Abstract
Amyloid β (Aβ) oligomers are derived from proteolytic cleavage of amyloid precursor protein (APP) and can impair memory and hippocampal long-term potentiation (LTP) in vivo and in vitro. They are recognized as the primary neurotoxic agents in Alzheimer's disease. The mechanisms underlying such toxicity on synaptic functions are complex and not fully understood. Here, we provide the first evidence that these mechanisms involve protein phosphatase 1 (PP1). Using a novel transgenic mouse model expressing human APP with the Swedish and Arctic mutations that render Aβ more prone to form oligomers (arcAβ mice), we show that the LTP impairment induced by Aβ oligomers can be fully reversed by PP1 inhibition in vitro. We further demonstrate that the genetic inhibition of endogenous PP1 in vivo confers resistance to Aβ oligomer-mediated toxicity and preserves LTP. Overall, these results reveal that PP1 is a key player in the mechanisms of AD pathology.
Keywords: impairment, PP1, oligomers, Aβ-peptide, Alzheimer's disease, LTP
Introduction
Recent progress in the field of Alzheimer's disease (AD) has provided evidence that amyloid β (Aβ) oligomers rather than β-amyloid plaques are the major toxic agents in the pathology of the disease (Selkoe, 2002). Aβ oligomers have been demonstrated to disrupt synaptic plasticity and impair memory (Lambert et al., 1998; Walsh et al., 2002; Oddo et al., 2003; Lesne et al., 2006) and are known to accumulate in the brain of AD patients (Takahashi et al., 2002). Therapeutic strategies developed to interfere with Aβ deposition and clearance have shown initial benefit in AD patients and rodent models (Hock et al., 2003; Klyubin et al., 2005; Schenk et al., 2005). Neutralization of Aβ toxicity, for instance, was found to reverse memory deficits in a mouse model of AD without altering β-amyloid plaque load (Dodart et al., 2002).
Many studies have attempted to determine the mechanisms by which Aβ assemblies disrupt synaptic plasticity and mediate their detrimental effect, but the actual pathways that couple Aβ toxicity to synaptic functions remain only partially understood. A recently identified mechanism implicates specific interactions between Aβ and neurotransmitter receptors such as the NMDA (Snyder et al., 2005; Kelly and Ferreira, 2006) and α-7 nicotinic acetylcholine receptors (Oddo and LaFerla, 2006). This mechanism was shown to recruit the Ca2+-dependent protein phosphatase calcineurin (PP2B) (Chen et al., 2002; Snyder et al., 2005; Kelly and Ferreira, 2006), a finding in line with the hypothesis that Ca2+ signaling is disrupted by Aβ neurotoxicity (Demuro et al., 2005).
To investigate whether other major signaling pathways downstream of the NMDA receptor are implicated in the mechanisms of Aβ toxicity in vivo, we generated a transgenic mouse model expressing a human amyloid precursor protein (APP) with the Swedish and Arctic mutations (arcAβ mice) that produces a form of Aβ more prone to yield Aβ oligomers (Knobloch et al., 2007). In these mice, expression of the mutant APP induces punctate intraneuronal Aβ deposition in several brain areas and severe behavioral deficits before the onset of extracellular β-amyloid plaque deposition. Using this model, we show here that the overproduction of Arctic Aβ is associated with an age-dependent impairment in hippocampal long-term potentiation (LTP) and synaptic plasticity in vitro that involves protein phosphatase 1 (PP1)-dependent mechanisms. Furthermore, we demonstrate that both the pharmacologic and genetic inhibition of PP1 in vitro and in vivo reverse the defect in synaptic plasticity induced by Aβ oligomers. These results strongly support a major role for PP1 in the mechanisms of Aβ oligomer-mediated toxicity and demonstrate its reversibility in adult animals.
Materials and Methods
Animals.
