Abstract
To characterize the organization and plasticity of the trigeminal reflex blink circuit, we interacted blink-evoking supraorbital (SO) and infraorbital (IO) nerve stimuli in alert rats. Stimulation of either trigeminal branch produced a short-lasting inhibition followed by a longer-lasting facilitation of blinks evoked by stimulating the other nerve. When IO stimulation evoked a smaller blink than SO stimulation (IO < SO), SO stimulation facilitated subsequent IO-evoked blinks more than IO stimulation facilitated SO-evoked blinks. When IO > SO, IO and SO stimulation exerted equivalent facilitation of subsequent reflex blinks. To investigate whether the blink circuit obeyed rules analogous to those governing the associative and spike timing-dependent plasticity exhibited by individual synapses, we compared the effects of 3600 simultaneous IO and SO pairings, asynchronous IO and SO pairings, or synchronous IO and SO pairings separated by 20 ms on temporal interactions between IO and SO inputs to the blink circuit. Simultaneous pairing of a weak IO and a strong SO strengthened the IO input to the blink circuit, whereas asynchronous pairing weakened the stronger input. When the pairing pattern made an afferent input arrive after blink circuit activity, it weakened that afferent input. Analogous to synaptic modifiability, the results revealed that blink-evoking stimuli acted as a “presynaptic input” and blink circuit activity acted as a “postsynaptic spike.” These mechanisms may create the maladaptive reorganization of trigeminal inputs in diseases such as hemifacial spasm.
Keywords: blink, supraorbital, trigeminal afferents, infraorbital, plasticity, trigeminal
Introduction
Although stimulating any branch of the trigeminal nerve evokes a reflex blink (Cruccu et al., 1986; Evinger et al., 1991; Ellrich et al., 1997; Esteban, 1999; Ohki and Takeuchi, 2002), the vigor of the blink depends on which branch is activated. Stimulation of the ophthalmic branch evokes the largest blink, whereas mandibular nerve stimulation generates the smallest blink (Gruart et al., 1995, 2000; Jaaskelainen, 1995a,b). This pattern indicates an activation gradient for inputs from the different branches of the trigeminal nerve to the blink circuit. Although this gradient may be set by the process of trigeminal development, two lines of evidence suggest that synaptic plasticity-like mechanisms continuously regulate the strength of inputs to second-order blink circuit neurons in the trigeminal nucleus. First, the sensory pathways of the trigeminal system exhibit significant modifiability throughout life (Nicolelis et al., 1998; Guido et al., 2001). Second, the presentation of high-frequency trigeminal stimuli to adult humans produces long-term potentiation- and long-term depression (LTD)-like effects in trigeminal reflex blinks (Mao and Evinger, 2001; Battaglia et al., 2006; Quartarone et al., 2006), and low-frequency stimulation also produces LTD-like modification of reflex blinks (Schorr and Ellrich, 2002). These data suggest that synaptic plasticity occurs in trigeminal blink circuits and may occur as early as the first trigeminal synapse.
Normal regulation of the ability of different trigeminal inputs to generate reflex blinks may use mechanisms analogous to synaptic associative plasticity. For example, simultaneous activation of a strong and a weak synapse strengthens the weak synapse (Hebb, 1949). With spike timing-dependent plasticity (STDP), a presynaptic input strengthens when it occurs immediately before the postsynaptic spike, and weakens when it occurs after the postsynaptic spike (Bi and Poo, 1998, 2001; Abbott and Nelson, 2000). If blink and synaptic associative plasticity are analogous, then simultaneous activation of a weak maxillary and a strong ophthalmic input to the blink circuit should enhance the ability of the maxillary stimulus to evoke a blink. Similarly, the ability of a trigeminal stimulus to evoke a blink should decrease if the afferent input arrives after blink circuit activity as with STDP.
If a mechanism in which trigeminal inputs form a “presynaptic” input and blink circuit activity acts as a “postsynaptic spike” continually regulates the strength of trigeminal inputs, then this process could produce the abnormal facial contractions of hemifacial spasm (HFS). HFS enables stimulation of the trigeminal ophthalmic branch to evoke contraction of the mouth muscle, orbicularis oris (Auger, 1979; Pavesi et al., 2003). In HFS, all the muscles on one side of the face simultaneously undergo involuntary contractions (Auger, 1979; Digre and Corbett, 1988), a pattern predicted to strengthen weak inputs to reflex circuits. Associative plasticity-like mechanisms strengthening the ophthalmic input to mouth reflex circuits may create this abnormal ability of ophthalmic nerve stimulation to activate mouth muscles. Thus, synaptic plasticity-like mechanisms that normally regulate blink circuits may sometimes create abnormal reflexes in disease states.
Materials and Methods
Five male Sprague Dawley rats (170–500 g) were used in these experiments. All experiments received previous approval by the State University of New York at Stony Brook Institutional Animal Care and Use Committee and were performed with strict adherence to all federal, state, and university regulations regarding the use of animals in research.
Under ketamine (90 mg/kg) and xylazine (10 mg/kg) general anesthesia, and using aseptic conditions, all rats were prepared for chronic recording of the orbicularis oculi EMG (OOemg) and stimulation of the supraorbital (SO) and infraorbital (IO) branches of the trigeminal nerve (Evinger et al., 1993). To record the OOemg, a pair of Teflon-coated wires (0.003 inch diameter bare, 0.0055 inch coated; number 791000; A-M Systems, Everett, WA) with 1 mm exposed at the tip were implanted in the orbicularis oculi (OO) muscle near the lateral and medial aspects of the eye. To stimulate the SO nerve, a nerve cuff consisting of a pair of Teflon-coated stainless-steel wires with the exposed wire (0.003 inch diameter bare, 0.0055 inch coated; A-M Systems number 791000) encased in Teflon tubing (1 mm diameter; number 163300; Small Parts, Miami, FL) were placed on the SO branch of the trigeminal nerve, just distal to the SO notch. An additional nerve cuff (2 mm diameter) was placed around the IO nerve caudal to the mystacial pad and anteroventral to the eye. Care was taken to spare extrinsic muscles overlying the IO branch. Wires were led subcutaneously to a connector embedded in a dental acrylic platform on the skull. The platform was attached to the skull by four stainless-steel screws. A silver wire connected to one of the stainless-steel screws served as ground. After surgery, all rats received buprenorphine analgesic (0.03 mg/kg) twice per day for 2 d. Rats were alert and eating within 24 h of the surgery, but at least 1 week passed before the experiments began.
We initially determined the threshold current (T) at which a 100 μs stimulus reliably evoked a blink for both the SO and IO branches of the trigeminal nerve. Using a 100 μs duration stimulus, the median threshold current was 0.26 mA for the SO stimulus and 0.33 mA for the IO stimulus for all subjects over all days tested. Over all rats and days tested, the median difference between the SO and IO threshold (IO − SO threshold) was 0.4 mA. All data were collected using stimulus intensities 2.5 times threshold (2.5T). Each experiment had three phases, prepairing, pairing, and postpairing. The prepairing and postpairing paradigms were identical. Both the IO and SO were stimulated on each trial but the timing between the stimuli varied between trials. The IO stimulus was presented from 100 ms before to 100 ms after the SO stimulus in 20 ms steps. Twenty milliseconds was the minimum interval for which there was rarely overlap between the end of the blink evoked by the first stimulus and the occurrence of the second stimulus (see Fig. 1B,D). A trial occurred every 20 ± 5 s. Postpairing data were collected 30 min after the end of the pairing paradigm. In the pairing paradigm, 3600 stimuli were presented at a 0.5 Hz rate. There were four pairing paradigms: (1) simultaneous repetitive pairing in which the IO and SO stimuli were delivered simultaneously; (2) “IO 20 SO pairing,” in which the IO always occurred 20 ms before the SO; (3) “SO 20 IO pairing,” in which the SO always occurred 20 ms before the IO; and (4) asynchronous repetitive pairing, in which the occurrence of the IO stimulus varied from 100 ms before to 100 ms after the SO in 20 ms steps. This series of 11 intervals was repeated 327 times. Not all rats were subjects in every pairing condition, but all rats were in more than one pairing paradigm.
Figure 1.
