Abstract
A variety of observations suggest that decreasing glycolysis and increasing levels of reduced glutathione, generated by metabolism of glucose through the pentose phosphate pathway, would have an anticonvulsant effect. Because fructose-1,6-bisphosphate (F1,6BP) shifts the metabolism of glucose from glycolysis to the pentose phosphate pathway, it was hypothesized to have anticonvulsant activity. The anticonvulsant activity of F1,6BP was determined in rat models of acute seizures induced by pilocarpine, kainic acid, or pentylenetetrazole. The efficacy of F1,6BP was compared with that of 2-deoxyglucose (2-DG; an inhibitor of glucose uptake and glycolysis), valproic acid (VPA), and the ketogenic diet. One hour before each convulsant, Sprague Dawley rats received either saline (as seizure controls), F1,6BP (0.25, 0.5 or 1 g/kg), 2-DG (0.25 or 0.5 g/kg), or VPA (0.3 g/kg). Additional animals received the ketogenic diet (starting at 20 or 60 d old). Time to seizure onset, seizure duration, and seizure score were measured in each group. F1,6BP had dose-dependent anticonvulsant activity in all three models, whereas VPA had partial efficacy. 2-DG was only effective in the pilocarpine model. The ketogenic diet had no effect in these models. F1,6BP was also partially effective when given at the first behavioral seizure after pilocarpine. Administration of sodium lactate, which bypasses the block in the glycolytic pathway, abolished the anticonvulsant activity of 2-DG in the pilocarpine model, but only decreased the efficacy of F1,6BP. These data demonstrate that F1,6BP has significant anticonvulsant efficacy.
Keywords: fructose-1,6-bisphosphate; seizures; pentose phosphate pathway; 2-deoxyglucose; ketogenic diet; valproic acid
Introduction
Glucose is the primary source of energy for the CNS. Imaging of children with Lennox–Gastaut and infantile spasms has shown decreased glucose utilization between seizures and excessive glycolysis immediately before, and during, seizures (Chugani and Chugani, 1999). In addition, a cerebral deficit in the reduced form of glutathione (GSH), which is an important free radical scavenger in the mammalian nervous system (Wu et al., 2004) and an endogenous anticonvulsant (Abe et al., 2000), has been shown in patients with partial seizures (Mueller et al., 2001). Oxidized glutathione is reduced by NADPH generated in the pentose phosphate pathway. The pentose phosphate pathway is an alternative pathway for glucose metabolism that generates NADPH for use in reductive biosynthesis.
Evidence suggests that the changes in glucose metabolism and decreased glutathione levels observed in the brains of patients with epilepsy favor the generation of each seizure. First, hyperglycemia has been associated with seizure activity (Schwechter et al., 2003; Lammouchi et al., 2004), whereas relative hypoglycemia has been shown to have an anticonvulsant effect (Greene et al., 2001). Second, the ketogenic diet (KD), which provides energy substrates for the brain that bypass glycolysis, has been shown to be an effective treatment for seizures (Freeman et al., 2007). Finally, animals with low levels of GSH have a low seizure threshold or spontaneous seizures (Wu et al., 2004).
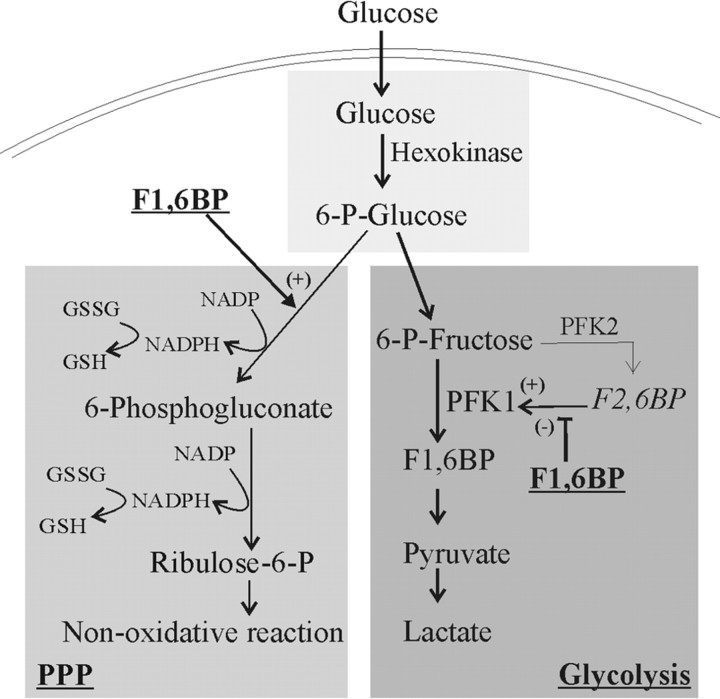
Fructose-1,6-bisphosphate (F1,6BP) has actions that suggest it may be an effective anticonvulsant (see Fig. 1). First, F1,6BP has been shown to increase flux of glucose into the pentose phosphate pathway (Kelleher et al., 1995; Espanol et al., 1998) and preserve cellular GSH levels (Vexler et al., 2003). Second, F1,6BP modulates the activity of phosphofructokinase-1 (PFK-1), which is the enzyme that controls the rate-limiting step in glycolysis. F1,6BP is a weak stimulator of PFK-1, but becomes inhibitory in the presence of fructose-2,6-bisphosphate (F2,6BP), a potent activator of PFK-1 (Heylen et al., 1982; Van Schaftingen, 1987). These data suggest that F1,6BP will slightly enhance basal glucose metabolism, but will prevent stimulation of glycolysis by F2,6BP. Diverting glucose from glycolysis toward the pentose phosphate pathway, thus increasing GSH levels while maintaining an energy source for the brain, should provide significant anticonvulsant efficacy.
