Abstract
Previous reports of tactile responses in human visual area MT/V5 have used complex stimuli, such as a brush stroking the arm. These complex moving stimuli are likely to induce imagery of visual motion, which is known to be a powerful activator of MT. The area described as “MT” in previous reports consists of at least two distinct cortical areas, MT and MST. Using functional magnetic resonance imaging, we separately localized human MT and MST and measured their response to vibrotactile stimuli unlikely to induce imagery of visual motion. Strong vibrotactile responses were observed in MST but not in MT. Vibrotactile responses in MST were approximately one-half as large as the response to visual motion and were distinct from those in another visual area previously reported to respond to tactile stimulation, the lateral occipital complex. To examine somatotopic organization, we separately stimulated the left and right hand and foot. No spatial segregation between hand and foot responses was observed in MST. The average response profile of MST was similar to that of somatosensory cortex, with a strong preference for the contralateral hand. These results offer evidence for the existence of somatosensory responses in MST, but not MT, independent of imagery of visual motion.
Keywords: multisensory, occipital, somatosensory, tactile, temporal, vibration, visual, visual motion
Introduction
The strategies used by the brain to integrate information from different sensory modalities are a question at the heart of cognitive neuroscience. One view is that all of sensory cortex is essentially multisensory (Ghazanfar and Schroeder, 2006). In support of this idea, functional neuroimaging studies have reported responses to tactile stimuli in regions of occipital lobe traditionally regarded as purely visual (Sathian et al., 1997; Amedi et al., 2001). More recently, two groups have reported responses to tactile stimuli in area MT, a region considered to be essential for processing visual motion (Hagen et al., 2002; Blake et al., 2004). In these studies, responses in MT were observed to complex moving tactile stimuli: a brush stroking the arm (Hagen et al., 2002) or a rotating three-dimensional globe (Blake et al., 2004). However, it is known that simply imagining a moving stimulus evokes activity in MT (Goebel et al., 1998) and that visual imagery in general is a powerful, specific activator of visual cortex (Ishai et al., 2000; O'Craven and Kanwisher, 2000). If in previous studies subjects engaged in visual motion imagery (such as imagining the movement of the brush as it traveled up and down the arm or the motion of the globe as it rotated), activation in MT could have been an indirect result of visual imagery rather than a direct result of somatosensory stimulation.
The standard MT localizers used in previous studies identify a region (which we henceforth refer to as MT+) (Beauchamp et al., 1997) that contains at least two distinct areas, MT and MST. In nonhuman primates, MT and MST have distinct functional specializations and different patterns of anatomical connectivity (Komatsu and Wurtz, 1988; Lewis and Van Essen, 2000). Therefore, we used anatomical and functional criteria to fractionate MT+ into MT and MST to separately measure tactile responses in each area. Because tactile responses have also been reported in the lateral occipital complex (LOC) (Amedi et al., 2001, 2002), a region that overlaps MT+ (Kourtzi et al., 2002), we also mapped the LOC in each subject to allow independent measurements of activity in MT, MST, and LOC.
We adopted two strategies to test whether somatosensory responses independent of visual imagery exist in MT+. First, we used vibrotactile stimuli delivered by piezoelectric bending elements to widely separated sites on the body surface. Because these stimuli do not contain any motion (real or apparent) relative to the body surface, although they do move perpendicular to the skin surface (a necessary precondition for activating mechanoreceptors), they are unlikely to induce the visual imagery of motion known to activate MT+. Second, we delivered vibrotactile stimulation to the ipsilateral and contralateral hands and feet of the subjects. If tactile activation in MT+ is produced by visual imagery of the tactile stimulus, the site of somatosensory stimulation should have relatively little effect. For instance, touching a rotating globe with the left hand or the right hand should induce similar amounts of visual motion imagery and concomitant activation in MT+. In contrast, one of the organizing principles of the somatosensory system is somatotopy, a map of the body surface. If MT+ tactile responses are not produced by imagery of visual motion, MT+ should show modulation by the body site of stimulation.
Materials and Methods
Subjects were recruited and informed consent was obtained in accordance with the University of Texas Committee for the Protection of Human Subjects. Four male participants and four female participants were scanned using a 3 tesla whole-body magnetic resonance (MR) scanner (Phillips Medical Systems, Bothell, WA). Anatomical images were collected using a magnetization-prepared 180° radio-frequency pulses and rapid gradient echo sequence optimized for gray–white matter contrast with 1-mm-thick sagittal slices and in-plane resolution of 0.938 × 0.938 mm. Functional images were collected using a gradient-recalled-echo echo-planar-imaging sequence sensitive to the blood-oxygen level-dependent (BOLD) signal. Thirty-three axial slices were collected in each 2 s repetition time (TR) with an echo time of 30 ms and a flip angle of 90°. Slice thickness was 3 mm, and in-plane resolution was 2.75 × 2.75 mm.
Vibrotactile stimulation.
Somatosensory stimuli were delivered to subjects using a custom-built apparatus (Yasar and Beauchamp, 2006). Five piezoelectric bending elements (Piezo Systems, Cambridge, MA) were attached, one each to the palm of the left and right hand, the sole of the left and right foot, and the right hip using nonslip silicon elastic bandages. A 200 Hz sinusoidal waveform was used to drive the piezoelectric bending elements (benders), based on behavioral data showing low detection thresholds at this frequency (Brisben et al., 1999).
The qualitative percept of stimulation was akin to holding a ringing cell phone set to “vibrate” mode, without any accompanying auditory percept. The vibration of the benders was inaudible because of its low sound pressure level, the high ambient noise of the MR scanner, and the hearing protection sound reducers worn by the subjects.
