Abstract
The neurobiological investigation of the placebo effect has shown that placebos can activate the endogenous opioid systems in some conditions. So far, the impact of this finding has been within the context of the clinical setting. Here we present an experiment that simulates a sport competition, a situation in which opioids are considered to be illegal drugs. After repeated administrations of morphine in the precompetition training phase, its replacement with a placebo on the day of competition induced an opioid-mediated increase of pain endurance and physical performance, although no illegal drug was administered. The placebo analgesic responses were obtained after two morphine administrations that were separated as long as 1 week from each other. These long time intervals indicate that the pharmacological conditioning procedure has long-lasting effects and that opioid-mediated placebo responses may have practical implications and applications. For example, in the context of the present sport simulation, athletes can be preconditioned with morphine and then a placebo can be given just before competition, thus avoiding administration of the illegal drug on the competition day. However, these morphine-like effects of placebos raise the important question whether opioid-mediated placebo responses are ethically acceptable in sport competitions or whether they have to be considered a doping procedure in all respects.
Keywords: placebo, pain, opioids, conditioning, sport, doping
Introduction
The recent advances in the neurobiology of the placebo effect have shown that the administration of a placebo (inert substance), along with verbal suggestions of clinical benefit, activates different neurotransmitters in the brain, like endogenous opioids (Levine et al., 1978; Amanzio and Benedetti, 1999; Zubieta et al., 2005; Wager et al., 2007) and dopamine (de la Fuente-Fernandez et al., 2001; Strafella et al., 2006), and is associated to neural changes at both the cortical and subcortical level (Petrovic et al., 2002; Benedetti et al., 2004; Wager et al., 2004; Kong et al., 2006; Matre et al., 2006; Price et al., 2007). Powerful placebo responses can be obtained after pharmacological preconditioning, whereby the repeated administration of a drug is replaced with an inert substance (Ader and Cohen, 1982; Benedetti et al., 2005; Colloca and Benedetti, 2005; Pacheco-Lopez et al., 2006). For example, the morphine-like effects of placebos after morphine preconditioning have been shown in the context of pain management (Amanzio and Benedetti, 1999).
Although these drug-like effects of placebos represent an interesting phenomenon in the clinical setting, they also have implications that have been ignored so far. One of these has to do with the use of drugs in sport competitions to boost physical performance. Among performance-boosting drugs, morphine is known to be a powerful analgesic that increases tolerance to pain, thereby improving physical performance [World Anti-Doping Agency (WADA), www.wada-ama.org]. The importance of opioid-mediated placebo responses consists in the fact that they can be exploited when one wants morphine-like effects without giving morphine. For example, in the context of pain management, it has been shown that morphine administration for 2 d in a row may induce robust placebo analgesic responses when morphine is replaced with a placebo on the third day (Amanzio and Benedetti, 1999). This raises the important question whether two morphine administrations separated several days or weeks from each other have similar powerful effects on subsequent placebo responses.
In sport competitions, this is particularly important because, according to the Prohibited Drugs List 2007 of the WADA, drugs can be divided into those that are prohibited at all times and those that are prohibited only during competition. For example, morphine is considered to be an illegal drug only during competition, whereas its use out of competition is legal. Therefore, one could conceive a precompetition conditioning with morphine and then its replacement with a placebo on the day of competition.
On the basis of these considerations, in the present study we simulated a sport competition, whereby four teams of 10 subjects each had to compete with each other in a competition of pain endurance. The four teams underwent different training procedures, with and without morphine, and then their performance on the day of competition was assessed. The possibility of evoking morphine-like, opioid-mediated, placebo responses during sport competitions highlights the impact of the neurobiological approach to the placebo effect on an important aspect of our society.
Materials and Methods
Subjects.
