A phenomenon familiar to clinicians treating patients with Parkinson's disease (PD) is kinesia paradoxica: astonishing displays of sudden mobility and agility by otherwise akinetic PD patients in instances of emergency (for instance, the case, perhaps apocryphal, in which an immobile patient suddenly leapt from his wheelchair to save a drowning man). Recently, Mazzoni et al. (2007) in their paper in The Journal of Neuroscience showed that such “normal” motor behavior amid the general motor symptoms of Parkinson's disease is not confined to extreme cases. Mazzoni et al. (2007) asked PD patients and healthy controls to move their arm to a prespecified target at different speeds [Mazzoni et al. (2007), their Fig. 1a (http://www.jneurosci.org/cgi/content/full/27/27/7105/F1)]. The accuracy and peak velocity of the movements were monitored and reported to the subjects after each trial [Mazzoni et al. (2007), their Fig. 1b,c (http://www.jneurosci.org/cgi/content/full/27/27/7105/F1)], with target distance and required speed range varying between experimental blocks. To complete a block subjects had to perform 20 movements within the required range. The question that interested Mazzoni et al. (2007) was whether the profound slowing of movement (bradykinesia) observed in PD might result from impaired decision-making and action selection, rather than from compensation for reduced accuracy of rapid movements.
This simple and elegant design revealed a multitude of interesting results. First, like healthy controls, PD patients were able to make movements at the required velocity in each condition. Moreover, when the accuracy and the kinematics of the 20 movements that met criteria were examined, PD patients were no different from healthy controls [Mazzoni et al. (2007), their Figs. 2 (http://www.jneurosci.org/cgi/content/full/27/27/7105/F2), 3 (http://www.jneurosci.org/cgi/content/full/27/27/7105/F3), 4 (http://www.jneurosci.org/cgi/content/full/27/27/7105/F4)].
In fact, a comparison of the accuracies of all movements of similar velocities (valid and invalid) from different blocks confirmed that patients were, in general, as accurate as controls [Mazzoni et al. (2007), their Fig. 5 (http://www.jneurosci.org/cgi/content/full/27/27/7105/F5)]. This result demonstrates convincingly that Parkinson's patients can display motor behavior that matches that of healthy controls in both speed and accuracy, even in mundane situations such as moving an arm to reach an arbitrary target.
However, Mazzoni et al. (2007) did find one major difference: PD patients made significantly more slow movements before reaching the criterion of 20 movements within the required speed range. This difference was most apparent when the task was difficult, that is, in blocks that required high speed relative to a small distance (rapid acceleration and deceleration of movement). For example, for a 6 cm target and a velocity requirement of 37–57 cm/s, PD patients made, on average, over 20 invalid (too slow) movements, whereas controls made only 10 [Mazzoni et al. (2007), their Fig. 6 http://www.jneurosci.org/cgi/content/full/27/27/7105/F6)]. Thus, although PD patients were capable of performing high-velocity movements, they performed these actions with lower probability. Figure 1 shows, for each block, the probability distributions from which movement velocities were chosen, as estimated from the peak velocities of all movements (valid and invalid). Although spanning the same range of velocities, the probability distribution used by PD patients was shifted to the left (i.e., it assigned higher probability to slower speeds) compared with that of controls.
Figure 1.
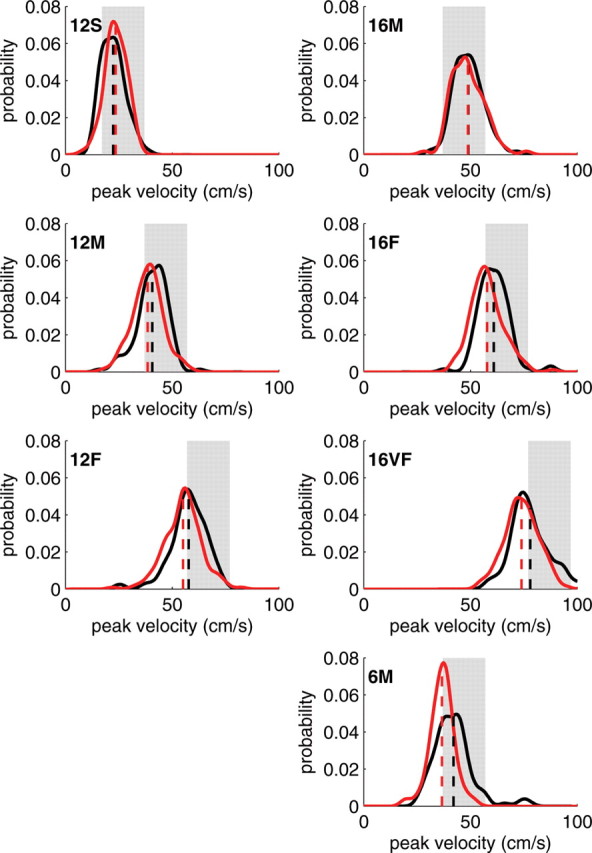
Velocity probability distributions of Parkinson's patients are shifted toward slower movements in all but the easiest conditions. Probability distribution functions for each condition were estimated using kernel density estimation based on all movements (valid and invalid) (see Mazzoni et al., 2007), and normalized [for non-normalized histograms, see Mazzoni et al. (2007), their Fig. 7 (http://www.jneurosci.org/cgi/content/full/27/27/7105/F7)]. In red are the estimated distributions for PD patients, in black are those for healthy controls. Shaded areas mark the required range of velocities in each condition [slow (S), 17–37 cm/s; medium (M), 37–57 cm/s; fast (F), 57–77 cm/s; very fast (VF), 77–97 cm/s], and numbers in bold (6,12, or 16) denote the distance to target in centimeters. Dashed line, Empirical mean. Data are courtesy of Dr. Pietro Mazzoni (Mazzoni et al., 2007)
If the slowing of behavior is not a result of compensation in terms of a speed-accuracy tradeoff, and if Parkinson's patients are physically able to perform the required actions at no loss of accuracy, why do they seem to “choose” to behave more slowly?
