Establishing precise neuronal connectivity in the mammalian cortex is essential for proper function. Many studies have suggested that neuronal activity plays an important role in specifying cortical circuits. The best characterized studies are of thalamocortical projections from the lateral geniculate nucleus to cortical layer 4, where cortical neurons are thought to go through an early phase of target selection dependent on molecular cues, followed by an activity-dependent mechanism, which then refines and maintains the connectivity (Katz and Crowley, 2002). In a recent The Journal of Neuroscience paper, Mizuno et al. (2007) add another piece to the puzzle by showing that neuronal activity in callosal neurons is necessary for the initial laminar-specific elaboration of their axons.
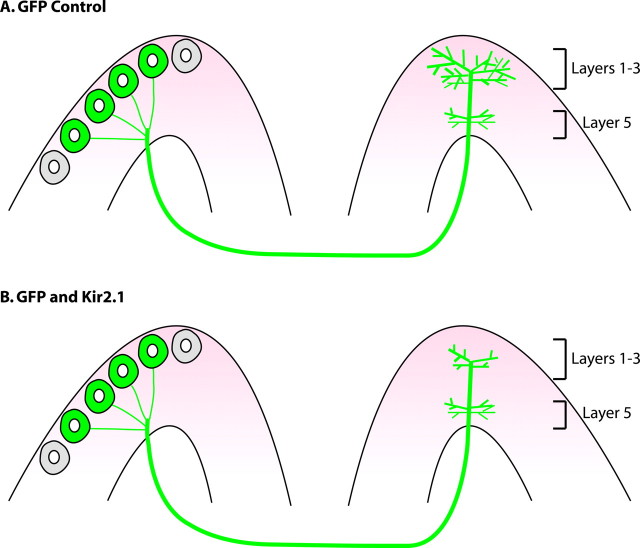
To investigate the wiring projections of callosal neurons, the authors used in utero electroporation to label layer 2/3 callosal axons with green fluorescent protein (GFP). Consistent with previous tracing studies, the authors observed that callosal projections in the contralateral hemisphere were restricted to a region between areas 17 and 18 with a strong enrichment of axon terminals in layers 1–3 and 5 (Fig. 1A) [Mizuno et al. (2007), their Fig. 1 (http://www.jneurosci.org/cgi/content/full/27/25/6760/F1)]. Thus, there is both a regional and laminar specificity in the innervation pattern of callosal neurons.
Figure 1.
Kir2.1 overexpression in layer 2/3 callosal neurons suppresses contralateral terminal axon density. A, In utero electroporation of layer 2/3 callosal neurons with GFP labels axons terminating in layers 1–3 and 5 in the contralateral hemisphere. B, Electroporation with Kir2.1 inhibits terminal axon growth in layers 1–3 but does not affect layer 5.
To determine the role of neuronal activity in callosal wiring, the authors electroporated the inwardly rectifying K+ channel Kir2.1 at embryonic day 15.5 to reduce neuronal activity in callosal neurons. This approach reduces the spontaneous activity of neurons, presumably by hyperpolarizing neurons (Johns et al., 1999). Indeed, in electrophysiological recordings of postnatal day 7 (P7) cortical slices, the mean firing rate of neurons expressing the Kir2.1 channel was ∼50% lower than in GFP-electroporated controls [Mizuno et al. (2007), their Fig. 3B (http://www.jneurosci.org/cgi/content/full/27/25/6760/F3)]. The authors saw a 50% decrease in GFP fluorescence in layers 1–3, whereas there was no difference in layer 5, for Kir2.1-electroporated cells compared with controls at P15 (Fig. 1B) [Mizuno et al. (2007), their Fig. 3C,D (http://www.jneurosci.org/cgi/content/full/27/25/6760/F3)]. Similarly, at earlier developmental time points (P10 and P13), Kir2.1-expressing axons were less dense than control axons in layers 1–3 [Mizuno et al. (2007), their Fig. 5C (http://www.jneurosci.org/cgi/content/full/27/25/6760/F5)]. This suggests that the reduced fluorescence was not caused by axon extension followed by retraction, but rather reduced initial axonal elaboration, at least on a global level.