ArcAβ mice, APPSwe/PS1 mice, and I-1* mutant mice were obtained by breeding as described previously (Holcomb et al., 1998; Genoux et al., 2002; Michalon et al., 2005; Knobloch et al., 2007). ArcAβ mice express human APP 695 carrying both the Swedish (K670N; M671L) and the Arctic (E693G) mutations in a single construct under the control of the prion protein promoter. APPSwe/PS1 mice carry a human APP with the Swedish mutation and a mutant presenilin 1 (M146L). I-1* mutant mice carry a transgene expressing an rtTA2 factor under the control of the calcium/calmodulin-dependent kinase II α (CaMKIIα) promoter and a transgene carrying a tetO promoter fused to I1* cDNA. Mice were kept under standard housing conditions on a reversed 12 h light/dark cycle with food and water ad libitum. Seven to nine days before the experiments, I-1* mutant mice and control littermates (carrying only the tetO-I-1* transgene) were fed doxycycline (West-Ward Pharmaceuticals, Eatontown, NJ) at 6 mg/g of food. All animal experiments were performed in accordance with guidelines of the Swiss veterinary cantonal office (licenses Nr 150/06 and 123/04).
Electrophysiological recordings.
Mice were anesthetized with isoflurane and then decapitated. Heads were immediately immersed in ice-cold freshly prepared artificial CSF (aCSF) for at least 2 min before brain extraction. Acute slices (400 μm thick) were prepared with a vibratome (VT 1000S; Leica Microsystems, Bannockburn, IL) in ice-cold gassed aCSF. Sections were incubated in aCSF at 34°C for 20 min and then kept at room temperature for at least 1 h before recording. Recording was performed in an interface chamber continuously flowed with aCSF at 1.1 ml/min. A monopolar electrode was placed in the Schaffer collaterals, and stimulation was applied at 0.033 Hz with stimulus intensity ranging from 20 to 80 μA, yielding evoked field EPSPs (fEPSPs) of 0.2–0.5 V. fEPSPs were recorded in the stratum radiatum using a borosilicate micropipette filled with aCSF. The signal was amplified with an Axopatch 200B amplifier (Molecular Devices, Union City, CA), digitized by a Digidata 1200 interface (Molecular Devices) and sampled at 10 kHz with Clampex 8.2 (Molecular Devices). aCSF was composed of the following (in mm): 119 NaCl, 11 d-glucose, 1.3 MgCl2.6H2O, 1.3 NaH2PO4, 2.5 KCl, 2.5 CaCl2, 26 NaHCO3, gassed with O2/CO2 (95/5%) at least 20 min before use and throughout the experiment. Baseline was recorded for a minimum of 20 min or until stable. Plasticity was then induced by stimulation with either 1 Hz for 15 min (900 pulses), 2 Hz for 10 min (1200 pulses), 5 Hz for 3 min (900 pulses), 10 Hz for 1.5 min (900 pulses), or 100 Hz for three trains of 1 s tetanus separated by 20 s. Data were analyzed by measuring the slope of individual fEPSPs at 1–1.5 ms after the stimulus pulse by linear fitting using Clampfit (Molecular Devices). The frequency-dependent plasticity curve (BCM curve) was built by determining mean fEPSP slopes over 10 min starting 35 min after stimulation (according to Jouvenceau et al., 2006).
Reverse transcription and quantitative real-time PCR.
Total RNA was isolated from frozen hippocampi of 6-month-old arcAβ mice and wild-type littermates (n = 5 for each) with TRIzol (Invitrogen, Carlsbad, CA) and cleaned up with RNeasy Mini kit (Qiagen, Valencia, CA). First-strand cDNA was synthesized using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen) according to the manufacturer's protocol. Real-time PCR was performed on TaqMan (ABI PRISM 7700 SDS) using SYBR Green (Applied Biosystems, Foster City, CA). Primer sequences were as follows: NMDA receptor subunit 2B (NR2B) forward (for.), AAGACAAGGGCCGATTCATG; reverse (rev.), GCAAAGGAGCTCTCACCAGC; CaMKII for., AGTCAGAGGAGACCCGCGT; rev., TGTGGAAGTGGACGATCTGC; glutamate receptor 1 (GluR1) for., CAATGTGGCAGGCGTGTTC; rev., TCGATTAAGGCAACCAGCATG; Syn.physin for., AAGGTGCTGCAGTGGGTCTTT; rev., CGAAGCTCTCCGGTGTAGCT; Zif268 for., CGAGAAGCCTTTTGCCTGTG; rev., TGGTATGCCTCTTGCGTTCA; Arc for., TGGAGGGAGGTCTTCTACCGT; rev., TATTTGCCGCCCATGGACT; β-actin for., TACTCTGTGTGGATCGGTGGC; rev., TGCTGATCCACATCTGCTGG; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for., GGCATCTTGGGCTACACTGAG; rev., CGAAGGTGGAAGAGTGGGAG.
