Abstract
Bradykinin (BK) is produced and acts at the site of injury and inflammation. In the CNS, migration of microglia toward the lesion site plays an important role pathologically. In the present study, we investigated the effect of BK on microglial migration. Increased motility of cultured microglia was mimicked by B1 receptor agonists and markedly inhibited by a B1 antagonist, but not by a B2 receptor antagonist. BK induced chemotaxis in microglia isolated from wild-type and B2-knock-out mice but not from B1-knock-out mice. BK-induced motility was not blocked by pertussis toxin but was blocked by chelating intracellular Ca2+ or by low extracellular Ca2+, implying that Ca2+ influx is prerequisite. Blocking the reverse mode of Na+/Ca2+ exchanger (NCX) completely inhibited BK-induced migration. The involvement of NCX was further confirmed by using NCX+/− mice; B1-agonist-induced motility and chemotaxis was decreased compared with that in NCX+/+ mice. Activation of NCX seemed to be dependent on protein kinase C and phosphoinositide 3-kinase, and resultant activation of intermediate-conductance (IK-type) Ca2+-dependent K+ currents (IK(Ca)) was activated. Despite these effects, BK did not activate microglia, as judged from OX6 staining. Using in vivo lesion models and pharmacological injection to the brain, it was shown that microglial accumulation around the lesion was also dependent on B1 receptors and IK(Ca). These observations support the view that BK functions as a chemoattractant by using the distinct signal pathways in the brain and, thus, attracts microglia to the lesion site in vivo.
Keywords: microglia, bradykinin, Na+/Ca2+ exchanger, Ca2+-dependent K+ current, chemotaxis, motility
Introduction
Activation of microglial cells, the resident macrophages of the brain, occurs rapidly after brain injury (Kreutzberg, 1996; Kim and Vellis, 2005). Activated microglia migrate rapidly to the affected sites of the CNS. The nonapeptide, bradykinin (BK), is produced in the brain during trauma and stroke, leading to an increase in blood–brain barrier permeability and an accumulation of leukocytes, and is therefore regarded as a mediator of inflammation and vasodepression (Abbott, 2000; Lehmberg et al., 2003). In the brain, BK has been assigned a neuroprotective role (Yasuyoshi et al., 2000; Noda et al., 2007a), especially via BK receptors in microglia (Noda et al., 2006, 2007a,b). BK receptors are coupled to multiple signal transduction pathways (Hall, 1992; Noda et al., 2004). One is mobilization of intercellular Ca2+ induced by inositol 1,4,5-trisphosphate (IP3) and activation of Ca2+-dependent K+ (KCa) channels (Higashida and Brown, 1988), inducing activation of phospholipase A2 and stimulation of arachidonic acid release (Welsh et al., 1988, Yanaga et al., 1991). BK receptors also couple to Gi/o-proteins, leading to mitogen-activated protein (MAP) kinase signal transduction (Liebmann, 2001). In unstimulated cultured microglia, expression of both B1 and B2 receptors was low, but strong immunolabeling of both B1 and B2 receptors was observed after the treatment of the cells with BK (Noda et al., 2007a). It was also reported that activation of BK receptors in microglia linked to activation of KCa channels. It is now well established that microglial cells can be neuroprotective or neurodestructive depending on the pathologic context and the type of stimulation (Pocock and Kettenmann, 2007). Our data indicate that BK triggers responses in microglia, which ultimately results in a protection of neurons, namely by inhibiting proinflammatory cytokine release and thus attenuating an inflammatory response (Noda et al., 2007a).
Microglia are abundant in all regions of the CNS. Under pathological conditions, activated microglia can phagocytose dead cells and clear cellular debris at the site of injury (Raivich et al., 1999; Stoll and Jander, 1999; Streit et al., 1999). There are several candidate molecules that serve as signals for pathologic events to microglia. As recently shown by Davalos et al. (2005), microglial processes rapidly respond to ATP, and ATP has been reported to be a chemoattractant for microglial cells (Honda et al., 2001). A variety of other signals stimulate microglial motility, such as cannabinoids (Walter et al., 2003), morphine (Takayama and Ueda., 2005), or the chemokine CCL21 (Rappert et al., 2002). Neuropeptides including BK are another family of candidates (Palkovits, 1995).
In the present study, we investigated the BK-induced increase in microglial migration and its underlying signaling cascade. The results indicate that BK can attract microglia to a lesion or inflammation sites, where BK is known to be upregulated, because it is a chemoattractant and increases microglial motility.
Materials and Methods
Cell culture.
The study was approved by the Animal Research Committee of Kyushu University and Max-Delbrück Center for Molecular Medicine and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Rat microglial cells were isolated from mixed cultures of cerebrocortical cells in postnatal day 3 Wistar rats (Kyudo, Kumamoto, Japan) as described previously (Noda et al., 2003). In brief, for rat microglia, the cerebral cortex was minced and treated twice with papain (90 U) and DNase (2000 U) at 37°C for 15 min. Dissociated cells were seeded into 300 cm2 plastic flasks at a density of 107 per 300 cm2 in Eagle's medium (MEM) with 0.17% NaHCO3 and 10% fetal calf serum (FCS) and maintained at 37°C in 10% CO2/90% air. Medium was changed twice per week. After 10–14 d, floating cells and weakly attached cells on the mixed primary cultured cell layer were obtained by gently shaking for 5–8 min. Microglial cells were then isolated as strongly adhering cells after unattached cells were removed. The purity of the microglial culture ranged from 95 to 99%, which was evaluated by staining with Griffonia simplicifolia isolectin B4, a marker for microglia.
Mouse microglial cells were isolated from total brain of newborn B1 receptor-knock-out (KO) (Pesquero et al., 2000), B2 receptor-knock-out (Milia et al., 2001), and wild-type control mice (C57BL/6J) as described previously (Prinz et al., 1999). In brief, cortical tissue was trypsinized for 2 min, dissociated with a fire-polished pipette, and washed twice. Mixed glial cells were cultured for 9–12 d in DMEM supplemented with 10% FCS, 2 mm l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin, with medium changes every third day. Microglial cells were then separated from the underlying astrocytic layer by gentle shaking of the flask for 1 h at 37°C in a shaker-incubator (100 rpm). The purity of microglia was >95%. Cells were used for experiments 1–5 d after plating. Cell media and supplements were purchased from Seromed/Biochrom (Berlin, Germany).
Motility experiment.
Rat cultured microglia were seeded on glass-bottom dishes (Matsunami, Osaka, Japan) at a density of 104 cells per dish. Motility of microglia under the control of temperature (37°C) and gas (10% CO2/90% air) was monitored with a time-lapse video microscopy system (Nikon Instec, Fukuoka, Japan). The video camera (Nikon inverted microscope, TE-2000-E) was controlled by Luminavision software (Mitani, Osaka, Japan). Images were acquired in 1 min intervals for 1 h and stored on a computer and analyzed by Dipp-Motion 2D (Ditect, Tokyo, Japan). Cell motility in response to BK and other chemicals was assessed by adding stock solution to the dish, diluting 100 times to get the final concentration. The diffusion of the drugs within the dish occurred rapidly and had distributed before the first image was taken, as verified by adding a dye.
Microchemotaxis assay.
BK-induced chemotaxis was tested using a 48-well microchemotaxis Boyden chamber (Neuroprobe, Bethesda, MD) (Nolte et al., 1997). Upper and lower wells were separated by polycarbonate filter (8 μm pore size; Poretics, Livermore, CA). Microglial cells (2–4 × 104) in 50 μl of serum-free MEM or DMEM medium were added to the upper wells, and the lower wells contained medium containing BK with or without other drugs. Culture medium (MEM or DMEM) was used as a control. The chamber was incubated at 37°C and 5% CO2 for 180 min. Cells remaining on the upper surface of the membrane were removed by wiping, and migrated cells were subjected to Diff-Quik stain (Sysmex, Kobe, Japan). The rate of microglial migration was calculated by counting cells in four random fields of each well using a 40× bright-field objective. The number of cells was ∼50–80 cells per field in control condition, and the number of fields in each condition was 92–152 (23–38 wells). The number of cells in each field was normalized to the average in control condition (100%).
Immunocytochemical analysis.