Figure 1.
Schematic illustration of glucose utilization through the glycolytic and the pentose phosphate pathways. The sites of action for F1,6BP are indicated. P, Phosphate; PPP, the pentose phosphate pathway; (+), stimulatory activity to the pathway or the enzyme; (−), inhibitory activity.
This study determined the anticonvulsant activity of F1,6BP in three rat models of acute seizures. The efficacy of F1,6BP was compared with the efficacy of 2-deoxyglucose (an inhibitor of glucose uptake and glycolysis), the ketogenic diet, which decreases glycolysis by forcing the body to use fat instead of glucose, and valproate (VPA), a commonly prescribed anticonvulsant drug.
Materials and Methods
All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication 8023; revised 1996) and with the approval of the local Animal Use Committee. Unless indicated, all chemicals were obtained from Sigma (St. Louis, MO). Male Sprague Dawley rats weighing 50–74 g (young) or 147–309 g (adult) were used in this study. Acute seizures were induced by kainic acid (KA; Ocean Produce, Shelburne, Nova Scotia, Canada), pilocarpine, or pentylenetetrazole (PTZ) as described previously (Lian et al. 2006). These models were chosen because they initiate seizures by different mechanisms and the seizures have a relatively gradual onset compared with seizures initiated by stimulation. There was no difference in the body weight of the animals receiving the different convulsants. For pilocarpine, kainic acid, and PTZ, the mean weights were 219 ± 5 g (range, 163–275 g), 215 ± 7 g (range, 183–258 g), and 216 ± 13 g (range, 147–309 g), respectively. The animals in the drug treatment groups were not different in weight from the animals in the control groups.
After administration of KA (10 mg/kg, i.p.), pilocarpine (scopolamine methyl bromide 1 mg/kg, s.c., followed 15 min later by 300 mg/kg, i.p., pilocarpine) or PTZ (50 mg/kg, i.p.) (Lian et al., 2006), animals were continuously monitored for seizure activity for at least 5 h after KA or pilocarpine and for 30 min after PTZ. Behavioral seizures were scored by an investigator blinded to the treatment. Latency to the first wet dog shake after KA, latency to the first forelimb clonus (after pilocarpine, kainic acid or PTZ), and the score and duration of seizures were measured. When at least 1 h had passed without any head bobbing (for pilocarpine) or any wet dog shakes (for KA), seizures were considered over. Status epilepticus lasting longer than 4 h (for KA) or 5 h (for pilocarpine) were assigned 4 and 5 h, respectively, for seizure duration. For animals receiving PTZ, the seizure duration was defined as the period of tonic-clonic seizures. EEG recording in the hippocampus was conducted as described previously (Lian and Stringer, 2004). After anesthesia, a recording electrode was placed in a burr hole centered at 3.0 mm posterior to bregma, 1.8 mm lateral to the midline, and then lowered 3.0 mm. A ground screw and wire was placed over the frontal region in another burr hole. This assembly was fixed to the skull with dental cement.
Each animal was assigned the score of the most severe seizure observed. The behavioral seizures induced by KA or pilocarpine were scored according to an adjusted version of the scale of Racine (Bough et al., 2002): stage 1, wet dog shakes after KA or trembling after pilocarpine; stage 2, head bobbing and stereotypes; stage 3, unilateral forelimb clonus; stage 4, bilateral forelimb clonus; stage 5, rearing and falling; stage 6, jumping and/or running followed by falling. Death within 24 h was assigned stage 7.