Data analysis was performed according to the ΔΔCt method, normalized to β-actin and GAPDH. Statistical analysis was performed on the ΔCt values using Student's t test.
Passive immunization.
Three-month-old arcAβ mice were immunized with a single intraperitoneal injection (10 mg/kg) of either purified 6E10 (Signet, Dedham, MA) or negative control antibody for mouse IgG1 (Lab Vision, Fremont, CA) 48 h before slice preparation.
Tautomycin application.
Slices from 3-month-old arcAβ mice, wild-type littermates, and APPSwe/PS1 mice were bathed in normal aCSF or aCSF containing 1 nm tautomycin (Sigma, St. Louis, MO) for at least 1 h before recording.
PP1 activity assay.
Activity of recombinant PP1 (New England Biolabs, Beverly, MA) and calcineurin (Biomol, Plymouth Meeting, PA) was measured with and without 1 nm tautomycin using a modified Biomol Green Assay kit (Biomol).
Western blot analysis of phospho-CaMKII levels.
Mice were perfused transcardially with ice-cold PBS, and hippocampi were dissected and immediately frozen in dry ice. Crude synaptic membrane fractions were prepared according to Genoux et al. (2002). Equal amount of proteins was loaded and separated by SDS-PAGE, transferred to a nitrocellulose membrane, and blocked in TBS containing 1% BSA and 0.1% Triton X-100. Primary antibodies [anti-phospho-CaMKII antibody, 1:1000 (Millipore, Billerica, MA); anti-total CaMKII antibody, 1:10,000 (Millipore); and anti-β-actin antibody, 1:5000 (Abcam, Cambridge, MA)] were incubated overnight at 4°C, and the corresponding secondary antibodies were incubated for 1.5 h at room temperature. Commercial ECL (GE Healthcare, Piscataway, NJ) was used for the detection of chemiluminescence. Densitometric analysis was performed using the free downloadable ImageJ software (http://rsb.info.nih.gov/ij/).
Aβ oligomer preparation and application.
Aβ oligomers were prepared according to Klein (2002). Synthetic Aβ1–42 (Bachem, King of Prussia, PA) was dissolved in Hexafluor2-propanol (HFIP), aliquoted, and kept at −80°C after evaporation of HFIP. Aβ oligomers were prepared freshly by dissolving the above peptide film with DMSO and diluting it into cold F12 medium without phenol red to yield a 100 μm stock. This preparation was incubated at 4°C for 24 h and centrifuged at 14,000 × g for 10 min at 4°C, and the supernatant was further used for electrophysiological experiments according to Wang et al. (2002). Slices were bathed for at least 1 h in aCSF containing either Aβ oligomers (1:200 diluted) or, as a control, phenol red-free F12 medium only (1:200 diluted). To prevent a washout of Aβ oligomers during recording, the aCSF used for perfusion contained a 1:400 dilution of Aβ oligomers/phenol red-free F12, respectively. Each Aβ oligomer preparation was used for one I-1* mutant mouse and a corresponding control in parallel, measured in a blinded manner.
Electron microscopy.
The Aβ oligomer preparation was controlled by electron microscopy. Five microliters of the above supernatant were adsorbed onto glow-discharged, 300-mesh carbon-coated Formvar grids for 2–3 min, negatively stained with 2% phosphorwolfram acid for 45 s, and viewed with a Philips CM12 scanning transmission electron microscope.
