Abstract
It is well established that the proteolytic processing of the β-amyloid precursor protein (APP) generates β-amyloid (Aβ), which plays a central role in the pathogenesis of Alzheimer's disease (AD). In contrast, the physiological role of APP and of its numerous proteolytic fragments and the question of whether a loss of these functions contributes to AD are still unknown. To address this question, we replaced the endogenous APP locus by gene-targeted alleles and generated two lines of knock-in mice that exclusively express APP deletion variants corresponding either to the secreted APP ectodomain (APPsα) or to a C-terminal (CT) truncation lacking the YENPTY interaction motif (APPΔCT15). Interestingly, the ΔCT15 deletion resulted in reduced turnover of holoAPP, increased cell surface expression, and strongly reduced Aβ levels in brain, likely because of reduced processing in the endocytic pathway. Most importantly, we demonstrate that in both APP knock-in lines the expression of APP N-terminal domains either grossly attenuated or completely rescued the prominent deficits of APP knock-out mice, such as reductions in brain and body weight, grip strength deficits, alterations in circadian locomotor activity, exploratory activity, and the impairment in spatial learning and long-term potentiation. Together, our data suggest that the APP C terminus is dispensable and that APPsα is sufficient to mediate the physiological functions of APP assessed by these tests.
Keywords: Alzheimer's disease, β-amyloid precursor protein, APPsα, physiological functions, knock-out, LTP
Introduction
One of the hallmarks of Alzheimer's disease (AD) is the deposition of the β-amyloid peptide (Aβ), derived from the β-amyloid precursor protein (APP), in the brains of AD patients (Selkoe, 2004). Whereas the mechanisms governing Aβ generation have been studied intensely, the physiological role of APP and the question of whether a loss of these functions contributes to AD remain unclear.
Two major obstacles complicate the analysis of APP functions in vivo. First, because APP is a member of a gene family including APP-like proteins (APLPs) APLP1 and ALPL2, functional redundancy may compensate for the loss of essential gene functions, e.g., in knock-out (KO) models. Indeed, the extensive structural similarities between APP and APLPs are also reflected at the functional level (Anliker and Müller, 2006). Previously, we and others documented that APP-KO mice exhibit growth and brain weight deficits, reduced grip strength, agenesis of the corpus callosum, hypersensitivity to seizures, defects in copper and lipid homeostasis, altered locomotor activity, and impaired spatial learning associated with impaired long-term potentiation (LTP) (Müller et al., 1994; Zheng et al., 1995; Li et al., 1996; Steinbach et al., 1998; Tremml et al., 1998; Dawson et al., 1999; Magara et al., 1999; Seabrook et al., 1999; White et al., 1999; Grimm et al., 2005). Like APP-KO mice, APLP-deficient mice proved viable, whereas combined APP−/−APLP2−/− or APLP1−/−APLP2−/− double KO (von Koch et al., 1997; Heber et al., 2000) and APP−/−APLP1−/−APLP2−/− triple mutants die postnatally, the latter exhibiting cranial dysplasias resembling human type II lissencephaly (Herms et al., 2004).
Second, functional diversity also may result from proteolytic processing of APP, leading to diverse extracellular and intracellular APP fragments (for review, see Selkoe, 2004). APP is a type I transmembrane protein and cleaved either by α-secretase within the Aβ region or by β-secretase at the N terminus of Aβ, leading to the secretion of the large soluble ectodomains APPsα and APPsβ. Intramembrane processing of β-secretase-cleaved APP C-terminal fragments by γ-secretase results in the production of Aβ and the APP intracellular domain (AICD). APPsα has been implicated in various cellular processes including neuroprotection, may serve a memory-enhancing function, and regulates neuronal excitability and synaptic plasticity (Turner et al., 2003; Reinhard et al., 2005; Zheng and Koo, 2006). Recently, the AICD has been shown to form a complex with Fe65/Tip60 and might regulate transcription (for review, see Selkoe, 2004). The nature of potential target genes, however, is still under debate (Hebert et al., 2006; Zheng and Koo, 2006). Most of the identified binding partners of APP interact with the cytoplasmic tail. Notably, the YENPTY motif that is conserved from Caenorhabditis elegans to mammalian APP/APLPs confers clathrin-mediated endocytosis and has been shown to bind several kinases as well as adaptor proteins, including mouse disabled homolog 1 (mDab1), Jun N-terminal kinase-interacting protein (JIP), X11/mints, and Fe65 family proteins. Although in vitro studies have shown that these interactions not only may modulate APP processing but also may mediate cell signaling, the in vivo relevance for both processing and physiology is still mainly unknown.
Here we have generated by gene targeting two lines of knock-in mice (KI) expressing different truncated APP variants. We introduced stop codons such that APPsα-KI mice express only secreted APPsα and APPΔCT15-KI mice lack the last 15 aa. These mice allowed us to test the requirement of different domains of APP for its physiological function. Here we report that the secreted APPsα domain is sufficient to rescue prominent abnormalities of APP-KO mice, including reductions in brain and body weight, grip strength deficits, and the impairment in spatial learning and LTP. Collectively, our data suggest that APPsα is sufficient to mediate the (postnatal) physiological functions of APP assessed by these tests.
Materials and Methods
Generation of mutant APPsa-KI and APPΔCT15-KI embryonic stem cells and mice.
The vector pEasyFlox was used as a backbone, and the arms of homology were inserted as detailed in the supplemental material (available at www.jneurosci.org). Electroporation and selection of embryonic stem (ES) cells was performed as previously described (Heber et al., 2000). Southern blotting was performed by using standard techniques, probing with a 0.6 kb PCR-generated fragment. Positive clones were identified and injected into blastocysts to generate germline-transmitting chimeras.
Quantitative PCR.
Total RNA was prepared from whole brains of 6- to 12-week-old mice, and quantitative real-time PCR was done as detailed in the supplemental material (available at www.jneurosci.org), using TaqMan probes overlapping the APP exon 9–10 boundary.
Tissue processing and Western blot analysis.
Western blot analysis of whole-brain homogenates of 6- to 12-week-old APPsα-KI and APPΔCT15-KI animals and wt littermates was performed as previously described (Heber et al., 2000). The fractionation of soluble APPs and cell-bound APP was performed as detailed in the supplemental material (available at www.jneurosci.org). Western blot signals were detected by enhanced chemiluminescence (Super Signal West Pico; Pierce, Rockford, IL); scanned and digitalized images were analyzed for quantification with AIDA (Advanced Image Data Analysis) software (Raytest USA, Wilmington, NC). Additional details are given in the supplemental material (available at www.jneurosci.org).
Antibodies.
For Western blots specific antibodies directed against the APP N terminus (22C11; 1:5000; Chemicon, Temecula, CA), the APP C terminus (B63; 1:1000), APLP1 (CT11; 1:1000; Calbiochem, La Jolla, CA), APLP2 (D2II; 1:2000; Calbiochem), and β-tubulin (MAB3408; 1:10,000; Chemicon) were used. For immunoprecipitation we used the polyclonal antibody AβNT, generated by immunizing rabbits with a peptide corresponding to the first 15 aa of murine Aβ.
Cell lines and metabolic labeling.
Mouse fibroblasts derived from APPsα-KI, APPΔCT15-KI, and wt littermates were obtained from embryonic day 14.5 embryos and immortalized with SV40Tag, using a retroviral vector. Details of metabolic labeling are given in the supplemental material (available at www.jneurosci.org).
Aβ measurements.
Mouse brains (age 9–12 months) were homogenized in 9 volumes of 0.2% diethylamine in 50 mm NaCl, using a Potter homogenizer. Homogenates were centrifuged at 100,000 × g for 1 h, and the supernatant was neutralized with a 1:10 volume of 50 mm Tris-HCl, pH 6.8. Aβx-40 and Aβx-42 ELISAs were performed by using the BNT-77/BA-27 (Aβx-40) and BNT-77/BC-05 (Aβx-42) system (Duff et al., 1996).
Behavioral analysis.
All behavioral procedures were approved by Swiss animal welfare authorities. Mice were housed in single cages and allowed to adapt to a reverse light cycle (lights off at 8:00 A.M.; on at 8:00 P.M.) for at least 1 week so that they could be tested during the dark phase of the cycle. A total of 94 mice (n = 13–17 per genotype), distributed over three cohorts (each containing approximately balanced numbers of male and female mice), were analyzed in a blinded manner in the following order of tests: water maze (age 2.42 ± 0.02 months), grip test (age 2.64 ± 0.02 months), light/dark box (age 3.2 ± 0.15 months), home cage activity recording (age 8.01 ± 0.14 months).
Home cage activity.
