Abstract
Neurons are continuously added to the brain throughout life, and these neurons must develop dendritic arbors and functional connections with existing neurons to be integrated into neuronal circuitry. The molecular mechanisms that regulate dendritic development of newborn neurons in the hippocampal dentate gyrus are still unclear. Here, we show that β-catenin is expressed in newborn granule neurons and in neural progenitor cells in the hippocampal dentate gyrus. Specific knock-out of β-catenin in newborn neurons, without affecting β-catenin expression in neural progenitor cells, led to defects in dendritic morphology of these newborn neurons in vivo. Majority of newborn neurons that cannot extend dendrites survive <1 month after they were born. Our results indicate that β-catenin plays an important role in dendritic development of postnatal-born neurons in vivo, and is therefore essential for the neurogenesis in the postnatal brain.
Keywords: conditional knock-out, β-catenin, dendritic development, newborns, dentate gyrus granule neurons, hippocampus
Introduction
Neurogenesis, the birth of new neurons, has been shown to occur throughout adulthood in certain regions of the mammalian brain. Specifically, neurons are continuously generated from neural progenitor cells (NPCs) in the subventricular zone (SVZ) of the lateral ventricles and subgranular zone (SGZ) of the hippocampal dentate gyrus (DG). Neurons generated in the SVZ migrate into the olfactory bulb and differentiate into granule and periglomerule interneurons (Alvarez-Buylla, 1997; Doetsch et al., 1999; Alvarez-Buylla and Garcia-Verdugo, 2002), whereas newborn neurons generated in the SGZ migrate into the dentate gyrus and differentiate into granule neurons (Eriksson et al., 1998; Gage, 2002; Taupin and Gage, 2002; van Praag et al., 2002; Kempermann et al., 2004; Kempermann et al., 2006) These postnatal-born neurons are electrically active and are capable of firing action potentials and receiving synaptic inputs (Markakis and Gage, 1999; Carlen et al., 2002; van Praag et al., 2002), suggesting that they are adequately integrated into existing neuronal circuitry. The finding that neurogenesis occurs in the postnatal mammalian CNS (Eriksson et al., 1998) has suggested promising therapeutic possibilities for treating CNS disease or injury, such as repairing CNS damage by NPC transplantation (Emsley et al., 2005; Taupin, 2005) or by inducing neurogenesis from endogenous NPCs (Magavi et al., 2000; Chen et al., 2004). To rebuild nerve circuitry and restore lost function, however, these newborn neurons must develop dendrites and axons that form connections with existing neurons to receive or send information.
Dendrites bear most of a neuron's synapses and are therefore the main input apparatus of a neuron. The development of a dendritic tree allows neurons to communicate with each other, and is therefore one of the crucially important steps in neurogenesis. Studies with isolated hippocampal granule neurons have provided growing evidence that dendritic morphology is regulated by extracellular signaling (McAllister et al., 1995; Redmond et al., 2000; Redmond and Ghosh, 2001; Whitford et al., 2002; Salama-Cohen et al., 2005), by intracellular molecules (Yu and Malenka, 2003), and by electrical activity (Wu et al., 1999, 2001; Chen and Ghosh, 2005) in vitro. However, the molecular mechanism that regulates dendritic development of newborn neurons in the postnatal brain remains a fascinating puzzle, and there is a lack of genetic models to study dendrite development in mammals in vivo. The intracellular protein β-catenin has been shown to play a critical role in dendritic morphology in cultured granule neurons isolated from hippocampal dentate gyrus (Yu and Malenka, 2003). Here, we demonstrated that β-catenin is expressed in neural progenitor cells and newborn dentate gyrus granule cells in the postnatal hippocampus. Because β-catenin null mice die during early embryonic development, the function of β-catenin in the postnatal brain is poorly understood. To study the role of β-catenin during differentiation of postnatal-born neurons, we have developed a mutant mouse to conditionally knock-out β-catenin in the postnatal-born neurons in the hippocampus. We observe a dramatic defect in dendritic arborization when β-catenin expression is interrupted, suggesting that this protein is involved in regulating dendritic development during neurogenesis in the postnatal brain in vivo.
Materials and Methods
Animal Care.
Mice were housed according to the principles outlined in the Guidelines for Care and Use of Experimental Animals. All procedures were approved by University of Kentucky, or by Massachusetts General Hospital/Harvard Medical School Institutional Animal) Care and Use Committees. The proopiomelanocortin (POMC-Cre) mouse line (B6.129) was kindly provided by Dr. B. B. Lowell at Beth Israel Deaconess Medical Center, Harvard Medical School (Boston, MA) to the Macklis laboratory and later transferred to the Chen Laboratory with permission. β-cateninflox/flox mice (B6.129-Ctnnb1tm2Kem/KnwJ) and Z/EG reporter mice [Tg(ACTB)-Bgeo/GFP021Lbe; stock #003920] were purchased from The Jackson Laboratory (Bar Harbor, ME).
Generation of POMC-Cre, β-cateninflox/flox mice.
The generation of POMC-Cre transgenic mice is detailed previously (Balthasar et al., 2004). POMC-Cre mice were bred to β-cateninflox/flox mice. All of the mice used in these studies were of mixed background, and littermates were used as controls. Animals were genotyped by PCR using genomic DNA extracted from mice tails using a Kit from Qiagen (Hilden, Germany). Primer sequences are as follows: 5′GAGATATCTTTAACCCTGATC 3′, 5′ TGGCTCAATGTCCTTCCTGG 3′ and 5′ CACATAAGCTGCATCGTTAAG 3′ for POMC-Cre, and 5′ AAGGTAGAGTGATGAAAGTTGTT 3′ and 5′ CACCATGTCCTCTGTCTATTC 3′ for β-cateninflox/flox.
Immunocytochemistry.
Animals were deeply anesthetized with an overdose of phenobarbital, and then perfused transcardially with cold 0.9% heparinized saline followed by fixative containing 4% paraformaldehyde (PFA) in PBS. Brains were postfixed overnight and cryoprotected with 30% sucrose for 48 h. Serial coronal sections (20–40 μm thick) were cut using a cryostat (Microm HM 500 M; Global Medical Instrumentation, Ramsey, MN) and stored at −80°C. Immunofluorescence was performed as follows. Sections were rinsed in PBS three times and incubated in blocking solution (0.1% Triton X-100, 1% bovine serum albumin, 5% normal goat serum in PBS) for 1 h at room temperature, followed by an overnight incubation with primary antibody at 4°C. Sections were then washed and incubated with the secondary antibody at room temperature for 2 h. After being treated with Hoechst 33258 or DAPI (4,6,diamidino-2-phenylindole) for 2 min, sections were washed with PBS three times and mounted using Fluorescentmount G. primary antibodies and their final concentrations were as follows: anti-Cre antibody (1:1000, rabbit; Covance, Princeton, NJ), anti-NeuN antibody (1:1000, mouse; Millipore, Temecula, CA), anti-GFAP antibody (1:100, mouse; Millipore), anti-doublecortin (Dcx) antibody (1:1000, guinea pig, Millipore), anti-BrdU antibody (1:400, rat; Accurate, Westbury, NJ), anti-GFP antibody (1:1000, rabbit and chick; Millipore), anti-β-catenin antibody (1:100, mouse; BD Biosciences, San Jose, CA), anti-Nestin antibody (1:50, rabbit; a gift from Dr. Jing, Shanghai Institute for Biological Science, Shanghai, China). Secondary antibodies from Jackson ImmunoResearch (West Grove, PA) were all applied at the same dilution of 1:1000. Cresyl violet was used for counterstaining.
