Abstract
Fragile X syndrome is a common form of inherited mental retardation and is caused by loss of fragile X mental retardation protein (FMRP), a selective RNA-binding protein that influences the translation of target messages. Here, we identify protein phosphatase 2A (PP2A) as an FMRP phosphatase and report rapid FMRP dephosphorylation after immediate group I metabotropic glutamate receptor (mGluR) stimulation (<1 min) in neurons caused by enhanced PP2A enzymatic activity. In contrast, extended mGluR activation (1–5 min) resulted in mammalian target of rapamycin (mTOR)-mediated PP2A suppression and FMRP rephosphorylation. These activity-dependent changes in FMRP phosphorylation were also observed in dendrites and showed a temporal correlation with the translational profile of select FMRP target transcripts. Collectively, these data reveal an immediate-early signaling pathway linking group I mGluR activity to rapid FMRP phosphorylation dynamics mediated by mTOR and PP2A.
Keywords: FMRP, fragile X, phosphorylation, mGluR, synaptic plasticity, PP2A
Introduction
Fragile X syndrome is the most common form of inherited mental retardation caused by the absence of a normally pan-neuronal RNA binding protein, fragile X mental retardation protein (FMRP) (Brown et al., 1998; Darnell et al., 2001; Schaeffer et al., 2001). Indeed, FMRP interacts with ∼4% of mammalian brain mRNAs, the functional consequence of which is presently known for few messages (Brown et al., 2001). For these, FMRP can suppress translation [e.g., FMRP interacts with the murine MAP1B or its Drosophila homolog Futsch, and both exhibit enhanced expression in mouse and fly Fmr1 knock-outs (KOs)] as expected in the absence of FMRP translational suppression (Zhang et al., 2001; Lu et al., 2004).
Consistent with its influence over translation, FMRP associates with polysomes, especially intriguing in dendritic spines, because abnormal spine structure is one morphological feature seen consistently in both fragile X patients and the mouse model (Corbin et al., 1997; Feng et al., 1997a,b; Stefani et al., 2004). This indicates a role for FMRP in synaptic protein synthesis supported by the effect of group I metabotropic glutamate receptor (mGluR1/5) signaling on FMRP synthesis and transport (Antar et al., 2004). Indeed, protein synthesis-dependent group I mGluR-induced long-term depression (LTD) is exaggerated in the Fmr1 knock-out mouse. Thus, loss of FMRP translational suppression may lead to excessive translation of LTD-required protein(s) (Bear et al., 2004). Compatible with this model, exaggerated group I mGluR-LTD is protein synthesis independent in the Fmr1 knock-out mouse, suggesting that FMRP loss leads to constitutive overexpression of LTD-required protein(s) possibly effected in part by upregulation of extracellular signal-regulated kinase (ERK) and phosphatidyl inositol 3-kinase (PI3K)–mammalian target of rapamycin (mTOR) pathways (Nosyreva and Huber, 2006). Because FMRP is thought to negatively balance group I mGluR-mediated translation, its absence may cause a neuronal phenotype of excessive mGluR signaling. Indeed, high doses of the group I mGluR agonist (RS)-3,5-dihydroxyphenylglycine (DHPG) in wild-type (WT) neurons result in a spine morphology similar to that in Fmr1-deficient neurons (Vanderklish and Edelman, 2002) and in converse experiments, mGluR5 antagonists rescued some phenotypes observed in both mouse and Drosophila models of fragile X (McBride et al., 2005; Yan et al., 2005).
Collectively, these data suggest that FMRP regulates local protein synthesis, but the regulation of FMRP itself is poorly understood. Posttranslational modifications are well-known modifiers of activity-induced protein synthesis (Routtenberg and Rekart, 2005), making the phosphorylation of a conserved serine of FMRP a prime regulatory candidate of FMRP function. We have suggested previously that phosphorylated FMRP may associate with stalled ribosomes (Ceman et al., 2003). Thus, FMRP dephosphorylation maybe a key regulatory step in activity-dependent protein synthesis. Here, we identify protein phosphatase 2A (PP2A) as a major FMRP phosphatase and demonstrate that group I mGluR stimulation (<1 min) effects rapid, FMRP dephosphorylation caused by increased PP2A activity, whereas stimulation >1 min leads to FMRP rephosphorylation through mTOR-dependent PP2A suppression. These changes in FMRP phosphorylation also occur in dendrites and correlate temporally with expression of an FMRP ligand, synapse-associated protein, synapse-associated protein 90/postsynaptic density-95 (PSD-95)-associated protein 3 (SAPAP3). Together, we reveal an immediate-early group I mGluR-mediated pathway resulting in dynamic FMRP phosphorylation changes mediated by mTOR and PP2A.
Materials and Methods
Metabolic labeling and immunoprecipitation analyses.
Metabolic labeling was performed as described by Ceman et al. (2003). L cells and neurons were plated in T75 tissue culture flasks at a density of 5 × 106 and 3 × 105 cells/flask, respectively. The L cells were rinsed twice in phosphate-free DMEM (Invitrogen, San Diego, CA) the following day and then labeled in phosphate-free DMEM supplemented with 5% dialysed FCS and 1 mCi/ml 32P orthophosphoric acid (GE Healthcare, Waukesha, WI) for 6–10 h, unless otherwise indicated. The immunoprecipitate (IP) was conducted as described by Ceman et al. (2003). Metabolic labeling in neurons was performed as above, except that the neurons were maintained in low serum medium before labeling, and each IP required cell lysates prepared from 2 T75 tissue culture flasks plated at 3 × 105 cells/flask. The cytoplasmic lysates were generated as described previously and processed for FMRP IPs as described by Brown et al. (2001).
Constructs and transfection.
The HA-tagged WT and L199P PP2Ac constructs were used as described by Evans et al. (1999). The Flag tagged WT-FMRP and S499A-FMRP constructs were described previously by Ceman et al. (2003). The green fluorescent protein (GFP)-tagged WT and S499A FMRP lentiviral vectors were cloned by and obtained from Stephanie Ceman (personal communication). A standard protocol was used for transient transfection (supplemental methods, available at www.jneurosci.org as supplemental material).
PP1 and PP2A enzyme kinetics assay.
Enzyme activity was measured as in the study by Mao et al. (2005) with 4 × 105 neurons/assay using a serine-threonine phosphatase assay kit (17–127; Upstate Biotechnology, Lake Placid, NY) and following the manufacturer directions. To IP PP1 or PP2A, rabbit antibodies against PP1δ (Upstate Biotechnology) or mouse antibodies against PP2Ac were added to a total of 150 μg/200 μl lysate, followed by 50% Protein A agarose/Sepharose bead slurry and incubation for 1–2 h at 4°C. Beads were washed three times with PBS, followed by a single wash in assay buffer before the phosphopeptide was added to a final concentration of 0.75 mm and incubated for 10 min at 30°C.
Statistical analyses of the PP1/2A enzymatic activity.
Three independent enzyme assays were performed, and the fold change at various time points was measured as the average absorbance value at: time = t (in minutes)/average absorbance, at t = 0. Two-tailed paired t tests were used to determine the significance of the fold change at t = 0.5 and t = 1, compared with t = 0.
Drugs and drug treatments.
