann–Whitney U test.
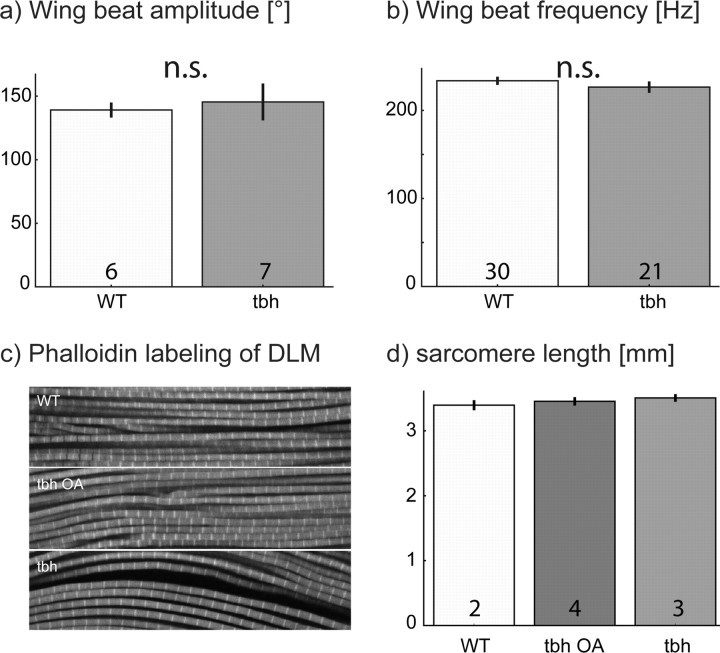
A number of flight motor system parameters do not differ between mutants and wild type, suggesting that the basic function and morphology of the flight apparatus is unaffected in TβHnM18 mutants. With regard to motor output, wing-beat amplitudes (Fig. 2a) and wing-beat frequencies are similar in TβHnM18 mutants and wild-type controls (Fig. 2b). On the muscular level, sarcomere length of the dorsal longitudinal flight muscle (DLM) flight is not affected in TβHnM18 mutants (Fig. 2c,d). Figure 2, c and d, includes a third group of flies, TβHnM18 mutants that were fed with octopamine to rescue the flight behavior phonotype (see below). Sarcomere lengths are similar in wild type, TβHnM18 mutants, and TβHnM18 mutants rescued by feeding octopamine. Within the CNS, the overall morphology of the DLM motoneurons MN1–MN5 appears similar between wild-type controls and TβHnM18 mutants as revealed by dye backfilling from the DLM flight muscle (data not shown). Consequently, the observed changes in flight behavior may be attributable to the acute changes in the titers of OA and TA (lack of OA and increase in TA) rather than to developmental defects. However, there may be differences in the number and strength of synaptic inputs or in the fine branching structure of flight motoneurons and interneurons, which were not subjected to this study. To further test whether the acute lack of OA in adults was a main cause for the observed flight behavior deficits, we conducted a number of rescue experiments.
Figure 2.
The development of the flight system is not impaired in TβH mutant flies. a shows the mean wing-beat amplitudes for wild-type (WT; light gray bar) and TβH mutant (tbh; dark gray bar) flies. b shows the mean wing-beat frequencies for wild-type (WT; light gray bar) and TβH mutant (tbh; dark gray bar) flies. c shows representative fields of view of DLM flight muscle fibers with phalloidin-labeled actin bands for wild-type (WT), TβH mutant (tbh), and TβH mutant flies that were fed with octopamine (tbh OA). d shows the mean sarcomere lengths for the three groups shown in c. Numbers in bars indicate numbers of animals. Error bars are SEMs. n.s., Not significant.
Manipulating octopamine and tyramine rescues flight initiation and maintenance
Rescuing the phenotype in TβHnM18 mutants is not a trivial task, because these flies not only lack OA but also show an eightfold increase in the concentration of the OA precursor TA. To adequately address this issue, we designed rescue experiments combining pharmacological and genetic techniques. For clarity, the tyramine and octopamine biosynthesis pathway is shown schematically in Figure 3e; genetic or pharmacological knockdowns as used throughout this study are indicated in light gray, and genetic or pharmacological rescues are indicated in dark gray. To oppose the effects of increased TA concentration, we fed the flies the selective competitive α2-adrenergic receptor antagonist YH, which has been demonstrated to block Drosophila tyramine receptors (TARs) (Arakawa et al., 1990; Saudou et al., 1990). To increase OA concentration in TβHnM18 mutants, we either fed the flies OA or induced TβH expression in all cells via an HS-inducible TβH transgene in the TβH null mutant genetic background. The following four permutations were tested as experimental groups: (1) TβHnM18; hsp–TβH + HS, (2) TβHnM18; hsp–TβH + HS + YH, (3) TβHnM18; hsp–TβH + YH, and (4) TβHnM18 + OA. The three negative control groups were TβH null mutants, TβH null mutant with a heat-shock-inducible TβH transgene kept at normal temperature, and TβH null mutants without inducible TβH transgene were exposed to the heat shock (TβHnM18, TβHnM18 hsp–TβH, and TβHnM18 + HS). The three control groups do not differ in any of the flight behavior parameters investigated (data not shown), and their data were thus pooled. The w+ strain serves as positive control (for strain genotype, see Materials and Methods).
Figure 3.