BrdU administration and cell counting.
The control and conditional knock-out (cKO) mice (3 for each) were given 3 bromodeoxyuridine (BrdU) injections a day (50 mg/kg in 0.9% saline, i.p.; Sigma, St. Louis, MO) with 4 h apart at age postnatal day 21 (P21). Four weeks after injection, the brains were fixed as described above. BrdU immunohistochemistry was performed simultaneously on sections from all intervals. Series of every sixth section (240 μm apart) through each hippocampus were processed. Free-floating sections were washed twice in PBS, incubated in 2N HCl (30 min at 37°C), and rinsed in 0.1 m borate buffer, pH 8.4 (10 min). Then the immunostaining was performed after the procedure described above. DG area contours were created, and the volumes were measured using BioQuant (Nashville, TN) system. BrdU-positive cell in the DG (the granule cell layer, including the subgranular zone) were counted under a fluorescent microscope at 40× through whole series of sections. BrdU-positive cells were expressed as average number per cubic millimeter.
Retrovirus-mediated gene transduction to label newborn neurons in the postnatal hippocampus.
Concentrated enhanced green fluorescent protein (eGFP)-expressing retroviral solution (1.6 × 108 pfu/ml) was prepared as reported previously (Zhao et al., 2006). Retroviruses (1 μl) were stereotactically injected into the hippocampus of 5-week-old mice. A Drummond Scientific (Broomall, PA) nanoinjector II was used to deliver retrovirus halfway between bregma and lamda, 1.7 mm lateral to midline, and at a depth of 1.9 mm. Ten days after injection, mice were anesthetized with an overdose of phenobarbital and perfused transcardially with heparinized 0.9% saline followed by 4% paraformaldehyde. Brains were postfixed with 4% paraformaldehyde and equilibrated in 30% sucrose for 48 h. Brain sections (40 μm) throughout the rostrocaudal extent of the hippocampus were processed for immunostaining to identify the newborn neurons with antibody against eGFP to show the distinct morphology of newborn neurons in the hippocampus.
Image analysis.
Images were acquired using a Zeiss (Oberkochen, Germany) conventional inverted microscopy system Axiovert 200 M equipped with ApoTome. Confocal images were acquired using a Zeiss LSM510 microscope. Dcx- positive cells in the dentate gyrus at different stages (early stage A and late stage B) were counted by eye, and a total of six sections were used in the same position from three mice. In our analysis, Dcx-positive cells with bipolar short process, located adjacent to SGZ with the axis of cell body parallel to SGZ, were considered as early stage A cells. Cells with long dendrites projecting close to or crossing the molecular layer, with axis of cell body perpendicular to SGZ, were considered as late stage B cells (see Fig. 7). Data were analyzed statistically using student t tests. For morphological analysis of eGFP-positive neurons, neurons (n > 30, from three mice) in the sections at the similar rostral and caudal positions in the control mice and cKO mice were sampled. The confocal settings were chosen so that the GFP fluorescence was within the brightness range required to clearly visualize the entire cell, and they were maintained for all experiments of the same category. Series of z-stack images were collected, encompassing all neuronal processes of each cell. Total number of branches, the longest dendrite length, the total neurite length and the average neurite length were determined for each cell. The experimenter was blind to genotype during sampling, image analysis, data collection, and statistical analysis. Statistical analyses were performed with Student's t test. Significance level was taken as p < 0.05.
Figure 7.
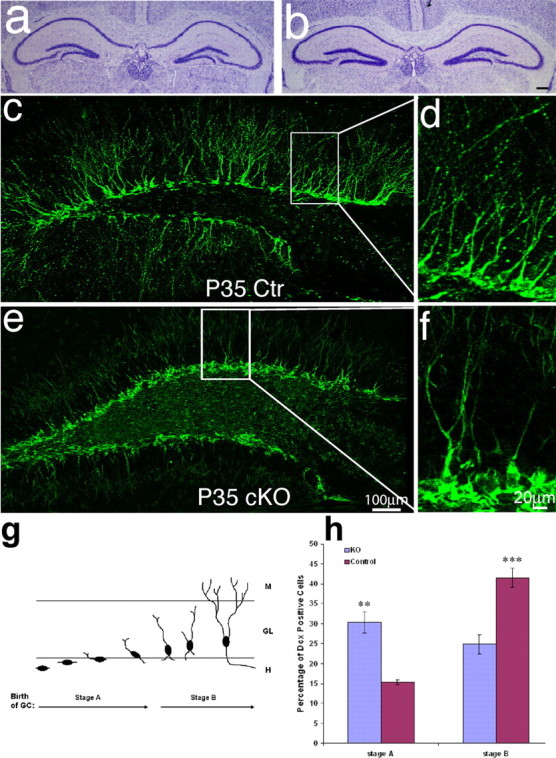
Dendrite malformation in mice lacking β-catenin in newborn granule neurons in the DG. a, b, Cresyl-violet staining for DG morphology. We did not find any obvious global malformation in DG between control and KO mice. c–f, Immunofluorescence labeling for Dcx (green) highly labeled the cell bodies and dendrites of newborn neurons. c, d, Dcx staining of control mice shows that most of the Dcx-positive newborn neurons develop long primary dendrites with extensive branching at the age of P35. e, f, The newborn neurons in the dentate gyrus of KO mice have no dendrites or have dendrites that lack branching. g, Diagram illustrating dendrite morphology in the early stages of neurogenesis in postnatal hippocampus. At stage A of neurogenesis, the axes of newborn neuron bodies are parallel to the granule cell layer but are becoming perpendicular to the granule cell layer. This is also the stage at which newborn neurons initiate dendrite outgrowth. At stage B of neurogenesis, newborn neurons extend dendrites, which approach the molecular layer and begin branching, thus developing a more complex dendrite morphology. h, Comparing the number of newborn neurons in stage A and late stage B, as determined by the length or morphology of dendrites, between the control mice and KO mice. Approximately 500 newborn neurons were counted and categorized as stage A or stage B. Scale bars: b, e, 100 μm; f, 20 μm.
Results
β-Catenin is expressed in newborn neurons of the postnatal dentate gyrus
To examine the expression of β-catenin in the postnatal hippocampus, we performed double immunostaining with antibody that recognize β-catenin and antibody that recognize Dcx, a marker for migrating newborn neurons. Brain sections from mice at P15 to adult were used for immunostaining. Using confocal microscopy, we found that β-catenin is expressed in most (>95%) of Dcx-positive cells (Fig. 1a–f) in the hippocampal DG from the brain at P25. β-Catenin is also expressed in the cells located in the SGZ under the Dcx-positive cell layer (Fig. 1a–f). Some of these β-catenin-positive newborn neurons are at the stage of extending their neurites toward the apical surface of the hippocampus (Fig. 1d–f, arrow), suggesting that β-catenin could be involved in dendrite development in these newborn neurons. In addition, β-catenin is expressed in the cytoplasm and is not detectable in the nucleus of newborn neurons (Fig. 1g–i). The β-catenin expression pattern in the cytoplasm is similar to its binding protein, cadherin, suggesting that β-catenin might be involved in the signaling of cadherin/catenin complex in the newborn neuron.
