Abstract
Dorsal stream visual processing is generally considered to underlie visually driven action, but when subjects grasp an object from memory, as visual information is not available, ventral stream characteristics emerge. In this study we use paired-pulse transcranial magnetic stimulation (TMS) to investigate the importance of the current visual input during visuomotor grasp. Previously, the amplitude of the paired-pulse motor evoked potentials (MEPs) in hand muscles before movement onset have been shown to predict the subsequent pattern of muscle activity during grasp. Specific facilitation of paired-pulse MEPs may reflect premotor–motor (PMC–M1) cortex connectivity. Here we investigate the paired-pulse MEPs evoked under memory-cued and visually driven conditions before grasping one of two possible target objects (a handle or a disc). All trials began with a delay period of 1200 ms. Then, a TMS pulse served as the cue to reach, grasp and hold the target object for 0.5 s. Total trial length was 5 s. Both objects were continually visible in both conditions, but the way in which the target object was designated differed between conditions. In the memory-cued condition, the target object was illuminated for the first 200 ms of the trial only. In the visually driven condition, the target object was illuminated throughout the 5 s trial. Thus, the conditions differed in whether or not the object to be grasped was designated at the time of movement initiation. We found that the pattern of paired-pulse MEP facilitation matched the pattern of object-specific muscle activity only for the visually driven condition. The results suggest that PMC–M1 connectivity contributes to action selection only when immediate sensory information specifies which action to make.
Keywords: transcranial magnetic stimulation, corticospinal, motor cortex, dorsal visual stream, grasping, affordance
Introduction
We can select the target object and initiate grasp based on memory for which object we wanted to retrieve, or we can make a similar movement in reaction to a visual cue that specifies the object. Different cortical structures are thought to underlie grasping under these two conditions. Whereas visually cued grasp appears to depend on “dorsal stream” cortical structures, memory-cued grasp involves the “ventral stream” (Milner and Goodale, 1995). In the premotor cortex (PMC), part of the dorsal stream, neurones are typically active in response to visual cues, whereas in the supplementary motor area (SMA), part of the ventral stream, neurons discharge for movements performed from memory, without visual guidance (Halsband et al., 1994). Even a short delay between visual presentation and motor response can disrupt on-line guidance via the dorsal stream (Westwood and Goodale, 2003). However, in these experiments in the memory-guided condition there was no visual input at the time of movement initiation. It is therefore uncertain whether the absence of visual information or grasping from memory is crucial in the ventral-dorsal stream dissociation. Here, we investigate whether the role of putative PMC–motor cortext (M1) connections depends on whether a grasping action is selected on the basis of current visual input, or from memory for a previously cued object.
Paired-pulse transcranial magnetic stimulation (TMS) has been used to examine putative PMC–M1 cortex connections before grasping (Cattaneo et al., 2005; Prabhu et al., 2007). The corticocortical inputs facilitated by paired-pulse TMS over M1 are thought to include those from PMC activated by visual presentation of graspable objects (Ziemann et al., 1998; di Lazzaro et al., 1999; Cerri et al., 2003; Shimazu et al., 2004; Cattaneo et al., 2005; Prabhu et al., 2007). This results in motor evoked potentials (MEPs) whose sizes predict the differential muscle activation required to grasp different objects (Cattaneo et al., 2005). The facilitation is related to grasp preparation, because it is absent when subjects just look at the object, or prepare arbitrary hand movements with equivalent muscle patterns (Cattaneo et al., 2005; Prabhu et al., 2007).