Results
Severe impairment in synaptic plasticity in arcAβ mice
To evaluate the effect of arcAβ on synaptic plasticity, we first measured hippocampal LTP in area CA1 in vitro by recording fEPSPs in acute slices from adult arcAβ mice and wild-type littermates after 100 Hz stimulation. LTP was severely impaired in slices from 3.5- and 7.5-month-old arcAβ mice (Fig. 1A,B) (p < 0.001; repeated-measurement ANOVA), with fEPSPs returning to baseline within 10 min after LTP induction. The LTP deficit was not caused by impaired synaptic transmission, because basal transmission was normal in the transgenic slices (Fig. 1A,B, inset). It did not result from a developmental defect caused by transgene expression either, because both LTP (Fig. 1C) and basal synaptic transmission (Fig. 1C, inset) were normal in slices from 1-month-old mice, consistent with the fact that Aβ accumulation is not detectable at this age despite high expression of mutant APP (Knobloch et al., 2007). We confirmed that the LTP deficit was caused by Aβ oligomers by showing that it could be reversed with a single dose of an antibody directed against the Aβ sequence (6E10) administered to the animals 48 h before LTP induction (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). The antibody treatment restored LTP in transgenic slices to ∼85% level of potentiation compared with slices from wild-type littermates. A control antibody of the identical IgG class against a nonrelated antigen failed to rescue LTP (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). In addition to a deficit in plasticity at high induction frequency (100 Hz for LTP), stimulation frequencies of 5 and 10 Hz also showed reduced fEPSP potentiation in slices from arcAβ mice compared with wild-type slices. Plasticity at 1 or 2 Hz stimulation frequency was not changed (Fig. 2D). These results reveal a deficit in plasticity across a wide range of stimulation frequencies with a shift of responses toward synaptic depression.
Figure 1.
CA1 hippocampal LTP is impaired in arcAβ mice. A, B, CA1 hippocampal LTP is severely impaired in slices from 3.5- and 7.5-month-old arcAβ mice (n = 5 tg, 5 wt mice) but basal transmission is normal (right inset). C, LTP and basal transmission (right inset) are normal in slices from 1-month-old arcAβ mice (n = 5 tg, 5 wt mice). Left insets, Individual fEPSP traces for tg and wt mice before (black) and after (red) LTP induction. Calibration: A, B, 0.4 mV, 5 ms; C, 0.6 mV, 5 ms. D, Real-time RT-PCR: normal mRNA expression of synaptophysin, NMDA and AMPA receptors, and CaMKII, but reduced arg3.1 (67%; range, 58–78%; n = 5 tg and 5 wt mice) and zif268 (52%; range, 45–61%) expression in the hippocampus of 6-month-old arcAβ mice. Error bars represent SEM. *p < 0.05. norm., Normalized; Synapto., synaptophysin; Tg, transgenic.
Figure 2.
In vitro inhibition of PP1 reverses Aβ oligomer-mediated LTP impairment. A, B, PP1 is inhibited by bath application of 1 nm tautomycin (tauto) in slices from 3-month-old arcAβ mice and wild-type littermates. Tautomycin fully reverses the LTP deficit in slices from tg mice (n = 5) but has no effect in wt (n = 5 mice). It also does not change basal transmission (right insets). Left insets, Individual fEPSP traces for tg and wt mice before (black) and after (red) LTP induction. Calibration: 0.3 mV, 5 ms. C, Phosphatase activity assays showing that 1 nm tautomycin specifically inhibits the activity of recombinant PP1 but not of recombinant calcineurin. D, Frequency–response curve showing mean fEPSP slopes over 10 min starting 35 min after stimulation with 1, 2, 5, 10, and 100 Hz in slices from tg and wt mice treated or not with tautomycin (n = 3–5 slices per condition and frequency). E, PP1 inhibition with 1 nm tautomycin also reverses the LTP deficit seen in 3-month-old APPSwe/PS1 mice, another mouse model of AD (n = 5 tg). Error bars represent SEM. CN, Calcineurin; Tg, transgenic; w/o, without.
