Abstract
Animals and humans maintain a definite body orientation in space during locomotion. Here we analyze the system for the control of body orientation in the lamprey (a lower vertebrate). In the swimming lamprey, commands for changing the body orientation are based on vestibular information; they are transmitted to the spinal cord by reticulospinal (RS) neurons. The aim of this study was to characterize the sensory-motor transformation performed by individual RS neurons. The brainstem–spinal cord preparation with vestibular organs was used. For each RS neuron, we recorded (1) its vestibular responses to turns in different planes and (2) responses in different motoneuron pools of the spinal cord to stimulation of the same RS neuron; the latter data allowed us to estimate the direction of torque (caused by the RS neuron) that will rotate the animal's body during swimming. For each of the three main planes (roll, pitch, and yaw), two groups of RS neurons were found; they were activated by rotation in opposite directions and caused the torques counteracting the rotation that activated the neuron. In each plane, the system will stabilize the orientation at which the two groups are equally active; any deviation from this orientation will evoke a corrective motor response. Thus, individual RS neurons transform sensory information about the body orientation into the motor commands that cause corrections of orientation. The closed-loop mechanisms formed by individual neurons of a group operate in parallel to generate the resulting motor responses.
Keywords: postural control, steering, locomotion, brainstem, reticulospinal neurons, motoneurons
Introduction
During locomotion, animals and humans maintain a definite body orientation in space. In higher vertebrates, studying the neuronal mechanisms for spatial orientation during locomotion is difficult because of the extreme complexity of the corresponding networks. Here we address these problems using a simpler animal “model,” the lamprey (a lower vertebrate, Cyclostome). The general structure of the CNS in this aquatic animal and the functional organization of its locomotor system are similar to those in higher vertebrates (Nieuwenhuys and ten Donkelaar, 1996). In the lamprey, however, the mechanisms for the control of spatial orientation can be investigated at the network and cellular levels (Orlovsky et al., 1999; Deliagina and Orlovsky, 2002).
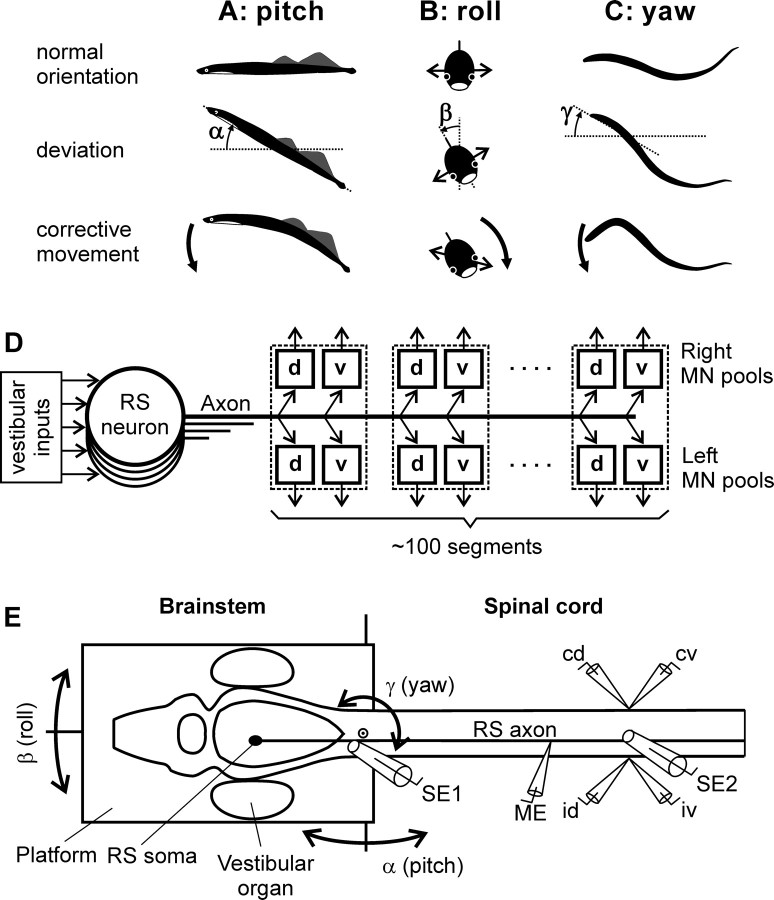
The lamprey swims because of the lateral body undulations that propagate in the rostrocaudal direction (Grillner and Kashin, 1976). During stationary swimming, orientation of the lamprey in the sagittal (pitch) and transversal (roll) planes (see Fig. 1 A,B) is stabilized by closed-loop control mechanisms driven by vestibular input (Deliagina et al., 1992a,b; Deliagina and Fagerstedt, 2000; Pavlova and Deliagina, 2002). Vestibular-driven mechanisms also contribute to stabilization of the direction of swimming in the horizontal (yaw) plane (see Fig. 1 C) (Karayannidou et al., 2007). Any deviations from the stabilized body orientation are reflected in vestibular signals, which cause corrective motor responses. In the pitch and yaw planes, the corrections occur because of the body bending in the corresponding plane (see Fig. 1 A,C) (Ullén et al., 1995; McClellan and Hagevik, 1997). In the roll plane, the corrections occur because of a change in the direction of locomotor body undulations, from lateral to oblique (see Fig. 1 B) (Zelenin et al., 2003a). These motor responses are caused by four motoneuron (MN) pools in each segment that innervate the dorsal and ventral parts of a myotome on the two sides, respectively (see Fig. 1 D) (Tretjakoff, 1927; Wannier et al., 1998).
Figure 1.
A–C, During regular swimming, the lamprey stabilizes its orientation in the sagittal (pitch) plane (A), transversal (roll) plane (B), and horizontal (yaw) plane (C). Deviations from the stabilized orientation in these planes (angles α, β, and γ, respectively) evoke corrective motor responses (large arrows) aimed at restoration of the initial orientation. D, Commands for correcting the orientation are formed on the basis of vestibular information and transmitted from the brainstem to the spinal cord by RS neurons; many RS axons reach the most caudal spinal segments. Motor output of each segment is generated by four MN pools controlling the dorsal and ventral parts of a myotome on the two sides (d and v pools). E, The brainstem–spinal cord preparation with vestibular organs was used for studying vestibular inputs to individual RS neurons and their motor effects. The preparation was positioned in a chamber and perfused with Ringer's solution. The brainstem with vestibular organs could be rotated around three axes: transverse (pitch), longitudinal (roll), and vertical (yaw). d-Glutamate was applied to the spinal cord to elicit fictive locomotion. Individual neurons were recorded from their axons in the spinal cord. To stimulate a neuron, positive current pulses were passed through the recording intracellular microelectrode (ME). Activity of MNs was recorded bilaterally in segment 30 by suction electrodes, from the dorsal and ventral branches of a ventral root (id, ipsilateral dorsal branch; iv, ipsilateral ventral; cd, contralateral dorsal; cv, contralateral ventral). Electrodes placed on the spinal cord surface (SE1 in segment 1 and SE2 in segment 30) recorded antidromic and orhtodromic RS spikes, respectively.
