Abstract
Cajal-Retzius (CR) cells, the earliest-born neurons in the neocortex, arise from discrete sources within the telencephalon, including the dorsal midline and the pallial–subpallial boundary (PSB). In particular, the cortical hem, a region of high bone morphogenetic proteins (BMPs) and Wnt (wingless-type MMTV integration site family) expression but lacking in Foxg1 (forkhead box G1) is a major source of CR neurons. Whether CR cells from distinct origins arise from disparate developmental processes or share a common mechanism is unclear. To elucidate the molecular basis of CR cell development, we assessed the role of both Foxg1 and dorsal midline signaling in the production of cortical hem- and PSB-derived CR cells. We demonstrate that the loss of Foxg1 results in the overproduction of both of these CR populations. However, removal of Foxg1 at embryonic day 13, although expanding the number of CR cells with a PSB phenotype, does not result in an expansion of BMPs or Wnts in the dorsomedial signaling center. Conversely, loss of the dorsal midline ligands as observed in Gli3 (glioma-associated oncogene homolog 3) mutants results in the loss of the cortical hem-derived CR character but does not affect the specification of PSB-derived CR cells. Hence, our findings demonstrate that, although the specification of cortical hem-derived CR cells is dependent on signaling from the dorsal midline, Foxg1 functions to repress the generation of both cortical hem- and PSB-derived CR cells.
Keywords: Foxg1, Cajal-Retzius cells, dorsal midline, pallial–subpallial boundary, BMPs, telencephalon
Introduction
Within the cerebral cortex, Cajal-Retzius (CR) cells are among the earliest-born subclass of neurons (Bayer and Altman, 1990) and, through their expression of the extracellular glycoprotein reelin (D'Arcangelo et al., 1995; Hirotsune et al., 1995), are vital for both cortical lamination and the maintenance of radial glia (Frotscher, 1998; Marin-Padilla, 1998; Super et al., 2000; Lyu and Wang, 2003; Meyer et al., 2004). Recent studies have suggested that CR cells arise from discrete focal domains within the telencephalon, including the cortical hem (Monuki et al., 2001; Meyer et al., 2002; Shinozaki et al., 2002; Takiguchi-Hayashi et al., 2004; Theil, 2005; Yoshida et al., 2006; Zhao et al., 2006) and the pallial–subpallial boundary (PSB) (Meyer et al., 2002; Bielle et al., 2005). Their disparate sites of origin and distinct patterns of gene expression raise the question of whether these populations are generated by a common molecular mechanism.
Previous work has shown that Foxg1 (forkhead box G1) plays a key role in determining the fate of CR cells. In the cerebral cortex, Foxg1 is expressed in a majority of cortical progenitors and neurons, with the exception of CR neurons (Tao and Lai, 1992; Hanashima et al., 2004). In the absence of Foxg1, cortical progenitors fail to generate later-born neurons and instead continue to produce CR cells. Moreover, after conditional inactivation of Foxg1, deep-layer progenitors revert to the production of CR cells (Hanashima et al., 2004; Shen et al., 2006). Foxg1 however has pleiotropic roles in telencephalic development (Xuan et al., 1995; Huh et al., 1999; Dou et al., 2000; Hanashima et al., 2002). One function of Foxg1 is to antagonize bone morphogenetic proteins (BMPs) and their signaling pathways (Dou et al., 2000; Seoane et al., 2004), and deletion of Foxg1 results in the expansion of dorsal signaling center in the telencephalon (Dou et al., 1999; Hanashima et al., 2002; Vyas et al., 2003; Muzio and Mallamaci, 2005). As BMP and Wnt (wingless-type MMTV integration site family) protein expression within the telencephalon precedes (Furuta et al., 1997) or coincides (Lee et al., 2000) with the specification of CR cells, one hypothesis is that Foxg1 may regulate CR cell fate through antagonizing BMP and Wnt signaling pathways (Muzio and Mallamaci, 2005). In this view, removal of Foxg1 function results in the overproduction of CR cells as a result of the concomitant expansion of dorsomedial signaling.
Here we examined the role of both Foxg1 and dorsal midline signaling in the production of both cortical hem- and PSB-derived CR cells. We demonstrate that the loss of Foxg1 results in the overproduction of both of these CR populations. Conversely, however, the late removal of Foxg1 in our conditional mutants does not result in an expansion of BMPs or Wnts in the dorsomedial signaling center and only results in the expansion of CR cells with a PSB phenotype. Consistent with these observations, loss of the dorsal midline ligands as observed in Gli3 (glioma-associated oncogene homolog 3) mutants results in the loss of the cortical hem-derived CR character but does not affect the specification of PSB-derived CR cells. We conclude that the extrinsic cues emanating from the dorsomedial signaling center are only required for the production cortical hem-derived CR subtype, whereas Foxg1 is required for suppressing CR cell fate in all contexts.
Materials and Methods
Mice.
Foxg1Cre/+ mice (Hebert and McConnell, 2000) were maintained in a Swiss Webster background and intercrossed to obtain homozygous Foxg1−/− null mutants. Conditional Foxg1 mutants were generated by crossing Foxg1tTA/+ mice to Foxg1lacZ/+:tetOFoxg1IRESlacZ double-heterozygote mice as described previously (Hanashima et al., 2004). Embryos hemizygous for Foxg1lacZ/tTA:tetOFoxg1IRESlacZ will be henceforth referred to as Foxg1tetOFoxg1 mice.
Gli3 (XtJ) mutant mice (The Jackson Laboratory, Bar Harbor, ME) were maintained as heterozygotes and interbred to obtain homozygous Gli3−/− mutants. Here we restricted our analysis to embryos that showed no neural tube closure defect (exencephaly).
The Bmpr1a floxed (Bmpr1aflox/+) allele and the Bmpr1b (Bmpr1bn/+) allele (Mishina et al., 1995; Ahn et al., 2001; Fernandes et al., 2007) were crossed onto a Foxg1Cre/+ background to obtain a forebrain-specific BMP type I receptor double knock-out (dKO). Mice heterozygous for the Foxg1Cre/+;Bmpr1aflox/+;Bmpr1bn/+ allele crossed with Bmpr1aflox/flox; Bmpr1bn/+ mice were collected, and we will refer to embryos with Foxg1Cre/+;Bmpr1aflox/flox;Bmpr1bn/n alleles as Bmpr1a/b dKOs. Embryos carrying the Foxg1Cre/+;Bmpr1aflox/+;Bmpr1bn/+ genotype from these crosses were used as controls. Both Foxg1tekOFoxg1 and Foxg1Cre/+;Bmpr1aflox/+;Bmpr1bn/+ mice were maintained in a mixed C57BL/6 background, in which Swiss Webster background was introduced to increase the litter size. Embryos were obtained from timed pregnancies, with noon of the plug date designated as embryonic day 0.5 (E0.5). Because these experiments were done on mice from a mixed background, in each case, we validated our results by confirming that they were consistent in at least three to five litters (n > 3 mutant embryos).
Doxycycline administration. To obtain Foxg1 conditional mutants, pregnant females from Foxg1lacZ/+: tetOFoxg1IRESlacZ and Foxg1tTA/+ crosses (Foxg1tetOFoxg1) were fed with 250 μl of 8 mg/ml doxycycline at E13 (referred to as Foxg1 E13KO). Mice were maintained with 2 mg/ml doxycycline in the drinking water with 5% sucrose. Embryos hemizygous for Foxg1tekOFoxg1 were referred to as Foxg1 E13KO and harvested at E14.5 and E16.5.
In situ hybridization and immunohistochemistry.