Adult rats received either D-F1,6BP, VPA (0.3 g/kg), 2-DG (0.25, 0.5 g/kg), or normal saline (vehicle) intraperitoneally followed 1 h later by one of the convulsants. The dose for VPA was based on experimental evidence that doses from 0.1 to 0.4 g/kg (i.p.) are effective in animal models (Bough and Eagles, 2001; Manent et al., 2007). Three doses of F1,6BP (0.25, 0.5, and 1 g/kg) were tested with this dosing schedule. Two additional groups were administered F1,6BP (0.5 or 1 g/kg; n = 5) after pilocarpine. In this experiment, the F1,6BP was given intraperitoneally at the first behavioral seizure, which was chewing movements of the jaw. Two additional sets of animals were fed the classic ketogenic diet (catalog #F3666; Bio-Serv, Frenchtown, NJ). One set of animals was given the diet beginning on postnatal day 22–26 [KD-young (Yng)] and maintained on this diet for 4 weeks. Thus, the seizures were tested in this group when the animals had reached approximately the same age (50–54 d) as the majority of the animals tested. The other set of animals started the diet as adults and remained on the diet for 10 d (KD-Adult). β-hydroxybutyrate levels (Clinical Pathology Laboratory, Texas Children's Hospital, Houston, TX) were confirmed to be elevated to levels previously reported (Bough et al., 1999) using an additional three animals in each diet group (control, 0.11–0.31 mmol/L; KD-Yng, 0.72–0.87 mmol/L; KD-Adult, 1.5–2.7 mmol/kg).
The latency to seizure onset, seizure score, and seizure duration were averaged across animals in each group. Comparisons between groups were done with an ANOVA with Bonferroni post hoc test. Statistical difference was defined as p < 0.05.
Results
Anticonvulsant activity of F1,6BP, 2-DG, valproate and the ketogenic diet in the pilocarpine model
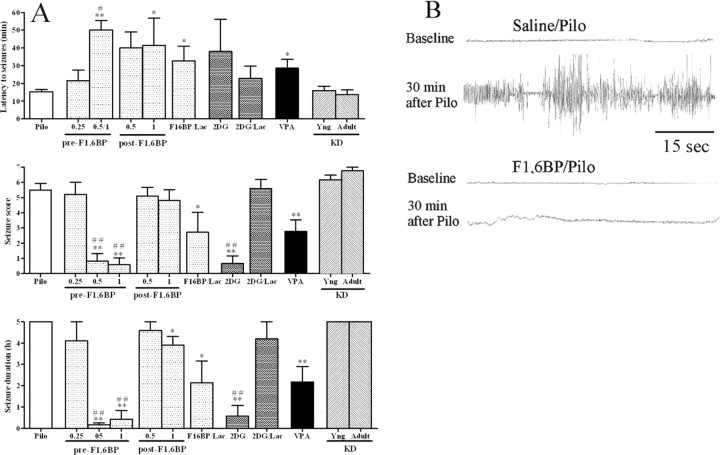
To begin to test the anticonvulsant activity of F1,6BP, pilocarpine, a cholinergic agonist, was used to produce the gradual onset of generalized seizures. All animals (n = 10) pretreated with saline followed by pilocarpine had generalized seizures lasting at least 5 h. The mean seizure score was 5.5 ± 0.4 (Fig. 2A). The mean latency to forelimb clonus was 14 ± 1 min. In addition, 4 of 10 animals died within 24 h.
Figure 2.
A, B, Anticonvulsant effect of F1,6BP in the pilocarpine model. One hour before the pilocarpine (300 mg/kg), animals received one of the following: saline (as seizure controls; Pilo), F1,6BP (0.25, 0.5, or 1 g/kg; pre-F1,6BP), F1,6BP (1 g/kg) plus lactate (0.5 g/kg) (F1,6BP/Lac), 2-DG (0.25 g/kg), 2-DG (0.25 g/kg) plus lactate (0.5 g/kg) (2-DG/Lac), VPA (0.3 g/kg), or ketogenic diet [starting at 20 d old (KD-Yng), or at 2 months of age (KD-Adult)]. Some animals received F1,6BP after the first behavioral seizure (post-F1,6BP). In A, the mean (± SEM) for each measured seizure parameter is shown for each treatment group. *p < 0.05, **p < 0.01 compared with Pilo; #p < 0.05, ##p < 0.01 vs VPA. In B, the hippocampal EEG 30 min after pilocarpine is presented from two animals: one pretreated with saline and one pretreated with F1,6BP (1 g/kg).
Pretreatment with F1,6BP had a dose-dependent anticonvulsant effect. The lowest dose (0.25 g/kg; n = 5) had no effect on the seizure parameters. In animals pretreated with 0.5 g/kg of F1,6BP, only 3 of 10 animals had a seizure score ≥3. In animals pretreated with 1 g/kg, 2 of 10 had a seizure score ≥3. In these five animals, the latency to the seizures was significantly increased. In animals pretreated with 0.5 or 1 g/kg F1,6BP, seizure duration and seizure score were significantly decreased. To identify electrographic seizures that do not have a behavioral component, hippocampal EEG recordings were conducted in two saline-pretreated rats and four rats pretreated with 1 g/kg F1,6BP followed by pilocarpine. The four rats treated with F1,6BP had no behavioral or electrographic seizures (Fig. 2B). To determine whether F1,6BP could alter the course of the pilocarpine-induced seizures once they had begun, either 0.5 or 1 g/kg (n = 5 for each dose) was administered at the very first sign of seizure activity, which was chewing movements. The higher dose (1 g/kg) significantly slowed the progression of the seizures as measured by an increase in the latency to forelimb clonus and decrease in seizure duration.