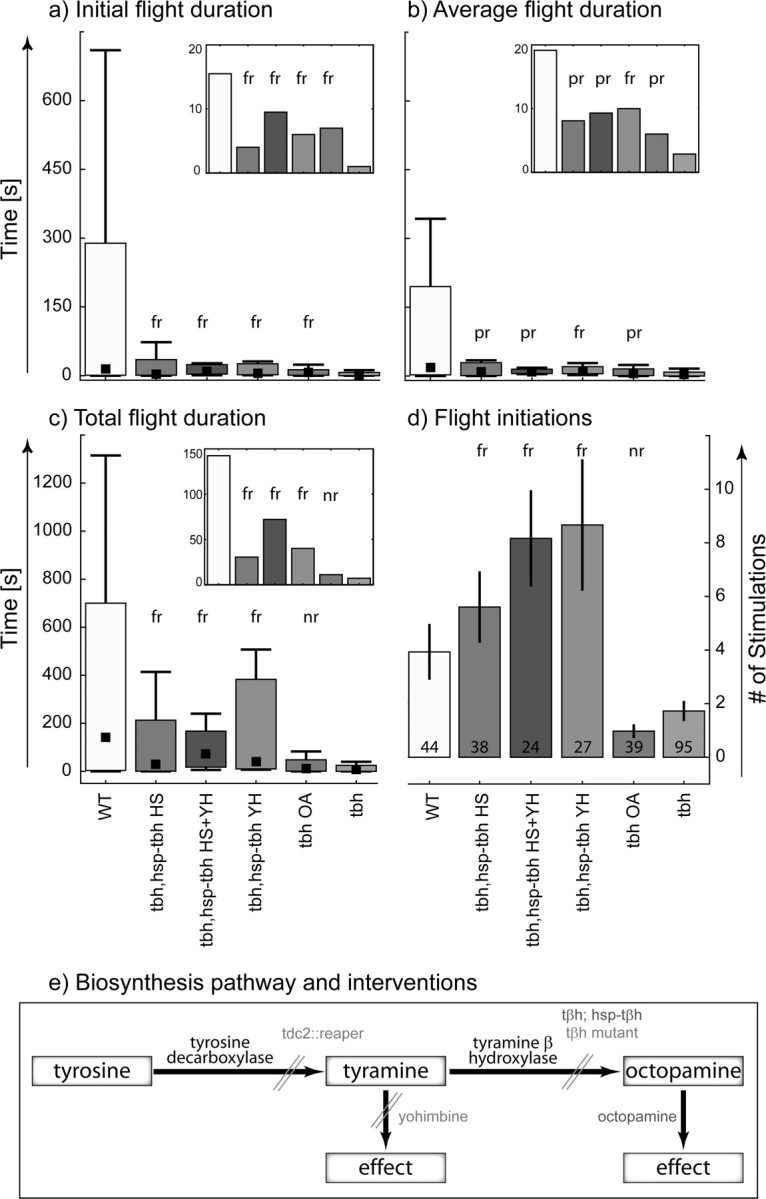
Different types of rescues of the TβHnM18 caused flight behavior phenotypes. For a–c, the black squares indicate the median, the boxes signify the 25 and the 75 percentiles, and the error bars range from the 15 to the 85 percentiles. To allow for a better between-group comparison, insets in a to c depict bar graphs of the respective medians at a higher y-axis resolution. a shows the duration of the initial flight bout for each experimental group, b shows the average duration of a flight bout for each group, c shows the total flight duration, and d shows the number of stimuli to which the flies responded with flight before they did not respond to three consecutive stimuli. fr, Full rescue; pr, partial rescue; nr, no rescue (for definition, see Materials and Methods). The experimental groups were wild-type flies (WT), a genetic rescue in which TβH expression in TβH mutant flies was induced in all cells via a heat-shock inducible TβH transgene in the TβH null mutant genetic background (tbh, hsp–tbh HS), a combined genetic and pharmacological rescue in which TβH expression was induced via a heat shock and in which the flies were also fed the tyramine receptor blocker yohimbine (tbh, hsp–tbh HS + YH), a pharmacological rescue in which TβH mutant flies containing the inducible TβH transgene received no heat shock but were fed yohimbine (tbh, hsp–tbh YH), a pharmacological rescue in which TβH mutant flies were fed octopamine (tbh OA), and TβH mutant flies (tbh). e shows the biosynthesis pathway of tyramine and octopamine from tyrosine. Genetic and pharmacological blocks are depicted in light gray. TA synthesis is blocked by killing all cells containing tyrosine decarboxylase by expressing reaper. OA synthesis is blocked in tyramine hydroxylase null mutants (TβHnM18). TARs are blocked by yohimbine. Rescues are depicted in dark gray. Octopamine levels were increased by either expressing tyramine hydroxylase under the control of a heat shock promoter or by feeding OA.
For the duration of the initial flight phase, we obtained a full rescue in all four experimental groups (Fig. 3a, see inset for comparison of medians only). Feeding YH and treating with HS in the same flies (HS + YH) yields the best rescue (median of 9; p < 0.001 compared with TβH flies, p = 0.464 compared with wild-type flies) followed by feeding YH only (median of 6; p = 0.001 compared with TβH flies, p = 0.284 compared with wild-type flies). Next are feeding OA (median of 8; p = 0.005 compared with TβH flies, p = 0.1 compared with wild-type flies) and HS only (median = 4; p = 0.013 compared with TβH flies, p = 0.169 compared with wild-type flies). In summary, blocking TA action pharmacologically, replacing OA genetically or pharmacologically, or combining TA and OA manipulations rescues the TβH phenotype with respect to the duration of the initial flight bout.
Average flight duration per stimulation is at least partially rescued in all experimental groups (Fig. 3b, see inset for comparison of medians). A full rescue is obtained only by feeding YH alone (median of 4; p < 0.001 compared with TβH flies, p = 0.114 compared with wild-type flies). Partial rescues can be achieved with HS + YH (median of 7; p < 0.001 compared with TβH flies, p = 0.047 compared with wild-type flies), with HS (median of 2; p = 0.025 compared with TβH flies, p = 0.032 compared with wild-type flies), and by feeding OA (median of 3; p = 0.025 compared with TβH flies, p = 0.015 compared with wild-type flies). In summary, a full rescue of the average flight duration in multiple subsequent flight bouts is achieved only by blocking TA receptors but not by replacing OA either genetically or pharmacologically.