Embryos were fixed in 4% paraformaldehyde and embedded in OCT. In situ hybridization was performed as described previously (Hanashima et al., 2002). cDNA probe templates were kindly provided by E. Grove (University of Chicago, Chicago, IL) (Wnt2b, Wnt3a, Wnt8b), B. Hogan (Duke University Medical Center, Durham, NC) (Bmp2, Bmp4, Bmp6), C. Abate-Shen (New Jersey–Robert Wood Johnson Medical School, New Brunswick, NJ) [Msx1 (msh homeobox homolog 1)], T. Curran (St. Jude Children's Hospital, Memphis, TN) (reelin), K. Campbell (Cincinnati Children's Hospital, Cincinnati, OH) [Dbx1 (developing brain homeobox 1)], S. Garel (Ecole Normale Superieure, Paris, France) [Ebf2 (early B-cell factor 2)], A. Joyner (Memorial Sloan-Kettering Cancer Center, New York, NY) [Cre (enterobacteria phage P1, cyclization recombinase)], Hatini et al. (1994) (Foxd1), and M. Gulisano (University of Catania, Catania, Italy) [Emx1, Emx2 (empty spiracles homologs 1 and 2)]. The monoclonal antibody for Reelin (CR50; 1:50) was a generous gift from Dr. M. Ogawa (RIKEN Brain Science Institute, Wako, Japan). The p73 antibody was applied at 1:100 (Santa Cruz Biotechnology, Santa Cruz, CA). Whole-mount in situ hybridization was performed as described previously (Wilkinson and Nieto, 1993).
Results
Cajal-Retizus cells of both cortical hem- and PSB-derived character are expanded in the absence of Foxg1
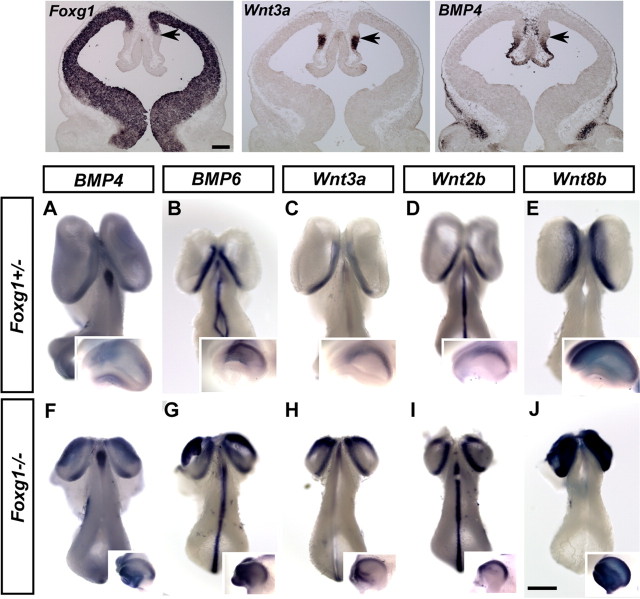
To elucidate the molecular basis of CR cell development, we first assessed whether Foxg1 represses CR cell production through antagonizing dorsomedial identity. We have therefore examined in detail whether the increased production of CR cells in Foxg1−/− nulls correlates with the expansion of the dorsomedial signaling center. In Foxg1−/− mutants, the onset of production of CR neurons is normal. However, the period during which CR neurons are produced in these mutants is prolonged, resulting in the supernumerary production of CR cells in the cortex by late embryogenesis (Hanashima et al., 2004). Foxg1 is known to antagonize BMPs (Dou et al., 1999). At E11.5, Foxg1 and BMP exhibit a complementary expression pattern within the telencephalon (Fig. 1). Bmp (Bmp4, Bmp6) and Wnt (Wnt3a, Wnt2b) expressing cells are normally restricted to the dorsomedial wall of the telencephalon demarcating the cortical hem (Fig. 1A–D), with the exception of Wnt8b whose expression expands farther into the lateral pallium (Fig. 1E). In the absence of Foxg1, the size of the telencephalon is smaller as a result of reduced proliferation of cortical progenitors (Xuan et al., 1995). However, both Bmp and Wnt expression are upregulated and expanded in the dorsomedial pallium (Fig. 1F–J). As a result, the expression of these genes in this mutant extends farther anteriorly into the rostral telencephalon but does not cover the entire pallium (Figs. 1F–J, 2F) (data not shown).
Figure 1.
Expansion of the dorsomedial signaling center in Foxg1−/− mutants. Top, Complementary expression of Foxg1 and Wnt3a, and BMP4 in E11.5 Foxg1+/− control embryos. Arrows indicate the boundary between the cortical hem and the isocortex. Scale bar, 200 μm. Bottom, Expression of multiple BMPs (BMP4, BMP6) and Wnts (Wnt3a, Wnt2b, Wnt8b) mRNA in E11.5 whole-mount embryos (dorsal view). A–E shows expression of these genes in the Foxg1+/− control embryos. F–J shows expression of the same genes in Foxg1−/− mutants. Figures in the insets show the lateral view of the telencephalon of each corresponding embryos (rostral is to the left). BMPs and Wnts are both expanded laterally and rostrally in the Foxg1−/− telencephalon. Scale bar, 1 mm.
Figure 2.
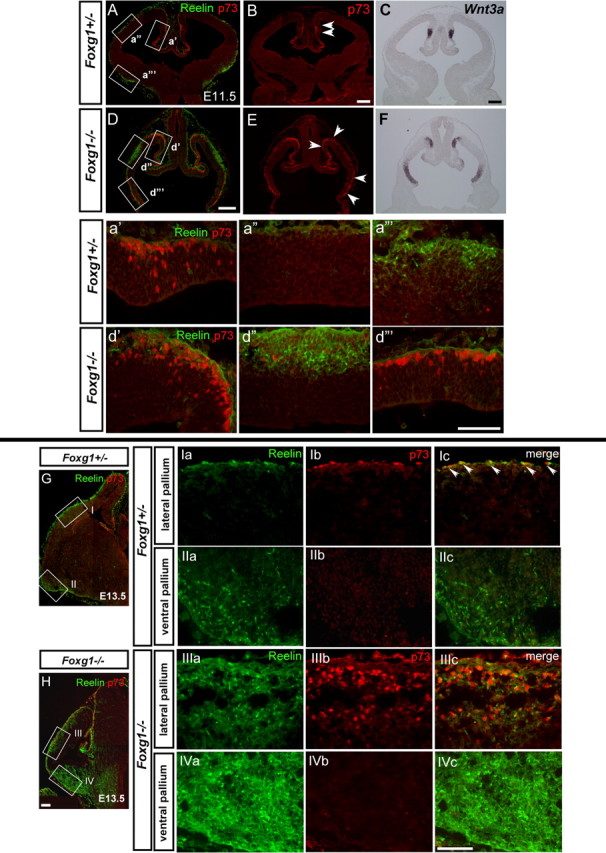
A, B, D, E, Both cortical hem- and PSB-derived CR cells are overproduced in the absence of Foxg1. Top, Expression of p73 (red) and Reelin (green) in E11.5 Foxg1+/− control (A, B) and Foxg1−/− (D, E) embryos. a′–d‴ show the enlarged view of the boxed region of medial (a′, d′), lateral (a″, d″), and ventral (a‴, d‴) pallium indicated in A and D, respectively. The ventricle is to the bottom, and the pial surface is to the top. The p73-expressing cells are detected in both the ventricular zone and postmitotic cells at E11.5. p73 marks CR cells before their expression of Reelin (a′). Reelin-expressing cells in the ventral pallium do not express p73 (a‴). C, F, Wnt3a mRNA expression in sections adjacent to those indicated in B and E. Arrowheads demarcate the boundary of p73-expressing cells at this stage. Scale bars: A–F, 200 μm; a′–d‴, 100 μm. Bottom, Reelin (green) and p73 (red) expression in E13.5 Foxg1+/− control (G, Ia–IIc) and Foxg1−/− (H, IIIa–IVc) embryos. Ia–Ic, IIIa–IIIc show the dorsal pallium, and IIa–IIc, IVa–IVc show the ventral pallium. Arrowheads in Ic indicate double p73/Reelin-positive cells in the marginal zone in Foxg1+/− controls. In Foxg1+/− cortex, p73 expressing CR cells are distributed in the lateral cortex (I), and many of them coexpress Reelin (Ib, Ic). In the Foxg1−/− cortex, many Reelin+ cells express p73 in the lateral pallium (IIIa–IIIc), but Reelin+ cells are p73 negative in the ventral pallium (IVa–IVc). Scale bar, 100 μm.