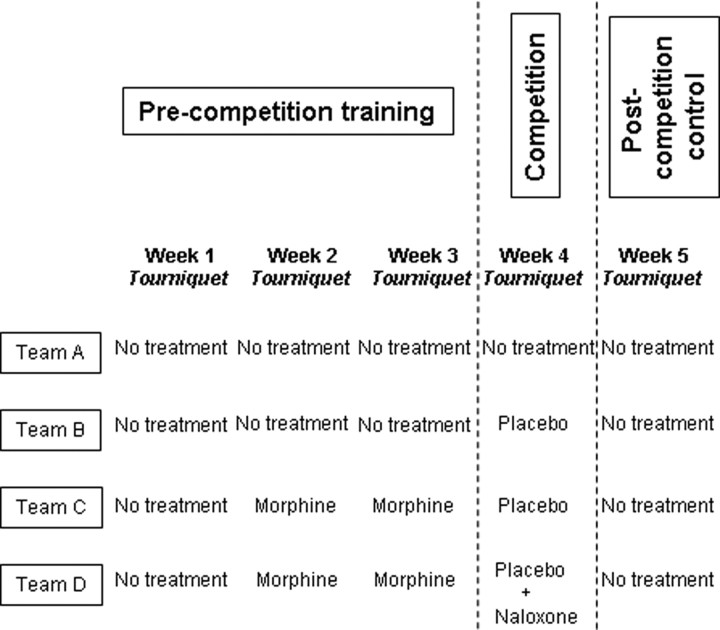
The subjects were healthy males who agreed to participate in one of the experimental groups after they signed an informed consent form in which the details of the experiment, including the drugs to be administered, were explained. In particular, the subjects were told that either morphine or naloxone would be administered at a given time, depending on the experimental group. None of them were training as a competitive athlete, but all the subjects engaged in recreational fitness training. As shown in Figure 1, we randomly assigned 10 subjects to team A (mean age, 24 ± 2.5 years; mean weight, 73.4 ± 4.1 kg; mean height, 178.4 ± 7.2 cm), 10 to team B (mean age, 23.4 ± 3.2 years; mean weight, 71.8 ± 6.3 kg; mean height, 177.1 ± 6.5 cm), 10 to team C (mean age, 24.5 ± 3.6 years; mean weight, 72.7 ± 5.8 kg; mean height, 176.5 ± 7.9 cm), and 10 to team D (mean age, 23.8 ± 2.6 years; mean weight, 72 ± 4.7 kg; mean height, 177.7 ± 6.9 cm). In Figure 1, it can also be seen that each team underwent a specific training procedure, as described in detail below.
Figure 1.
Experimental simulation of the competition. The precompetition training, the competition day, and the postcompetition control are shown for each group.
One week before the beginning of the precompetition training sessions, the subjects underwent a clinical examination, including an electrocardiogram, to ascertain their physical conditions and to rule out main diseases. All the subjects were informed that they had to abstain from consuming coffee, tea, and caffeine-containing drinks for 48 h before each training session, as well as alcohol and any medication. Before beginning each session, the subjects were given a standardized meal, which consisted of orange juice, toast and muffins, whose energy content was ∼2000 KJ.
Drugs and double-blind procedure.
Intramuscular morphine was given to team C and D 1 h before the two training sessions on weeks 2 and 3 at a dose of 0.14 mg/kg, and the subjects were told that an increase in pain tolerance was expected. Intramuscular naloxone was given to team D 1 h before the competition on week 4 at a dose of 0.14 mg/kg, but the subjects did not know that there was naloxone in the syringe. Drugs were administered according to a randomized double-blind design in which neither the subject nor the experimenter knew what drug was being administered. To do this, either the active drug or saline solution was given. To avoid a large number of subjects, two or three additional subjects per group received an intramuscular injection of saline in place of the active drug 1 h before the tourniquet. These subjects were not included in the study because they were used only to allow the double-blind design, as described previously by Benedetti et al. (2003, 2006). Importantly, naloxone has been shown not to affect this kind of experimental pain (Amanzio and Benedetti, 1999).
Precompetition training.