An ultrapure sinusoidal oscillator and high-gain amplifier (both from Krohn-Hite, Brockton, MA) generated the waveform, which was delivered to the benders by a relay box under computer control. All stimuli were synchronized to the scanner via a transistor–transistor logic pulse sent every TR by the MR scanner to a PC running Presentation software (Neurobehavioral Systems, Albany, CA). Large piezoelectric benders (6.6 cm long and 3.3 cm wide) were used to stimulate a large territory of mechanoreceptors to produce a maximal BOLD functional magnetic resonance imaging (fMRI) response to the vibrotactile stimuli. Because fMRI measures neural activity integrated over time, we attempted to maximize the evoked BOLD response by delivering stimuli throughout each 2 s trial. Because both central and peripheral adaptation is observed to vibrotactile stimulation (Leung et al., 2005; Tommerdahl et al., 2005), a pulsed design was used (four repetitions of 250 ms ON/250 ms OFF). The driving voltage delivered to the benders was 50 V, producing a displacement of 0.5 mm. Before each experiment, the amplitude of each element was individually adjusted using the relay box potentiometers (in the range of 40–50 V, 0.4–0.5 mm) to equate the perceived intensity across benders. This served as a rough control for differences in efficacy caused by small differences in the placement, attachment, or manufacture of individual benders.
Visual stimulus presentation.
The visual stimuli were back-projected from a liquid crystal display projector (Sony Electronics, San Diego, CA) onto a Lucite screen (Da-Lite, Warsaw, IN) and viewed through a mirror attached to the MR head coil. The visual stimuli for the MT/MST localizer consisted of low-contrast random dots presented in the left or right hemifields (Huk et al., 2002). The dots moved radially in or out on sequential trials with slightly varying speeds (randomly chosen from 3–5°/s) to minimize adaptation. The visual stimuli for the LOC localizer consisted of photographs and scrambled photographs from a variety of object categories.
fMRI experimental design.
The visual area localizer experiments were conducted using a block design. There were eight 30 s blocks in each 4 min scan series, with each block containing 20 s of stimulation (10 trials) of a single stimulation type followed by 10 s of baseline. Regressors were created by convolving the timing of each type of stimulation block with a gamma-variate function to account for the BOLD hemodynamic response function.
The vibrotactile experiments were conducted using a rapid event-related design. There were 110 2 s stimulation trials in each 5 min scan series: 25 for each of left and right hand and foot and 10 catch (hip) trials (see below, Behavioral Task). Interspersed between stimulation trials were intervals of fixation (80 s total). An optimal stimulus sequence generator (optseq2) (Dale, 1999) generated a pseudorandom ordering for the different trial types and fixation, resulting in a range of interstimulus intervals from 0 to 12 s. Event-related data were analyzed using the finite-impulse response (FIR) method, as implemented in the 3dDeconvolve program in the AFNI software package (Cox, 1996). Tent-shaped regressors were created for each of the nine images (18 s window with a TR of 2 s) after trial onset. Because a rapid event-related design was used, at any point in the MR time series the image intensity contained contributions from the overlapping responses evoked by many previous stimuli. By assuming linearity and time invariance, the FIR method deconvolved the overlapping responses into separate responses, one for each stimulus type, that were equivalent to those that would be measured with a slow event-related design.
In the general linear model, regressors of no interest consisted of the motion estimates from volume registration and polynomial regressors to account for baseline shifts and linear drifts in each scan series. The ratio of variance accounted for by the stimulus regressors and regressors of no interest to the regressors of no interest alone was used to calculate an F-ratio and associated significance for each voxel.
Cortical surface models.
Individual cortical surface models were created using FreeSurfer software (Fischl et al., 1999). Surfaces were visualized and average cortical surface models created using SUMA software (Argall et al., 2006). To visualize activity buried in sulcal depths, the surface was partially inflated using 500 iterations of a smoothing algorithm. Anatomical features on the partially inflated surface were visualized by colorizing the surface with a sulcal depth index derived from the distance of each node from the brain's bounding ellipsoid (Van Essen, 2004).
Identified visual areas.
To locate area MT and MST, anatomical and functional criteria were used (Tootell et al., 1995; Beauchamp et al., 1997, 2001; Dumoulin et al., 2000; Huk et al., 2002). First, an MT+ region of interest (ROI) was created containing voxels showing a significant response to left or right hemifield stimulation (p < 10−6) in the ascending portion of posterior inferior temporal sulcus (pITS). Second, contiguous voxels that showed no significant response to ipsilateral visual stimulation (p > 0.05) were classified as MT; these voxels were concentrated on the posterior bank of the pITS in the posterior and ventral portion of the MT+ ROI. Contiguous voxels that showed a significant response to ipsilateral visual stimulation (p < 0.05) were classified as MST; these voxels were concentrated on the anterior bank of the pITS. To locate the LOC, voxels showing a significant response to real or scrambled photographs (p < 10−6) were identified, followed by a second stage of thresholding to find only voxels with a significant (p < 0.05) preference for real versus scrambled photographs (Grill-Spector et al., 2001; Beauchamp, 2005). All active voxels directly adjacent to the localizer-defined visual regions (i.e., within 3 mm) were included in the ROI under the assumption that these were likely to represent regions of MT, MST, or LOC not mapped by the localizer because they represented peripheral parts of the visual field not accessible with our visual stimulation apparatus.
Voxels were classified as being somatosensory responsive if any combination of left and right hand or foot regressors showed a significant (p < 0.01) effect. Group average somatosensory time series were created by calculating the average time series in each individual hemisphere, reordering the time series so that right (contralateral) hand and foot responses in left hemisphere were averaged with left (contralateral) responses in right hemisphere, and ipsilateral responses in left hemisphere were averaged with ipsilateral responses in right hemisphere, averaging across hemispheres to create individual subject time series, and then collapsing into a grand mean. In each individual, the MST time series was created from only those voxels that showed a significant vibrotactile response. In many hemispheres, there were no voxels in MT with significant vibrotactile responses, so the time series was created from all voxels in the MT ROI.
