Abstract
Dopamine is involved in multiple neural functions including motor control, reward and motivational processing, learning and reinforcement, and cognitive attention. Dopamine binds to two distinct classes of receptors, namely D1 and D2, to exert these functions. Various endogenous substances regulate dopamine signaling, although their physiological functions are not fully understood. Here, we examined the role of prostaglandin E2 (PGE2) and one of its receptors, EP1, in dopaminergic function in the striatum. EP1 was expressed in both preprodynorphin-containing D1 and preproenkephalin-containing D2 neurons, and PGE2 was produced in striatal slices in response to both D1 and D2 dopamine receptor stimulation. EP1-deficient mice exhibited significant suppression of hyperlocomotion induced by cocaine or SKF81297 (6-chloro-2,3,4,5-tetrahydro-1-phenyl-1H-3-benzazepine hydrobromide), a D1 agonist, and significant attenuation of catalepsy induced by raclopride, a D2 antagonist. Despite these behavioral defects, the extracellular concentration of dopamine was not suppressed in the striatum of EP1-deficient mice, and the densities of D1 and D2 receptors in the striatum were not different between the two genotypes. Stimulation of the D1 receptor induced phosphorylation of dopamine and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) at Thr34 in striatal slices, and the addition of indomethacin, a PG synthesis inhibitor, attenuated the D1 agonist-induced increase in DARPP-32–Thr34 phosphorylation. The further addition of an EP1 agonist restored the indomethacin-attenuated phosphorylation. Furthermore, both D1- and D2-mediated changes in the DARPP-32–Thr34 phosphorylation were attenuated in EP1−/− slices. These results suggest that PGE2 is formed in response to dopamine receptor stimulation in the striatum and amplifies both D1 and D2 receptor signaling via EP1.
Keywords: EP1, DARPP-32, D1, D2, dopamine, prostaglandin, striatum
Introduction
Dopamine is implicated in various CNS functions including motor control, emotional processing, learning and reinforcement, and cognitive attention (Graybiel et al., 1994; Holland and Gallagher, 1999; Di Chiara, 2002; Wise, 2004; Everitt and Robbins, 2005). Dopaminergic neurons are located in several midbrain structures in mammals, such as substantia nigra pars compacta and ventral tegmental area, and innervate the striatum, the prefrontal cortex, and limbic structures such as the amygdala (Dahlström and Fuxe, 1964; Fallon and Moore, 1978). Dopamine exerts its functions by binding to two distinct classes of G-protein-coupled receptors (GPCRs), namely the D1 receptors comprising the D1 and D5 subtypes and the D2 receptors comprising the D2, D3, and D4 subtypes (Missale et al., 1998). D1 and D2 receptors mediate specific functions through distinct intracellular signaling cascades (Missale et al., 1998; Nicola et al., 2000; Greengard, 2001; Nestler, 2005) and different patterns of expression (Goldman-Rakic, 1996; Gerfen, 2004). Furthermore, various neural pathways such as those of glutamate, serotonin, and acetylcholine as well as humoral factors such as adenosine interact with dopaminergic pathways and critically modulate D1 and D2 receptor signaling (Svenningsson et al., 2004). Elucidating neural and humoral networks surrounding dopaminergic neurons and regulation is therefore important for understanding functions of the dopaminergic nervous system as well as neurological and psychiatric disorders caused by its dysfunction.
We have been studying the physiological roles of prostagladins (PGs). PGs including PGD2, PGE2, PGF2α, PGI2, and thromboxane A2 (TXA2) are a family of lipid mediators that are widely formed throughout our body and elicit versatile functions in a variety of processes including vascular homeostasis, inflammation, and reproduction (Narumiya et al., 1999). PGs bind to eight types and subtypes of GPCRs to exert their actions; they are the PGD receptor (DP), the four subtypes of PGE receptor (EP1, EP2, EP3, and EP4), the PGF receptor (FP), the PGI receptor (IP), and the TXA receptor (TP). Because of their specific localization and intracellular signaling pathways, each receptor can mediate a unique profile of actions in the body (Narumiya et al., 1999). The function of PGs in CNS has thus far been studied mostly in relation to sickness behaviors (Elmquist et al., 1997; Turnbull and Rivier, 1999). Our previous studies on each EP subtype-deficient mice individually revealed that EP3 mediates febrile responses, and that both EP1 and EP3 are required for the neuroendocrine stress response (Ushikubi et al., 1998; Matsuoka et al., 2003). EP1-deficient mice further exhibit enhanced aggression and impaired cliff avoidance, indicating that the PGE2–EP1 signaling controls impulsive behaviors under environmental and social stress (Matsuoka et al. 2005). Although these mice show enhanced dopamine turnover in the striatum and frontal cortex and administration of dopamine receptor antagonists suppresses their impulsivity, they do not show hyperlocomotion. Here, we have analyzed the role of EP1 in the dopaminergic signaling in the striatum and related behaviors. Our data suggest that PGE2 is formed in response to dopamine receptor stimulation in the striatum and regulates signaling and function of both D1 and D2 receptors via EP1.
Materials and Methods
Mice.
Mice lacking the Ptger1 gene encoding EP1 (EP1−/−) were generated and backcrossed >10 generations to the C57BL/6CrSlc background as described previously (Matsuoka et al., 2005). Male littermates 2–3 months of age having EP1−/− and wild-type genotypes derived from the same EP1+/− parents were used for each comparison group in all the experiments, except those in Figure 6 and supplemental Figure 2 (available at www.jneurosci.org as supplemental material), in which age-matched, male C57BL/6CrSlc mice (SLC, Shizuoka, Japan) were used as the wild-type control. All animal care and use was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee of Kyoto University Graduate School of Medicine or that of Kurume University Graduate School of Medicine.
Figure 6.