The duration of total flight (Fig. 3c) can be fully rescued by HS + YH (median of 72; p < 0.001 compared with TβH flies, p = 0.259 compared with wild-type flies), by only feeding YH (median of 40; p < 0.001 compared with TβH flies, p = 0.441 compared with wild-type flies), and by HS (median of 30; p = 0.002 compared with TβH flies, p = 0.076 compared with wild-type flies) but not by supplementing OA alone (median of 11; p = 0.163 compared with TβH flies, p = 0.005 compared with wild-type flies). Total flight duration is the product of the number of flight initiations times the average time of the flight bouts. The average time of the flight bouts is partially rescued by feeding OA (Fig. 3b), but the number of responses (flight initiations) is not rescued by feeding OA to TβH flies (Fig. 3d).
The responsiveness to stimulation (Fig. 3d) was fully rescued by feeding YH (median of 10; p < 0.001 compared with TβH flies, p = 0.083 compared with wild-type flies) and by HS (median of 8,1; p = 0.021 compared with TβH flies, p = 0.599 compared with wild-type flies). Feeding OA only did not rescue this phenotype (p = 0.994 over TβH flies, p = 0.053 over wild-type flies) but even caused a slight but nonsignificant decrease in the responsiveness to stimulation. HS + YH-treated animals responded to stimulation even more often than wild-type flies (median of 9.3; p < 0.001 compared with TβH flies, p = 0.028 compared with wild-type flies).
This complex set of full and partial rescues depending on OA and TA manipulation demonstrates that flight behavior depends on OA and on TA. One possibility is that OA and TA each act on different aspects of the flight machinery, such as sensory sensitivity, muscle metabolism, or CPG activation. Alternatively, OA and TA might act antagonistically on similar aspects of motor behavior, and thus, the absolute levels of one modulator are not important, but the relative levels of both modulators influence flight behavior. In a first test of the latter hypothesis, we ablated all neurons synthesizing TA from tyrosine by expressing the apoptosis-inducing gene reaper under control of the dTdc2 promotor (for details, see Materials and Methods). The dTdc2 gene codes for the neural version of two TDC enzymes converting tyrosine to TA.
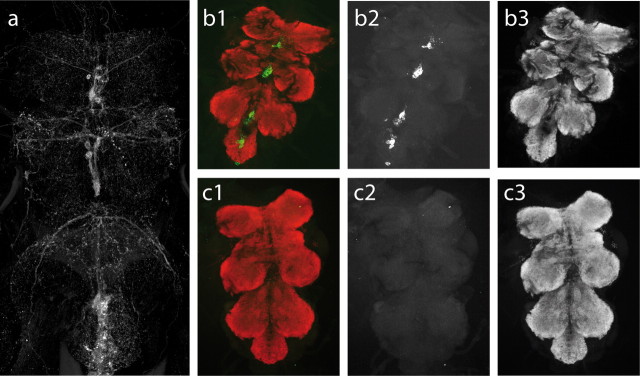
Because TA is the precursor of OA, dTdc2 expresses in all neurons containing TA or OA, as can be visualized by expressing eGFP under the control of dTdc2 and enhancing the eGFP signal by anti-GFP immunocytochemistry (Fig. 4a). Cell bodies of dTdc2 neurons are located in the midlines of each thoracic and each abdominal neuromere, bilateral symmetric processes of efferent unpaired median neurons can clearly be seen, and a large number of finer aminergic processes with numerous varicosity-like structures can be visualized within the CNS (Fig. 4a).
Figure 4.
Genetic ablation of all tyraminergic and octopaminergic neurons. a, Visualization of all tyraminergic and octopaminergic neurons in the thoracic and abdominal ventral nerve cord by expressing 2xeGFP under the control of Tdc2 and enhancing the signal by anti-GFP immunocytochemistry. To test the effectiveness of neuron ablation by targeted ectopic expression of the cell death gene reaper, animals expressing either only GFP or GFP together with reaper were subjected to standard immunohistochemistry. Animals expressing only GFP reveal the expression pattern typical of Tdc2 neurons. b1 shows double labels of the ventral nerve cord for Tdc2 neurons labeled by targeted expression of eGFP (green) and all synapses labeled with bruchpilot antibody Nc82 (Kittel et al., 2006) (red) to visualize presynaptic active zones in the neuropil regions. b2 and b3 show the Tdc2 and the Nc82 signal separately as grayscale images. b1–b3 show a ventral nerve cord from heterozygous progeny of dTdc2–Gal4 crossed with y w P{w+mC=UAS–2xEGFP}. c, Gal4-driven apoptosis was induced by crossing dTdc2–Gal4 with w;; P{UAS-rpr}/TM3 Sb, and eGFP was from y w P{w+mC=UAS–2xEGFP}. No GFP expression can be detected in animals with targeted expression of both GFP and reaper to these OA/TA cells (c1), but Nc82 immunostaining appears unaffected in these animals (c3), demonstrating effective and specific ablation.
Expressing the apoptosis signal reaper under the control of dTdc2 causes a complete and specific ablation of TA- and OA-containing neurons (Fig. 4b,c). This genetic ablation of all neurons releasing TA or OA also leads to a profound decrease in all four behavioral parameters studied compared with control strains (Fig. 5). The genetic controls were parent dTdc2–Gal4 and UAS–reaper strains. The white-eyed w1118 strain was also chosen as control line, because it is the original nonrecombinant line from which the dTdc2–Gal4 and the UAS–reaper strains have been created. dTdc2–Gal4 and UAS–reaper flies were backcrossed with white flies, and the progeny were used as heterozygous controls. The three control groups did not differ in flight behavior (data not shown), and their data were pooled (Fig. 5). Similar to knocking out OA only in TβHnM18 mutants (Fig. 1), ablating all TA and OA neurons drastically decreased the initial flight duration (Fig. 5a), the flight duration per stimulation (Fig. 5b), and extended flight (Fig. 5c, Total flight duration). Moreover, the mutants resumed flight less often after stimulation compared with control animals (Fig. 5d). However, it is noteworthy that flies with all TA- and OA-containing neurons ablated were still able to fly, and wing-beat frequencies were normal. In summary, in flies without TA- or OA-containing neurons, flight initiation and maintenance are affected in a similar manner to flies lacking OA but having increased TA levels.