Pretreatment with 2-DG was also effective against pilocarpine-induced seizures, decreasing seizure duration and seizure score. After treatment with 2-DG at 0.25 g/kg (n = 6), only one animal had a stage 3 seizure. Pretreatment with VPA (0.3 g/kg, i.p.; n = 9) significantly reduced the mean seizure score and duration, but not to the extent of F1,6BP and 2-DG. In these animals, six of nine had forelimb clonus and one died. The ketogenic diet had no effect on any measured seizure parameters. In the group that received the ketogenic diet for 4 weeks (KD-Yng; n = 6), two of six died within 24 h. Those that received the ketogenic diet for 10 d as adults (n = 4) all had severe clonus and three died within 24 h.
Effect of exogenous lactate on the anticonvulsant efficacy of F1,6BP and 2-DG
F1,6BP and 2-DG both reduce metabolism of glucose through the glycolytic pathway, but F1,6BP also increases the flux of glucose through the pentose phosphate pathway. This increase may contribute to the anticonvulsant efficacy of F1,6BP. To test this hypothesis, exogenous sodium lactate (0.5 g/kg, i.p.) was administered 30 min after F1,6BP (1 g/kg, i.p.) or 2-DG (0.25 g/kg, i.p.). Thirty minutes later, the animals received pilocarpine (300 mg/kg, i.p.). Lactate should provide a substrate for the glycolytic pathway beyond the point of inhibition by either F1,6BP or 2-DG (Fig. 1).
In animals pretreated with F1,6BP and lactate, three of seven had at least stage 3 seizures and an increase in latency to seizures (Fig. 2A). The seizure score and seizure duration were significantly decreased compared with the seizure control group. In animals pretreated with 2-DG and lactate, severe seizures (stage 4–5) were noted in all animals (n = 5). The seizure score and duration were not different from those in the seizure control group. These data demonstrate that lactate abolishes the anticonvulsant action of 2-DG, but only reduces the efficacy of F1,6BP.
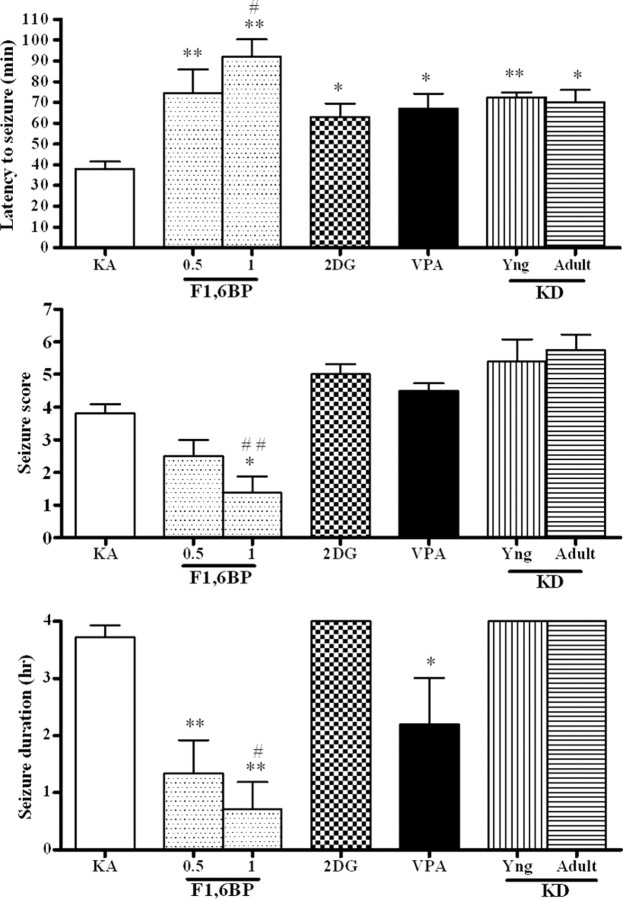
Anticonvulsant activity of F1,6BP, 2-DG, valproate, and the ketogenic diet in the kainic acid model
To determine whether F1,6BP is effective in other models, additional animals were given kainic acid, a glutamate receptor agonist, to induce partial seizures with secondary generalization. In animals pretreated with saline followed by KA (n = 10), one had only wet dog shakes (stage 1) (Fig. 3) and the remainder had severe seizures (at least stage 3). The latency to the first wet dog shake was 38 ± 4 min, the latency to the first forelimb clonus was 58 ± 2 min, values for seizure score and duration were 3.7 ± 0.3 and 3.7 ± 0.2 h, respectively. No animals died. F1,6BP had a dose-dependent effect on the seizures. At 0.25 g/kg (n = 6), F1,6BP had no effect. At 0.5 or 1 g/kg, F1,6BP significantly delayed the onset of seizures, and decreased the seizure score and seizure duration. Two of eight animals pretreated with 0.5 g/kg had no seizures, three had mild seizures (wet dog shakes or head bobbing), and the remaining three had severe seizures (mean seizure score 2.5 ± 0.5). Three of eight pretreated with 1 g/kg had no seizures, three had mild seizures (wet dog shakes or head bobbing), and two had severe seizures, for an average seizure score of 1.4 ± 0.5 for the entire group. The mean latency to first forelimb clonus in this group was 105 ± 7 min, which was statistically different from the control group.