Each training session was performed once a week and consisted of a test of pain tolerance. Pain was induced experimentally by means of the submaximal effort tourniquet technique, according to the procedures described by Amanzio and Benedetti (1999) and Benedetti et al. (2006). Briefly, the subject reclined on a bed, his or her nondominant forearm was extended vertically, and venous blood was drained by means of an Esmarch bandage. A sphygmomanometer was placed around the upper arm and inflated to a pressure of 300 mmHg. The Esmarch bandage was maintained around the forearm, which was lowered on the subject's side. After this, the subject started squeezing a hand spring exerciser 12 times while his or her arm rested on the bed. Each squeeze was timed to last 2 s, followed by a 2 s rest. The force necessary to bring the handles together was 7.2 kg. This type of ischemic pain increases over time very quickly, and the pain becomes unbearable after ∼14 min (Amanzio and Benedetti, 1999; Benedetti et al., 2006). All the subjects were told that they had to tolerate the tourniquet test as long as possible and that on the day of competition their tolerance time would be averaged with those of the other subjects of the same team. The winner was the team that showed the highest mean tolerance time. To make the subjects tolerate the pain as long as possible, the tolerance times were taken with steps of 30 s (15, 15.5, 16, 16.5, 17, 17.5 min, and so on), and the subjects were told that they had to complete a full step to increase their scores. In other words, if a subject resisted 16 min and 29 s, his tolerance time was 16, whereas if he resisted 16 min and 31 s, his tolerance time was 16.5.
Team A underwent a precompetition training without the use of any pharmacological substance (Fig. 1). The subjects of this team were trained once a week with the submaximal effort tourniquet test. They had to resist as much as possible and the training was repeated three times for 3 weeks in a row. Team B was trained in the same way as team A. In contrast, team C was trained with morphine. In fact, the subjects of this team received morphine intramuscularly 1 h before the training session, and this procedure was run once a week for 2 weeks in a row in the precompetition phase (Fig. 1). Team D underwent exactly the same precompetition training procedure as team C.
Competition.
On the day of the competition (Fig. 1), team A tried to tolerate the tourniquet test as much as possible, as it did in the precompetition phase. In contrast, team B was given a placebo (saline solution; intramuscularly) 1 h before the competition, along with the verbal suggestions that it was morphine. Thus, team B expected an increase in pain tolerance. Team C was given the same placebo as team B, along with the verbal suggestions that it was the same morphine of the previous weeks. Thus, the difference between team C and B was that team C was preconditioned with morphine in the precompetition phase whereas team B was not. Team D received a placebo as well. However, in the syringe there was naloxone, but the subjects were told that it was the same morphine of the previous weeks. A pain tolerance test was also performed 1 week after the competition (Fig. 1) to see whether everything returned to the precompetition baseline.
Statistical analysis.
As the experimental design involves both a between- and a within-subjects design, statistical analysis was performed by means of one way ANOVA and ANOVA for repeated measures, followed by the post hoc Student–Newman–Keuls test for multiple comparisons and Dunnett test for comparisons between a control group and different experimental groups. In addition, correlations were performed by using linear regression analysis. Comparisons between regression lines was performed by means of the global coincidence test and a slope comparison t test. Data are presented as mean ± SD and the level of significance is p < 0.05.
Results
By averaging the tolerance times across the subjects, the “winner” was team C, as the mean pain tolerance on the day of competition was 20.8 ± 3.3 min, whereas it was 16.7 ± 2.5 min for team B, 15.7 ± 1.7 min for team A, and 15.4 ± 2.9 min for team D. The raw data are shown in Tables 1–4 for each group along with the within-group analysis. As shown in Figure 2, placebo administration on the day of competition produced an increase in pain tolerance both in teams B (post hoc ANOVA Student–Newman–Keuls, q(36) = 7.503; p < 0.01) and C (q(36) = 16.878; p < 0.001), but the morphine preconditioned team C showed a larger placebo effect than team B (F(1,18) = 9.81; p < 0.007). Therefore, morphine preconditioning was crucial for inducing the largest placebo responses. In team C, the effect of the placebo was smaller than that of morphine (q(36) = 6.631; p < 0.01).
Table 1.