Figure 5.
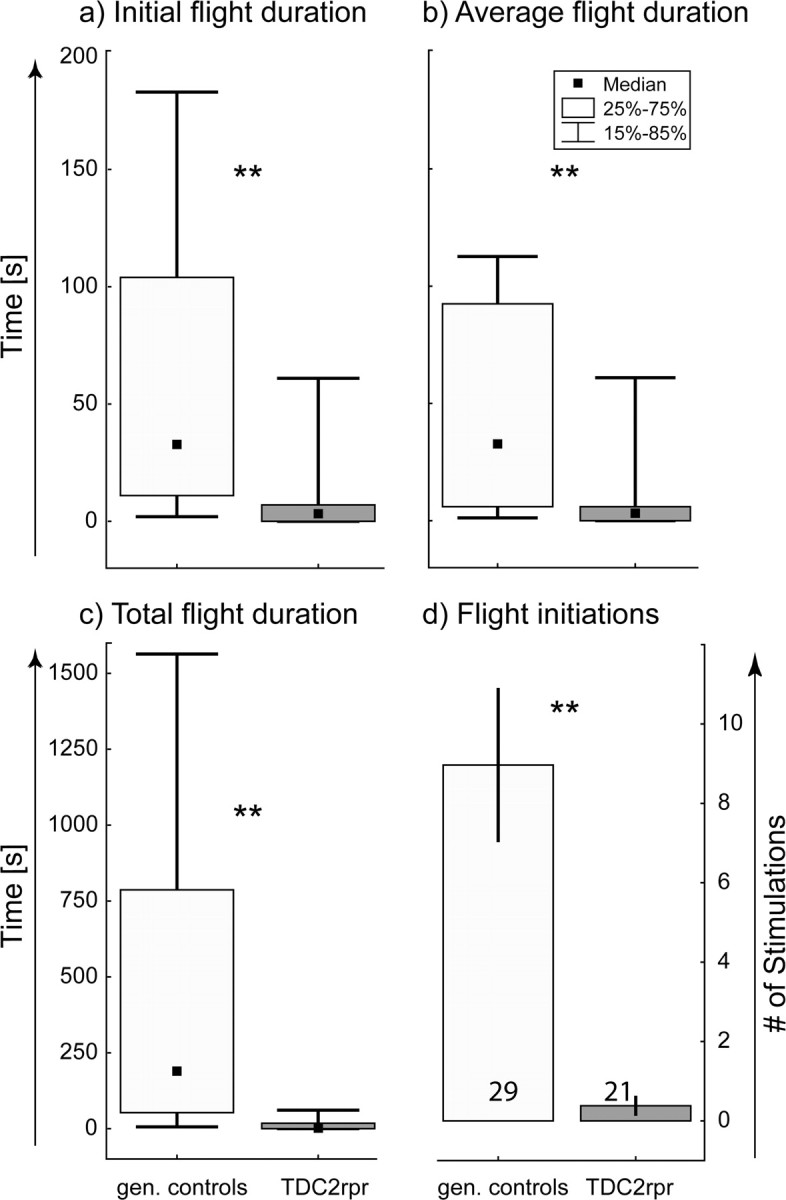
Genetic ablation of all tyraminergic and octopaminergic neurons decreases flight initiation and maintenance. For a–c, the black squares indicate the median, the boxes signify the 25 and the 75 percentiles, and the error bars range from the 15 to the 85 percentiles. a shows the flight duration until the first stop in control flies (gen. controls; light gray bar) and for flies expressing reaper under the control of TDC2 (TDC2rpr; dark gray bar). b indicates the mean duration of all flight bouts for control and TDC2rpr flies. c shows the total flight duration for control and TDC2rpr flies. d shows the mean number of stimuli to which control and TDC2rpr responded with flight bouts before they did not respond to three consecutive stimuli (error bars are SEMs). **p < 0.01, Mann–Whitney U test.
At first glance, it appears contradictory that TβHnM18 mutants can be rescued by blocking TA receptors, but flies without OA and without TA show behavioral phenotypes similar to TβHnM18 mutants. This result clearly opposes the interpretation that OA and TA simply act antagonistically on the same targets, but it might be explained by dose effects and different sites of action (see Discussion). However, we further tested the effects of TA on flight behavior in flies with normal OA and TA levels by pharmacological block of TA action.
We compared initial flight (Fig. 6a), mean flight bout duration (Fig. 6b), total flight duration (Fig. 6c), and the number of stimulations causing flight (Fig. 6d) in wild-type flies that were fed with yohimbine and wild-type controls that were fed with sucrose solution only. Feeding yohimbine yields the most effective rescues of flight initiation and maintenance in TβHnM18 mutants (Fig. 3). However, none of these flight parameters is different among sucrose-fed and yohimbine-fed wild-type flies (Fig. 6). Consequently, flight initiation and maintenance do not depend strictly on the relative levels of OA and TA but are affected by some concerted interaction of both amines. Depleting OA and increasing TA impairs flight motor behavior, as does ablation of all OA- and TA-containing neurons. In OA-depleted flies with increased TA, flight initiation and maintenance can be rescued either by restoring OA levels or blocking TA action. In contrast, blocking TA action in flies with normal OA and TA levels does not affect any of the flight motor behavior parameters measured in this study.
Figure 6.