Figure 1.
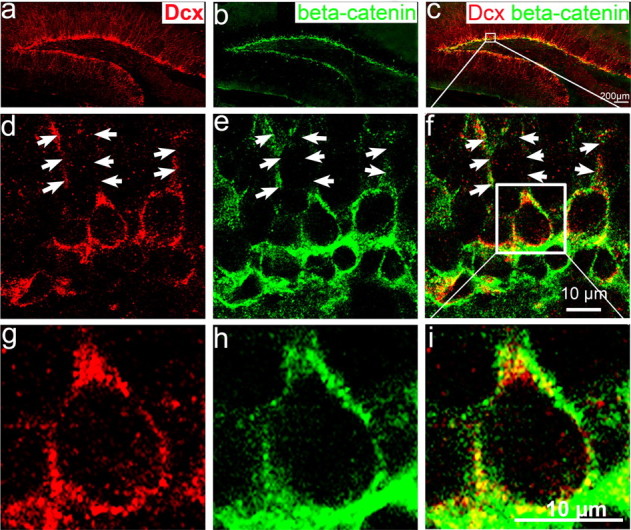
β-Catenin is expressed in postnatal dentate gyrus and colocalizes with Dcx-positive newborn neurons at the inner granular cell layer. Immunohistochemistry of hippocampal sections from wild-type mouse brain at P25 labeled with antibodies to the newborn neuronal marker Dcx (red) and to β-catenin (green). a, Dcx positive-cells located in the inner cell layer of dentate gyrus. b, β-Catenin is highly expressed in the SGZ and inner granular cell layer. c, Merged image of a and b showing that β-catenin is expressed in Dcx-positive newborn neurons adjacent to the SGZ. d, e, Confocal images of single focal section further shows that Dcx (d) and β-catenin (e) are highly expressed in the cytoplasm and in the processes of newborn neurons (arrows). f, Merged image of e and d showing that β-catenin colocalizes with the Dcx-positive newborn neurons in the dentate gyrus. g–i, Enlarged view of single newborn neuron within the white box in f showed that Dcx-positive newborn neurons (g) expresses β-catenin (h) in the cytoplasm (i), but not detectable in the nucleus.
Conditional knock-out of β-catenin in newborn neurons in the postnatal dentate gyrus
To study the function of β-catenin in newborn neurons in the DG, we used the Cre/Loxp system to selectively knock-out the β-catenin gene in newborn neurons in the postnatal DG, without affecting embryonic brain development. Excision of the β-catenin coding sequence in the brain was accomplished by crossing β-cateninflox/flox (B6.129-Ctnnb1tm2Kem/KnwJ; The Jackson Laboratory) with POMC-Cre transgenic mice generated in the laboratories of J. K. Elmquist and B. B. Lowell (Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA) (Balthasar et al., 2004). In POMC-Cre mice, the expression of the Cre recombinase is driven by the POMC promoter (Balthasar et al., 2004). Because the expression of Cre recombinase determines the temporal, spatial, and cell-type specificity of targeted gene deletion, we carefully analyzed the expression pattern of Cre recombinase in these animals.
To assess the spatial pattern of Cre expression in this line of POMC-Cre transgenic mice, we performed immunohistological analysis with an antibody against Cre recombinase. Our results show that Cre recombinase is expressed in several brain regions including the DG (Fig. 2a–d), the hypothalamus and the intermediate lobe of the pituitary gland (data not shown). This Cre expression pattern is similar to the pattern observed in another line of POMC-Cre transgenic mice that was generated in the same laboratory (Balthasar et al., 2004). However, we observed some differences as well. In this specific line of mice, we observed that Cre is highly expressed in the DG. The Cre-expressing cells in the DG of POMC-Cre transgenic mice are immediately adjacent to the SGZ, suggesting that these Cre-expressing cells might be newborn neurons (Fig. 2). We describe studies to determine the cell-type specificity of Cre expression below.
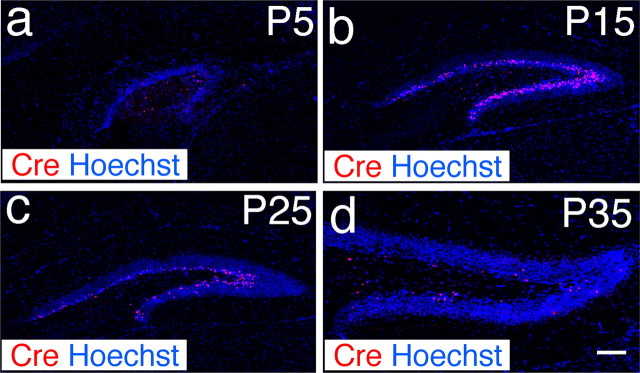
Figure 2.
Expression of Cre recombinase in the hippocampal dentate gyrus of POMC-Cre transgenic mice. a, Cre (red) is expressed in a few cells in the inner granule cell layer in 5-d-old (P5) transgenic mice. b–d, The number of Cre-expressing cells increases in P15 mice (b), then decreases at P25 (c) and remains low in P35 mice (d). Nuclei are stained with Hoechst 33258 in blue to show the dentate gyrus. Scale bar, 100 μm.
To determine the temporal pattern of Cre expression in the DG, we analyzed Cre recombinase expression immunohistologically in brain sections from POMC-Cre transgenic mice at various ages, including embryonic day 13 (E13), E15, E18, E21, P1, P5, P10, P15, P25, P35, and P63. We did not detect any Cre-positive cells in the embryonic brain, indicating that Cre recombinase is expressed only in the postnatal brain. We also did not detect any Cre expression in the DG of the POMC-Cre mice at P1. Increasing levels of Cre recombinase expression were detected in the DG beginning from P5 (Fig. 2a), with peak Cre expression detected at P15 (Fig. 2b). Expression levels then decreased dramatically at P25 (Fig. 2c), and remained at these low levels of expression after P35 (Fig. 2d). The temporal pattern of Cre expression correlates with the generation of new neurons in the DG. These findings further suggested that these Cre expression cells might be newborn neurons in the DG.