Tolerance times (in minutes) for each subject and statistical analysis within groups for team A
| Subject | Precompetition training |
Competition |
Postcompetition |
||
|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 (control) | |
| 1 | 13 | 14.5 | 14 | 16 | 11 |
| 2 | 11.5 | 13 | 14 | 14 | 12 |
| 3 | 10 | 10.5 | 13 | 14.5 | 12 |
| 4 | 18 | 17 | 16.5 | 17.5 | 15 |
| 5 | 19.5 | 18.5 | 18 | 19.5 | 17 |
| 6 | 17 | 17 | 18.5 | 16.5 | 15 |
| 7 | 15 | 16.5 | 15.5 | 17 | 15.5 |
| 8 | 15.5 | 14 | 14.5 | 16 | 13 |
| 9 | 13 | 13.5 | 11 | 14 | 12 |
| 10 | 14 | 12.5 | 11.5 | 13.5 | 10.5 |
| Mean ± SD | 14.6 ± 2.9 | 14.7 ± 2.5 | 14.6 ± 2.5 | 15.7 ± 1.7 | 13.3 ± 2.2 |
Repeated-measures ANOVA across the 5 weeks: F(4,36)= 6.892; p < 0.001. Student–Newman–Keuls: week 5 versus week 1, q(36)= 4.074, p < 0.05; week 5 versus week 2, q(36)= 4.225, p < 0.05; week 5 versus week 3, q(36)= 4.074, p < 0.05; week 5 versus week 4, q(36)= 7.394, p < 0.01. The other comparisons are not significant.
Table 2.
Tolerance times (in minutes) for each subject and statistical analysis within groups for team B
| Subject | Precompetition training |
Competition |
Postcompetition |
||
|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 (placebo) | Week 5 (control) | |
| 1 | 17.5 | 17 | 17 | 18.5 | 13 |
| 2 | 13 | 14.5 | 14 | 16 | 15 |
| 3 | 11.5 | 12 | 14.5 | 16.5 | 12.5 |
| 4 | 12.5 | 13.5 | 15 | 15.5 | 13 |
| 5 | 18 | 19 | 18 | 21 | 16.5 |
| 6 | 10 | 12 | 11 | 14 | 12.5 |
| 7 | 13 | 13 | 12.5 | 13.5 | 11 |
| 8 | 11.5 | 13 | 11.5 | 14.5 | 10 |
| 9 | 19 | 19.5 | 17 | 19.5 | 15.5 |
| 10 | 16.5 | 17.5 | 17 | 18.5 | 16 |
| Mean ± SD | 14.2 ± 3.2 | 15.1 ± 2.9 | 14.7 ± 2.5 | 16.7 ± 2.5 | 13.5 ± 2.2 |
Repeated-measures ANOVA across the 5 weeks: F(4,36)= 13.202, p < 0.001. Student–Newman–Keuls: week 4 versus week 1, q(36)= 7.503, p < 0.01; week 2 versus week 5, q(36)= 4.802, p < 0.05; week 4 versus week 2, q(36)= 4.952, p < 0.05; week 3 versus week 5, q(36)= 3.751, p < 0.05; week 4 versus week 3, q(36)= 6.002, p < 0.01; week 4 versus week 5, q(36)= 9.754, p < 0.01. The other comparisons are not significant.
Table 3.
Tolerance times (in minutes) for each subject and statistical analysis within groups for team C
| Subject | Precompetition training |
Competition |
Postcompetition |
||
|---|---|---|---|---|---|
| Week 1 | Week 2 (morphine) | Week 3 (morphine) | Week 4 (placebo) | Week 5 (control) | |
| 1 | 15 | 25 | 27 | 22 | 13 |
| 2 | 14 | 22 | 23 | 21 | 13 |
| 3 | 18 | 27.5 | 27 | 24 | 16.5 |
| 4 | 11.5 | 18 | 21 | 16.5 | 13 |
| 5 | 11 | 19.5 | 19 | 17.5 | 10.5 |
| 6 | 17.5 | 28.5 | 27.5 | 24 | 15 |
| 7 | 16 | 28 | 29 | 26.5 | 14 |
| 8 | 11.5 | 17.5 | 20.5 | 18 | 11 |
| 9 | 13 | 22 | 21.5 | 19.5 | 12 |
| 10 | 10.5 | 20 | 20 | 19 | 11 |
| Mean ± SD | 13.8 ± 2.7 | 22.8 ± 4.2 | 23.5 ± 3.7 | 20.8 ± 3.3 | 12.9 ± 1.9 |
Repeated-measures ANOVA across the 5 weeks: F(4,36)= 148.791; p < 0.001. Student–Newman–Keuls: week 3 versus week 1, q(36)=23.509, p < 0.001; week 2 versus week 4, q(36)=4.822, p < 0.05; week 3 versus week 4, q(36)=6.631, p < 0.01; week 2 versus week 5, q(36)=23.871, p < 0.001; week 3 versus week 5, q(36)=25.679, p < 0.001; week 4 versus week 1, q(36)=16.878, p < 0.001; week 2 versus week 1, q(36)=21.701, p < 0.001; week 4 versus week 5, q(36)=19.049, p < 0.001. The other comparisons are not significant.