Effects of an EP1-selective agonist and of EP1 deficiency on dopamine D1 and D2 receptor-mediated changes in DARPP-32 Thr34 phosphorylation in striatal slices. A, Effect of indomethacin on D1 receptor-stimulated DARPP-32 Thr34 phosphorylation in striatal slices from wild-type mice. After incubation for 30 min with or without 100 μm indomethacin (Indo), slices were incubated for additional 5 min with 1 μm SKF81297 (SKF) or vehicle. Typical immunoblots for detection of phospho-Thr34 DARPP-32 are shown in top panels. The levels of phospho-Thr34 DARPP-32 were normalized to values obtained from untreated slices (a.u., arbitrary units). B, Effects of an EP1 agonist, ONO-DI-004, on D1 receptor stimulated DARPP-32 Thr34 phosphorylation in PG-depleted slices. Striatal slices, prepared from indomethacin-injected mice, were incubated with 100 μm indomethacin for 60 min to block PG synthesis. In the continued presence of indomethacin, the slices were incubated with 0, 1, 10, or 100 μm ONO-DI-004 for 10 min, and SKF81297 (1 μm) was added at 5 min of incubation. C, Effects of a D1 receptor agonist on DARPP-32 Thr34 phosphorylation in striatal slices from wild-type and EP1-deficient mice. Striatal slices, prepared from wild-type (BL/6) and EP1-deficient (EP1−/−) mice, were incubated with 1 μm SKF81297 for 0, 2, or 5 min. The numbers of slices used in experiments A–C are shown below each column. D, Effects of a D2 receptor agonist, quinpirole, on DARPP-32 Thr34 phosphorylation in striatal slices from wild-type and EP1-deficient mice. Striatal slices, prepared from wild-type (+/+) and EP1-deficient (−/−) mice, were incubated with either vehicle or quinpirole (1, 10, 100, or 1000 nm) for 5 min. The data were normalized to values from vehicle-treated slices in each genotype. n = 5–9. Error bars indicate SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
In situ hybridization and immunohistochemistry.
In situ hybridization was performed using the standard method with an 35S-labeled antisense riboprobe matching the entire length of mouse EP1 cDNA as described previously (Furuyashiki et al., 1999). Control slides were incubated with the radioactive probe in the presence of a 200 times excess amount of nonradioactive riboprobe from the same template. Immunohistochemistry was performed as described previously (Lee et al., 1997) with modifications. Under deep anesthesia with sodium pentobartiturate injected intraperitoneally (60 mg/kg; Dainippon Pharmaceutical, Osaka, Japan), mice were perfused transcardially with 0.9% sodium chloride in 5 mm phosphate buffer (PBS; pH 7.4), followed by 4% formaldehyde in 0.1 m sodium phosphate, pH 7.4. After incubation in 30% sucrose in PBS for cryoprotection, brains were cut at coronal planes at 20 μm thickness on a microtome frozen at −20°C. The brain sections were incubated for 36 h at 4°C with a rabbit polyclonal antibody to EP1 (1:250; 101740; Cayman Chemical, Ann Arbor, MI), followed by incubation with HRP-conjugated goat anti-rabbit IgG (1:200 dilution; 111-035-144; Jackson ImmunoResearch, West Grove, PA) for 1 h at room temperature. The incubation with antibodies was performed in PBS containing 0.25% Triton X-100, 0.25% λ-carrageenan, 10% goat serum, and 1% BSA, and followed by a rinse with PBS containing 0.1% Triton X-100. For double staining, the sections were further incubated overnight at 4°C with a guinea pig polyclonal antibody to preprodynorphin (PPD) (1:200 dilution; AB5519, Chemicon, Temecula, CA) or that to preproenkephalin (PPE) (1:200 dilution; a gift from Dr. Takeshi Kaneko, Kyoto University, Kyoto, Japan) (Lee et al., 1997). Signals for EP1 and PPD/PPE were visualized with fluorescein-conjugated Tyramide (PerkinElmer, Boston, MA) and Cy3-conjugated anti-guinea pig IgG antibody (1:500 dilution; 706-165-148; Jackson ImmunoResearch), respectively. Fluorescence images were acquired on a LSM510 laser-scanning confocal microscope (Zeiss, Oberkochen, Germany). The specificity of the EP1 signal was examined by blocking with an epitope peptide as well as on tissues from EP1-deficient mice (see Results).
Perfusion of striatal slices and quantification of PGE2.
Striatal slices of 300 μm thickness were prepared from wild-type mice as described previously (Nishi et al., 1997). The slices were loaded onto the chambers and perfused with Krebs-HCO3− buffer (124 mm NaCl, 4 mm KCl, 26 mm NaHCO3, 1.5 mm CaCl2, 1.25 mm KH2PO4, 1.5 mm MgSO4, 10 mm d-glucose, pH 7.4) saturated with 95% O2 and 5% CO2 at a flow rate of 0.5 ml/min. Some animals and their slices were treated with indomethacin, a cyclooxygenase (COX) inhibitor that blocks the production of PGs. For the indomethacin-treated controls, slices were prepared from wild-type mice administered with 5 mg/kg indomethacin (Nacalai Tesque, Kyoto, Japan) intraperitoneally 30 min before decapitation, and were perfused with Krebs-HCO3− buffer containing 1 mm indomethacin. After equilibration for 60 min, the perfusing buffer was changed to Krebs-HCO3− buffer containing either vehicle or indicated concentrations of 6-chloro-2,3,4,5-tetrahydro-1-phenyl-1H-3-benzazepine hydrobromide (SKF81297) (Sigma-Aldrich, St. Louis, MO), or quinpirole (Sigma-Aldrich), or both. After the slices were exposed to the drug-containing buffer, perfusates were collected for 5 min in chilled tubes containing 600 μl of ethanol and 700 μl of 0.1N hydrochloric acid. [5,6,8,11,12,14,15(n)-3H]PGE2 (7.4 TBq/mmol; Amersham Biosciences, Piscataway, NJ) (10,000 cpm) was then added to the samples as a tracer for monitoring recovery, and the samples were centrifuged at 3000 × g for 10 min at 4°C. The supernatants were applied to preconditioned Sep-Pak plus cartridges (WAT0200515; Waters, Milford, MA), which were washed serially with 15% ethanol, petroleum ether, and, finally, 5 ml of methyl formate as described previously (Powell, 1980). The methyl formate fraction was recovered in a glass vial and stored at −80°C until use. Methyl formate was then evaporated, and the residues were suspended in 150 μl of water/methyl formate (80:20, v/v). Samples were then subjected to HPLC on a Cosmosil 5C18 column (4.6 × 150 mm; Nacalai Tesque) with water/acetonitrile/acetic acid (67:33:0.01, v/v/v). Fractions corresponding to the elution position of authentic PGE2 were collected, and, after evaporation, residues were suspended in 200 μl volume of an assay buffer (0.1 m phosphate buffer, pH 7.5, containing 0.9% bovine serum albumin and 0.5% kathon). The PGE2-like immnoreactivity was then determined using an enzyme immunoassay (EIA) kit (GE Healthcare, Piscataway, NJ).
Behavioral analysis.