Behavioral task.
To ensure that subjects remained alert and attentive throughout the experiment, we used a catch trial design adapted from magnetoencephalography experimental designs. Infrequently (10 trials per 5 min scan series), the piezoelectric bender affixed to the subject's hip (the catch stimulus) would be activated. This signaled the subject to make an eye movement to a visual target (white fixation crosshairs) placed in the upper right corner of the display screen, which was otherwise blank except for the target. BOLD data from catch trials and any false alarm trials were analyzed separately from all other trials, so that oculomotor and visual brain activations produced by eye movements would not contaminate the activations measured in hand and foot trials. The visual display, including the target, remained constant at all times. With the exception of catch trials, subjects were not required to make any overt or covert behavioral responses.
Behavioral response collection.
In all experiments, an MR-compatible eye-tracking system (Applied Science Laboratories, Bedford, MA) was used to monitor the subjects' behavioral state and record behavioral responses. A brief training session and calibration of the eye tracker was performed before the start of scanning, and recalibration was performed as needed throughout the scanning session. A window was created around the visual target with a 100 pixel margin, and a response was scored if the subject's eye position entered this response window at any time during a trial. On average, there was less than one false alarm and less than one miss in each 5 min scan series. Across subjects, the average saccadic reaction time during catch trials was 573 ± 118 (SD) ms.
Results
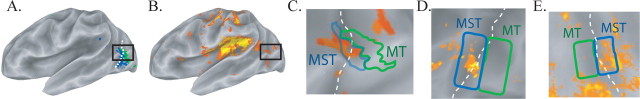
To localize area MT and MST, BOLD fMRI was used to measure brain activity as subjects viewed moving random dots presented in the left or right visual field (Huk et al., 2002). Visual motion-responsive voxels were mapped to inflated cortical surface models (Fig. 1A). Within motion-responsive cortex, MST was identified as the area in the ascending limb of the posterior inferior temporal sulcus that responded strongly to ipsilateral visual stimulation, and MT was identified as the caudally adjacent area on the cortical surface that did not respond to ipsilateral visual stimulation.
Figure 1.
BOLD responses to visual and tactile stimulation (A–C, single subject; D–E, group data). A, Lateral view of the partially inflated left hemisphere. Brain areas responsive to visual motion that showed a response to ipsilateral stimulation (MST; blue) or no response to ipsilateral stimulation (MT; green) are shown. Dashed white line shows the fundus of the ascending limb of the posterior inferior temporal sulcus. B, Brain areas in the same subject responding to vibrotactile stimulation (orange-to-yellow color scale). C, Composite map showing MST (blue outline) and MT (green outline) overlaid on tactile activation map (enlarged view of black outlined region in A and B). D, Random-effects group average map (n = 8 subjects) showing location of MST and MT relative to tactile activation in left hemisphere (anterior is left). E, Group average composite map for right hemisphere (anterior is right).
Somatosensory brain regions were identified with a rapid event-related fMRI experiment in which the palms and soles of the left and right hands and feet were stimulated with brief vibrotactile pulses (Burton et al., 2004). As shown in Figure 1B, vibrotactile stimulation evoked a vigorous response concentrated in the parietal operculum, the location of secondary somatosensory cortex and other somatosensory association areas (S2+).
Vibrotactile responses were also observed in “visual” regions of lateral occipitotemporal cortex. To determine the spatial relationship between tactile responses and the location of identified visual areas, composite activation maps were created for an individual subject (Fig. 1C) and for the group activation map (Fig. 1D,E).
Tactile responses were consistently observed in MST but not in MT. To quantify the relative areas of tactile activation in MT and MST, we counted the number of suprathreshold nodes in the group average cortical surface model. In the left hemisphere, 2% of MT nodes and 49% of MST nodes showed significant tactile activation, whereas in the right hemisphere, 7% of nodes in MT and 43% of nodes in MST were significantly active.
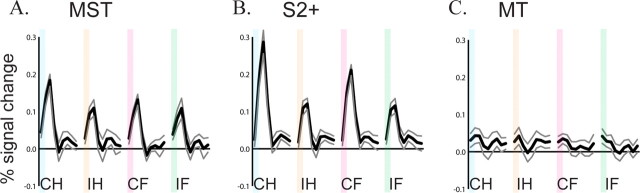
To more closely examine MST tactile responses, we calculated the average evoked response from all MST voxels that showed significant tactile responses (Fig. 2A). The temporal profile of the evoked response followed the classical BOLD hemodynamic response function, with a sharp peak 4 s after stimulus onset (2 s after stimulus offset) followed by a return to baseline within 4 s. There was a strong dependence on the location of stimulation. The largest response was to stimulation of the contralateral hand (mean of first and second time points, 0.16%) with smaller but still significant responses to stimulation of the ipsilateral hand and both feet (0.10, 0.11, and 0.09%, respectively). To measure the significance of these differences, we performed an ANOVA across subjects using two factors, body part of stimulation (hand vs foot) and side of stimulation (ipsilateral vs contralateral) with percentage MR signal change as the dependent variable. There was a significant preference for contralateral versus ipsilateral stimulation (p = 0.006) and for hand versus foot stimulation (p = 0.04) but no interaction between them (p = 0.11). For comparison with MST, we also calculated the average response in S2+ (Fig. 2B). The dynamics of the evoked hemodynamic response appeared similar to MST, with a peak 4 s after stimulus onset. As in MST, there was a significant preference for contralateral compared with ipsilateral stimulation (p = 10−10) and hand compared with foot stimulation (p = 0.02). In contrast to the strong tactile response observed in MST and S2+, the average time series from all voxels in MT showed only a weak response to tactile stimulation (Fig. 2C).