The increase in CR cells in the lateral pallium of Foxg1−/− mutants (Hanashima et al., 2004), a region beyond the territory of BMP and Wnt expression, led to the question of whether this region gives rise to a distinct subtype of CR cells. Notably, CR neuron subtypes can be molecularly distinguished by distinct gene expression patterns (Meyer et al., 2004; Yamazaki et al., 2004; Bielle et al., 2005). For instance, p73 is an early marker for CR neurons derived from the cortical hem, and its expression is observed in these cells before the initiation of reelin expression during development. At E11.5, CR cells from the dorsal midline are p73 positive (p73+)/reelin negative (reelin−) (Fig. 2a′), although reelin is already highly expressed in CR cells arising from the PSB at this age (Fig. 2a‴). In mutants that lack p73, cortical hem-derived CR cells are lost but the PSB-derived CR population is unaffected (Meyer et al., 2004). Indeed, whereas the majority of reelin-positive CR cells in the lateral pallium coexpress p73 (Fig. 2Ia–Ic) at E13.5, p73 expression is excluded from the PSB-derived CR cells populating the ventral pallium at this stage (Fig. 2IIa–IIc). Conversely, these CR cells express Ebf2, which is specifically upregulated in the ventral pallial CR cell population (supplemental Fig. S1, available at www.jneurosci.org as supplemental material) (Yamazaki et al., 2004).
The ability to distinguish between CR cells arising from the dorsal midline versus the PSB prompted us to investigate whether the ectopic and supernumerary CR cells in the Foxg1−/− nulls were all cortical hem derivatives that coexpress p73 and reelin. As in control animals, the expression of p73+ cells did not overlap with that of reelin+ cells at this early stage (Fig. 2D,d′–d‴). At E11.5, both the p73−/reelin+ CR cells within the lateral pallium (Fig. 2D,d″) and the p73+/reelin− CR cells within the dorsomedial pallium (Fig. 2E,d′) are present. However, it should be noted that, because of the loss of ventral regions in Foxg1−/− mutants, dorsomedial areas are extended rostroventrally at this stage (Figs. 1F–J, 2d‴). As a result, the expression of p73+ cells appear discontinuous when cut in coronal section as demonstrated in Figure 2, E and F. Either whole-mount analysis or sagittal sections reveals the p73 expression domain to be continuous along the rostral pole (data not shown). Notably, cortical hem-derived (p73+/reelin−) CR cells are expanded in a manner that correlates with the expanded Wnt3a expression observed in these mutants (Fig. 2B,E, arrowheads, C,F). Importantly the overproduction of CR cells only becomes evident in Foxg1−/− during mid-neurogenesis. At E13.5, both the number of PSB-derived and cortical hem-derived CR cells was expanded in the Foxg1−/− mutants (Fig. 2IIIc,IVc). In the lateral pallium, the majority of reelin+ cells coexpressed p73 (Fig. 2IIIa–IIIc), whereas in the ventral pallium, CR cells only expressed reelin and did not express p73 (Fig. 2IVa–IVc). Thus, the expanded dorsomedial signaling center in Foxg1−/− directly correlates with cortical hem-derived CR cell overproduction. Moreover, loss of Foxg1 results in expansion of both cortical hem- and PSB-derived CR cell character.
Only PSB-derived CR cells are overproduced during conditional inactivation of Foxg1
The removal of Foxg1 throughout the entire cortical progenitor domain from the onset of CR neurogenesis resulted in expansion of both cortical hem- and PSB-derived CR subtypes. Therefore, we next tested whether we can specifically bias the overproduction toward the PSB-derived CR subtype by expanding the competence window of CR cell production without altering dorsomedial signaling. To achieve this, we used a conditional approach (Hanashima et al., 2004) to remove Foxg1 function in vivo at later development stages (E13), a time point when the cortical hem structure is already well established. Removal of Foxg1 function at this stage results in resumption of CR cell production (Fig 3J,j′), with ectopic and supernumerary CR cells being produced throughout the mediolateral extent of the cortex. To assess whether dorsomedial signaling is altered in these mutants, we examined the expression of BMPs as well as a downstream target of BMP proteins, Msx1, and the cortical hem marker Wnt3a. After inactivation of Foxg1 at E13, multiple dorsomedial ligands, Wnt3a, BMP4, and BMP6, as well as Msx1, expression were restricted to their normal distribution within the cortical hem (Fig. 3C,D) (supplemental Fig. S2, available at www.jneurosci.org as supplemental material), choroid plexus (Msx1 expression in Fig. 3d′), and fimbria (Wnt3a and Msx1 expression in Fig. 3H,I), when examined at early (E14.5) and late (E16.5) time points. Furthermore, the graded expression of both Emx1 and Emx2 (medialhigh to laterallow) were maintained in these mutants (supplemental Fig. S2, available at www.jneurosci.org as supplemental material). Hence, ablation of Foxg1 during this period, unlike null mutants, does not result in an obvious respecification of the lateral cortical progenitor domain to a dorsomedial fate. These results demonstrate that, on removal of Foxg1 at E13, cortical progenitors are competent to produce supernumerary numbers of CR cells in the absence of any apparent expansion of dorsomedial signals.
Figure 3.
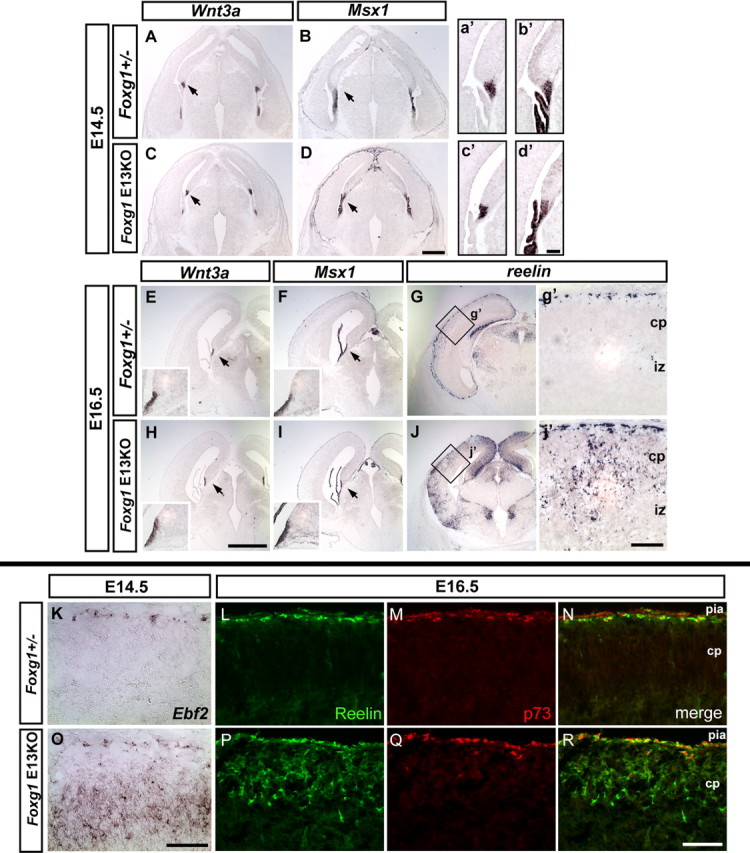
Dorsomedial signaling is not expanded in Foxg1 E13 conditional mutants. A–D, Top, Coronal sections showing Wnt3a and Msx1 expression in E14.5 Foxg1tTA/+ heterozygote control (A, B) and Foxg1 conditional (removed at E13) mutants (C, D). Arrows indicate that the expression of both genes is restricted to the cortical hem and the choroid plexus. a′–d′ show an enlarged view of the region indicated in A–D. Middle, Wnt3a, Msx1, and reelin expression at E16.5. E–G show Foxg1tTA/+ control embryos. In H–J, Foxg1 conditional (removed at E13) mutants are shown. Insets in E, F, H, and I show an enlarged view of the region indicated by the arrows. The increased production of CR cells is indicated by the expansion in reelin expression in both the medial and lateral cortex of Foxg1 conditional (removed at E13) mutants (J), whereas expression is confined to the marginal zone in the lateral cortex in Foxg1tTA/+ control mice (G). g′ and j′ show an enlarged view of the boxed region in G and J, respectively. Bottom, Expression of Ebf2 (K, O), Reelin (L, P), and p73 (M, Q) in Foxg1tTA/+ (K–N) and Foxg1 E13 conditional mutants (O–R). Pia is to the top. The ectopic and supernumerary CR cells in the cortical plate express reelin but do not coexpress p73 in the conditional mutants (R). Note the background staining in the pia in L–N, which do not represent CR cells. cp, Cortical plate; iz, intermediate zone. Scale bars: A–D, E–J, 0.5 mm; a′–d′, g′, j′, K–R, 100 μm.