In the lamprey, commands for changing the body orientation are transmitted from the brainstem to the spinal cord mainly by reticulospinal (RS) neurons with uncrossed axons (see Fig. 1 D) (Brodin et al. 1988; Bussières, 1994; Deliagina et al., 2002). These command neurons, because of their vestibular inputs (see Fig. 1 D), respond to rotation of the animal in different planes. In each of the main planes (pitch, roll, yaw), there are two groups of RS neurons responding to rotation in opposite directions (Deliagina et al., 1992a; Deliagina and Fagerstedt, 2000; Pavlova and Deliagina, 2002; Karayannidou et al., 2007). A hypothesis was advanced that each of these two groups causes rotation of the animal in the direction opposite to the initial turn (which activated the neurons) and the system will stabilize the orientation with equal activities of the two antagonistic groups (Deliagina et al., 1993, 2006). In a separate set of experiments, motor effects of individual RS neurons were investigated. The effects were similar along the entire axon, and they could be characterized by a combination of influences on the four MN pools in any segment. Approximately 20 combinations were found (Zelenin et al., 2001, 2003b).
The aim of the present study was, by combining these two approaches, to characterize both vestibular inputs and motor effects of individual command neurons. The results of this study confirmed the hypothesis advanced by Deliagina et al. (1993, 2006).
Materials and Methods
Experiments were performed on the in vitro preparation dissected from adult lampreys (Ichthyomyzon unicuspis). All experiments were approved by the local ethical committee (Norra Djurförsöksetiska Nämnden). The experimental design is shown schematically in Figure 1 E. The brainstem, vestibular organs, and the rostral part of the spinal cord (∼40 segments) were isolated together with the cranium and notochord. Segments 11–40 of the notochord were firmly pinned to the bottom of the experimental chamber. The cranium was pinned to a small plate that could be rotated around three axes: longitudinal, transverse, and vertical ones. The dissection and experiments were performed in cold (8–10°C) oxygenated Ringer's solution for lampreys (Grillner et al., 1981).
Individual RS axons were recorded intracellularly in the left and right sides of the spinal cord at the level of segments 20–25. Vestibular responses of RS neurons were examined with trapezoidal turns (90° peak to peak) in the pitch (α), roll (β), and yaw (γ) plane (Fig. 1 E). A change in position lasted for ∼1 s, and each position was maintained for ∼2 s. Rotation of the brainstem did not cause instability of recordings.
Motor effects (functional spinal projections) of RS neurons were determined by using a technique of synchronous accumulation of responses of spinal MNs to spikes in individual RS neurons. Single spikes in a neuron were evoked by positive current pulses (pulse period, 100 ms; pulse duration, 8–20 ms; current up to 20 nA) passed through the intracellular microelectrode (Fig. 1 E, ME). Arrival of an RS spike to segment 30 was monitored by a surface electrode (Fig. 1 E, SE2). Another electrode (Fig. 1 E, SE1) was positioned in segment 1 and detected the antidromic spike to make sure that the recorded axon projected from the brainstem. The activity of spinal MNs was recorded bilaterally in segment 30 by four suction electrodes, from the dorsal and ventral branches of the ventral roots. In the brainstem–spinal cord preparation, the spinal networks usually have a low level of activity at rest. To activate the networks, we elicited the neural correlate of locomotion (fictive swimming) by application of d-glutamate (0.5–1 mm) to the spinal cord (Grillner et al., 1981, 1995). After testing of the motor effects, the Ringer's solution with d-glutamate was replaced with the solution without d-glutamate.
For each individual RS neuron, a post-RS spike histogram was generated for the spikes of MNs recorded in the dorsal and ventral branches of the left and right ventral roots (see Figs. 2 B, 3 B, 4 B, 7 B). The moment of RS spike occurrence at the stimulated site of the axon was taken as the origin of the time axis in the histogram. Typically, responses to a few thousand RS spikes (up to 20 min of stimulation at 10 Hz, i.e., up to 12,000 RS spikes) were used for generation of a histogram [for details of the technique, see Fetz and Cheney 1980 and Zelenin et al. 2001]. A high-frequency “noise” in the histograms was reduced by “filtering” (i.e., weighted averaging across seven neighboring bins centered around the current one) (for details, see Zelenin et al., 2003b). The histogram provided information about the effects of RS spikes on the motor output averaged across the locomotor cycle. In the previous study, it was shown that spinal MNs respond to single RS spikes in that half of the swim cycle when they are activated by the spinal locomotor central pattern generator and do not respond when they are inhibited (Zelenin et al., 2001).
Figure 2.
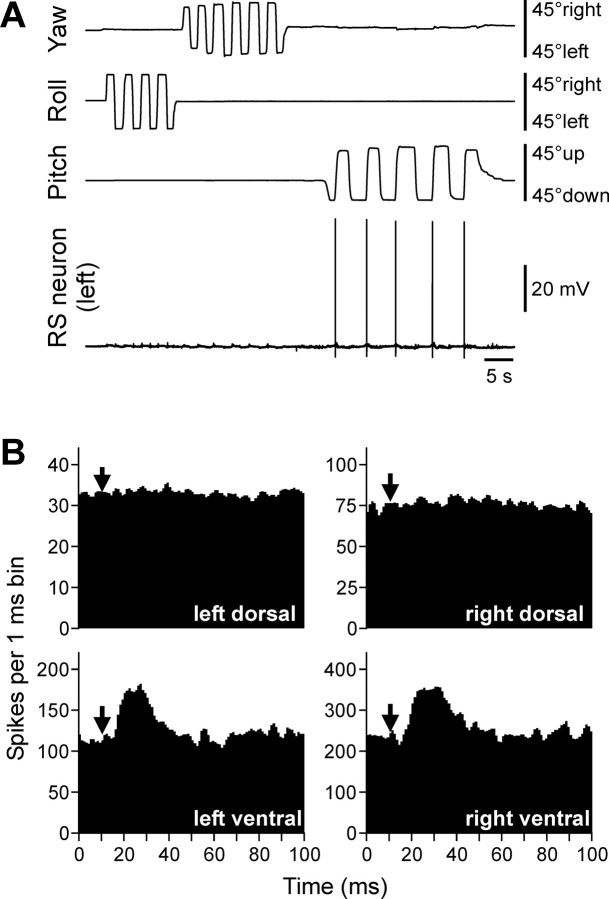
An RS neuron that contributed only to stabilization of the pitch angle. A, The neuron responded to nose-up pitch tilts only. B, The RS spike-triggered averaging has shown that the neuron evoked excitation in both ventral branches of ventral roots and did not influence both dorsal branches. The arrows in the histograms indicate the time of arrival of the RS spike to segment 30 (where motor output was monitored).
Figure 3.