Figure 2.
Time course of average evoked BOLD response (n = 8 subjects) to vibrotactile stimulation in MST (A), S2+ (B), and MT (C). Plots show evoked response to contralateral hand (CH), ipsilateral hand (IH), contralateral foot (CF), and ipsilateral foot (IF) stimulation. Colored bars illustrate 2 s stimulus duration. Black lines show mean signal change for 16 s after stimulus onset, and gray lines show ±1 SEM.
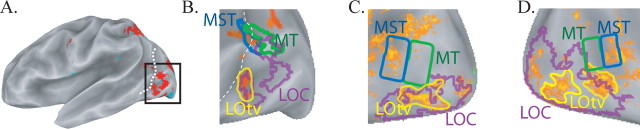
Another well studied visual region is the LOC, defined as occipitotemporal cortex that prefers real visual images to scrambled visual images (Malach et al., 1995). In a study in which subjects manipulated real objects, tactile responses were reported in a subregion of the LOC, labeled LOtv (tactile–visual) (Amedi et al., 2001). There is overlap between parts of the LOC and MT+ (Kourtzi et al., 2002). This raises the question of whether the tactile MST responses observed in our study correspond to LOtv or represent a distinct focus of tactile activity. To answer this question, brain activity was measured as subjects viewed photographs and scrambled photographs. Cortex preferring real to scrambled photographs (the LOC) covered a large portion of lateral and ventral occipitotemporal lobes (Fig. 3A). An overlap map was created to show the relationship between LOC and other regions of interest (Fig. 3B). Superior portions of the LOC overlapped with inferior portions of MT. Tactile responses were observed in posterior and anterior portions of the LOC, corresponding to the previous descriptions of LOtv (Amedi et al., 2001, 2002). In the single-subject and group maps (Fig. 3), the more anterior portion of LOtv was near the inferior border of MT. Therefore, the relatively few nodes in MT with a significant response to tactile stimulation likely share membership with LOtv. Tactile responses in MST were anterior and superior to LOtv and constituted a distinct focus of activity.
Figure 3.
Relationship between tactile responses in MST and LOC (A, B, single subject; C, D, group data). A, Brain regions, in red, showing greater response to real pictures compared with scrambled controls in a single-subject left hemisphere. Dashed white line shows the fundus of the ascending limb of the posterior inferior temporal sulcus. B, Enlarged view of posterior lateral cortex (black region outlined in A). Shown is a composite map of LOC (outlined in purple), MST (blue), and MT (green) overlaid on tactile responses (orange). Portions of LOC, labeled as LOtv and outlined in yellow, responded to tactile stimulation. C, Group composite map showing identified visual areas overlaid on tactile activation in the left hemisphere (anterior is left). D, Group composite map of right hemisphere (anterior is right).
Previous studies of tactile responses in MT+ and LO have used only hand stimulation. To search for somatotopy in lateral occipital temporal cortex, we constructed individual and group maps of the contrast of contralateral hand versus contralateral foot stimulation (Fig. 4). The parietal operculum contained regions responsive to hand and foot stimulation organized in a mirror-symmetric manner, corresponding to the expected somatotopic organization of multiple representations of the body surface (Disbrow et al., 2000; Eickhoff et al., 2007). We did not observe somatotopic organization in MST. Most MST voxels preferred hand stimulation or showed no significant preference. We quantified the lack of somatotopy in MT+ as follows. If an area is somatotopic, there should be a spatial segregation between voxels that respond to hand stimulation and voxels that respond to foot stimulation. Mathematically, this can be quantified as the ratio between the number of voxels that respond to both hand and foot stimulation and the number of voxels that respond to either hand or foot stimulation. We calculated a relatively high ratio of 0.24 for MT+, indicating weak somatotopy (Young et al., 2004).
Figure 4.
Location of hand- and foot-preferring regions (A–C, single subject; D–F, group data). A, Lateral view of right hemisphere. The color scale shows regions with a significant response to tactile stimulation and a preference for contralateral (left) foot stimulation (blue color scale), contralateral (left) hand stimulation (orange color scale), or no preference (green). The same color scale is used for A–F. B, Enlarged view of posterior lateral cortex (black region outlined in A). C, Enlarged view of operculum (white region outlined in A). A mirror-symmetric organization of foot- and hand-responsive areas was observed. D, Lateral view of group average dataset. E, Enlarged view of posterior cortex. F, Enlarged view of operculum.
Our analyses were conducted without any spatial smoothing to prevent the possibility that smoothing would blur activity from our visual areas of interest (MT, MST, and LOC), all of which are in close proximity. However, to obtain a global picture of brain areas responsive to vibrotactile stimulation, we performed a traditional SPM-style analysis in which a coarse (8 mm) Gaussian filter was applied to each individual subject's data before intersubject averaging. Then, a clustering technique was used to find the largest areas of activation on the group average cortical surface map. The results of this analysis are shown in Figure 5 and Table 1. As expected, the spatial smoothing blurred together discrete activation foci (S2+, STS, MST, and LOC) into a single large patch of activation. The second largest area of activation was observed dorsally, in primary somatosensory cortex (S1) and adjacent areas, especially in a region in the postcentral sulcus. On the medial wall of the hemisphere, tactile responses were found in the supplementary motor area (Lim et al., 1994) and posteriorly in the medial portion of S1.
Figure 5.
Group map of cortical tactile activations with spatial smoothing (8 mm full-width half-maximum Gaussian kernel). A, Lateral view of left hemisphere. B, Medial view of left hemisphere. C, Superior view of both hemispheres. D, Lateral view of right hemisphere. E, Medial view of right hemisphere. Outline shows selected regions. SMA, Supplementary motor area, located in the medial superior frontal cortex. Light green dashed line shows the fundus of the central sulcus, and dark green dashed line shows the fundus of the postcentral sulcus.