To identify the specific cell types that express Cre recombinase in the DG, we chose brain sections from POMC-Cre mice at P15 and performed double immunostaining using antibodies against Cre recombinase in conjunction with different cell-type-specific markers. The DG contains various types of cells, including NPCs in the SGZ, migrating newborn neurons, mature granule neurons, and astrocytes. We chose widely used cell-type-specific antibodies against Nestin, Dcx, NeuN, and GFAP to label NPCs, migrating newborn neurons, mature granule neurons, and astrocytes, respectively. We found that Cre protein was not expressed in NeuN-positive, mature neurons (Fig. 3a1–a4) or in GFAP-positive astrocytes (supplemental Fig. 1a–d), but was highly expressed in Dcx-positive migrating newborn neurons (Fig. 3b1–b4). These Cre-expressing cells were located immediately above the Nestin-positive cell layer (Fig. 3c1–c4), but did not colocalize with Nestin-positive cells. We further analyzed and documented Cre expression in the POMC-Cre mice using a confocal microscope. The confocal images clearly show that Cre is expressed in Dcx-positive newborn neurons (supplemental Fig. 2a,b, available at www.jneurosci.org as supplemental material), but not in Nestin-positive NPCs. Although, in some instances we observed Nestin-positive processes near Cre-expressing cells (supplemental Fig. 2c, available at www.jneurosci.org as supplemental material). To be absolutely sure that none of these Cre-expressing cells were NPCs, we performed a single injection of the thymidine analog BrdU to POMC-Cre mice at P15 and killed the animals 12 h after injection. The brains were collected for double immunostaining using an antibody against BrdU and an antibody against Cre. BrdU integrates into the chromosomes and labels proliferating NPCs. If Cre is expressed in proliferating, Nestin-positive NPCs, we would expect to observe BrdU-positive NPCs expressing Cre. However, we did not find any Cre-expressing cells colabeled with BrdU. Once again, the Cre-positive cells always localized above the BrdU-positive NPC cell layer (supplemental Fig. 2d, available at www.jneurosci.org as supplemental material). In a handful of cells in which Cre-positive cells appeared to contain a BrdU signal (supplemental Fig. 2d, available at www.jneurosci.org as supplemental material), we performed three-dimensional reconstruction of the confocal images and found that the Cre signal is simply superimposed on top of the BrdU signal (supplemental Fig. 2e, available at www.jneurosci.org as supplemental material). Therefore, in this strain of POMC-Cre transgenic mice, Cre is not expressed in proliferating NPCs, but only in postmitotic, newly generated neurons. When breeding this POMC-Cre mouse with another mouse carrying the floxed β-catenin allele, β-catenin should only be deleted in the newborn neurons in the DG, whereas the β-catenin gene in the NPCs should not be affected.
Figure 3.
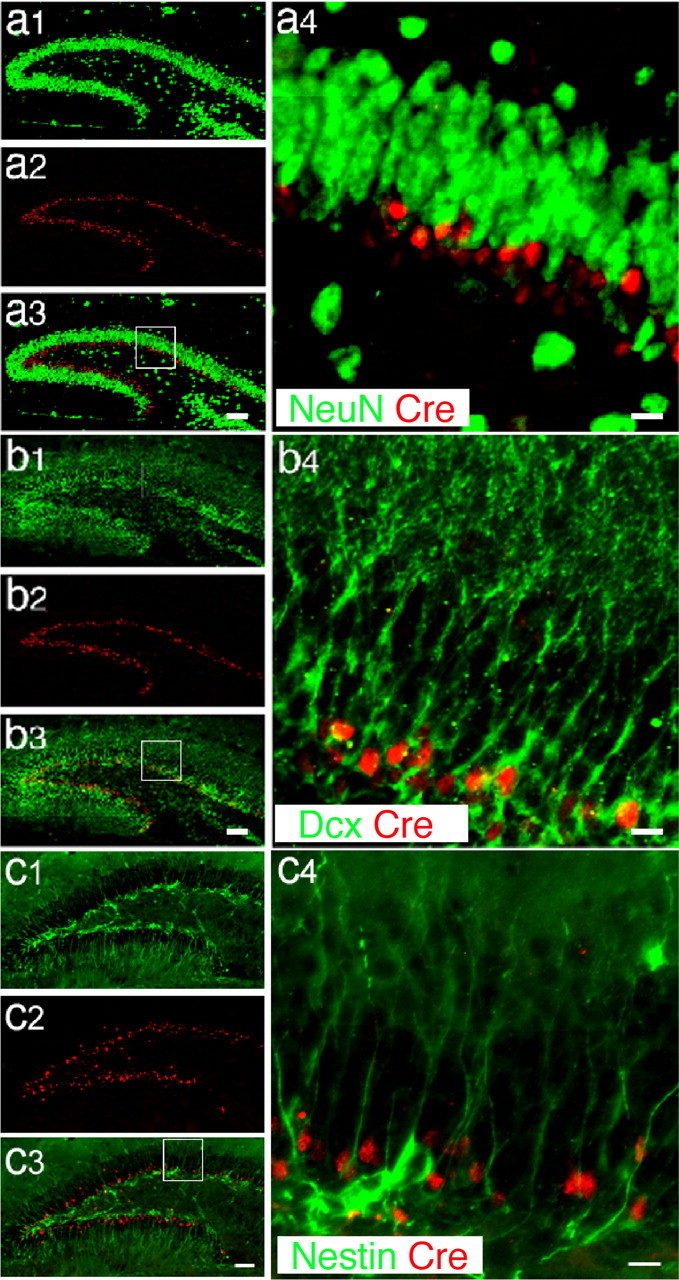
Cell-type specificity of Cre expression in POMC-Cre transgenic mice. a1–a4, Immunohistochemistry of hippocampal sections from POMC-Cre mice at P15, labeled using antibodies for Cre (red) and a neuron specific marker, NeuN (green). NeuN-positive granular neurons (a1; green) and Cre-positive newborn neurons (a2; red) are located in the postnatal dentate gyrus. In a3, the merged image of a1 and a2 shows that Cre-positive cells are located in the inner granular layer and are adjacent to NeuN-positive mature granular neurons. a4 is an amplified view of a3 of Cre (red) and NeuN (green) double staining to show that Cre is not expressed in NeuN positive cells. b1–b4, Immunohistochemistry of hippocampal sections using antibodies for Dcx (green), a marker of migrating newborn neurons, and Cre (red). The Dcx-positive neurons (b1; green) are located in the same layer as Cre positive cells (b2; red). The merged image (b3) and the enlarged view (b4) of these pictures show that Cre is highly expressed in Dcx-positive newborn neurons in the dentate gyrus. c1–c4, Immunofluorescence labeling for Nestin (green), a marker of neural progenitor cells, and Cre (red). The Nestin-positive cell bodies (green; b1, b3, and b4) are located below the Cre-positive cell layer (red; c2, c3, and c4). The enlarged view (c4) of the merged image (c3) shows that the Nestin is highly expressed in the processes of neural progenitors, but is very weakly expressed in the cell bodies. We do not see any colocalization of Nestin signals and Cre signals. These results suggest that Cre is specifically expressed in the Dcx positive-newborn neurons in the postnatal dentate gyrus. Scale bars: a3, b3, c3, 100 μm; a4, b4, c4, 20 μm.