Rat primary cultured microglia were fixed with 4% paraformaldehyde, treated with a primary antibody against activated microglia, OX6 (1:250) (PharMingen, San Diego, CA) together with goat serum (10% in volume) for overnight at room temperature, washed four times, and then incubated with the Alexa Fluor 594-conjugated goat anti-mouse (1:200) antibody (Invitrogen, Carlsbad, CA) together with goat serum (10% in volume) at room temperature for 6 h. Series of images were examined with a confocal laser-scanning microscope (LSM510; Carl Zeiss, Oberkochen, Germany). The fluorescence intensity was measured by Photoshop (Adobe Systems, San Jose, CA).
Lesion model mice.
Male C57BL/6 mice (8–12 weeks old) and BK1 and BK2 receptor-knock-out mice (8–12 weeks) were anesthetized with pentobarbital (12.5 mg/kg) and placed in a stereotactic apparatus. A 2-μl Hamilton syringe with a 26 or 23 gauge needle was inserted into the right cortex through a small hole drilled through the skull. For cortex lesion, the microsyringe was inserted into brains of knock-out or wild-type mice at the following coordinates; anterior, 1 mm; lateral, 2.2 mm; and dorsoventral, 4 mm. For injection of charybdotoxin (CTX) (0.1 μm) or PBS (both in 1 μl volume) in C6BL/6J mice (Kyudo, Saga, Japan), the needle was lifted for 1 mm. n = 3–4 animals were used per experimental group. Eighteen hours after making lesion by insertion of microsyringe or microinjection of CTX or PBS, each mouse was anesthetized with pentobarbital (12.5 mg/kg) and transcardially perfused with 20 ml each of 10 U/ml heparin and 0.5% procaine in PBS and 4% paraformaldehyde. Then the brain was removed, postfixed for 3 h, and cryoprotected for 24 h in PBS containing 20% sucrose and 0.05% sodium azide.
Immunofluorescent labeling with microglia.
The brain was sliced into 20 μm in thickness using a cryostat, and the sections were placed on a slide glass precoated with silane and stored at −80°C until the time to use. The coronal sections were cut from part of the cortex. The sections were permeabilized with 0.3% Triton X-100 (Sigma, St. Louis, MO) in PBS for 15 min and blocked in PBS containing 1% BSA and 5% normal donkey serum (Jackson ImmunoResearch, West Grove, PA) for 60 min at room temperature. The brain sections were incubated overnight at 4°C with primary antibody, rabbit anti-Iba1 antibody (1:500; Wako, Richmond, VA), and incubated for 5 h with Alexa Fluor 488 goat anti-rabbit IgG (1:250; Invitrogen) at room temperature. Every treatment was followed by washing three times for 5 min with PBS. Sections were mounted in the Permafluor aqueous mounting medium (Thermo Fisher Scientific, Waltham, MA) and were analyzed with a confocal laser-scanning microscope (LSM510 Meta; Carl Zeiss).
Quantification of microglia number around the lesion site.
Microglia in the sections containing lesion were immunohistochemically stained with anti-Iba-1 as mentioned above. The number of microglia in 60–80 areas of 100 × 100 μm around the lesion site in five slices per brain was counted, and the averaged number per 104 μm2 for each brain was obtained.
Statistical analysis.
The results are expressed as the mean ± SEM. The data were compared with Student's t test or one-way ANOVA followed by the Scheffé or Dunnett's test, using software package Statview 5.0J. Values of p < 0.05 were considered statistically significant.
Drugs and reagents.
BK was from Peptide Institute (Osaka, Japan) or Sigma. ATP, Griffonia simplicifolia isolectin B4 3′, and 4′-dichlorobenzamil hydrochloride were from Sigma. Charybdotoxin, iberiotoxin, apamin, staurosporine, wortmannin, chelerythrine chloride, and PD98059 (2′-amino-3′-methoxyflavone) were from Sigma. Des-Arg9-[Leu8]-bradykinin, Hoe140, Des-Arg9-bradykinin, and Des-Arg10-kallidin were from Peptide Institute (Osaka, Japan). Pertussis toxin (PTX) was from List Biological Laboratories (Campbell, CA). KB-R7943 (2-(2-(4-(4-nitrobenzyloxy)phenyl)ethyl)isothiourea, methane sulfonate) was from EMD Biosciences (San Diego, CA). Gadolinium oxide was from Ishizu Seiyaku (Osaka, Japan). SN-6 was provided by Senju Pharmaceutical (Kobe, Japan).
Results
Bradykinin-induced motility of microglia is mediated by B1 receptors but not by B2 receptors
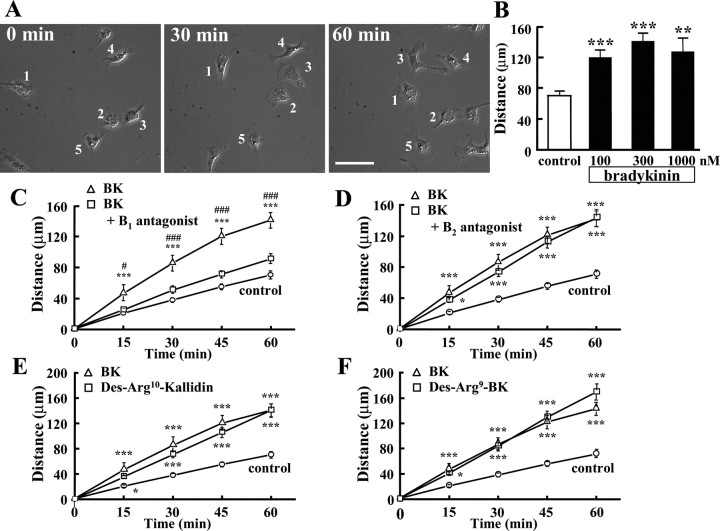
To examine the motility of microglia, individual cells were monitored by time-lapse video microscopy for 1 h. Figure 1A shows images of cultured microglial cells at 0, 30, and 60 min after addition of 300 nm BK. A significant increase in microglial motility was observed already after 15 min (data not shown). The increase in microglial motility could be monitored for up to 18 h (data not shown). The average distance of microglial migration determined within 1 h increased significantly in the presence of BK. However, there was no significant difference between 100, 300, and 1000 nm BK (Fig. 1B).
Figure 1.
Motility of BK-stimulated microglial cells. A, Microglial cells were stimulated with 300 nm BK and were recorded using time-laps imaging system every 5 min. The images show at 0, 30, and 60 min after application of BK. B, Effects of different concentration of BK on microglial motility. Total distance (in micrometers) of microglial cells for 1 h was significantly increased by BK at 100, 300, and 1000 nm. **p < 0.01; ***p < 0.001. Effects of bradykinin receptor antagonist and agonist. C, B1 receptor antagonist, Des-Arg9-[Leu8]-bradykinin (1 μm), inhibited BK-induced microglial motility. ***p < 0.001, significantly different from control. #p < 0.05, ###p < 0.001, significantly different from B1 antagonist. D, B2 receptor antagonist, Hoe140 (1 μm), did not inhibit BK-induced microglial motility. ***p < 0.001. E, Total distance (in micrometers) of microglial migration for 1 h was increased by B1 agonist, Des-Arg10-kallidin (300 nm). F, Another B1 agonist, Des-Arg9-bradykinin (300 nm), also increased microglial motility. *p < 0.05; ***p < 0.001.
To determine the type of BK receptor mediating BK-induced microglial motility, we tested the effect of selective antagonists for B1 and B2 receptor on BK-induced motility. Figure 1, C and D, shows the effects of Des-Arg9-[Leu8]-bradykinin, a B1 receptor antagonist, and Hoe140, a B2 receptor antagonist, on the BK-induced increase in microglial motility. BK-induced microglial motility was decreased by ∼70% by the B1 antagonist (Fig. 1C), but was not affected by the B2 antagonist (Fig. 1D). This represents that BK-induced microglial motility was caused by the activation of B1 receptors.
Consistent with the previous results, two B1 agonists, Des-Arg9-bradykinin and Des-Arg10-kallidin, mimicked the response of BK (Fig. 1E,F). These results indicate that B1 receptors, but not B2 receptors, are responsible for BK-induced increase in microglial motility.