DHPG and 2-methyl-6-(phenylethynyl)-pyridine hydrochloride (MPEP) were purchased from Tocris Cookson (Bristol, UK). Neuron cultures were treated with the drugs in HBSS (Invitrogen). At the end of the drug treatment, the cells were quickly washed with ice-cold PBS (pH 7.4, Ca+2 free) and placed immediately on ice. The cell monolayer was rapidly scraped into ice-cold lysis buffer.
Neuron cultures.
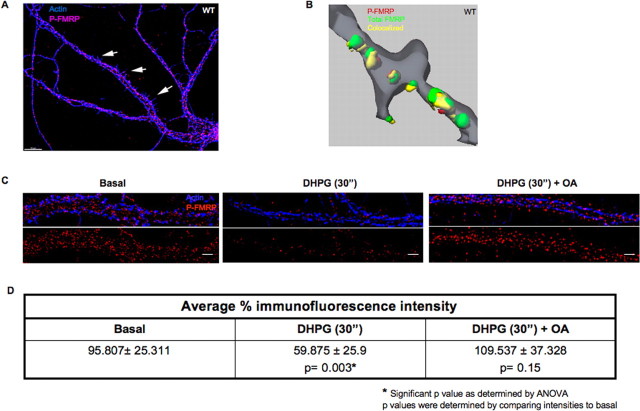
For biochemical assays, cryopreserved rat embryonic hippocampal neurons were obtained from Cambrex Biosciences (Walkersville, MD) and plated in T75 flasks treated with poly-l-lysine (Sigma, St. Louis, MO) in borate buffer. The cells were maintained in culture for 2 d in Eagle's minimum essential medium (Invitrogen) supplemented with 10% dialysed fetal calf serum, 10% horse serum, and 0.2–0.3 μg of recombinant basic FGF (Invitrogen) per flask. For the next 3 d, the neuronal cell population was enriched using 30 μm d-AP-5 (AG Scientific, San Diego, CA). For immunostaining (see Fig. 4), 14 d in vitro (DIV) embryonic hippocampal neuron cultures (supplemental methods, available at www.jneurosci.org as supplemental material) were fixed and stained with rabbit anti-phospho-FMRP (P-FMRP) raised to the phosphopeptide NSEAS*NAS*ETES*DHRDE (S* denotes phosphorylated serine), and the secondary staining was done as in the study by Antar et al. (2004). For assays in Figure 4, C and D, 14 DIV neuron cultures were stimulated with 50 μm DHPG (Tocris Cookson) for 30 s, with or without pretreatment with 10 nm okadaic acid (OA) before fixation with 4% buffered formaldehyde and stained for immunofluorescence (IF) as above.
Figure 4.
Rapid FMRP dephosphorylation was also detected in dendrites after DHPG-mediated group I mGluR activation. A, Phosphorylated FMRP is distributed throughout neuronal dendrites and spines. Wild-type rat hippocampal neurons fixed, labeled with a rabbit antibody to the phosphopeptide NSEAS*NAS*ETES*DHRDE (S* denotes phosphorylated serine) revealing abundant P-FMRP (red) in dendrites and spines (arrows) in the form of granules; actin (blue) was used as a cytoskeletal marker. B, 3-D reconstructed isosurfaces of double labeling assays from 14 DIV, wild-type rat hippocampal neurons showing the total FMRP (green), P-FMRP (red), and colocalized (yellow) granules in a distal dendrite stained with phalloidin (gray). C, Representative images of distal dendrites from hippocampal neurons at steady state, treated with 50 μm DHPG for 30 s in the presence/absence of 10 nm OA before fixation and immunofluorescence. D, Average percentage of immunofluorescence intensity of P-FMRP present in the distal dendrites was quantified using IP laboratory software. The table shows that percentage of basal and DHPG-treated (±OA) P-FMRP intensities in distal dendrites, and the SD was calculated using 10 neurons each from three different experiments, and the p value was found to be significant (p < 0.005) as measured by ANOVA.
Image acquisition and processing.
Neurons were imaged using a 60× Plan Neofluor objective and a 120 W metal-halide lamp (X-Cite) and Photometrics (Huntington Beach, CA) cooled CCD on a Nikon Eclipse 300 inverted microscope. Images acquired in Z series were deconvolved using the Autoquant software (Bitplane, Zurich, Switzerland) and a three-dimensional (3-D) blind algorithm.
Densitometric analyses.
Data from the Adobe Photoshop images (Adobe Systems, San Jose, CA) of three different experiments were normalized to tubulin and then calibrated and graphed using KaleidaGraph software version 3.5.
Antibodies and Western blot analyses.
For Western blotting seen in Figure 5A, 50 μg of total protein was loaded/sample, whereas in supplemental Fig. 3A (available at www.jneurosci.org as supplemental material), ∼100 μg of synaptosomal extracts were generated in a buffer containing 50 mm TrisCl, pH 7.5, 1% NP40, 100 mm NaCl, 5 mm MgCl2, and protease inhibitors, electrophoretically resolved, transferred onto polyvinylidene difluoride membranes, and probed with the P-FMRP-specific antibody (used at 1:300). The antibodies used are listed in detail in the supplemental data (at www.jneurosci.org as supplemental material).
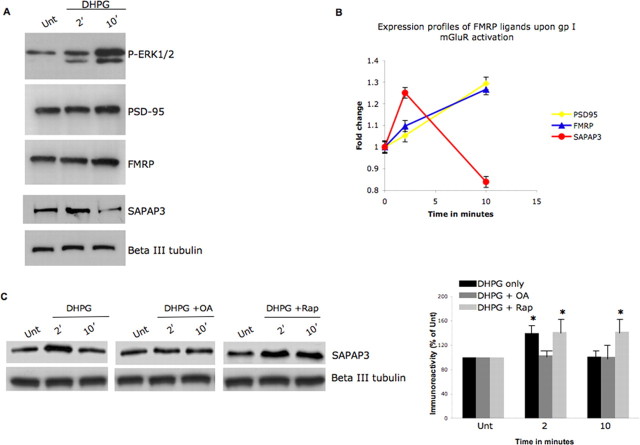
Figure 5.
Group I mGluR activity-dependent FMRP phosphorylation correlates with translation of FMRP ligand SAPAP3. A, Neurons were treated with 100 μm DHPG for 0 [untreated (Unt.)], 2, or 10 min, and protein expression levels of the FMRP ligands were examined by Western blotting cytoplasmic cell lysates. A synapse associated protein, SAPAP3, shows changes in protein expression that correlate with the time course of FMRP phosphorylation. B, Densitometry analyses of the changes in FMRP ligand expression in the presence of 100 μm DHPG in primary hippocampal neurons. Error bars represent the SD between sample triplicates in the assay. C, Neurons were treated with 100 μm DHPG for 0 (untreated), 2, or 10 min in the presence of DHPG alone/DHPG stimulation in the presence of 10 nm OA or 20 μm rapamycin. SAPAP3 protein expression levels as measured by Western blotting of cytoplasmic cell lysates show PP2A activity-sensitive changes in protein expression. In the right panel, SAPAP3 immunoreactivity was normalized to β-III tubulin under the conditions studied, calculated as ± SEM, and represented as a histogram (n = 4; the asterisk denotes significance compared with untreated with a Student's t test, *p < 0.05).