Home cage activity was recorded as described previously (Madani et al., 2003) with the use of a cage rack equipped with one passive infrared sensor per mouse (ActiviScope; New Behavior, Zurich, Switzerland). The sensors detected any locomotion and remained silent only when the mice were sleeping or grooming. Recording started after a habituation period of at least 18 h, and circadian profiles were calculated by averaging data from at least 4 recording days.
Spatial learning, light/dark box, and grip strength tests.
Place navigation, light/dark box, and grip strength were measured as detailed in the supplemental material (available at www.jneurosci.org).
Electrophysiology.
In vitro extracellular recordings were performed on acute hippocampal slices of individuals characterized behaviorally. The following animals were studied: APP-KO, n = 6, and wt littermates, n = 5 (age 9–12 months); APPsα-KI, n = 4, and wt littermates, n = 5 (12–15 months); APPΔCT15-KI, n = 6, and wt littermates, n = 4 (age 12–15 months). Details on slice preparation and electrophysiology are given as supplemental material (available at www.jneurosci.org).
Results
Generation of APPsα-KI and APPΔCT15-KI mice
Transgenic approaches based on random integration of expression vectors may fall short of matching the endogenous spatiotemporal expression pattern because of the modulating effects of neighboring chromatin regions. Therefore, we used gene targeting in ES cells to replace the endogenous APP locus with modified APP alleles. We generated two lines of KI mice that express under control of the endogenous chromosomal APP promoter either exclusively (1) APPsα or (2) APPΔCT15, a C-terminal deletion mutant lacking the last 15 aa of APP harboring the YENPTY motif. Using the targeting strategy depicted in Figure 1, we have introduced a stop codon into APP exon 16 behind the α-secretase site at position QK612, flanked by a SV40 polyA site to ensure efficient polyadenylation of the mRNA (APPsα-KI). We have chosen as the long arm of homology an intronic fragment downstream of exon 17 to guarantee that homologous recombination also will result in a deletion of the endogenous APP transmembrane domain encoded by exon 17 (Fig. 1B). It should be noted that because of the knock-in approach APP-truncated variants can be studied on an APP null background, that these variants are expressed by the endogenous promoter, and that all splice sites are unaffected by our strategy, thus leading to the expression of the endogenous pattern of APP isoforms.
Figure 1.
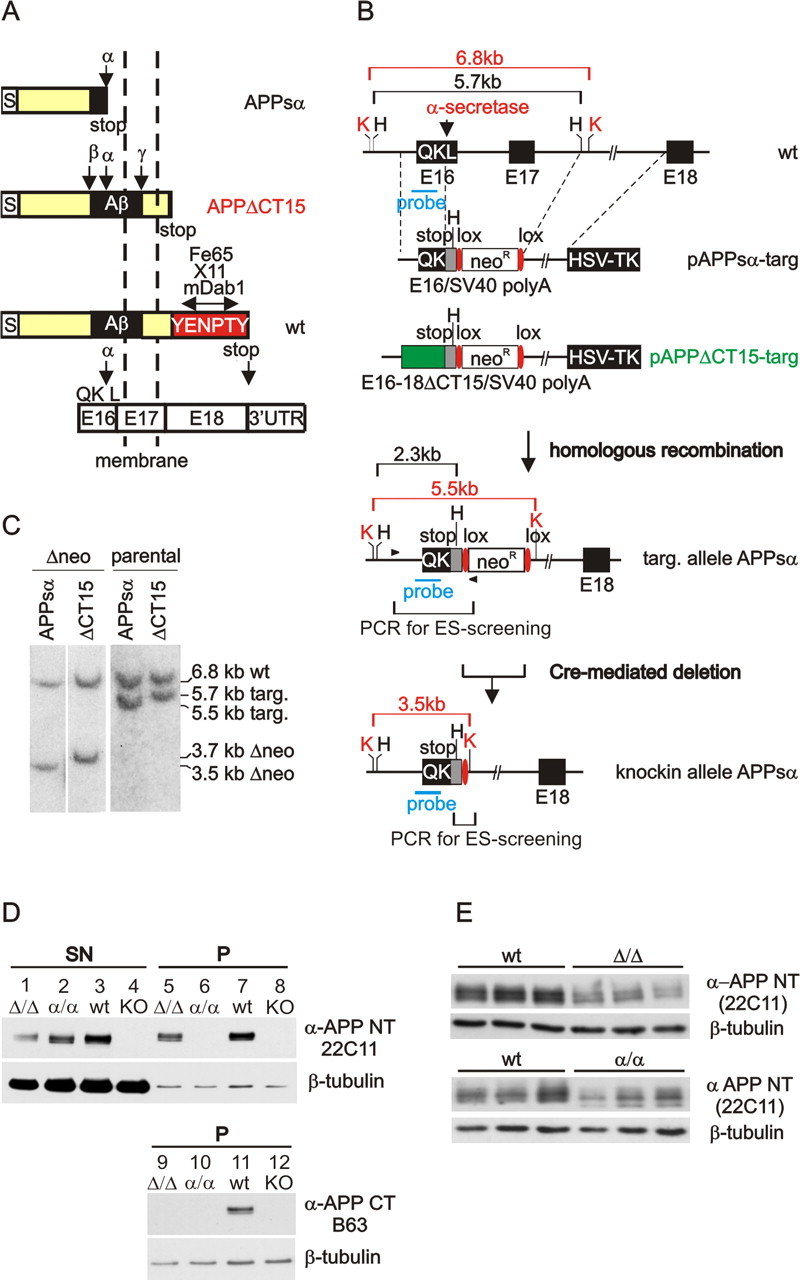
Generation of knock-in mice. A, Schematic representation (not to scale) of APP-KI variants APPsα-KI and APPΔCT15-KI. Open boxes represent exons (E16, E17, E18) encoding the APP C terminus; S, signal peptide. B, The short arm of pAPPsα-targ encompasses exon 16 into which a stop codon was introduced behind the α-secretase site. In pAPPΔCT15-targ the coding sequence of exon 16 was fused to APP695-cDNA sequences up to amino acid 680 (E16–18ΔCT15; green box) flanked by a stop codon. The stop codon was followed by a SV40 polyA site (gray box) and a floxed neo gene for selection in ES cells. The long arm of homology consists of a 6.2 kb intronic fragment downstream of exon 17. Subsequently, the neo gene was deleted by transient expression of Cre-recombinase (B, bottom). K, KpnI; H, HindIII. C, Correct gene targeting and removal of the neo gene (Δneo) was confirmed by Southern blotting with a 0.6 kb probe overlapping exon 16 (B, probe). KpnI digestion of ES cell DNA revealed expected bands of 6.8 kb (wt), 3.5 kb (APPsα-allele), and 3.7 kb (APPΔCT15 allele). D, Western blot analysis (N-terminal antibody 22C11) of soluble APPs and membrane-bound holoAPP from adult brain revealed in pellet fractions (P) from wt mice the typical set of bands expected for holoAPP (lane 7) and a similar set of bands for APPΔCT15-KI mice (lane 5). No membrane-bound APP was detected in pellet fractions of APPsα-KI mice (lane 6). In supernatant fractions (SN) soluble APPs derived from APPsα-KI brain co-migrated with APPs produced in wt or APPΔCT15-KI brains (lanes 1–3). As expected, no signal was detectable with a C-terminal antibody for either KI variant (lanes 9, 10). As a negative control APP-KO brain was used (lanes 4, 8, 12), and β-tubulin staining was performed to monitor loading. E, Western blot analysis of whole-brain homogenates of APP-KI mutants (25 μg of protein/lane), using an N-terminal anti-APP antibody (22C11). Note that quantification revealed for APPΔCT15-KI brain a significant reduction of APP expression. A similar, but not significant, trend was observed for APPsα-KI mice (see Results for details).
To generate APPΔCT15-KI mice, we fused the coding sequence of APP exon 16 to APP695-cDNA sequences up to amino acid 680, followed by a stop codon (Fig. 1B, middle). Homologous recombination events were identified by PCR and confirmed by Southern blot analysis, using KpnI-digested DNA (Fig. 1C). The resulting mutant ES cells were injected into blastocysts and gave rise to germline-transmitting chimeras. Intercrosses of heterozygous APPsα-KI and APPΔCT15-KI mice led to homozygous mutant animals at the expected Mendelian frequencies.