We next examined whether the expanded CR population in Foxg1 E13 conditional mutants represent a biased production of CR subtypes. We used p73 as the marker for cortical hem-derived subtype, as well as Ebf2, which is specifically expressed in the ventral pallial CR cell population (supplemental Fig. S1, available at www.jneurosci.org as supplemental material) (Takiguchi-Hayashi et al., 2004). In control animals at E16.5, both p73 and reelin expression was confined to CR cells restricted to the marginal zone (Fig. 3L–N). Notably, the number of p73-expressing CR cells within the marginal zone of Foxg1 conditional mice was comparable with that seen in controls (Fig. 3Q,R). In contrast, in E13 Foxg1 conditional mutants, the ectopic and supernumerary CR cells in the cortical plate expressed reelin (Fig. 3P) but did not coexpress p73. Furthermore, Ebf2 expression was upregulated in newly-born neurons after Foxg1 removal in the E13 mutant cortices (Fig. 3O) (supplemental Fig. S3, available at www.jneurosci.org as supplemental material), whereas its expression was restricted to the marginal zone in controls (Fig. 3K). These results demonstrate that, in the absence of overt expansion in dorsomedial signaling, inactivation of Foxg1 function selectively elongates the competence window for PSB-derived (but not hem-derived) CR cell production.
Downregulation of Foxg1 in the absence of dorsomedial signaling center results in overproduction of PSB-derived CR cells and the loss of the cortical hem-derived CR subtype
The biased overproduction of PSB-derived CR cells in E13 Foxg1 conditional mutants suggests that Foxg1 is responsible for suppressing the competence of CR neuron production in general, regardless of their origin. However, in Foxg1 conditional mutants, the dorsomedial signaling center still persists, and whether this contributes to the supernumerary production of CR cells is unclear. We therefore examined whether downregulation of Foxg1 expression can specifically expand the number of PSB-derived CR cells in the absence of dorsomedial signaling. This also allowed us to assess whether the dorsomedial signaling center is itself dispensable for the production of CR cells. We addressed this question by using the extra-toesJ (XtJ) mutant, which carries an intragenic deletion of the Gli3 gene (Hui and Joyner, 1993; Rallu et al., 2002). Specifically, Gli3 homozygotes fail to establish the entire BMP- and Wnt-expressing dorsomedial signaling center (Grove et al., 1998; Tole et al., 2000). Unexpectedly, however, the complete removal of Gli3 in the absence of Foxg1 (i.e., Foxg1−/−Gli3−/− mice) results in loss of telencephalic identity (supplemental Fig S4, available at www.jneurosci.org as supplemental material). This at least partially appears to be attributable to a requirement for Gli3 for the maintenance of Foxg1 expression. In these mice, cells expressing Foxg1 (as assessed through Cre expression in Foxg1Cre/Cre;Gli3−/− mice) acquire a mixed telencephalic–diencephalic character. This was revealed by the overlap of Cre expression driven from the Foxg1 locus and Foxd1 expression, a gene normally restricted to the diencephalon (Hatini et al., 1994; Herrera et al., 2004). These results suggest that Gli3 is required for the maintenance of telencephalic identity in the absence of Foxg1. Consistent with this, the level of dorsal Foxg1 expression is affected by the level of Gli3. In Gli3 mutants, Foxg1 and Cre transcripts were reduced specifically in the dorsal but not ventral telencephalon in both Foxg1+/+ (Foxg1 transcript) and Foxg1−/− null backgrounds (Cre transcript). As shown in supplemental Figure S5 (available at www.jneurosci.org as supplemental material), removing one copy of Gli3 significantly affected the dorsal expression of the Cre transcript in the Foxg1Cre/Cre null background, and loss of both copies of Gli3 resulted in a reduction of the expression of Foxg1 in a Foxg1+/+ wild-type background. We therefore assessed whether the observed downregulation of Foxg1 expression in Gli3−/− cortices alters the production and specification of CR cells in these mutants.
In E10.5 and E13.5 Gli3+/− heterozygous embryos, as in wild types, the expression of multiple Wnt genes (Wnt3a, Wnt2b, Wnt5a) is observed within the medial region of the cortical hem, (Fig. 4A,B) (Grove et al., 1998) In contrast, in Gli3−/− homozygous embryos, the expression of Wnt3a, Wnt2b, and Wnt5b is undetectable at either of these ages (Fig. 4E,F and data not shown) (Grove et al., 1998). The only Wnt gene that persists in the cortex of Gli3−/− homozygotes is Wnt8b (Fig. 4G), whose expression normally extends beyond the cortical hem (Fig. 4C). As expected, CR cells are detected throughout the pial surface in Gli3+/− heterozygote control animals (Fig. 4D). Surprisingly, in the Gli3−/− homozygous mutants, although the architecture of the dorsal telencephalon is perturbed because of the lack of the dorsal signaling center, reelin-positive CR cells were detected throughout the surface of the telencephalon (Fig. 4H). Hence, although their distribution is irregular because of the general perturbation of the cortex in these mutants, CR neurons were observed in these mutants (Fig. 4H). Because the Gli3 mutants lack the dorsomedial signaling center, we assessed whether these CR neurons represent a specific CR subtype. Indeed, this CR cell population did not express p73, suggesting that they do not possess cortical hem-derived CR character (Fig. 4j′–j‴). We therefore examined whether this population represents the PSB-derived subtype. In E10.5 Gli3 homozygous mice, the pallial–subpallial boundary as judged by Dbx1 expression appears to be normal (supplemental Fig. 4S, available at www.jneurosci.org as supplemental material), and, in these mice, the earliest reelin-positive neurons are detected in the domain flanking this region as observed in Gli3+/− control animals (Fig. 4R,T). Furthermore, by E14.5, Dbx1 expression in the progenitor cells within the ventricular zone (Fig. 4X) and Ebf2 expression in early differentiating cells (Fig. 4Y) were specifically expanded in the dorsal telencephalon in the Gli3−/− mutants, and upregulated reelin expression in postmitotic neurons indicated production of CR cells in this region (Fig. 4Z). These results suggest that downregulation of Foxg1 expression seen in Gli3−/− mutants specifically results in the expansion of PSB-derived CR cells. Together, these results support the hypothesis that Foxg1 is required to suppress CR cells possessing a distinct regional character, whereas the acquisition of the cortical hem-derived subtype is solely dependent on the presence of dorsomedial signaling.