Results
Protein phosphatase 2A is a major FMRP phosphatase
To identify the phosphatase acting on FMRP, we examined the most thoroughly characterized neuronal serine-threonine phosphatases: protein phosphatase 1, 2A, and 2B (Honkanen et al., 1990; Winder and Sweatt, 2001). Individual phosphatase activities were inhibited pharmacologically by applying microcystin, OA, and deltamethrin at enzyme IC50 values of 0.1, 0.5, and 0.2 nm, respectively. At these concentrations, deltamethrin is PP2B-specific and OA is PP2A-specific, whereas microcystin inhibits both PP1 and 2A (Honkanen et al., 1990; Namboodiripad and Jennings, 1996; Dounay and Forsyth, 2002).
We initially evaluated the candidate FMRP phosphatases by metabolic labeling of FMRP with 32P orthophosphate in previously described mouse L cell lines expressing Flag-tagged FMRP (WT) or the Flag tag (VC) alone (Ceman et al., 2003). Cell lysates prepared in the presence of the phosphatase inhibitors (applied individually, as a mixture) were used to IP FMRP using the Flag tag. Subsequent autoradiogram analyses detected a band of the appropriate molecular size, ∼80 kDa, indicating that FMRP phosphorylation was sustained by the phosphatase inhibitor mixture; unlabeled FMRP detected by Western blotting was used as an IP control (Fig. 1A). Okadaic acid (0.5 nm) by itself resulted in similar P-FMRP amounts as seen with the mixture, whereas microcystin (0.1 nm) showed reduced P-FMRP levels. Inhibiting PP2B alone using deltamethrin failed to yield any detectable P-FMRP (Fig. 1A). These data implicated PP2A as an FMRP phosphatase.
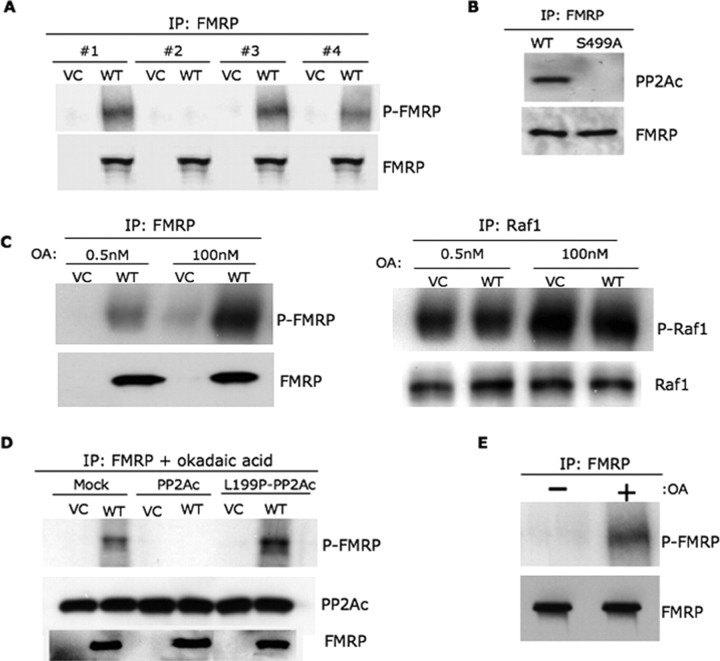
Figure 1.
PP2A is a major FMRP phosphatase in non-neuronal cells. A, Pharmacological inhibitors of serine-threonine phosphatase reveal PP2A as a candidate FMRP phosphatase. L cells stably expressing the Flag-tag (VC) or Flag-FMRP (WT) were used in metabolic labeling assays with 32P orthophosphate in the presence of the phosphatase inhibitors deltamethrin, OA, and microcystin, either applied individually (# 2–4, respectively) or as a mixture (# 1). P-FMRP was identified by autoradiography, and Western blotting for FMRP was used as a loading control. B, Only wild-type FMRP (WT) and not nonphosphorylated FMRP (S499A) was found to coimmunoprecipitate with the catalytic subunit of PP2A, PP2Ac. Western blotting for FMRP was used as a loading control. C, FMRP becomes hyperphosphorylated after treatment with 100 nm OA compared with 0.5 nm OA (left panel) in a manner similar to well-known PP2A substrate, Raf1 (right panel). Western blotting for FMRP and Raf1 were used as loading controls. D, WT or dominant-negative mutant (L199P) of PP2Ac, the catalytic subunit of PP2A, was transfected into L cells; untransfected L cells were used as a control (mock). Metabolic labeling analyses were conducted in the presence of 0.5 nm OA, and Western blotting for PP2Ac was used as a transfection control. E, PP2A is a major FMRP phosphatase in primary hippocampal neurons. Metabolic labeling assays were conducted in primary rat hippocampal neurons in the presence/absence of 0.5 nm OA, as indicated, and IPs were conducted using FMRP antibodies. Western blotting for FMRP was used as a loading control. All the biochemical assays were repeated three times, unless otherwise stated.
Consistent with the previous work showing phosphatase subunits associated with their target proteins (Thelin et al., 2005), we successfully immunoprecipitated both the PP2A catalytic and regulatory subunits (PP2Ac and PR65, respectively) with FMRP from L cell lysates (Lechward et al., 2001) (supplemental Fig. 1A, available at www.jneurosci.org as supplemental material). Importantly, this association between PP2Ac and FMRP was absent in IPs from lysates of L cells stably expressing Flag-S499AFMRP, which contains a serine to alanine change at position 499 and represents nonphosphorylated FMRP (Ceman et al., 2003) (Fig. 1B). Next, we conducted hyperphosphorylation assays to determine phosphatase specificity using the phosphoprotein amount recovered as a direct correlate of the phosphatase inhibitor concentration. As shown in Figure 1C (left panel), more P-FMRP was recovered after exposure to 100 nm OA compared with 0.5 nm, mimicking the phosphorylation pattern of Raf1, a bona fide PP2A substrate (Fig. 1C, right panel) (Bottorff et al., 1995; Adams et al., 2005). However, it should be noted that okadaic acid at 100 nm inhibits both PP2A and PP1. Thus, we tested FMRP phosphorylation by causing a specific molecular disruption of PP2A by using a PP2A dominant-negative mutant, L199P (Evans et al., 1999; Kins et al., 2001) (Fig. 1D). Metabolic labeling assays were conducted with the catalytic subunits of wild-type PP2A or L199P-PP2A (PP2Ac and L199P-PP2Ac) transiently transfected into VC and Flag-FMRP (WT) stable cell lines; mock transfected cell lines were used as a control. Transfected WT PP2Ac did not allow detection of P-FMRP either in the presence (Fig. 1D) or absence (supplemental Fig. 2, available at www.jneurosci.org as supplemental material) of OA. However, P-FMRP amounts recovered increased with L199P-PP2Ac compared with the mock-transfected culture even in the presence of 0.5 nm OA. Thus, disrupting PP2A specifically using a dominant-negative mutant increased FMRP phosphorylation. All these findings argue for PP2A as a major FMRP phosphatase in non-neuronal cells.
To determine whether this held true in neuronal cells, we performed metabolic labeling in primary hippocampal neurons and showed that P-FMRP was only recovered in the presence of 0.5 nm OA (Fig. 1E). Additionally, both PP2Ac and PR65 coimmunoprecipitated with FMRP in these neurons (supplemental Fig. 1B, available at www.jneurosci.org as supplemental material). These data validate PP2A as a major FMRP phosphatase in primary neurons.