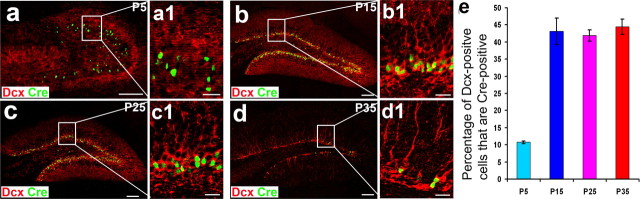
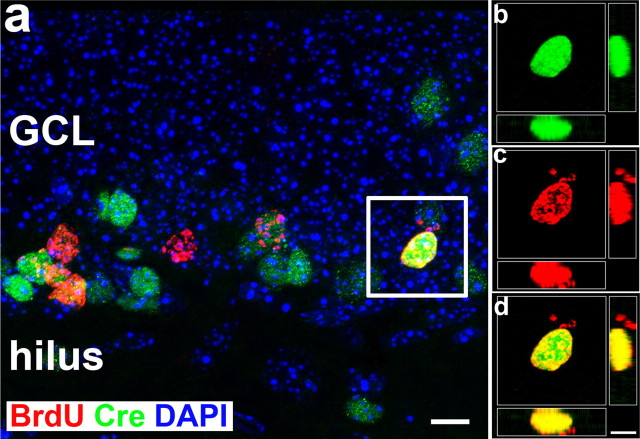
To determine the percentage of newborn neurons expressing Cre recombinase, we analyzed Cre recombinase expression and Dcx-positive newborn neurons in brain sections from POMC-Cre transgenic mice at various ages, including P5, P15, P25, and P35 using double immunostaining with antibodies against Cre and Dcx (Fig. 4). We found that Dcx-positive newborn neurons were increasingly generated from P5 to P15, and significantly decreased after 1 month. When we quantify the percentages of Dcx-positive newborn neurons expressing Cre recombinase, we found that there are 10.76 ± 0.37 of newborn neurons expressing Cre recombinase at P5 (Fig. 4e). The percentages of Dcx-positive newborn neurons expressing Cre recombinase increases to 43.13 ± 3.93 (Fig. 4e) at P15, and then maintains the similar percentages at P25 (41.97 ± 1.65) (Fig. 4e) and P35 (44.43 ± 2.23) (Fig. 4e). Because the Dcx-positive newborn neurons were born at the different dates, to determine the age of newborn neurons expressing Cre, the duration of Cre expression, and the percentages of newborn neurons at different ages expressing Cre recombinase, we administrated BrdU to transgenic mice (3 weeks old) that subsequently were perfused 3 d, 11 d, and 30 d after injection. We chose an injection paradigm (i.p. 50 mg/kg, three times for 1 d; interval, 6 h) (Cameron and McKay, 2001; Hayes and Nowakowski, 2002; Chen et al., 2004) to label the newborn neurons that were born at the date of BrdU administration. Confocal microscopy was performed to confirm that BrdU-labeled newborn cells express Cre (Fig. 5a–d). Quantification showed that 10.42% of the BrdU-positive cells were colabeled with Cre 3 d after the BrdU injection (n = 3; >500 BrdU-positive cells counted) and 69.75% of the BrdU-positive cells were colabeled with Cre 11 d after the BrdU injection; however, we did not observe any BrdU-positive cells were colabeled with Cre 30 d after the BrdU injection. These results suggest that the Cre expression in the newborn neurons are transient, beginning at the early age (day 3), lasting at least 11 d, and turning off before 30 d after they were born. Together, the high colocalization between BrdU and Cre at 11 d after the BrdU injection suggests that most, if not all, newborn granule neurons transiently express Cre recombinase.
Figure 4.
Quantification of newborn neurons expressing Cre recombinase. a–d, Immunostaining to show newborn neurons (Dcx-expressing cells; red) and Cre-expressing cells (green) in the dentate gyrus at p5 (a), p15 (b), p25 (c), and p35 (d). Scale bars: 100 μm. a1–d1, Enlarged views of a–d, respectively within the white boxes. Scale bars, 20 μm. e, The quantification of Cre-positive cells that were colocalized with Dcx at different ages (P5, 10.77 ± 0.37%; P15, 43.13 ± 3.93%; P25, 41.97 ± 1.66%; P35, 44.43 ± 2.23%; n > 5000 cells, from six sections).
Figure 5.
Cre recombines is expressed in BrdU-labeled newborn cells in the postnatal dentate gyrus (3 weeks old). a, Immunostaining to show BrdU-labeled newborn cells (red) and Cre-expressing cells (green) in the postnatal hippocampal dentate gyrus. b–d, High magnification of confocal image showing colocalization of Cre and BrdU within the white box in a from x, y, and z axes. Scale bars, 10 μm. GCL, Granule cell layer.
To further assess whether Cre expressed in newborn neurons can functionally delete a floxed target gene in vivo, we asked whether expression of a reporter was observed in Cre-expressing cells from our POMC-Cre:ROSA26 (The Jackson Laboratory) (Soriano, 1999) and POMC-Cre:Z/EG reporter mice [Tg(ACTB)-Bgeo/GFP021Lbe, stock #003920; The Jackson Laboratory] (Novak et al., 2000), in which LacZ or eGFP are expressed after Cre-mediated deletion of loxP-flanked stop codons (Novak, 2000). These mice should express β-galactosidase (β-gal) or eGFP in newborn neurons in the dentate gyrus, because these cells express the Cre recombinase. We stained brains from these double transgenic mice at P35 with X-gal or performed immunohistological analysis using an antibody against eGFP to characterize the regional and temporal pattern of LacZ and eGFP expression. LacZ staining or eGFP immunostaining showed that LacZ (data not shown) and eGFP (Fig. 6a,b) were expressed in the postnatal DG, suggesting that the transgenic Cre could functionally mediate the deletion of a floxed-targeted gene in vivo. The eGFP-positive cell layer (Fig. 6b) was much wider than the Cre expressing cell layer shown previously (Fig. 2). This is because the expression of Cre is transient whereas chromosomal reorganization is permanent: Cre is only expressed in the Dcx-positive newborn neurons and disappears in the NeuN-positive mature granule neurons, whereas eGFP expression begins when Cre mediates the targeted gene deletion in Dcx-positive newborn neurons and remains even after the newborn neurons differentiate into mature granule neurons. To further determine the percentage of total neurons in DG expressing eGFP, we stained the brain section from POMC-Cre;eGFP double transgenic mice at various ages including P5, P15, P25, P35, and P63 with antibodies against Cre (data not shown) and eGFP. The quantification shows that 12.5% (±1.04) of total DG neurons expresses eGFP at P5. The percentage increases to 39.1 ± 0.93% at P15, and then increase slightly after P15, to 42.5 ± 10.66% at P25, 44.9 ± 1.0% at P35, and 49.1 ± 0.57% at P63 (Fig. 6e). Because the majority of the neurons born at ∼P5 does not express Cre (Fig. 4e) or eGFP (Fig. 6e). Our findings suggested that a low percentage of newborn neurons express Cre (Fig. 4e) and eGFP (Fig. 6e) at the early postnatal dentate gyrus. The percentage of newborn neurons that express Cre (Fig. 2) or eGFP increases dramatically from P15 (Fig. 6); the transgenic Cre could functionally mediate the deletion of a floxed-targeted gene in vivo and ∼40–45% of neurons in DG are recombined in POMC-Cre transgenic mice.
Figure 6.