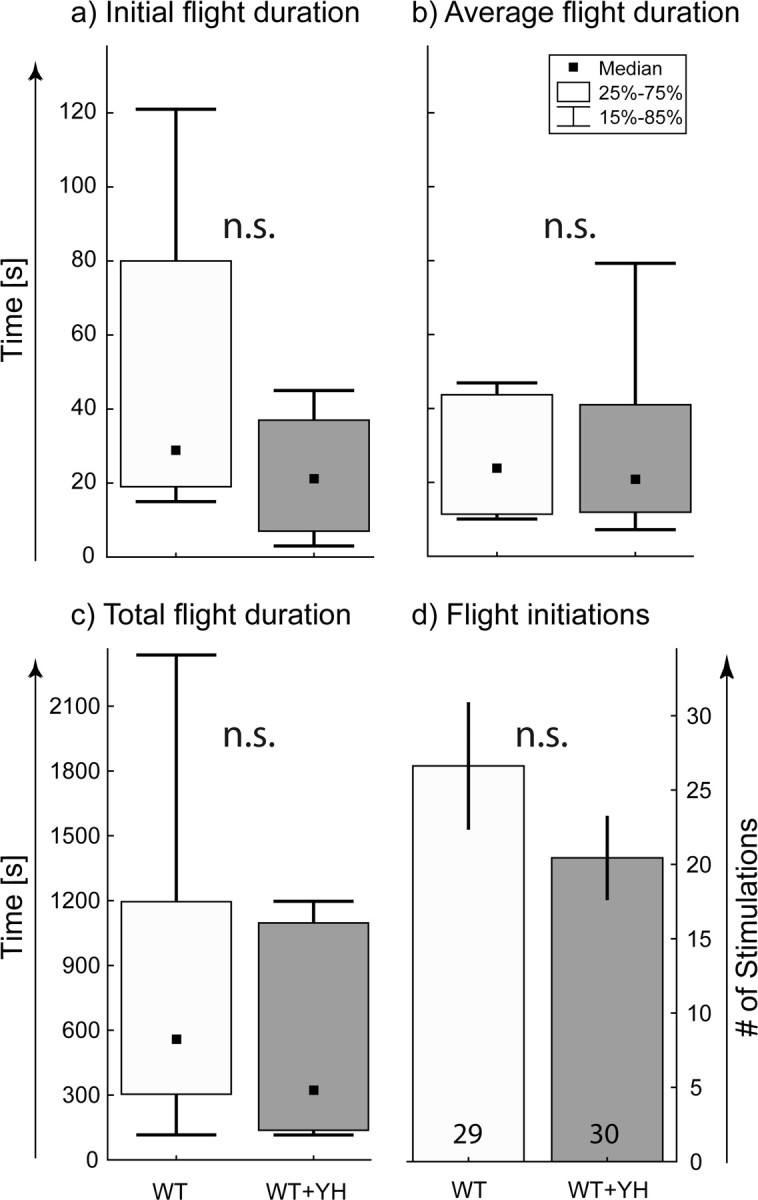
Blocking TA receptors in wild-type flies does not affect flight behavior. For a–c, the black squares indicate the median, the boxes signify the 25 and the 75 percentiles, and the error bars range from the 15 to the 85 percentiles. a shows the flight duration until the first stop in control wild-type flies fed with sucrose (WT; light gray bar) and for wild-type flies fed with the TA receptor blocker yohimbine (WT + YH; dark gray bar). b indicates the average duration of flight bouts for WT control and WT + YH flies. c shows the total flight duration for WT control and WT + YH flies. d shows the mean number of stimuli to which WT control and WT + YH responded with flight bouts before they did not respond to three consecutive stimuli (error bars are SEMs). n.s. indicates that no significant differences were found, Mann–Whitney U test.
Discussion
OA is not required for flight initiation
Flies lacking OA and having increased TA levels (TβH null mutants) show a profound decrease in flight initiation and maintenance compared with wild-type controls. Five lines of evidence suggest that morphology, kinematics, and development of the flight machinery are not impaired in TβH mutants: (1) wing-beat frequencies, (2) wing-beat amplitudes, (3) flight muscle structure (length of myofibrils), and (4) the number and overall dendritic structure of flight motoneurons are unaffected in TβH mutants, and (5) the behavioral phenotype can acutely be rescued in adult flies. Although acute application of OA is sufficient to elicit flight in a number of different insect preparations (Sombati and Hoyle, 1984; Claassen and Kammer, 1986; Stevenson and Kutsch, 1987; Duch and Pflueger, 1999), OA is not necessary for the initiation of flight in Drosophila but modulates flight initiation and maintenance. Even flies without any OA/TA-containing neurons can fly. Therefore, OA is either not a necessary natural signal for flight initiation or Drosophila flight initiation is a unique case.
Concerted action of OA and TA on flight behavior
A novel finding is that flies lacking OA and with TARs blocked show wild-type-like flight behavior. It is important to note that the TβH phenotype comprises OA knock-out plus eightfold increased TA levels. Pharmacological blockade of TARs yields the most efficient rescue of the TβH mutants, even outscoring replacement of OA by heat-shock plus TAR blockade. However, blocking TARs in wild-type flies does not increase flight initiation or maintenance. This indicates that TA inhibits flight behavior only at abnormally high TA levels. Furthermore, with regard to flight maintenance, the inhibitory effects of TA take place only at low OA levels, because OA replacement without affecting the TA system also yields rescues of the initial and the average flight bout durations. In contrast, the responsiveness to stimulation is rescued best by blocking TA. Therefore, flight initiation is most likely inhibited by high TA levels, regardless of the OA levels. Accordingly, feeding TβH mutants OA does not rescue flight initiation but restoring tyramine-β-hydroxylase activity by heat shock does, because only the latter manipulation decreases the levels of TA by conversion of TA into OA. Therefore, the most parsimonious interpretation is that OA is necessary for flight maintenance, and TA acts most likely as an inhibitor, especially for flight initiation at high concentrations.