To examine whether candidate genes involved in synaptic signaling may underlie the impairment in plasticity induced by Aβ oligomers, we measured the level of expression of several candidate genes by real-time reverse transcription (RT)-PCR. Hippocampal expression of synaptophysin, a synaptic vesicle protein involved in neurotransmitter release, the NR2B and GluR1 subunits of the NMDA and AMPA receptors critical for glutamatergic neurotransmission, or CaMKII, a major protein kinase necessary for the induction of LTP (Lisman et al., 2002), was not altered in arcAβ mice (Fig. 1D). However, because intracellular Ca2+ signaling is altered by Aβ oligomers, it is likely that Ca2+-dependent posttranslational processes such as protein phosphorylation are perturbed. Therefore, we examined whether phosphorylation of CaMKII is changed in arcAβ mice. Western blot analyses revealed a decrease in the level of phosphorylated CaMKII in synaptic membranes from arcAβ mice (supplemental Fig. 2, available at www.jneurosci.org as supplemental material), suggesting a perturbation in protein kinase/phosphatase pathways. Furthermore, the expression of transcription factors rapidly activated after neuronal activity and required for LTP, arg3.1/arc and zif268 (Worley et al., 1991; Link et al., 1995; Guzowski et al., 2000), was significantly reduced in arcAβ mice (Fig. 1D) [p < 0.05; t test; mean and range percentage change versus wild type (wt): arg3.1, 67% (58–78%); zif268, 52% (45–61%)]. These results therefore indicate that Aβ oligomers alter protein phosphorylation and the activation of immediate early genes (IEGs).
In vitro inhibition of PP1 reverses Aβ oligomer-mediated plasticity impairment
Because PP1 is a major protein phosphatase in neurons that negatively regulates intracellular signaling downstream from the NMDA receptor (including CaMKII) and modulates hippocampal LTP (Jouvenceau et al., 2006; Mansuy and Shenolikar, 2006), we examined whether it might be involved in the deficit in plasticity induced by Aβ oligomers. PP1 was inhibited pharmacologically in acute hippocampal slices from arcAβ mice and wild-type littermates using the selective inhibitor tautomycin (1 nm), and the effect on plasticity was examined across several stimulation frequencies. Remarkably, PP1 inhibition was found to fully reverse the deficit in plasticity observed at 5, 10, or 100 Hz in arcAβ slices (Fig. 2A,D) (p < 0.05; repeated-measurement ANOVA) but had no effect on plasticity in wild-type slices (Fig. 2B) (p = 0.3; repeated-measurement ANOVA). This rescuing effect resulted specifically from PP1 inhibition and did not involve the protein phosphatase calcineurin because only PP1 activity was significantly inhibited by 1 nm tautomycin (Fig. 2C), confirming that PP1-dependent mechanisms are involved in the plasticity deficit.
To confirm the rescuing effect of PP1 inhibition observed in arcAβ mice, we examined whether it could be reproduced in another transgenic mouse model of AD. We tested the well established APPSwe/PS1 model that expresses human APP carrying the Swedish mutation and a mutated Presenilin1 and exhibits an early AD pathology associated with marked behavioral deficits (Holcomb et al., 1998). Similarly to arcAβ mice, CA1 hippocampal LTP was severely impaired in 3-month-old APPSwe/PS1 animals and was significantly rescued by PP1 inhibition (1 nm tautomycin) (Fig. 2E) (p < 0.05; repeated-measurement ANOVA). The rescue was however less pronounced than in arcAβ mice, possibly because of higher production of the more toxic Aβ1–42 as a result of the presenilin mutation in APPSwe/PS1. Together, these results strongly suggest that PP1 is a critical factor in the course of Aβ-mediated toxicity.