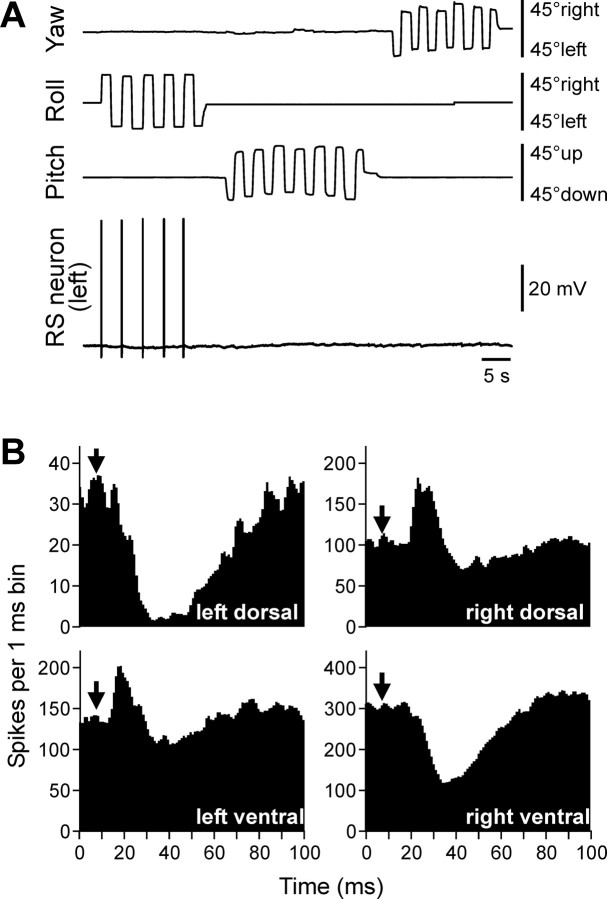
An RS neuron that contributed only to stabilization of the roll angle. A, The neuron responded to right (contralateral) roll tilts only. B, The neuron evoked excitation in the left (ipsilateral) ventral and right (contralateral) dorsal branches of the ventral roots and inhibition in the right ventral and left dorsal branches.
Figure 4.
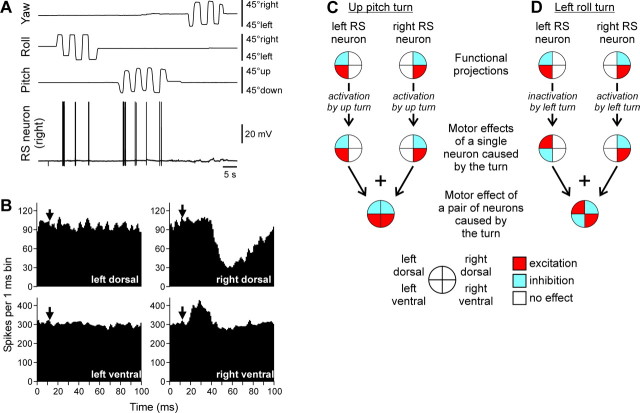
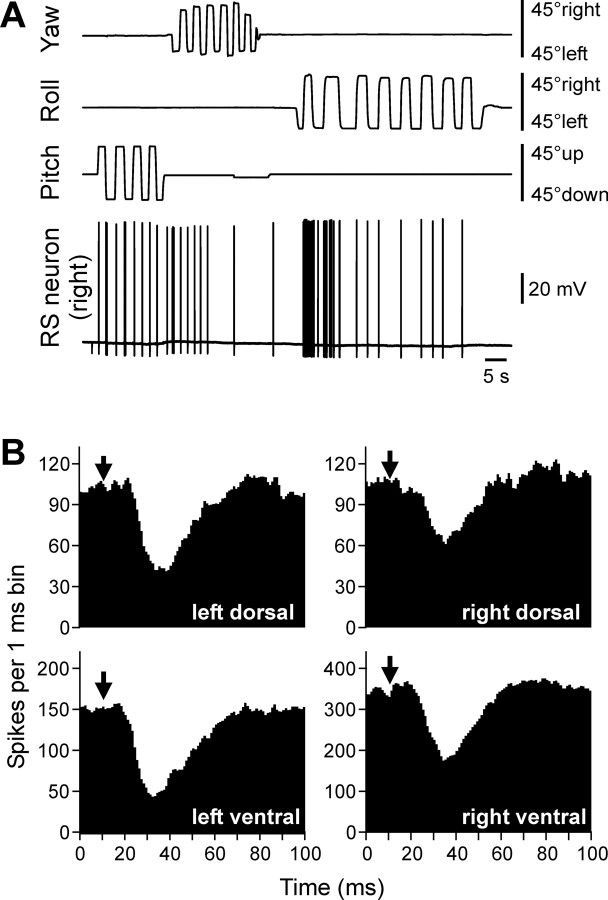
An RS neuron that contributed to stabilization of the roll and pitch angles. A, The neuron responded to the left (contralateral) roll tilts and nose-up pitch tilts. B, The neuron excited the ventral branch and inhibited the dorsal branch of the right (ipsilateral) ventral root. C, D, Motor effects of two symmetrical neurons of this type caused by rotation in the pitch plane (C) or roll plane (D). The patterns of motor effects are shown as circle diagrams, with the quadrants representing the MN pools projecting to the corresponding parts of myotomes. Different colors designate the type of effect (red, excitation; blue, inhibition; white, no effect). See Results for explanations.
Figure 7.
An RS neuron with non-specific vestibular inputs and motor effects. A, The neuron fired spikes in response to tilts in any direction. B, The neuron inhibited all MNs.
Results
In this study, 121 RS neurons were recorded from 31 animals, and their responses to vestibular input (rotation in the roll, pitch, and yaw planes), as well as their influences on the spinal motor output (on the activity of four MN pools in segment 30), were analyzed. Axonal conduction velocities ranged from 1.75 to 4.84 m/s (3.21 ± 0.72 m/s; mean ± SD). This high velocity as well as the long extent of the recorded fibers strongly suggest that they were the larger RS axons (Deliagina and Fagerstedt, 2000).
Specificity of both vestibular responses and functional projections was observed in 84 RS neurons. These neurons responded to rotation in one plane, in two planes, or even in all three planes. In a given plane, they could be activated by rotation in only one direction. The neurons exhibited no tonic activity, thus no inhibitory effects of vestibular stimulation could be observed. These RS neurons also had specific functional projections (i.e., their activity resulted in a combination of excitation and inhibition in the four MN pools that would cause rotation of the body during swimming). The characteristics of vestibular inputs and motor effects of these 84 neurons, as well as correlations between inputs and motor effects, will be considered below.
Three representative examples of vestibular responses and motor effects in individual RS neurons are shown in Figures 2 –4. Responses to tilts consisted of a single spike (Fig. 2 A) or a burst of spikes (see Fig. 4 A) generated during and shortly after rotation. Figure 2 A presents the left RS neuron that was activated by nose-up pitch tilts but did not respond to nose-down tilts; rotation in the roll and yaw planes was not effective. This neuron exerted excitatory influences on the MNs projecting to the ventral branches of the right and left ventral roots that innervate ventral muscles and did not influence the activity of MNs projecting to the dorsal branches (Fig. 2 B). During swimming, this pattern of influences would produce ventral bending of the body (Fig. 1 A, bottom) and would cause the nose-down rotation of the animal (Fig. 1 A, bottom, arrow). Thus, the motor effect of this RS neuron counteracts the turn that caused activation of the neuron.