Group I mGluR stimulation enhances PP2A activity within 60 s and results in rapid FMRP dephosphorylation
Synaptic signaling through group I mGluRs has been found recently to influence PP2A activity in primary neuron cultures (Mao et al., 2005). Having recognized PP2A as an FMRP phosphatase, we examined FMRP phosphorylation after chemical group I mGluR stimulation both in the presence/absence of OA (Fig. 2A, top and bottom panels). We performed metabolic labeling followed by FMRP immunoprecipitations in primary hippocampal neurons after exposure to either 100 μm DHPG, a group I mGluR agonist, or 10 μm MPEP, an mGluR5 antagonist, for 10 or 30 min; untreated neurons were used as a control. In the presence of 0.5 nm OA, DHPG stimulation at both time points markedly increased FMRP phosphorylation, whereas MPEP treatment reduced P-FMRP levels relative to the DHPG-untreated cultures (Fig. 2A, top panel). We also investigated P-FMRP in the absence of OA and found that DHPG-untreated neurons revealed no detectable P-FMRP in the absence of okadaic acid presumably because of steady-state PP2A activity in the cell lysates (Fig. 2A, bottom panel). Strikingly, DHPG applied for 5, 10, or 30 min sustained P-FMRP even in the absence of OA. This observation is consistent with previous work, showing that group I mGluR stimulation can suppress PP2A activity (Mao et al., 2005). Collectively, these data indicate that FMRP phosphorylation is group I mGluR activity dependent.
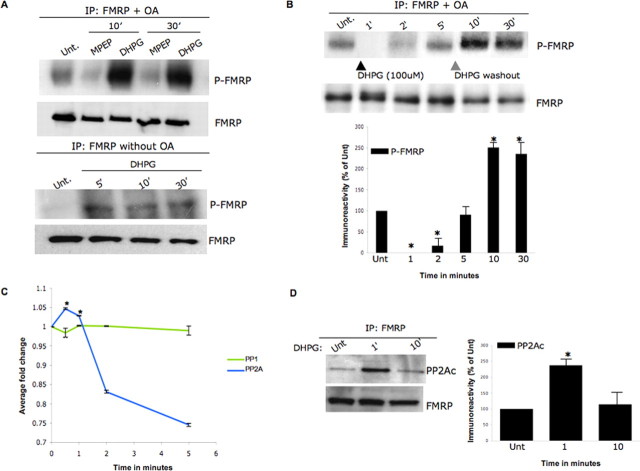
Figure 2.
FMRP phosphorylation is dependent on group I mGluR and PP2A activity. A, FMRP phosphorylation is group I mGluR activity dependent. Primary hippocampal neurons were treated with 100 μm DHPG (group I mGluR agonist) or 10 μm MPEP (mGluR5 antagonist) for the time points indicated in the presence/absence of 0.5 nm OA (top and bottom panels, respectively) and analyzed by metabolic labeling; a DHPG-untreated (Unt.) sample was used as control. Western blotting for FMRP was used as a loading control. B, Time course study of FMRP phosphorylation in primary neurons reveals that FMRP is rapidly dephosphorylated after 1 min of (100 μm) DHPG-mediated group I mGluR stimulation in the presence of 0.5 nm OA. FMRP phosphorylation was monitored at 1, 2, 5, 10, and 30 min, and DHPG was washed out after 5 min for physiological relevance, as indicated by the gray arrowhead. Western blotting for FMRP was used as a loading control. In the bottom panel, P-FMRP signal was normalized to FMRP in the IPs, calculated as ± SEM, and represented as a histogram (n = 3; the asterisk denotes significance compared with untreated with a Student's t test, *p < 0.05). C, PP2A enzyme activity profiling after DHPG treatment. Primary hippocampal neurons were treated for 0.5, 1, 2, and 5 min with 100 μm DHPG; an untreated sample was used as a control. Neurons were harvested to examine enzyme activities of both PP1 and 2A after IPs using the serine-threonine phosphatase assay kit (Upstate Biotechnology). Error bars represent the SD between the three independent experiments, and asterisks indicate that the fold change as measured by two-tailed paired t tests at the 0.5 and 1 min time points (when compared with time point 0) was highly significant (<0.005). D, Protein phosphatase 2A association with FMRP is sensitive to group I mGluR activity changes. Western blot analyses of FMRP IPs from primary hippocampal neurons were conducted in the presence of DHPG (100 μm) applied for 1 or 10 min; a DHPG untreated sample was used as a control. As a control, the samples were probed for FMRP. In the right panel, PP2Ac immunoreactivity was normalized to FMRP in the IPs, calculated as ±SEM, and represented as a histogram (n = 4; the asterisk denotes significance compared with untreated with a Student's t test, *p < 0.05).
Nevertheless, the observed pattern of FMRP phosphorylation is inconsistent with our hypothesis that phosphorylated FMRP may cause translational suppression, because synaptic stimulation is known to enhance translation (Raymond et al., 2000; Ceman et al., 2003). To determine whether we missed an early wave of FMRP dephosphorylation, we analyzed FMRP phosphorylation immediately after DHPG stimulation (Fig. 2B, top and bottom panels). Remarkably, neurons exposed to DHPG for 1 min revealed no detectable P-FMRP compared with the DHPG-untreated cultures. By 2 min, some P-FMRP is apparent, and by 10 min (5 min after washout), FMRP is heavily rephosphorylated, a pattern that is maintained for at least 25 min after washout. We confirmed these dynamics of FMRP phosphorylation in the absence of OA (Fig. 3A). Together, these data indicate that immediately after group I mGluR activation, FMRP shows dynamic changes in phosphorylation. Also, it is possible that immediate FMRP dephosphorylation after synaptic stimulation may release its translational suppression for a brief period, after which FMRP phosphorylation is quickly restored.
Figure 3.
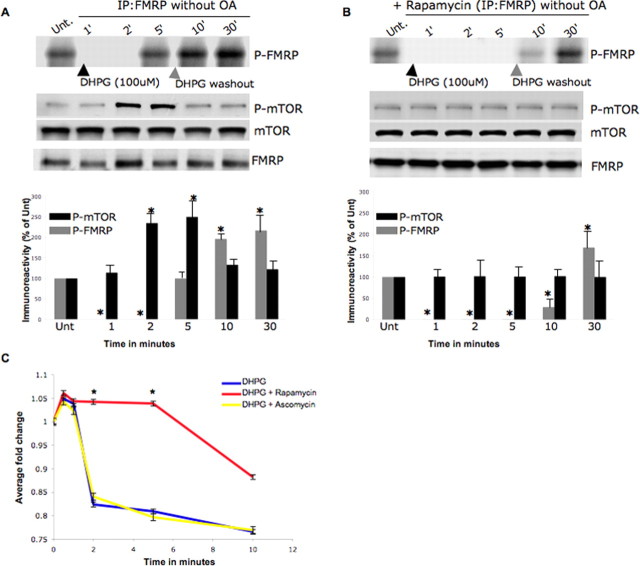
Extended (1–5 min) group I mGluR stimulation results in FMRP phosphorylation though mTOR-dependent PP2A suppression. A, B, Time course study of FMRP phosphorylation in the presence of 100 μm DHPG alone or after 20 μm rapamycin treatment (B). Metabolic labeling assays conducted in primary neurons reveal a delay in FMRP phosphorylation. FMRP phosphorylation was monitored at 1, 2, 5, 10, and 30 min, and DHPG was washed out after 5 min for physiological relevance, as indicated by the gray arrowhead. The DHPG-untreated (Unt.) samples contained 0.5 nm OA so that basal P-FMRP could be detected; Western blotting for FMRP was used as an IP control. In the bottom panels, P-FMRP signal was normalized to FMRP in the IPs, and P-mTOR was normalized to total mTOR, calculated as ± SEM and represented as a histogram (n = 3; the asterisk denotes significance compared with untreated with a Student's t test, *p < 0.05). C, PP2A enzyme activity profiling in the presence/absence of mTOR activation. Primary hippocampal neurons were treated with 20 μm rapamycin or an inactive chemical analog, ascomycin, and followed by treatment for 0.5, 1, 2, 5, and 10 min with 100 μm DHPG/DHPG stimulation; a DHPG-untreated sample was used as a control. Neurons were harvested to examine enzyme activities of PP2A after IPs using the serine-threonine phosphatase assay kit. Error bars represent the SD between three independent experiments, and asterisks indicate that the fold change as measured by two-tailed paired t tests at the 2 and 5 min time points between DHPG plus rapamycin (red) and DHPG alone (blue) was highly significant (<0.005).