Inhibition of endogenous PP1 in vivo confers resistance to Aβ oligomer-mediated toxicity
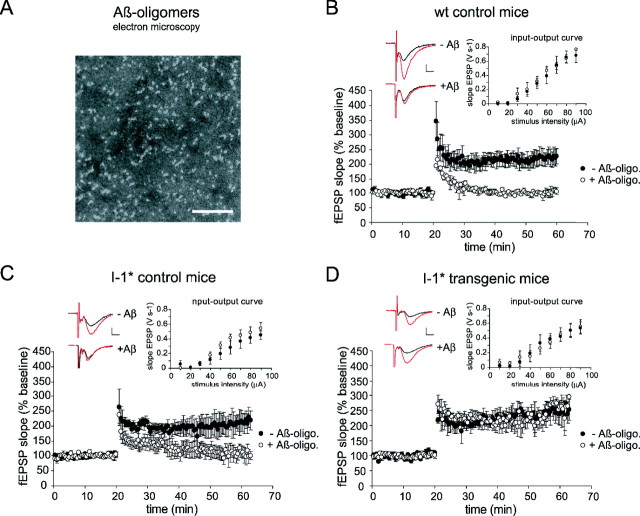
To assess whether the detrimental effect of PP1 is also reversible in vivo, we examined whether inhibition of endogenous PP1 before exposure to Aβ oligomers confers resistance to Aβ oligomer-mediated toxicity. We took advantage of a transgenic mouse model in which PP1 is selectively inhibited (by 40–60%) in forebrain neurons by expression of a constitutively active form of the PP1 inhibitor-1 (I-1*) (Genoux et al., 2002; Michalon et al., 2005). Acute slices from adult I-1* transgenic mice were exposed to Aβ oligomers produced from synthetic Aβ1–42. This preparation of Aβ oligomers contained globular Aβ assemblies of 7–12 nm diameter (Fig. 3A), as previously described (Klein, 2002). To verify that our preparation of Aβ oligomers had the reported inhibitory effect on LTP (Wang et al., 2002), we bathed slices from wild-type mice in aCSF containing Aβ oligomers (0.5 μm of the initial peptide) or normal aCSF. As expected, Aβ oligomers strongly impaired the induction of LTP in slices from wild-type mice, and fEPSPs returned to baseline within 10 min after tetanic stimulation (Fig. 3B). Likewise, Aβ oligomers impaired LTP in slices from I-1* control littermates (Fig. 3C) (p < 0.05; repeated-measurement ANOVA). However, in slices from I-1* transgenic mice, Aβ oligomers did not prevent the induction of LTP, which was robust and stable (Fig. 3D) (p = 0.6; repeated-measurement ANOVA comparing LTP with and without Aβ oligomers). These results therefore indicate that PP1 inhibition in vivo confers protection against Aβ oligomer-mediated toxicity.
Figure 3.
Endogenous PP1 inhibition in vivo confers resistance to Aβ oligomer-mediated toxicity. A, Electron microscopy of Aβ oligomer preparation produced from synthetic Aβ1–42 (according to Klein, 2002) showing globular Aβ assemblies of 7–12 nm after 24 h incubation at 4°C. Scale bar, 200 nm. B, Confirmation of Aβ oligomer-mediated toxicity in wt mice. Aβ oligomers were bath applied to wt slices for at least 1 h. Aβ oligomers impair LTP (n = 3–4; 3 slices with Aβ oligomers and 4 control slices). Right inset, Basal transmission was not affected. Left insets, Individual fEPSP traces with and without Aβ oligomer application before (black) and after (red) LTP induction. Calibration: 0.6 mV, 5 ms. C, D, Aβ oligomers are bath applied for at least 1 h to slices from I-1* transgenic mice and control littermates. Aβ oligomers impair LTP in I-1* control mice (n = 5) but not in I-1* transgenic mice (n = 5). Right inset, Basal transmission is not affected. Left insets, Individual fEPSP traces with and without application of Aβ oligomers before (black) and after (red) LTP induction. Calibration: 0.6 mV, 5 ms. Error bars represent SEM. oligo., Oligomer.
Discussion
This study demonstrates that the inhibitory effects of Aβ oligomers on hippocampal LTP in the adult brain are reversible and that normal LTP can be restored by PP1 inhibition in vitro in independent mouse models of AD (arcAβ mice and APPSwe/PS1 mice). It also reveals that in addition to LTP induced by 100 Hz stimulation, Aβ oligomers perturb synaptic plasticity in the hippocampus at intermediate frequencies (5 and 10 Hz) and induce a general shift in synaptic responses toward depression that, similar to LTP, can be fully reversed by PP1 inhibition. Endogenous PP1 inhibition in vivo is further shown to prevent the impairment of LTP induced by Aβ oligomers, providing strong evidence that PP1 is a critical player in the mechanisms of Aβ oligomer-mediated toxicity.