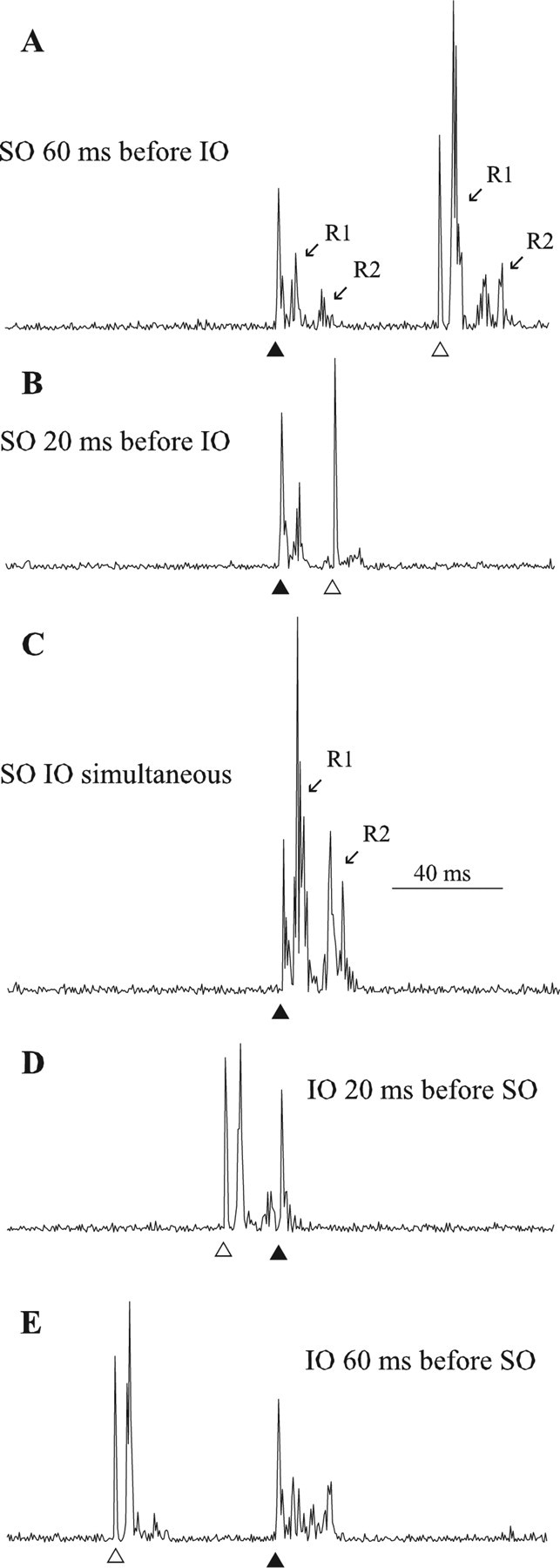
Temporal interaction of SO- and IO-evoked blinks. A, SO stimulus (▴) presented 60 ms before IO stimulus (▵). B, SO stimulus (▴) presented 20 ms before IO stimulus (▵). C, SO stimulus and IO stimulus presented simultaneously (▴). D, IO stimulus (▵) presented 20 ms before SO stimulus (▴). E, IO stimulus (▵) presented 60 ms before SO stimulus (▴). Each trace is the rectified OOemg of a single trial from the same rat.
Blinks were measured from the rectified OOemg signal using laboratory-developed software. OOemg signals were amplified (A-M Systems; model 1700; four-channel differential amplifier), filtered at 0.3–1 kHz, collected at 4 kHz per channel (DT 2831; Data Translation, Marlboro, MA; 12-bit analog-to-digital resolution), and stored for off-line analysis. The OOemg latency, duration, and amplitude were determined by marking the start and end of the R1 and R2 components of the blink and the stimulus artifact. Blink amplitude was determined by integrating the rectified OOemg activity between the beginning and end of each component of the blink (Evinger et al., 1991; Gruart et al., 1995; Pellegrini et al., 1995). Blink latency was determined as the time between the stimulus and the onset of OOemg activity for each component. Blink duration was defined as the time from the onset to the end of OOemg activity for each blink component.
To compare data among experiments and animals, normalized blink amplitude was calculated as the amplitude of the response to the second stimulus divided by the amplitude of the response to the stimulus when it was presented alone (control). The control blink amplitude in each experiment corresponded to the median blink amplitude for blinks that occurred at least 60 ms before the other. For example, control blink amplitude for IO-evoked blinks was the median of all IO-evoked blinks occurring 100, 80, and 60 ms before the SO-evoked blink. In the cases in which both SO and IO stimuli were presented simultaneously, the blink obtained was normalized to the sum of the control IO- and SO-evoked blinks. Because the 3600 pairing trials decreased the amplitude of control blinks, we used median amplitude of control blinks obtained after pairing to normalize postpairing data. Normalized values >1 denoted that the first stimulus facilitated the blink evoked by the second stimulus and values <1 indicated that the first stimulus suppressed the blink elicited by the second stimulus. Not including pairing, the data set from the five rats included >4000 individual trials.
Statistical tests of significance (p < 0.05) were performed with SPSS software (SPSS, Chicago, IL) using a one-way repeated-measures ANOVA with a least significant difference post hoc test or an independent-samples t test. Data are presented as the mean ± SEM.
Results
Stimulation of either the SO or IO nerve evoked a short latency, R1 response, sometimes followed by a longer-latency, R2 component (Fig. 1A) in the lid closing OO muscle. Like SO-evoked blinks in other nonprimate species, R1 was the dominant component of the rat blink and contributed significantly to lid closure (Pellegrini et al., 1995; LeDoux et al., 1997). In our rats, SO and IO stimuli evoked R1 responses with mean latencies of 4.2 ± 0.03 and 4.6 ± 0.07 ms, respectively. R2 responses occurred with mean latencies of 13.7 ± 0.09 and 13.2 ± 0.09 ms for the SO and IO stimuli, respectively. R2 responses occurred on 25 ± 3 and 44 ± 5% of the trials after SO and IO stimulation, respectively. Across all rats, the mean R1 duration produced by SO and IO stimuli was 7.3 ± 0.04 and 7.8 ± 0.07 ms, and mean R2 duration was 9.3 ± 0.09 and 9.5 ± 0.12 ms, respectively. Thus, activation of either the ophthalmic or maxillary branch of the trigeminal nerve evoked blinks with similar patterns of OO muscle activity. The simplest interpretation of this reflex pattern was that both the ophthalmic and maxillary trigeminal afferents innervated the same blink circuit. To characterize the relative strength of the IO and SO inputs to the blink circuit, we investigated the effects of temporal interactions between the IO and SO stimuli on the reflex blink magnitude of the R1 component.
Temporal interactions
Both SO and IO stimuli modulated subsequent blinks similarly when the IO stimulus was presented from 100 ms before to 100 ms after the SO stimulus in 20 ms steps (Figs. 1, 2). With an interstimulus interval (ISI) of 20 ms between the IO and SO, the R1 OOemg activity evoked by the second stimulus was smaller than the control R1 OOemg activity (Fig. 1B,D). For example, the SO-evoked blink was smaller when the IO stimulus occurred 20 ms before the SO stimulus (Fig. 1D, ▴) compared with SO-evoked blinks without previous IO modulation (Fig. 1A, ▴). In contrast, when the ISI between the IO and SO was 60 ms, the first stimulus facilitated the blink evoked by the second stimulus (Fig. 1A,E). For example, the IO-evoked blink was larger when the SO stimulus occurred 60 ms before the IO (Fig. 1A, ▵) than when the IO stimulus occurred first (Fig. 1E, ▵). To compare blinking between animals and experiments quantitatively, blink amplitude in each experiment was normalized to the median amplitude of the blinks that occurred at least 60 ms before the second stimulus and plotted as a function of time after the occurrence of the first stimulus (Fig. 2).
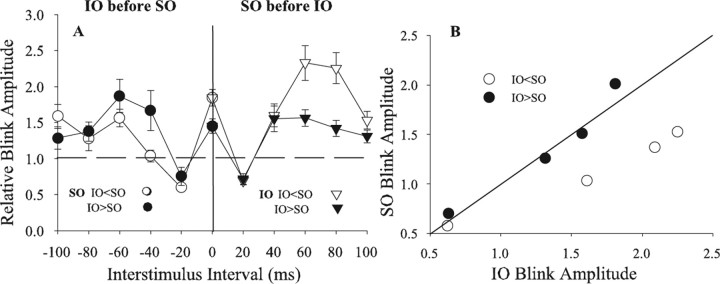
Figure 2.
Temporal interactions between SO- and IO-evoked blinks as a function of relative control blink size. A, Mean (±SEM) normalized R1 OOemg amplitudes of the SO-evoked blink as a function of the interstimulus interval by which the IO stimulus preceded the SO stimulus when the control IO-evoked blink was smaller (IO < SO; ○) or larger (IO > SO; ·) than the control SO-evoked blink (left side of graph). Mean (±SEM) normalized R1 OOemg amplitudes of the IO-evoked blink as a function of the interstimulus interval by which the SO stimulus preceded the IO stimulus when the control IO-evoked blink was smaller (IO < SO; ▿) or larger (IO > SO; ▾) than the control SO-evoked blink (right side of graph). B, Mean normalized SO-evoked blink amplitude (SO Blink Amplitude) when preceded by an IO stimulation as a function of mean normalized IO-evoked blink amplitude (IO Blink Amplitude) when preceded by an SO stimulus for interstimulus intervals from 20 to 80 ms when the control IO-evoked blink was smaller (IO < SO; ○) or larger (IO > SO; ·) than the control SO-evoked blink. The solid line is the unity line. Each data point is the average of at least 30 trials.