To ascertain whether alterations in PP2A activity mediate activity-dependent FMRP phosphorylation dynamics, we conducted endpoint enzymatic assays on PP2A or PP1 (control) from DHPG-treated neurons using a synthetic phosphopeptide substrate (Fig. 2C). We found that PP2A activity increased with statistical significance within 30 s of DHPG exposure falling below baseline by 2 min, agreeing with the time course of FMRP phosphorylation. The suppression of PP2A after 2 min of DHPG exposure has been reported previously (Mao et al., 2005), but the early, transient increase in enzymatic activity after synaptic stimulation apparently escaped detection in the study. To further assess the link between activity-dependent changes in FMRP phosphorylation and PP2A, we performed IPs with FMRP in the presence of DHPG for either 1 or 10 min; an untreated sample was used as control (Fig. 2D, left and right panels). These time points were chosen as FMRP was dephosphorylated at 1 min and phosphorylated at 10 min in the presence of DHPG. We found that the catalytic subunit of PP2A, PP2Ac, showed an increased association with FMRP at 1 min compared with 10 min (Fig. 2D, left and right panels). Thus, the increase in association of PP2Ac and FMRP concurs temporally with the period of maximal PP2A activity and presumably occurs with the substrate, P-FMRP. These data further strengthen the argument that the modest PP2A activation (∼5%) seen at 1 min in enzymatic assays may well be enough for the rapid FMRP dephosphorylation seen in Figure 2B. Our data reveal a novel, immediate, and transient burst of PP2A activity after group I mGluR stimulation that precedes the established decline in PP2A activity. Also, PP2A-sensitive phosphoproteins, including FMRP, may experience a transient unphosphorylated state (Figs. 2B, 3A) after mGluR stimulation.
Group I mGluR stimulation beyond 1 min leads to mTOR-mediated PP2A suppression and FMRP phosphorylation
Group I mGluR stimulation is known to activate mTOR signaling, which regulates synaptic protein synthesis (Daw et al., 2002). Moreover, mTOR is known to suppress PP2A activity in the presence of growth factor agonists, viz. epidermal growth factor (Peterson et al., 1999; Van Kanegan et al., 2005). Because FMRP phosphorylation was found to be both group I mGluR and PP2A activity dependent, we investigated the role of mTOR signaling in this process (Fig. 3A,B). We measured P-FMRP in a time course analysis after DHPG stimulation with or without preincubation with 20 μm rapamycin, an mTOR inhibitor (using a DHPG-untreated but OA-treated sample as control). In the absence of OA (Fig. 3A, top and bottom panels), P-FMRP is detected at 5 min of DHPG stimulation, retaining the previously noted temporal pattern thereafter (the lack of OA in these experiments prevented detection of P-FMRP at 2 min). DHPG stimulation after preincubation with rapamycin delayed FMRP phosphorylation, with low levels of P-FMRP being detectable only at 10 min (Fig. 3B, top and bottom panels). To confirm rapamycin efficacy, we monitored phospho-mTOR and total mTOR levels. As anticipated, we found mTOR phosphorylation increased at 2 and 5 min after DHPG treatment, a pattern absent in the presence of rapamycin; in both cases, total mTOR levels were unchanged (Fig. 3A,B). Thus, DHPG-dependent mTOR activation at 2 and 5 min corresponds with PP2A suppression and the reappearance of FMRP phosphorylation.
Because mTOR can suppress PP2A in the presence of growth factor agonists (Peterson et al., 1999), we tested whether it mediates the PP2A suppression after DHPG exposure for 2 and 5 min. Accordingly, we performed endpoint PP2A activity assays on primary neurons in the presence of DHPG either preincubated with rapamycin or its inactive analog ascomycin (Fig. 3C). PP2A activity was determined at 0.5, 1, 2, 5, and 10 min after DHPG application, with the last time point inclusive of a 5 min washout of DHPG; an untreated sample served as control. Group I mGluR stimulation after rapamycin treatment prolonged PP2A activation, whereas the presence of ascomycin/DHPG alone showed the previously reported activation at 0.5 and 1 min followed by suppression at 2 and 5 min (Fig. 3C). Thus, group I mGluR stimulation can activate mTOR and suppress PP2A in a time course consistent with the reappearance of FMRP phosphorylation.
Group I mGluR activity-dependent changes in FMRP phosphorylation are dynamic in dendrites
Although the relative increases in both PP2A enzymatic activity and association with FMRP after synaptic stimulation reported above was significant, the change in activity was ∼5%. One explanation might be that the increased PP2A activity is limited primarily to the dendritic compartment and that change was substantially diluted in the whole cell lysates. Moreover, lysate preparations, as used above, may disrupt protein–protein associations that normally limit PP2A activity, necessitating the use of OA to study basal P-FMRP in our biochemical analyses. We therefore examined FMRP phosphorylation using immunocytochemistry in neurons, paying particular attention to the dendrites. We immunostained primary hippocampal neurons with an antibody raised against the phosphorylated serine 499 of FMRP described previously (Ceman et al., 2003) (Stephanie Ceman, personal communication). Antibody recognition of P-FMRP was ascertained by Western blotting synaptosomes from wild-type and Fmr1 knock-out mice (supplemental Fig. 3A, available at www.jneurosci.org as supplemental material). Wild-type synaptosomes (supplemental Fig. 3A, top panel, available at www.jneurosci.org as supplemental material) revealed a band of the expected size of ∼80 kDa, that: (1) comigrated with a band stained by anti-FMRP, (2) was absent after treatment with alkaline phosphatase, and (3) increased by ∼25% in the presence of OA (FMRP controls showed equal protein loading in all cases) (supplemental Fig. 3A, middle panel, available at www.jneurosci.org as supplemental material). Probing wild-type and Fmr1 knock-out total lysates/synaptosomes using the phospho-FMRP antibody showed a band at ∼80 kDa using this antibody (supplemental Fig. 3A, middle and right panels, available at www.jneurosci.org as supplemental material) along with a faint, higher, cross-reacting band. The band at ∼80 kDa was confirmed to be FMRP after reprobing the blot with anti-FMRP (supplemental Fig. 3A, right bottom panel, available at www.jneurosci.org as supplemental material). To further ascertain whether the antibody specifically recognizes serine 499 phosphorylation in FMRP via immunocytochemistry, we used L cells stably transfected with either WT or S499A Flag-FMRP; cells transfected with the vector alone were used as control (Ceman et al., 2003). The use of stable cell lines allowed analysis of the P-FMRP antibody against physiological levels of WT or S499A Flag-FMRP, because these cells have extremely low endogenous FMRP levels. Moreover, small epitope tags (e.g., Flag) are known to affect fusion protein conformation minimally. We found modest background staining (supplemental Fig. 3B, top right panel, available at www.jneurosci.org as supplemental material) with the P-FMRP antibody in the L cells expressing vector alone (supplemental Fig. 3B, top left panel, available at www.jneurosci.org as supplemental material). Importantly, cells transfected with Flag-S499AFMRP, which contains a serine to alanine change at position 499 and does not get phosphorylated, showed no increase in P-FMRP staining above background (supplemental Fig. 3B, middle right panel, available at www.jneurosci.org as supplemental material) despite efficient transfection (supplemental Fig. 3B, middle left panel, available at www.jneurosci.org as supplemental material). Quantitative analysis of the IF signals demonstrated that the P-FMRP antibody does not recognize the nonphosphorylated S499A mutant when compared with the vector control. In contrast, Flag-WTFMRP showed significantly increased P-FMRP staining (supplemental Fig. 3B, bottom right and histogram, available at www.jneurosci.org as supplemental material). Transfected cells were confirmed by increased Flag staining (supplemental Fig. 3B, bottom left panel, available at www.jneurosci.org as supplemental material). These data show that the phospho-FMRP antibody specifically recognizes phosphorylation of FMRP at serine 499 despite modest nonspecific reactivity and the cytological data obtained from dendrites would primarily represent phosphorylation changes at serine 499.