Expression of APP-KI variants and Aβ generation
The primary goal of this study was to assess which domains of APP are crucial for its in vivo function(s). To this end we first examined the expression of APP-KI variants from the gene-targeted loci. Western blot analysis of soluble APPs and membrane-bound holoAPP from adult homozygous KI mice is depicted in Figure 1D. Incubation with an anti-APP antibody (22C11) directed against an N-terminal epitope revealed in pellet fractions from wt mice the typical set of bands expected for holoAPP (corresponding to immature and glycosylated mature APP) and a similar set of bands for APPΔCT15-KI mice (Fig. 1D, lanes 5, 7). Because of the minor difference in molecular weight no apparent size difference was resolvable between the wt and the APPΔCT15 protein variant. Notably, for APPsα-KI mice no membrane-bound APP was detected in pellet fractions (Fig. 1D, lane 6), whereas in supernatant fractions soluble APPs derived from APPsα-KI brain was prominently expressed and co-migrated with APPs produced in wt or APPΔCT-KI brains (Fig. 1D, lanes 1–3). This indicates that, although not generated by α-secretase processing of holoAPP, APPsα can be produced and secreted in KI brains at high levels. Importantly, no signal could be detected with an antibody raised against the APP C terminus for either KI variant (Fig. 1D, lanes 9, 10), indicating a deletion of this epitope and corroborating the functionality of the knock-in approach.
We examined expression levels of mutant APP in APP-KI brains at the mRNA and protein levels. To assess whether introduction of the SV40 polyA cassette might have altered the steady-state level of APP mRNA in KI mice, we performed quantitative real-time PCR on whole-brain RNA of adult mice (6–12 weeks of age), which indicated that mRNA levels were reduced to 65 ± 12% (p < 0.001; Student's t test) in APPΔCT15 mutants and to 67 ± 10% (p < 0.001; Student's t test) in APPsα mice as compared with wt littermates. Similarly, Western blot analysis of APP from whole-brain homogenates (without additional fractionation) (Fig. 1E) revealed for APPΔCT15-KI mice a reduction to ∼57 ± 11% of wt littermate levels (p < 0.001; Student's t test). For APPsα-KI mice a similar trend was observed; however, differences did not reach statistical significance (p ≥ 0.05; Student's t test). For all samples APP expression levels were normalized to β-tubulin stainings. Thus the reduced expression of the APP-KI protein variants can be attributed mainly to reduced APPsα and APPΔCT15 mRNA levels, which may be due to the SV40 polyA cassette. Expression of the related APP family members APLP1 and APLP2 was unaltered in both KI mutants (data not shown), indicating no compensatory upregulation.
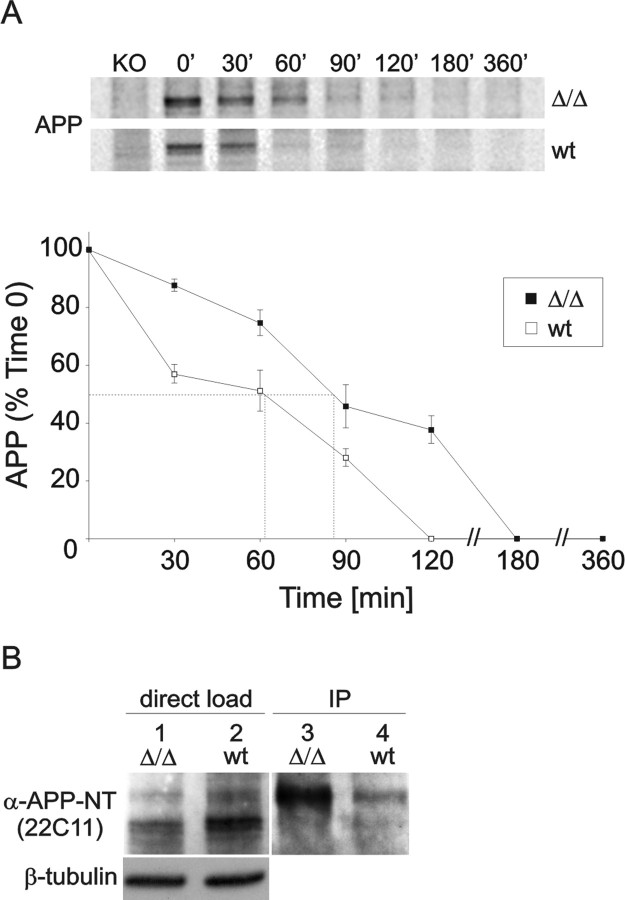
We then investigated whether the deletion of the C-terminal 15 aa (containing a prominent protein interaction motif as well as an endosomal internalization site) affects endogenous APP processing and analyzed Aβ levels in whole-brain extracts by sandwich ELISA. Because APPΔCT15 protein variants were expressed at reduced levels, we corrected the raw values accordingly. Nevertheless, APPΔCT15-KI mice showed a reduction of Aβ40 to 34 ± 16% (p < 0.001; Student's t test) of wt littermate level. Likewise, Aβ42 levels were reduced similarly to 44 ± 8% (p < 0.001; Student's t test). To assess whether reduced Aβ generation is attributable to reduced processing by the endocytic pathway, we studied the turnover of endogenous full-length APP by metabolic labeling. Mouse embryonic fibroblasts (MEFs) derived from wt or APPΔCT15-KI mice were pulse-labeled for 15 min and chased for 0, 30, 60, 90, 120, 180, and 360 min. APP was recovered by immunoprecipitation with AβNT, an antibody directed against the N terminus of the murine Aβ region. Whereas wt APP showed a half-life of ∼60 min (Fig. 2A), the turnover of APPΔCT15 from mutant MEFs was reduced markedly, as revealed by a prolonged half-life of ∼90 min (Fig. 2A). To assess the cell surface pool of APP directly, we performed biotinylation experiments. Indeed, APP surface expression in APPΔCT15 MEFs was ∼2.5-fold increased as compared with APP wt MEFs (Fig. 2B). Together, our data obtained with the APPΔCT15 variant expressed from the endogenous APP locus are consistent with previous studies that used similar APP mutants in transfected cell lines (Perez et al., 1999) and suggest that reduced endocytosis leads to reduced amyloidogenic processing.
Figure 2.
Turnover of full-length APP and cell surface expression. A, Turnover of endogenous cell-bound APP. APPΔCT15-KI and wt MEFs were pulse-labeled with [35S]methionine/cysteine for 15 min and chased for the indicated time intervals. At time 0, APP consists predominantly of immature N-glycosylated species. Both the N-glycosylated and mature N- and O-glycosylated species are abundant at 30 min for both cell types. After a 60–90 min chase period the APP level is reduced dramatically in wt MEFs and below detection level at 120 min. In contrast, in APPΔCT15-KI MEFs the APP expression is reduced to only 40% after a 120 min chase period. Half-life was determined by quantitating the results as indicated by dotted lines (B). All values are presented as the means ± SEM. Differences were detected with two-tailed Student's t test, accepting p < 0.05 as significant. B, Biotinylation of surface APP. APPΔCT15-KI and wt MEFs were surface-biotinylated for 30 min. Cell lysate (15 μg/lane) was loaded directly onto a 10% Tris-tricine gel (lanes 1, 2) or was immunoprecipitated (350 μg/immunoprecipitate) by using NeutrAvidin-agarose beads (lanes 3, 4). APP was detected with the use of an N-terminal anti-APP antibody (22C11), revealing a considerable increase of surface APP in APPΔCT15-KI cells when compared with wt controls.
The growth and brain weight deficit of APP-KO mice is rescued in APP-KI mice
Finally, we addressed the primary question of whether the phenotype of APP-KI mice would be identical to that of APP-deficient mice (which would indicate a requirement of the APP C terminus and/or anchorage within the membrane) or whether and to what extent the APP deletion variants might rescue the various deficits of APP-KO mice. Previous analysis of APP-KO mice had revealed a phenotype characterized by the following features: (1) reduced postnatal brain and body weight, (2) behavioral abnormalities such as reduced grip strength and locomotor alterations, and (3) reduced spatial learning associated with an LTP deficit in aged mice (Müller et al., 1994; Zheng et al., 1995; Li et al., 1996; Dawson et al., 1999; Magara et al., 1999; Seabrook et al., 1999). For all studies the APP-KO, APPsα-KI, APPΔCT15-KI mice, and their respective wt littermate controls were obtained from heterozygous intercrosses (e.g., APP+/− × APP+/−; APPα/+ × APPα/+).