Acute and chronic psychostimulant effects of cocaine were tested as described previously (Hikida et al., 2001). For acclimation to the injection procedure, mice were injected intraperitoneally with saline and placed in a transparent, plastic test chamber (22 × 32 × 13.5 cm3) for 10 min once a day on days 1 and 2. From day 3 to day 7, mice were administered cocaine (10 or 20 mg/kg; Takeda Pharmaceutical, Osaka, Japan) or vehicle (0.9% sodium chloride) and placed in the test chamber for 10 min once a day. For SKF81297-induced hyperlocomotion (Xu et al., 1994), mice were habituated to a transparent, plastic test chamber (46 × 46 × 30.3 cm3) for 1 h. They then received intraperitoneal injection of SKF81297 (2.5 or 5 mg/kg) or vehicle (0.9% sodium chloride) and were immediately returned to the test chamber for an additional 1 h observation period. Locomotor activity was measured based on interruption of infrared beams (Melquest, Toyama, Japan). Raclopride-induced catalepsy was measured as described previously (Pertwee, 1972). Mice were injected intraperitoneally with raclopride (0.25, 0.5, 1, and 2 mg/kg; Sigma-Aldrich) or vehicle (0.9% sodium chloride). Behavior was video-recorded for 5 min from 30 min after drug injection, and the duration of catalepsy was determined post hoc. Catalepsy was defined as the total motionless time on the wire ring. All behavioral tests were performed and analyzed by experimenters blinded to the mouse genotype. A total volume of intraperitoneal injection was set at 8 ml/kg by adjusting drug concentrations and matched across individuals in the same comparison.
Whole-animal studies.
Mice were injected intraperitoneally with vehicle (0.9% sodium chloride), cocaine (10 mg/kg), or SKF81297 (2.5 mg/kg) at 15 min before decapitation. After decapitation, the heads of mice were immediately immersed in liquid nitrogen for 30 s. Rostral parts of the frozen heads were cut to the level of the striatum on dry ice, and striatal tissues were punched out and stored at −80°C until use. For quantification of PGE2, frozen striata were weighed, and extracted by homogenization of the tissues in ethanol containing 0.1 m hydrochloric acid. The extracts were then mixed with a tracer of [3H]PGE2 and subjected to quantification. Alternatively, frozen striata were used for analysis of dopamine and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) phosphorylation as described below.
Analysis of DARPP-32 phosphorylation.
Experiments were performed as described previously (Nishi et al., 1997). After decapitation, the brain was rapidly removed, and coronal striatal slices were prepared at 350 μm thickness using a vibrating blade microtome, VT1000S (Leica Microsystems, Nussloch, Germany). The slices were preincubated at 30°C for 60 min in Krebs-HCO3− buffer saturated with 95% O2 and 5% CO2, and were incubated with drugs as specified in each experiment. ONO-DI-004 was obtained from Ono Pharmaceutical (Osaka, Japan). To terminate the incubation, slices were frozen on dry ice, and stored at −80°C until use. For immunoblot, the tissues were sonicated in boiling 1% SDS, and boiled for 10 min. Each sample of 100 μg protein was subjected to SDS-PAGE (10% polyacrylamide gels), and separated proteins were transferred to nitrocellulose membranes (Nishi et al., 1997). The membranes were incubated with mAb-23, a monoclonal antibody to Thr34-phosphorylated DARPP-32 (1:750 dilution) (Snyder et al., 1992). The membrane was then incubated with a goat anti-mouse Alexa 680-linked IgG (1:5000; Invitrogen, Eugene, OR) or a goat anti-mouse IRDye 800-linked IgG (1:5000; Rockland, Gilbertsville, PA). Antibody binding was quantified with the use of an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE). For each experiment, values were normalized to those of control slices without D1 or D2 stimulation. To determine the total amount of DARPP-32 in samples, C24-5a monoclonal antibody to DARPP-32 (1:22,500 dilution) (Hemmings et al., 1984), which is not phosphorylation state-specific, was used for reblotting the membrane. None of the experimental manipulations in the present study altered the total amount of DARPP-32.
Statistical analysis.
All data are shown as means ± SEM. Comparison of two groups was analyzed using unpaired two-tailed Student's t test. One-way or two-way ANOVA was performed for each comparison followed by Tukey's or Bonferroni's post hoc tests for evaluation of pairwise group differences. A value of p < 0.05 was considered statistically significant. The analyses were performed by Prism 4.0 software (GraphPad, San Diego, CA).
Results
EP1 is expressed in both D1- and D2-containing neurons in the striatum
We first examined expression and localization of EP1 in the striatum. In situ hybridization using radioactive EP1 antisense riboprobe exhibited silver grains diffusely distributed over cell bodies of a large number of cells in both ventral and dorsal striatum, namely the nucleus accumbens (NAc) and the caudate putamen (CPu). The size and shape of these cells indicate that they are striatal neurons. Most of the grains over these cells were abolished by the addition of an excess amount of cold riboprobes (Fig. 1A), indicating expression of EP1 in a majority of neurons in the striatum. We then confirmed this finding by immunostaining. Because the EP1 immunoreactivity was not of a high amount, we amplified the antigen–antibody complex by the fluorescein-conjugated Tyramide-HRP system. The amplified signals exhibited both diffuse and punctate accumulation in the cytoplasm and proximal processes of a majority of neuron-like cells in the striatum (Fig. 1B). In addition, small dot-like signals forming ill defined networks were found over the tissue. Most of the former signals and not the latter were absent in the striatum of EP1-deficient mice, suggesting the former represent EP1 receptor immunoreactivity and the latter background. These results suggested that EP1 is expressed and present in a number of neurons in the striatum. Over 90% of striatal neurons in rodents are GABAergic projection neurons called medium spiny neurons (MSNs) (Gerfen, 2004). MSNs are composed of at least two neurochemically distinct populations: those expressing D1 receptors and PPD and others expressing D2 receptors and PPE (Gerfen and Young, 1988; Lee et al., 1997). We therefore conducted double immunohistochemistry for EP1 and these marker proteins. Double staining for EP1 and PPD or EP1 and PPE showed that signals for EP1 colocalized with those for PPD in some neurons and with those for PPE in other neurons, suggesting that EP1 is expressed in both PPD-containing D1 and PPE-containing D2 MSNs (Fig. 1C). Quantitative analysis revealed that, in the CPu, EP1 was expressed in 61% of PPD-positive neurons and 55% of PPE-positive neurons and that, in the NAc, EP1 was expressed in 71% of PPD-positive neurons and 79% of PPE-positive neurons.
Figure 1.