Figure 4.
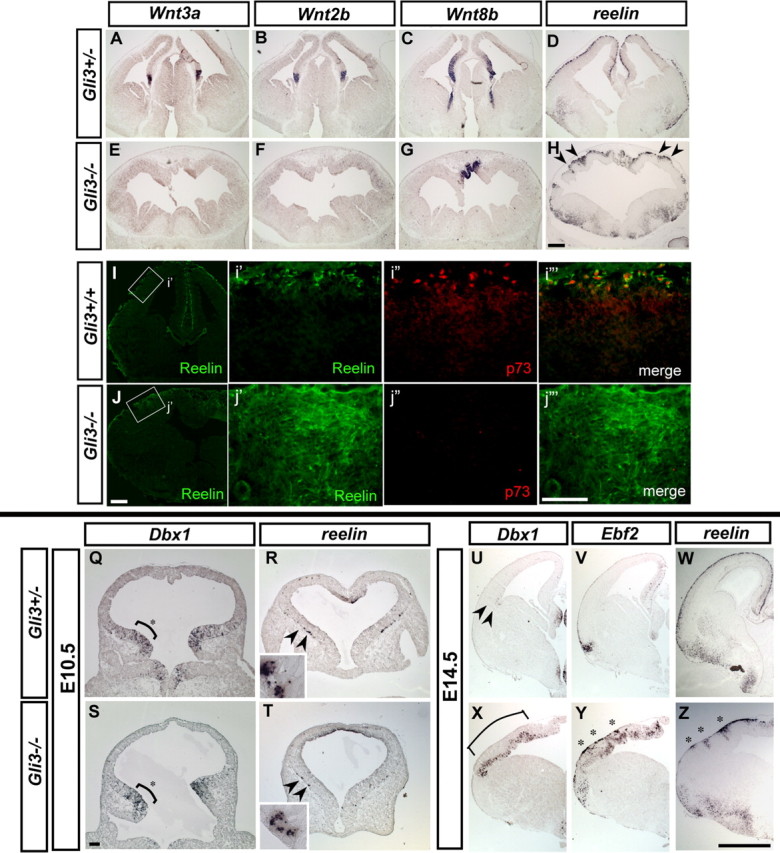
Supernumerary production of Cajal-Retzius cells in the absence of the cortical hem. A–G, Coronal sections showing the expression of Wnt3a, Wnt2b (cortical hem Wnts), and Wnt8b in E13.5 Gli3+/− control embryos (A–C) and Gli3−/− mutants (E–G). Expression of Wnt3a and Wnt2b expression is absent in the dorsal midline of Gli3−/− mutants (E, F), whereas Wnt8b expression is restricted the medial most dorsal telencephalon (G). D and H show reelin expression, indicating the presence of CR cells in both control (D) and Gli3−/− mutant mice (H). However, reelin-expressing CR cells are clustered rather than being evenly distributed throughout the cortex in Gli3−/− mutants (shown at arrowheads). I, J, Expression of Reelin and p73 in control and Gli3−/− cortex. i′–i‴ and j′-j‴ show an enlarged view of the boxed region indicated in I and J (pial surface is to the top). Many Reelin+ are detected in the dorsal pallium in Gli3−/− mutants (J, j′), and most of these do not express p73 (j″, j‴). Scale bars: A–J, 200 μm; i′–j‴, 100 μm. Q–T, PSB is preserved in Gli3−/− mutants, as indicated by the normal expression of Dbx1 (Q, S). CR cells are present in both wild-type and mutant embryos as indicated by reelin expression (R, T) in Gli3+/− control (Q, R) and Gli3−/− (S, T) E10.5 embryos. Dbx1 is expressed in the ventrolateral telencephalon (shown at asterisk), which is not perturbed in the Gli3−/− mutants (S). The earliest reelin-expressing CR cells were detected in the ventral telencephalon at this stage in both control and Gli3−/− mice. R and T show sections that are situated anterior to the Dbx1-expressing regions shown in Q and S. Insets show an enlarged view of reelin-expressing cells as indicated with arrowheads. U–Z, Expression of Dbx1, Ebf2, and reelin in E14.5 control (U–W) and Gli3−/− (X–Z) mice. Arrowheads in U indicate the restricted expression of Dbx1 within the corticostriatal boundary in controls, whereas Dbx1 expression is expanded in Gli3−/− nulls (X). Asterisk indicates the cluster of supernumerary CR cells in the Gli3−/− cortices. Scale bars: Q–T, 100 μm; U–Z, 0.5 mm.
BMP signaling is not required for the production of hem-derived CR neurons
Our results demonstrated that dorsomedial signaling is not required for the production of CR cells in general, but the specification of cortical hem-derived CR cells appear to depend on signals emanating from the cortical hem. We therefore tested whether BMP signaling specifically might account for the loss of specification of cortical hem-derived CR cell subtype in Gli3 mutants, by ablating BMP signaling throughout the entire cortical progenitor domain.
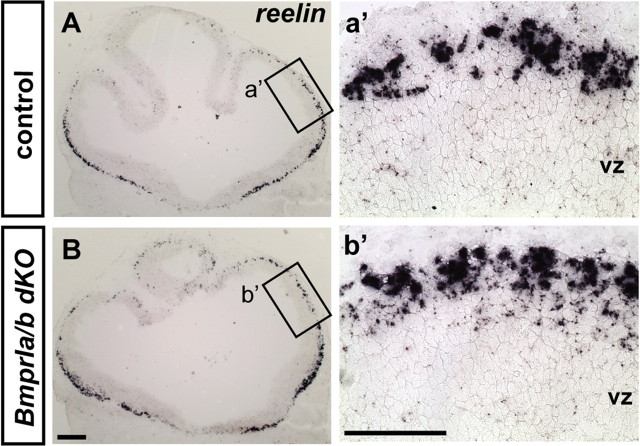
In the telencephalon, two type I BMP receptors are known to be expressed, Bmpr1a and Bmpr1b (Hebert et al., 2002). Although Bmpr1a is the major type I BMP receptor expressed in the telencephalon, conditional removal of Bmpr1a in the telencephalon does not have a significant effect on the development of the cortical hem structure and does not abolish all BMP signaling (Hebert et al., 2002). Moreover, expression of the Bmpr1b gene, which is restricted to the dorsal neural tube, is unaffected in Bmpr1a conditional mutants (Hebert et al., 2002). Therefore, to ablate BMP signaling throughout all cortical progenitor cells, we removed both type I BMP receptors in the telencephalon by crossing the Foxg1Cre/+;Bmpr1aflox/+;Bmpr1b−/+ allele to the Bmpr1aflox/flox; Bmpr1b−/+ mice (Fernandes et al., 2007). Our previous fate-mapping experiments indicate that, in these mice, loss of BmprIa/Ib receptors should be complete by E8.5 (Hebert and McConnell, 2000; Fernandes et al., 2007). Given that CR cells are produced >1 d later (Hevner et al., 2001), we believe that residual BMP signaling before E8.5 is unlikely to affect CR production. Embryos that lack both Bmpr1a and Bmpr1b did not survive beyond E11.5, and the mutants displayed a reduction in the size of the telencephalon. However, reelin-expressing CR cells were detected throughout the entire telencephalon (Fig. 5B,b′). To confirm that BMP signaling is abolished in these double mutants, we assessed the expression of a downstream effector of BMP signaling, Msx1. Consistent with BMP signaling being lost in the telencephalon of these mutants, we observed extinction of Msx1 expression in the cortex (data not shown). It remains possible that, as a result of Alk2 (activin receptor-like kinase 2) function (i.e., the sole remaining BMPRI receptor in these mutants), some diminished level of BMP signaling may be retained in the cortical hem of these mutants.
Figure 5.