Immunofluorescence and digital imaging on steady-state cultured hippocampal neurons using the phospho-FMRP antibody revealed P-FMRP in the cell body extending throughout the dendrite and into spines in the form of granules as indicated by white arrows; actin was used as a cytoskeletal marker (Fig. 4A). Colocalization between P-FMRP and total FMRP was studied by double immunolabeling assays. A Z-series of images was acquired, deconvolved to remove out-of-focus light, and subjected to volume rendering in a 3-D analysis (Imaris) (Fig. 4B). Quantification using automated thresholding revealed that >50% of the total FMRP signal (green) colocalized with P-FMRP (red; colocalization in yellow). The zip code binding protein, which is not known to colocalize with FMRP, was used as a control (supplemental Fig. 3A, left bottom panel, available at www.jneurosci.org as supplemental material). Our data show that >50% FMRP is phosphorylated at steady state in intact neurons, suggesting a physiological role for FMRP phosphorylation. To ascertain activity-dependent modulation of FMRP phosphorylation, we immunostained P-FMRP 30 s after group I mGluR stimulation and observed a clear reduction in signal from the distal dendrites. Importantly, in the presence 10 nm OA, the P-FMRP levels did not change despite DHPG stimulation (Fig. 4C). Further quantification of average percentage fluorescence intensity of FMRP phosphorylation showed that the reduced P-FMRP levels at 30 s after synaptic stimulation was statistically significant from either steady-state levels or stimulated neurons in the presence of OA (Fig. 4D). These findings confirm our biochemical data showing the rapid changes in FMRP phosphorylation after group I mGluR stimulation and demonstrate that these changes also occur within dendrites.
Enhanced translation of FMRP target mRNAs immediately after DHPG-mediated synaptic stimulation
Previous biochemical studies have strongly suggested the involvement of FMRP in translation (Laggerbauer et al., 2001) and the observation of FMRP within and at the base of spines in apparent proximity of polysomes suggest a role for FMRP in local protein synthesis (Feng et al., 1997b). Additionally, we previously showed data consistent with phospho-mimic FMRP associating with ribosomes that failed to run-off messages, perhaps stalled, whereas nonphosphorylated FMRP appeared run-off messages with actively translating polysomes (Ceman et al., 2003). Because synaptic activity has been shown to stimulate local protein synthesis (Raymond et al., 2000) and we show above that synaptic stimulation modulates FMRP phosphorylation, we asked whether synaptic activity influences translation of selected FMRP target messages. Using primary hippocampal neurons, we performed Western blot analyses on a small subset of FMRP ligands after DHPG treatment at 2 and 10 min using unstimulated cells as control (Fig. 5A). We used a phospho-specific ERK1/2 antibody as a positive control to monitor group I mGluR stimulation and observed the previously reported increase in ERK1/2 phosphorylation at 2 min of stimulation (Mao et al., 2005). PSD-95, previously shown to display an FMRP-dependent increase in protein abundance after synaptic stimulation, showed the expected modest increase at 10 min after stimulation as did FMRP itself, another established FMRP ligand message (Todd et al., 2003; Weiler et al., 2004) (Fig. 5A). We also examined SAPAP3, a PSD-95-associated protein, the mRNA of which primarily localizes to glutamatergic spines and was previously identified as a putative FMRP target (Brown et al., 2001; Kindler et al., 2001); verified here by showing that SAPAP3 mRNA coimmunoprecipitates with FMRP in neuronal lysates (supplemental Fig. 4A, available at www.jneurosci.org as supplemental material). Western blot analysis of the SAPAP3 showed a marked increase at 2 min of DHPG stimulation followed by a drop within 10 min (Fig. 5A), despite equivalent SAPAP3 mRNA abundance at all three time points (Fig. S4B, available at www.jneurosci.org as supplemental material). Densitometry analyses further confirmed that this limited yet informative collection of FMRP target mRNAs indeed showed higher expression levels after group I mGluR stimulation (Fig. 5B). Although these data do not directly assess the role of FMRP phosphorylation in target message translation, the temporal SAPAP3 protein expression pattern closely corresponds with the dynamics of FMRP phosphorylation shown in Figure 2B above. We therefore examined SAPAP3 expression at 2 min of DHPG stimulation in the presence/absence of 100 nm OA (Fig. 5C, left and right panels). At this OA concentration, FMRP was found to be hyperphosphorylated (supplemental Fig. 5, available at www.jneurosci.org as supplemental material), and the temporal rise in SAPAP3 protein was not observed even after group I mGluR activation. To show a more direct functional consequence to modulating FMRP phosphorylation, we used a human hippocampal neuronal cell line, HT22 (gift from Pam Maher, Salk Institute, San Diego, CA) (Morimoto and Koshland, 1990; Maher and Davis, 1996; Sagara and Schubert, 1998) and investigated the temporal effect of group I mGluR activation after both an Fmr1 small interfering RNA (siRNA)-mediated knockdown to reduce endogenous FMRP and a transient transfection of either Flag-FMRP or Flag-S499AFMRP (supplemental Fig. 6, available at www.jneurosci.org as supplemental material). The HT-22 cells express group I mGluRs, FMRP, PP2A, and SAPAP3 (data not shown) and recapitulate the temporal pattern of FMRP phosphorylation in the presence of DHPG application (supplemental Fig. 6A, available at www.jneurosci.org as supplemental material). After optimizing the Fmr1 siRNA knockdown, we found the levels of FMRP were greatly reduced with a final siRNA concentration of 25 nm used for 2 d as revealed by Western blotting of samples (done in triplicate) for FMRP using eIF4E as an internal control (supplemental Fig. 6B, available at www.jneurosci.org as supplemental material). Following the successful knockdown of endogenous FMRP, either WT or S499A Flag-FMRP was transiently transfected into the HT-22 cells to replace endogenous FMRP, and the downstream translational effects on SAPAP3 were analyzed in the presence of DHPG (100 μm) for either 2 or 10 min on day 4 (supplemental Fig. 6C, left and right panels, available at www.jneurosci.org as supplemental material). We found that WT-Flag FMRP maintained the SAPAP3 expression pattern seen previously in Figure 5A. However, both in the presence of nonphosphorylated (S499A Flag-FMRP) or in the absence of FMRP, SAPAP3 did not show reduced expression at 10 min. Thus, the group I mGluR-mediated increase in SAPAP3 abundance is PP2A dependent and corresponds with the temporal pattern of FMRP phosphorylation. Although other PP2A-sensitive influences on translation could also account for this, these data are certainly consistent with P-FMRP influencing SAPAP3 translation after synaptic stimulation.