Localization of EP1 in the striatum. A, In situ hybridization in the dorsal (CPu) and ventral (NAc) striatum. Signals with the EP1 antisense riboprobes are indicated by arrows. No significant signals were detected in an excess of nonradioactive riboprobes from the same template (Cold Excess). B, Immunofluorescence. Signals for EP1 detected in the soma and proximal processes (indicated by arrows) of cells in the dorsal (CPu) and ventral (NAc) striatum of wild-type mice are reduced to the background level in the striatum from EP1-deficient mice. ac, Anterior commissure. C, Double staining for EP1 and PPD (top panels) or EP1 and PPE (bottom panels). Signals for EP1 and PPD or PPE are shown in green (left panels) and red (middle panels), respectively, and the merged images are shown in right. The arrowheads indicate neurons showing colocalization of the two signals. Scale bars, 20 μm.
Prostaglandin E2 is produced in striatal slices in response to dopamine receptor stimulation
The above findings indicate a possibility that PGE2 is synthesized and acts on EP1 in the striatum. To test this hypothesis, we prepared striatal slices from wild-type mice, incubated them with either vehicle, a D1 agonist, SKF81297, or a D2 agonist, quinpirole, and examined production of PGE2. PGE2 was extracted from the reaction medium by the use of a Sep-Pak plus cartridge and purified by reversed-phase HPLC, and the PGE2-like immunoreactivity in the HPLC fraction was measured by EIA. Authenticity of the PGE2-like immunoreactivity was further verified by its decrease in samples obtained from indomethacin-treated mice and incubated with indomethacin. The addition of either SKF81297 or quinpirole significantly increased PGE2 production in the striatal slices, and this increase was abolished by treatment with indomethacin (Fig. 2A,B). Furthermore, when 3 μm each of SKF81297 and quinpirole were added together, the PGE2 production under this condition was about the sum of those induced by each compound alone (Fig. 2C). These results suggest that PGE2 is produced in the striatum in response to both D1 and D2 dopamine receptor stimulation and the effect of D1 and D2 receptor stimulation is additive.
Figure 2.
Prostaglandin E2 is produced in striatal slices in response to dopamine receptor stimulation. Striatal slices were prepared from wild-type mice, perfused with Krebs-HCO3−, and stimulated with either vehicle (control), a D1 agonist, SKF81297 (10 μm) (SKF) (A), or a D2 agonist, quinpirole (10 μm) (Qui) (B), or both (3 μm each) (C) for 5 min. The amount of PGE2 in the perfusates was measured by HPLC-linked enzyme immunoassay kit as described in Materials and Methods. As alternative controls, striatal slices were prepared from indomethacin-injected mice, pretreated with 1 mm indomethacin (Indo) for 60 min, and stimulated with SKF81297 or quinpirole in the continued presence of indomethacin. The numbers of mice used in experiments are shown below each column. Error bars indicate SEM. *p < 0.05; **p < 0.01.
EP1 deficiency alters behaviors mediated by dopaminergic signaling in the striatum
To elucidate the function of EP1 in the striatum in vivo, we used acute cocaine injection. Acute administration of cocaine leads to hyperlocomotion, which is mediated primarily by high synaptic concentration of dopamine because of blockade of dopamine transporters in the striatum (Giros et al., 1996; Pierce and Kalivas, 1997). We used wild-type and EP1-deficient littermates from heterozygous mating, and, after acclimation to injection procedures over 2 d, injected either saline or cocaine (10 or 20 mg/kg) intraperitoneally to these mice. We then tested their locomotor activity in the next 10 min. Cocaine induced hyperlocomotion in wild-type mice in a dose-dependent manner. EP1-deficient mice also showed a dose-dependent response to cocaine injection, but hyperlocomotion in these mice was significantly reduced compared with that found in wild-type mice, whereas the locomotor activity on saline injection did not differ between the genotypes (Fig. 3A). We next examined whether EP1 is also involved in behavioral sensitization induced by repeated cocaine exposure (Pierce and Kalivas, 1997). Cocaine (10 mg/kg) was injected intraperitoneally once a day into wild-type and EP1-deficient littermates over 5 consecutive days from day 3 to day 7. Daily exposure to cocaine induced sensitization (i.e., a gradual increase in locomotor response to each-day injection of the same dose of cocaine in both wild-type and EP1-deficient mice). Furthermore, after 2 week abstinence, both genotypes similarly showed retention of previous sensitization. However, the EP1-deficient mice persistently showed significantly less locomotor activity in response to each day cocaine injection over the entire test period (Fig. 3B). These data suggest that EP1 is not involved in sensitization to cocaine per se, but participates in elicitation of acute locomotor activity induced by each injection of cocaine. We next injected a D1-selective agonist, SKF81297, and examined effects of the EP1 deficiency on hyperlocomotion induced by D1 receptor stimulation (Bordi and Meller, 1989). SKF81297 stimulated locomotion of wild-type mice in a dose-dependent manner, which lasted over 1 h after injection (Fig. 4). Compared with wild-type mice, EP1-deficient mice showed reduced hyperlocomotion in response to SKF81297. The reduction in EP1-deficeint mice was statistically significant at the dose of 2.5 mg/kg SKF81297, and became less apparent at the higher dose. These findings indicate that PGE2 is formed in vivo in the striatum in response to D1 receptor stimulation and modulates mouse behavior induced by this signaling. To confirm this hypothesis, we treated wild-type mice for 15 min with 2.5 mg/kg SKF81297. Mice were then decapitated, and production of PGE2 was measured in the striatum punched out from frozen brain. We found that a considerable amount of PGE2 was present already under basal conditions, but that administration of SKF81297 significantly increased PGE2 production in this brain region (1.98 ± 0.10 and 2.38 ± 0.10 pg/mg of tissue for control and SKF81297-treated mice, respectively; n = 4 for each group; p = 0.034). A similar treatment with cocaine (10 mg/kg) tended to increase striatal PGE2 production (2.08 ± 0.08 pg/mg of tissue; n = 4; p = 0.485), but the increase was not statistically significant compared with the vehicle-treated control group.
Figure 3.
A, Suppression of cocaine-induced hyperlocomotion in EP1-deficient mice. Wild-type (+/+) and EP1-deficient (−/−) mice were injected with either vehicle or 10 or 20 mg/kg cocaine, and locomotor activity was measured over 10 min. The numbers of animals are shown in each column. *p < 0.05. B, Reduced hyperlocomotion in EP1-deficient mice during and after sensitization with repeated cocaine injections. Wild-type (+/+; n = 11) and EP1-deficient mice (−/−; n = 13) were administered vehicle once a day on days 1 and 2, and then 10 mg/kg cocaine from day 3 to day 7. After 2 weeks of cocaine-free period, the mice were challenged again with 10 mg/kg cocaine on day 20. The locomotor activity was measured over 10 min after each injection. The data at 10 mg/kg in A were adopted from those at day 3 in B. A difference between the genotypes was maintained over the entire testing periods (F(1,4) = 4.925; #p = 0.037). Error bars indicate SEM.