BK-induced microglial chemotaxis in B2- but not B1-knock-out mice
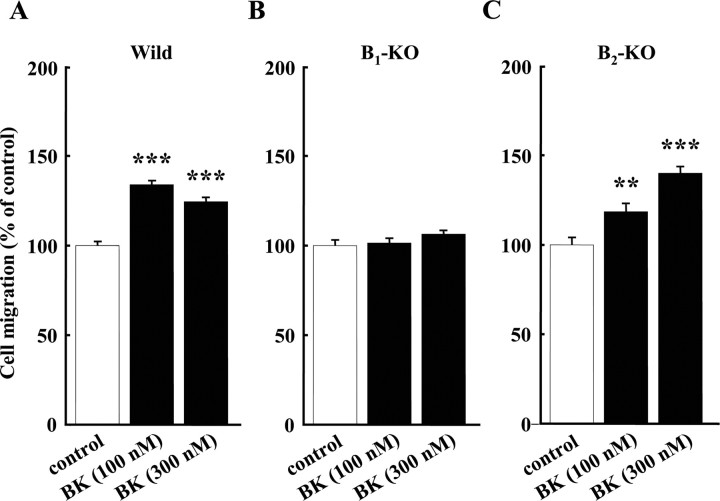
To confirm the pharmacological data, we used B1 and B2 receptor-KO mice and studied the BK-induced chemotaxis of microglia using a microchemotaxis (Boyden) chamber. In wild-type mice, BK at concentrations of 100 and 300 nm significantly increased microglial migration. The relative increases in migration by 100 and 300 nm BK were 134 ± 1.89% (n = 152) and 124 ± 1.99% (n = 148), respectively (Fig. 2A).
Figure 2.
Microglial chemotaxis in three genotypes of bradykinin receptors. A, BK-induced microglial chemotaxis in wild-type (Wild) mice. B, In B1 receptor-knock-out mice (B1-KO), 100 and 300 nm BK did not induce chemotaxis. C, In B2 receptor knock-out mice (B2-KO), BK at either concentration induced microglial chemotaxis. **p < 0.005; ***p < 0.001.
In B1-KO mice, 100 and 300 nm BK did not increase microglial migration (Fig. 2B). In contrast, in B2-KO mice, microglial migration induced by BK was similar to that in wild-type mice. BK increased cell migration to 118 ± 4.12% (n = 96) and 140 ± 3.26% (n = 92) with 100 and 300 nm BK, respectively (Fig. 2C).
Gi/o-protein was not involved in BK-induced microglial motility
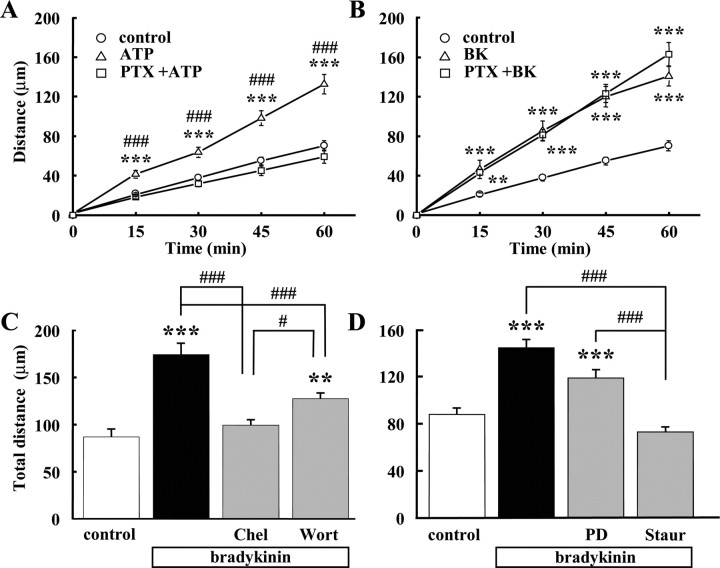
The ATP-increased microglial migration via P2Y12 receptor is coupled to a PTX-sensitive Gi/o-protein (Honda et al., 2001). BK receptors have also been reported to couple to Gi/o-protein, leading to MAP kinase signaling (Liebmann, 2001). Therefore, we investigated whether Gi/o-protein was involved in BK-induced increase in microglial motility. As a positive control, the ATP-induced increase in microglial motility could be completely inhibited by pretreatment with PTX for 12 h (Fig. 3A), as reported previously (Honda et al., 2001). In contrast, pretreatment with PTX for 12 h did not affect the BK-induced increase in microglial motility (Fig. 3B). These results suggest that PTX-sensitive G-proteins (Gi/o) are not involved in the BK-induced increase in microglial motility.
Figure 3.
Effects of PTX and inhibitors of protein kinases. A, Total distance (in micrometers) of microglial migration for 1 h of microglial cells was increased by ATP. Pretreatment of PTX (50 ng/ml) for 12 h (with additional 50 ng/ml PTX before application of ATP) almost completely blocked the effect of ATP. ***p < 0.001, significantly different from control. ###p < 0.001, significantly different from ATP+PTX. B, PTX did not affect BK (300 nm)-induced microglial motility (n = 20). ***p < 0.001. C, BK-induced microglial motility was inhibited by pretreatment of PKC inhibitor, chelerythrine (Chel; 10 μm), for 24 h. A part of BK-induced microglial motility was inhibited by pretreatment of PI3 kinase inhibitor, wortmannin (Wort; 1 μm), for 24 h. ***p < 0.001; ###p < 0.001. D, BK-induced microglial motility was inhibited by pretreatment of PKC inhibitor, staurosporine (Staur; 10 nm) for 24 h. However, BK-induced microglial motility was not affected by pretreatment of MAP/ERK inhibitor, PD98059 (PD; 10 μm), 24 h. ***p < 0.001; ###p < 0.001.
PKC and PI3K but not MAP/ERK signaling were involved in BK-induced microglial motility
BK receptors were reported to couple to Gq/11 protein and its downstream contains several protein kinases such as protein kinase C (PKC) (Higashida and Brown, 1988) and phosphoinositide 3-kinase (PI3K). The PKC inhibitors, staurosporine and chelerythrine, inhibited the increase in BK-induced microglial motility (Fig. 3C,D). BK-induced microglial motility was partially inhibited by wortmannin, a PI3K inhibitor (Fig. 3C), suggesting that PI3K was also involved in the increase in microglial migration induced by BK.
On the other hand, BK-induced microglial motility was not affected by PD98059, a MAP kinase/extracellular signal-regulated kinase (ERK) inhibitor (Fig. 3D). These results suggested that PKC-induced phosphorylation and PI3K were important for BK-induced microglial motility.
Intracellular and extracellular Ca2+ were important for BK-induced microglial migration
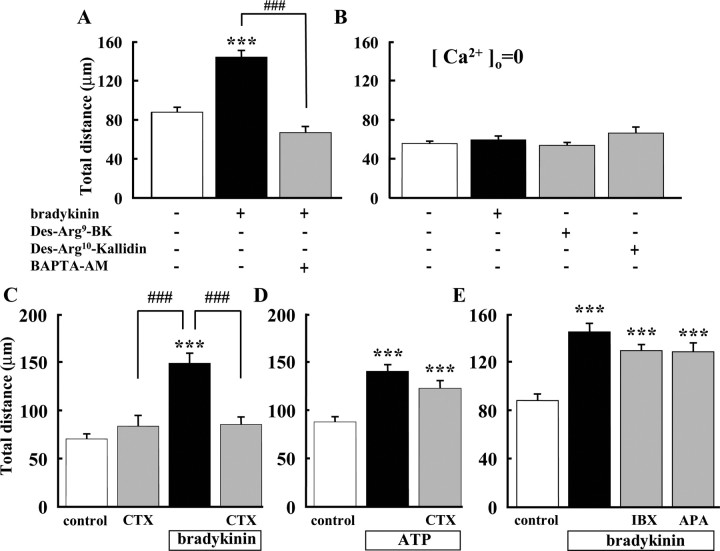
Microglial motility in the presence of BK was significantly inhibited when the cells were preincubated with 10 μm BAPTA-AM, an intracellular Ca2+ chelator (Fig. 4A). In nominally free extracellular Ca2+ solution, the basal level of microglial motility was reduced and was no longer affected by either BK or the B1 agonists Des-Arg9-bradykinin (300 nm) and Des-Arg10-kallidin (300 nm) (Fig. 4B). These results indicate that Ca2+ influx and the subsequent increase in intracellular Ca2+ are necessary for BK-induced microglial migration.
Figure 4.