Parkinson's disease has long been associated with the progressive death of midbrain dopaminergic neurons and a consequent lack of dopamine in the basal ganglia, specifically in areas of the striatum that are implicated in motor control and movement initiation. More recently, dopamine in the basal ganglia has been tightly linked to reinforcement learning via reward prediction error signals (Montague et al., 1996). Can a reinforcement learning deficiency cause motor slowing?
Based on their experimental results, and building on a previous reinforcement learning model of response speed (Niv et al., 2007), Mazzoni et al. (2007) hypothesized that the slowness of movement in PD may be attributable to higher sensitivity to movement energy costs as a result of loss of dopamine. However, according to the model, optimal choice of movement time (or action latency) should take into account not only motor and energetic costs, but also the “opportunity cost” of devoting time to one action and not to others. This suggests another possible source of bradykinesia: because the steady-state net rate of rewards per unit time quantifies the cost of time, the model shows that decreasing the net rate of rewards will influence the pace of all actions such that they will be performed more slowly. Importantly, it has been suggested that the net rate of rewards is represented by tonic levels of dopamine in the striatum (Niv et al., 2007). Thus, loss of dopamine may cause bradykinesia not through a speed/accuracy tradeoff, but rather by affecting decision making through an enhancement of the costs of movement or through a distorted value of time itself.
Mazzoni et al.'s (2007) study highlights an important characteristic of dopamine-dependent decision making, namely, its implicit nature. Subjects were given full instructions and feedback, allowing them to explicitly (or consciously) appreciate that faster responding would, overall, reduce both time and motor efforts. Despite this, the dopamine-dependent cost/benefit computation “chose” slower responses, which had the detrimental effect of necessitating more movements.
Interestingly, the tendency to perform actions that are too slow was more apparent in difficult conditions [Mazzoni et al. (2007), their Fig. 9 (http://www.jneurosci.org/cgi/content/full/27/27/7105/F9)], in line with results from dopamine-depleted rats, which showed more prominent response-rate impairments for more difficult tasks (Salamone and Correa, 2002). This may reflect stronger reliance on implicit (“habitual”) dopamine-dependent mechanisms of response choice when tasks are more difficult, whereas simple tasks may be more amenable to explicit (“goal-directed”) mechanisms that are relatively dopamine-independent. It may also reflect a learning deficiency: daily movements typically fall within a certain comfortable range of accelerations and velocities, with which subjects are likely to have had much experience. The difficult conditions were less similar to highly practiced movements, perhaps necessitating online learning through feedback. Because this learning presumably relies on dopaminergic signaling in the basal ganglia (Montague et al., 1996), impaired learning in PD patients may explain why they performed worse in these conditions.
To shed additional light on the relationship between dopamine depletion and bradykinesia, it would be interesting to study how behavior in this paradigm differs between patients in their on and off therapy states, and in different stages of the disease. This is especially pressing given that the patients in this study were in the early stages of the disease [Mazzoni et al. (2007), their Table 1 (http://www.jneurosci.org/cgi/content/full/27/27/7105/T1)], and half were taking dopamine agonists at the time of testing. Of course, abnormal neural activity is observed in the basal ganglia even with dopamine replacement treatment (Heimer et al., 2006), complicating interpretations regarding the role of dopamine. Another question for future research is whether PD also affects the profile of motor actions such that there is a more limited repertoire of movements available to PD patients [as suggested by Figs. 3 (http://www.jneurosci.org/cgi/content/full/27/27/7105/F3), 4c,d (http://www.jneurosci.org/cgi/content/full/27/27/7105/F4) from Mazzoni et al. (2007)], perhaps as a result of less “exploratory” behavior caused by loss of dopamine.
In summary, Mazzoni et al. (2007) provide evidence that the movement deficits characteristic of Parkinson's disease reflect not a purely motor impairment, but rather an aberrant implicit decision-making process. Indeed, parkinsonism has been described previously as a “paralysis of the will”; it is not that PD patients cannot move, it is that their dopamine circuitry does not “want” to.
Footnotes
Editor's Note: These short reviews of a recent paper in the Journal, written exclusively by graduate students or postdoctoral fellows, are intended to mimic the journal clubs that exist in your own departments or institutions. For more information on the format and purpose of the Journal Club, please see http://www.jneurosci.org/misc/ifa_features.shtml.
This work was supported by the Human Frontiers Science Program (Y.N.) and by a Ms. Lily Safra Interdisciplinary Center for Neural Computation graduate student fellowship (M.R.). We are grateful to Pietro Mazzoni, Peter Dayan, Nathaniel Daw, and Hagai Bergman for helpful comments.
References
- Heimer G, Rivlin-Etzion M, Bar-Gad I, Goldberg JA, Haber SN, Bergman H. Dopamine replacement therapy does not restore the full spectrum of normal pallidal activity in the 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine primate model of parkinsonism. J Neurosci. 2006;26:8101–8114. doi: 10.1523/JNEUROSCI.5140-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni P, Hristova A, Krakauer JW. Why don't we move faster? Parkinson's disease, movement vigor, and implicit motivation. J Neurosci. 2007;27:7105–7116. doi: 10.1523/JNEUROSCI.0264-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci. 1996;16:1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology (Berl) 2007;191:507–520. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]