Figure 4.
Suppression of hyperlocomotion induced by D1 receptor stimulation in EP1-deficient mice. After a 1 h habituation, wild-type (+/+) and EP1-deficient (−/−) mice were injected intraperitoneally with either saline or 2.5 or 5 mg/kg SKF81297, and the locomotor activity was measured for 60 min. The numbers of animals are shown in each column. Error bars indicate SEM. *p < 0.05; **p < 0.01; ***p < 0.001 for indicated comparison.
Given expression of EP1 in not only D1-containing MSNs but also D2-containig MSNs, we next examined effects of EP1 deficiency on a behavioral paradigm induced by a D2-selective drug. To this end, we tested catalepsy induced by a D2-selective antagonist, raclopride (Pertwee, 1972). The addition of raclopride dose-dependently increased immobility time (i.e., catalepsy) in wild-type mice. Although raclopride also dose-dependently increased catalepsy in EP1-deficient mice, this response was significantly suppressed in the EP1-deficient mice at a dose of 0.25 mg/kg (Fig. 5).
Figure 5.
Suppression of catalepsy induced by D2 receptor inhibition in EP1-deficient mice. Wild-type (+/+) and EP1-deficient (−/−) mice were injected intraperitoneally with either saline or 0.25, 0.5, 1.0, or 2.0 mg/kg raclopride, and immobility time was measured for 5 min from 30 min after the injection. The numbers of animals are shown in each column. Error bars indicate SEM. *p < 0.05; **p < 0.01.
Extracellular dopamine concentration and ligand binding properties of D1 and D2 receptors in the striatum of EP1-deficient mice
Neurotransmitter signaling depends on both release of a neurotransmitter from the presynaptic terminal and its binding to the cognate receptor at the postsynaptic site. We analyzed whether EP1 deficiency affects either of these processes. We first addressed the presynaptic mechanism by measuring the extracellular dopamine content in the ventral striatum of wild-type and EP1-deficient mice by microdialysis. The striatum was perfused in vivo with artificial CSF through a microdialysis probe, and the content of dopamine recovered in the effluent was determined. The dopamine content in the dialysates from EP1-deficient mice tended to be higher than that from wild-type mice, although there was no statistically significant difference (supplemental Fig. 1A, available at www.jneurosci.org as supplemental material). The dopamine contents from both genotypes of animals increased after acute cocaine injection (10 mg/kg), the condition in which a significantly reduced hyperlocomotion in EP1-deficient mice was observed (Fig. 3). When normalized to the baseline level before cocaine injection, both groups exhibited a similar rise in the dopamine content (supplemental Fig. 1B, available at www.jneurosci.org as supplemental material). Although we did not determine absolute concentration of dopamine by equilibrium microdialysis, these results suggest that the EP1 deficiency did not reduce the synaptic concentration of dopamine compared with that in wild-type mice before and after the cocaine injection.
We next examined ligand binding properties of D1 and D2 receptors. The membrane fraction was prepared from the striatum of wild-type and EP1-deficient mice, and subjected to radioligand binding assay using [3H]SCH23390 and [3H]spiperone for D1 and D2 receptors, respectively (Glowinski and Iversen 1966; List and Seeman, 1981; Charifson et al., 1988) (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). The [3H]SCH23390 binding exhibited a single class of binding; the Kd and Bmax values were 0.421 ± 0.080 and 0.555 ± 0.063 nm (p = 0.266) and 1311 ± 125 and 1495 ± 221 fmol/mg protein (p = 0.489) for wild-type and EP1-deficient mice, respectively. The [3H]spiperone binding exhibited two classes of binding, high-affinity binding and low-affinity binding, which apparently represent D2 and 5-HT2, respectively. The Kd and Bmax values of the former binding were 0.212 ± 0.057 and 0.286 ± 0.132 nm (p = 0.634) and 287 ± 48 and 347 ± 106 fmol/mg protein (p = 0.633) for wild-type and EP1-deficient mice, respectively, and the Kd and Bmax values of the latter binding are 1.37 ± 0.61 and 1.19 ± 0.48 pm (p = 0.829) and 420 ± 194 and 389 ± 243 fmol/mg protein (p = 0.926) for wild-type and EP1-deficient mice, respectively.
EP1 augments both D1- and D2-mediated effects on DARPP-32 Thr34 phosphorylation in striatal slices
Because the above analysis could not reveal significant differences in dopamine release or binding properties of D1 and D2 receptors in the striatum between wild-type mice and EP1-deficient mice, we next examined the downstream signaling of D1 and D2 receptors. To address this issue, we used striatal slices and examined DARPP-32 phosphorylation at Thr34, a critical downstream step in D1 and D2 receptor signaling (Svenningsson et al., 2004). Treatment of the slices with SKF81297 increased the level of phospho-Thr34 DARPP-32 (Fig. 6A), as previously shown (Nishi et al., 2000). We then examined the effect of indomethacin on the SKF81297-induced increase in this phosphorylation. Treatment of slices with indomethacin significantly attenuated the SKF81297-induced increase in DARPP-32 Thr34 phosphorylation (Fig. 6A), indicating the involvement of endogenously formed PGs in D1 receptor signaling in the striatum. Consistently, when an EP1-selective agonist, ONO-DI-004, was added to the indomethacin-treated slices, ONO-DI-004 restored the SKF81297-induced increase in DARPP-32 Thr34 phosphorylation in a concentration-dependent manner (Fig. 6B), whereas ONO-DI-004 alone did not affect DARPP-32 Thr34 phosphorylation (data not shown).
We next examined the effect of SKF81297 on DARPP-32 phosphorylation in EP1-deficient mice. Treatment of striatal slices with SKF81297 for 2 min stimulated DARPP-32 Thr34 phosphorylation by approximately threefold in slices from both wild-type and EP1-deficient mice. However, the subsequent increase in the Thr34 phosphorylation observed in slices from wild-type mice (by approximately sixfold at 5 min of incubation) was blunted in the slices from EP1-deficient mice (Fig. 6C). These results suggest that SKF81297 stimulates PGE2 production and that the PGE2 produced acts on EP1 to augment D1 receptor signaling in striatal slices in a positive-feedback manner.