Figure 3.
Anticonvulsant effect of F1,6BP in the kainic acid model. One hour before the kainic acid (10 mg/kg), animals received one of the following: saline (as seizure controls; KA), F1,6BP (0.5 or 1 g/kg), 2-DG (0.25 g/kg), VPA (0.3 g/kg), or ketogenic diet [starting at 20 d old (KD-Yng), or at 2 months of age (KD-Adult)]. The mean (± SEM) for each measured seizure parameter is shown for each treatment group. *p < 0.05, **p < 0.01 vs KA; #p < 0.05, ##p <0.01 vs VPA.
2-DG at 0.25 g/kg (n = 6) delayed the appearance of the first wet dog shake, but not the first forelimb clonus, and had no effect on the other parameters. At 0.5 g/kg, 2-DG had no additional activity (n = 3) (data not shown). Although animals pretreated with VPA (0.3 g/kg, i.p.; n = 6) had severe seizures with a seizure score of 4.5 ± 0.2, VPA significantly delayed the appearance of wet dog shakes, but not the appearance of forelimb clonus and decreased the duration of seizures. The ketogenic diet only delayed the appearance of wet dog shakes, but not the appearance of forelimb clonus. All animals treated with the ketogenic diet (n = 5, KD-Yng; n = 4, KD-Adult) had severe seizures and three died within 24 h.
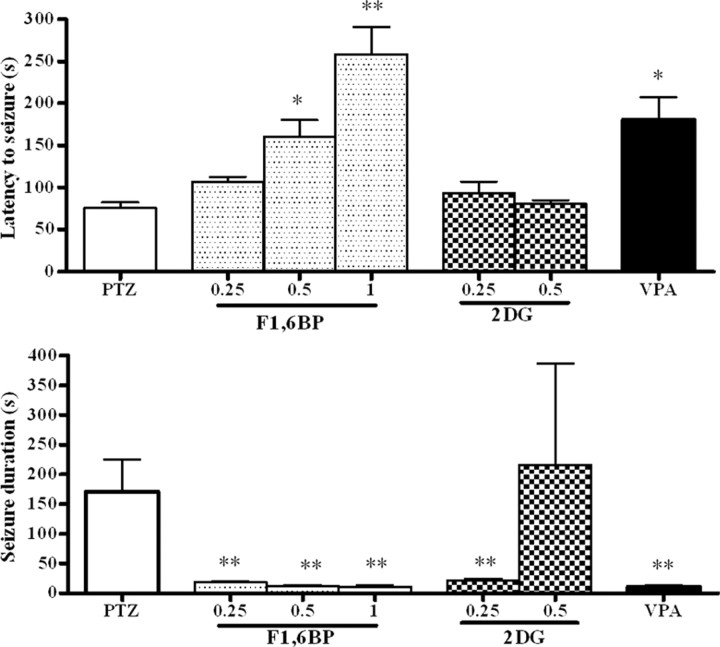
Anticonvulsant activity of F1,6BP, 2-DG, valproate, and the ketogenic diet in the PTZ model
To further test the anticonvulsant activity of F1,6BP, PTZ, a GABA antagonist, was used to induce a single generalized seizure. All rats (n = 9) pretreated with saline had generalized tonic-clonic seizures after PTZ (Fig. 4). The latency to generalized tonic-clonic seizures was 76 ± 6 s and the duration of the seizures was 170 ± 54 s. F1,6BP had a dose-dependent effect on the latency to the seizures (0.25 g/kg, n = 6, latency, 107 ± 6 s; 0.5 g/kg, n = 6, latency, 161 ± 16 s; 1 g/kg, n = 7, latency, 259 ± 32 s). All doses reduced the seizure duration to the same degree (0.25 g/kg, n = 6, duration, 18 ± 2 s; 0.5 g/kg, n = 6, duration, 12 ± 1 s; 1 g/kg, n = 7, duration, 11 ± 3 s). Three of eight animals who received 1 g/kg F1,6BP had no seizures.
Figure 4.