For all rats, an ISI of 20 ms suppressed the amplitude of the second blink, whereas ISIs of 40–80 ms facilitated the second blink relative to control blinks (Fig. 2A) (SO, F(5,554) = 12.5, p < 0.001; IO, F(5,637) = 25.4, p < 0.001). Presenting an IO stimulus 20 ms before the SO significantly suppressed the SO-evoked blink (p < 0.01), whereas IO presentation 60 ms before the SO stimulus significantly facilitated SO-evoked blinks (Fig. 2A, left side of graph) (p < 0.001). Similarly, an SO stimulus presented 20 ms before the IO stimulus significantly suppressed the IO-evoked blink (p < 0.01). At all other ISIs except 100 ms, however, the SO stimulus significantly facilitated the IO-evoked blinks (Fig. 2A, right side of graph) (p < 0.001). It was unlikely that blink facilitation resulted from summation of blink inputs to OO motoneurons because the blink evoked by the first stimulus was over before the start of the blink evoked by the second stimulus. Indeed, the only interval at which a temporally overlapping drive to OO motoneurons could occur, 20 ms, suppressed the second blink (Fig. 1B,D).
The ability of one stimulus to potentiate blinks evoked by the other stimulus depended on the relative strength of the IO and SO stimuli (Fig. 2). For example, if the control IO stimulus evoked a smaller blink than the control SO stimulus (IO < SO), then the SO stimulus produced significantly more facilitation of IO-evoked blinks (Fig. 2A, ▿, right side of graph) than the IO exerted on SO-evoked blinks (Fig. 2A, ○, left side of graph) (F(5,180) = 8.77; p < 0.02). In experiments in which the control IO-evoked blink was larger than the control SO-evoked blink (IO > SO), however, there was no significant difference between the facilitation produced by the IO and SO stimuli (Fig. 2A, ·, ▾) (p > 0.05). This result indicated that the SO input dominated the blink circuit when IO < SO. When IO > SO, however, the IO stimulus matched SO influence on the blink circuit, but the IO stimulus did not dominate the blink circuit. Consistent with this interpretation, an SO stimulus preceding the IO generated significantly more facilitation of IO-evoked blinks at the 60 ms (t(73) = 3.1; p < 0.01) and 80 ms (t(71) = 3.8; p < 0.001) ISIs when IO < SO (Fig. 2A, ▿) than in experiments in which IO > SO (Fig. 2A, ▾). Supporting the proposal that IO control of the blink circuit did not exceed SO control of the circuit, there was no significant difference between IO modulation of SO-evoked blinks, regardless of whether IO < SO (Fig. 2A, ○) or IO > SO (Fig. 2A, ·).
The simplest interpretation of these data is that the primary afferent volleys of both the IO and SO bring a number of neurons within the trigeminal blink circuit to discharge, but that both inputs are too weak to activate all of the neurons that they innervate. In these latter neurons, IO and SO stimuli only generate a long-lasting, subthreshold depolarization. Thus, control blink amplitude is a function of the number of trigeminal blink neurons driven beyond threshold by the primary afferent volley. The “effective strength” of the afferent volley, however, is the number of blink circuit neurons generating spikes plus the number of neurons with subthreshold depolarization. The second blink is facilitated because arrival of the second primary afferent volley acts on many already depolarized blink circuit neurons. This subthreshold depolarization enables inputs too weak to bring the postsynaptic neuron to spike threshold by themselves to drive some fraction of the depolarized neurons to threshold. Adding the activity of these additional neurons to the blink circuit potentiates the blink amplitude relative to control blinks. Thus, the effective strength of the first primary afferent volley determines the amount of blink facilitation elicited by the second afferent volley. Based on this interpretation, an SO stimulus produces significantly more facilitation of IO-evoked blinks than the IO stimulus generates for SO-evoked blinks when IO < SO (Fig. 2A). The blink suppression at the 20 ms ISI is consistent with the primary afferent volley activating interneurons that inhibit second-order trigeminal neurons producing feedforward inhibition.
Simultaneous stimulation of the IO and SO nerves is a unique case of the temporal interaction paradigm. The synchronized IO and SO primary afferent volleys should bring many blink circuit neurons to threshold that would only have generated subthreshold depolarization by stimulation of either the IO or SO nerve alone. Thus, the relative number of discharging and subthreshold depolarized neurons determines the blink facilitation produced by combined IO and SO stimulation. If both IO and SO stimulation evoke large blinks, then there will be relatively few subthreshold neurons for the combined IO and SO stimuli to activate. In this case, the sum of the IO and SO control blink amplitudes will only be slightly smaller than the blink amplitude evoked by combined IO and SO stimuli. In contrast, when the IO stimulus evokes a small blink relative to that evoked by the SO stimulus, then the blink evoked by the combined IO and SO stimulation will be much larger than the sum of the control blinks because the combined stimulus causes a large number of the neurons only depolarized by individual IO stimuli to discharge. Consistent with this interpretation, simultaneous IO and SO stimulation evoked a blink that was 1.9 times larger than the sum of the control IO- and SO-evoked blinks in experiments in which IO < SO (Fig. 2A, ○, 0 time point). When IO > SO, however, simultaneous IO and SO stimulation evoked a blink only 1.5 times larger than the sum of the control IO- and SO-evoked blinks, a significantly smaller facilitation than when IO < SO (Fig. 2A, ·) (t(74) = 2.66; p < 0.01).
If the SO input produced more depolarization of the blink circuit than the IO input, then the SO afferent volley should facilitate IO-evoked blinks at all temporal pairings more than IO stimulation facilitates subsequent SO-evoked blinks. To test this prediction, we plotted the SO-evoked blink amplitude at the 20–80 ms ISIs against the IO-evoked blink amplitude at the same intervals for the experiments in which IO < SO (Fig. 2B, ○). The position of these data points below the unity line indicates that an SO stimulus produced more facilitation of IO-evoked blinks than an IO stimulus generated for SO-evoked blinks. For experiments in which IO > SO, however, the data points were near the unity line, indicating that the effective strength of SO and IO stimuli on the blink circuit was equivalent although control IO-evoked blinks were larger than control SO-evoked blinks (Fig. 2B, ·).
Simultaneous repetitive pairing of IO and SO stimuli
The temporal interaction data demonstrate that both the ophthalmic and maxillary branches of the trigeminal nerve innervate the blink circuit but that the effective strength of the IO afferent volley never exceeds and is usually weaker than that of the SO. Extending an associative model of synaptic plasticity (Hebb, 1949) to the blink circuit predicts that simultaneous presentation of a strong SO and a weak IO input to the blink circuit will strengthen the IO input to the blink circuit. This increased IO input strength will reveal itself as an enhanced facilitation of subsequent SO-evoked blinks in the temporal interaction paradigm. To test this hypothesis, 3600 simultaneous IO and SO stimuli were presented at 0.5 Hz to four rats: “simultaneous repetitive pairing.”