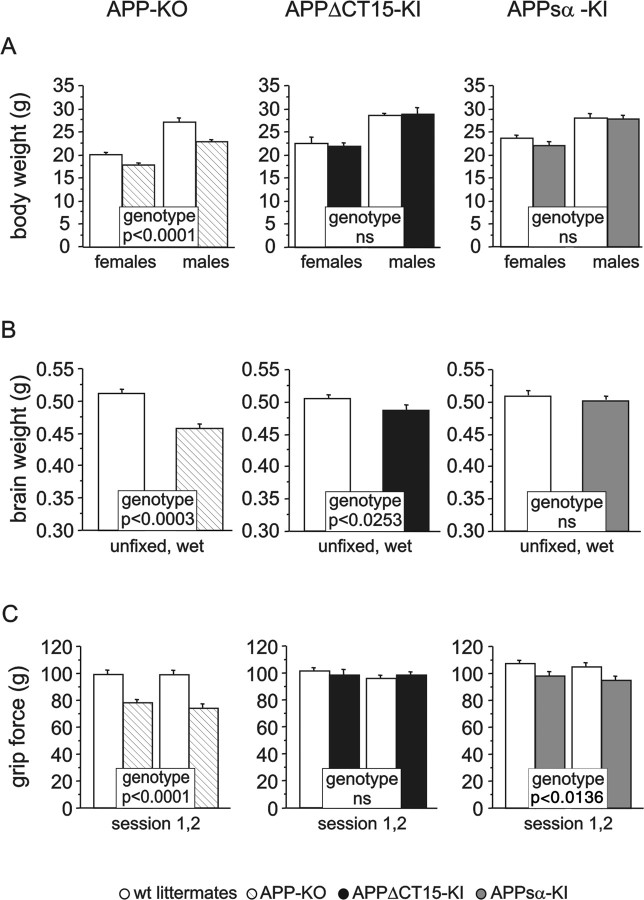
We have shown previously that APP-KO mice show a postnatal somatic growth deficit that emerges in the second postnatal week and is maximal at 4 weeks postnatally. Here the brain and body weights were analyzed in parallel to the various behavioral tests. Body weights were studied at 2.4 months of age, and we observed a reduction of ∼15% in female and 19% in male APP-KO mice (Fig. 3A), thus confirming our previous results (Müller et al., 1994; Magara et al., 1999). In contrast, not only APPΔCT15-KI mice but also the APPsα-KI line showed normal body weights (Fig. 3A). Brain weights were determined after completion of the behavioral analysis at 10–12 months of age. As expected, APP-KO mice displayed a strong reduction of wet brain weight by 11% (Fig. 3B). This effect was corrected completely in the APPsα-KI line and grossly attenuated in the APPΔCT15-KI mutants, who displayed a reduction by 3.6% (Fig. 3B). Likewise, histopathological analysis of KO and KI mutants (as assessed by cresyl violet, hematoxylin/eosin, synaptophysin, mitogen-activated protein kinase 2, and glial fibrillary acidic protein staining at 11 months) did not show any apparent abnormalities as compared with wt littermate controls (data not shown).
Figure 3.
Somatic development and grip strength. A, Body weight measured at 2.4 months before water maze testing was reduced in APP-KO (13 KO and 15 wt) regardless of gender, but not in APPΔCT15-KI (17 KI and 16 wt) nor in APPsα-KI (17 KI and 16 wt) mice. ANOVA: genotype × line, F(2,58) = 5.1 (p < 0.0092); genotype × line × gender, F(2,58) = 1.3, ns. APP-KO: genotype, F(1,16) = 31.2 (p < 0.0001); genotype × gender, F(1,16) = 2.7, ns. APPΔCT15-KI: genotype, F(1,21) = 0.1, ns; genotype × gender, F(1,21) = 0.1, ns. APPsα-KI: genotype, F(1,21) = 0.7, ns; genotype × gender, F(1,21) = 0.8, ns. B, Brain weight at 10–12 months of age was strongly reduced in APP-KO mice (9 KO and 10 wt). This effect was attenuated in the APPΔCT15-KI (12 KI and 10 wt) and fully corrected in the APPsα-KI line (12 KI and 10 wt). ANOVA: genotype × line, F(1,29) = 4.9 (p < 0.0151). APP-KO: genotype, F(1,9) = 31.4 (p < 0.0003). APPΔCT15-KI: genotype, F(1,12) = 6.5 (p < 0.0253). APPsα-KI: genotype, F(1,8) = 0.3, ns. C, Grip strength was strongly reduced in APP-KO mice (13 KO and 15 wt) in both test sessions on consecutive days. This effect was fully corrected in the APPΔCT15-KI (17 KI and 16 wt) and attenuated in the APPsα-KI line (17 KI and 16 wt). ANOVA: genotype × line, F(2,58) = 9.0 (p < 0.0004); genotype × line × session, F(2,58) = 0.7, ns. APP-KO: genotype, F(1,16) = 52.4 (p < 0.0001); genotype × session, F(1,16) = 0.7, ns. APPΔCT15-KI: genotype, F(1,21) = 0.4, ns; genotype × session, F(1,21) = 0.3, ns. APPsα-KI: genotype, F(1,21) = 7.3 (p < 0.0136); genotype × session, F(1,21) = 1.3, ns.
APP-KI mutants rescue the grip strength deficit, altered circadian locomotor activity, exploratory activity, and learning deficit of APP-KO mice
First we assessed a potential grip strength deficit in KI mice, because we had previously observed this as a robust abnormality of APP-KO mutants. As predicted, APP-KO mice revealed, on average, a grip strength deficit of 23% (Fig. 3C). In the APPΔCT15-KI line grip strength was not statistically different from wt littermates, and APPsα-KI mice showed a slight deficit of 9.2% (Fig. 3C).
Spontaneous home cage activity was monitored after habituation in individual cages equipped with infrared sensors (Madani et al., 2003). APP-KO mice showed massively overshooting activity during the beginning of the dark phase but were less active than wt controls toward the end of the dark phase. In contrast, levels and circadian distribution of activity of both KI mutants were indistinguishable from controls (Fig. 4A). We also analyzed mice in the light/dark box paradigm that assesses potential differences toward aversive stimuli and exploratory activity. Regardless of genotype and line, all mice spent significantly more than 50% of their time in the dark compartment of a light/dark box, confirming the aversiveness of the brightly illuminated compartment (data not shown). The number of transitions between the two compartments was significantly smaller in APP-KO mice, indicative of reduced exploratory activity under aversive conditions. This effect was corrected fully in both KI lines (Fig. 4B).
Figure 4.
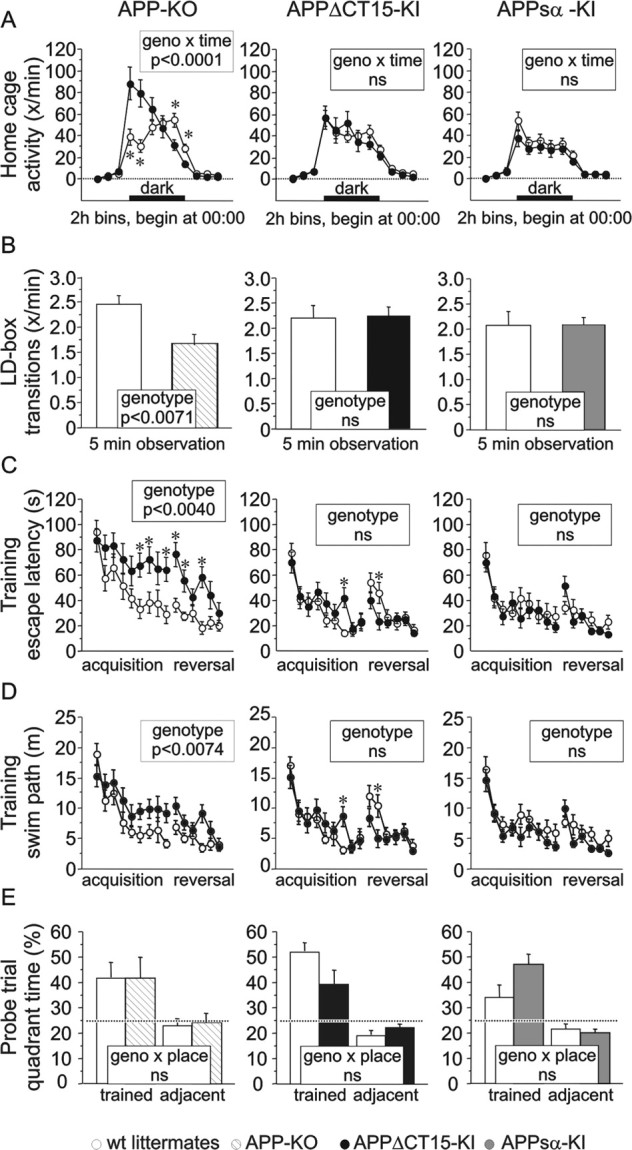
Home cage activity, explorative activity, and spatial learning. A, In their home cages the APP-KO mice (13 KO and 14 wt) showed overshooting activity during the beginning of the dark phase. This effect was corrected in the APPΔCT15-KI (15 KI and 12 wt) as well as in the APPsα-KI line (15 KI and 18 wt). ANOVA: genotype × line, F(2,73) = 1.0, ns; genotype × time × line, F(22,803) = 3.8 (p < 0.0001). APP-KO: genotype, F(1,23) = 2.8, ns; genotype × time, F(11,253) = 10.3 (p < 0.0001). APPΔCT15-KI: genotype, F(1,23) = 0.1, ns; genotype × time, F(11,253) = 0.5, ns. APPsα-KI: genotype, F(1,27) = 0.1, ns; genotype × time, F(11,297) = 0.5, ns. B, In the light/dark box APP-KO mice showed significantly fewer transitions between the two compartments (F(1,24) = 9.8; p < 0.0071), indicative of reduced exploratory activity under aversive conditions. In contrast, both KI lines were statistically indistinguishable from wt littermate controls (APPΔCT15-KI, F(1,29) = 0.1, ns; APPsα-KI, F(1,29) = 0.1, ns). C, In the water maze place navigation task APP-KO mice (13 KO and 15 wt) took significantly longer than wt controls to find the hidden platform toward the end of acquisition training and during most of reversal training. Performance was similar to controls at the beginning and at the very end of testing. Neither APPΔCT15-KI (17 KI and 16 wt) nor APPsα-KI (17 KI and 16 wt) mice were impaired. APPΔCT15-KI had longer escape latencies during trial block 7 but were better than controls during trial block 11. ANOVA: genotype × line, F(2,58) = 6.8 (p < 0.0022); genotype × time, F(14,812) = 2.3 (p < 0.0051). APP-KO: genotype, F(1,16) = 11.3 (p < 0.0040); genotype × time, F(14,224) = 1.7 (p < 0.0662). APPΔCT15-KI: genotype, F(1,21) = 0.2, ns; genotype × time, F(14,294) = 1.9 (p < 0.0275). APPsα-KI: genotype, F(1,21) = 0.1, ns; genotype × time, F(14,294) = 1.0, ns. D, APP-KO mice also swam longer distances to reach the platform, whereas the two KI lines were unimpaired according to this criterion. APPΔCT15-KI had longer swim paths during trial block 7 but were better than controls during trial block 11. ANOVA: genotype × line, F(2,58) = 4.6 (p < 0.0146); genotype × time, F(14,812) = 2.0 (p < 0.0170). APP-KO: genotype, F(1,16) = 9.4 (p < 0.0074); time, F(14,224) = 13.8 (p < 0.0001); genotype × time, F(14,224) = 1.5, ns. APPΔCT15-KI: genotype, F(1,21) = 0.1, ns; time, F(14,294) = 10.9 (p < 0.0001); genotype × time, F(14,294) = 1.8 (p < 0.0376). APPsα-KI: genotype, F(1,21) = 1.0, ns; time, F(14,294) = 9.4 (p < 0.0001); genotype × time, F(14,294) = 0.8, ns. E, Probe trial performance was normal in all three lines. Time (percentage) spent in the trained quadrant was compared with average time (percentage) spent in the left and right adjacent quadrants. ANOVA: genotype × place × line, F(2,58) = 0.9, ns. APP-KO: place, F(1,16) = 4.8 (p < 0.0433); genotype × place, F(1,16) = 1.0, ns. APPΔCT15-KI: place, F(1,21) = 20.6 (p < 0.0002); genotype × place, F(1,16) = 1.3, ns. APPsα-KI: place, F(1,21) = 9.5 (p < 0.0056); genotype × place, F(1,21) = 0.3, ns.