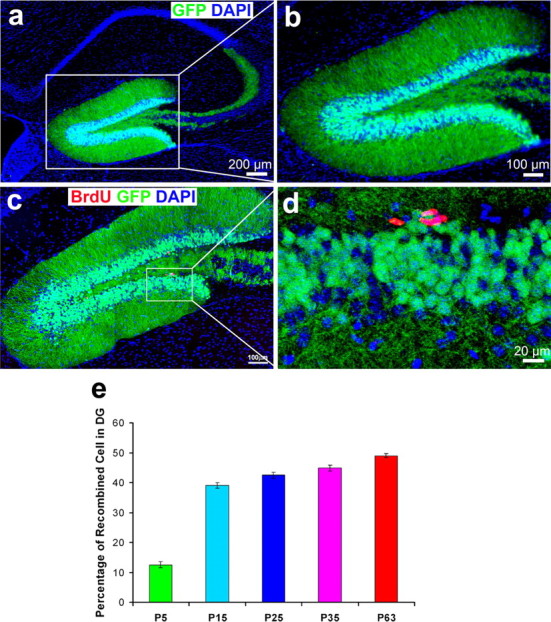
EGFP reporter (green) is expressed only in granule neurons not in proliferating neural progenitor cells (BrdU-positive cells; red). Immunohistochemistry of POMC-Cre;eGFP double transgenic mice (P35), in which eGFP was expressed after Cre-mediated deletion of a loxP-flanked stop codon. A, Immunofluorescence staining for eGFP (green) to show that the Cre recombinase can functionally delete the targeted gene fragment and induce the expression of eGFP in vivo. eGFP is highly expressed in granular neurons, including in dendrites and axons in the dentate gyrus. b, An enlarged view of a shows eGFP expression in the granular neurons of dentate gyrus. c, Double immunofluorescence staining for eGFP (green) and BrdU (red), a marker of proliferating cells, shows that BrdU-positive cells are located below the eGFP-positive cell layer. There are no eGFP and BrdU double-labeled cells in the dentate gyrus. d, An enlarged image of c. Scale bars: a, 200 μm; b, c, 100 μm; d, 20 μm. e, Quantification of neurons in DG expressing eGFP in the POMC-Cre;eGFP (Z/EG) double transgenic mice at various ages.
To further rule out the possibility that Cre-mediated gene deletion occurs in NPCs, we again performed a single injection of BrdU to pulse-label the proliferating neural progenitor cells in the POMC-Cre:eGFP (Z/EG) mouse at the age of P35 and perfuse the mice 12 h after injection. If Cre-mediated gene deletion had occurred in NPCs, we should have been able to detect BrdU and eGFP double-positive cells. As shown in Figure 6, c and d, the eGFP-positive cells are located above the BrdU-positive cell layer. We found no eGFP and BrdU double-positive cells. This finding suggests, once again, that Cre is not expressed in NPCs. In summary, the Cre recombinase driven by the POMC promoter was specifically expressed in newborn neurons in the postnatal DG, and Cre expression in POMC-Cre mice was effective at mediating a floxP-flank gene deletion in newborn neurons in the postnatal DG. It would be feasible to use this POMC-Cre transgenic mouse model to conditionally knock out the β-catenin gene in the DG from P5 onward without affecting embryonic development of the hippocampus.
To conditionally knock out β-catenin in newborn neurons in the postnatal DG, mice with a floxed β-catenin allele (β-cateninflox/flox) were bred with POMC-Cre transgenic mice. Mice with the genotype POMC-Cre;β-cateninflox/flox are homozygous cKO; in these mice, the Cre recombinase should mediate the deletion of both β-catenin alleles in newborn neurons. The expression of β-catenin in POMC-Cre or β-cateninflox/flox should not be affected, because these mice either do not have floxed β-catenin allele or do not express Cre recombinase. Double immunostaining using antibodies against β-catenin and an antibody against Cre was used to assess the expression of β-catenin protein in the DG at P25. Again, we found that in control mice the β-catenin protein was expressed in the cytoplasm of Cre-positive cells and the cell layer below it, which is in the subgranular zone where the NPCs were located (supplemental Fig. 3a–d, available at www.jneurosci.org as supplemental material). In cKO mice, the expression of β-catenin was eliminated specifically in Cre-positive neurons (supplemental Fig. 3e–h, available at www.jneurosci.org as supplemental material), but the expression of β-catenin in the subgranular zone, which is right below the Cre-positive cell layer, was not affected (supplemental Fig. 3e–h, available at www.jneurosci.org as supplemental material). Although the NPCs and newborn neurons were located immediately adjacent to each other, only the expression of β-catenin in the newly born neurons was affected. These results indicate exquisite specificity of β-catenin deletion in newborn neurons in the postnatal DG.
Conditional interruption of β-catenin expression results in severe dendrite malformation in newborn neurons in the postnatal DG
To study the function of β-catenin in dendrite development in newborn neurons in the postnatal DG, we examined the neuroanatomy of mice lacking β-catenin in newborn neurons. First, we performed cresyl violet staining to analyze the gross anatomy of brains from homozygous POMC-Cre;β-cateninflox/flox and control mice ages 5 weeks old. We did not see any obvious global malformation in the hippocampus in the cKO mice (Fig. 7a,b). To specifically identify newly born neurons and visualize their dendrites, we immunostained brain sections from mice at P35 using antibody against Dcx (Nacher et al., 2001; Brown et al., 2003; Rao and Shetty, 2004). We found that conditional knock out of the β-catenin gene resulted in a defect in dendrite development in newborn neurons in the postnatal DG (Fig. 7c–f). Whereas a high percentage of Dcx-positive newborn neurons had developed long, branched dendrites oriented toward the molecular layer in the hippocampus of control mice (Fig. 7c–d), the newborn neurons in cKO mice had no dendrites or only very short dendrites with less branches (Fig. 7e–f).
To further characterize this defect in dendritic morphology, we analyzed the morphology of newborn neurons lacking β-catenin. Neurogenesis in the postnatal DG has been divided into five stages, based on morphological characteristics (Zhao et al., 2006): stage A polarization, migration, initial axonal, and dendrite growth; stage B1, mainly neurite growth; stage B2, axons enter CA3 area; stage C, spine growth; and stage D, structural modification on spines. Because migrating newborn neurons expressing Dcx have not reached their functional location and have not yet developed spines or synapses, they are mostly at morphological stages A and B (Fig. 7g). We quantified the proportion of Dcx-positive newborn neurons at each stage in six similar sections from three cKO and three control mice at the age of P35. All Dcx-positive cells with full morphologies in the dentate gyrus were counted. The cell with full morphologies was defined as a cell with intact cell body, easily identified nuclear, and processes. The cells without full morphologies were not counted. In control mice, we found that 15.3% of Dcx-positive newborn neurons were in neurogenesis early stage A (lacking dendrites or bearing only short dendrites), and 41.5% were in late stage B (with long and complicated dendrite morphology) (Fig. 7c,d). In contrast, 30.4% of Dcx-positive newborn neurons were in neurogenesis stage A and only 24.9% were in stage B in the cKO mice (Fig. 7e,f). This result reflects an increase of 15% in Dcx-positive neurons in stage A (**p < 0.01) (Fig. 7h) and a 14.7% decrease in neurons in stage B (***p < 0.001) (Fig. 6h) in cKO mice, indicating that neurons lacking β-catenin are developmentally stalled in the early phases of dendrite development. This result indicates that β-catenin plays a crucial role in dendrite elongation and branching during neurogenesis in the postnatal dentate gyrus.