Cajal-Retzius cells are produced in the absence of BMP signaling. A, B, Reelin expression in both control (A) and Foxg1Cre/+; Bmpr1B−/−; Bmpr1Aflox/flox (BmprIa/b dKO) mice (B) at E10.5 (coronal view). Embryos carrying the Foxg1Cre/+;Bmpr1aflox/+;Bmpr1bn/+ genotype are referred to as controls. a′ and b′ show the enlarged view of the boxed region indicated in A and B, respectively. vz, Ventricular zone. Scale bars: A, B, 200 μm; a′, b′, 100 μm.
We further examined whether the specification of cortical hem-derived subtype of CR cells requires BMP signaling, by delineating the subtype of CR cells produced in the Bmpr1a/b compound null mice. Although at E10.5 there is only limited differentiation of CR neurons, both p73+ and p73− CR cells were observed in these mutants (supplemental Fig. S6, available at www.jneurosci.org as supplemental material). This result implies that signals other than BMPs are responsible for the specification of the cortical hem-derived CR subtype. Together, these data demonstrate that BMP signaling subsequent to E8.5 is not essential for the development of CR cells in general, further supporting our conclusion that the dorsomedial signaling is itself dispensable for the production of CR cells.
Discussion
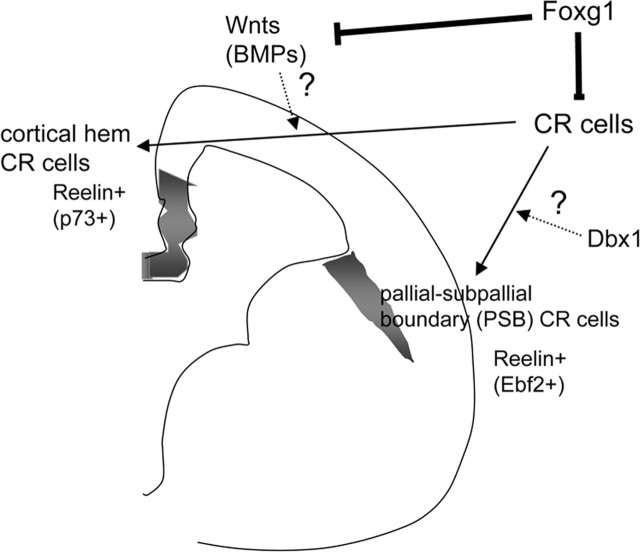
In this study, we demonstrated that Foxg1 is essential for suppressing CR cell fate arising from multiple progenitor domains within the cortex. CR cells originating from different areas of the telencephalon, specifically the cortical hem and the PSB, possess distinct molecular character. Here we show that the loss of Foxg1 results in the overproduction of both of these CR populations. Notably, the supernumerary production of CR cells on conditional late removal of Foxg1 gene function occurs without any overt change in the organization or signaling of the dorsal midline. In addition, neither loss of BMP signaling nor removal of the dorsal midline signaling center prevented the generation of CR cells. These results suggest that, although Foxg1 can interact and antagonize dorsomedial signaling (Dou et al., 2000; Hanashima et al., 2002; Vyas et al., 2003; Seoane et al., 2004; Muzio and Mallamaci, 2005), the ability of Foxg1 to suppress CR cells appears to be independent of its repression of BMP and Wnt signaling (Fig. 6).
Figure 6.
Model of the genetic interactions mediating CR cell development in the telencephalon. The current study demonstrates that Foxg1 antagonizes both CR cell fate and dorsomedial signaling center (BMPs, Wnts), but these two events can be dissociated. Moreover, the loss of Foxg1 gene function results in overproduction of both cortical hem- and PSB-derived CR cell subtypes, demonstrating that it is universally involved in the production of CR cells, regardless of their site of origin. However, although Foxg1 is essential to repress CR cell production in all contexts, specification of distinct subclasses may depend on local cues such as Wnt proteins (in the Hem) or Dbx1 expression (in the PSB).
In contrast, distinct subtypes of CR cells appear to depend on local signaling cues to acquire a regional molecular character. Indeed, the late removal of Foxg1, in which the dorsomedial signaling is not augmented, results in only expansion of CR cells with a PSB phenotype. Conversely, removal of the dorsal midline ligands, although not preventing the generation of CR cells per se, does result in the loss of cortical hem-derived CR character. Hence, in addition to a general suppression of CR cells by Foxg1, distinct subtypes of CR cells appear to depend on local signaling cues to acquire a character in accordance with their site of origin.
The role of Foxg1 and dorsal midline signaling in CR production
Our studies have shown that CR cells are produced in the absence of dorsal midline ligands. Although the currently accepted notion is that CR cells primarily arise from the cortical hem (Monuki et al., 2001; Meyer et al., 2002; Shinozaki et al., 2002; Takiguchi-Hayashi et al., 2004; Theil, 2005, Yoshida et al., 2006; Zhao et al., 2006), what allows the generation of this early class of neurons in this region has not been elucidated. Our data demonstrates that the two leading candidates, BMP and Wnt secreted proteins, do not appear to be necessary for the production of CR cells. Instead, specific elimination of Foxg1 expression in this area appears to create a zone permissive for the generation of CR cells. Notably, although BMP is known to antagonize Foxg1 expression, in Bmpr1a/Bmpr1b double mutants the expression of Foxg1 is still absent from the dorsal midline (Fernandes et al., 2007). This suggests that either BMP signaling is not required for the removal of Foxg1 from this area or that transient BMP signaling in these mutants (before the deletion of the BMP type 1 receptors by the Foxg1Cre driver) is sufficient to permanently remove Foxg1 expression from this region. However, it formally remains a possibility that Alk2, although it is not known to be expressed in the cortical hem, results in residual BMP signaling in this region. We also cannot rule out the possibility that BMP signaling before early recombination and concomitant loss of Bmpr1a and Bmpr1b receptors (at approximately E8.5) (Hebert and McConnell 2000; Fernandes et al., 2007) may be sufficient to instruct both CR cell fate and cortical hem-derived subtype. However, CR progenitors undergo several cell divisions after the recombination event before exiting the cell cycle (at approximately E10.5–E11.5) (Hevner et al., 2003). This suggests that, if BMP signaling acts to specify CR cells, this occurs long before they become postmitotic.
Intrinsic determinants for CR cell specification
The correlation between changes in the expression of Foxg1 expression and alterations in the number of CR cells is intriguing. Specifically, mutants that alter the number of CR cells are consistently associated with a shift in the expression boundary of Foxg1. For instance, in the absence of Lhx2 (LIM homeobox 2), the number of reelin-expressing marginal zone cells are expanded, and this is accompanied by the loss of Foxg1 expression in the dorsal telencephalon (Monuki et al., 2001).
Although Foxg1 is clearly a negative regulator of CR cells, what are the intrinsic determinants that specify this population? Positive regulators of CR cells have yet to be elucidated. Two candidate genes are Emx1 and Emx2, which may function in the dorsal telencephalon to direct CR cell production (Mallamaci et al., 2000; Shinozaki et al., 2002; Bishop et al., 2003). However, in the Emx1/2 double mutants, the expression of Foxg1 expands to the dorsomedial telencephalon, and the Foxg1-negative telencephalic region fails to establish (Shinozaki et al., 2004), which may account for the loss of CR cells. Furthermore, Emx1 is expressed in cortical hem-derived CR populations but not in the PSB-derived CR cells (Bielle et al., 2005). Hence, the Emx genes rather than positively acting in the induction of CR cells may function indirectly through their influence on Foxg1 expression. Interestingly, it has been suggested recently that the cortical hem itself may be derived from a progenitor pool distinct from those that contribute to the rest of the telencephalon (Kimura et al., 2005). It is plausible that acquiring CR cell identity and telencephalic character is mutually exclusive. Thus, Foxg1, in bestowing telencephalic character on progenitor pools, may simultaneously repress their intrinsic competence to produce CR cells.