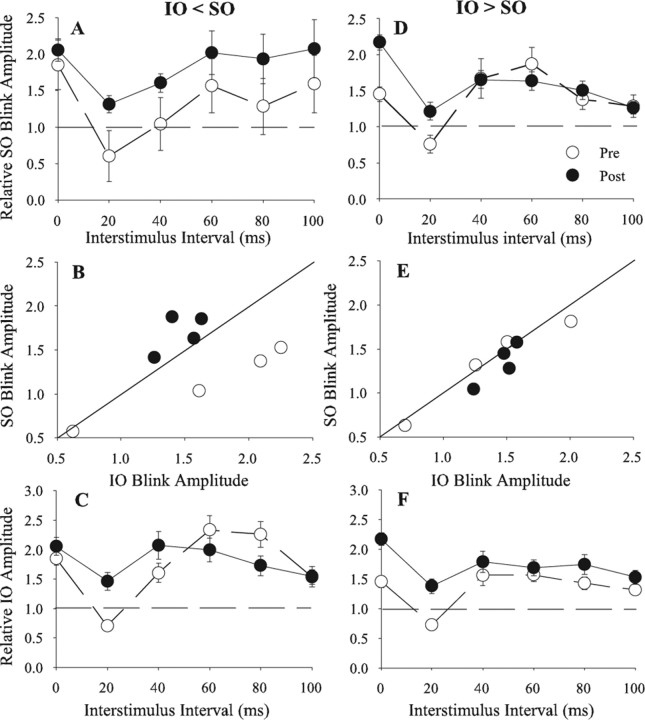
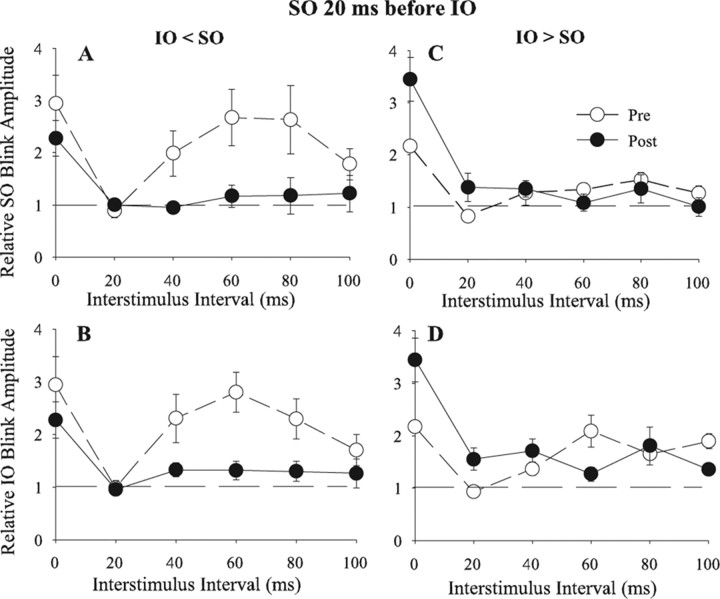
For experiments in which the initial control IO blink amplitude was smaller than control SO blink amplitude (IO < SO), simultaneous repetitive pairing significantly enhanced IO facilitation of subsequent SO-evoked blinks at all intervals (Fig. 3A) (F(9,343) = 5.409; p < 0.04). Plotting the SO-evoked blink amplitude as a function of the IO-evoked blink amplitude for each ISI before (Fig. 3B, ○) and after (Fig. 3B, ·) simultaneous repetitive pairing showed an upward shift of the data points to lie on or above the unity line. This shift indicated that simultaneous repetitive pairing increased the effective strength of the IO afferent volley until it equaled or exceeded the effective strength of SO stimulation. Simultaneous repetitive pairing of the weak IO with a strong SO input to the blink circuit, however, did not modify the SO input to the blink circuit. There was no significant change in the facilitation of IO-evoked blinks by SO stimuli for ISIs >20 ms when IO < SO (Fig. 3C) (p > 0.05). Nor was there a significant change in the size of blinks evoked by simultaneous IO and SO stimulation after simultaneous repetitive pairing (pre, 1.8 ± 0.1; post, 2.1 ± 0.2) (Fig. 3A,C) (t(63) = 1.09; p > 0.05). These results indicated that repetitively pairing the weak IO input with a strong SO input strengthened the effective strength of the IO input to the blink circuit, but did not significantly modify the effective strength of the SO input.
Figure 3.
Temporal interactions between SO- and IO-evoked blinks before and after repeated simultaneous pairing of IO and SO stimuli. A, D, Mean (±SEM) normalized R1 OOemg amplitude of the SO-evoked blink (Relative SO Blink Amplitude) as a function of the interstimulus interval between the IO and SO stimuli before (Pre; ○) and after (Post; ·) repeated simultaneous pairing of IO and SO stimuli when the control IO-evoked blink was smaller (IO < SO; A) or larger (IO > SO; D) than the control SO-evoked blink. B, E, Mean normalized SO-evoked blink amplitude (Relative SO Blink Amplitude) when preceded by an IO stimulation as a function of mean normalized IO-evoked blink amplitude (Relative IO Blink Amplitude) when preceded by an SO stimulus for interstimulus intervals from 20 to 80 ms when the control IO-evoked blink was smaller (IO < SO; B) or larger (IO > SO; E) than the control SO-evoked blink before (○) and after (·) simultaneous SO and IO pairing. The solid line is the unity line. C, F, Mean (±SEM) normalized R1 OOemg amplitude of the IO-evoked blink (Relative IO Blink Amplitude) as a function of the interstimulus interval between the IO and SO stimuli before (Pre; ○) and after (Post; ·) simultaneous IO and SO pairing when the control IO-evoked blink was smaller (IO < SO; C) or larger (IO > SO; F) than the control SO-evoked blink. Each point is the average of data from at least 30 trials.
When IO > SO, simultaneous repetitive pairing failed to modify the ability of either the IO or the SO to facilitate subsequent blinks at ISIs >20 ms (Fig. 3D,F) (p > 0.05). Before simultaneous repetitive pairing, plotting the SO-evoked against IO-evoked blink data at the 20–80 ms ISIs showed the points to lie around the unity line (Fig. 3E, ○). Simultaneous repetitive pairing did not alter this relationship (Fig. 3E, ·). This result further illustrated that simultaneous repetitive pairing failed to modify the effective strength of IO and SO inputs to the blink circuit when their strength was nearly equal. Similar to synaptic plasticity (Bienenstock et al., 1982; Abbott and Nelson, 2000; Abraham et al., 2001), it appeared easier to strengthen a weak input to the blink circuit than to augment a strong input. In contrast to the results at other intervals, there was significant potentiation of blinks evoked by simultaneous IO and SO stimulation from 1.5 ± 0.1 to 2.2 ± 0.1 (Fig. 3D,F) (t(97) = 4.95; p < 0.001). This increase probably resulted from our procedure to estimate blink facilitation at the 0 ms interval. The 3600 pairings decreased control IO- and SO-evoked blink amplitude by ∼50%, presumably resulting from the IO and SO stimuli activating a smaller number of blink circuit neurons. In this habituated condition, the increased number of depolarized neurons available for activation enabled the simultaneous IO and SO stimulation to evoke a blink much larger than the sum of the control blinks.
At the 20 ms ISI, simultaneous repetitive pairing converted the suppression to facilitation for all conditions (Fig. 3A,C,D,F). IO suppression of SO-evoked blinks was converted to facilitation when IO < SO (Fig. 3A) (t(58) = −5.54; p < 0.001) and when IO > SO (Fig. 3D) (t(86) = −2.55; p < 0.02). Likewise, SO suppression of IO-evoked blinks was converted to facilitation when IO < SO (Fig. 3C) (t(59) = −4.92; p < 0.001) and when IO > SO (Fig. 3F) (t(89) = −9.9; p < 0.001). The simplest interpretation of these data was that simultaneous repetitive pairing significantly reduced the strength of the feedforward inhibition so that only the second-order neuron depolarization from primary afferent input remained. This result may be analogous to a decrease in postsynaptic inhibition caused by repetitive coincident activation of inhibitory synapses and postsynaptic depolarization (Woodin et al., 2003).
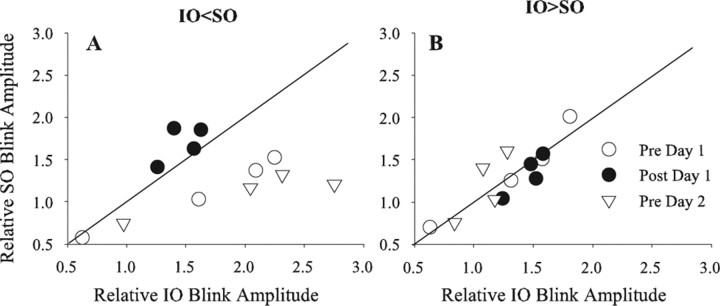
If associative plasticity mechanisms engendered by normal facial movements continuously regulate the strength of trigeminal afferent inputs to the blink circuit, then the 2 h of simultaneous repetitive pairings in our study should not produce permanent modifications because the rats experienced 22 h of normal blink patterns in that 24 h period. When IO < SO, simultaneous repetitive pairing increased the strength of IO afferent inputs to match those of SO afferent inputs [Fig. 4A, Pre Day 1 (○) to Post Day 1 (·)]. Twenty-four hours later, however, relative IO strength returned to the levels present before simultaneous repetitive pairing [Fig. 4A, Pre Day 2 (▿)]. When IO > SO, neither simultaneous repetitive pairing [Fig. 4B, Pre Day 1 (○) to Post Day 1 (·)] nor 22 h of normal blinking altered relative IO strength [Fig. 4B, Pre Day 2 (▿)].
Figure 4.