This interpretation is further supported by ablating all OA/TA neurons by expressing the apoptosis factor reaper in these cells. Flies without OA/TA neurons show the same massive changes in flight behavior as TβH mutants. Therefore, genetic ablation of all TA/OA-containing neurons does not phenocopy genetic ablation of the OA-producing enzyme paired with pharmacological block of TA action. How can these seemingly contradictory results be explained? Clearly, the pharmacological treatment with yohimbine is effective; it fully rescues the mutant phenotype. The ablation of the OA/TA neurons is equally effective, ruling out methodological flaws. However, yohimbine does most likely not block all TA action, whereas genetic ablation of all TA-containing neurons does. Thus, the action of TA presumably follows a bell-shaped curve, with its presence necessary for normal flight but hindering flight initiation and maintenance at high concentration. OA is required most likely for flight maintenance because feeding it to TβH mutants fully rescues normal flight maintenance. However, OA supplementation in the food might also exert rescuing effects in TβH mutants by downregulating TA via feedback inhibition. In summary, the most compelling explanation for the data are that OA is boosting flight maintenance, low levels of TA are required for flight maintenance and initiation, and inhibitory TA actions fall in place at high TA and low OA levels.
TA as neurotransmitter/modulator
Our finding that OA and TA are involved in regulating flight emphasizes the role of TA as an independent neurotransmitter in invertebrates. Further supporting this role, tyramine-like immunoreactivity has been demonstrated in non-octopaminergic cells of Caenorhabditis elegans and locusts (Stevenson and Spoerhase-Eichmann, 1995; Donini and Lange, 2004; Alkema et al., 2005). Moreover, at least one Drosophila amine receptor is specific for TA and does not cross-react with OA (Cazzamali et al., 2005). Furthermore, OA and TA receptor distributions in the insect CNS differ considerably from each other [J. Erber (Technical University Berlin, Berlin, Germany), personal communication]. Functionally, exogenous TA increases chloride conductances in Drosophila malphigian tubules (Blumenthal, 2003), alters body wall muscle excitatory junction potentials (Kutsukake et al., 2000), and can rescue cocaine sensitization in Drosophila (McClung and Hirsh, 1999). In mammals, the physiological roles for trace amines such as TA and OA are mostly unknown, but they have been implicated in a variety of neurological disorders (Branchek and Blackburn, 2003), and receptors specific for TA have been identified (Borowsky et al., 2001). In invertebrates, a role of endogenous TA as an important transmitter/modulator has been shown for Drosophila locomotor (Saraswati et al., 2004; this study) and olfactory avoidance (Kutsukake et al., 2000) behavior, as well as for C. elegans motor behavior (Alkema et al., 2005).
Sites of OA and TA action
Previous studies suggested that OA acts as a potent, direct stimulator of flight muscle metabolism (Wegener, 1996; Mentel et al., 2003). Accordingly, we expected that especially prolonged flight would be affected in TβH mutants, attributable to insufficient fuel supply. In contrast, all flight parameters are similarly affected in TβH mutants. The initial flight bout duration is decreased ∼40 times, and the total flight duration is decreased ∼30 times in TβH mutants. Moreover, flight behavior changes in TβH mutants are rescued by blocking TA action alone, leaving OA levels unaltered. This is hard to reconcile with direct effects of OA on flight metabolism and would require independent effects of OA and TA on flight metabolism. These considerations render metabolism unlikely as the site of action for OA. Therefore, amine effects on Drosophila flight initiation and maintenance are more likely to be mediated by effects on the nervous system.
Two main OA/TA effects on flight behavior can be observed: maintenance of flight and the probability of initiating flight. In principle, both could be controlled by aminergic action on the CPG and/or on the fly's sensory system. It is well established that OA acts on the CPG in a number of insect species (Sombati and Hoyle, 1984; Claassen and Kammer, 1986; Stevenson and Kutsch, 1987), but central actions of TA are not known. OA has also been reported to increase the responsiveness of flight-associated sensory cells in insects (Ramirez and Orchard, 1990), and TA could conceivably reduce excitability of sensory neurons as Drosophila TARs activate chloride currents (Cazzamali et al., 2005).
Motor behavior specificity of combined amine effects
OA and TA have been implicated as agonist and antagonist, respectively, controlling locomotor behavior in Drosophila larvae (Saraswati et al., 2004; Fox et al., 2006) and in C. elegans (Alkema et al., 2005). This raises the possibility of a general, opponent OA/TA control of locomotor behavior in invertebrates. Our results make it unlikely that OA and TA simply act antagonistically on the same targets because, with regard to flight initiation and maintenance, OA and TA probably have different sites of action and TA effects are important only at high TA and low OA levels. Nevertheless, in some preliminary experiments, we tested whether TβHnM18 mutant adults show also walking behavior deficits. Neither the overall motor activity per unit time nor the number of walking bouts differed between wild-type and TβHnM18 mutant flies. However, we found a slight but statistically significant reduction in walking speed in TβHnM18 mutants (data not shown). These findings indicate that aminergic modulation by OA and TA does not act generally on locomotor performance but specifically affects different aspects of motor behaviors.
In summary, the emerging picture is that, for some motor behaviors, the concerted interaction of specific biogenic amines is more important than the concentration of single amines (Scheiner et al., 2002; Schwaerzel et al., 2003; Saraswati et al., 2004; Alkema et al., 2005; Fox et al., 2006; Fussnecker et al., 2006). The current study is the first to suggest that the antagonistic actions of OA and TA are not a general feature of all invertebrate locomotor behaviors but specifically affect distinct aspects of different motor behaviors. It provides evidence that OA and TA do not simply act antagonistically on the same targets but most likely mediate their effects on motor performance by affecting different targets in a dose-dependent manner. The next steps toward understanding amine function for motor behavior is to determine their sites of action during behavior. One possibility addressing this question is to combine pharmacological and genetic rescues and test immunocytochemically where the OA and TA levels are restored in which rescue procedure, how behavior is affected in these different manipulations, and where the various subtypes of TA and OA receptors are localized. Ultimately, a complete understanding of the mechanism by which various modulators interact on different parts of the brain and other tissues to control motor behavior will require a large number of targeted manipulations of each individual circuit component separately.
Footnotes
This work was supported by the German Science Foundation (C.D., H.J.P.). We are grateful for the help of Fernando Vonhoff with our behavioral experiments. We thank Drs. R. B. Levine (University of Arizona, Tucson, AZ) and J. A. Mustard (Arizona State University, Tempe, AZ) for many critical comments on this manuscript and Marinus de Bruyne and Wernher Fouquet for discussions concerning fly genetics.