Table 4.
Tolerance times (in minutes) for each subject and statistical analysis within groups for team D
| Subject | Precompetition training |
Competition |
Postcompetition |
||
|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 (placebo plus naloxone) | Week 5 (control) | |
| 1 | 18.5 | 28 | 29 | 19 | 17 |
| 2 | 11 | 22 | 20 | 12 | 11 |
| 3 | 14 | 23.5 | 21.5 | 15 | 10.5 |
| 4 | 17.5 | 27.5 | 27 | 17 | 16 |
| 5 | 10 | 19 | 18.5 | 12 | 11 |
| 6 | 12.5 | 21 | 20 | 14 | 12 |
| 7 | 16.5 | 27 | 28.5 | 18 | 15.5 |
| 8 | 19 | 25 | 26 | 19.5 | 18 |
| 9 | 11.5 | 18 | 18 | 12 | 11 |
| 10 | 15 | 22.5 | 21.5 | 16 | 14 |
| Mean ± SD | 14.5 ± 3.2 | 23.3 ± 3.5 | 23 ± 4.2 | 15.4 ± 2.9 | 13.6 ± 2.8 |
Repeated-measures ANOVA across the 5 weeks: F(4,36)=173.045; p < 0.001. Student–Newman–Keuls: week 3 versus week 1, q(36)= 23.255, p < 0.001; week 2 versus week 4, q(36)= 21.742, p < 0.001; week 3 versus week 4, q(36)= 20.779, p < 0.001; week 2 versus week 5, q(36)= 26.833, p < 0.001; week 3 versus week 5, q(36)= 25.870, p < 0.001; week 4 versus week 5, q(36)= 5.091, p < 0.01; week 2 versus week 1, q(36)= 24.219; p < 0.001. The other comparisons are not significant.
Figure 2.
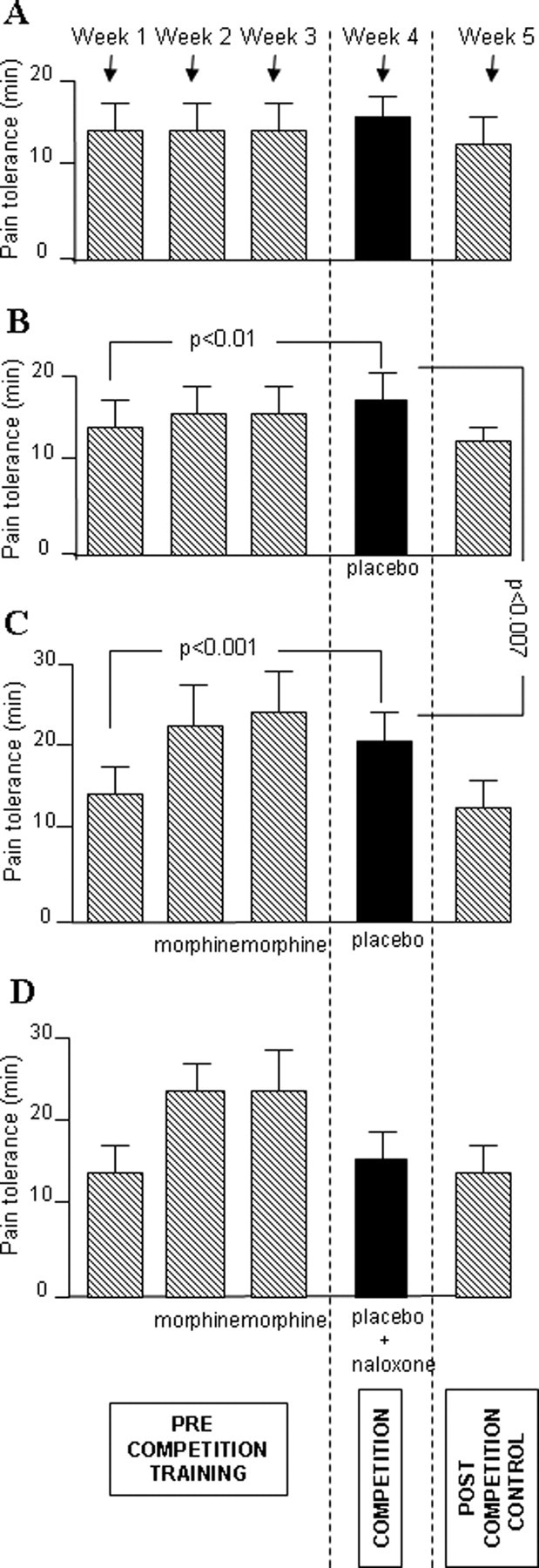
A–D, Mean pain endurance times (±SD) in the precompetition training phase, on the day of competition, and in the postcompetition control. It can be seen that, on the day of competition, a significant increase in performance occurred after placebo administration compared with baseline in both groups B and C, whereas in group A and D, no increase in performance occurred. The performance of team C was significantly better than that of group B.