A different RS neuron is shown in Figure 3. This left RS neuron responded to right (contralateral) roll tilts but did not respond to left tilts or to rotation in the pitch and yaw planes (Fig. 3 A). This neuron exerted excitatory influences on the MNs projecting to the ipsilateral ventral and contralateral dorsal branches and inhibitory influences on the ipsilateral dorsal and contralateral ventral MNs (Fig. 3 B). During swimming, this RS neuron would cause a change in the direction of locomotor body undulations, from lateral to oblique (along the ipsilateral ventral–contralateral dorsal diagonal axis) (Fig. 1 B, bottom). Such a change in the plane of undulations would cause a torque in the roll plane (Fig. 1 B, bottom, arrow) rotating the body, with its ipsilateral side moving down (Zelenin et al., 2003a). Thus, the motor effect of this RS neuron counteracts the turn that caused activation of the neuron.
The RS neurons shown in Figures 2 and 3 responded to rotation in only one of the three main planes. In contrast, the right RS neuron shown in Figure 4 A responded both to left (contralateral) roll tilts and to nose-up pitch tilts but did not respond to turns in other directions or to rotation in the yaw plane. This RS neuron excited the ipsilateral ventral MNs and inhibited the ipsilateral dorsal MNs (Fig. 4 B). To understand the role of this RS neuron, one should take into account that the RS system in the lamprey is symmetrical (Rovainen, 1967; Nieuwenhuys, 1972), suggesting that each RS neuron on one side has a counterpart (mirror image) on the other side, and they should be considered together. For the neurons responding to rotation in more than one plane, the resultant motor effect will depend on the type of applied vestibular stimulus. If the body rotates nose up, both right and left neurons of the type shown in Figure 4 (each affecting only ipsilateral MNs) will be activated (Deliagina et al., 1992a; Pavlova and Deliagina, 2002), and their collective effect will be bilateral excitation of the ventral MNs and bilateral inhibition of the dorsal MNs (Fig. 4 C). As a result, the nose-down pitch torque will be produced, counteracting the initial nose-up rotation. In contrast, tilting the body in the roll plane to the left will excite the right RS neuron (Deliagina et al., 1992a; Deliagina and Fagerstedt, 2000). That will lead to an increase in activity of the right ventral MNs and decrease in activity of the right dorsal MNs (Fig. 4 D). The homologous left RS neuron will receive an inhibitory input during the left roll turn [as demonstrated for roll-related RS neurons (Deliagina and Pavlova, 2002)]. If this neuron is tonically active [like many other RS neurons during locomotion (Zelenin, 2005)], its activity will be reduced. That will lead to an increase in activity of the left dorsal MNs and a decrease in activity of the left ventral MNs (Fig. 4 D). Together, these right and left RS neurons will produce excitation of the right ventral and left dorsal MNs and inhibition of the right dorsal and left ventral MNs (Fig. 4 D). This will lead to the left roll torque counteracting the initial left turn (Zelenin et al., 2003a). The same analysis can be done for the right roll turn. Thus, in response to a roll turn, two symmetrical RS neurons of this type would cause a change in the direction of locomotor body undulations, from lateral to oblique (along the ipsilateral dorsal–contralateral ventral diagonal axis). Such a change in the plane of undulations would cause a torque in the roll plane counteracting the initial roll turn.
A close correlation between the input and output characteristics was found in all 84 RS neurons exhibiting both specific vestibular responses and specific functional projections. These results are summarized in Figure 5. The RS neurons are divided into nine groups according to their vestibular inputs. The circle diagrams show the patterns of functional projections of individual RS neurons [i.e., the effects of the neurons on the activity of the four MN pools (Fig. 1 D) supplying dorsal and ventral parts of myotomes on the two sides, ipsilateral and contralateral to the axon position].
Figure 5.
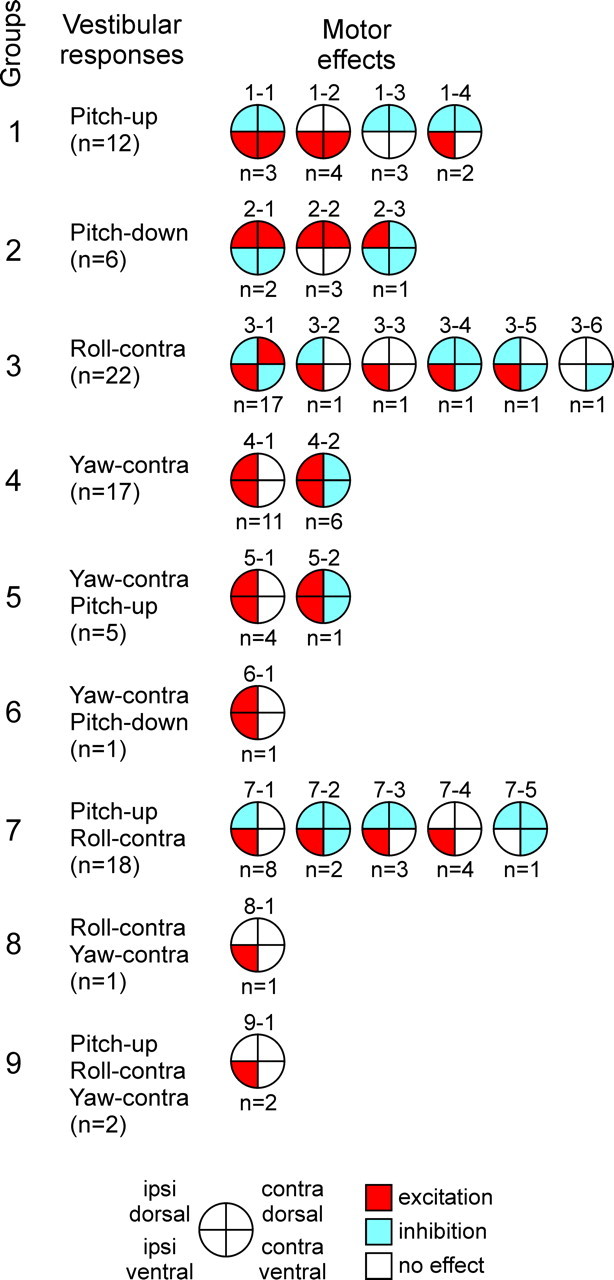
Relationships between vestibular responses and motor effects in individual RS neurons (n = 84). The neurons were grouped according to their inputs (vestibular responses). Groups 1–4 responded to turns in only one plane, groups 5–8 responded to turns in two planes, and group 9 responded to turns in all three planes. For each group, the patterns of motor effects produced by its neurons are shown as circle diagrams, with the quadrants representing the MN pools projecting to the corresponding parts of myotomes. Different colors designate the type of effect (red, excitation; blue, inhibition; white, no effect). Numbers below the diagrams show the number of neurons with a given pattern. contra, Contralateral; ipsi, ipsilateral.