Spatial navigation was assessed in a water maze place navigation task that included an acquisition phase as well as a reversal phase, with the platform moved to the opposite quadrant. APP-KO mice performed similarly to controls at the beginning but took approximately twice as long to find the platform toward the end of acquisition training and also were impaired during most of the reversal phase (Fig. 4C). Only at the very end of training did they reach a performance level similar to controls. No such impairment was observed in KI lines (Fig. 4C). Swim speed was reduced in APP-KO mice by 19% (13 KO 0.168 ± 0.008 m/s; 15 wt, 0.207 ± 0.007 m/s; genotype, F(1,16) = 15.7; p < 0.001), reflecting the muscle weakness already evident from grip strength deficits. The KI lines showed only minor, but not significant, reductions, with APPΔCT15-KI being 4.6% slower than controls [17 KI, 0.228 ± 0.006 m/s; 16 wt, 0.239 ± 0.007 m/s; genotype, F(1,21) = 3.8, not significant (ns)] and APPsα-KI being 9.0% slower than controls (17 KI, 0.214 ± 0.008 m/s; 16 wt, 0.235 ± 0.008 m/s; genotype, F(1,21) = 4.1, ns). The amount of time spent floating passively was very small (1.42 ± 1.12 s) and did not differ across groups. The swim speed reduction of APP-KO mice accounted only partially for their performance deficit during training, as evidenced by the fact that they also swam considerably longer distances to find the platform (Fig. 4D) and also were impaired according to speed-insensitive measures of spatial accuracy such as Whishaw's error (average percentage path outside the beeline corridor) (KO, 80.3 ± 1.1%; wt, 76.0 ± 1.3%; F(1,16) = 4.8; p < 0.0442). In contrast, APPΔCT15-KI were unimpaired according to swim path length (Fig. 4D) and also earned Whishaw's error scores typical for the genetic background used in this study (KI, 75.2 ± 1.4%; wt, 73.6 ± 1.7%; genotype, F(1,21) = 1.2, ns). Similarly, APPsα-KI showed path lengths (Fig. 4D) and Whishaw's error scores (KI, 72.6 ± 1.9%; wt, 77.8 ± 1.3%; genotype, F(1,21) = 2.3, ns) indistinguishable from controls. The reduced training performance of APP-KO mice may be explained at least in part by wall hugging, which was increased on average by 100% in APP-KO mice (KO, 15.0 ± 4.0%; wt, 7.3 ± 1.1%; F(1,16) = 4.6; p < 0.0469) but was unchanged in APPΔCT15-KI (KI, 9.6 ± 1.4%; wt, 11.9 ± 1.8%; F(1,21) = 0.1, ns) and APPsα-KI (KI, 7.1 ± 1.6%; wt, 10.5 ± 1.2%; F(1,21) = 1.5, ns). During the probe trial APP-KO mice as well as both KI lines showed a significant preference for the trained platform quadrant, regardless of genotype (Fig. 4E). In summary, both KI mutants (including the APPsα-producing line) performed like wt controls and thus rescued the spatial navigation deficit of APP-KO mice.
Impaired LTP in aged APP-KO mice is rescued in APP-KI mutants
To determine whether the phenotype of the APP-KO and -KI lines in learning tasks correlates with alterations of synaptic plasticity, we performed electrophysiological recordings on acute hippocampal slices of the behaviorally tested individuals. First we tested for potential changes in basal synaptic transmission as such, focusing on field EPSP (fEPSP) size and paired pulse facilitation. In both KI lines, as well as in KO mice, basal synaptic transmission (expressed as the strength of responses to excitatory synaptic stimulation) was unaltered, as well as presynapse functionality, which was assessed by paired pulse facilitation (data not shown).
A classic model to study the cellular foundation of learning and memory in mammals is hippocampal LTP. In line with previous studies (Dawson et al., 1999; Seabrook et al., 1999) we observed that deletion of the APP gene causes a deficit in LTP in old age, paralleling the behavioral deficits. APP-KO mice showed a defect in the rate of induction and maintenance of LTP in the CA3/CA1 pathway of the hippocampus (Fig. 5). We recorded fEPSPs from 9- to 12-month-old mice and, after 20 min of baseline recording, induced LTP by theta burst stimulation (TBS; 10 trains of four pulses at 100 Hz with an interburst interval of 200 μs, repeated 3×). Slices from APP-KO mice showed a significantly reduced potentiation 60 min after the TBS [wt mice, 212 ± 14.5 (n = 15); APP-KO mice, 142.8 ± 9.5 (n = 15); p < 0.0013] (Fig. 5A). To assess whether LTP deficits observed for APP-KO mice might be attributable to functions mediated by the APP C terminus, we induced LTP in hippocampal slices of aged (12–15 months old) individuals of the two KI lines. We noticed that in this set of experiments the maximal potentiation obtained in wt mice was less than in wt animals of the APP-KO experiments, likely because of the increased age of KI mice. Yet, importantly, when we directly compared KI mice and their wt littermate controls (as had been done for the KO line), no LTP deficits were detectable in KI mice (Fig. 5B,C). Unlike APP-KO mice, aged APPsα-KI mice showed unaltered LTP responses relative to their wt littermates after 60 min [wt, 141.97 ± 5.25 (n = 22); KI, 136.63 ± 6.96 (n = 35)]. The same was true for aged mice of the APPΔCT15 line [wt, 131.92 ± 6.07 (n = 16); KI, 124.94 ± 6.45 (n = 22)]. Although the absolute value of potentiation of ∼150% was somewhat lower when compared with the APP-KO/wt recordings, the sensitivity was still high enough to detect potential differences of at least 15% between KI mice and their internal wt littermate controls (ANOVA; p < 0.05). These results thus parallel the normal behavioral performance of KI mice, indicating again a rescue effect of both truncated APP polypeptides.