Temporal interaction of IO and SO stimuli 1 d after simultaneous IO and SO pairing. A, Mean normalized SO-evoked blink amplitude when preceded by an IO stimulation as a function of mean normalized IO-evoked blink amplitude when preceded by an SO stimulus for interstimulus intervals from 20 to 80 ms when the control IO-evoked blink was smaller than the control SO-evoked blink (IO < SO). B, Mean normalized SO-evoked blink amplitude (Relative SO Blink Amplitude) when preceded by an IO stimulation as a function of mean normalized IO-evoked blink amplitude (Relative IO Blink Amplitude) when preceded by an SO stimulus for interstimulus intervals from 20 to 80 ms when the control IO-evoked blink was larger than the control SO-evoked blink (IO > SO). Data points are before pairing on day 1 (○), immediately after simultaneous pairing on day 1 (·), and before pairing on day 2 (▿). The solid line is the unity line. Each point is the mean of data from at least 20 trials from three rats.
Repetitive pairing of IO and SO stimuli with a 20 ms ISI
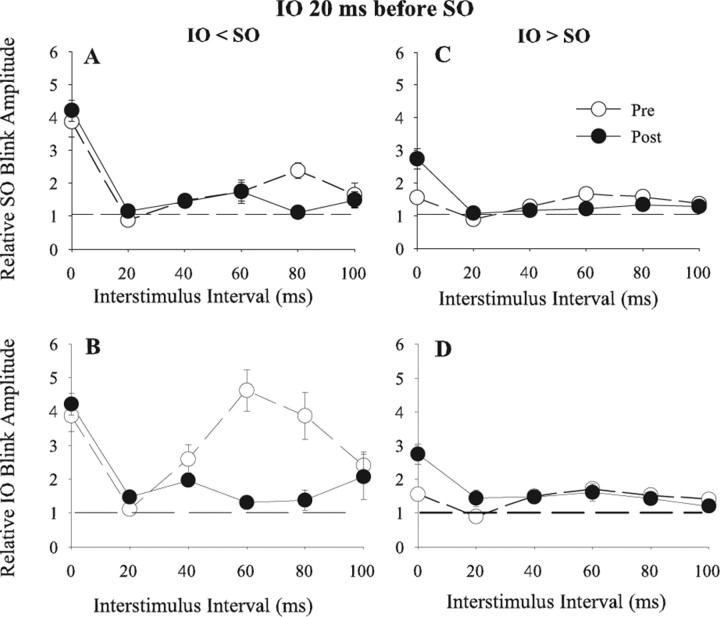
Temporal interactions between the IO and SO inputs suggest that reflex blinking exhibits STDP. Simultaneous presentation of the IO and SO stimuli potentiates blink circuit activity, whereas separating the delivery of the two stimuli by 20 ms suppresses the response of the blink circuit to the second stimulus (Figs. 1, 2). If STDP-like changes occur for IO and SO inputs to the blink circuit, then repetitive pairing of the two inputs using a 20 ms ISI should reduce the effective strength of the second stimulus because it arrives after blink circuit activity. A decrease in effective input strength will reveal itself as a reduced facilitation of subsequent blinks in the temporal interaction paradigm. To test this hypothesis, we presented 3600 trials in which the IO preceded the SO by 20 ms (two rats) (IO 20 SO pairing) or 3600 trials in which the IO followed the SO by 20 ms (two rats) (SO 20 IO pairing).
As predicted from STDP-like mechanisms, when the SO stimulus occurred 20 ms after the IO (IO 20 SO pairing), there was a significant reduction in the ability of the SO to modulate IO-evoked blinks at 60 (t(19) = 5.7; p < 0.001) and 80 (t(16) = 3.8; p < 0.001) ms ISIs when IO < SO (Fig. 5B). Reversing the pairing order so that the SO preceded the IO (SO 20 IO pairing) significantly reduced IO modulation of SO evoked blinks at 40 (t(15) = 2.7; p < 0.02), 60 (t(17) = 2.6; p < 0.02), and 80 (t(12) = 2.3; p < 0.05) ms ISIs when IO < SO (Fig. 6A). Consistent with STDP-like mechanisms, IO 20 SO pairing produced no significant change in IO modulation of SO-evoked blinks (Fig. 5A) (p > 0.05) except at the 80 ms ISI (t(17) = 6.5; p < 0.001) when IO < SO. SO 20 IO pairing, however, significantly reduced SO modulation of IO-evoked blinks when IO < SO at 40 (t(17) = 2.3; p < 0.05), 60 (t(16) = 4.1; p < 0.001), and 80 (t(17) = 2.5; p < 0.05) ms ISIs (Fig. 6B). Neither pairing paradigm modified IO or SO facilitation of subsequent blinks when IO > SO (Figs. 5C,D, 6C,D).
Figure 5.
Temporal interactions between SO- and IO-evoked blinks before and after pairing in which the IO preceded the SO by 20 ms. A, C, Mean (±SEM) normalized R1 OOemg amplitude of the SO-evoked blink (Relative SO Blink Amplitude) as a function of the interstimulus interval between the IO and SO stimuli before (Pre; ○) and after (Post; ·) repetitive IO 20 SO pairing when the control IO-evoked blink was smaller (IO < SO; A) or larger (IO > SO; C) than the control SO-evoked blink. B, D, Mean (±SEM) normalized R1 OOemg amplitude of the IO-evoked blink (Relative IO Blink Amplitude) as a function of the interstimulus interval between the IO and SO stimuli before (Pre; ○) and after (Post; ·) repetitive IO 20 SO pairing when the control IO-evoked blink was smaller (IO < SO; B) or larger (IO > SO; D) than the control SO-evoked blink. Each point is the average of data from at least 10 trials.
Figure 6.
Temporal interactions between SO- and IO-evoked blinks before and after pairing in which the SO preceded the IO by 20 ms. A, C, Mean (±SEM) normalized R1 OOemg amplitude of the SO-evoked blink (Relative SO Blink Amplitude) as a function of the interstimulus interval between the IO and SO stimuli before (Pre; ○) and after (Post; ·) repetitive SO 20 IO pairing when the control IO-evoked blink was smaller (IO < SO; A) or larger (IO > SO; C) than the control SO-evoked blink. B, D, Mean (±SEM) normalized R1 OOemg amplitude of the IO-evoked blink (Relative IO Blink Amplitude) as a function of the interstimulus interval between the IO and SO stimuli before (Pre; ○) and after (Post; ·) repetitive SO 20 IO pairing when the control IO-evoked blink was smaller (IO < SO; B) or larger (IO > SO; D) than the control SO-evoked blink. Each point is the average of data from at least 10 trials.
Although separating the IO and SO stimuli by 20 ms did not modify the ability of the first stimulus to facilitate subsequent blinks when IO > SO, both IO 20 SO and SO 20 IO pairing significantly increased the amplitude of the blink evoked by simultaneous IO and SO stimulation when IO > SO (Figs. 5C,D, 6C,D) (0 ms ISI). IO 20 SO pairing significantly facilitated blinks evoked by simultaneous stimulation of the IO and SO from 1.6 ± 0.1 to 2.7 ± 0.3 (t(67) = −3.51; p < 0.001) (Fig. 5C,D). SO 20 IO pairing significantly increased the amplitude of blinks evoked by simultaneous IO and SO stimulation from 2.2 ± 0.1 to 3.4 ± 0.4 (Fig. 6C,D) (t(17) = −3.33; p < 0.001). In experiments in which IO < SO, however, neither pairing paradigm significantly altered the amplitude of blinks evoked by simultaneous IO and SO stimulation (Figs. 5A,B, 6A,B) (p > 0.05).