Group 1 neurons were activated by the upward pitch tilt (a neuron with pattern 1-2 is shown in Fig. 2). Group 2 neurons were activated by the downward pitch tilt. Group 3 neurons were activated by the contralateral roll turn (a neuron with pattern 3-1 is shown in Fig. 3). Group 4 neurons were activated by the contralateral yaw turn. Group 5 and 6 neurons were activated (1) by the contralateral yaw turn and (2) by the upward pitch tilt (group 5) or by the downward pitch tilt (group 6). Group 7 neurons were activated by the upward pitch tilt and by the contralateral roll tilt (a neuron with pattern 7-1 is shown in Fig. 4). Group 8 and 9 neurons were activated by the contralateral roll tilt and by the contralateral yaw turn; in addition, group 9 neurons were activated by the upward pitch tilt.
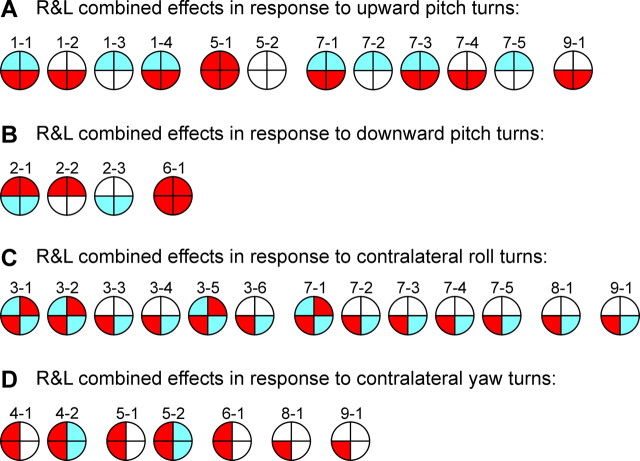
Most of the patterns shown in Figure 5 were observed in the axons recorded on the right and on the left sides (except for the patterns observed just once). We will consider motor effects of these “functional pairs” caused by turns in different planes (Fig. 6).
Figure 6.
The combined effects of right (R) and left (L) RS neurons producing the same pattern in response to upward pitch turns (A), downward pitch turns (B), roll turns (C), and yaw turns (D). Designations are as in Figure 5. See Results for explanations.
Turn upward in the pitch plane activates both right and left RS neurons of groups 1, 5, 7, and 9. The combined effect of the neurons of group 1 is excitation of ventral MNs (Fig. 6 A, pattern 1-2), inhibition of dorsal MNs (Fig. 6 A, pattern 1-3), or simultaneous excitation of ventral MNs and inhibition of dorsal MNs (Fig. 6 A, patterns 1-1 and 1-4). Note that because patterns 1-1, 1-2, and 1-3 are symmetrical in relation to the sagittal plane, the right and left RS neurons with these patterns produce the same effects. Pattern 1-4 is not symmetrical (Fig. 5), and the right neuron with this pattern excites the right ventral MNs and inhibits all dorsal MNs, whereas the left neuron with this pattern excites the left ventral MNs and inhibits all dorsal MNs. The combined effect of right and left pattern 1-4 neurons is excitation of all ventral MNs and inhibition of all dorsal MNs.
The combined effect of the right and left pattern 5-1 neurons is excitation of all MNs (Fig. 6 A, pattern 5-1). The effects of the right and left pattern 5-2 neurons (causing ipsilateral excitation and contralateral inhibition) (Fig. 5) effectively cancel each other (Fig. 6 A, pattern 5-2).
Similarly, it is easy to demonstrate that the combined effects of the right and left neurons from groups 7 and 9 in response to the upward turn are excitation of ventral MNs (Fig. 6 A, patterns 7-4 and 9-1), inhibition of dorsal MNs (Fig. 6 A, patterns 7-2 and 7-5), or simultaneous excitation of ventral MNs and inhibition of dorsal MNs (Fig. 6 A, patterns 7-1 and 7-3). Note that the combined effect of the right and left neurons with pattern 7-5 (inhibition of all MNs, except for the ipsilateral ventral ones) (Fig. 5) is inhibition of all MNs, both dorsal and ventral. However, inhibition of dorsal MNs comes from both right and left RS neurons, whereas ventral MNs are inhibited by only the right or only the left RS neuron, so that dorsal inhibition is stronger. To emphasize this dorsoventral asymmetry, we show no inhibition of ventral MNs in the pattern of combined right and left effects (Fig. 6 A, pattern 7-5).
In summary, a regular effect of groups 1, 5, 7, and 9 (observed in 32 of 37 neurons) is the pitch-down torque (Table 1). Only the neurons of group 5 produce no torque. The neurons with pattern 5-1 provide excitation to all MNs.
Table 1.
Correlations between sensory inputs to RS neurons and their motor effects
| Rotation | Pitch-up | |||
|---|---|---|---|---|
| Groups activated | 1 | 5 | 7 | 9 |
| Regular effect (32 of 37) | Pitch-down | No | Pitch-down | Pitch-down |
| Additional effects (0 of 37) | No | No | No | No |
A similar analysis was done for the downward pitch turn, contralateral roll turn, and contralateral yaw turn. The results are shown in Figure 6 B–D. The population of RS neurons, activated by the downward pitch turn, includes groups 2 and 6. A regular effect of these groups (observed in six of seven neurons) (Table 2) is the pitch-up torque. The neurons of group 2 cause the torque because of excitation of dorsal MNs (Fig. 6 B, pattern 2-2), inhibition of ventral MNs (Fig. 6 B, pattern 2-3), or the combination of both (Fig. 6 B, pattern 2-1). The right and left neurons of group 6 in combination produce no torque but an excitation of all MNs (Fig. 6 B, pattern 6-1).
Table 2.
Correlations between sensory inputs to RS neurons and their motor effects
| Rotation | Pitch-down | |
|---|---|---|
| Groups activated | 2 | 6 |
| Regular effect (6 of 7) | Pitch-up | No |
| Additional effects (0 of 7) | No | No |
RS neurons of groups 3, 7, 8, and 9 change their activity during roll turns. The neurons contralateral to the turn are excited, whereas the neurons ipsilateral to the turn are inhibited (Deliagina and Pavlova, 2002). Inhibition of the neurons that are tonically active during swimming (Zelenin, 2005) effectively inverts their effects (Fig. 4 D). A combined effect of right and left RS neurons of these groups (observed in all 43 neurons) (Table 3) is the roll-ipsilateral torque. It is usually attributable to a centrally symmetrical oblique pattern of activity of MNs (Fig. 6 C, patterns 3-1, 3-2, 3-5, and 7-1). In the other cases, the oblique pattern is not centrally symmetrical (Fig. 6 C, patterns 3-3, 3-4, 3-6, 7-2, 7-3, 7-4, 7-5, 8-1, and 9-1). These neurons cause a combination of the roll torque counteracting the initial roll turn (regular effect) and the yaw torque (additional effect, observed in 16 of 43 neurons) (Table 3).