Although the mechanisms of Aβ action are not fully understood, our results provide evidence that, in addition to involving PP1-dependent processes, they also implicate CaMKII and IEGs. CaMKII, arg3.1, and zif268 are traditionally known as key molecules in the mechanisms of LTP and memory formation (Jones et al., 2001; Lisman et al., 2002; Tzingounis and Nicoll, 2006) and have also been implicated in β-amyloid pathology (Dickey et al., 2003; Lin et al., 2004). The novel correlation that our results reveal between these molecules and PP1 suggests that they may be downstream targets of PP1-dependent pathways perturbed by Aβ oligomers. This may involve direct dephosphorylation of CaMKII by PP1, because CaMKII is a known target of PP1 (Strack et al., 1997) and could account for the alteration in plasticity in our model, which is severe, despite normal expression of NMDA and AMPA receptors and synaptophysin. Interestingly, PP1 inhibition in vivo has been shown to increase CaMKII phosphorylation, shift hippocampal plasticity toward potentiation (Jouvenceau et al., 2006), and reverse cognitive deficits in aged mice (Genoux et al., 2002). The present results significantly extend these previous findings by demonstrating the relevance of these PP1-dependent pathways in the mechanisms of AD. The present findings further underscore the implication of dysfunctional protein phosphorylation in AD pathology and highlight the importance of protein phosphatases in this pathology. The importance of their contribution is clearly reflected by the fact that the deficits in plasticity associated with the pathology are fully reversible, just like protein phosphorylation is. Because PP1 is a major protein phosphatase in neuronal cells, multiple targets and pathways are likely to be modified, such as phosphorylation or trafficking of glutamate receptors, as recently shown in primary neurons and organotypic brain slices from AD mouse models (Snyder et al., 2005; Hsieh et al., 2006).
Finally, the severe LTP impairment observed in young arcAβ mice (3 months) when no β-amyloid plaques are detectable supports the hypothesis that oligomeric Aβ species are major contributors to synaptic failure in AD (for review, see Rowan et al., 2003). The rescue of LTP achieved with a single dose of an antibody directed against the Aβ sequence confirms this hypothesis and suggests that such antibodies may be useful in immunotherapeutic strategies against AD (Rowan et al., 2005). In our model, the reduced arg3.1 and zif268 expression occurs before any detectable extracellular β-amyloid deposition, supporting the evidence that disruption of synaptic plasticity is an early event in the course of the disease. Overall, these results point to PP1 as a promising target for the development of potential therapeutic approaches against Aβ-mediated toxicity in AD.
Footnotes
This work was supported in part by Swiss National Science Foundation Grant 3200B0-112616, the National Center for Competence in Research (NCCR) “Neuronal Plasticity and Repair,” and European Union contract LSHM-CT-2003-503330 (APOPIS). The laboratory of I.M.M. was supported by the University Zurich, the Swiss Federal Institute of Technology Zurich, the Swiss National Science Foundation, NCCR “Neural Plasticity and Repair,” Human Frontier Science Program, the Slack-Gyr Foundation, and the Bitterlin Foundation. We thank Dr. Sandor Vizi for preparing the I-1* transgenic mice and Karen Duff and Karen Ashe for providing the original PS1 mouse line and the Swedish mouse line. We acknowledge the laboratory of electron microscopy (University of Zurich) for the support with electron microscopy.