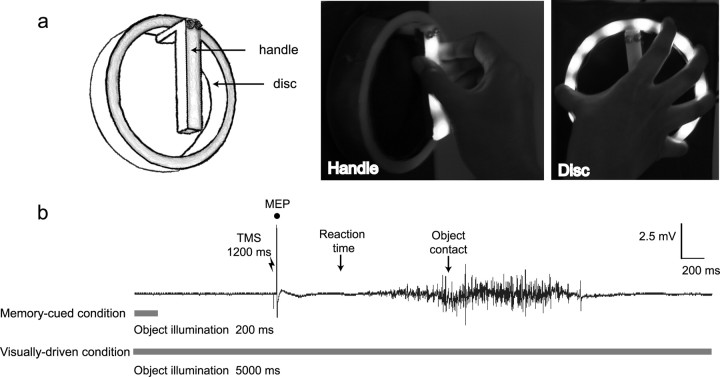
In the current experiment we compared the effects of designating the object to be grasped either by continuous visual information or by memory cues. Based on previous work (Hasbroucq et al., 1997, 1999; Touge et al., 1998; Cattaneo et al., 2005; Prabhu et al., 2007), we predicted that the PMC–M1 network would facilitate paired-pulse MEPs relative to single-pulse MEPs during grasp preparation in an object and muscle specific manner. Because the “dorsal stream” is preferentially involved in on-line visuomotor control, we predicted that such facilitation would be stronger when current visual input, as opposed to memory cues, specified which object to grasp. Two objects, a handle and a disc (see Fig. 1A), were continually visible. All trials began with a delay period of 200 ms. In the memory-cued condition the target object was illuminated for 200 ms only at the start of the delay period. Subjects had to remember the target object when the illumination ceased, and prepare to grasp it. A “go” signal instructing them to begin the grasping movement occurred 1200 ms after the start of the trial. In the visually driven condition, the target object was illuminated throughout the trial. Therefore, the current visual input designated the target object at the time of the go signal, making reliance on memory unnecessary (Fig. 1B). Only in the visually driven condition was there object-specific modulation of the MEPs. The results are in favor of PMC–M1 connectivity being modulated only when on-line sensory information is available.
Figure 1.
A, B, Target objects (A) and muscle activity recorded from the ADM of the right-hand illustrating the experimental protocol (B). Subjects were seated in a dimly lit room. Two objects, a handle and a disc were visible throughout the experiment (A). LEDs embedded in the objects enabled them to be illuminated independently. The target object was illuminated for 200 ms after the start of the trial in the visually driven block, or remained illuminated throughout the trial (5 s) in the visually driven block. Subjects were asked to fixate on the target object and grasp the object as soon as the go cue, TMS at 1200 ms, was delivered. During the intertrial interval (3 s), there was no object illumination.
Materials and Methods
Subjects.
Twelve right-handed healthy volunteers (eight female, four male; mean age, 25.7 years; SD, ±4.85) participated in this study after giving informed written consent. The experiment was performed in compliance with relevant institutional guidelines and approved by the local ethics committee.
Transcranial magnetic stimulation.
TMS pulses were delivered using two Magstim (Whitland, UK) 200 stimulators through one figure-of-eight TMS coil (7 cm diameter). The coil handle was at 45° to the midline, pointing laterally and backwards with a posterior current. Stimuli were applied to the “hotspot” on the scalp over the left primary motor cortex characterized as where a low threshold MEP could be evoked from both first dorsal interosseous (1DI) and abductor digiti minimi (ADM) of the right-hand. Stimuli were either single-pulse [130% resting motor threshold (RMT)] (Rossini et al., 1994) or paired-pulse (130% and 90% RMT for the first and second stimulus, respectively). The interstimulus interval (ISI) for paired-pulse TMS was 2.5 ms, which had been found previously to show object by muscle facilitation (Cattaneo et al., 2005; Prabhu et al., 2007). MEP and EMG activity were recorded using bipolar (belly tendon) surface EMG electrodes on the two muscles. EMG was sampled at 4 kHz and high-pass filtered in hardware with a cutoff of 3 Hz.
Experimental procedure.
Subjects were seated in a dimly lit room with their right-hand resting pronated on a waist level home pad, to the right of the body midline. In front of the subjects were two Perspex objects mounted on a vertical board, a ring (12 cm diameter, 2 cm deep) inside which was a vertically orientated handle (9 cm high, 5 cm deep) (Fig. 1A). Computer controlled light-emitting diodes were embedded in the objects allowed each object to be independently illuminated. Touch sensitive electronic circuits were used to measure the times of home-pad release and object contact.
The experiment consisted of two counterbalanced blocks of 40 trials. Trials started with object illumination, either for 200 ms, in one block, or 5 s, for the other block (Fig. 1B). The sound and sensation of TMS delivered 1200 ms after object illumination served as a cue to reach out and grasp whichever object was or had been illuminated. There were 10 trials per object for both single-pulse and paired-pulse stimulation. Objects and TMS stimulus conditions were presented in random order. Subjects were asked to fixate on the target object and grasp the object as soon as the GO signal was delivered. Before the experiment, subjects performed a training block of 12 trials in which they were given feedback on premovement EMG levels and reaction time (RT). Training was repeated until subjects could perform the task satisfactorily.