Extracellular Ca2+ and activation of intermediate-conductance IK(Ca) was important for BK-induced microglial motility. A, Microglial motility in the presence of BK was significantly inhibited by pretreatment with BAPTA-AM (10 μm) for 20 min. ***p < 0.001; ###p < 0.001. B, In the absence of extracellular Ca2+ ([Ca2+]o = 0), total distance (in micrometers) of microglial migration was not affected by BK (300 nm) (n = 19), Des-Arg9-bradykinin (300 nm) (n = 18), or Des-Arg10-kallidin (300 nm) (n = 18). C, BK-induced increase in microglial motility was inhibited by CTX (0.1 μm). D, ATP (50 μm)-induced microglial motility was not affected by CTX (0.1 μm). E, Iberiotoxin (IBX) and apamin (APA), LK-type (large-conductance) and SK-type (small-conductance) IK(Ca) blockers, respectively, had no effect on BK-induced microglial motility. ***p < 0.001; ###p < 0.001.
Activation of intermediate-conductance Ca2+-activated K+ channels was involved in BK-induced microglial motility
As an effector for downstream signaling by increased intracellular Ca2+, activation of Ca2+-dependent K+ channels was tested using specific channel blockers. In the presence of 0.1 μm CTX, a blocker of Ca2+-dependent K+ currents (IK(Ca)), BK did not increase microglial motility (Fig. 4C). This result suggests that activation of IK(Ca) is required for BK-induced increase in microglial motility. In contrast, ATP-induced microglial motility was not affected by CTX (Fig. 4D).
Because CTX is a blocker for both intermediate-conductance (IK-type) and large-conductance (LK-type) IK(Ca), we used a specific blocker for the LK-type, iberiotoxin, and, in addition, a specific blocker for small-conductance (SK-type) IK(Ca), apamin. Figure 4E shows that BK-induced microglial motility was not affected by iberiotoxin nor apamin. These results suggest that the IK-type IK(Ca) is probably responsible for BK-induced microglial migration.
Reverse-mode (Ca2+ entry mode) of NCX as a mechanism of Ca2+ influx
To elucidate the mechanism of extracellular Ca2+ influx, we tested the influence of Na+–Ca2+ exchanger (NCX) activity on BK-induced migration, because NCX is involved in the regulation of intracellular Ca2+ concentration ([Ca2+]i), either with forward-mode (Ca2+ extrusion) or reverse mode (Ca2+ influx). In the CNS, three isoforms (NCX1–3) and several splicing variants are expressed in neurons, oligodendrocytes, astrocytes (Quednau et al., 1997; He et al., 1998), and microglia (Nagano et al., 2004). In pathological conditions such as interferon-γ (IFN-γ) or nitric oxide exposure, the activity and expression of NCX increased in microglia, causing Na+-dependent Ca2+ uptake (Matsuda et al., 2006). These results imply that NCX-induced Ca2+ influx plays a key role in microglial function.
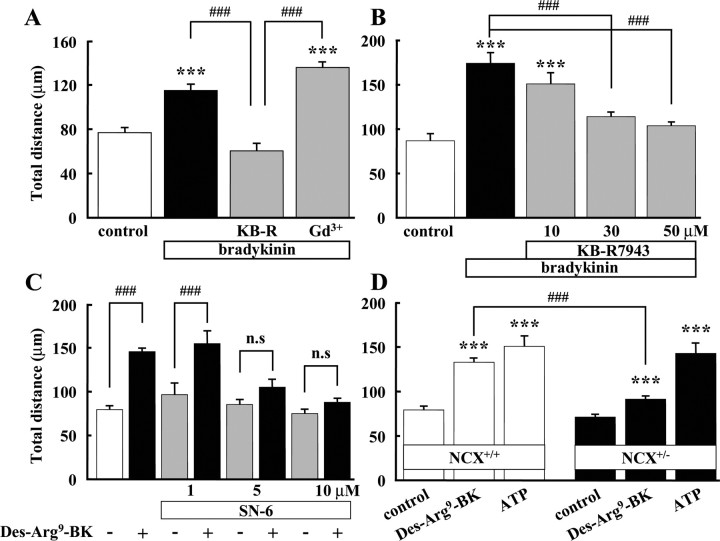
To examine whether Ca2+ influx via NCX is involved in BK-induced microglial motility, we used two benzyloxyphenyl inhibitors, KB-R7943 and SN-6, specific inhibitors of NCX in reverse mode (Iwamoto et al., 1996a, 2004). BK-induced microglial motility was completely inhibited by 30 and 50 μm KB-R7943 (Fig. 5B). On the other hand, BK-induced microglial motility was not affected by gadolinium (Gd3+), an inhibitor of capacitative calcium entry channels (Fig. 5A). Furthermore, we used SN-6, a specific NCX inhibitor, more than KB-R7943 (Iwamoto, 2004). Des-Arg9-BK (B1 agonist)-induced microglial motility completely inhibited by 5 and 10 μm SN-6 (Fig. 5C). Next, we used heterozygous NCX knock-out mice (NCX+/−), because the homozygous (NCX−/−) deletion is lethal (Wakimoto et al., 2000). Using NCX+/− mice, 300 nm Des-Arg9-BK-induced microglial motility was significantly attenuated compared with the one from wild-type (NCX+/+) mice. On the other hand, ATP-induced microglial motility showed no difference between wild-type and NCX+/− mice (Fig. 5D). These results suggest that Ca2+ influx via NCX is prerequisite for BK- and B1 agonist-induced microglial motility.
Figure 5.
Activation of reverse mode of Na+/Ca2+ exchanger was important for bradykinin-induced microglial motility. A, BK-induced microglial motility was inhibited by KB-R7943, a specific inhibitor of reverse-mode NCX. BK-induced microglial motility was not affected by gadolinium (Gd3+, 100 μm), an inhibitor of capacitative Ca2+ channels. ***p < 0.001; ###p < 0.001. B, BK-induced microglial motility was inhibited by KB-R7943 dose dependently. ***p < 0.001; ###p < 0.001. C, B1 agonist (Des-Arg9-BK)-induced microglial motility was inhibited by SN-6 (5 and 10 μm), a more specific inhibitor of reverse-mode NCX. D, B1 agonist (Des-Arg9-BK)-induced microglial motility in wild-type (NCX+/+) mice (open bar) and NCX knock-out (NCX+/−) mice (filled bar). In NCX+/+ mice, 300 nm Des-Arg9-BK induced microglial motility was 1.7-fold increased, whereas in NCX+/− mice, Des-Arg9-BK-induced microglial motility was only 1.3-fold increased. ***p < 0.001; ###p < 0.001. n.s., No significant difference.
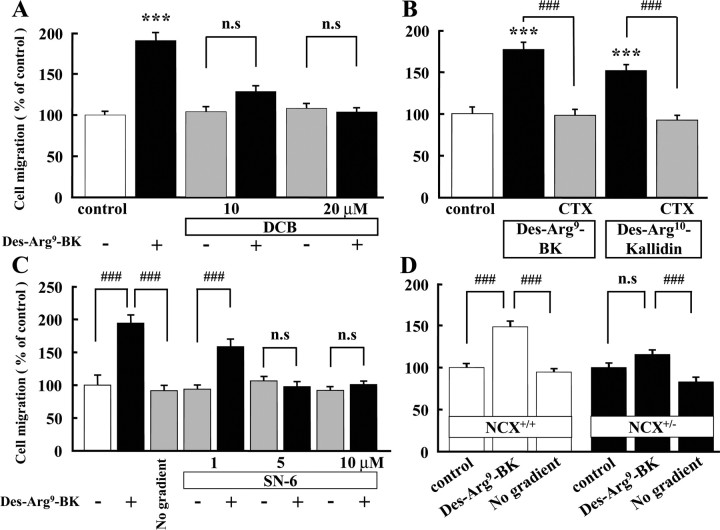
We also tested the involvement of NCX and activation of IK(Ca) in BK- and B1 agonist-induced chemotaxis using the Boyden chamber. Another NCX inhibitor, 3′,4′-dichlorobenzamil (DCB) (Plasman et al., 1991, Krasznai et al., 2006), inhibited BK-induced chemotaxis at concentrations of 10 and 20 μm, to the control level (Fig. 6A). DCB alone did not affect the microglial chemotaxis.
Figure 6.