The effect on laminar targeting is likely attributable to the suppression of neuronal activity in callosal neurons, because exogenous Kir2.1 expression did not affect neuronal cell fate, migration, neuron viability, developmental timing, axon extension, or region-specific axon targeting [Mizuno et al. (2007), their Fig. 4 (http://www.jneurosci.org/cgi/content/full/27/25/6760/F4)]. The specific effect of activity on layers 1–3 is interesting, because it suggests that the reduction of activity does not prevent axon growth, but instead affects layer-specific cues for axon elaboration.
Analysis at a single-axon level is needed to characterize whether retraction occurs. Future experiments could determine whether there is activity-based competition between callosal neurons, similar to that observed in the zebrafish tectum (Hua et al., 2005). One could simultaneously label Kir2.1-expressing as well as non-Kir2.1-expressing callosal axons and determine whether non-Kir2.1-expressing axons elaborate beyond their normal sizes.
To test the requirement for postsynaptic activity in callosal axon wiring, the authors transfected callosal axons from one hemisphere with GFP and the other hemisphere with Kir2.1 and DsRed2. Presumably GFP+ callosal axons should innervate Kir2.1-expressing neurons in the other hemisphere. When compared with control animals, there was no difference in callosal axon density in Kir2.1-expressing neurons [Mizuno et al. (2007), their Fig. 7 (http://www.jneurosci.org/cgi/content/full/27/25/6760/F7)]. The authors conclude that postsynaptic activity is not necessary for proper callosal axon innervation. However, this experiment is difficult to interpret because of several caveats mentioned by the authors, most notably that Kir2.1 was only expressed in approximately one-half of the postsynaptic neurons. The remaining neurons that did not express Kir2.1 might provide sufficient activity-dependent cues that are important postsynaptically for callosal innervation.
Human mutations in Kir2.1 perturb skeletal and cardiac muscle function (Plaster et al., 2001). The consequences of mutations in Kir2.1 in the brain are unknown. Mizuno et al. (2007) tested whether exogenous expression of mutant forms of Kir2.1 affected callosal wiring. Three of these mutations have dominant-negative activity in heterologous systems, and one has a gain-of-function effect (Plaster et al., 2001). Although electroporation of the dominant-negative mutant channels did not affect wiring of callosal axons, the gain-of-function mutant had defects similar to exogenous expression of Kir2.1 [Mizuno et al. (2007), their Fig. 6A,B (http://www.jneurosci.org/cgi/content/full/27/25/6760/F6)]. This suggests that humans with this gain-of-function mutation may have defects in callosal neuron projections.
In summary, Mizuno et al. (2007) show that mouse layer 2/3 callosal axons require activity for initial establishment of axonal elaboration in layers 1–3. This study provides additional evidence for a role of neuronal activity in early target selection decisions, contrasting with the well established requirement of neuronal activity for refining and maintaining axonal connections (Katz and Crowley, 2002; Innocenti and Price, 2005). The authors establish a genetically amenable system to study cortical wiring in vivo, thus providing an entryway to dissect the molecular mechanisms by which activity affects layer specificity in the cortex.
Footnotes
Editor's Note: These short reviews of a recent paper in the Journal, written exclusively by graduate students or postdoctoral fellows, are intended to mimic the journal clubs that exist in your own departments or institutions. For more information on the format and purpose of the Journal Club, please see http://www.jneurosci.org/misc/ifa_features.shtml.
References
- Hua JY, Smear MC, Baier H, Smith SJ. Regulation of axon growth in vivo by activity-based competition. Nature. 2005;434:1022–1026. doi: 10.1038/nature03409. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nat Rev Neurosci. 2005;6:955–965. doi: 10.1038/nrn1790. [DOI] [PubMed] [Google Scholar]
- Johns DC, Marx R, Mains RE, O'Rourke B, Marban E. Inducible genetic suppression of neuronal excitability. J Neurosci. 1999;19:1691–1697. doi: 10.1523/JNEUROSCI.19-05-01691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Crowley JC. Development of cortical circuits: lessons from ocular dominance columns. Nat Rev Neurosci. 2002;3:34–42. doi: 10.1038/nrn703. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Hirano T, Tagawa Y. Evidence for activity-dependent cortical wiring: formation of interhemispheric connections in neonatal mouse visual cortex requires projection neuron activity. J Neurosci. 2007;27:6760–6770. doi: 10.1523/JNEUROSCI.1215-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaster NM, Tawil R, Tristani-Firouzi M, Canun S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL, Jr, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell. 2001;105:511–519. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]