Discussion
Despite intensive research into FMRP function, little is known about the regulation of FMRP function. Aside from recent reports of arginine methylation of FMRP (Denman, 2002; Stetler et al., 2006), phosphorylation of a highly conserved serine residue appears to be a likely candidate for a functional posttranslational modification of FMRP. Ceman et al. (2003) reported data consistent with FMRP phosphorylation influencing translation of target messages. If this interpretation were correct, dephosphorylation may signal the release of translational suppression by FMRP, a widely accepted function of FMRP (Laggerbauer et al., 2001; Mazroui et al., 2002). Thus, the FMRP phosphatase may be a key modulator of FMRP function.
Above, we showed multiple lines of evidence for PP2A as a major FMRP phosphatase in both neuronal and non-neuronal cells. Studies using a battery of phosphatase inhibitors lead to the most parsimonious conclusion that PP2A is responsible for FMRP dephosphorylation. Immunoprecipitation analyses revealed that PP2A failed to associate with stably expressed nonphosphorylated S499A-FMRP. Crucially, disrupting PP2A by a well-known dominant-negative mutant yielded increased FMRP phosphorylation.
PP2A is a prominent neuronal serine-threonine phosphatase and dephosphorylates a number of proteins, including ERK1/2 (Lechward et al., 2001). Phosphorylation of ERK1/2 increases after group I mGluR stimulation, and PP2A inhibition mimics this effect. Indeed, previous studies show that DHPG-mediated group I mGluR activation inhibits PP2A activity reaching maximal inhibition at 10 min after stimulation (Mao et al., 2005). We also find that FMRP becomes heavily phosphorylated 10 min after group I mGluR activation. Although this finding agrees with group I mGluR activity-dependent FMRP phosphorylation being mediated by PP2A inhibition, it conflicted with one model of FMRP function at the synapse where P-FMRP would suppress translation of key mRNAs until synaptic stimulation leads to a wave of local protein synthesis. Under this model, we expected FMRP dephosphorylation after group I mGluR stimulation.
Because previous studies examined activity-dependent PP2A activity at 2 min after DHPG stimulation, we measured FMRP phosphorylation at earlier time points. Remarkably, we found that the pool of FMRP, which exhibited partial phosphorylation in resting neuronal cultures, was completely dephosphorylated at 1 min after DHPG stimulation and only partly rephosphorylated by 2 min. Additionally, there was increased association of PP2A and FMRP at the time point when the enzyme was found to be most catalytically active. This suggests that PP2A may exhibit an early, transient, and previously unknown burst of activity after group I mGluR stimulation, which influences FMRP phosphorylation. Indeed, we developed a modified protocol (supplemental methods, available at www.jneurosci.org as supplemental material) to allow us to reproducibly measure PP2A activity at earlier time points and found that PP2A enzymatic activity increased significantly as early as 30 s after DHPG exposure. At 2 min poststimulation, PP2A activity declined as reported previously.
This DHPG-mediated decline in PP2A activity was tightly linked to mTOR, because treatment with rapamycin, an mTOR inhibitor, delayed PP2A suppression. Enzymatic studies in the presence of rapamycin provided the mechanistic insight that at both 2 and 5 min of DHPG exposure, PP2A showed extended activation following the time course of FMRP phosphorylation under the same conditions. Notably, mTOR inhibition did not abolish FMRP phosphorylation completely, suggesting a possible role for an as yet unidentified FMRP kinase. It is possible that such an activity-dependent FMRP kinase helps balance PP2A activity to maintain phosphorylation status of FMRP or that rapid, early activation of PP2A competes/blocks the kinase activity enabling the temporal pattern of phosphorylation and the downstream translational consequences. In such a case, we would expect that a transgenic mouse expressing nonphosphoryated, S499A FMRP would share some of the phenotypic features of the Fmr1 knock-out mouse. Further mechanistic analyses would be required to follow up this coupling of FMRP, a regulator of translation, with the well-studied mTOR translation pathway, a critical component of synaptic signaling. At present, these data present the first line of evidence for mTOR-mediated PP2A suppression after group I mGluR stimulation revealing rapid, reversible FMRP phosphorylation.
Immunocytochemistry in intact neurons allowed the steady-state detection of FMRP phosphorylation presumably because of the maintenance of cellular compartmentalization. We confirmed the same and ensured that the developmental stage of the primary neurons used (14 DIV for immunostaining vs 5 DIV for metabolic labeling) does not contribute to the detection of steady-state FMRP phosphorylation by examining FMRP phosphorylation in the presence/absence of okadaic acid both in the 5 and 14 DIV cultures (supplemental Fig. 7, available at www.jneurosci.org as supplemental material). These data further validated the cytological analyses of FMRP phosphorylation in dendrites using an antibody developed against P-FMRP. Steady-state imaging in wild-type neurons revealed >50% of FMRP in dendrites was phosphorylated, suggesting that FMRP phosphorylation is physiologically relevant in neurons. A significant reduction in P-FMRP was observed after 30 s of DHPG-mediated group I mGluR stimulation. Strikingly, the presence of okadaic acid maintained basal levels of P-FMRP even in the presence of group I mGluR activity. Moreover, okadaic acid treatment in synaptoneurosomes after stimulation increased P-FMRP by ∼25% validating the argument that the modest effects we assayed in whole-cell lysates become more pronounced in the dendritic compartment.
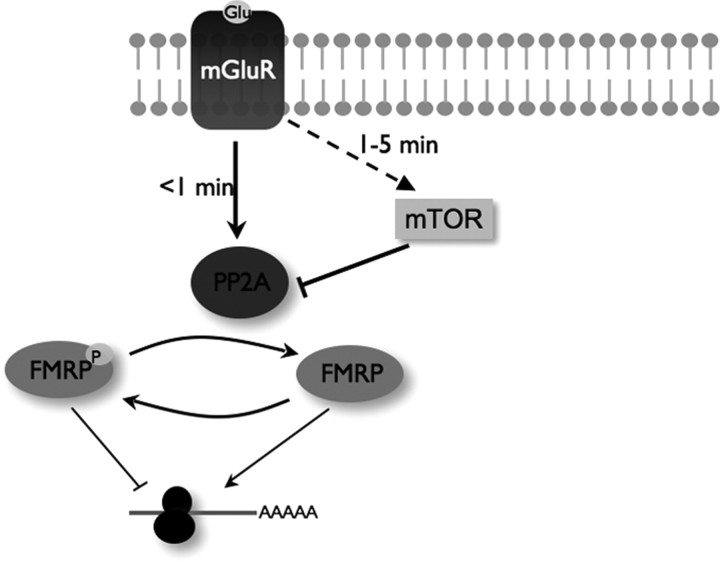
Our findings reconcile the model of FMRP function with PP2A activity, introducing a dynamic, regulatory feature to FMRP function. We propose that PP2A is activated immediately after group I mGluR stimulation leading to transient FMRP dephosphorylation, possibly releasing some P-FMRP-sensitive target messages from translational suppression. By 10 min, however, PP2A is inhibited through mTOR, and FMRP is rephosphorylated suppressing translation (Fig. 6). This possibility is supported by examination of SAPAP3 protein levels. The SAPAP3 message is predominantly dendritic and was identified as an FMRP target experimentally in mouse brain (Brown et al., 2001). Additionally, we verified SAPAP3 mRNA as an FMRP ligand by detecting its presence in FMRP immunoprecipitates. As shown above, SAPAP3 protein increased at 2 min, followed by a decline, closely paralleling the temporal profile of FMRP phosphorylation. And, the decrease in SAPAP3 expression at 10 min is abolished after exposure to rapamycin. The effect of DHPG-mediated changes in FMRP phosphorylation was confirmed in a more direct manner in neuronal cell lines by depleting the endogenous FMRP using siRNA-mediated knockdown and transiently expressing either WT or S499A Flag-FMRP to replace the endogenous FMRP. It will be interesting to determine the half-life of SAPAP3 and what role such dynamic changes in protein abundance play in synaptic function.