Data analysis.
EMG activity from each grasp trial was high-pass filtered in software with a cutoff of 40 Hz, and then rectified. Integrated EMG activity was calculated for the hand preshaping phase, 300 ms preceding object contact. EMG activity for each trial was normalized to that subject's average EMG in that muscle across grasp of both objects. Normalizing relative to the pooled-object EMG in this way meant that EMG values for the handle were no longer independent from EMG values for the disc. Therefore, to test for specific involvement of each muscle in grasping each object, we performed paired t tests on each block comparing ADM (handle) and 1DI (handle). To test whether EMG varied between the visually driven and memory-cued conditions, an ANOVA was performed with the additional within-subjects factor of cue type.
To measure RT, the timing of home-pad release was calculated for all combinations of object and TMS stimulus conditions. Repeated measures ANOVAs were performed using the within-subjects factors of cue type (visually driven vs memory-cued) and object (handle vs disc).
EMG traces for each trial were visually inspected, and trials with detectable background EMG during the 100 ms before TMS were rejected. The criterion level for detecting background EMG was generally set to 0.15 mV peak-to-peak, but was occasionally reduced to a more conservative level of 0.09 mV when there was evidence of any sustained EMG activity on a particular trial. A second rater independently reinspected a subset of 40 trials, and the agreement between the two raters was 100%. Peak-to-peak amplitude of the MEPs was measured for all combinations of object, muscle, TMS stimulus and illumination conditions. Statistical analysis was performed on a facilitation ratio (paired-pulse MEP/single-pulse MEP) calculated within subjects for each object and muscle. An ANOVA was performed for the within-subjects factors of cue type (visually driven vs memory-cued), object, and muscle and then for each muscle for the factor of cue type. Percentage MEP facilitation values were used to illustrate the contributions of single- and paired-pulse TMS to the MEP facilitation ratio. To provide a measure of object specificity, we divided each subject's average MEP for each object by the sum of the average MEP for both objects, and expressed this as a percentage. A value of 50% indicates no object specification. This measure was calculated separately for each muscle, cue type, and TMS condition (single- and paired-pulse).
Results
Muscle activity during preshaping of the hand clearly differed when grasping the handle compared with the disc (Fig. 2B). ADM, which abducts and flexes the little finger, showed strong activity for the disc, and minimal activity for the handle, whereas activity in 1DI, which abducts and flexes the index finger, did not vary between the two grasps (object by muscle interaction, p < 0.001). Muscle activity and RTs did not differ between visually driven and memory-cued conditions (all p values >0.1). The mean RT in the visually driven condition was 386 ± 110 (SD) ms compared with 373 ± 129 ms for the memory-cued condition.
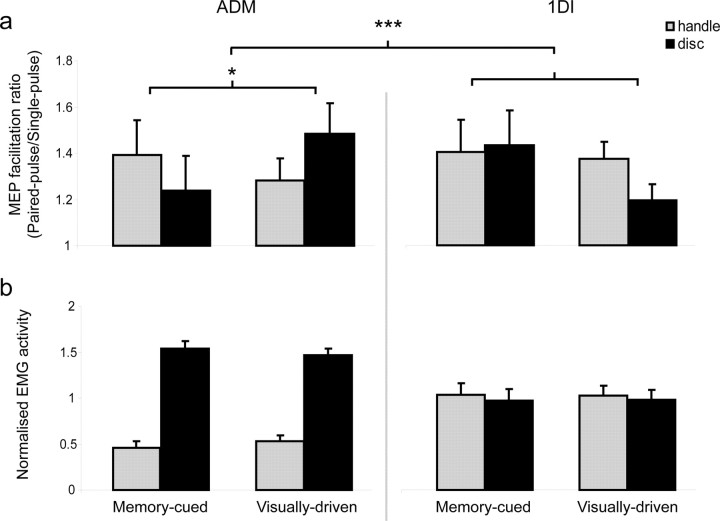
Figure 2.
A, B, Average MEP facilitation (A) and normalized average EMG activity (B) from the two blocks (n = 12 subjects). A, Paired-pulse (ISI, 2.5 ms)/single-pulse MEP facilitation ratio for both muscles in visually driven and memory-cued conditions. B, Integrated rectified EMG activity during hand preshaping during the 300 ms preceding object contact. EMG levels were normalized across conditions for each subject to remove individual differences in mean EMG level and highlight differences between conditions. *p < 0.05; ***p < 0.001.