Activation of D2 receptors is known to suppress DARPP-32 phosphorylation at Thr34 (Nishi et al., 1997). Consistent with previous reports, treatment of striatal slices with quinpirole decreased DARPP-32 Thr34 phosphorylation in a concentration-dependent manner (Fig. 6D). The ability of quinpirole to decrease DARPP-32 Thr34 phosphorylation was significantly attenuated at 10 nm in slices from EP1-deficient mice compared with that in slices from wild-type mice, although the effects of quinpirole were similar at higher concentration. These results suggest that the PGE2–EP1 signaling augments D2 receptor signaling, leading to the suppression of DARPP-32 phosphorylation at Thr34. Together, our data suggest that EP1 interacts with and augments both D1 and D2 receptor signaling in striatal neurons.
Attenuation of cocaine or D1 agonist-induced DARPP-32 Thr34 phosphorylation in the striatum of EP1-deficient mice in vivo
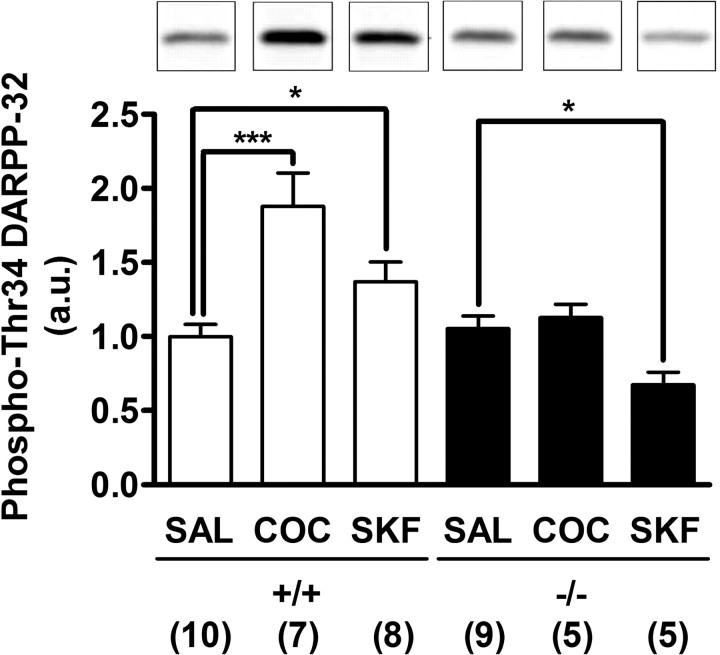
To investigate the role of PGE2–EP1 signaling in the regulation of DARPP-32 phosphorylation in vivo, wild-type and EP1-deficient mice were treated for 15 min with either cocaine (10 mg/kg) or SKF81297 (2.5 mg/kg), conditions in which stimulation of locomotor activity was attenuated in EP1-deficient mice (Figs. 3, 4). In wild-type mice, administration of both cocaine and SKF81297 significantly increased the level of DARPP-32 Thr34 phosphorylation in the striatum (Fig. 7). In contrast, in EP1-deficient mice, administration of cocaine did not increase and administration of SKF81297 decreased DARPP-32 Thr34 phosphorylation. These results indicate that the PGE2–EP1 pathway plays an essential role in the regulation of dopaminergic signaling in the striatum in vivo.
Figure 7.
Attenuation of DARPP-32 Thr34 phosphorylation induced by cocaine or a D1 receptor agonist in the striatum of EP1-deficient mice in vivo. Wild-type (+/+) and EP1-deficient (−/−) mice were injected (intraperitoneally) with vehicle (0.9% sodium chloride) (SAL), cocaine (10 mg/kg) (COC), or a D1 receptor agonist, SKF81297 (2.5 mg/kg) (SKF). Fifteen minutes after drug injection, the mice were decapitated. The striatum was dissected from frozen brain, and the level of phospho-Thr34 DARPP-32 was analyzed. Typical immunoblots for detection of phospho-Thr34 DARPP-32 are shown in the top panels. The data were normalized to values from saline-treated samples in each genotype (a.u., arbitrary units). The numbers of mice used in experiments are shown below each column. Error bars indicate SEM. *p < 0.05; ***p < 0.001.
Discussion
In this study, we used EP1-deficient mice and an EP1-selective agonist, and examined whether the PGE2–EP1 pathway regulates dopaminergic signaling and function in the striatum. We found that EP1 is expressed in more than one-half of MSNs in the striatum, that PGE2 is produced in the striatum in response to stimulation with a D1 or D2 agonist, and that the PGE2–EP1 pathway facilitates both D1 and D2 signaling as assayed by DARPP-32 Thr34 phosphorylation in vitro in striatal slices. The in vivo relevance of these in vitro findings was underscored by significant attenuation of cocaine- or D1 agonist-induced hyperlocomotion, D2 antagonist-induced catalepsy, and cocaine-induced rise of DARPP-32 Thr34 phosphorylation in situ in the striatum in EP1-deficient mice. These findings together strongly suggest that PGE2 functions as a critical modulator of the dopaminergic system in the striatum. Neurotransmitters such as glutamate, GABA, acetylcholine, and serotonin, neuromodulators such as adenosine, and neuropeptides such as opioids and cholecystokinin are known to regulate dopamine signaling in the striatum. However, the role of prostaglandins has been overlooked. Our present findings suggest that PGE2–EP1 signaling should be added to this growing list of regulators of dopaminergic signaling.
PGs including PGE2 are produced from arachidonic acid released from the cell membrane by sequential catalysis of COXs and respective isomerases. There are two COX isoforms, COX-1, which is constitutively expressed, and COX-2, which is induced on demand. Berke et al. (1998) found that COX-2 expression is induced by D1 receptor stimulation in the striatum treated with 6-hydroxydopamine, and suggested that this is part of a genetic adaptation mechanism associated with dopamine depletion. Given that COX-2 expression lasts over hours to days (Smith et al., 2000), the PGE2–EP1 signaling we found as a physiological regulator of dopamine signaling may function also as a long-term adaptive, paracrine-like mechanism to amplify dopamine signaling in the striatum under some pathological conditions.
Why has this seemingly very important modulatory action of PGE2 been overlooked in the dopamine research field? One plausible reason is that COX inhibitors, such as aspirin and indomethacin, are generally without effects on dopaminergic functions such as motor control. These results suggest a possibility of other PG-dependent pathway(s) opposed to the PGE2–EP1 pathway operating in the striatum. Previously, Horton (1964) reported that intracerebroventricular administration of PGE2 resulted in catalepsy in cats, and Schwarz et al. (1982) reported that intrastriatal injection of PGE2 inhibited apomorphine-induced circling in mice. Interestingly, these PGE2 actions appear to antagonize dopamine signaling, opposite to the facilitatory function of the PGE2–EP1 signaling we found here. This may indicate that other EP subtypes that potentially antagonize the effect of EP1 are present in the striatum. The presence of such opposing pathways is seen in PGs working in other systems such as cardiovascular homeostasis and regulation of allergy (Kobayashi et al., 2004; Kunikata et al., 2005).