Development and diversity of CR cell subtypes
The existence of multiple origins of CR cells also raises the question as to what determines this cell type. In this regard, how the PSB-derived CR subtype (Bielle et al., 2005) is generated is also unclear. Again the loss of Foxg1 function may provide a common link, because removal of Foxg1 also results in overproduction of CR cells characteristic of this region. Although we have not observed obvious exclusion of Foxg1 expression in the PSB progenitors attributable to the lack of appropriate detection method, it is plausible that CR progenitors arising from this domain are committed in their fate before the onset of Foxg1 expansion in the telencephalon. Notably as with CR cells in other areas, Foxg1 expression is excluded from reelin-expressing CR cells in this region, suggesting that these cells actively suppress the Foxg1 promoter during differentiation. Another possibility is that, rather than Foxg1 per se, corepressors that interact with Foxg1 may be specifically excluded from this region during early neurogenesis. It is possible that extrinsic cues emanating from the distinct sources of CR cells does not direct the specification of CR cells but simply provide differentiation cues to the early progenitors, thereby allowing CR cells to be generated in these restricted areas of the developing pallium before the expansion of Foxg1 expression.
Recently, it has been reported that, like CR cells, oligodendrocytes arise from multiple origins within the forebrain, including the medial ganglionic eminence, the lateral/caudal ganglionic eminence, and later on in the cortex itself. Restricted ablation studies demonstrated that oligodendrocytes arising from distinct sources are capable of compensating for the loss of oligodendrocytes generated from other sources (Kessaris et al., 2006). Thus, a global mechanism for ensuring the appropriate levels of oligodendrocyte production must exist. Rather than, or perhaps in addition to, a compensatory feedback loop, sufficient oligodendrocyte production appears to be safeguarded by an excess of production, which is later resolved. A similar neuronal overproduction may occur within CR populations, in which the rapid and widespread generation of CR cells ensures an adequate supply of CR cells for proper development.
Do CR cells arising from distinct spatial origins, once intermingled within the marginal zone, exhibit the same function regardless of their origin, or do subclasses of CR neurons serve distinctive roles in directing migration and circuitry maturation within the different regions of the cortex? Although some studies have suggested diversity in the dendritic morphology and axonal projections of CR cells, physiologically, all CR cells appear to share similar membrane properties and firing patterns (Radnikow et al., 2002). Furthermore, removal or perturbation of CR cells from the cortical hem (Meyer et al., 2004; Yoshida et al., 2006) or the PSB (Bielle et al., 2005) does not result in nearly as pronounced defects as that seen in reeler mutants. Ablation of cortical hem-derived CR cells with a Wnt3a-driven diphteria toxin-A (DTA) line or p73−/− mutants results in normal cortical lamination, with a limited perturbation in the organization of the hippocampal structure. Conversely, loss of PSB-derived CR cells using a Dbx1-drived DTA line results in the reduction of CR cell number in a region-specific manner, but the cortex is also laminated properly with a mild defect in the cytoarchitecture of the lateral cortex. That a relatively small number of CR cells can compensate for the loss of the majority of CR cells suggests that, with regards to their most profound requirements for cortical development, CR neurons derived from different sources share common properties. Recent gene expression profiling of CR cells have identified a number of genes that are expressed in these neurons (Garcia-Frigola et al., 2004; Kuvbachieva et al., 2004; Yamazaki et al., 2004). These studies may provide the means to identify the molecular pathways that CR cells use during neocortical development. Similarly, combinatorial fate-mapping and cell ablation studies will no doubt help elucidate whether CR cells are better considered as a single population or rather represent distinct subclasses. Regardless of their origin, Foxg1 plays a crucial role in suppressing the identity of this specific class (Fig. 6).
Footnotes
This work was supported by National Institutes of Health–National Institute of Neurological Disorders and Stroke Grant R01NS032993 (G.F.) and National Institute of Mental Health Grants R01MH068469 (G.F.) and R01MH070596 (J.M.H.). We thank the members of the Fishell and Hebert laboratories for their many helpful suggestions. We also thank Stewart Anderson for critical reading of this manuscript. cDNA probe templates were kindly provided by E. Grove (Wnt2b, Wnt3a, and Wnt8b), B. Hogan (Bmp2, Bmp4, and Bmp6), C. Abate-Shen (Msx1), T. Curran (reelin), K. Campbell (Dbx1), S. Garel (Ebf2), A. Joyner (Cre), E. Lai (Foxd1), and M. Gulisano (Emx1 and Emx2). The monoclonal antibody for Reelin (CR50; 1:50) was a generous gift from Dr. M. Ogawa.
References
- Ahn K, Mishina Y, Hanks M, Behringer R, Crenshaw BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development. 2001;128:4449–4461. doi: 10.1242/dev.128.22.4449. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Development of layer I and the subplate in the rat neocortex. Exp Neurol. 1990;107:48–62. doi: 10.1016/0014-4886(90)90062-w. [DOI] [PubMed] [Google Scholar]
- Bielle F, Griveau A, Narboux-Neme N, Vigneau S, Sigrist M, Arber S, Wassef M, Pierani A. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–1012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Garel S, Nakagawa Y, Rubenstein JL, O'Leary DD. Emx1 and Emx2 cooperate to regulate cortical size, lamination, neuronal differentiation, development of cortical efferents, and thalamocortical pathfinding. J Comp Neurol. 2003;457:345–360. doi: 10.1002/cne.10549. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- Dou C, Lee J, Liu B, Liu F, Massague J, Xuan S, Lai E. BF-1 interferes with transforming growth factor beta signaling by associating with Smad partners. Mol Cell Biol. 2000;20:6201–6211. doi: 10.1128/mcb.20.17.6201-6211.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou CL, Li S, Lai E. Dual role of brain factor-1 in regulating growth and patterning of the cerebral hemispheres. Cereb Cortex. 1999;9:543–550. doi: 10.1093/cercor/9.6.543. [DOI] [PubMed] [Google Scholar]
- Fernandes M, Gutin G, Alcorn H, McConnell SK, Hébert JM. Mutations in the BMP pathway in mice supports the existence of two molecular classes of holoprosencephaly. Development. 2007 doi: 10.1242/dev.004325. in press. [DOI] [PubMed] [Google Scholar]
- Frotscher M. Cajal-Retzius cells, Reelin, and the formation of layers. Curr Opin Neurobiol. 1998;8:570–575. doi: 10.1016/s0959-4388(98)80082-2. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–2212. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- Garcia-Frigola C, Burgaya F, Calbet M, Lopez-Domenech G, de Lecea L, Soriano E. A collection of cDNAs enriched in upper cortical layers of the embryonic mouse brain. Brain Res Mol Brain Res. 2004;122:133–150. doi: 10.1016/j.molbrainres.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- Hanashima C, Shen L, Li SC, Lai E. Brain factor-1 controls the proliferation and differentiation of neocortical progenitor cells through independent mechanisms. J Neurosci. 2002;22:6526–6536. doi: 10.1523/JNEUROSCI.22-15-06526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanashima C, Li SC, Shen L, Lai E, Fishell G. Foxg1 suppresses early cortical cell fate. Science. 2004;303:56–59. doi: 10.1126/science.1090674. [DOI] [PubMed] [Google Scholar]