B1 agonist-induced microglial chemotaxis, determined by Boyden chamber, was dependent on NCX and IK(Ca) activity. A, Des-Arg9-bradykinin (300 nm)-induced microglial chemotaxis was inhibited by DCB (10 and 20 μm), an inhibitor of NCX. ***p < 0.001; ###p < 0.001. B, CTX (0.1 μm) inhibited microglial chemotaxis induced by B1 agonists, Des-Arg9-bradykinin (300 nm) and Des-Arg10-kallidin (300 nm). ***p < 0.001; ###p < 0.001. C, Des-Arg9-bradykinin (300 nm)-induced microglial chemotaxis was inhibited by SN-6 (5 and 10 μm). ***p < 0.001; ###p < 0.001. D, Des-Arg9-bradykinin (300 nm) induced microglial chemotaxis in wild-type (NCX+/+) mice (open bar), but not in NCX knock-out (NCX+/−) mice or when Des-Arg9-BK was added to both upper and lower wells to eliminate the concentration gradient (No gradient). ###p < 0.001. n.s., No significant difference.
The importance of activation of IK(Ca) was also confirmed in BK-induced chemotaxis. B1 agonists, Des-Arg9-bradykinin (300 nm) and 300 nm Des-Arg10-kallidin, increased chemotaxis in cultured rat microglia by 177 ± 8.41% (n = 16) and 152 ± 6.76% (n = 16), respectively. These increases in chemotaxis were completely blocked by 0.1 μm CTX (Fig. 6B).
Furthermore, we used NCX+/− mice and SN-6 in chemotaxis. Des-Arg9-BK-induced microglial chemotaxis was completely inhibited by 5 and 10 μm SN-6 (Fig. 6C). In wild-type mice, microglial chemotaxis induced by Des-Arg9-BK was similar to that in cultured rat microglia. In contrast, in NCX+/− mice, 300 nm Des-Arg9-BK did not induce microglial chemotaxis (Fig. 6D). Des-Arg9-BK-induced microglial chemotaxis was almost the same as the control level when Des-Arg9-BK was added to both sides of the membrane (lower and upper well) to eliminate the concentration gradient (no gradient).
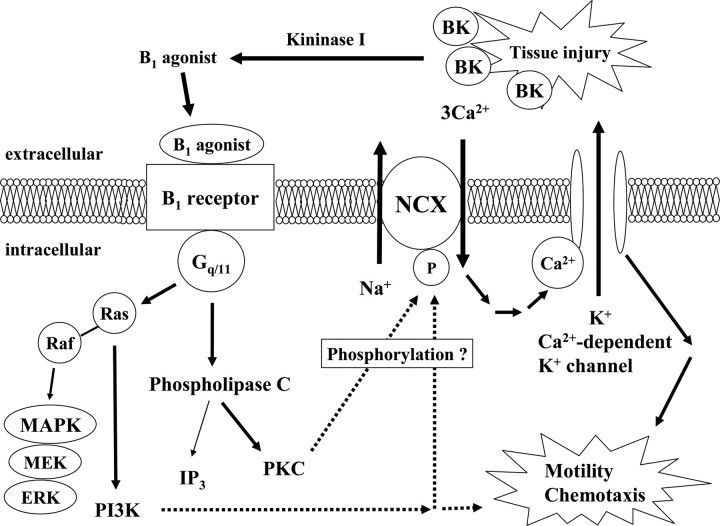
Signal cascade from B1 receptor in microglia
The above in vitro experiments using cultured microglia suggest the signaling cascade for BK-induced microglial migration shown in Figure 7. Thus, BK (B2 agonist) is enzymatically metabolized to B1 agonist by kininogen, which in turn, working on B1 receptors, coupled to Gq/11 proteins, activates PKC and PI3K, phosphorylates and activates NCX, inducing influx of extracellular Ca2+, activates IK(Ca), and then induces microglial migration by hyperpolarization or some other mechanisms.
Figure 7.
Possible signal transduction caused by activation of B1 receptor. Bradykinin is produced at site of tissue injury. Bradykinin is rapidly degraded by peptidases known as kininase I, which cleave off C-terminal arginine (Arg9) to generate Des-Arg9-bradykinin. Activation of the B1 receptor and coupling to Gq/11 results in the activation of PLCβ and PKC. PKC can phosphorylate NCX and increase NCX activity and subsequent mobilization of intracellular Ca2+. Increase in intercellular Ca2+ can activate Ca2+-dependent K+ channels. Resulting hyperpolarization may induce microglial motility and chemotaxis. Activation of the B1 agonist also couples the B1 receptor to MAPK signaling, which involves activation of Ras and consecutive stimulation of PI3K. PI3K was involved in a part of microglial motility.
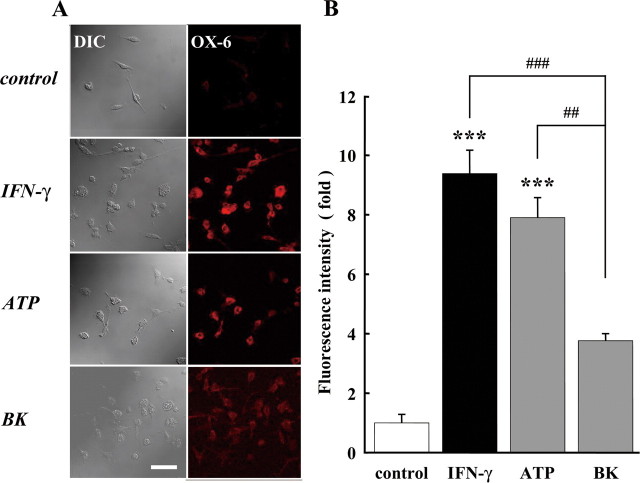
Bradykinin did not induce OX6 expression in microglia
Activated microglia are known to express the class 2 major histocompatibility complex (MHC-2). MHC-2 expression as determined by OX6 immunoreactivity was used as indicator for microglial activation (Majed et al., 2006). As a positive control, microglial cells were treated with IFN-γ (50 U/ml) and ATP (50 μm) (Fig. 8A). The fluorescence intensity of OX6 was significantly increased by IFN-γ and ATP, but not by BK (Fig. 8B) This indicates that BK did not induce microglial activation. We also tested the involvement of Rho and cyclic adenosine-ribose(cADPR), but they were not involved (supplemental Fig. 1, available at www.jneurosci.org as supplemental material).
Figure 8.
Bradykinin did not stimulate MHCII expression. A, The cells were treated with 50 U/ml IFN-γ, 50 μm ATP, or 300 nm BK for 24 h. Differential interference contrast (DIC) image and immunofluorescence stained with anti-OX6 antibody (labeled with Alexa Fluor 568; red) are shown. Scale, 100 μm. B, The relative fluorescence intensity of OX6 immunoreactivity. The intensity of fluorescence after application of IFN-γ, ATP, or BK stimulation was normalized to the one in untreated (control) microglia. ***p < 0.001; ##p < 0.01; ###p < 0.001.
Bradykinin acts as an inducer of microglial migration in vivo
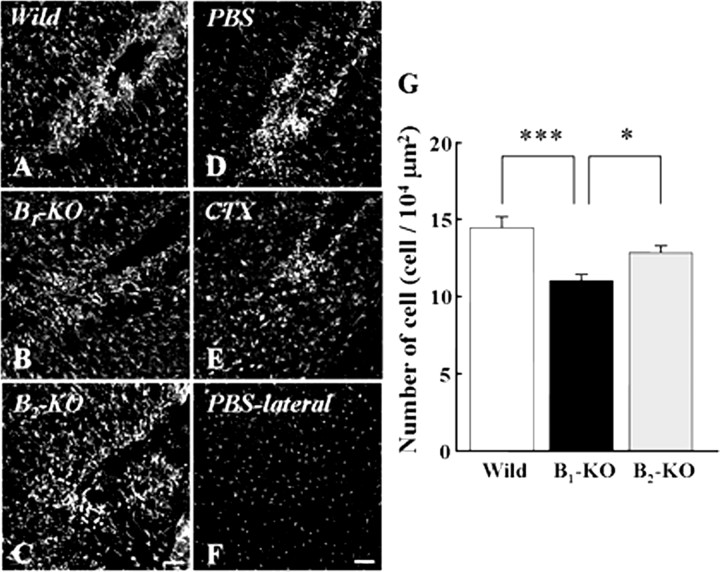
It is important to know whether the phenomena described above occur in vivo, i.e., whether BK produced at the site of injury attracts microglia for either neuroprotection or cleaning up debris. Therefore, we compared microglial density 18 h after a focal stab wound injury in normal and B1- and B2-deficient adult mice (Fig. 9A–C). Because BK is not the only mediator produced at the lesion site, BK-induced microglial accumulation would be only partial. Nonetheless, microglial accumulation in B1-knock-out mice was significantly decreased compared with that in wild-type mice. In wild-type mice, the average microglial density at the lesion site was 14.43 ± 0.69 (n = 3) in the 104 μm2 inspected areas, whereas in the same region at the contralateral side, it was 2.73 ± 0.17. In B1-knock-out mice, microglial accumulation was significantly decreased (11.03 ± 0.35, n = 4) compared with wild-type mice (Fig. 9G). Microglial density at the contralateral side was 2.19 ± 0.21 in the B1-knock-out mice. In B2-knock-out mice, microglial density was 12.83 ± 0.40 at the lesion site and 2.29 ± 0.28 at the contralateral side (n = 3).