References
- Chen QS, Wei WZ, Shimahara T, Xie CW. Alzheimer amyloid beta-peptide inhibits the late phase of long-term potentiation through calcineurin-dependent mechanisms in the hippocampal dentate gyrus. Neurobiol Learn Mem. 2002;77:354–371. doi: 10.1006/nlme.2001.4034. [DOI] [PubMed] [Google Scholar]
- Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- Dickey CA, Loring JF, Montgomery J, Gordon MN, Eastman PS, Morgan D. Selectively reduced expression of synaptic plasticity-related genes in amyloid precursor protein + presenilin-1 transgenic mice. J Neurosci. 2003;23:5219–5226. doi: 10.1523/JNEUROSCI.23-12-05219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418:970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, de Quervain DJ, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O'Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, Charnay P, Bozon B, Laroche S, Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Jouvenceau A, Hedou G, Potier B, Kollen M, Dutar P, Mansuy IM. Partial inhibition of PP1 alters bidirectional synaptic plasticity in the hippocampus. Eur J Neurosci. 2006;24:564–572. doi: 10.1111/j.1460-9568.2006.04938.x. [DOI] [PubMed] [Google Scholar]
- Kelly BL, Ferreira A. beta-Amyloid-induced dynamin 1 degradation is mediated by N-methyl-d-aspartate receptors in hippocampal neurons. J Biol Chem. 2006;281:28079–28089. doi: 10.1074/jbc.M605081200. [DOI] [PubMed] [Google Scholar]
- Klein WL. Abeta toxicity in Alzheimer's disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem Int. 2002;41:345–352. doi: 10.1016/s0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- Klyubin I, Walsh DM, Lemere CA, Cullen WK, Shankar GM, Betts V, Spooner ET, Jiang L, Anwyl R, Selkoe DJ, Rowan MJ. Amyloid beta protein immunotherapy neutralizes Abeta oligomers that disrupt synaptic plasticity in vivo. Nat Med. 2005;11:556–561. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- Knobloch M, Konietzko U, Krebs DC, Nitsch RM. Intracellular Abeta and cognitive deficits precede beta-amyloid deposition in transgenic arcAbeta mice. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2006.06.019. in press. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Lin KF, Chang RC, Suen KC, So KF, Hugon J. Modulation of calcium/calmodulin kinase-II provides partial neuroprotection against beta-amyloid peptide toxicity. Eur J Neurosci. 2004;19:2047–2055. doi: 10.1111/j.0953-816X.2004.03245.x. [DOI] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Mansuy IM, Shenolikar S. Protein serine/threonine phosphatases in neuronal plasticity and disorders of learning and memory. Trends Neurosci. 2006;29:679–686. doi: 10.1016/j.tins.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Michalon A, Koshibu K, Baumgartel K, Spirig DH, Mansuy IM. Inducible and neuron-specific gene expression in the adult mouse brain with the rtTA2S-M2 system. Genesis. 2005;43:205–212. doi: 10.1002/gene.20175. [DOI] [PubMed] [Google Scholar]
- Oddo S, LaFerla FM. The role of nicotinic acetylcholine receptors in Alzheimer's disease. J Physiol (Paris) 2006;99:172–179. doi: 10.1016/j.jphysparis.2005.12.080. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Rowan MJ, Klyubin I, Cullen WK, Anwyl R. Synaptic plasticity in animal models of early Alzheimer's disease. Philos Trans R Soc Lond B Biol Sci. 2003;358:821–828. doi: 10.1098/rstb.2002.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan MJ, Klyubin I, Wang Q, Anwyl R. Synaptic plasticity disruption by amyloid beta protein: modulation by potential Alzheimer's disease modifying therapies. Biochem Soc Trans. 2005;33:563–567. doi: 10.1042/BST0330563. [DOI] [PubMed] [Google Scholar]
- Schenk DB, Seubert P, Grundman M, Black R. A beta immunotherapy: lessons learned for potential treatment of Alzheimer's disease. Neurodegener Dis. 2005;2:255–260. doi: 10.1159/000090365. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Strack S, Barban MA, Wadzinski BE, Colbran RJ. Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. J Neurochem. 1997;68:2119–2128. doi: 10.1046/j.1471-4159.1997.68052119.x. [DOI] [PubMed] [Google Scholar]
- Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H, Greengard P, Gouras GK. Intraneuronal Alzheimer abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol. 2002;161:1869–1879. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis AV, Nicoll RA. Arc/Arg3.1: linking gene expression to synaptic plasticity and memory. Neuron. 2006;52:403–407. doi: 10.1016/j.neuron.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Wang HW, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B, Viola KL, Klein WL, Stine WB, Krafft GA, Trommer BL. Soluble oligomers of beta amyloid (1–42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002;924:133–140. doi: 10.1016/s0006-8993(01)03058-x. [DOI] [PubMed] [Google Scholar]
- Worley PF, Christy BA, Nakabeppu Y, Bhat RV, Cole AJ, Baraban JM. Constitutive expression of zif268 in neocortex is regulated by synaptic activity. Proc Natl Acad Sci USA. 1991;88:5106–5110. doi: 10.1073/pnas.88.12.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]