References
- Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46:247–260. doi: 10.1016/j.neuron.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Arakawa S, Gocayne JD, McCombie WR, Urquhart DA, Hall LM, Fraser CM, Venter JC. Cloning, localization, and permanent expression of a Drosophila tyramine receptor. Neuron. 1990;2:342–354. doi: 10.1016/0896-6273(90)90047-j. [DOI] [PubMed] [Google Scholar]
- Baier A, Wittek B, Brembs B. Drosophila as a model organism for the neurobiology of aggression. J Exp Biol. 2002;205:1233–1240. doi: 10.1242/jeb.205.9.1233. [DOI] [PubMed] [Google Scholar]
- Blenau W, Baumann A. Aminergic signal transduction in invertebrates: focus on tyramine and octopamine receptors. Recent Res Dev Neurochem. 2003;6:225–240. [Google Scholar]
- Blumenthal EM. Regulation of chloride permeability by endogenously produced tyramine in the Drosophila malphigian tubule. Am J Cell Physiol. 2003;284:C718–C728. doi: 10.1152/ajpcell.00359.2002. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S. Trace amines: identification of a family a mammalian G protein-coupled receptors. Proc Natl Acad Sci USA. 2001;98:8866–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchek TA, Blackburn TP. Trace amines receptors as targets for novel therapeutics: legend, myth and fact. Curr Opin Pharmacol. 2003;3:90–97. doi: 10.1016/s1471-4892(02)00028-0. [DOI] [PubMed] [Google Scholar]
- Cazzamali G, Klaerke DA, Grimmelikhuijzen CJP. A new family of insect tyramine receptors. Biochem Biophys Res Comm. 2005;2:1189–1196. doi: 10.1016/j.bbrc.2005.10.058. [DOI] [PubMed] [Google Scholar]
- Claassen DE, Kammer AE. Effects of octopamine, dopamine, and serotonin on production of flight motor output by thoracic ganglia of Manduca sexta. J Neurobiol. 1986;17:1–14. doi: 10.1002/neu.480170102. [DOI] [PubMed] [Google Scholar]
- Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem. 2005;280:14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- Donini A, Lange AB. Evidence for a possible neurotransmitter/neuromodulator role of tyramine on the locust oviduct. J Insect Physiol. 2004;50:351–361. doi: 10.1016/j.jinsphys.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Duch C, Pflueger HJ. DUM neurons in locust flight: a model system for amine-mediated peripheral adjustments to the requirements of a central motor program. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1999;184:489–499. [Google Scholar]
- Edwards JS. The central nervous control of insect flight. J Exp Biol. 2006;209:4411–4413. doi: 10.1242/jeb.02592. [DOI] [PubMed] [Google Scholar]
- Evans PD, O'Shea M. An octopaminergic neurone modulates neuromuscular transmission in the locust. Nature. 1979;270:257–259. doi: 10.1038/270257a0. [DOI] [PubMed] [Google Scholar]
- Fox LE, Soll DR, Wu CF. Coordination and modulation of locomotion pattern generators in Drosophila larvae: effects of altered biogenic amine levels by the tyramine β hydroxylase mutation. J Neurosci. 2006;26:1486–1498. doi: 10.1523/JNEUROSCI.4749-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fussnecker BL, Smith BH, Mustard JA. Octopamine and tyramine influence the behavioral profile of locomotor activity in the honey bee (Apis mellifera) J Insect Physiol. 2006;52:1083–1092. doi: 10.1016/j.jinsphys.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Markram H, De Schutter E, Silberberg G, LeBeau FE. Microcircuits in action—from CPGs to neocortex. Trends Neurosci. 2005;28:525–533. doi: 10.1016/j.tins.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Halfon MS, Gisselbrecht S, Lu J, Estrada B, Keshishian H, Michelson AM. New fluorescent protein reporters for use with the Drosophila Gal4 expression system and for vital detection of balancer chromosomes. Genesis. 2002;34:135–138. doi: 10.1002/gene.10136. [DOI] [PubMed] [Google Scholar]
- Hoyle G. Generation of motor activity and control of behaviour: the role of the neuromodulator octopamine and the orchestration hypothesis. In: Kerkut GA, Gilbert L, editors. Comparative insect physiology, biochemistry and pharmacology. Vol 5. Toronto: Pergamon; 1985. pp. 607–621. [Google Scholar]
- Kiehn O, Kullander K. Central pattern generators deciphered by molecular genetics. Neuron. 2004;41:317–321. doi: 10.1016/s0896-6273(04)00042-x. [DOI] [PubMed] [Google Scholar]
- Kittel RJ, Wichman C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, Pawlu C, Kellner RR, Willig KI, Hell SW, Buchner E, Heckmann M, Sigrist SJ. Bruchpilot promotes active zone assembly, calcium channel clustering, and vesicle release. Science. 2006;312:1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- Kobayashi EA. Role of catecholamine signaling in brain and nervous system functions: new insights from mouse molecular genetic study. J Investig Dermatol Symp Proc. 2001;6:115–121. doi: 10.1046/j.0022-202x.2001.00011.x. [DOI] [PubMed] [Google Scholar]
- Kravitz EA, Huber R. Aggression in invertebrates. Curr Opin Neurobiol. 2003;13:736–743. doi: 10.1016/j.conb.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Kutsukake M, Komatsu A, Yamamoto D, Ishiwa-Chigusa S. A tyramine receptor gene mutation causes a defective olfactory behavior in Drosophila melanogaster. Gene. 2000;245:31–42. doi: 10.1016/s0378-1119(99)00569-7. [DOI] [PubMed] [Google Scholar]