Anticonvulsant effect of F1,6BP in the PTZ model. One hour before PTZ (50 mg/kg), animals received one of the following: saline (as seizure controls; PTZ), F1,6BP (0.25, 0.5, or 1 g/kg), 2-DG (0.25 or 0.5 g/kg), or VPA (0.3 g/kg). The mean (± SEM) for each measured seizure parameter is shown for each treatment group. *p < 0.05; **p < 0.01 compared with PTZ.
All animals that received 2-DG had seizures and the seizure latency was not increased (n = 5 for both 0.25 and 0.5 g/kg). The seizures were significantly shortened by the 0.25 g/kg dose. In animals pretreated with VPA (0.3 g/kg; n = 8), two animals had no generalized tonic-clonic seizures and six had a significantly longer seizure latency (185 ± 27 s). VPA also significantly decreased the seizure duration (9 ± 4 s).
Discussion
This study demonstrates that F1,6BP, a regulator of glucose utilization by inhibition of glycolysis and enhancement of metabolic flux through the pentose phosphate pathway, has anticonvulsant efficacy against acute seizures triggered by a cholinergic agonist (pilocarpine), a glutamate receptor agonist (kainic acid), and a GABA antagonist (PTZ). F1,6BP was also able to significantly modify the pilocarpine-induced seizures when administered after the seizures had begun. 2-DG (an inhibitor of glycolysis) had some efficacy in these models, but was not as consistently effective as F1,6BP. The ketogenic diet had limited efficacy. Our data with the ketogenic diet are consistent with that in the literature for activity against acute seizures induced by kainic acid (Bough et al., 2002; Noh et al., 2003), PTZ, or other animal models of seizures (Nylen et al., 2005). Although additional work is needed to elucidate the mechanism of F1,6BP, these results suggest that F1,6BP may be an effective anticonvulsant agent. The finding that F1,BP is effective in all models tested may be attributable to its action on a final common pathway in epileptogenesis that is independent of the mechanism of seizure initiation.
It is possible that the reduction in glycolysis (metabolism of glucose to pyruvate) is responsible for the anticonvulsant action of F1,6BP. The ketogenic diet, which forces the body to use fat instead of carbohydrates, has been used to manage refractory epilepsy in children (Freeman et al., 2007). Previously, a decrease in glycolysis has been suggested to be the mechanism of this diet (Greene et al., 2001, 2003). 2-DG was reported previously to have anticonvulsant activity (Garriga-Canut et al., 2006). 2-DG blocks glucose uptake and also inhibits glycolysis by inhibiting hexokinase, the enzyme that phosphorylates glucose (Bissonnette et al., 1996). In the present experiments, exogenous lactate was given to provide substrate for cells beyond the point of inhibition in the glycolytic pathway (Fig. 1). The anticonvulsant action of 2-DG was completely reversed by lactate suggesting that the inhibition of glycolysis underlies its anticonvulsant action. The effect of F1,6BP was only partially reversed. This is presumably related to the ability of F1,6BP to increase flux of glucose into the pentose phosphate pathway (Kelleher et al., 1995; Espanol et al., 1998) and increase levels of GSH (Vexler et al., 2003), a potent endogenous anticonvulsant (Abe et al., 2000). Addition of lactate would not alter this action of F1,6BP. Together these data suggest that decreasing glycolysis pharmacologically might be an effective anticonvulsant mechanism.
Clinical testing will be needed to determine whether F1,6BP has activity and can be safely used in patients with epilepsy. F1,6BP has been administered to humans with no reported toxicity. It has been safely used in patients with myocardial damage (Munger et al., 1994), ischemic heart disease (Pasotti et al., 1989; Liu et al., 1998), ischemic stroke (Karaca et al., 2002), and during coronary artery bypass graft surgery (Riedel et al., 2004). It has also been found to be safe in trials with healthy volunteers in doses from 5 to 15 g (Ripari and Pieralisi, 1988; Markov et al., 2000). However, intravenous administration of F1,6BP has been shown to have an LD50 in rats of 1,068 mg/kg (Nunes et al., 2003). There are no reports of F1,6BP testing in humans with epilepsy.
If F1,6BP, the ketogenic diet and 2-DG are all altering seizure susceptibility by an action on glycolysis, then one might predict that F1,6BP would have fewer side effects. It has been hypothesized that the efficacy of the ketogenic diet is attributable to the reduction in glucose availability (Greene et al., 2003) and 2-DG inhibits glucose uptake (Bissonnette et al., 1996). Therefore, both of these treatments would result in an overall decrease in glucose utilization (including through the pentose phosphate pathway). F1,6BP shifts metabolism of glucose from the glycolytic pathway to the pentose phosphate pathway in astrocytes (Kelleher et al., 1995). This would provide two apparently beneficial effects: reducing glycolysis and increasing glutathione production. A decrease in overall glucose utilization by the ketogenic diet may impair cognitive function (Zhao et al., 2004). Additionally, subcutaneous administration of 0.3 μmol of 2-DG to a chick has been shown to inhibit memory consolidation (Gibbs and Summers, 2002). Because F1,6BP allows glucose utilization, it may result in less cognitive impairment making it a suitable candidate for clinical use as an anticonvulsant.