Although team D received morphine preconditioning whereas team A and B did not, no difference was present between team D and A and between team D and B, as shown by the between-groups analysis in Table 5. Similarly, in team D there was no significant increase in tolerance time on the day of competition compared with baseline. Thus, naloxone abolished the morphine preconditioning effects, which indicates the activation of endogenous opioids after placebo administration. Figure 2 also shows that tolerance times returned to the precompetition baseline in all cases.
Table 5.
Statistical analysis of the mean tolerance times ± SD between groups on the day of competition
| Team C (20.8 ± 3.3) vs team A (15.7 ± 1.7) | F(1,18)=18.88; p < 0.001 |
| Team C vs team B (16.7 ± 2,5) | F(1,18)=9.81; p < 0.007 |
| Team C vs team D (15.4 ± 2.9) | F(1,18)=15.11; p < 0.002 |
The other comparisons are not significant.
In teams C and D, we also measured the percentage increase in performance after morphine administration and after placebo administration, and correlated the individual increases in performance after placebo with those after morphine (Fig. 3). A correlation was present in team C for both the first (r = 0.781; p < 0.008) and second (r = 0.752; p < 0.015) morphine administration, and this correlation was modified by naloxone in team D. In fact, in team D, although a correlation was present for both the first (r = 0.641; t(8) = 2.363; p < 0.05) and the second (r = 0.655; t(8) = 2.458; p < 0.04) morphine injection, there was a significant difference between the regression lines of group C and D for the first morphine injection (global coincidence test, F(2,16) = 90.212, p < 0.001; slope comparison, t(16) = 2.620, p < 0.02) and the second morphine injection (global coincidence test, F(2,16) = 55.278, p < 0.001; slope comparison, t(16) = 2.070, p < 0.05). To summarize, the larger the performance increase after morphine, the larger the performance increase after placebo. This correlation, albeit present, was changed by naloxone.
Figure 3.