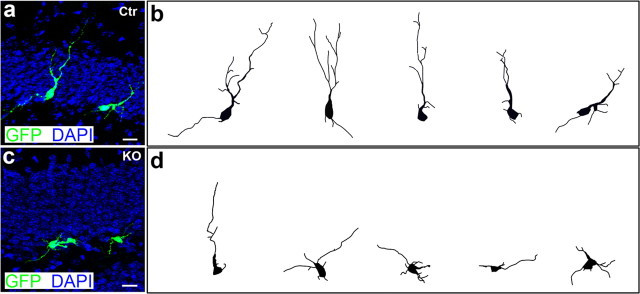
The previous morphological analysis was based on Dcx immunostaining. To further confirm the effects of β-catenin on dendrite development and to rule out the possibility that knock-out of β-catenin affects Dcx expression, we used retrovirus-mediated gene transduction to label newly born neurons in control and cKO mice, and repeated our morphological analysis in the adult hippocampus (Zhao et al., 2006). Retroviruses containing a GFP expression construct were injected into control and cKO mice at the age of 5 weeks old and subsequently perfused after injection. GFP expression was visualized using an antibody. The retrovirus led to strong eGFP expression in the newborn neurons (Fig. 8). Ten days after retrovirus injection, the newborn neurons (n > 30) in the control mice migrated into the inner granule layer. These neurons were appropriately polarized, containing elaborate dendrites with branches oriented toward the molecular layer, and morphologically resembled granule neurons (Fig. 8a,b). In the β-catenin knock-out mice, in contrast, the newborn neurons (n > 30) either did not have obvious dendrites or had very short dendrites (Fig. 8c,d). Additional quantification analysis indicates that newborn neurons in the cKO mice have significantly less number of neurites (control, 8.77 ± 0.44; β-catenin cKO, 5.37 ± 0.42; p < 0.0001) and shorter dendrite length than newborn neurons in the control mice. We assessed the longest neurite length, total neurite length, and average neurite length of each neuron. The longest neurite length in control is 75.58 ± 4.74 μm, whereas in β-catenin cKO it is 37.2 ± 3.45 μm (n > 30; p < 0.0001); the total neurite length in control is 172.47 ± 12.36 μm, and in β-catenin cKO is 81.89 ± 6.94 μm (n > 30; p < 0.0001). The average neurite length in control is 19.44 ± 0.86 μm, and in β-catenin cKO is 15.23 ± 0.78 (n > 30; p < 0.001). We also performed immunostaining to confirm that β-catenin expression in eGFP-expressing newborn neurons is diminished in cKO mice (supplemental Fig. 4, available at www.jneurosci.org as supplemental material). These results confirm that β-catenin is required for dendrite development of newborn neurons in the postnatal DG.
Figure 8.
Dendritic morphologies of newly generated neurons labeled by retrovirus-mediated eGFP expression. eGFP-expressing retroviruses were injected into the hippocampus to label newly generated neurons in control and β-catenin KO mice at 5 weeks old. a, Examples of eGFP-labeled newborn neurons in control mice 10 d after retrovirus injection. b, Drawings of representative neurons in control mice. c, Examples of eGFP-labeled newborn neurons in β-catenin KO mice 10 d after retrovirus injection. d, Drawings of representative neurons in β-catenin KO mice. Scale bars, 20 μm.
To determine the long-term survival of postnatal born neurons in the DG of cKO mice, we traced the fate of postnatal born neurons by injecting BrdU into the control mice and cKO at P21 (n = 3). The mice were allowed to survive for 28 d after BrdU injection before being killed to detect the survival of BrdU-positive cells in the dentate gyrus. Our result showed that there are 458 ± 7.1/mm3 of BrdU-positive cells in the DG of control mice; in contrast, there are only 236 ± 9.9/mm3 of BrdU-positive cells in the DG of cKO mice. The BrdU-positive cells in cKO DG mouse is obvious less in control one (p < 0.01). This result suggest that the newborn neurons with β-catenin mutant die before morphological mature.
Discussion
β-Catenin is involved in regulating many of the cellular features of the developing CNS, and it can affect neuronal morphology in vitro, but less is known about the function of β-catenin during normal neurogenesis in the adult. β-catenin is continuously expressed in neural stem cells and in newborn neurons in the adult brain, suggesting that it might play a role in postnatal precursor/stem cell fate determination and neuronal development. Indeed, it has been shown that β-catenin enhances the differentiation of neural precursors into neurons in the adult hippocampus (Lie et al., 2005). However, the function of β-catenin in the newborn neurons is mostly unknown. To investigate the function of β-catenin in the development of postnatal-born neurons, we crossed strains of mice to generate mice that lacked β-catenin exclusively in newly born neurons in the postnatal DG. This was achieved by expressing the Cre recombinase downstream of the POMC promoter, a protein that is activated in neurons shortly after their differentiation from precursors within the DG. Morphological analysis indicates that POMC-Cre mice express Cre recombinase in the dentate gyrus shortly after the precursors commit to becoming neurons, as they begin expressing Dcx. Furthermore, the BrdU tracing experiment suggests that most, if not all, newborn granule neurons transiently express Cre recombinase (Fig. 5). This result is consistent with a report on a POMC-GFP transgenic mouse (Overstreet et al., 2004). Cre recombinase expression caused deletion of β-catenin in newly differentiated neurons from mice containing a floxed β-catenin gene. Newborn neurons lacking β-catenin expressed the immature neuronal marker doublecortin and successfully completed early differentiation events, but failed to develop normal dendritic morphologies, as indicated by the appearance of short but incompletely formed dendritic arbors (Figs. 7, 8), and did not mature into hippocampal granule neurons. When we traced the fate of those postnatal born neurons using BrdU labeling, we found that newborn neurons that cannot extend dendrites survive <1 month after they were born. These results indicate that β-catenin is important for the development of the dendritic arbor in postnatal-born neurons of the dentate gyrus. Because the efficiency of recombination is very high, it is likely that the effect of β-catenin on dendritic development is autonomic. However, it will be interesting to analyze where conditional knock-out of newborn neurons in the dentate gyrus modifies the neural stem cell fate determination in the subgranular zone or the development of existing newborn neurons that were born before POMC promoter activation.
β-Catenin is a signaling molecule that both mediates gene transcription of the Wnt pathway (Dickinson and McMahon, 1992; Nusse and Varmus, 1992; Ille and Sommer, 2005) and stabilizes adhesive interactions through its interactions with cadherins (Schuman and Murase, 2003; Wheelock and Johnson, 2003; Abe et al., 2004; Hatzfeld, 2005; Salinas and Price, 2005). It will be interesting to understand which signaling pathways are involved in regulating dendrite development. Although cadherin and β-catenin were first identified as the proteins responsible for calcium-dependent cell–cell interactions, as the molecules involved in these interactions were characterized it became clear that cadherin and β-catenin have multiple functions during neuronal development, including dendrite morphogenesis and synapse formation. In the cytoplasm, β-catenin binds to the C terminus of the cadherin family of cell–cell adhesion molecules and stabilizes the actin cytoskeleton (Huber et al., 1997). Dendrite morphogenesis is a highly dynamic process that involves constant extension and retraction of branches, presumably through changes in the actin and microtubule cytoskeleton of dendrites. β-Catenin-mediated cadherin signaling might therefore be involved in dendritic morphogenesis by regulating the dynamics of actin formation. It has been shown that β-catenin promoted dendrite formation in cultured hippocampal neurons by a transcription-independent mechanism (Yu and Malenka, 2003). Our data show that β-catenin was highly expressed in the cytoplasm of newly born neurons in vivo, whereas we failed to detect β-catenin in the nuclei of doublecortin positive neurons by immunocytochemistry. Our data seems support the conclusion from in vitro study that the effect of β-catenin on dendrite formation in hippocampal neurons is independent of its function in the nucleus.