Table 1.
Clustered-node analysis of the group average surface activation map shown in Figure 5
| Area (mm2) | Mean t statistic | Maximum t statistic | Coordinates of peak activity | Location of peak activity | |
|---|---|---|---|---|---|
| Left hemisphere | |||||
| Lateral occipital–temporal–parietal | 4545 | 4.9 | 18.2 | (−54, −49, 14) | Posterior superior temporal sulcus |
| Postcentral gyrus | 1834.25 | 4.4 | 8.7 | (−15, −51, 59) | Postcentral gyrus and sulcus |
| Right hemisphere | |||||
| Lateral occipital–temporal–parietal | 2908 | 4.8 | 12.7 | (40, −69, −2) | Lateral occipital (MST) |
| Postcentral gyrus | 1588 | 4.7 | 17.5 | (21, −52, 65) | Postcentral gyrus |
Subjects were not required to make any behavioral responses to the hand or foot vibrotactile stimuli, and little activation was observed in area MT. Because MT is known to be modulated by spatial attention (Treue and Maunsell, 1996; Beauchamp et al., 1997; O'Craven et al., 1997), this raises the question of whether attending specifically to the vibrotactile stimulation would have resulted in increased activity in MT. To address this question, in a control experiment on a single subject, stimulation was delivered to different locations on the hand, and the subject made a behavioral response depending on the pattern of stimulation. As shown in Figure 6B, despite attention directed to the site of stimulation, activation was observed in MST but not in MT.
Figure 6.
A, Brain areas responsive to visual motion that showed a response to ipsilateral visual stimulation (MST; blue) or no response to ipsilateral stimulation (MT; green) in a control subject (enlarged view of posterior left hemisphere). B, Composite map showing location of area MST and MT in the same subject overlaid on tactile activation map for an experiment in which all stimuli were delivered to the contralateral (right) hand. C, Results of a separate control experiment comparing responses to somatosensory and visual stimulation. Shown is an average time series from MST in a single subject for contralateral hand and ipsilateral hand (CH and IH, respectively) and foot (CF and IF, respectively) stimulation and for an average of six types of visual stimulation consisting of moving point-light figures (VIS).
A second question concerns the relative amplitude of somatosensory and visual responses in MST. Because the visual localizer stimuli used a block design (20 s of stimulation), whereas the somatosensory experiment used a rapid event-related design (2 s of stimulation), it was not possible to directly compare the amplitudes of response. Therefore, in another control experiment on a single subject, a rapid event-related design was used to present point-light displays of biological motion, a stimulus known to evoke strong responses in MT+ (Beauchamp et al., 2003; Peelen et al., 2006). Consistent with amplitudes observed in previous studies, MST showed a 0.54% response to moving points. In the same subject, the MST response amplitude to vibrotactile stimulation of the contralateral hand was 0.26%, approximately one-half as large (Fig. 6C).
Postmortem human brain studies have identified multiple cytoarchitectonically defined areas in the neighborhood of S1 and S2 (Geyer et al., 1999; Grefkes et al., 2001; Eickhoff et al., 2006a,b, 2007). To determine which of these areas were active in our study, we created an intersubject volume average and overlaid it on the publicly available standard-space map of cytoarchitectonic areas (Fig. 7). Near the superior portion of the central sulcus, activity was observed in areas 1, 2, and 3b, areas collectively referred to as the primary somatosensory cortex or S1. In the parietal operculum, activity was observed in OP1 (possible homolog of area SII), OP2 (possible homolog of PIVC), OP3 (possible homolog of area VS) and OP4 (possible homolog of area PV). To obtain a quantitative measure of which opercular areas were most active, we calculated the fraction of voxels within each region that exceeded our significance threshold of p < 0.01. A majority of voxels in OP1 (59.9%) and OP2 (59.8%) were significantly active, with a smaller fraction of active voxels in OP3 (19.1%) and OP4 (21.7%).
Figure 7.
Relationship between cytoarchitectonically defined areas and somatosensory vibrotactile activation. A, Cytoarchitectonic regions in and near S1 from the Anatomy Toolbox (z = 54 mm) (Geyer et al., 1999; Grefkes et al., 2001; Eickhoff et al., 2005). B, Average somatosensory activation (orange-to-yellow color scale) under cytoarchitectonic boundaries (colored outlines). C, Cytoarchitectonic regions in and near S2 (z = 18 mm) (Eickhoff et al., 2006a,b). D, Average somatosensory activations under cytoarchitectonic boundaries.
Discussion
These experiments demonstrate that simple vibrotactile stimuli evoke robust BOLD fMRI responses in MST but not in MT. A potential source for vibrotactile responses in MST is a projection from the ventral intraparietal area (VIP). In nonhuman primates, VIP receives input from hand and arm regions of S2, and in turn VIP projects to MST (Lewis and Van Essen, 2000). Connections from macaque VIP to MT are much sparser than those from VIP to MST, perhaps reflecting the weaker vibrotactile responses in MT than MST observed in the present study. Although the connectivity of human MST is uncertain, studies using diffusion tensor tractography (Rushworth et al., 2006) and functional effective connectivity (Peltier et al., 2007) offer promising avenues of exploration. Additional support for VIP contributions to MST activation comes from a recent fMRI study, which proposed that the human homolog of VIP lies in the postcentral sulcus (Sereno and Huang, 2006). Consistent with this proposal, we observed a strong focus of somatosensory activity in the mediolateral regions of the postcentral sulcus (Fig. 7). However, the homology between monkey and human parietal cortex is far from settled (Grefkes and Fink, 2005), and other parietal areas must also be investigated as possible anatomical sources for tactile responses in MST.