- Leitch B, Judge S, Pitman RM. Octopaminergic modulation of synaptic transmission between an identified sensory afferent and flight motoneuron in the locust. J Comp Neurol. 2003;462:55–70. doi: 10.1002/cne.10698. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Curr Biol. 2001;11:R986–R996. doi: 10.1016/s0960-9822(01)00581-4. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D, Schulz DJ, Taylor AL. Invertebrate central pattern generation moves along. Curr Biol. 2005;6:R685–R699. doi: 10.1016/j.cub.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Matheson T. Octopamine modulates the responses and presynaptic inhibition of proprioceptive sensory neurones in the locust Schistocerca gregaria. J Exp Biol. 1997;200:1317–1325. doi: 10.1242/jeb.200.9.1317. [DOI] [PubMed] [Google Scholar]
- McClung C, Hirsh J. The trace amine tyramine is essential for sensitization to cocaine in Drosophila. Curr Biol. 1999;9:853–860. doi: 10.1016/s0960-9822(99)80389-3. [DOI] [PubMed] [Google Scholar]
- Mentel T, Duch C, Stypa H, Wegener G, Mueller U, Pflueger HJ. Central modulatory neurons control fuel selection in flight muscle of migratory locust. J Neurosci. 2003;23:1109–1113. doi: 10.1523/JNEUROSCI.23-04-01109.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastirioti M, Linn CE, Jr, White K. Characterization of Drosophila tyramine β-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci. 1996;16:3900–3911. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya Y, Kutsukake M, Chigusa SI, Komatsu A. A trace amine, tyramine, functions as a neuromodulator in Drosophila melanogaster. Neurosci Lett. 2002;329:324–328. doi: 10.1016/s0304-3940(02)00596-7. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- Orchard I, Ramirez JM, Lange AB. A multifunctional role for octopamine in locust flight. Annu Rev Entomol. 1993;38:227–249. [Google Scholar]
- Popova NK. From genes to aggressive behavior: the role of the serotonergic system. BioEssays. 2006;28:495–503. doi: 10.1002/bies.20412. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Orchard I. Octopaminergic modulation of the forewing stretch receptor in the locust, Locusta migratoria. J Exp Biol. 1990;149:255–279. [Google Scholar]
- Ramirez JM, Pearson KG. Octopaminergic modulation of plateau potentials in the flight system of the locust. Brain Res. 1991;549:332–337. doi: 10.1016/0006-8993(91)90477-d. [DOI] [PubMed] [Google Scholar]
- Riemensperger T, Voller T, Stock P, Buchner E, Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol. 2005;16:1741–1747. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Roeder T. Octopamine in invertebrates. Prog Neurobiol. 1999;59:533–561. doi: 10.1016/s0301-0082(99)00016-7. [DOI] [PubMed] [Google Scholar]
- Saraswati S, Fox LE, Soll DR, Wu CF. Tyramine and octopamine have opposite effects on the locomotion of Drosophila larvae. J Neurobiol. 2004;58:425–441. doi: 10.1002/neu.10298. [DOI] [PubMed] [Google Scholar]
- Saudou F, Amlaiky N, Plassat JL, Borelli E, Hen R. Cloning and characterization of Drosophila tyramine receptor. EMBO J. 1990;9:3611–3617. doi: 10.1002/j.1460-2075.1990.tb07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner R, Plückhahn S, Oney B, Blenau W, Erber J. Behavioral pharmacology of octopamine, tyramine, and dopamine in honey bees. Behav Brain Res. 2002;136:545–553. doi: 10.1016/s0166-4328(02)00205-x. [DOI] [PubMed] [Google Scholar]
- Scholtissen B, Verhey FR, Steinbusch HW, Leentjens AF. Serotonergic mechanisms in Parkinson's disease: opposing results from preclinical and clinical data. J Neural Transm. 2006;113:59–73. doi: 10.1007/s00702-005-0368-3. [DOI] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombati S, Hoyle G. Generation of specific behaviors in a locust by local release into neuropil of the natural neuromodulator octopamine. J Neurobiol. 1984;15:481–506. doi: 10.1002/neu.480150607. [DOI] [PubMed] [Google Scholar]
- Stevenson PA, Kutsch W. A reconsideration of the central pattern generator concept for locust flight. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1987;161:115–129. [Google Scholar]
- Stevenson PA, Spoerhase-Eichmann U. Localization of octopaminergic neurons in insects. Comp Biochem Physiol A Physiol. 1995;110:203–215. doi: 10.1016/0300-9629(94)00152-j. [DOI] [PubMed] [Google Scholar]
- Stevenson PA, Dyakonova V, Rillich J, Schildberger K. Octopamine and experience-dependent modulation of aggression in crickets. J Neurosci. 2005;25:1431–1441. doi: 10.1523/JNEUROSCI.4258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C, Fricker AD, Devi LA, Gomes I. Mechanisms of action of antidepressants: from neurotransmitter systems to signaling pathways. Cell Signal. 2005;17:549–557. doi: 10.1016/j.cellsig.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Dürrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, Wichmann C, Kittel R, Sigrist SJ, Buchner E. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Wegener G. Flying insects: model systems in exercise physiology. Experientia. 1996;52:404–412. doi: 10.1007/BF01919307. [DOI] [PubMed] [Google Scholar]
- White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- Wilson DM. The central nervous control of locust flight. J Exp Biol. 1961;38:471–490. [Google Scholar]
- Wilson DM. Central nervous mechanisms for the generation of rhythmic behavior in arthropods. Symp Soc Exp Biol. 1966;20:199–228. [PubMed] [Google Scholar]
- Wilson DM, Wyman RJ. Motor output patterns during random and rhythmic stimulation of locust thoracic ganglia. Biophys J. 1965;5:121–143. doi: 10.1016/s0006-3495(65)86706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Schnitzler A, Agapite J, Schwartz LM, Steller H, Nambu JR. Cooperative functions of the reaper and head involution defective genes in the programmed cell death of Drosophila central nervous system midline cells. Proc Natl Acad Sci USA. 1997;94:5131–5136. doi: 10.1073/pnas.94.10.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]