Asynchronous repetitive IO and SO pairing
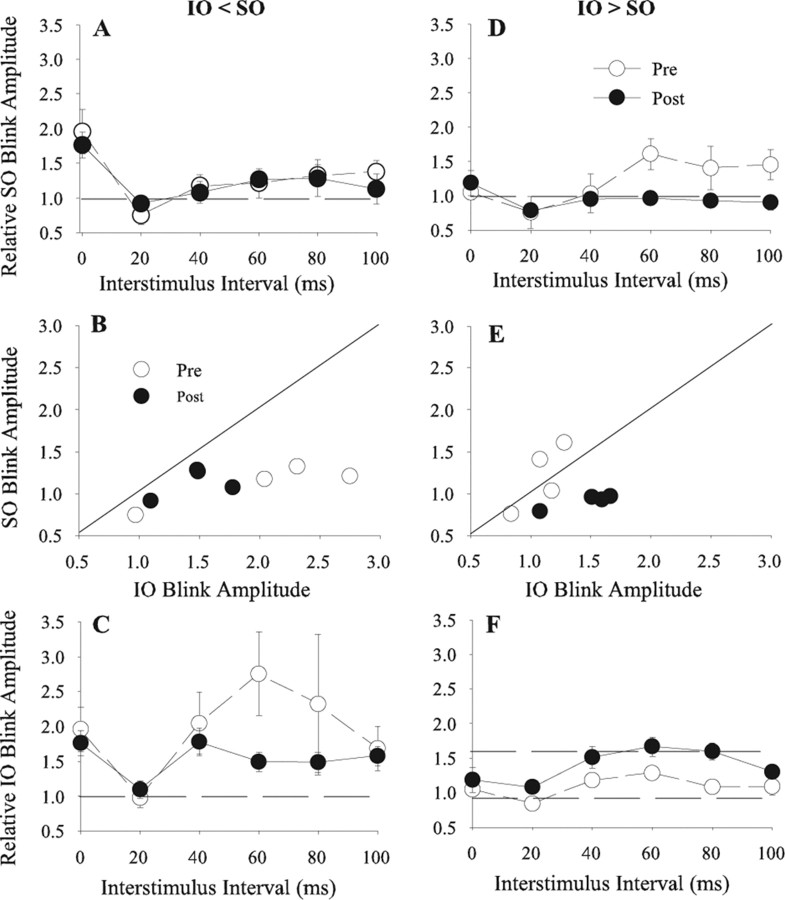
As demonstrated by the lack of significant change in the temporal interactions between IO and SO, the repetitive simultaneous and 20 ms ISI pairing paradigms did not modify IO or SO effective strength when IO > SO. Analogous to synaptic physiology (Abbott and Nelson, 2000), the high effective strength of both IO and SO inputs in the IO > SO condition probably made them more difficult to strengthen further. If STDP-like processes occur in the blink circuit, then it should be possible to weaken the effective strength of strong IO and SO inputs by making the afferent inputs occur irregularly relative to blink circuit activity. This loss of effective strength will reveal itself as a decrease in the ability of the afferent input to facilitate subsequent blinks in the temporal interaction paradigm. Because it should be difficult to reduce the effective strength of weak IO and SO inputs (Abbott and Nelson, 2000), the effective strength of the weak afferent volley should not change significantly. To test this hypothesis, three rats received 3600 presentations of the IO and SO stimuli at 0.5 Hz, but the occurrence of the IO stimulus varied from 100 ms before to 100 ms after the SO stimulus in 20 ms steps: “asynchronous repetitive pairing.”
As predicted, asynchronous repetitive pairing had little effect on the ability of the less effective afferent volley to modify the blink circuit but significantly reduced the ability of the stronger stimulus to affect the blink circuit (Fig. 7). When IO < SO, asynchronous repetitive pairing significantly reduced the ability of the SO stimulus to facilitate IO-evoked blinks at the 60 ms (t(50) = 4.13; p < 0.001) and 80 ms (t(47) = 3.78; p < 0.001) ISIs (Fig. 7C) but did not significantly change IO facilitation of SO-evoked blinks (p > 0.05) (Fig. 7A). When IO > SO, there was a significant reduction in IO facilitation of SO-evoked blinks at the 60 ms (t(49) = 3.53; p < 0.001) and 80 ms (t(45) = 3.41; p < 0.001) ISIs (Fig. 7D), and a significant increase in SO facilitation of IO-evoked blinks at the 20 ms (t(41) = −2.76; p < 0.009), 40 ms (t(46) = −2.49; p < 0.02), 60 ms (t(43) = −2.07; p < 0.05), and 80 ms (t(48) = −2.40; p < 0.02) ISIs (Fig. 7F). This postpairing pattern is similar to that of the prepairing condition when IO < SO (Fig. 2A, SO before IO). For two of the three experiments in which IO > SO before pairing, asynchronous repetitive pairing altered the relative size of the control blinks so that the control IO became smaller than the control SO. Consistent with the conversion of a before IO > SO condition to an after asynchronous repetitive pairing IO < SO, the relationship between IO and SO blink amplitude fell below the unity line (Fig. 7E, ·), as occurs with prepairing conditions when IO < SO (Figs. 2B, ○; 7B, ○). These data indicated that asynchronous repetitive pairing reduced the effective strength of the IO primary afferent volley more than that of the SO input. Unlike all other pairing paradigms, asynchronous repetitive pairing did not significantly modify the facilitation of blinks evoked by combined IO and SO stimuli regardless of initial IO strength (IO < SO, t(50) = 0.45, p > 0.05; IO > SO, t(47) = −1.23, p > 0.05). Asynchronous repetitive pairing also failed to modify the suppression at the 20 ms ISI in any condition except for SO modulation of IO blinks when initial IO > SO (Fig. 7F).
Figure 7.
Temporal interactions between SO- and IO-evoked blinks before and after asynchronous IO and SO pairing. A, D, Mean (±SEM) normalized R1 OOemg amplitude of the SO-evoked blink (Relative SO Blink Amplitude) as a function of the interstimulus interval between the IO and SO stimuli before (Pre; ○) and after (Post; ·) asynchronous IO and SO pairing when the control IO-evoked blink was smaller (IO < SO; A) or larger (IO > SO; D) than the control SO-evoked blink. B, E, Mean normalized SO-evoked blink amplitude (Relative SO Blink Amplitude) when preceded by an IO stimulation as a function of mean normalized IO-evoked blink amplitude (Relative IO Blink Amplitude) when preceded by an SO stimulus for interstimulus intervals from 20 to 80 ms when the control IO-evoked blink was smaller (IO < SO; B) or larger (IO > SO; E) than the control SO-evoked blink before (○) and after (·) repetitive asynchronous SO and IO pairing. The solid line is the unity line. C, F, Mean (±SEM) normalized R1 OOemg amplitude of the IO-evoked blink (Relative IO Blink Amplitude) as a function of the interstimulus interval between the IO and SO stimuli before (Pre; ○) and after (Post; ·) repetitive asynchronous IO and SO pairing when the control IO-evoked blink was smaller (IO < SO; C) or larger (IO > SO; F) than the control SO-evoked blink. Each point is the average of data from at least 20 trials.
Discussion
The data revealed that plasticity of the IO and SO inputs to the trigeminal blink reflex followed rules analogous to those demonstrated by individual synapses. As predicted from associative plasticity (Hebb, 1949; Paulsen and Sejnowski, 2000; Blair et al., 2001; Woodin et al., 2003), simultaneous repetitive pairing of a weak IO and a strong SO input strengthened the weaker input to the blink circuit (Fig. 3). As with STDP-like mechanisms (Bi and Poo, 1998; Abbott and Nelson, 2000; Paulsen and Sejnowski, 2000; Bi, 2002), strengthening or weakening an input depended on the time it occurred relative to activation of the trigeminal blink reflex circuit. An afferent input arriving during blink circuit activity was strengthened (Fig. 3), whereas an afferent input arriving after blink circuit activity was weakened (Figs. 5, 6). A mechanism analogous to metaplasticity postulated to prevent synapses from being stuck at one end of their range (Bienenstock et al., 1982; Abbott and Nelson, 2000; Abraham et al., 2001) was also present in the blink circuit. It was much easier to strengthen a weak input than a strong one (Figs. 3, 5, 6), and asynchronous repetitive stimulation relative to blink circuit activity weakened strong more than weak inputs (Fig. 7).
This interpretation of the pairing data depends on spinal trigeminal neurons receiving inputs from both the ophthalmic and maxillary branches of the trigeminal nerve. Although many spinal trigeminal neuron receptive fields appear to respond to stimulation of only one trigeminal branch, sensitization (Burstein et al., 1998; Bartsch and Goadsby, 2003) or blocking GABA receptors (Takeda et al., 2000) reveal that a single trigeminal neuron responds to both ophthalmic and maxillary trigeminal nerve branches. In the absence of sensitization, wide dynamic range trigeminal neurons in the blink circuit respond to cornea stimulation and mechanical stimulation of the periorbital and snout skin (Carstens et al., 1998; Hirata et al., 2000; Henriquez and Evinger, 2007). Thus, the presence of both ophthalmic and maxillary inputs to trigeminal neurons provides a potential substrate for associative plasticity and STDP-like mechanisms for reflex blinks.
Temporal interactions between the IO and SO inputs to the blink circuit (Figs. 1, 2) further characterized trigeminal blink circuit organization. Stimulation of one trigeminal branch produced a short-lasting suppression followed by a longer-lasting facilitation of blinks evoked by the other branch of the trigeminal nerve. The most straightforward interpretation of these data was that a trigeminal primary afferent volley generated a long-lasting EPSP in second-order blink circuit neurons and activated inhibitory interneurons that produced a shorter duration, feedforward IPSP on second-order spinal trigeminal neurons. Lo et al. (1999) demonstrated this pattern in principal trigeminal nucleus neurons of rats in which trigeminal nerve stimulation produced monosynaptic EPSPs lasting ∼100 ms and disynaptic IPSPs lasting ∼40 ms in second-order trigeminal neurons. Although plasticity occurring at the trigeminal afferent synapses is the simplest explanation of our data, it is possible that the interactions we describe occur at multiple trigeminal sites and the reticular formation.