One of the unique characteristics of the PGE2–EP1 pathway is that this pathway modulates both D1 and D2 signaling positively. Given that there is a very low degree of overlap of D1 and D2 receptor in MSNs (Gerfen et al., 1990; Hersch et al., 1995), and that EP1 is present both in D1 and D2 neurons, it is most likely that the PGE2 acts directly on EP1 of each neuron type, either D1 or D2, and augments its signaling. It is well known that D1 and D2 receptors have opposing actions on the activity of adenylyl cyclase: activation of D1 receptors increases and that of D2 receptors suppresses cAMP formation, and, consequently, Thr34 phosphorylation of DARPP-32 via PKA. Given that activation of EP1 results in a rise in intracellular Ca2+ ion (Watabe et al., 1993) and that a rise in intracellular Ca2+ ion generally induces dephosphorylation of DARPP-32 Thr34 (Svenningsson et al., 2004), the effects of EP1 stimulation on D1 and D2 signaling cannot be readily explained by their signal transduction. An indication as to the mechanism of EP1 action is the fact that effects of the EP1 deficiency can be overridden by increasing concentration of agonists (Figs. 3, 4, 6D) and that such an effect is seen also in the phenotype induced by an antagonist (Fig. 5). These findings suggest that EP1 may modulate ligand-binding activity of D1 and D2 receptors. One possibility is that intracellular Ca2+ increase might affect desensitization of D1 and D2 receptors by suppressing G-protein-coupled receptor kinases as suggested previously for the D1 receptor (Tiberi et al., 1996; Iacovelli et al., 1999). An alternative possibility is that EP1 receptors may modulate D1 and D2 ligand binding via receptor–receptor interaction. It is known that D2 receptors (Fuxe et al., 1998; Rocheville et al., 2000) interact physically with other GPCRs such as the adenosine A2A receptor and the somatostatin SSTR5 receptor, and that hetero-oligomerization modulates ligand binding and signaling of the D2 receptor. Similar oligomer formation may occur between EP1 and D1 and EP1 and D2 receptors to increase the binding affinities of the receptors for dopamine agonists and antagonists. This hypothesis may well explain the features of EP1 action observed here. However, we failed to detect significant differences in binding properties of each type of dopamine receptor between wild-type and EP1-deficient mice. Careful examination of the change of ligand binding activity using heterologous expression systems may reveal modulation of D1 and D2 receptor binding by EP1.
Although we found in this study that EP1 amplifies dopamine signaling, we previously reported that EP1 deficiency increases dopamine turnover in the striatum and the frontal cortex, and that EP1 apparently functions to suppress dopamine-dependent aggressive behavior (Matsuoka et al., 2005). In our preliminary work, we found that EP1 facilitates GABAergic neurotransmission in substantia nigra pars compacta and negatively modulates the activity of dopaminergic neurons (our unpublished observation). EP1 may also function in the orbitofrontal cortex and amygdala, the brain areas implicated in controlling impulsive aggression (Davidson et al., 2000). Localization of EP1 in the latter was already reported (Matsuoka et al., 2003). EP1 might therefore have distinct effects on multiple dopaminoceptive areas, each of which mediate unique motor control, emotional, or cognitive functions.
If EP1 does indeed regulate dopaminergic signaling differently at different sites, it would offer a pharmacological means to modulate various dopaminergic functions in unique patterns. For instance, EP1 agonists may augment dopaminergic activity only in the striatum, thus possibly circumventing psychotic symptoms associated with classical Parkinson therapy using levodopa (Factor et al., 1995). Similarly, treatment of schizophrenic patients with EP1 agonists may suppress dopaminergic functions in the prefrontal cortex and limbic structures without extrapyramidal side effects, which still remain a common complication in antipsychotic therapy (Leucht et al., 2003). Our findings have not only demonstrated that the PGE2–EP1 pathway provides a modulatory mechanism of dopamine function in the striatum but also presented a novel strategic concept in development of drugs modulating dopaminergic pathways.
Footnotes
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, a grant from the National Institute of Biomedical Innovation of Japan, and a grant from ONO Research Foundation. We thank Prof. Takeshi Kaneko (Faculty of Medicine, Kyoto University, Kyoto, Japan) for antibodies to preproenkephalin, Ono Pharmaceutical Company (Osaka, Japan) for ONO-DI-004, Drs. Zhi-Li Huang and Yoshihiro Urade (Osaka Bioscience Institute, Osaka, Japan) for advice on microdialysis experiments, and Drs. Eri Segi and Yasuhiro Tanaka for discussion and comments. We also thank Teruhisa Fujiwara for animal care and breeding and Kimiko Nonomura, Yurie Kitagawa, and Tae Arai for assistance.