However, small amount of β-catenin below the detection level by immunostaining in the nucleus may drive the expression of Wnt-responsive gene expression. Therefore, more studies need to be done to determine which signaling pathway is involved in regulating dendritic development of the postnatal-born neurons in vivo.
Elucidating the mechanisms that regulate the development of dendrites in newly generated neurons in the postnatal brain is crucial for understanding how new neurons mature and integrate into the existing neural network, and could also provide insights into how alterations in this process contribute to disease.
Footnotes
This work was supported by the Kentucky Head and Spinal Cord Injury Research Trust (J.C.). Pilot work on the development of the β-catenin conditional knock-out mice was performed in the laboratory of J. D. Macklis and funded by National Institutes of Health Grants NS 45523, NS 49553, and NS 41590 (J.D.M.). P.A. was partially supported by a Claflin Distinguished Scholar Award and by the Amyotrophic Lateral Sclerosis Association. We thank Drs. Joel K. Elmquist, Bradford B. Lowell, and Roberto Coppari for providing POMC-Cre transgenic mice, and Drs. Edward Hall and George Smith for critical reading of this manuscript.
References
- Abe K, Chisaka O, Van Roy F, Takeichi M. Stability of dendritic spines and synaptic contacts is controlled by alpha N-catenin. Nat Neurosci. 2004;7:357–363. doi: 10.1038/nn1212. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A. Neurogenesis in the adult brain: prospects for brain repair. In: Gage FH, Christen Y, editors. Isolation, characterization and utilization of CNS stem cells. New York: Springer; 1997. pp. 87–100. [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Carlen M, Cassidy RM, Brismar H, Smith GA, Enquist LW, Frisen J. Functional integration of adult-born neurons. Curr Biol. 2002;12:606–608. doi: 10.1016/s0960-9822(02)00771-6. [DOI] [PubMed] [Google Scholar]
- Chen J, Magavi SS, Macklis JD. Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc Natl Acad Sci USA. 2004;101:16357–16362. doi: 10.1073/pnas.0406795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ghosh A. Regulation of dendritic development by neuronal activity. J Neurobiol. 2005;64:4–10. doi: 10.1002/neu.20150. [DOI] [PubMed] [Google Scholar]
- Dickinson ME, McMahon AP. The role of Wnt genes in vertebrate development. Curr Opin Genet Dev. 1992;2:562–566. doi: 10.1016/s0959-437x(05)80172-8. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci USA. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld M. The p120 family of cell adhesion molecules. Eur J Cell Biol. 2005;84:205–214. doi: 10.1016/j.ejcb.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Hayes NL, Nowakowski RS. Dynamics of cell proliferation in the adult dentate gyrus of two inbred strains of mice. Brain Res Dev Brain Res. 2002;134:77–85. doi: 10.1016/s0165-3806(01)00324-8. [DOI] [PubMed] [Google Scholar]
- Huber O, Krohn M, Kemler R. A specific domain in alpha-catenin mediates binding to beta-catenin or plakoglobin. J Cell Sci. 1997;110:1759–1765. doi: 10.1242/jcs.110.15.1759. [DOI] [PubMed] [Google Scholar]
- Ille F, Sommer L. Wnt signaling: multiple functions in neural development. Cell Mol Life Sci. 2005;62:1100–1108. doi: 10.1007/s00018-005-4552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Chesler EJ, Lu L, Williams RW, Gage FH. Natural variation and genetic covariance in adult hippocampal neurogenesis. Proc Natl Acad Sci USA. 2006;103:780–785. doi: 10.1073/pnas.0510291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- Nacher J, Crespo C, McEwen BS. Doublecortin expression in the adult rat telencephalon. Eur J Neurosci. 2001;14:629–644. doi: 10.1046/j.0953-816x.2001.01683.x. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Novak K. Lost in the FOG. Nat Med. 2000;6:864. doi: 10.1038/78615. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE. Wnt genes. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- Overstreet LS, Hentges ST, Bumaschny VF, de Souza FS, Smart JL, Santangelo AM, Low MJ, Westbrook GL, Rubinstein M. A transgenic marker for newly born granule cells in dentate gyrus. J Neurosci. 2004;24:3251–3259. doi: 10.1523/JNEUROSCI.5173-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Redmond L, Ghosh A. The role of Notch and Rho GTPase signaling in the control of dendritic development. Curr Opin Neurobiol. 2001;11:111–117. doi: 10.1016/s0959-4388(00)00181-1. [DOI] [PubMed] [Google Scholar]
- Redmond L, Oh SR, Hicks C, Weinmaster G, Ghosh A. Nuclear Notch1 signaling and the regulation of dendritic development. Nat Neurosci. 2000;3:30–40. doi: 10.1038/71104. [DOI] [PubMed] [Google Scholar]
- Salama-Cohen P, Arevalo MA, Meier J, Grantyn R, Rodriguez-Tebar A. NGF controls dendrite development in hippocampal neurons by binding to p75NTR and modulating the cellular targets of notch. Mol Biol Cell. 2005;16:339–347. doi: 10.1091/mbc.E04-05-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas PC, Price SR. Cadherins and catenins in synapse development. Curr Opin Neurobiol. 2005;15:73–80. doi: 10.1016/j.conb.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Schuman EM, Murase S. Cadherins and synaptic plasticity: activity-dependent cyclin-dependent kinase 5 regulation of synaptic beta-catenin-cadherin interactions. Philos Trans R Soc Lond B Biol Sci. 2003;358:749–756. doi: 10.1098/rstb.2002.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Taupin P. Adult neurogenesis in the mammalian central nervous system: functionality and potential clinical interest. Med Sci Monit. 2005;11:RA247–252. [PubMed] [Google Scholar]
- Taupin P, Gage FH. Adult neurogenesis and neural stem cells of the central nervous system in mammals. J Neurosci Res. 2002;69:745–749. doi: 10.1002/jnr.10378. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MJ, Johnson KR. Cadherin-mediated cellular signaling. Curr Opin Cell Biol. 2003;15:509–514. doi: 10.1016/s0955-0674(03)00101-7. [DOI] [PubMed] [Google Scholar]
- Whitford KL, Marillat V, Stein E, Goodman CS, Tessier-Lavigne M, Chedotal A, Ghosh A. Regulation of cortical dendrite development by Slit-Robo interactions. Neuron. 2002;33:47–61. doi: 10.1016/s0896-6273(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Wu GY, Zou DJ, Rajan I, Cline H. Dendritic dynamics in vivo change during neuronal maturation. J Neurosci. 1999;19:4472–4483. doi: 10.1523/JNEUROSCI.19-11-04472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nat Neurosci. 2001;4:151–158. doi: 10.1038/83976. [DOI] [PubMed] [Google Scholar]
- Yu X, Malenka RC. Beta-catenin is critical for dendritic morphogenesis. Nat Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]