In a meta-analysis of 57 functional imaging studies of S2, Eickhoff et al. (2006b) found somatosensory activation in four cytoarchitectonically defined regions of parietal operculum (OP1–OP4), with the highest probability of activation in OP1 (putative S2). Consistent with this analysis, we also observed activation in each cytoarchitectonic region, with the highest fraction of active voxels in OP1/S2. There are likely to be important functional differences between the cytoarchitectonic subdivisions of the parietal operculum. In particular, OP2 may be equivalent to PIVC, the parietoinsular vestibular cortex. Our study found strong vibrotactile activation in OP2/PIVC, suggesting that multisensory convergence of tactile and vestibular information may occur in this region. Vibration applied to the neck can induce changes in steering of locomotion (Bove et al., 2001) and perceived body orientation (Karnath et al., 1994), demonstrating the behavioral relevance of vestibular–tactile integration. Additional studies examining the response of OP2 to the tactile and vestibular cues produced during natural behaviors, such as head movements (Petit and Beauchamp, 2003), will be important to better understand the functional differences between the cytoarchitectonic subdivisions of the operculum.
Connections from many parietal regions to MST are considered to be “top-down” in the neuroanatomical sense, because they receive feedforward projections from MST and send feedback projections to MST, placing them higher in the visual processing hierarchy than MST (Felleman and Van Essen, 1991). Top-down has a quite different meaning in the psychological literature, in which it is used to describe perceptual phenomena that have a strong cognitive component, as opposed to bottom-up perceptual processes that are the direct result of sensory input. Although somatosensory responses in MST may arise from perceptual processing (bottom-up in the psychological sense), their potential substrates (e.g., VIP-to-MST feedback projections) is neuroanatomically top-down. We separately consider cognitive and perceptual processes that may be related to MST vibrotactile responses without labeling them as bottom-up or top-down.
The first cognitive factor that we consider is visual imagery. Previous studies reporting tactile activation in MT+ have used complex stimuli (such as a brush stroking the arm) and tasks (such as discriminating the direction of motion of a rotating sphere by touch) likely to induce imagery of visual motion. In our experiment, the piezoelectric vibrator was stationary relative to the body surface, so that there was no external cue to evoke imagery of visual motion. Imagery of the body site of stimulation is another possibility. In this view, tactile activation in MST is primarily an epiphenomenon of imagining the visual appearance of the touch or the body part being touched. However, imagery is an active process engaged by demanding tasks, making this possibility unlikely: because there was no behavioral task for hand or foot stimulation in our study, there was no reason for subjects to engage in imagery. Furthermore, it is highly unlikely that over the hundreds of trials in our rapid event-related design, the subject would reliably (and in a time-locked manner) engage in visual imagery of the stimulus or the body part that was stimulated. Finally, it is also unlikely that imagining the visual appearance of the hand evokes stronger responses in MST than imagining the visual appearance of the foot, which is what was measured. Despite this evidence against an imagery explanation, we cannot definitively rule out a contribution of visual imagery without additional studies.
The second cognitive factor that we consider is attention. MT+ is part of the “attentional network” that shifts spatial attention to exogenous cues (Corbetta et al., 1998; Beauchamp et al., 2001). Tactile stimuli might produce a shift in the focus of attention to the body location of the stimulus. However, like visual imagery, shifting attention is a resource-demanding process. As argued for imagery, without a task requiring them to shift attention, it seems likely that subjects would habituate over the course of the hundreds of trials of passive stimulation.
We next consider perceptual processes that may explain MST activation. We now turn to more likely processes. The first perceptual process that may be an explanation for tactile MST activity is spatial transformation. An fMRI study suggests that anterior regions of MT+ (likely MST) code space in a spatiotopic (body-centered) as opposed to retinotopic reference frame (d'Avossa et al., 2007). MST might be part of a brain network, including parietal areas such as VIP, that transform somatotopic touches on the body surface to spatiotopic coordinates. The second perceptual process that may explain tactile activation in MST is sensorimotor integration. Temporary inactivation of MST interferes with visually guided hand movements as well as smooth-pursuit eye movements (Ilg and Schumann, 2007), and there are anatomical connections between MST and hand motor areas (Marconi et al., 2001). Our finding that MST responds more strongly to hand than foot stimulation supports a link between MST and eye–hand coordination (Whitney et al., 2007). Integrating visual and tactile signals in MST may be important for enabling the complex dynamics necessary to track and grasp a moving object. A third perceptual process that may explain tactile responses in MST is involvement in purely somatosensory processing. Just as MST is important for perceiving the direction and speed of visual stimuli (Celebrini and Newsome, 1995), it may also be important for computing the direction and speed of tactile stimuli (Hagen et al., 2002; Blake et al., 2004). Although our vibrotactile stimuli were stationary relative to the body surface, they vibrated at a high frequency perpendicular to the skin surface. MT+ responds to visual flicker (Tootell et al., 1995), which could be considered analogous to stationary vibrotactile stimulation. Therefore, responses in MST to simple vibrotactile stimulation do not rule out the involvement of MST in tactile motion processing.
Activity in MT and MST may also be dependent on the behavioral task. Although we did not observe MT activity in response to passive vibrotactile stimulation, it is possible that other kinds of tactile stimuli and tasks, such as direction discrimination of a moving tactile stimulus, could evoke MT activity. However, a recent study demonstrated that passive presentation of a moving tactile stimulus activated only anterior regions of MT+ (likely corresponding to MST) in normal controls (Ricciardi et al., 2007). Additional studies comparing the responses of MT and MST to stationary and moving tactile stimuli with different behavioral tasks will be important to determine the functional role of tactile responses in MT+.