The time course of blink suppression was a striking difference between pairing blink evoking stimuli from different branches of the trigeminal nerve and paired stimulation of the same branch of the trigeminal nerve. With a pair of SO stimuli, the first stimulus suppressed the blink evoked by the second stimulus for periods of 300 ms in rats (Powers et al., 1997) and >1000 ms in humans (Berardelli et al., 1985b; Eekhof et al., 1996; Powers et al., 1997). Pairing electrical cornea and SO stimulation also caused long-lasting suppression of the blink evoked by the second stimulus in humans, although this suppression was not as long-lasting as occurs with SO–SO pairing (Berardelli et al., 1985a). An air puff stimulus combining cornea and SO stimulation suppressed blinks evoked by a second air puff stimulus for 50 ms in the cat (Gruart et al., 1995). Interacting reflex blinks evoked by different modalities (e.g., SO and acoustic stimuli), however, produced a short-latency suppression followed by a longer latency facilitation (Powers et al., 1997) and anesthetizing the SO did not affect light-evoked blinks (Trigo et al., 1999). Thus, a trigeminal reflex blink appeared only to initiate long-lasting suppression of reflex blinks evoked by the same trigeminal afferents.
The available data indicate that the short-duration suppression and long-duration facilitation of the blink circuit created by interacting the IO and SO branches of the trigeminal nerve occurs in the spinal trigeminal complex, whereas the long-lasting blink suppression produced by pairing SO inputs happens in the reticular formation portion of the trigeminal reflex blink circuit. First, primary afferents generate the appropriate pattern of synaptic activity in second-order trigeminal neurons to account for the pattern of suppression and facilitation seen with IO and SO temporal interactions (Lo et al., 1999). Second, single-unit recordings of second-order corneal responsive neurons in the blink circuit show that there is no decrease in the response to a second corneal stimulus, although the blink evoked by the second cornea stimulus is significantly smaller (Henriquez and Evinger, 2007). Thus, suppression of the blink evoked by the second of two identical stimuli must not occur in the second-order trigeminal neurons, but probably occurs within the reticular part of the blink circuit (Dauvergne et al., 2001; Zerari-Mailly et al., 2001, 2003).
Individuals with HFS exhibit involuntary, unilateral contractions of all of the muscles innervated by the ipsilateral facial nerve. The most prevalent initial cause of HFS is arterial compression of the facial nerve at its root exit zone (Moller and Jannetta, 1984; Nielsen, 1984a,b; Digre and Corbett, 1988). The disease begins with OO contractions and gradually spreads to other facial muscles (Auger, 1979; Digre and Corbett, 1988). Hypotheses explaining the symptoms of HFS based on pulsatile arterial compression include ephaptic transmission after demyelination (Nielsen, 1984a), chronic orthodromic activation of facial motoneurons that increases motoneuron excitability (Moller and Jannetta, 1984; Moller, 1987), and elevated motoneuron excitability created by axotomy (Ferguson, 1978). Our study reveals a mechanism that offers a more comprehensive explanation for the symptoms of HFS than previous proposals. The arterial pulsation activating the facial nerve generates simultaneous contraction of multiple facial muscles. This muscle contraction creates a synchronized burst of afferent feedback into the trigeminal system. If the long-term simultaneous pairing of trigeminal inputs produced by this abnormal pattern uses the associative and STDP-like mechanisms that normally regulate the strength of afferent inputs to trigeminal reflex circuits, then the trigeminal afferent feedback from facial muscle cocontraction should strengthen the weaker inputs and reduce blink suppression (Fig. 3). The abnormal strengthening of weak inputs into trigeminal reflex circuits accounts for two other characteristics of HFS, synkinesis and lateral spread. Synkinesis is an inappropriate activation of a muscle not normally excited by stimulation of that branch of the trigeminal nerve (Auger, 1979). For example, SO stimulation evoking a contraction of the orbicularis oris muscle of the mouth with a latency similar to OOemg activation is common in HFS (Auger, 1979; Kim and Fukushima, 1984; Eekhof et al., 2000). This synkinetic pattern is exactly what should occur if associative and STDP-like mechanisms regulate the strength of afferent inputs onto reflex circuits. The normally weak SO input to trigeminal mouth reflexes becomes stronger and suppression is lost, enabling SO stimulation to evoke orbicularis oris contractions. Clinically, lateral spread appears as a long-latency activation of a muscle produced by cutaneous stimulation of a branch of the facial nerve not innervating that muscle (Nielsen, 1984b). Lateral spread may actually be a variant of synkinesis because cutaneous stimulation of facial nerves in human patients excites trigeminal afferents in the skin as well as the deeper motor nerves. Cutaneous stimulation exciting trigeminal afferents will activate reflex circuits. Thus, HFS may co-opt the associative plasticity and STDP-like mechanisms that normally regulate the strength of afferent inputs into trigeminal reflex circuits to produce pathological movements.
Footnotes
This work was supported by National Eye Institute Grant EY07391 (C.E.). We thank Drs. Frans vanderWerf and Alice Powers for their critical comments on previous versions of this manuscript and Donna Schmidt for technical assistance.
References
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3(Suppl):1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Mason-Parker SE, Bear MF, Webb S, Tate WP. Heterosynaptic metaplasticity in the hippocampus in vivo: a BCM-like modifiable threshold for LTP. Proc Natl Acad Sci USA. 2001;98:10924–10929. doi: 10.1073/pnas.181342098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger RG. Hemifacial spasm: clinical and electrophysiologic observations. Neurology. 1979;29:1261–1272. doi: 10.1212/wnl.29.9_part_1.1261. [DOI] [PubMed] [Google Scholar]
- Bartsch T, Goadsby PJ. Increased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura mater. Brain. 2003;126:1801–1813. doi: 10.1093/brain/awg190. [DOI] [PubMed] [Google Scholar]
- Battaglia F, Ghilardi MF, Quartarone A, Bagnato S, Girlanda P, Hallett M. Impaired long-term potentiation-like plasticity of the trigeminal blink reflex circuit in Parkinson's disease. Mov Disord. 2006;21:2230–2233. doi: 10.1002/mds.21138. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Cruccu G, Manfredi M, Rothwell JC, Day BL, Marsden CD. The corneal reflex and the R2 component of the blink reflex. Neurology. 1985a;35:797–801. doi: 10.1212/wnl.35.6.797. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Day BL, Marsden CD. Pathophysiology of blepharospasm and oromandibular dystonia. Brain. 1985b;108:593–608. doi: 10.1093/brain/108.3.593. [DOI] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Bi GQ. Spatiotemporal specificity of synaptic plasticity: cellular rules and mechanisms. Biol Cybern. 2002;87:319–332. doi: 10.1007/s00422-002-0349-7. [DOI] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- Carstens E, Kuenzler N, Handwerker HO. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J Neurophysiol. 1998;80:465–492. doi: 10.1152/jn.1998.80.2.465. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Agostino R, Berardelli A, Manfredi M. Excitability of the corneal reflex in man. Neurosci Lett. 1986;63:320–324. doi: 10.1016/0304-3940(86)90378-2. [DOI] [PubMed] [Google Scholar]
- Dauvergne C, Pinganaud G, Buisseret P, Buisseret-Delmas C, Zerari-Mailly F. Reticular premotor neurons projecting to both facial and hypoglossal nuclei receive trigeminal afferents in rats. Neurosci Lett. 2001;311:109–112. doi: 10.1016/s0304-3940(01)02150-4. [DOI] [PubMed] [Google Scholar]
- Digre K, Corbett JJ. Hemifacial spasm: differential diagnosis, mechanism, and treatment. Adv Neurol. 1988;49:151–176. [PubMed] [Google Scholar]