Table 3.
Correlations between sensory inputs to RS neurons and their motor effects
| Rotation | Roll-contra | |||
|---|---|---|---|---|
| Groups activated | 3 | 7 | 8 | 9 |
| Regular effect (43 of 43) | Roll-ipsi | Roll-ipsi | Roll-ipsi | Roll-ipsi |
| Additional effects (16 of 43) | Yaw-ipsi | Yaw-ipsi | Yaw-ipsi | Yaw-ipsi |
contra, Contralateral; ipsi, ipsilateral.
Finally, the neurons of groups 4–6, 8, and 9 are excited with the contralateral yaw turns (Table 4). The contralateral counterparts of these neurons do not change their activity (Karayannidou et al., 2007). A regular effect of these groups (observed in all 26 neurons) is the ipsilateral yaw torque. The neurons of groups 4–6 cause the torque because of excitation of the ipsilateral MNs, which can be combined with inhibition of the contralateral MNs (Fig. 6 D). The neurons of groups 8 and 9 excite ipsilateral ventral MNs and thus cause a combination of ipsilateral yaw torque (regular effect) with ipsilateral roll torque and downward pitch torque (Table 4, additional effects).
Table 4.
Correlations between sensory inputs to RS neurons and their motor effects
| Rotation | Yaw-contra | ||||
|---|---|---|---|---|---|
| Groups activated | 4 | 5 | 6 | 8 | 9 |
| Regular effect (26 of 26) | Yaw-ipsi | Yaw-ipsi | Yaw-ipsi | Yaw-ipsi | Yaw-ipsi |
| Additional effects (3 of 26) | No | No | No | Roll-ipsi Pitch-down | Roll-ipsi Pitch-down |
contra, Contralateral; ipsi, ipsilateral.
Besides the RS neurons described above, which had both specific vestibular inputs and specific functional projections (n = 84), we recorded a group of RS neurons (n = 36) that did not have specific functional projections. They either inhibited all four MN pools (n = 10), as illustrated in Figure 7 B, or excited all MN pools (n = 2), or produced no effect on the MN activity (n = 24). Their vestibular responses were diverse. Some of them did not respond to vestibular stimulation (n = 10), some had nonspecific responses (n = 13), as illustrated in Figure 7 A, and the others could respond specifically to turns in one, two, or all three planes (n = 13). We also recorded one RS neuron that had specific functional projections (it caused excitation of the ipsilateral ventral MNs only) but lacked specific vestibular responses. One cannot exclude, however, that this neuron could be activated by stronger vestibular stimulation or by some non-vestibular stimuli (e.g., visual or tactile).
The majority of patterns of motor effects observed in the present study were also found in the previous study (Zelenin et al., 2001). However, patterns 1-2, 1-4, 2-3, 3-5, and 7-5 were found for the first time. Some of the patterns recorded previously were not encountered in this work [patterns B2, E3, F2, F3, and G1 described by Zelenin et al. (2001)]. It seems, therefore, possible that some other patterns remained not revealed in these studies.
Discussion
The population of RS neurons constitutes an essential part of the closed-loop control system responsible for maintenance of a certain body orientation in the swimming lamprey (Deliagina et al., 2000, 2006). The RS neurons receive information about body orientation from the vestibular system (Deliagina et al., 1992a,b). To change (correct) the orientation, the RS neurons send commands to the spinal motor networks causing a specific motor effect (Zelenin et al., 2001, 2003b). In the present study, we correlated vestibular inputs to individual RS neurons and their motor effects (functional projections) and have thus characterized the sensory-motor transformation performed by these neurons. A collective effect of RS neurons provides a good explanation of the orientation control during locomotion.
Sensory inputs to RS neurons
We have analyzed 84 RS neurons that had both specific vestibular inputs and specific patterns of functional projections. When tested by rotation in the three main planes (roll, pitch, and yaw), most of the neurons (68%) (Fig. 5, groups 1–4) responded to turns in only one plane. In the pitch plane, groups 1 and 2 were activated by the nose-up and nose-down tilts, respectively. In the roll plane, group 3 included right neurons that responded to the left roll tilt and left neurons that responded to the right tilt. Roll and pitch responses in RS neurons had usually only a dynamic component (activity during rotation). It should be noted that, in intact lampreys, these responses contain also a static component (i.e., a position-dependent activity of RS neurons) (Deliagina and Fagerstedt, 2000; Pavlova and Deliagina, 2002). Rotation in the yaw plane affected group 4 neurons, including right neurons that responded dynamically to the left yaw turn and left neurons that responded to the right turn [these responses were similar to those in intact animals (Karayannidou et al., 2007)]. Only 30% of RS neurons responded to turns in two planes (groups 5–8) (see also Pavlova and Deliagina, 2002), and 2% responded to turns in three planes (group 9).
On the basis of these findings, one can suggest that the control of orientation in the three main orthogonal planes is performed by three separate mechanisms, and three populations of RS neurons form three channels that transmit commands to the MN pools for correcting the orientation in each of the planes. This suggestion corresponds well to the fact that the lamprey is capable of stabilizing its orientation in one plane (e.g., in the roll plane) while performing various maneuvers in other planes (e.g., in the pitch or yaw planes) (Ullén et al., 1995). It seems also likely that combined rotation in two or three main planes will cause activation of the specific (one-plane) subpopulations of RS neurons, as well as of the neurons responding to turns in two or three planes (groups 5–9).
Motor effects of RS neurons
Individual RS neurons exerted specific excitatory and/or inhibitory effects on the spinal MNs, which were revealed using the spike-triggered averaging technique (see Materials and Methods and Fig. 1 E). In contrast to higher vertebrates (Drew et al., 1986), a contribution of single RS neurons to the spinal motor output in the lamprey was noticeable. A change in the mean frequency of MNs attributable to RS influences was ∼75% on average (Figs. 2 –4, 7) (see also Zelenin et al., 2001). Taking into account that each RS neuron exerts a uniform effect on the segmental motor output along the entire length of its axon (Zelenin et al., 2001, 2003b), one can conclude that activation of a single RS neuron substantially affects the configuration and movement of a considerable part of the body.
The effects of individual RS neurons on the segmental motor output were very diverse (see also Zelenin et al., 2001). In total, 17 patterns of functional projections have been found in the present study (Fig. 5). Some patterns would cause body flexion in the pitch plane (Fig. 2 B) or in the yaw plane, which is necessary for correcting the body orientation in these planes during swimming. Other patterns would result in a change in the plane of locomotor body undulations (from lateral to diagonal) (Fig. 3 B), which is necessary for correcting the body orientation in the roll plane. There were patterns that would have mixed effects, such as the body flexion in more than one plane (Fig. 4 B). One can suggest that the diversity of motor effects of RS neurons allow the CNS, by recruiting different combinations of neurons, to easily form numerous muscle synergies necessary for a wide variety of behaviors.