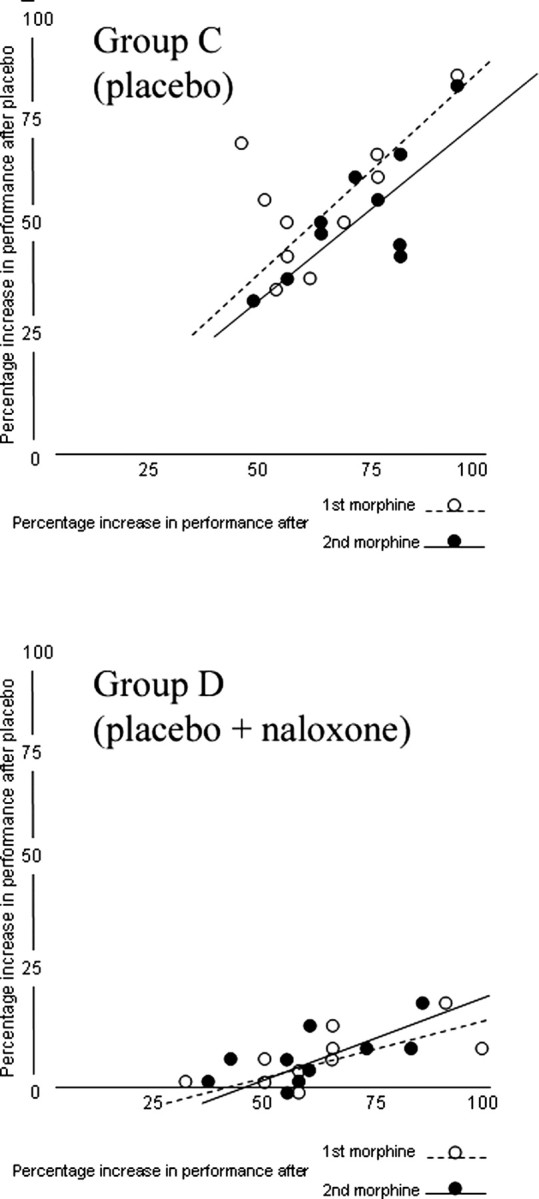
The correlation between the percentage increase in performance after the first and second injection of morphine and the percentage increase in performance after placebo in group C and D. A correlation was present after placebo administration in group C, and this correlation was modified by naloxone in group D.
Discussion
The present study demonstrates that a pharmacological preconditioning, with morphine given twice at intervals as long as 1 week, can induce robust placebo analgesic responses when morphine is replaced with a placebo. It should also be noted that placebo administration without previous morphine conditioning (team B) induced a small but significant increase in pain endurance, which indicates smaller effects when a placebo is given for the first time compared with its administration after pharmacological conditioning. In a previous study (Amanzio and Benedetti, 1999), we showed that the administration of morphine for two consecutive days may induce substantial placebo responses when the placebo is given on the third day. Thus the present study shows that long time lags between two consecutive administrations of morphine and the administration of the placebo are not very different from short time lags, at least in the range of days/weeks. This indicates that the pharmacological conditioning procedure has long-lasting effects.
The occurrence of placebo analgesic responses after these long time intervals of morphine administration represents an important aspect of placebo responsiveness. In fact, as already shown in a nonpharmacological conditioning paradigm (Colloca and Benedetti, 2006), conditioning effects may last several days. Therefore, the role of previous experience in placebo responsiveness appears to be very important and substantial: only two exposures to morphine, once a week, are enough to affect the magnitude of placebo analgesia.
It should be noted that, whereas the mean placebo response across all subjects showed a complete blockade by naloxone (Fig. 2D), a detailed analysis of the percentage increase in performance showed that a correlation between morphine and placebo was still present after naloxone treatment, albeit altered (Fig. 3). This suggests the possible contribution of nonopioid mechanisms in the placebo response.
The power of pharmacological preconditioning on placebo responsiveness has of course very practical implications and applications, not only in the context of pain management, as previously investigated in detail (Amanzio and Benedetti, 1999), but also on several aspects of our society. In the present study we wanted to simulate one of these social aspects, i.e., sport, whereby the problem of reproducing morphine-like effects without morphine administration represents a very important and timely topic. In fact, according to the procedure we used in our experiments, a performance-boosting drug might be given before competition and the drug-mimicking effects of placebo exploited during competition, thus avoiding the administration of the illegal drug on the day of competition. Although we did not assess the plasma concentration of morphine on the day of competition after placebo administration, the short half-life of morphine warrants that neither drug nor its metabolites were present 1 week after the last administration of morphine. Therefore, an anti-doping test would have been negative.