Footnotes
This work was supported in part by the National Science Foundation Cognitive Neuroscience Initiative Grants 0642801 (T.R.) and 0642532 (M.S.B.). National Institutes of Health Grant S10 RR19186 provided partial funding for the purchase of the 3T scanner. M.S.B. designed and conducted the experiments, analyzed the data, and wrote this manuscript. N.E.Y. built the tactile stimulation apparatus. N.K. created the cortical surface models. T.R. helped construct the eye-tracking and visual stimulus presentation systems. Eszter Zavodszky and Allison Mackey collected and analyzed the eye movement data. Ashley Kingon created the average cortical surface models. Dona Murphey provided valuable comments on this manuscript. We thank Ziad Saad and Robert Cox for their tireless development of SUMA and AFNI. Vips Patel of the University of Texas MR Imaging Center assisted with MR data collection.
References
- Amedi A, Malach R, Hendler T, Peled S, Zohary E. Visuo-haptic object-related activation in the ventral visual pathway. Nat Neurosci. 2001;4:324–330. doi: 10.1038/85201. [DOI] [PubMed] [Google Scholar]
- Amedi A, Jacobson G, Hendler T, Malach R, Zohary E. Convergence of visual and tactile shape processing in the human lateral occipital complex. Cereb Cortex. 2002;12:1202–1212. doi: 10.1093/cercor/12.11.1202. [DOI] [PubMed] [Google Scholar]
- Argall BD, Saad ZS, Beauchamp MS. Simplified intersubject averaging on the cortical surface using SUMA. Hum Brain Mapp. 2006;27:14–27. doi: 10.1002/hbm.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS. Statistical criteria in FMRI studies of multisensory integration. Neuroinformatics. 2005;3:93–114. doi: 10.1385/NI:3:2:093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Cox RW, DeYoe EA. Graded effects of spatial and featural attention on human area MT and associated motion processing areas. J Neurophysiol. 1997;77:516–520. doi: 10.1152/jn.1997.78.1.516. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Petit L, Ellmore TM, Ingeholm J, Haxby JV. A parametric fMRI study of overt and covert shifts of visuospatial attention. NeuroImage. 2001;14:310–321. doi: 10.1006/nimg.2001.0788. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. FMRI responses to video and point-light displays of moving humans and manipulable objects. J Cogn Neurosci. 2003;15:991–1001. doi: 10.1162/089892903770007380. [DOI] [PubMed] [Google Scholar]
- Blake R, Sobel KV, James TW. Neural synergy between kinetic vision and touch. Psychol Sci. 2004;15:397–402. doi: 10.1111/j.0956-7976.2004.00691.x. [DOI] [PubMed] [Google Scholar]
- Bove M, Diverio M, Pozzo T, Schieppati M. Neck muscle vibration disrupts steering of locomotion. J Appl Physiol. 2001;91:581–588. doi: 10.1152/jappl.2001.91.2.581. [DOI] [PubMed] [Google Scholar]
- Brisben AJ, Hsiao SS, Johnson KO. Detection of vibration transmitted through an object grasped in the hand. J Neurophysiol. 1999;81:1548–1558. doi: 10.1152/jn.1999.81.4.1548. [DOI] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ, McLaren DG. Cortical activity to vibrotactile stimulation: an fMRI study in blind and sighted individuals. Hum Brain Mapp. 2004;23:210–228. doi: 10.1002/hbm.20064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celebrini S, Newsome WT. Microstimulation of extrastriate area MST influences performance on a direction discrimination task. J Neurophysiol. 1995;73:437–448. doi: 10.1152/jn.1995.73.2.437. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Avossa G, Tosetti M, Crespi S, Biagi L, Burr DC, Morrone MC. Spatiotopic selectivity of BOLD responses to visual motion in human area MT. Nat Neurosci. 2007;10:249–255. doi: 10.1038/nn1824. [DOI] [PubMed] [Google Scholar]
- Disbrow E, Roberts T, Krubitzer L. Somatotopic organization of cortical fields in the lateral sulcus of Homo sapiens: evidence for SII and PV. J Comp Neurol. 2000;418:1–21. doi: 10.1002/(sici)1096-9861(20000228)418:1<1::aid-cne1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Dumoulin SO, Bittar RG, Kabani NJ, Baker CL, Jr, Le Goualher G, Bruce Pike G, Evans AC. A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cereb Cortex. 2000;10:454–463. doi: 10.1093/cercor/10.5.454. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Schleicher A, Zilles K, Amunts K. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex. 2006a;16:254–267. doi: 10.1093/cercor/bhi105. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K. The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex. 2006b;16:268–279. doi: 10.1093/cercor/bhi106. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Grefkes C, Zilles K, Fink GR. The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cereb Cortex. 2007;17:1800–1811. doi: 10.1093/cercor/bhl090. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schleicher A, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. NeuroImage. 1999;10:63–83. doi: 10.1006/nimg.1999.0440. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Schroeder CE. Is neocortex essentially multisensory? Trends Cogn Sci. 2006;10:278–285. doi: 10.1016/j.tics.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Goebel R, Khorram-Sefat D, Muckli L, Hacker H, Singer W. The constructive nature of vision: direct evidence from functional magnetic resonance imaging studies of apparent motion and motion imagery. Eur J Neurosci. 1998;10:1563–1573. doi: 10.1046/j.1460-9568.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. J Anat. 2005;207:3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K. Human somatosensory area 2: observer-independent cytoarchitectonic mapping, interindividual variability, and population map. NeuroImage. 2001;14:617–631. doi: 10.1006/nimg.2001.0858. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Res. 2001;41:1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Hagen MC, Franzen O, McGlone F, Essick G, Dancer C, Pardo JV. Tactile motion activates the human middle temporal/V5 (MT/V5) complex. Eur J Neurosci. 2002;16:957–964. doi: 10.1046/j.1460-9568.2002.02139.x. [DOI] [PubMed] [Google Scholar]