Sensory-motor transformation by RS neurons
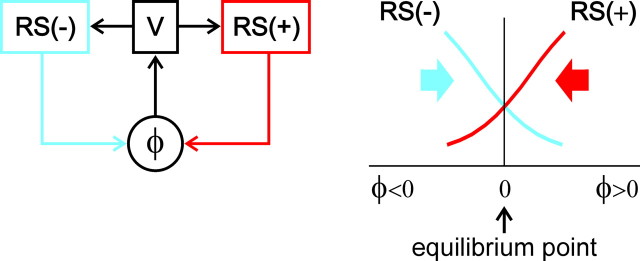
A strong correlation was found in the present study between the vestibular responses of RS neurons and their motor effects (Fig. 5, Tables 1 –4). If a neuron was activated by a turn of the animal in a given plane, this neuron most often caused a torque opposing the turn (and never caused a torque augmenting the turn). Besides this major effect, a small proportion of neurons caused additional effects (i.e., torques in other planes). These findings strongly suggest that any deviation of the body from the stabilized orientation in the pitch, roll, and yaw planes will result in activation of a specific population of RS neurons. These RS neurons will modify the ongoing locomotor output and will cause the torque that counteracts the change in orientation. Two populations of RS neurons, with opposite reactions to rotation and with opposite motor effects, constitute an essential part of the orientation control mechanism for each of the main planes (Fig. 8). Operation of this mechanism is shown schematically in the graph, in which the abscissa is the turn angle in a given plane (φ) and the ordinate is the activity (motor effects) of two antagonistic populations, RS(+) and RS(−). Any change in the angle results in an increase in activity in one population and in a decrease in the other. The two populations tend to rotate the lamprey in the opposite directions (Fig. 8, arrows). The dominating population causes a corrective motor response aimed at returning the animal to the initial position. The stabilized orientation (equilibrium point of the system) is the angle at which the activities of the two populations and, therefore, their motor effects are equal to each other. Thus, results of the present study strongly supported the conceptual model of the control system for spatial orientation, with two antagonistic vestibular reflexes in each of the main planes (Deliagina and Fagerstedt, 2000; Pavlova and Deliagina, 2002; Karayannidou et al., 2007).
Figure 8.
The principle of stabilization of body orientation. The controlled variable φ is the pitch, roll, or yaw angle. Information about φ is delivered to RS neurons by the vestibular system (V). Two populations of neurons, RS(+) and RS(−), with opposite reactions to rotation and with opposite motor effects (arrows), constitute an essential part of the orientation control mechanism for each of the main planes. A deviation of φ from its stabilized value (φ = 0) toward positive or negative values will result in activation of a specific population of neurons, RS (+) (shown in red) or RS(−) (shown in blue), respectively. The activated RS neurons will cause a corrective motor response that counteracts the change in orientation and tends to return the animal to the initial position. In the pitch and roll planes, vestibular input provides information about the absolute value of φ. In these planes, the stabilized orientation (equilibrium point of the system) is the angle at which the activities of the two populations and therefore their motor effects are of the same magnitude. In the yaw plane, the vestibular system provides information only about dynamic changes of φ. To maintain a definite yaw angle, vestibular input should be supplemented by input from other sensory systems (e.g., visual) signaling the absolute value of φ.
It should be noted that, in the control mechanisms for the pitch and roll planes, the corresponding RS neurons receive vestibular signals about orientation in the gravity field (absolute values of tilt angles), which allow the system to maintain certain angles in these planes. In contrast, turns in the horizontal plane do not affect the animal's orientation in relation to the gravity vector. For that reason, the yaw control mechanism receives vestibular signals only about dynamic changes of the yaw orientation but not about the absolute value of the yaw angle. As a result, the yaw control mechanism can reduce rapid but not slow deviations from the swim trajectory. Thus, maintenance of a certain direction of swimming requires participation of other sensory inputs (e.g., visual) signaling the absolute yaw angle value.
Some RS neurons, besides causing a motor pattern for correcting the orientation in the plane of perturbation, generated the commands that caused torques in other plane(s) (Tables 1 –4, additional effects). This finding can be related to a different strategy used by the lamprey for large-scale turns, which may include supplementary movements in other planes (Ullén et al., 1995). Another possibility is that these RS neurons participate not only in the orientation control but also in other forms of behavior that require different motor coordinations.
Besides the RS neurons with specific functional projections, some neurons inhibited all four MN pools (Fig. 7 B) or excited all MN pools. Their possible function could be inactivation or activation of the spinal locomotor networks, respectively. Some RS neurons produced no effect on the MN activity. Their function is not clear. It should also be noted that smaller RS neurons as well as vestibulospinal neurons were not recorded in this study, and their contribution to the orientation control remains unknown.
To conclude, in the present study, a command system for spatial orientation in a vertebrate animal was for the first time analyzed in detail, with characterization of both sensory inputs and motor effects of individual command neurons. It was shown that transformation of the spatial information into the corrective motor commands has two steps. First, a population of command neurons in the brainstem, with specific spatial sensitivity, is activated by sensory signals. Second, this population of neurons, because of their spinal projections, activates a specific group of muscles that causes correction of orientation. A similar functional organization of the system for equilibrium and steering control was described previously for the flying locust (Wilson, 1968; Hensler, 1988; Rowell, 1988; Hensler and Rowell, 1990) and for the swimming mollusc Clione (Deliagina et al., 1998, 1999). In Clione, the command neurons are also driven by gravitational organs. In contrast, the command neurons in the locust are driven by visual input. These studies jointly demonstrate a striking similarity in the principal design of the spatial orientation mechanisms across vertebrates and invertebrates, as well as across swimming and flying animals.
Footnotes
This work was supported by the Swedish Research Council (Section of Natural Science and Technology and Section of Medicine) and the Gösta Fraenkels Foundation. We thank Dr. R. Hill for critical review of this manuscript.