In light of the distinction between drugs that are prohibited during and/or out of competition, the preconditioning procedure may be deemed ethical and legal for drugs that are prohibited only during competition, like morphine. In fact, according to the Prohibited Drugs List 2007 of the WADA (www.wada-ama.org), the training procedures of teams C and D should be considered legal because athletes are allowed to assume narcotics out of competition. However, they could also be considered illegal because morphine administration was aimed at conditioning the subjects for subsequent replacement with a placebo, which was supposed to show morphine-like effects during the competition. In addition, it will be crucial to understand whether those drugs that are prohibited at all times, both during and out of competition, show similar effects on placebo responsiveness.
In addition to the mechanisms of placebo responsiveness and the preconditioning effects of morphine, this study raises important ethical questions: do opioid-mediated placebo effects during competitions have to be considered a doping procedure? Should we consider morphine conditioning in the training phase ethical and legal? This issue is not easy to be resolved and will need both an ethical and legal discussion. Although we are aware that the experimental conditions of the present study do not represent a real competitive event, but a pain challenge paradigm, the increase in pain endurance after the placebo is real and robust and has key attributes relevant to situations encountered in sport competitions. For example, our model of tonic ischemic arm pain represents a long-lasting painful stimulation that is likely to be encountered in real long-lasting sport activities. Therefore, if the conditioned subjects of this study engaged in a real sport activity, they would tolerate pain for a longer time.
From both an ethical and a semantic perspective, it is worth emphasizing that the present work, with its experimental approach and its legal/ethical implications, shows how the neurobiological approach to the investigation of the placebo effect is paying dividends, both as new knowledge of its mechanisms and as implications for the clinic and the society. Doping is a matter of great public concern today, and we should be aware that, if a procedure like that described in the present study is performed, illegal drugs in sport would be neither discoverable nor would they violate the antidoping rules.
Footnotes
This work was supported by grants from Istituto San Paolo di Torino and from Regione Piemonte.
References
- Ader R, Cohen N. Behaviorally conditioned immunosuppression and murine systemic lupus erythematosus. Science. 1982;215:1534–1536. doi: 10.1126/science.7063864. [DOI] [PubMed] [Google Scholar]
- Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19:484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious expectation and unconscious conditioning in analgesic, motor and hormonal placebo/nocebo responses. J Neurosci. 2003;23:4315–4323. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Colloca L, Torre E, Lanotte M, Melcarne A, Pesare M, Brgamasco B, Lopiano L. Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat Neurosci. 2004;7:587–588. doi: 10.1038/nn1250. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK. Neurobiological mechanisms of the placebo effect. J Neurosci. 2005;25:10390–10402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Amanzio M, Vighetti S, Asteggiano G. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci. 2006;26:12014–12022. doi: 10.1523/JNEUROSCI.2947-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, Benedetti F. Placebos and painkillers: is mind as real as matter? Nat Rev Neurosci. 2005;6:545–552. doi: 10.1038/nrn1705. [DOI] [PubMed] [Google Scholar]
- Colloca L, Benedetti F. How prior experience shapes placebo analgesia. Pain. 2006;124:126–133. doi: 10.1016/j.pain.2006.04.005. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science. 2001;293:1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26:381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Gordon NC, Fields HL. The mechanisms of placebo analgesia. Lancet. 1978;2:654–657. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- Matre D, Casey KL, Knardahl S. Placebo-induced changes in spinal cord pain processing. J Neurosci. 2006;26:559–563. doi: 10.1523/JNEUROSCI.4218-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Lopez G, Engler H, Niemi MB, Schedlowski M. Expectations and associations that heal: immunomodulatory placebo effects and its neurobiology. Brain Behav Immun. 2006;20:430–446. doi: 10.1016/j.bbi.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia: imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127:63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Ko JH, Monchi O. Therapeutic application of transcranial magnetic stimulation in Parkinson's disease: the contribution of expectation. NeuroImage. 2006;31:1666–1672. doi: 10.1016/j.neuroimage.2006.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn KL, Rose RM, Cohen JD. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta JK. Placebo effects on human {micro}-opioid activity during pain. Proc Natl Acad Sci USA. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on μ-opioid receptors. J Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]