Footnotes
This work was supported by a grant from The Epilepsy Research Foundation (X.Y.L.) and by National Institutes of Health–National Institute of Neurological Disorders and Stroke Grant NS039941 (J.L.S.).
References
- Abe K, Nakanishi K, Saito H. The possible role of endogenous glutathione as an anticonvulsant in mice. Brain Res. 2000;854:235–238. doi: 10.1016/s0006-8993(99)02269-6. [DOI] [PubMed] [Google Scholar]
- Bissonnette P, Gagne H, Blais A, Berteloot A. 2-Deoxyglucose transport and metabolism in Caco-2 cells. Am J Physiol. 1996;270:153–162. doi: 10.1152/ajpgi.1996.270.1.G153. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Eagles DA. Comparison of the anticonvulsant efficacies and neurotoxic effects of valproic acid, and the ketogenic diet. Epilepsia. 2001;42:1345–1353. doi: 10.1046/j.1528-1157.2001.08901.x. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Valiyil R, Han FT, Eagles DA. Seizure resistance is dependent upon age and calorie restriction in rats fed a ketogenic diet. Epilepsy Res. 1999;35:21–28. doi: 10.1016/s0920-1211(98)00125-9. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Gudi K, Han FT, Rathod AH, Eagles DA. An anticonvulsant profile of the ketogenic diet in the rat. Epilepsy Res. 2002;50:313–325. doi: 10.1016/s0920-1211(02)00086-4. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Chugani DC. Basic mechanisms of childhood epilepsies: studies with positron emission tomography. Adv Neurol. 1999;79:883–891. [PubMed] [Google Scholar]
- Espanol MT, Litt L, Hasegawa K, Chang LH, Macdonald JM, Gregory G, James TL, Chan PH. Fructose-1,6-bisphosphate preserves adenosine triphosphate but not intracellular pH during hypoxia in respiring neonatal rat brain slices. Anesthesiology. 1998;88:461–472. doi: 10.1097/00000542-199802000-00025. [DOI] [PubMed] [Google Scholar]
- Freeman JM, Kossoff EH, Hartman AL. The ketogenic diet: one decade later. Pediatrics. 2007;119:535–543. doi: 10.1542/peds.2006-2447. [DOI] [PubMed] [Google Scholar]
- Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, Morrison JF, Ockuly J, Stafstrom C, Sutula T, Roopra A. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9:1382–1387. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Summers RJ. Effects of glucose and 2-deoxyglucose on memory formation in the chick: interaction with beta(3)-adrenoceptor agonists. Neuroscience. 2002;114:69–79. doi: 10.1016/s0306-4522(02)00229-4. [DOI] [PubMed] [Google Scholar]
- Greene AE, Todorova MT, McGowan R, Seyfried TN. Caloric restriction inhibits seizure susceptibility in epileptic EL mice by reducing blood glucose. Epilepsia. 2001;42:1371–1378. doi: 10.1046/j.1528-1157.2001.17601.x. [DOI] [PubMed] [Google Scholar]
- Greene AE, Todorova MT, Seyfried TN. Perspectives on the metabolic management of epilepsy through dietary reduction of glucose and elevation of ketone bodies. J Neurochem. 2003;86:529–537. doi: 10.1046/j.1471-4159.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- Heylen A, Van Schaftingen E, Hers HG. The stimulation of phosphofructokinase from human erythrocytes by fructose-2,6-bisphosphate. FEBS Lett. 1982;143:141–143. doi: 10.1016/0014-5793(82)80291-3. [DOI] [PubMed] [Google Scholar]
- Karaca M, Kilic E, Yazici B, Demir S, de la Torre JC. Ischemic stroke in elderly patients treated with a free radical scavenger-glycolytic intermediate solution: a preliminary pilot trial. Neurol Res. 2002;24:73–80. doi: 10.1179/016164102101199567. [DOI] [PubMed] [Google Scholar]
- Kelleher JA, Chan PH, Chan TY, Gregory GA. Energy metabolism in hypoxic astrocytes: protective mechanism of fructose-1,6-bisphosphate. Neurochem Res. 1995;20:785–792. doi: 10.1007/BF00969690. [DOI] [PubMed] [Google Scholar]