- Hatini V, Tao W, Lai E. Expression of winged helix genes, BF-1 and BF-2, define adjacent domains within the developing forebrain and retina. J Neurobiol. 1994;25:1293–1309. doi: 10.1002/neu.480251010. [DOI] [PubMed] [Google Scholar]
- Hebert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol. 2000;222:296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- Hebert JM, Mishina Y, McConnell SK. BMP signaling is required locally to pattern the dorsal telencephalic midline. Neuron. 2002;35:1029–1041. doi: 10.1016/s0896-6273(02)00900-5. [DOI] [PubMed] [Google Scholar]
- Herrera E, Marcus R, Li S, Williams SE, Erskine L, Lai E, Mason C. Foxd1 is required for proper formation of the optic chiasm. Development. 2004;131:5727–5739. doi: 10.1242/dev.01431. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JL. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Neogi T, Englund C, Daza RA, Fink A. Cajal-Retzius cells in the mouse: transcription factors, neurotransmitters, and birthdays suggest a pallial origin. Brain Res Dev Brain Res. 2003;141:39–53. doi: 10.1016/s0165-3806(02)00641-7. [DOI] [PubMed] [Google Scholar]
- Hirotsune S, Takahara T, Sasaki N, Hirose K, Yoshiki A, Ohashi T, Kusakabe M, Murakami Y, Muramatsu M, Watanabe S, Nakao K, Katsuki M, Hayashizaki Y. The reeler gene encodes a protein with an EGF-like motif expressed by pioneer neurons. Nat Genet. 1995;10:77–83. doi: 10.1038/ng0595-77. [DOI] [PubMed] [Google Scholar]
- Huh S, Hatini V, Marcus RC, Li SC, Lai E. Dorsal-ventral patterning defects in the eye of BF-1-deficient mice associated with a restricted loss of shh expression. Dev Biol. 1999;211:53–63. doi: 10.1006/dbio.1999.9303. [DOI] [PubMed] [Google Scholar]
- Hui CC, Joyner AL. A mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat Genet. 1993;3:241–246. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J, Suda Y, Kurokawa D, Hossain ZM, Nakamura M, Takahashi M, Hara A, Aizawa S. Emx2 and Pax6 function in cooperation with Otx2 and Otx1 to develop caudal forebrain primordium that includes future archipallium. J Neurosci. 2005;25:5097–5108. doi: 10.1523/JNEUROSCI.0239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuvbachieva A, Bestel AM, Tissir F, Maloum I, Guimiot F, Ramoz N, Bourgeois F, Moalic JM, Goffinet AM, Simonneau M. Identification of a novel brain-specific and Reelin-regulated gene that encodes a protein colocalized with synapsin. Eur J Neurosci. 2004;20:603–610. doi: 10.1111/j.1460-9568.2004.03473.x. [DOI] [PubMed] [Google Scholar]
- Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- Lyu YL, Wang JC. Aberrant lamination in the cerebral cortex of mouse embryos lacking DNA topoisomerase IIbeta. Proc Natl Acad Sci USA. 2003;100:7123–7128. doi: 10.1073/pnas.1232376100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallamaci A, Mercurio S, Muzio L, Cecchi C, Pardini CL, Gruss P, Boncinelli E. The lack of Emx2 causes impairment of Reelin signaling and defects of neuronal migration in the developing cerebral cortex. J Neurosci. 2000;20:1109–1118. doi: 10.1523/JNEUROSCI.20-03-01109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Padilla M. Cajal-Retzius cells and the development of the neocortex. Trends Neurosci. 1998;21:64–71. doi: 10.1016/s0166-2236(97)01164-8. [DOI] [PubMed] [Google Scholar]
- Meyer G, Perez-Garcia CG, Abraham H, Caput D. Expression of p73 and Reelin in the developing human cortex. J Neurosci. 2002;22:4973–4986. doi: 10.1523/JNEUROSCI.22-12-04973.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Cabrera Socorro A, Perez Garcia CG, Martinez Millan L, Walker N, Caput D. Developmental roles of p73 in Cajal-Retzius cells and cortical patterning. J Neurosci. 2004;24:9878–9887. doi: 10.1523/JNEUROSCI.3060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- Monuki ES, Porter FD, Walsh CA. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32:591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- Muzio L, Mallamaci A. Foxg1 confines Cajal-Retzius neuronogenesis and hippocampal morphogenesis to the dorsomedial pallium. J Neurosci. 2005;25:4435–4441. doi: 10.1523/JNEUROSCI.4804-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radnikow G, Feldmeyer D, Lubke J. Axonal projection, input and output synapses, and synaptic physiology of Cajal-Retzius cells in the developing rat neocortex. J Neurosci. 2002;22:6908–6919. doi: 10.1523/JNEUROSCI.22-16-06908.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rallu M, Machold R, Gaiano N, Corbin JG, McMahon AP, Fishell G. Dorsoventral patterning is established in the telencephalon of mutants lacking both Gli3 and Hedgehog signaling. Development. 2002;129:4963–4974. doi: 10.1242/dev.129.21.4963. [DOI] [PubMed] [Google Scholar]
- Seoane J, Le HV, Shen L, Anderson SA, Massague J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–223. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE, Temple S. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Miyagi T, Yoshida M, Miyata T, Ogawa M, Aizawa S, Suda Y. Absence of Cajal-Retzius cells and subplate neurons associated with defects of tangential cell migration from ganglionic eminence in Emx1/2 double mutant cerebral cortex. Development. 2002;129:3479–3492. doi: 10.1242/dev.129.14.3479. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yoshida M, Nakamura M, Aizawa S, Suda Y. Emx1 and Emx2 cooperate in initial phase of archipallium development. Mech Dev. 2004;121:475–489. doi: 10.1016/j.mod.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Super H, Del Rio JA, Martinez A, Perez-Sust P, Soriano E. Disruption of neuronal migration and radial glia in the developing cerebral cortex following ablation of Cajal-Retzius cells. Cereb Cortex. 2000;10:602–613. doi: 10.1093/cercor/10.6.602. [DOI] [PubMed] [Google Scholar]
- Takiguchi-Hayashi K, Sekiguchi M, Ashigaki S, Takamatsu M, Hasegawa H, Suzuki-Migishima R, Yokoyama M, Nakanishi S, Tanabe Y. Generation of reelin-positive marginal zone cells from the caudomedial wall of telencephalic vesicles. J Neurosci. 2004;24:2286–2295. doi: 10.1523/JNEUROSCI.4671-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W, Lai E. Telencephalon-restricted expession of BF-1, a new member of the HNF-3/fork head gene family, in the developing rat brain. Neuron. 1992;8:957–966. doi: 10.1016/0896-6273(92)90210-5. [DOI] [PubMed] [Google Scholar]
- Theil T. Gli3 is required for the specification and differentiation of preplate neurons. Dev Biol. 2005;286:559–571. doi: 10.1016/j.ydbio.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Tole S, Ragsdale CW, Grove EA. Dorsoventral patterning of the telencephalon is disrupted in the mouse mutant extra-toes(J) Dev Biol. 2000;217:254–265. doi: 10.1006/dbio.1999.9509. [DOI] [PubMed] [Google Scholar]
- Vyas A, Saha B, Lai E, Tole S. Paleocortex is specified in mice in which dorsal telencephalic patterning is severely disrupted. J Comp Neurol. 2003;466:545–553. doi: 10.1002/cne.10900. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- Xuan S, Baptista CA, Balas G, Tao W, Soares VC, Lai E. Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron. 1995;14:1141–1152. doi: 10.1016/0896-6273(95)90262-7. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Sekiguchi M, Takamatsu M, Tanabe Y, Nakanishi S. Distinct ontogenic and regional expressions of newly identified Cajal-Retzius cell-specific genes during neocorticogenesis. Proc Natl Acad Sci USA. 2004;101:14509–14514. doi: 10.1073/pnas.0406295101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Assimacopoulos S, Jones KR, Grove EA. Massive loss of Cajal-Retzius cells does not disrupt neocortical layer order. Development. 2006;133:537–545. doi: 10.1242/dev.02209. [DOI] [PubMed] [Google Scholar]
- Zhao C, Guan W, Pleasure SJ. A transgenic marker mouse line labels Cajal-Retzius cells from the cortical hem and thalamocortical axons. Brain Res. 2006;1077:48–53. doi: 10.1016/j.brainres.2006.01.042. [DOI] [PubMed] [Google Scholar]