References
- Brodin L, Grillner S, Dubuc R, Ohta Y, Kasicki S, Hökfelt T. Reticulospinal neurons in lamprey: transmitters, synaptic interactions and their role during locomotion. Arch Ital Biol. 1988;126:317–345. [PubMed] [Google Scholar]
- Bussières N. Etude Anatomique et Functionnelle. Montreal: University of Montreal; 1994. Les Systemes Descendants chez la Lamproie. [Google Scholar]
- Deliagina TG, Fagerstedt P. Responses of reticulospinal neurons in intact lamprey to vestibular and visual inputs. J Neurophysiol. 2000;83:864–878. doi: 10.1152/jn.2000.83.2.864. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN. Comparative neurobiology of postural control. Curr Opin Neurobiol. 2002;12:652–657. doi: 10.1016/s0959-4388(02)00376-8. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Pavlova EL. Modifications of vestibular responses of individual reticulospinal neurons in the lamprey caused by a unilateral labyrinthectomy. J Neurophysiol. 2002;87:1–14. doi: 10.1152/jn.00315.2001. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN, Grillner S, Wallén P. Vestibular control of swimming in lamprey. II. Characteristics of spatial sensitivity of reticulospinal neurons. Exp Brain Res. 1992a;90:489–498. doi: 10.1007/BF00230931. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN, Grillner S, Wallén P. Vestibular control of swimming in lamprey. III. Activity of vestibular afferents. Convergence of vestibular inputs on reticulospinal neurons. Exp Brain Res. 1992b;90:499–507. doi: 10.1007/BF00230932. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Grillner S, Orlovsky GN, Ullén F. Visual input affects the response to roll in reticulospinal neurons of the lamprey. Exp Brain Res. 1993;95:421–428. doi: 10.1007/BF00227134. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Arshavsky YI, Orlovsky GN. Control of spatial orientation in a mollusc. Nature. 1998;393:172–175. doi: 10.1038/30251. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN, Selverston AI, Arshavsky YI. Neuronal mechanisms for the control of body orientation in Clione. I. Spatial zones of activity of different neuron groups. J Neurophysiol. 1999;82:687–699. doi: 10.1152/jn.1999.82.2.687. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Zelenin PV, Fagerstedt P, Grillner S, Orlovsky GN. Activity of reticulospinal neurons during locomotion in the freely behaving lamprey. J Neurophysiol. 2000;83:853–863. doi: 10.1152/jn.2000.83.2.853. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Zelenin PV, Orlovsky GN. Encoding and decoding of reticulospinal commands. Brain Res Rev. 2002;40:166–177. doi: 10.1016/s0165-0173(02)00199-6. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN, Zelenin PV, Beloozerova IN. Neural bases of postural control. Physiology. 2006;21:216–225. doi: 10.1152/physiol.00001.2006. [DOI] [PubMed] [Google Scholar]
- Drew T, Dubuc R, Rossignol S. Discharge patterns of reticulospinal and other reticular neurons in chronic, unrestrained cats walking on a treadmill. J Neurophysiol. 1986;55:375–401. doi: 10.1152/jn.1986.55.2.375. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J Neurophysiol. 1980;44:751–772. doi: 10.1152/jn.1980.44.4.751. [DOI] [PubMed] [Google Scholar]
- Grillner S, Kashin S. On the generation and performance of swimming in fish. In: Herman RM, Grillner S, Stein PSG, Stuart DF, editors. Neural control of locomotion. New York: Plenum; 1976. pp. 181–202. [Google Scholar]
- Grillner S, McClellan A, Sigvardt K, Wallén P, Wilén M. Activation of NMDA-receptors elicits “fictive locomotion” in lamprey spinal cord in vitro . Acta Physiol Scand. 1981;113:549–551. doi: 10.1111/j.1748-1716.1981.tb06937.x. [DOI] [PubMed] [Google Scholar]
- Grillner S, Deliagina T, Ekeberg Ö, el Manira A, Hill RH, Lansner A, Orlovsky GN, Wallén P. Neural networks controlling locomotion and body orientation in lamprey. Trends Neurosci. 1995;18:270–279. [PubMed] [Google Scholar]
- Hensler K. The pars intercerebralis neuron PI(2)5 of locusts—convergent processing of inputs reporting head movements and deviations from straight flight. J Exp Biol. 1988;140:511–533. [Google Scholar]
- Hensler K, Rowell CHF. Control of optomotor responses by descending deviation detector neurons in intact flying locusts. J Exp Biol. 1990;149:191–205. [Google Scholar]
- Karayannidou A, Orlovsky GN, Zelenin PV, Deliagina TG. Responses of reticulospinal neurons in the lamprey to lateral turns. J Neurophysiol. 2007 doi: 10.1152/jn.00912.2006. in press. [DOI] [PubMed] [Google Scholar]
- McClellan AD, Hagevik A. Descending control of turning locomotor activity in larval lamprey: neurophysiology and computer modeling. J Neurophysiol. 1997;78:214–228. doi: 10.1152/jn.1997.78.1.214. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R. Topological analysis of the brain stem of the lamprey Lampetra fluviatilis . J Comp Neurol. 1972;145:165–177. doi: 10.1002/cne.901450204. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, ten Donkelaar HR. The central nervous system of vertebrates. Berlin: Springer; 1996. [Google Scholar]
- Orlovsky GN, Deliagina TG, Grillner S. From mollusc to man. Oxford: Oxford UP; 1999. Neuronal control of locomotion. [Google Scholar]
- Pavlova EL, Deliagina TG. Responses of reticulospinal neurons in intact lamprey to pitch tilt. J Neurophysiol. 2002;88:1136–1146. doi: 10.1152/jn.2002.88.3.1136. [DOI] [PubMed] [Google Scholar]
- Rovainen CM. Physiological and anatomical studies on large neurons of central nervous system of the sea lamprey (Petromyzon marinus). I. Muller and Mautner cells. J Neurophysiol. 1967;30:1000–1023. doi: 10.1152/jn.1967.30.5.1000. [DOI] [PubMed] [Google Scholar]
- Rowell CHF. Mechanisms of flight steering in locusts. Experientia. 1988;44:389–395. [Google Scholar]
- Tretjakoff D. Das nervensystem des flussnevnauges. Z Wiss Zool. 1927;129:359–452. [Google Scholar]
- Ullén F, Deliagina TG, Orlovsky GN, Grillner S. Spatial orientation of lamprey. 1. Control of pitch and roll. J Exp Biol. 1995;198:665–673. doi: 10.1242/jeb.198.3.665. [DOI] [PubMed] [Google Scholar]
- Wannier T, Deliagina TG, Orlovsky GN, Grillner S. Differential effects of reticulospinal system on locomotion in lamprey. J Neurophysiol. 1998;80:103–112. doi: 10.1152/jn.1998.80.1.103. [DOI] [PubMed] [Google Scholar]
- Wilson DM. Inherent asymmetry and reflex modulation of the locust flight motor pattern. J Exp Biol. 1968;48:631–641. doi: 10.1242/jeb.48.3.631. [DOI] [PubMed] [Google Scholar]
- Zelenin PV. Activity of individual reticulospinal neurons during different forms of locomotion in the lamprey. Eur J Neurosci. 2005;22:2271–2282. doi: 10.1111/j.1460-9568.2005.04395.x. [DOI] [PubMed] [Google Scholar]
- Zelenin PV, Grillner S, Orlovsky GN, Deliagina TG. Heterogeneity of the population of command neurons in the lamprey. J Neurosci. 2001;21:7793–7803. doi: 10.1523/JNEUROSCI.21-19-07793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenin PV, Grillner S, Orlovsky GN, Deliagina T. The pattern of motor coordination underlying the roll in the lamprey. J Exp Biol. 2003a;206:2557–2566. doi: 10.1242/jeb.00451. [DOI] [PubMed] [Google Scholar]
- Zelenin PV, Pavlova EL, Grillner S, Orlovsky GN, Deliagina TG. Comparison of the motor effects of individual vestibulo- and reticulospinal neurons on dorsal and ventral myotomes in lamprey. J Neurophysiol. 2003b;90:3161–3167. doi: 10.1152/jn.00555.2003. [DOI] [PubMed] [Google Scholar]