Figure 9.
Bradykinin acts as an inducer of microglial migration in vivo. A–C, B1 receptors play an important role in migration and accumulation of microglia. Microglial cells around lesion were stained immunohistochemically with anti-Iba-1 in wild-type (A), B1-knock-out (B), and B2-knock-out mice (C). D, E, Ca2+-dependent K+ channels play an important role in migration and accumulation of microglia. Injection of CTX inhibited the number of microglia around lesion (E) compared with that in control (PBS-injected; D). F, Microglial distribution at contralateral cortex in control brain (D). G, The number of microglia around lesion was decreased in B1-knock-out mice and CTX-injected brain compared with the one in wild-type and B2-knock-out mice and control (PBS-injected) brain, respectively. Numbers of cells are represented as mean ± SEM. *p < 0.05; ***p < 0.001. Scale bar, 100 μm.
We also examined whether blocking the downstream components of BK-induced signal transduction also inhibited the microglial migration. Injection of CTX (0.1 μm, 1 μl) significantly inhibited microglial migration to the lesion site compared with that in control (injection of PBS) (Fig. 9D,E). The average numbers of microglia around the lesion in PBS and CTX-injected brain were 14.62 ± 0.63 (n = 3) and 10.62 ± 0.60 (n = 3) per 104 μm2, respectively (Fig. 9G), indicating that CTX impairs the accumulation at the lesion site. At the contralateral side (Fig. 9F), microglial density was 2.13 ± 0.19 in CTX-injected and 2.23 ± 0.16 in PBS-injected animals.
Discussion
Bradykinin induced microglial migration by stimulating B1 receptors
In the brain, BK receptors are expressed in all major cell types, neurons (Delmas et al., 2002), astrocytes, oligodendrocytes (Ritchie et al., 1987; Gimpl et al., 1992; Hosli et al., 1992; Lin and Chuang, 1992; Stephens et al., 1993), and microglial cells (Noda et al., 2003). Two subtypes of BK receptors have been distinguished, termed B1 and B2 receptors. B1 receptor mRNA is expressed at very low levels in microglia under normal conditions, but is upregulated after application of BK (Noda et al., 2003, 2007a). In the present study, we provide evidence that the BK-induced migration, both motility and chemotaxis, is mediated by B1, but not by B2 receptors, because the response can be mimicked with B1-specific agonists and blocked by B1-specific antagonists. This was supported by results obtained using B1- and B2-deficient mouse lines. Combined with a chemotactic response, a general increase in motility will result in a faster accumulation of the cells at the lesion site.
The signal cascade of B1 receptor activation
We have obtained evidence that BK would be produced after lesion in vivo and identified several steps in the intracellular signaling cascade followed by B1 receptor activation in vitro, as shown in Figure 7. The first step is the production of BK or kallidin at the lesion site. These are rapidly degraded by peptidases known as kininases, because the half-life of BK is reported to be <30 s in plasma (Kariya et al., 1982). The degraded BK becomes B1 agonist and activates the B1 receptor linked to a PTX-independent G-protein, presumably Gq/11. Activation of members of the Gq/11 family is involved in phosphoinositide (PI) turnover and the mobilization of intracellular Ca2+ (Noda et al., 2004). Although BK triggered a transient increase in intracellular Ca2+ (Noda et al., 2007b) and a transient activation of Ca2+-dependent K+ currents (IK(Ca)) in microglia (Noda et al., 2003), some observations indicate that the long-term effect of BK on migration is caused by a different signaling mechanism. This is based on the observations that (1) the population of cells showing the transient (IP3-induced) increase in intracellular Ca2+ or IK(Ca) were ∼20%, whereas >80% of microglial cells showed BK-induced migration; and (2) BK-induced migration depends on extracellular Ca2+ levels (Fig. 4).
Our data indicate that reverse-mode operation of NCX provides the influx of Ca2+. This Ca2+ influx would in turn induce a more persistent activation of IK(Ca). Persistent IK(Ca) activation appears to be an important step in stimulating migration, because blocking IK(Ca) completely inhibited BK-induced migration (Figs. 4, 6B). A similar observation was reported for lysophosphatidic acid-induced microglial migration: it also depended on the activity of intermediate-conductance IK(Ca) (Schilling et al., 2002; Eder, 2005). One possibility is that the hyperpolarization induced by IK(Ca) increases the driving force (the electrochemical gradient) for Ca2+ influx and thus enhances the intracellular Ca2+ signal required to stimulate cell migration.
We found that activation of the kinases PKC and PI3K are important in mediating the effects of bradykinin. This may be attributed to phosphorylation of NCX, because PKC can phosphorylate NCX, and most, but not all, studies have reported PKC-mediated increase in NCX activity (Vigne et al., 1988; Iwamoto et al., 1996b). The involvement of PKC and tyrosine kinase in controlling NCX activity in microglia has previously been reported by Matsuda et al. (2006). PI3K is also involved in reverse mode of NCX activity, as observed in neonatal cardiomyocytes (McDowell et al., 2004). Because activation of PKC and PI3K could be also a downstream consequence of PI turnover, our results suggest that B1-induced activation of PKC, and presumably also PI3K, was linked to activation of NCX in microglia.
Distinct mechanisms of microglial migration induced by BK and ATP
Our data showed that microglial migration induced by BK and that induced by ATP were caused by different mechanisms. BK- and lysophosphatidic acid-induced migration was controlled by a Gi/o-protein-independent pathway, leading to an activation of IK(Ca), whereas ATP-induced migration was via a Gi/o-protein-dependent but not IK(Ca)-dependent pathway. Another difference between ATP and BK was that BK did not significantly induce membrane ruffling in microglia (data not shown), as observed with ATP (Honda et al., 2001). Furthermore, ATP and IFN-γ, but not BK, increased the expression of OX6 (Fig. 8), a parameter of microglial activation. These differences indicate that ATP and BK control distinct microglial functions in pathological conditions such as lesion and inflammation.
New signaling by bradykinin in microglia
BK is widely accepted as a signaling substance that mediates pain and inflammation, whereas the role of BK in the normal, healthy CNS still remains unknown. Recently, a neuroprotective role for BK after a neurotoxic insult has been reported (Yasuyoshi et al., 2000; Noda et al., 2007a). Because microglial cells are the primary immune effector cells in the brain and highly dynamic surveillants of brain parenchyma (Nimmerjahn et al., 2005), BK would be an important mediator to attract microglial cells to the site of lesion. Interestingly, the link between bradykinin and microglial migration is mediated by B1 but not by B2 receptors. B1 receptors are upregulated in microglia in response to activation [e.g. by lipopolysaccharide (LPS)]. A similar upregulation of B1 receptor subtype was observed in other cells treated with LPS, cytokine, heat stress, or endotoxin (Galizzi et al., 1994; Campos et al., 1996; Nicolau et al., 1996; Lagneux and Ribuot, 1997; Busser et al., 1998; Schremmer-Danninger et al., 1998; Wille et al., 2001). Another interesting observation was the involvement of NCX operating in reverse mode leading to Ca2+ influx. Although the physiological function of NCX is assumed to be Ca2+ extrusion, Matsuda et al. (2006) provide evidence that Ca2+ uptake via NCX operating in reverse mode may play a role for microglial function under pathological conditions. The signaling cascade of BK and its coupling to NCX might be quite distinct in microglia compared with other cell types. Our results indicate that BK is an important signaling substance that affects microglial functions via activation of B1 receptors. This information could contribute to the treatments against infectious diseases, inflammation, trauma, or neurodegeneration accompanying inflammation.