MEP facilitation at the time of the go signal, 200–640 ms before grasp, predicted the subsequent muscle activation pattern in the visually driven condition, but not in the memory-cued condition (object by muscle by cue type interaction, p < 0.001). Thus, in the visually driven condition, ADM showed an increase of the paired-pulse/single-pulse facilitation ratio before grasping the disc compared with the handle (object by cue type interaction, p < 0.05) (Fig. 2A). In contrast, facilitation of the ADM MEP was reduced when grasping the disc compared with the handle for the memory-cued condition. 1DI activity was comparable when grasping the disc and the handle, and MEP facilitation ratios did not vary significantly with object and cue type (object by cue type interaction p > 0.09). Thus, although the grasp-related muscle activity was similar in visually driven and memory-cued conditions, ADM MEP facilitation only reflected the subsequent muscle activation when there was visual specification of the object at the time of the cue to grasp. This change in MEP facilitation ratio before grasping arose from a contrasting pattern of paired- and single-pulse responses in the visually driven condition.
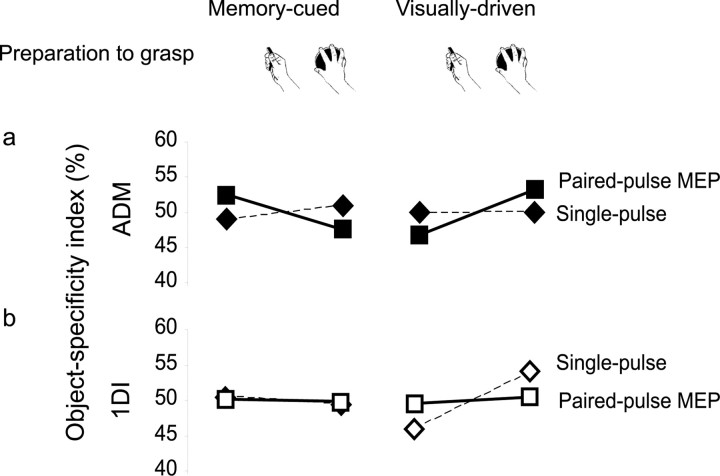
To reveal this pattern more clearly, we computed an object-specificity index for each combination of TMS condition, muscle, and cue type. We divided the average MEP for each object by the average MEP for both objects, and expressed the result as a percentage. Notice that these indices must sum to 100%, and the hypothesis of no object specificity predicts an index value of 50%. Because the index values for the disc and handle are perfectly inversely correlated, we performed ANOVA analysis on the object-specificity index for the disc only, using factors of muscle, cue type, and TMS condition. Only the three-way interaction of these factors was significant (p = 0.003). Follow-up two-way ANOVA showed no effects for 1DI (all p > 0.165), but an interaction between cue type and TMS condition for ADM (p = 0.001). This arose because paired-pulse MEPs in ADM showed greater object specificity in the direction of the subsequent grasp-related EMG activity in visually driven than in the memory-guided condition (paired t test, p = 0.021). Single-pulse MEPs showed a nonsignificant effect in the opposite direction (p > 0.5). To summarize, object-specific MEP facilitation was restricted to paired-pulse TMS stimulation of ADM when the target object was visually designated throughout the period before grasping.
Discussion
Previously, we suggested that paired-pulse TMS at specific interstimulus intervals reflected excitability of cortical-cortical inputs to the M1 hand area during motor preparation (Cattaneo et al., 2005; Prabhu et al., 2007). In those studies, the object-specific pattern of muscle activity for an impending grasp was related to the pattern of MEP facilitation during preparation. These new results show that this modulation occurs only if the object is specified by current visual input at the moment of grasp initiation. In contrast, when subjects have to remember the target object, even for only 1 s, a different neural network for motor preparation seems to be used, and the relation between MEP facilitation and impending EMG is broken. The patterns of MEP facilitation in our visually guided conditions were again highly object specific, because they were only seen in ADM. This muscle showed object-specific EMG activity in the subsequent grasp, for both visually guided and memory-guided conditions. The modulation involved suppression of the single-pulse MEP in ADM, coupled with a facilitation of the MEP to paired-pulse TMS (Fig. 3) in visually guided conditions only. Our result suggests that the predictive relation between MEP facilitation and muscle activity applies only in specific sensory conditions of sustained visual input. Crucially, these sensory conditions are consistent with the known properties of the dorsal visuomotor stream in general, and the premotor cortex in particular. Therefore, our result is consistent with the view that paired-pulse MEP facilitation reflects interactions between PMC and MI (Cattaneo et al., 2005), although it does not conclusively prove that PMC is involved.