References
- Berke JD, Paletzki RF, Aronson GJ, Hyman SE, Gerfen CR. A complex program of striatal gene expression induced by dopaminergic stimulation. J Neurosci. 1998;18:5301–5310. doi: 10.1523/JNEUROSCI.18-14-05301.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi F, Meller F. Enhanced behavioral stereotypies elicited by intrastriatal injection D1 and D2 dopamine agonists in intact rats. Brain Res. 1989;504:276–283. doi: 10.1016/0006-8993(89)91368-1. [DOI] [PubMed] [Google Scholar]
- Charifson PS, Wyrick SD, Hoffman AJ, Simmons RM, Bowen JP, McDougald DL, Mailman RB. Synthesis and pharmacological characterization of 1-phenyl-, 4-phenyl-, and 1-benzyl-1,2,3,4-tetrahydroisoquinolines as dopamine receptor ligands. J Med Chem. 1988;31:1941–1946. doi: 10.1021/jm00118a012. [DOI] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K. Evidence for the existence of monoamine neurons in the central nervous system. I. Demonstration of monoamines in cell bodies of brainstem neurons. Acta Physiol Scand. 1964;64(Suppl):232–278. [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Scammell TE, Saper CB. Mechanisms of CNS responses to systemic immune challenge: the febrile response. Trends Neurosci. 1997;20:565–570. doi: 10.1016/s0166-2236(97)01138-7. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1491. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Factor SA, Molho ES, Podskalny GD, Brown D. Parkinson's disease: drug-induced psychiatric states. Adv Neurol. 1995;65:115–138. [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain—IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978;180:545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Furuyashiki T, Fujisawa K, Fujita A, Madaule P, Uchino S, Mishina M, Bito H, Narumiya S. Citron, a Rho-target, interacts with PSD-95/SAP-90 at glutamatergic synapses in the thalamus. J Neurosci. 1999;19:109–118. doi: 10.1523/JNEUROSCI.19-01-00109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Ferré S, Zoli M, Agnati LF. Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res Rev. 1998;26:258–273. doi: 10.1016/s0165-0173(97)00049-0. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Basal ganglia. In: Paxinos G, editor. The rat nervous system. Ed 3. London: Elsevier; 2004. pp. 455–508. [Google Scholar]
- Gerfen CR, Young WS., III Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988;460:161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Glowinski J, Iversen LL. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966;13:655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci USA. 1996;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Greengard P, Tung HY, Cohen J. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kaneko S, Isobe T, Kitabatake Y, Watanabe D, Pastan I, Nakanishi S. Increased sensitivity to cocaine by cholinergic cell ablation in nucleus accumbens. Proc Natl Acad Sci USA. 2001;98:13351–13354. doi: 10.1073/pnas.231488998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational process. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Horton EW. Actions of prostaglandins E1, E2 and E3 on the central nervous system. Br J Pharmacol. 1964;22:189–192. doi: 10.1111/j.1476-5381.1964.tb01558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovelli L, Sallese M, Mariggiò S, De Blasi A. Regulation of G-protein-coupled receptor kinase subtypes by calcium sensor proteins. FASEB J. 1999;13:1–8. doi: 10.1096/fasebj.13.1.1. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Tahara Y, Matsumoto M, Iguchi M, Sano H, Murayama T, Arai H, Oida H, Yurugi-Kobayashi T, Yamashita JK, Katagiri H, Majima M, Yokode M, Kita T, Narumiya S. Roles of thromboxane A2 and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J Clin Invest. 2004;114:784–794. doi: 10.1172/JCI21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunikata T, Yamane H, Segi E, Matsuoka T, Sugimoto Y, Tanaka S, Tanaka H, Nagai H, Ichikawa A, Narumiya S. Suppression of allergic inflammation by prostaglandin E receptor subtype EP3. Nat Immunol. 2005;6:524–531. doi: 10.1038/ni1188. [DOI] [PubMed] [Google Scholar]
- Lee T, Kaneko T, Taki K, Mizuno N. Preprodynorphin-, preproenkephalin-, and preprotachykinin-expressing neurons in the rat neostriatum: an analysis by immunocytochemistry and retrograde tracing. J Comp Neurol. 1997;386:229–244. doi: 10.1002/(sici)1096-9861(19970922)386:2<229::aid-cne5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Leucht S, Wahlbeck K, Hamann J, Kissling W. New generation antipsychotics versus low-potency conventional antipsychotics: a systematic review and meta-analysis. Lancet. 2003;361:1581–1589. doi: 10.1016/S0140-6736(03)13306-5. [DOI] [PubMed] [Google Scholar]
- List SJ, Seeman P. Resolution of dopamine and serotonin receptor components of [3H]spiperone binding to rat brain regions. Proc Natl Acad Sci USA. 1981;78:2620–2624. doi: 10.1073/pnas.78.4.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Furuyashiki T, Bito H, Ushikubi F, Tanaka Y, Kobayashi T, Muro S, Satoh N, Kayahara T, Higashi M, Mizoguchi A, Shichi H, Fukuda Y, Nakao K, Narumiya S. Impaired adrenocorticotropic hormone response to bacterial endotoxin in mice deficient in prostaglandin E receptor EP1 and EP3 subtypes. Proc Natl Acad Sci USA. 2003;100:4132–4137. doi: 10.1073/pnas.0633341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Furuyashiki T, Yamada K, Nagai T, Bito H, Tanaka Y, Kitaoka S, Ushikubi F, Nabeshima T, Narumiya S. Prostaglandin E receptor EP1 controls impulsive behavior under stress. Proc Natl Acad Sci USA. 2005;102:16066–16071. doi: 10.1073/pnas.0504908102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Nestler E. Is there a common molecular pathway for addiction. Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Nishi A, Snyder GL, Greengard P. Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J Neurosci. 1997;17:8147–8155. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Bibb JA, Snyder GL, Higashi H, Nairn AC, Greengard P. Amplification of dopaminergic signaling by a positive feedback loop. Proc Natl Acad Sci USA. 2000;97:12840–12845. doi: 10.1073/pnas.220410397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. The ring test: a quantitative method for assessing the “cataleptic” effect of cannabis in mice. Br J Pharmacol. 1972;46:753–763. doi: 10.1111/j.1476-5381.1972.tb06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Powell WS. Rapid extraction of oxygenated metabolites of arachidonic acid from biological samples using octadecylsilyl silica. Prostaglandins. 1980;20:947–957. doi: 10.1016/0090-6980(80)90144-6. [DOI] [PubMed] [Google Scholar]
- Rocheville M, Lange DC, Kumar U, Patel SC, Patel RC, Patel YC. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science. 2000;288:154–157. doi: 10.1126/science.288.5463.154. [DOI] [PubMed] [Google Scholar]
- Schwarz RD, Uretsky NJ, Bianchine JR. Prostaglandin inhibition of apomorphine-induced circling in mice. Pharmacol Biochem Behav. 1982;17:1233–1237. doi: 10.1016/0091-3057(82)90126-5. [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Girault JA, Chen JY, Czernik AJ, Kebabian JW, Nathanson JA, Greengard P. Phosphorylation of DARPP-32 and protein phosphatase inhibitor-1 in rat choroid plexus: regulation by factors other than dopamine. J Neurosci. 1992;12:3071–3083. doi: 10.1523/JNEUROSCI.12-08-03071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Tiberi M, Nash SR, Bertrand L, Lefkowitz RJ, Caron MG. Differential regulation of dopamine D1A receptor responsiveness by various G protein-coupled receptor kinases. J Biol Chem. 1996;271:3771–3778. doi: 10.1074/jbc.271.7.3771. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, Kobayashi T, Hizaki H, Tuboi K, Katsuyama M, Ichikawa A, Tanaka T, Yoshida N, Narumiya S. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature. 1998;395:281–284. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- Watabe A, Sugimoto Y, Honda A, Irie A, Namba T, Negishi M, Ito S, Narumiya S, Ichikawa A. Cloning and expression of cDNA for a mouse EP1 subtype of prostaglandin E receptor. J Biol Chem. 1993;268:20175–20178. [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Xu M, Moratalla R, Gold LH, Hiroi N, Koob GF, Graybiel AM, Tonegawa S. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell. 1994;79:729–742. doi: 10.1016/0092-8674(94)90557-6. [DOI] [PubMed] [Google Scholar]