Figure 5.
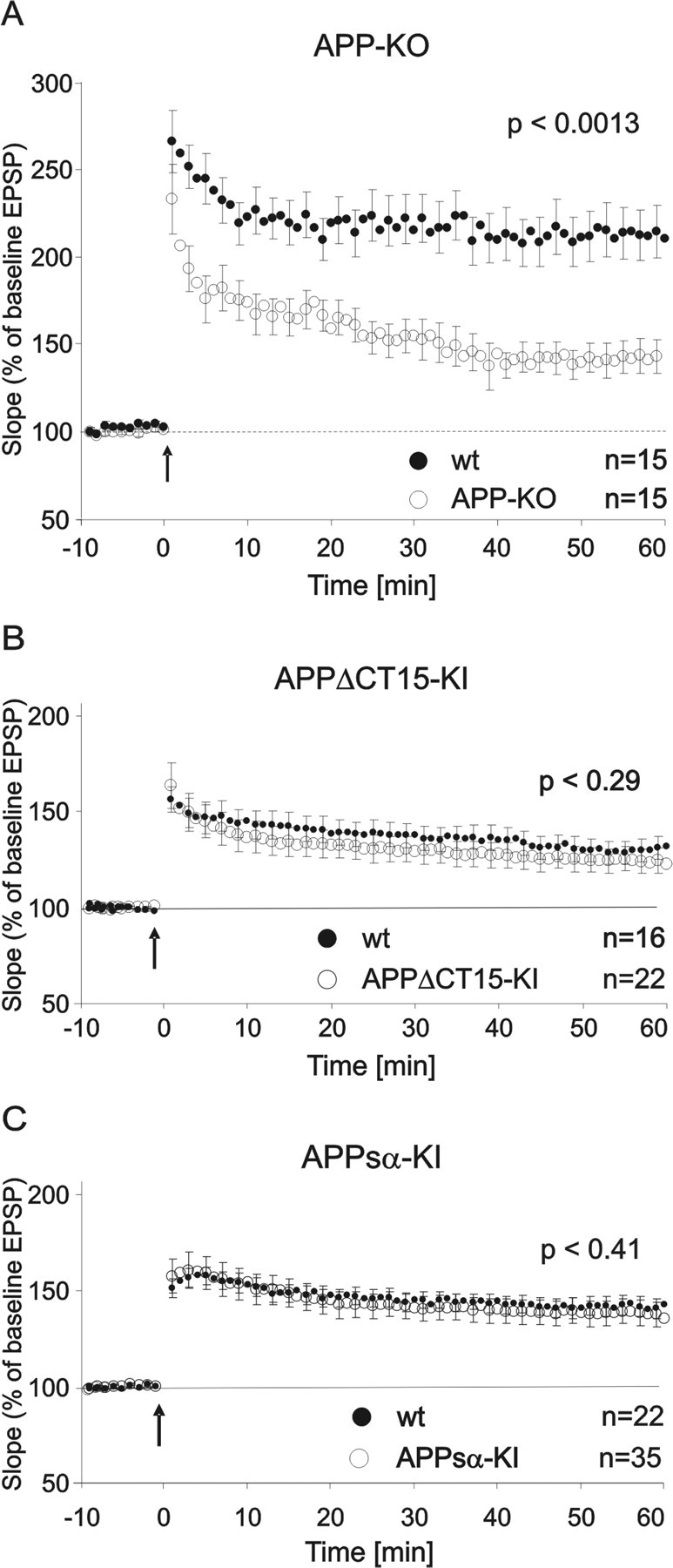
LTP analysis in APP-KI. LTP of fEPSPs was induced in hippocampal slices by TBS (10 trains of 4 pulses at 100 Hz with an interburst interval of 200 μs, repeated 3×) after 20 min of 0.1 Hz test stimulation (arrow). A, Aged APP-KO mice (9–12 months) showed significantly lower rates of induction and maintenance of LTP than their wt littermates, expressed as a percentage of the mean slope before LTP induction (baseline). On testing for 60 min after TBS, the APP-KO mice showed a significantly reduced potentiation [wt, 212 ± 14.5 (n = 15); APP-KO, 142.8 ± 9.5 (n = 15); p < 0.0013]. B, Unlike APP-KO mice, aged APPΔCT15-KI mice (12–15 months) did not have LTP deficits relative to their wt littermates after 60 min [wt, 131.92 ± 6.07 (n = 16); APPΔCT15-KI, 124.94 ± 6.45 (n = 22)]. C, Neither did aged APPsα-KI mice show deficits in induction or maintenance of LTP after 60 min when compared with wt control animals [wt, 141.97 ± 5.25 (n = 22); APPsα-KI, 136.63 ± 6.96 (n = 35); filled circles, wt animals; open circles, KI animals]. All data are presented as the means ± SEM; Student's t test.
Discussion
In this study we have analyzed APP functional domains by using a reverse genetic in vivo approach and by introducing stop codons into the chromosomal APP locus in such a way that two different C-terminally truncated APP variants are expressed. We then compared the phenotype of KI mice with that of completely APP-deficient mice to delineate which portion of the APP molecule or which proteolytic fragments are essential for the various in vivo functions. We initially hypothesized that mice expressing the APPΔCT15 variant, which is still processed and expressed at the cell surface, might resemble more closely the wt phenotype, whereas mice expressing solely APPsα might exhibit some deficits. The key finding of this study is that (although expressed at a slightly reduced level) both KI mutants grossly attenuate or completely rescue the defects observed in adult APP-KO mice. This finding is rather surprising because APP resembles a cell surface receptor. The numerous adaptor protein interactions with the APP C terminus rather suggested an APP signaling function in its full-length configuration or via AICD-mediated transcriptional regulation (for review, see Reinhard et al., 2005). Thus, at least with regard to the (postnatal) APP functions assessed by the tests used here, we have to refocus our attention to secreted APPsα that seems to be sufficient for normal brain weight and somatic growth, normal grip strength development, normal spontaneous home cage activity, normal exploratory activity, as well as normal spatial learning and LTP. This conclusion is further supported by recent studies from C. elegans, showing that expression of extracellular APL-1 fragments rescue the APL-1 KO phenotype (Hornsten et al., 2007). Future experiments will show whether APPsα might also be sufficient to rescue defects underlying the lethal phenotype of APP−/−APLP2−/− mutants.
Our data suggest that the APP C terminus is dispensable for the physiological functions described above. It is also clear, however, that it is crucial for APP processing and trafficking within the cell. Here we provide in vivo evidence that lack of the last 15 aa of endogenous APP markedly reduces Aβ production. Reduced Aβ levels in brain were paralleled by a prolonged half-life of full-length APP in MEFs, increased cell surface expression, and thus reduced endocytosis. Although we have not studied APP cleavage products in detail, we consider reduced endosomal β/γ-cleavage of APPΔCT15 as the most likely cause for reduced Aβ generation, because our data are well in agreement with previous in vitro studies that used truncated APP constructs in transfected cell lines (Koo and Squazzo, 1994; Perez et al., 1999; Chyung and Selkoe, 2003). Reduced endocytosis leading to reduced Aβ production might be attributable to the lack of the endocytic YENP internalization signal itself (Perez et al., 1999) and/or attributable to the lack of protein interactions with the YENPTY motif, including Fe65. Interestingly, Fe65 links APP in a trimeric complex to the low density lipoprotein receptor-related protein LRP (Pietrzik et al., 2004), and genetic deficiency of LRP in LRP-KO cells reduces APP endocytosis (Pietrzik et al., 2002) as observed here for the APPΔCT15 deletion. Recently, studies have suggested that Aβ, in addition to its neurotoxic effects, also may play a beneficial physiological role, e.g., by limiting neuronal excitation (Kamenetz et al., 2003). From our study we can exclude that deficits observed for APP-KO mice are attributable to the lack of Aβ, because these deficits were rescued in APPsα-KI mice.
With regard to the growth and brain weight deficit that can be rescued by APPsα, it is interesting to note that in vitro APPsα exerts proliferative effects on thyrocytes and keratinocytes as well as certain fibroblast lines (Saitoh et al., 1989). More recently, Caillé et al. (2004) have demonstrated that APPsα functions synergistically with epidermal growth factor as a growth factor for neuronal progenitor cells in the subventricular zone of adult mice (Caille et al., 2004). However, we and others (Phinney et al., 1999; Herms et al., 2004; Priller et al., 2006) have not found major neuronal loss, at least in the cortex or hippocampus of adult APP-KO mice, and at present the mechanism underlying the brain weight deficit of APP-KO mice that emerges in the first postnatal weeks is unclear.