- Huk AC, Dougherty RF, Heeger DJ. Retinotopy and functional subdivision of human areas MT and MST. J Neurosci. 2002;22:7195–7205. doi: 10.1523/JNEUROSCI.22-16-07195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg UJ, Schumann S. Primate area MST-l is involved in the generation of goal-directed eye and hand movements. J Neurophysiol. 2007;97:761–771. doi: 10.1152/jn.00278.2006. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Haxby JV. Distributed neural systems for the generation of visual images. Neuron. 2000;28:979–990. doi: 10.1016/s0896-6273(00)00168-9. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Sievering D, Fetter M. The interactive contribution of neck muscle proprioception and vestibular stimulation to subjective “straight ahead” orientation in man. Exp Brain Res. 1994;101:140–146. doi: 10.1007/BF00243223. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Wurtz RH. Relation of cortical areas MT and MST to pursuit eye movements. I. Localization and visual properties of neurons. J Neurophysiol. 1988;60:580–603. doi: 10.1152/jn.1988.60.2.580. [DOI] [PubMed] [Google Scholar]
- Kourtzi Z, Bulthoff HH, Erb M, Grodd W. Object-selective responses in the human motion area MT/MST. Nat Neurosci. 2002;5:17–18. doi: 10.1038/nn780. [DOI] [PubMed] [Google Scholar]
- Leung YY, Bensmaia SJ, Hsiao SS, Johnson KO. Time-course of vibratory adaptation and recovery in cutaneous mechanoreceptive afferents. J Neurophysiol. 2005;94:3037–3045. doi: 10.1152/jn.00001.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000;428:112–137. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Lim SH, Dinner DS, Pillay PK, Luders H, Morris HH, Klem G, Wyllie E, Awad IA. Functional anatomy of the human supplementary sensorimotor area: results of extraoperative electrical stimulation. Electroencephalogr Clin Neurophysiol. 1994;91:179–193. doi: 10.1016/0013-4694(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RB. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci USA. 1995;92:8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi B, Genovesio A, Battaglia-Mayer A, Ferraina S, Squatrito S, Molinari M, Lacquaniti F, Caminiti R. Eye-hand coordination during reaching. I. Anatomical relationships between parietal and frontal cortex. Cereb Cortex. 2001;11:513–527. doi: 10.1093/cercor/11.6.513. [DOI] [PubMed] [Google Scholar]
- O'Craven KM, Kanwisher N. Mental imagery of faces and places activates corresponding stimulus-specific brain regions. J Cogn Neurosci. 2000;12:1013–1023. doi: 10.1162/08989290051137549. [DOI] [PubMed] [Google Scholar]
- O'Craven KM, Rosen BR, Kwong KK, Treisman A, Savoy RL. Voluntary attention modulates fMRI activation in human MT/MST. Neuron. 1997;18:591–598. doi: 10.1016/s0896-6273(00)80300-1. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Wiggett AJ, Downing PE. Patterns of fMRI activity dissociate overlapping functional brain areas that respond to biological motion. Neuron. 2006;49:815–822. doi: 10.1016/j.neuron.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Peltier S, Stilla R, Mariola E, LaConte S, Hu X, Sathian K. Activity and effective connectivity of parietal and occipital cortical regions during haptic shape perception. Neuropsychologia. 2007;45:476–483. doi: 10.1016/j.neuropsychologia.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Petit L, Beauchamp MS. Neural basis of visually guided head movements studied with fMRI. J Neurophysiol. 2003;89:2516–2527. doi: 10.1152/jn.00988.2002. [DOI] [PubMed] [Google Scholar]
- Ricciardi E, Vanello N, Sani L, Gentili C, Scilingo EP, Landini L, Guazzelli M, Bicchi A, Haxby JV, Pietrini P. The effect of visual experience on the development of functional architecture in hMT+ Cereb Cortex. 2007 doi: 10.1093/cercor/bhm018. in press. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Johansen-Berg H. Connection patterns distinguish 3 regions of human parietal cortex. Cereb Cortex. 2006;16:1418–1430. doi: 10.1093/cercor/bhj079. [DOI] [PubMed] [Google Scholar]
- Sathian K, Zangaladze A, Hoffman JM, Grafton ST. Feeling with the mind's eye. NeuroReport. 1997;8:3877–3881. doi: 10.1097/00001756-199712220-00008. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Huang RS. A human parietal face area contains aligned head-centered visual and tactile maps. Nat Neurosci. 2006;9:1337–1343. doi: 10.1038/nn1777. [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Hester KD, Felix ER, Hollins M, Favorov OV, Quibrera PM, Whitsel BL. Human vibrotactile frequency discriminative capacity after adaptation to 25 Hz or 200 Hz stimulation. Brain Res. 2005;1057:1–9. doi: 10.1016/j.brainres.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, Rosen BR, Belliveau JW. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci. 1995;15:3215–3230. doi: 10.1523/JNEUROSCI.15-04-03215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treue S, Maunsell JHR. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. Surface-based approaches to spatial localization and registration in primate cerebral cortex. NeuroImage 23. 2004;(Suppl 1):S97–S107. doi: 10.1016/j.neuroimage.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Whitney D, Ellison A, Rice NJ, Arnold D, Goodale M, Walsh V, Milner D. Visually guided reaching depends on motion area MT+ Cereb Cortex. 2007 doi: 10.1093/cercor/bhl172. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasar NE, Beauchamp MS. A variable-amplitude multichannel vibrotactile somatosensory stimulator for fMRI. Soc Neurosci Abstr. 2006;32:804–25. [Google Scholar]
- Young JP, Herath P, Eickhoff S, Choi J, Grefkes C, Zilles K, Roland PE. Somatotopy and attentional modulation of the human parietal and opercular regions. J Neurosci. 2004;24:5391–5399. doi: 10.1523/JNEUROSCI.4030-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]