Figure 3.
Object-specific facilitation of MEPs before memory-cued and visually driven grasp. An index of object specificity is calculated by dividing the average MEP for each object by the average MEP for both objects, and expressing the result as a percentage. Notice that these indices must sum to 100%, and the hypothesis of no object specificity predicts a value of 50%. Data are shown for single- (dashed line, diamond symbols) and paired-pulse (solid line, squares) TMS, for ADM (upper row) and 1DI (lower row). Note that object specificity of the MEP follows object specificity of EMG (Fig. 2) only for paired-pulse MEPs in the ADM muscle in the visually driven condition.
Neuronal recording studies of object coding in ventral PMC (PMv) (Murata et al., 1997; Raos et al., 2006; Umilta et al., 2007) are consistent with facilitation of the paired-pulse MEPs for grasping objects (Cattaneo et al., 2005; Prabhu et al., 2007). A large proportion of PMv neurones recorded in nonhuman primates show object- and grasp-specific firing peaks both on initial presentation of the target object, followed by a further peak of firing on movement initiation (Murata et al., 1997; Umilta et al., 2007). This phasic pattern suggests PMv does not maintain a “memory” of the target object, but relies on its continued visibility throughout the delay period, similar to the visually driven condition tested here. Previously, we have shown that task-related modulation of the paired-pulse MEP was abolished when stimulation was 400 ms or earlier from the imperative cue or if the time of the go signal was unpredictable (Prabhu et al., 2007). This indicates that the visuomotor grasping circuit does not modulate M1 outputs throughout the period from object presentation until the moment of grasp execution. Rather, inputs to M1 that facilitate grasp show raised excitability in the period immediately before grasp is executed. We suggest that the parietal-premotor circuit may prepare and then maintain grasp motor programs during the delay period, forwarding them to primary motor cortex only at the time they are finally needed for action. Our results suggest that these processes required sustained visual representation of the grasped object. Other premotor structures, such as the dorsal PMC (Wise and Mauritz, 1985) and SMA (Halsband et al., 1994) may be involved in memory-cued grasp. Indeed, both ablation (Passingham, 1988) and single-unit selectivity (Halsband et al., 1994) studies support the view of two independent circuits for motor preparation converging on the primary motor cortex as a common path for motor execution (Sherrington 1947). A circuit based on the dorsal premotor cortex would be used for externally cued action, whereas a circuit based on the SMA may be used for internally generated or memory-guided action. Our result is consistent with this dissociation.
The absence of a task-related facilitation of the MEPs in the memory-cued condition cannot be explained by lack of visual information about the object, because both handle and disc were continuously visible. Nor could sustained illumination in the visually driven condition influence MEPs indirectly, for example by modulating attention. Although, the sustained illumination in our visually driven condition could lead to subjects being more aroused, or attending more selectively to the target object, than in the memory-cued condition, for two reasons we think this is unlikely. First, any such effect should produce shorter RT in visually driven than in memory-cued conditions. A within-subjects repeated-measures ANOVA with factors of cue type and object showed no significant main effects or interaction (all p values > 0.1). Second, attentional effects cannot easily explain the stronger suppression of the single-pulse MEP in visually driven compared with memory-cued conditions. Instead, our results suggest a distinction between two modes of selection for object-oriented action: an “internally guided” mode in which stored memories specify which action to make, and a visually driven mode which selects actions on the basis of current sensory information. Our results show task-related enhancement of putative PMC–M1 connectivity for visually driven but not memory-based grasp preparation. Premotor–motor connectivity reflects immediate information about an object and its affordance, but does not maintain this information, even over short intervals. In our study, a 1 s delay between object specification and action was sufficient to abolish the object-specific paired-pulse effects.
Footnotes
This work was supported by the Biotechnology and Biological Science Research Council. We thank Thomas Brochier, Ed Bye and Victor Baller.