Previously, experiments that used anti-APP antibodies to block protein function or antisense oligonucleotides have supported a role for APP in learning and memory (Turner et al., 2003), although the interpretation has been hampered by the fact that the antibodies used cannot distinguish between APPs and full-length APP and also may have cross-reacted with APLPs. Here we have shown that the defect in Morris water maze acquisition learning of adult APP-KO mice is corrected in mice expressing solely either APPsα or APPΔCT15 (which is processed readily by α-secretase). During training APP-KO mice displayed considerably delayed learning, evidenced by less rapid reductions in escape latency and swim path length. The overall performance deficit resulted from a combination of a true learning deficit (as evidenced by increased thigmotaxis, less efficient orientation toward the platform, and increased swim path length) and a motor impairment (as shown by reduced swim speed). APP-KI variants completely rescued the learning deficit and attenuated the physical impairment to a level that no longer had a detectable effect on training performance. APP deficiency did, however, not affect spatial memory per se as evidenced by normal probe trial scores of the relatively young (2.4 months old) APP-KO mice studied here. Because animals that have undergone water maze testing once are not naive any longer, we could not assess whether aged mice would display, in addition to learning deficits, impairments in memory as observed in previous studies (Phinney et al., 1999; Seabrook et al., 1999). Instead, we studied synaptic plasticity in aged mice to assess potentially correlated impairments. In summary, our behavioral data provide compelling genetic evidence that APPsα is the principal APP fragment that is limiting in the compromised spatial learning performance of APP-KO mice. This is consistent with and extends previous studies showing that exogenous recombinant APPsα (recAPPsα) injected into the brain of wt mice can enhance memory in certain behavioral paradigms (Meziane et al., 1998; Bour et al., 2004). In this regard it is interesting that recently the infusion of recAPPsα and ADAM10 overexpression was shown to lead to increased cortical synaptogenesis (Bell et al., 2007).
As a novel phenotype we report here that APP-KO mice display altered circadian locomotor activity with increased agitation at the onset of the dark period, reminiscent of the altered rhythms of activity and restlessness observed in AD patients (Reisberg et al., 1987). Again, this deficit is rescued in APP-KI mice, suggesting that a deficit in APPsα levels may contribute to the noncognitive behavioral symptoms in AD. APP-KO mice are known to exhibit reduced activity when placed in a novel environment (Zheng et al., 1995). This was confirmed in the light/dark box test of the present study in which the number of light/dark transitions was reduced in APP-KO mice. Again this deficit was rescued fully in both KI lines.
APP release may occur constitutively but also is induced by neuropeptides and neuronal activity, by agonist-mediated stimulation of neurotransmitter receptors (Turner et al., 2003), and after the in vivo induction of LTP in the dentate gyrus of anesthetized rats (Fazeli et al., 1994). In addition, preincubation of hippocampal slices with APPsα has been shown to facilitate (high frequency-induced) LTP in the CA1 region and to modulate long-term depression (Ishida et al., 1997). However, up to now the role of endogenously produced APPsα for induction and maintenance of LTP has remained elusive. Our study now corroborates that the LTP defect observed for APP-KO mice is attributable to a loss of physiological APPsα functions and not attributable to other confounding effects, including reactive gliosis and axodendritic abnormalities as previously hypothesized (Seabrook et al., 1999). Interestingly, both memory and LTP deficits that occur as a consequence of APP (and thus APPsα) deficiency are age-dependent (Dawson et al., 1999; Seabrook et al., 1999), suggesting that compensatory mechanisms that may mask the APPsα deficit in young mice are lost during the aging process. Intriguingly, reduced APPs levels not only have been found in the CSF of aged rats, which correlated with spatial memory deficits (Anderson et al., 1999), but also were found to be reduced in the CSF of affected members of a familial case of AD (Almkvist et al., 1997). Moreover, reduced CSF levels of APPsα, α-secretase ADAM10 protein level, and activity are prominent features of sporadic AD cases (Lannfelt et al., 1995; Sennvik et al., 2000; Colciaghi et al., 2002; Tyler et al., 2002). Together, these studies and our data highlight the functional importance of endogenous APPsα for learning and neuronal plasticity as well as circadian locomotor activity. This suggests that loss of APPsα may contribute more directly to the development of symptoms and the pathogenesis of AD than previously anticipated and lends additional support to the concept of increasing α-secretase activity as an AD treatment.
Footnotes
This work was supported by Deutsche Forschungsgemeinschaft Grant MU1457/4-3 (U.C.M.), Bundesministerium für Bildung und Forschung Grant 01GS0469 (U.C.M.), and grants from the Thyssen Foundation (to U.C.M. and M.K.), the Swiss National Science Foundation, and the National Center of Competence in Research, Neural Plasticity and Repair. We thank Heinrich Betz for continuous support; Paul Mathews for advice regarding APPsα detection; Bart De Strooper for providing the B63 antibody; Jakob Tschäpe for help with art work; and Nicole Fürst, Franziska Stöcklin, and Inger Drescher for excellent technical assistance.
References
- Almkvist O, Basun H, Wagner SL, Rowe BA, Wahlund LO, Lannfelt L. Cerebrospinal fluid levels of alpha-secretase-cleaved soluble amyloid precursor protein mirror cognition in a Swedish family with Alzheimer disease and a gene mutation. Arch Neurol. 1997;54:641–644. doi: 10.1001/archneur.1997.00550170111022. [DOI] [PubMed] [Google Scholar]
- Anderson JJ, Holtz G, Baskin PP, Wang R, Mazzarelli L, Wagner SL, Menzaghi F. Reduced cerebrospinal fluid levels of alpha-secretase-cleaved amyloid precursor protein in aged rats: correlation with spatial memory deficits. Neuroscience. 1999;93:1409–1420. doi: 10.1016/s0306-4522(99)00244-4. [DOI] [PubMed] [Google Scholar]
- Anliker B, Müller U. The functions of the mammalian amyloid precursor protein and related amyloid precursor-like proteins. Neurodegener Dis. 2006;3:239–246. doi: 10.1159/000095262. [DOI] [PubMed] [Google Scholar]
- Bell KF, Zheng L, Fahrenholz F, Cuello AC. ADAM-10 over-expression increases cortical synaptogenesis. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2006.11.004. in press. [DOI] [PubMed] [Google Scholar]
- Bour A, Little S, Dodart JC, Kelche C, Mathis C. A secreted form of the beta-amyloid precursor protein (sAPP695) improves spatial recognition memory in OF1 mice. Neurobiol Learn Mem. 2004;81:27–38. doi: 10.1016/s1074-7427(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Caillé I, Allinquant B, Dupont E, Bouillot C, Langer A, Müller U, Prochiantz A. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development. 2004;131:2173–2181. doi: 10.1242/dev.01103. [DOI] [PubMed] [Google Scholar]
- Chyung JH, Selkoe DJ. Inhibition of receptor-mediated endocytosis demonstrates generation of amyloid beta-protein at the cell surface. J Biol Chem. 2003;278:51035–51043. doi: 10.1074/jbc.M304989200. [DOI] [PubMed] [Google Scholar]
- Colciaghi F, Borroni B, Pastorino L, Marcello E, Zimmermann M, Cattabeni F, Padovani A, Di Luca M. α-Secretase ADAM10 as well as αAPPs is reduced in platelets and CSF of Alzheimer disease patients. Mol Med. 2002;8:67–74. [PMC free article] [PubMed] [Google Scholar]
- Dawson GR, Seabrook GR, Zheng H, Smith DW, Graham S, O'Dowd G, Bowery BJ, Boyce S, Trumbauer ME, Chen HY, van der Ploeg LH, Sirinathsinghji DJ. Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience. 1999;90:1–13. doi: 10.1016/s0306-4522(98)00410-2. [DOI] [PubMed] [Google Scholar]
- Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-Tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-β 42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- Fazeli MS, Breen K, Errington ML, Bliss TV. Increase in extracellular NCAM and amyloid precursor protein following induction of long-term potentiation in the dentate gyrus of anaesthetized rats. Neurosci Lett. 1994;169:77–80. doi: 10.1016/0304-3940(94)90360-3. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Grimm HS, Patzold AJ, Zinser EG, Halonen R, Duering M, Tschäpe JA, De Strooper B, Müller U, Shen J, Hartmann T. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat Cell Biol. 2005;7:1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- Heber S, Herms J, Gajic V, Hainfellner J, Aguzzi A, Rülicke T, von Kretzschmar H, von Koch C, Sisodia S, Tremml P, Lipp HP, Wolfer DP, Müller U. Mice with combined gene knock-outs reveal essential and partially redundant functions of amyloid precursor protein family members. J Neurosci. 2000;20:7951–7963. doi: 10.1523/JNEUROSCI.20-21-07951.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert SS, Serneels L, Tolia A, Craessaerts K, Derks C, Filippov MA, Müller U, De Strooper B. Regulated intramembrane proteolysis of amyloid precursor protein and regulation of expression of putative target genes. EMBO Rep. 2006;7:739–745. doi: 10.1038/sj.embor.7400704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herms J, Anliker B, Heber S, Ring S, Fuhrmann M, Kretzschmar H, Sisodia S, Müller U. Cortical dysplasia resembling human type 2 lissencephaly in mice lacking all three APP family members. EMBO J. 2004;23:4106–4115. doi: 10.1038/sj.emboj.7600390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsten A, Lieberthal J, Fadia S, Malins R, Ha L, Xu X, Daigle I, Markowitz M, O'Connor G, Plasterk R, Li C. APL-1, a Caenorhabditis elegans protein related to the human beta-amyloid precursor protein, is essential for viability. Proc Natl Acad Sci USA. 2007;04:1971–1976. doi: 10.1073/pnas.0603997104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A, Furukawa K, Keller JN, Mattson MP. Secreted form of beta-amyloid precursor protein shifts the frequency dependency for induction of LTD and enhances LTP in hippocampal slices. NeuroReport. 1997;8:2133–2137. doi: 10.1097/00001756-199707070-00009. [DOI] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Koo EH, Squazzo SL. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- Lannfelt L, Basun H, Wahlund LO, Rowe BA, Wagner SL. Decreased alpha-secretase-cleaved amyloid precursor protein as a diagnostic marker for Alzheimer's disease. Nat Med. 1995;1:829–832. doi: 10.1038/nm0895-829. [DOI] [PubMed] [Google Scholar]
- Li ZW, Stark G, Götz J, Rülicke T, Gschwind M, Huber G, Müller U, Weissmann C. Generation of mice with a 200-kb amyloid precursor protein gene deletion by Cre recombinase-mediated site-specific recombination in embryonic stem cells. Proc Natl Acad Sci USA. 1996;93:6158–6162. doi: 10.1073/pnas.93.12.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani R, Kozlov S, Akhmedov A, Cinelli P, Kinter J, Lipp HP, Sonderegger P, Wolfer DP. Impaired explorative behavior and neophobia in genetically modified mice lacking or overexpressing the extracellular serine protease inhibitor neuroserpin. Mol Cell Neurosci. 2003;23:473–494. doi: 10.1016/s1044-7431(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Magara F, Müller U, Li ZW, Lipp HP, Weissmann C, Stagljar M, Wolfer DP. Genetic background changes the pattern of forebrain commissure defects in transgenic mice underexpressing the β-amyloid precursor protein. Proc Natl Acad Sci USA. 1999;96:4656–4661. doi: 10.1073/pnas.96.8.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meziane H, Dodart JC, Mathis C, Little S, Clemens J, Paul SM, Ungerer A. Memory-enhancing effects of secreted forms of the beta-amyloid precursor protein in normal and amnestic mice. Proc Natl Acad Sci USA. 1998;95:12683–12688. doi: 10.1073/pnas.95.21.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U, Cristina N, Li ZW, Wolfer DP, Lipp HP, Rülicke T, Brandner S, Aguzzi A, Weissmann C. Behavioral and anatomical deficits in mice homozygous for a modified beta-amyloid precursor protein gene. Cell. 1994;79:755–765. doi: 10.1016/0092-8674(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Perez RG, Soriano S, Hayes JD, Ostaszewski B, Xia W, Selkoe DJ, Chen X, Stokin GB, Koo EH. Mutagenesis identifies new signals for β-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Aβ42. J Biol Chem. 1999;274:18851–18856. doi: 10.1074/jbc.274.27.18851. [DOI] [PubMed] [Google Scholar]
- Phinney AL, Calhoun ME, Wolfer DP, Lipp HP, Zheng H, Jucker M. No hippocampal neuron or synaptic bouton loss in learning-impaired aged beta-amyloid precursor protein-null mice. Neuroscience. 1999;90:1207–1216. doi: 10.1016/s0306-4522(98)00645-9. [DOI] [PubMed] [Google Scholar]
- Pietrzik CU, Busse T, Merriam DE, Weggen S, Koo EH. The cytoplasmic domain of the LDL receptor-related protein regulates multiple steps in APP processing. EMBO J. 2002;21:5691–5700. doi: 10.1093/emboj/cdf568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzik CU, Yoon IS, Jaeger S, Busse T, Weggen S, Koo EH. Fe65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. J Neurosci. 2004;24:4259–4265. doi: 10.1523/JNEUROSCI.5451-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priller C, Bauer T, Mitteregger G, Krebs B, Kretzschmar HA, Herms J. Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci. 2006;26:7212–7221. doi: 10.1523/JNEUROSCI.1450-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard C, Hebert SS, De Strooper B. The amyloid-beta precursor protein: integrating structure with biological function. EMBO J. 2005;24:3996–4006. doi: 10.1038/sj.emboj.7600860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg B, Borenstein J, Salob SP, Ferris SH, Franssen E, Georgotas A. Behavioral symptoms in Alzheimer's disease: phenomenology and treatment. J Clin Psychiatry. 1987;48(Suppl):9–15. [PubMed] [Google Scholar]
- Saitoh T, Sundsmo M, Roch JM, Kimura N, Cole G, Schubert D, Oltersdorf T, Schenk DB. Secreted form of amyloid beta protein precursor is involved in the growth regulation of fibroblasts. Cell. 1989;58:615–622. doi: 10.1016/0092-8674(89)90096-2. [DOI] [PubMed] [Google Scholar]
- Seabrook GR, Smith DW, Bowery BJ, Easter A, Reynolds T, Fitzjohn SM, Morton RA, Zheng H, Dawson GR, Sirinathsinghji DJ, Davies CH, Collingridge GL, Hill RG. Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology. 1999;38:349–359. doi: 10.1016/s0028-3908(98)00204-4. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Cell biology of protein misfolding: the examples of Alzheimer's and Parkinson's diseases. Nat Cell Biol. 2004;6:1054–1061. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- Sennvik K, Fastbom J, Blomberg M, Wahlund LO, Winblad B, Benedikz E. Levels of alpha- and beta-secretase-cleaved amyloid precursor protein in the cerebrospinal fluid of Alzheimer's disease patients. Neurosci Lett. 2000;278:169–172. doi: 10.1016/s0304-3940(99)00929-5. [DOI] [PubMed] [Google Scholar]
- Steinbach JP, Müller U, Leist M, Li ZW, Nicotera P, Aguzzi A. Hypersensitivity to seizures in beta-amyloid precursor protein-deficient mice. Cell Death Differ. 1998;5:858–866. doi: 10.1038/sj.cdd.4400391. [DOI] [PubMed] [Google Scholar]
- Tremml P, Lipp HP, Müller U, Ricceri L, Wolfer DP. Neurobehavioral development, adult open field exploration and swimming navigation learning in mice with a modified beta-amyloid precursor protein gene. Behav Brain Res. 1998;95:65–76. doi: 10.1016/s0166-4328(97)00211-8. [DOI] [PubMed] [Google Scholar]
- Turner PR, O'Connor K, Tate WP, Abraham WC. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity, and memory. Prog Neurobiol. 2003;70:1–32. doi: 10.1016/s0301-0082(03)00089-3. [DOI] [PubMed] [Google Scholar]
- Tyler SJ, Dawbarn D, Wilcock GK, Allen SJ. α- and β-secretase: profound changes in Alzheimer's disease. Biochem Biophys Res Commun. 2002;299:373–376. doi: 10.1016/s0006-291x(02)02635-9. [DOI] [PubMed] [Google Scholar]
- von Koch CS, Zheng H, Chen H, Trumbauer M, Thinakaran G, van der Ploeg LH, Price DL, Sisodia SS. Generation of APLP2 KO mice and early postnatal lethality in APLP2/APP double KO mice. Neurobiol Aging. 1997;18:661–669. doi: 10.1016/s0197-4580(97)00151-6. [DOI] [PubMed] [Google Scholar]
- White AR, Reyes R, Mercer JF, Camakaris J, Zheng H, Bush AI, Multhaup G, Beyreuther K, Masters CL, Cappai R. Copper levels are increased in the cerebral cortex and liver of APP and APLP2 knockout mice. Brain Res. 1999;842:439–444. doi: 10.1016/s0006-8993(99)01861-2. [DOI] [PubMed] [Google Scholar]
- Zheng H, Koo EH. The amyloid precursor protein: beyond amyloid. Mol Neurodegener. 2006;1:5. doi: 10.1186/1750-1326-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Jiang M, Trumbauer ME, Sirinathsinghji DJ, Hopkins R, Smith DW, Heavens RP, Dawson GR, Boyce S, Conner MW, Stevens KA, Slunt HH, Sisodia SS, Chen HY, van der Ploeg LH. β-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell. 1995;81:525–531. doi: 10.1016/0092-8674(95)90073-x. [DOI] [PubMed] [Google Scholar]