Footnotes
This work was supported by Grants-in-Aid for Scientific Research of Japan Society for Promotion of Science; Research Grant in Priority Area Research of the Ministry of Education, Culture, Sports, Science and Technology, Japan; Grants-in-Aid for Scientific Research of the Ministry of Health, Labour and Welfare, Japan; and the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation. We thank M. Ueda (Nikon Instec, Fukuoka, Japan) and S. Sato (Ditect, Tokyo, Japan) for their technical support on motility experiments, Drs. S. Kohsaka and K. Ohsawa (National Center of Neurology and Psychiatry, Tokyo, Japan) for their help in microchemotaxis assay, Drs. R. Glass and M. Synowitz (Max-Delbrück-Center, Berlin, Germany) and Dr. H. Sugimori (Kyushu University, Fukuoka, Japan) for technical assistance of lesion model We also thank Prof. D. A. Brown (University College London, London, UK), Prof. H. Higashida (Kanazawa University, Ishikawa, Japan) for valuable suggestions.
References
- Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busser BW, Hammond TG, Bjorling DE, Saban R. Lipopolysaccharide upregulates bradykinin 1 receptors in the isolated mouse bladder. J Urol. 1998;160:2267–2273. doi: 10.1097/00005392-199812010-00098. [DOI] [PubMed] [Google Scholar]
- Campos MM, Souza GE, Calixto JB. Upregulation of B1 receptor mediating des-Arg9-BK-induced rat paw oedema by systemic treatment with bacterial endotoxin. Br J Pharmacol. 1996;117:793–798. doi: 10.1111/j.1476-5381.1996.tb15262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Delmas P, Wananerbercq N, Abogadie FC, Mistry M, Brown DA. Signaling microdomains define the specificity of receptor-mediated InsP3 pathways in neurons. Neuron. 2002;14:209–220. doi: 10.1016/s0896-6273(02)00641-4. [DOI] [PubMed] [Google Scholar]
- Eder C. Regulation of microglial behavior by ion channel activity. J Neurosci Res. 2005;81:314–321. doi: 10.1002/jnr.20476. [DOI] [PubMed] [Google Scholar]
- Galizzi JP, Bodinier MC, Chapelain B, Ly SM, Coussy L, Giraud S, Neliat G, Jean T. Up-regulation of [3H]-des Arg10-kallidin binding to the bradykinin B1 receptor by interleukin-1 beta in isolated smooth muscle cells: correlation with B1 agonist-induced PG12 production. Br J Pharmacol. 1994;113:389–394. doi: 10.1111/j.1476-5381.1994.tb17001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Walz W, Ohlemeyer C, Kettenmann H. Bradykinin receptors in cultured astrocytes from neonatal rat brain are linked to physiological responses. Neurosci Lett. 1992;144:139–142. doi: 10.1016/0304-3940(92)90735-p. [DOI] [PubMed] [Google Scholar]
- Hall JM. Bradykinin receptors: pharmacological properties and biological roles. Pharmacol Ther. 1992;56:131–190. doi: 10.1016/0163-7258(92)90016-s. [DOI] [PubMed] [Google Scholar]
- He S, Ruknudin A, Bambrick LL, Jon Lederer W, Schulze DH. Isoform-specific regulation of the Na+/Ca2+ exchanger in rat astrocytes and neurons by PKC. J Neurosci. 1998;18:4833–4841. doi: 10.1523/JNEUROSCI.18-13-04833.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida H, Brown DA. Ca2+-dependent K+ channels in neuroblastoma hybrid cells activated by intracellular inositol trisphosphate and extracellular bradykinin. FEBS Lett. 1988;238:395–400. doi: 10.1016/0014-5793(88)80519-2. [DOI] [PubMed] [Google Scholar]
- Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosli L, Hosli E, Kaeser H, Lefkovits M. Colocalization of receptors for vasoactive peptides on astrocytes of cultured rat spinal cord and brain stem: electrophysiological effects of atrial and brain natriuretic peptide, neuropeptide Y and bradykinin. Neurosci Lett. 1992;148:114–116. doi: 10.1016/0304-3940(92)90817-q. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Watano T, Shigekawa M. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J Biol Chem. 1996a;271:22391–22397. doi: 10.1074/jbc.271.37.22391. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Pan Y, Wakabayashi S, Imagawa T, Yamanaka HI, Shigekawa M. Phosphorylation-dependent regulation of cardiac Na+/Ca2+ exchanger via protein kinase C. J Biol Chem. 1996b;271:13609–13615. doi: 10.1074/jbc.271.23.13609. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Inoue Y, Ito K, Sakaue T, Kita S, Katsuragi T. The exchanger inhibitory peptide region-dependent inhibition of Na+/Ca2+ exchange by SN-6 [2-[4-(4-nitrobenzyloxy)benzyl]thiazolidine-4-carboxylic acid ethyl ester], a novel benzyloxyphenyl derivative. Mol Pharmacol. 2004;66:45–55. doi: 10.1124/mol.66.1.45. [DOI] [PubMed] [Google Scholar]
- Kariya K, Yamauchi A, Hattori S, Tsuda Y, Okada Y. The disappearance rate of intraventricular bradykinin in the brain of the conscious rat. Biochem Biophys Res Commun. 1982;107:1461–1466. doi: 10.1016/s0006-291x(82)80163-0. [DOI] [PubMed] [Google Scholar]
- Kim SU, de Vellis J. Microglia in health and disease. J Neurosci Res. 2005;81:302–313. doi: 10.1002/jnr.20562. [DOI] [PubMed] [Google Scholar]
- Krasznai Z, Krasznai ZT, Morisawa M, Bazsane ZK, Hernadi Z, Fazekas Z, Tron L, Goda K, Marian T. Role of the Na+/Ca2+ exchanger in calcium homeostasis and human sperm motility regulation. Cell Motil Cytoskeleton. 2006;63:66–76. doi: 10.1002/cm.20108. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lagneux C, Ribuot C. In vivo evidence for B1-receptor synthesis induction by heat stress in the rat. Br J Pharmacol. 1997;121:1045–1046. doi: 10.1038/sj.bjp.0701305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmberg J, Beck J, Baethmann A, Uhl E. Bradykinin antagonists reduce leukocyte-endothelium interactions after global cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:441–448. doi: 10.1097/01.WCB.0000052280.23292.35. [DOI] [PubMed] [Google Scholar]
- Liebmann C. Bradykinin signalling to MAP kinase: cell-specific connections versus principle mitogenic pathways. Biol Chem. 2001;382:49–55. doi: 10.1515/BC.2001.008. [DOI] [PubMed] [Google Scholar]
- Lin WW, Chuang DM. Regulation of bradykinin-induced phosphoinositide turnover in cultured cerebellar astrocytes: possible role of protein kinase C. Neurochem Int. 1992;21:573–579. doi: 10.1016/0197-0186(92)90090-e. [DOI] [PubMed] [Google Scholar]
- Majed HH, Chandran S, Niclou SP, Nicholas RS, Wilkins A, Wing MG, Rhodes KE, Spillantini MG, Compston A. A novel role for Sema3A in neuroprotection from injury mediated by activated microglia. J Neurosci. 2006;26:1730–1738. doi: 10.1523/JNEUROSCI.0702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Nagano T, Takemura M, Baba A. Topics on the Na+/Ca2+ exchanger responses of Na+/Ca2+ exchanger to interferon gamma and nitric oxide in cultured microglia. J Pharmacol Sci. 2006;102:22–26. doi: 10.1254/jphs.fmj06002x4. [DOI] [PubMed] [Google Scholar]
- McDowell SA, McCall E, Matter WF, Estridge TB, Vlahos CJ. Phosphoinositide-3-kinase regulates excitation-contraction coupling in neonatal cardiomyocytes. Am J Physiol Heart Circ Physiol. 2004;286:H796–H805. doi: 10.1152/ajpheart.00546.2003. [DOI] [PubMed] [Google Scholar]
- Milia AF, Gross V, Plehm R, De Silva JA, Jr, Bader M, Luft FC. Normal blood pressure and renal function in mice lacking the bradykinin B(2) receptor. Hypertension. 2001;37:1473–1479. doi: 10.1161/01.hyp.37.6.1473. [DOI] [PubMed] [Google Scholar]