- Lammouchi T, Zoghlami F, Ben Slamia L, Grira M, Harzallah MS, Benammou S. Epileptic seizures in non-ketotic hyperglycemia. Neurophysiol Clin. 2004;34:183–187. doi: 10.1016/j.neucli.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Lian XY, Stringer JL. Inhibition of aconitase in astrocytes increases the sensitivity to chemical convulsants. Epilepsy Res. 2004;60:41–52. doi: 10.1016/j.eplepsyres.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Lian XY, Zhang Z, Stringer JL. Anticonvulsant and neuroprotective effects of ginsenosides in rats. Epilepsy Res. 2006;70:244–256. doi: 10.1016/j.eplepsyres.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Liu J, Li L, Wang Y, Chang Y. Clinical study of fructose 1,6-diphosphate on myocardial ischemia/reperfusion injuries. Hunan Yi Ke Da Xue Xue Bao. 1998;23:476–478. [PubMed] [Google Scholar]
- Manent JB, Jorquera I, Mazzucchelli I, Depaulis A, Perucca E, Ben-Ari Y, Represa A. Fetal exposure to GABA-acting antiepileptic drugs generates hippocampal and cortical dysplasias. Epilepsia. 2007;48:684–693. doi: 10.1111/j.1528-1167.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- Markov AK, Neely WA, Didlake RH, Terry IIIJ, Causey A, Lehan PH. Metabolic responses to fructose-1,6-diphosphate in healthy subjects. Metabolism. 2000;49:698–703. doi: 10.1053/meta.2000.6249. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Trabesinger AH, Boesiger P, Wieser HG. Brain glutathione levels in patients with epilepsy measured by in vivo (1)H-MRS. Neurology. 2001;57:1422–1427. doi: 10.1212/wnl.57.8.1422. [DOI] [PubMed] [Google Scholar]
- Munger MA, Botti RE, Grinblatt MA, Kasmer RJ. Effect of intravenous fructose-1,6-diphosphate on myocardial contractility in patients with left ventricular dysfunction. Pharmacotherapy. 1994;14:522–528. [PubMed] [Google Scholar]
- Noh HS, Kim YS, Lee HP, Chung KM, Kim DW, Kang SS, Cho GJ, Choi WS. The protective effect of a ketogenic diet on kainic acid-induced hippocampal cell death in the male ICR mice. Epilepsy Res. 2003;53:119–128. doi: 10.1016/s0920-1211(02)00262-0. [DOI] [PubMed] [Google Scholar]
- Nunes FB, Gaspareto PB, Santos RC, de Assis M, Graziottin CM, Biolchi V, Filho JC, Lunardelli A, Avila LD, Pires MG, Wachter PH, DeOliveira JR. Intravenous toxicity of fructose-1,6-bisphosphate in rats. Toxicol Lett. 2003;143:73–81. doi: 10.1016/s0378-4274(03)00075-4. [DOI] [PubMed] [Google Scholar]
- Nylen K, Likhodii S, Abdelmalik PA, Clarke J, Burnham WM. A comparison of the ability of a 4:1 ketogenic diet and a 6.3:1 ketogenic diet to elevate seizure thresholds in adult and young rats. Epilepsia. 2005;46:1198–1204. doi: 10.1111/j.1528-1167.2005.71204.x. [DOI] [PubMed] [Google Scholar]
- Pasotti C, Nicrosini S, Fiori G. Effects of fructose-1,6-diphosphate in patients with chronic ischemic heart disease. Echocardiographic study. Riv Eur Sci Med Farmacol. 1989;11:315–320. [PubMed] [Google Scholar]
- Riedel BJ, Gal J, Ellis G, Marangos PJ, Fox AW, Royston D. Myocardial protection using fructose-1,6-diphosphate during coronary artery bypass graft surgery: a randomized, placebo controlled clinical trial. Anesth Analg. 2004;98:20–29. doi: 10.1213/01.ANE.0000094336.97693.90. [DOI] [PubMed] [Google Scholar]
- Ripari P, Pieralisi G. Effects of fructose-1,6-diphosphate on heart rate, ventilation, oxygen consumption and endurance performance. Pharmatherapeutica. 1988;5:249–255. [PubMed] [Google Scholar]
- Schwechter EM, Veliskova J, Velisek L. Correlation between extracellular glucose and seizure susceptibility in adult rats. Ann Neurol. 2003;53:91–101. doi: 10.1002/ana.10415. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E. Fructose-2,6-bisphosphate. Adv Enzymol. 1987;59:315–395. doi: 10.1002/9780470123058.ch7. [DOI] [PubMed] [Google Scholar]
- Vexler ZS, Wong A, Francisco C, Manabat C, Christen S, Tauber M, Ferriero DM, Gregory G. Fructose-1,6-bisphosphate preserves intracellular glutathione and protects cortical neurons against oxidative stress. Brain Res. 2003;960:90–98. doi: 10.1016/s0006-8993(02)03777-0. [DOI] [PubMed] [Google Scholar]
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Stafstrom CE, Fu DD, Hu Y, Holmes GL. Detrimental effects of the ketogenic diet on cognitive function in rats. Pediatr Res. 2004;55:498–506. doi: 10.1203/01.PDR.0000112032.47575.D1. [DOI] [PubMed] [Google Scholar]