References
- Cattaneo L, Voss M, Brochier T, Prabhu G, Wolpert DM, Lemon RN. A cortico-cortical mechanism mediating object-driven grasp in humans. Proc Natl Acad Sci USA. 2005;102:898–903. doi: 10.1073/pnas.0409182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerri G, Shimazu H, Maier MA, Lemon RN. Facilitation from ventral premotor cortex of primary motor cortex outputs to macaque hand muscles. J Neurophysiol. 2003;90:832–842. doi: 10.1152/jn.01026.2002. [DOI] [PubMed] [Google Scholar]
- di Lazzaro V, Rothwell JC, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P. Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli applied to human motor cortex. Exp Brain Res. 1999;129:494–499. doi: 10.1007/s002210050919. [DOI] [PubMed] [Google Scholar]
- Halsband U, Matsuzaka Y, Tanji J. Neuronal activity in the primate supplementary, pre-supplementary and premotor cortex during externally and internally instructed sequential movements. Neurosci Res. 1994;20:149–155. doi: 10.1016/0168-0102(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Kaneko H, Akamatsu M, Possamai CA. Preparatory inhibition of cortico-spinal excitability: a transcranial magnetic stimulation study in man. Brain Res Cogn Brain Res. 1997;5:185–192. doi: 10.1016/s0926-6410(96)00069-9. [DOI] [PubMed] [Google Scholar]
- Hasbroucq T, Kaneko H, Akamatsu M, Possamai CA. The time-course of preparatory spinal and cortico-spinal inhibition: an H-reflex and transcranial magnetic stimulation study in man. Exp Brain Res. 1999;124:33–41. doi: 10.1007/s002210050597. [DOI] [PubMed] [Google Scholar]
- Milner AD, Goodale MA. Oxford UP: 1995. The visual brain in action. [Google Scholar]
- Murata A, Fadiga L, Fogassi L, Gallese V, Raos V, Rizzolatti G. Object representation in the ventral premotor cortex (area F5) of the monkey. J Neurophysiol. 1997;78:2226–2230. doi: 10.1152/jn.1997.78.4.2226. [DOI] [PubMed] [Google Scholar]
- Passingham RE. Premotor cortex and preparation for movement. Exp Brain Res. 1988;70:590–596. doi: 10.1007/BF00247607. [DOI] [PubMed] [Google Scholar]
- Prabhu G, Voss M, Brochier T, Cattaneo L, Haggard P, Lemon R. Excitability of human motor cortex inputs prior to grasp. J Physiol (Lond) 2007;581:189–201. doi: 10.1113/jphysiol.2006.123356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raos V, Umilta MA, Murata A, Fogassi L, Gallese V. Functional properties of grasping-related neurons in the ventral premotor area F5 of the macaque monkey. J Neurophysiol. 2006;95:709–729. doi: 10.1152/jn.00463.2005. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, Maertens de Noordhout A, Marsden CD, Murray NMF, Rothwell J, Swash M, Tomberg C. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Sherrington C. Cambridge, UK: Cambridge UP; 1947. The integrative action of the nervous system. [Google Scholar]
- Shimazu H, Maier MA, Cerri G, Kirkwood PA, Lemon RN. Macaque ventral premotor cortex exerts powerful facilitation of motor cortex outputs to upper limb motoneurons. J Neurosci. 2004;24:1200–1211. doi: 10.1523/JNEUROSCI.4731-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touge T, Taylor JL, Rothwell JC. Reduced excitability of the cortico-spinal system during the warning period of a reaction time task. Electroencephalogr Clin Neurophysiol. 1998;109:489–495. doi: 10.1016/s0924-980x(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Umilta MA, Brochier TG, Spinks RL, Lemon RN. Simultaneous recording of macaque premotor and primary motor cortex neuronal populations reveals different functional contributions to visuomotor grasp. J Neurophysiol. 2007 doi: 10.1152/jn.01094.2006. in press. [DOI] [PubMed] [Google Scholar]
- Westwood DA, Goodale MA. Perceptual illusion and the real-time control of action. Spat Vis. 2003;16:243–254. doi: 10.1163/156856803322467518. [DOI] [PubMed] [Google Scholar]
- Wise SP, Mauritz KH. Set-related neuronal activity in the premotor cortex of rhesus monkeys: effects of changes in motor set. Proc R Soc Lond B Biol Sci. 1985;223:331–354. doi: 10.1098/rspb.1985.0005. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol (Lond) 1998;511:181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]