- Nagano T, Kawasaki Y, Baba A, Takemura M, Matsuda T. Up-regulation of Na+/Ca2+ exchanger activity by interferon-γ in cultured rat microglia. J Neurochem. 2004;90:784–791. doi: 10.1111/j.1471-4159.2004.02511.x. [DOI] [PubMed] [Google Scholar]
- Nicolau M, Feltrin MR, Regoli D. Induction of bradykinin B1 hypotensive receptor in rats by lipopolysaccharide. Can J Physiol Pharmacol. 1996;74:337–340. [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Noda M, Kariura Y, Amano T, Manago Y, Nishikawa K, Aoki S., Wada K. Expression and function of bradykinin receptors in microglia. Life Sci. 2003;72:1573–1581. doi: 10.1016/s0024-3205(02)02449-9. [DOI] [PubMed] [Google Scholar]
- Noda M, Kariura Y, Amano T, Manago Y, Nishikawa K, Aoki S, Wada K. Kinin receptors in cultured rat microglia. Neurochem Int. 2004;45:437–442. doi: 10.1016/j.neuint.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Noda M, Kettenmann H, Wada K. Anti-inflammatory effects of kinins via microglia in the central nervous system. Biol Chem. 2006;387:167–171. doi: 10.1515/BC.2006.022. [DOI] [PubMed] [Google Scholar]
- Noda M, Kariura Y, Pannasch U, Nishikawa K, Wang L, Seike T, Ifuku M, Kosai Y, Wang B, Nolte C, Aoki S, Kettenmann H, Wada K. Neuroprotective role of bradykinin due to the attenuation of pro-inflammatory cytokine release from activated microglia. J Neurochem. 2007a;101:397–410. doi: 10.1111/j.1471-4159.2006.04339.x. [DOI] [PubMed] [Google Scholar]
- Noda M, Sasaki K, Ifuku M, Wada K. Multifunctional effects of bradykinin on glial cells in relation to potential anti-inflammatory effects. Neurochem Int. 2007b;51:185–191. doi: 10.1016/j.neuint.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Nolte C, Kirchhoff F, Kettenmann H. Epidermal growth factor is a motility factor for microglial cells in vitro: evidence for EGF receptor expression. Eur J Neurosci. 1997;9:1690–1698. doi: 10.1111/j.1460-9568.1997.tb01526.x. [DOI] [PubMed] [Google Scholar]
- Palkovits M. Neuropeptide messenger plasticity in the CNS neurons following axotomy. Mol Neurobiol. 1995;10:91–103. doi: 10.1007/BF02740669. [DOI] [PubMed] [Google Scholar]
- Pesquero JB, Araujo RC, Heppenstall PA, Stucky CL, Silva JA, Jr, Walther T, Oliveira SM, Pesquero JL, Paiva AC, Calixto JB, Lewin GR, Bader M. Hypoalgesia and altered inflammatory responses in mice lacking kinin B1 receptors. Proc Natl Acad Sci USA. 2000;97:8140–8145. doi: 10.1073/pnas.120035997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasman PO, Lebrun P, Cragoe EJ, Jr, Herchuelz A. Inhibition of Na/Ca exchange in pancreatic islet cells by 3′,4′-dichlorobenzamil. Biochem Pharmacol. 1991;41:1759–1768. doi: 10.1016/0006-2952(91)90181-4. [DOI] [PubMed] [Google Scholar]
- Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Prinz M, Kann O, Draheim HJ, Schumann RR, Kettenmann H, Weber JR, Hanisch UK. Microglial activation by components of gram-positive and -negative bacteria: distinct and common routes to the induction of ion channels and cytokines. J Neuropath Exp Neurol. 1999;58:1078–1089. doi: 10.1097/00005072-199910000-00006. [DOI] [PubMed] [Google Scholar]
- Quednau BD, Nicoll DA, Philipson KD. Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2 and NCX3 in rat. Am J Physiol. 1997;272:C1250–C1261. doi: 10.1152/ajpcell.1997.272.4.C1250. [DOI] [PubMed] [Google Scholar]
- Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res Rev. 1999;30:77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- Rappert A, Biber K, Nolte C, Lipp M, Schubel A, Lu B, Gerard NP, Gerard C, Boddeke HW, Kettenmann H. Secondary lymphoid tissue chemokine (CCL21) activates CXCR3 to trigger a Cl− current and chemotaxis in murine microglia. J Immunol. 2002;168:3221–3226. doi: 10.4049/jimmunol.168.7.3221. [DOI] [PubMed] [Google Scholar]
- Ritchie T, Cole R, Kim HS, de Vellis J, Noble EP. Inositol phospholipid hydrolysis in cultured astrocytes and oligodendrocytes. Life Sci. 1987;41:31–39. doi: 10.1016/0024-3205(87)90553-4. [DOI] [PubMed] [Google Scholar]
- Schilling T, Repp H, Richter H, Koschinski A, Heinemann U, Dreyer F, Eder C. Lysophospholipids induce membrane hyperpolarization in microglia by activation of IKCa1 Ca2+-dependent K+ channels. Neuroscience. 2002;109:827–835. doi: 10.1016/s0306-4522(01)00534-6. [DOI] [PubMed] [Google Scholar]
- Schilling T, Stock C, Schwab A, Eder C. Functional importance of Ca2+-activated K+ channels for lysophosphatidic acid-induced microglial migration. Eur J Neurosci. 2004;19:1469–1474. doi: 10.1111/j.1460-9568.2004.03265.x. [DOI] [PubMed] [Google Scholar]
- Schremmer-Danninger E, Offner A, Siebeck M, Roscher AA. B1 bradykinin receptors and carboxypeptidase M are both upregulated in the aorta of pigs after LPS infusion. Biochem Biophys Res Commun. 1998;243:246–252. doi: 10.1006/bbrc.1997.7999. [DOI] [PubMed] [Google Scholar]
- Stephens GJ, Marriott DR, Djamgoz MB, Wilkin GP. Electrophysiological and biochemical evidence for bradykinin receptors on cultured rat cortical oligodendrocytes. Neurosci Lett. 1993;153:223–226. doi: 10.1016/0304-3940(93)90327-h. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Takayama N, Ueda H. Morphine-induced chemotaxis and brain-derived neurotrophic factor expression in microglia. J Neurosci. 2005;25:430–435. doi: 10.1523/JNEUROSCI.3170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne P, Breittmayers J-P, Deval D, Frelin C, Lazdunski M. The Na+/Ca2+ antiporter in aortic smooth muscle cells. Characterization and demonstration of an activation by phorbol esters. J Biol Chem. 1988;263:8078–8083. [PubMed] [Google Scholar]
- Wakimoto K, Kobayashi K, Kuro-O M, Yao A, Iwamoto T, Yanaka N, Kita S, Nishida A, Azuma S, Toyoda Y, Omori K, Imahie H, Oka T, Kudoh S, Kohmoto O, Yazaki Y, Shigekawa M, Imai Y, Nabeshima Y, Komuro I. Targeted disruption of Na+/Ca2+ exchanger gene leads to cardiomyocyte apoptosis and defects in heartbeat. J Biol Chem. 2000;27:36991–36998. doi: 10.1074/jbc.M004035200. [DOI] [PubMed] [Google Scholar]
- Wang JY, Wang J, Golovina VA, Li L, Platoshyn O, Yuan JX. Role of K(+) channel expression in polyamine-dependent intestinal epithelial cell migration. Am J Physiol Cell Physiol. 2000;278:C303–314. doi: 10.1152/ajpcell.2000.278.2.C303. [DOI] [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh C, Dubyak G, Douglas JG. Relationship between phospholipase C activation and prostaglandin E2 and cyclic adenosine monophosphate production in rabbit tubular epithelial cells. Effects of angiotensin, bradykinin, and arginine vasopressin. J Clin Invest. 1988;81:710–719. doi: 10.1172/JCI113376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille PR, Vitor R, Gabilan NH, Nicolau M. Plasma extravasation mediated by lipopolysaccharide-induction of kinin B1 receptors in rat tissues. Mediators Inflamm. 2001;10:163–167. doi: 10.1080/09629350124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaga F, Hirata M, Koga T. Evidence for coupling of bradykinin receptors to a guanine-nucleotide binding protein to stimulate arachidonate liberation in the osteoblast-like cell line, MC3T3–E1. Biochim Biophys Acta. 1991;1094:139–146. doi: 10.1016/0167-4889(91)90001-e. [DOI] [PubMed] [Google Scholar]
- Yasuyoshi H, Kashii S, Zhang S, Nishida A, Yamauchi T, Honda Y, Asano Y, Sato S, Akaike A. Protective effect of bradykinin against glutamate neurotoxicity in cultured rat retinal neurons. Invest Ophthalmol Vis Sci. 2000;41:2273–2278. [PubMed] [Google Scholar]