- Eekhof JL, Aramideh M, Bour LJ, Hilgevoord AA, Speelman HD, Ongerboer de Visser BW. Blink reflex recovery curves in blepharospasm, torticollis spasmodica, and hemifacial spasm. Muscle Nerve. 1996;19:10–15. doi: 10.1002/(SICI)1097-4598(199601)19:1<10::AID-MUS2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Eekhof JL, Aramideh M, Speelman JD, Devriese PP, Ongerboer De Visser BW. Blink reflexes and lateral spreading in patients with synkinesia after Bell's palsy and in hemifacial spasm. Eur Neurol. 2000;43:141–146. doi: 10.1159/000008153. [DOI] [PubMed] [Google Scholar]
- Ellrich J, Bromm B, Hopf HC. Pain-evoked blink reflex. Muscle Nerve. 1997;20:265–270. doi: 10.1002/(SICI)1097-4598(199703)20:3<265::AID-MUS1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Esteban A. A neurophysiological approach to brainstem reflexes. Blink reflex. Neurophysiol Clin. 1999;29:7–38. doi: 10.1016/S0987-7053(99)80039-2. [DOI] [PubMed] [Google Scholar]
- Evinger C, Manning KA, Sibony PA. Eyelid movements. Mechanisms and normal data. Invest Ophthalmol Vis Sci. 1991;32:387–400. [PubMed] [Google Scholar]
- Evinger C, Basso MA, Manning KA, Sibony PA, Pellegrini JJ, Horn AK. A role for the basal ganglia in nicotinic modulation of the blink reflex. Exp Brain Res. 1993;92:507–515. doi: 10.1007/BF00229040. [DOI] [PubMed] [Google Scholar]
- Ferguson JH. Hemifacial spasm and the facial nucleus. Ann Neurol. 1978;4:97–103. doi: 10.1002/ana.410040202. [DOI] [PubMed] [Google Scholar]
- Gruart A, Blazquez P, Delgado-Garcia JM. Kinematics of spontaneous, reflex, and conditioned eyelid movements in the alert cat. J Neurophysiol. 1995;74:226–248. doi: 10.1152/jn.1995.74.1.226. [DOI] [PubMed] [Google Scholar]
- Gruart A, Schreurs BG, del Toro ED, Delgado-Garcia JM. Kinetic and frequency-domain properties of reflex and conditioned eyelid responses in the rabbit. J Neurophysiol. 2000;83:836–852. doi: 10.1152/jn.2000.83.2.836. [DOI] [PubMed] [Google Scholar]
- Guido W, Lo FS, Erzurumlu RS. Synaptic plasticity in the trigeminal principal nucleus during the period of barrelette formation and consolidation. Brain Res Dev Brain Res. 2001;132:97–102. doi: 10.1016/s0165-3806(01)00283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. New York: Wiley; 1949. The organization of behavior: a neuropsychological theory. [Google Scholar]
- Henriquez VM, Evinger C. The three-neuron corneal reflex circuit and modulation of second-order corneal responsive neurons. Exp Brain Res. 2007;179:691–702. doi: 10.1007/s00221-006-0826-7. [DOI] [PubMed] [Google Scholar]
- Hirata H, Takeshita S, Hu JW, Bereiter DA. Cornea-responsive medullary dorsal horn neurons: modulation by local opioids and projections to thalamus and brain stem. J Neurophysiol. 2000;84:1050–1061. doi: 10.1152/jn.2000.84.2.1050. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen SK. Blink reflex with stimulation of the mental nerve. Methodology, reference values, and some clinical vignettes. Acta Neurol Scand. 1995a;91:477–482. doi: 10.1111/j.1600-0404.1995.tb00449.x. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen SK. Electrophysiological study of blink reflex in humans: differences in mental and supraorbital nerves. Acta Physiol Scand. 1995b;154:143–150. doi: 10.1111/j.1748-1716.1995.tb09896.x. [DOI] [PubMed] [Google Scholar]
- Kim P, Fukushima T. Observations on synkinesis in patients with hemifacial spasm. Effect of microvascular decompression and etiological considerations. J Neurosurg. 1984;60:821–827. doi: 10.3171/jns.1984.60.4.0821. [DOI] [PubMed] [Google Scholar]
- LeDoux MS, Lorden JF, Weir AD, Smith JM. Blink reflex to supraorbital nerve stimulation in the cat. Exp Brain Res. 1997;116:104–112. doi: 10.1007/pl00005730. [DOI] [PubMed] [Google Scholar]
- Lo FS, Guido W, Erzurumlu RS. Electrophysiological properties and synaptic responses of cells in the trigeminal principal sensory nucleus of postnatal rats. J Neurophysiol. 1999;82:2765–2775. doi: 10.1152/jn.1999.82.5.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao JB, Evinger C. Long-term potentiation of the human blink reflex. J Neurosci. 2001;21(RC151):1–4. doi: 10.1523/JNEUROSCI.21-12-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller AR. Hemifacial spasm: ephaptic transmission or hyperexcitability of the facial motor nucleus? Exp Neurol. 1987;98:110–119. doi: 10.1016/0014-4886(87)90076-8. [DOI] [PubMed] [Google Scholar]
- Moller AR, Jannetta PJ. On the origin of synkinesis in hemifacial spasm: results of intracranial recordings. J Neurosurg. 1984;61:569–576. doi: 10.3171/jns.1984.61.3.0569. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Katz D, Krupa DJ. Potential circuit mechanisms underlying concurrent thalamic and cortical plasticity. Rev Neurosci. 1998;9:213–224. doi: 10.1515/revneuro.1998.9.3.213. [DOI] [PubMed] [Google Scholar]
- Nielsen VK. Pathophysiology of hemifacial spasm: I. Ephaptic transmission and ectopic excitation. Neurology. 1984a;34:418–426. doi: 10.1212/wnl.34.4.418. [DOI] [PubMed] [Google Scholar]
- Nielsen VK. Pathophysiology of hemifacial spasm: II. Lateral spread of the supraorbital nerve reflex. Neurology. 1984b;34:427–431. doi: 10.1212/wnl.34.4.427. [DOI] [PubMed] [Google Scholar]
- Ohki M, Takeuchi N. Objective evaluation of infraorbital nerve involvement in maxillary lesions by means of the blink reflex. Arch Otolaryngol Head Neck Surg. 2002;128:952–955. doi: 10.1001/archotol.128.8.952. [DOI] [PubMed] [Google Scholar]
- Paulsen O, Sejnowski TJ. Natural patterns of activity and long-term synaptic plasticity. Curr Opin Neurobiol. 2000;10:172–179. doi: 10.1016/s0959-4388(00)00076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavesi G, Cattaneo L, Chierici E, Mancia D. Trigemino-facial inhibitory reflexes in idiopathic hemifacial spasm. Mov Disord. 2003;18:587–592. doi: 10.1002/mds.10405. [DOI] [PubMed] [Google Scholar]
- Pellegrini JJ, Horn AK, Evinger C. The trigeminally evoked blink reflex. I. Neuronal circuits. Exp Brain Res. 1995;107:166–180. doi: 10.1007/BF00230039. [DOI] [PubMed] [Google Scholar]
- Powers AS, Schicatano EJ, Basso MA, Evinger C. To blink or not to blink: inhibition and facilitation of reflex blinks. Exp Brain Res. 1997;113:283–290. doi: 10.1007/BF02450326. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Sant'Angelo A, Battaglia F, Bagnato S, Rizzo V, Morgante F, Rothwell JC, Siebner HR, Girlanda P. Enhanced long-term potentiation-like plasticity of the trigeminal blink reflex circuit in blepharospasm. J Neurosci. 2006;26:716–721. doi: 10.1523/JNEUROSCI.3948-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorr A, Ellrich J. Long-term depression of the human blink reflex. Exp Brain Res. 2002;147:549–553. doi: 10.1007/s00221-002-1248-9. [DOI] [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Matsumoto S. Change in mechanical receptive field properties induced by GABA(A) receptor activation in the trigeminal spinal nucleus caudalis neurons in rats. Exp Brain Res. 2000;134:409–416. doi: 10.1007/s002210000514. [DOI] [PubMed] [Google Scholar]
- Trigo JA, Gruart A, Delgado-Garcia JM. Role of proprioception in the control of lid position during reflex and conditioned blink responses in the alert behaving cat. Neuroscience. 1999;90:1515–1528. doi: 10.1016/s0306-4522(98)00539-9. [DOI] [PubMed] [Google Scholar]
- Woodin MA, Ganguly K, Poo MM. Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl− transporter activity. Neuron. 2003;39:807–820. doi: 10.1016/s0896-6273(03)00507-5. [DOI] [PubMed] [Google Scholar]
- Zerari-Mailly F, Pinganaud G, Dauvergne C, Buisseret P, Buisseret-Delmas C. Trigemino-reticulo-facial and trigemino-reticulo-hypoglossal pathways in the rat. J Comp Neurol. 2001;429:80–93. doi: 10.1002/1096-9861(20000101)429:1<80::aid-cne7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Zerari-Mailly F, Dauvergne C, Buisseret P, Buisseret-Delmas C. Localization of trigeminal, spinal, and reticular neurons involved in the rat blink reflex. J Comp Neurol. 2003;467:173–184. doi: 10.1002/cne.10917. [DOI] [PubMed] [Google Scholar]