Figure 6.
Model depicting a novel, early signaling cascade linking group I mGluR stimulation to FMRP phosphorylation through mTOR and PP2A. Immediate (<1 min) DHPG-induced group I mGluR activation results in rapid FMRP dephosphorylation mediated by increased PP2A activity correlating with a burst of translation (FMRP ligands). Extended group I mGluR activation (1–5 min) reveals that FMRP is rephosphorylated by mTOR-dependent PP2A suppression.
The early signaling through FMRP phosphorylation effecting translational changes downstream presented here is supported by recent work at somewhat later time points of group I mGluR activation (Muddashetty et al., 2007). They show that early mGluR activation at 5 min in WT synaptoneurosomes stimulates PSD-95 synthesis as analyzed by metabolic labeling and analysis of RNA levels in polysome gradients. In contrast, synaptoneurosomes from Fmr1 KO failed to show recruitment of PSD-95 mRNA into polysomes or synthesis of PSD-95 protein after 5 min of DHPG treatment. These data are consistent with a novel molecular mechanism for rapid translation activation as described in the present study. The model of FMRP function suggested above is consistent with previous observations that FMRP can suppress translation of target transcripts (Brown et al., 2001; Mazroui et al., 2002). The absence of FMRP results in excess translation of target mRNAs, such as MAP1B in mice or its Drosophila ortholog Futsch (Zhang et al., 2001; Lu et al., 2004). Synaptic plasticity requires local protein synthesis of preexisting messages and electrophysiological measures of plasticity in FMRP-deficient mice, such as mGluR1/5-induced LTD, are exaggerated, consistent with the overexpression of LTD-required protein(s) (Huber et al., 2000, 2002). Indeed, hippocampal mGluR5-induced LTD, which is sensitive to protein synthesis inhibitors in wild-type mice, is not sensitive to the same inhibitors in FMRP-deficient mice, suggesting the preexistence and constitutive expression of key protein(s) (Nosyreva and Huber, 2006). We propose it is in the translational regulation of these key and, as yet unidentified, LTD-requiring proteins that FMRP plays a critical role in and phosphorylation of FMRP may regulate this process. Also, this regulation of FMRP is via a novel immediate-early signaling cascade, described above, linking group I mGluR activity to dynamic FMRP phosphorylation mediated by mTOR and PP2A.
Footnotes
This work was supported by National Institutes of Health Grant HD20521 (S.T.W.), Baylor-Emory Fragile X Center Grants HD24064 (S.T.W.), CA57327 (D.C.P.), NS051127 (G.J.B.), and HD41591 (S.C.), and a FRAXA postdoctoral fellowship (U.N.). We thank Julie Mowrey (Warren laboratory), Sameer Patel (Pallas laboratory), and Xiaodi Yao (Bassell laboratory) for technical assistance and Kathryn Garber and Ray Dingledine for helpful discussion on this manuscript.
References
- Adams DG, Coffee RL, Jr, Zhang H, Pelech S, Strack S, Wadzinski BE. Positive regulation of Raf1-MEK1/2-ERK1/2 signaling by protein serine/threonine phosphatase 2A holoenzymes. J Biol Chem. 2005;280:42644–42654. doi: 10.1074/jbc.M502464200. [DOI] [PubMed] [Google Scholar]
- Antar LN, Afroz R, Dictenberg JB, Carroll RC, Bassell GJ. Metabotropic glutamate receptor activation regulates fragile x mental retardation protein and FMR1 mRNA localization differentially in dendrites and at synapses. J Neurosci. 2004;24:2648–2655. doi: 10.1523/JNEUROSCI.0099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bottorff D, Stang S, Agellon S, Stone JC. RAS signalling is abnormal in a c-raf1 MEK1 double mutant. Mol Cell Biol. 1995;15:5113–5122. doi: 10.1128/mcb.15.9.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V, Small K, Lakkis L, Feng Y, Gunter C, Wilkinson KD, Warren ST. Purified recombinant Fmrp exhibits selective RNA binding as an intrinsic property of the fragile X mental retardation protein. J Biol Chem. 1998;273:15521–15527. doi: 10.1074/jbc.273.25.15521. [DOI] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, Darnell JC, O'Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren ST. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Ceman S, O'Donnell WT, Reed M, Patton S, Pohl J, Warren ST. Phosphorylation influences the translation state of FMRP-associated polyribosomes. Hum Mol Genet. 2003;12:3295–3305. doi: 10.1093/hmg/ddg350. [DOI] [PubMed] [Google Scholar]
- Corbin F, Bouillon M, Fortin A, Morin S, Rousseau F, Khandjian EW. The fragile X mental retardation protein is associated with poly(A)(+) mRNA in actively translating polyribosomes. Hum Mol Genet. 1997;6:1465–1472. doi: 10.1093/hmg/6.9.1465. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–499. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Daw MI, Bortolotto ZA, Saulle E, Zaman S, Collingridge GL, Isaac JT. Phosphatidylinositol 3 kinase regulates synapse specificity of hippocampal long-term depression. Nat Neurosci. 2002;5:835–836. doi: 10.1038/nn903. [DOI] [PubMed] [Google Scholar]
- Denman RB. Methylation of the arginine-glycine-rich region in the fragile X mental retardation protein FMRP differentially affects RNA binding. Cell Mol Biol Lett. 2002;7:877–883. [PubMed] [Google Scholar]
- Dounay AB, Forsyth CJ. Okadaic acid: the archetypal serine/threonine protein phosphatase inhibitor. Curr Med Chem. 2002;9:1939–1980. doi: 10.2174/0929867023368791. [DOI] [PubMed] [Google Scholar]
- Evans DR, Myles T, Hofsteenge J, Hemmings BA. Functional expression of human PP2Ac in yeast permits the identification of novel C-terminal and dominant-negative mutant forms. J Biol Chem. 1999;274:24038–24046. doi: 10.1074/jbc.274.34.24038. [DOI] [PubMed] [Google Scholar]
- Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM. Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci. 1997a;17:1539–1547. doi: 10.1523/JNEUROSCI.17-05-01539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Absher D, Eberhart DE, Brown V, Malter HE, Warren ST. FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol Cell. 1997b;1:109–118. doi: 10.1016/s1097-2765(00)80012-x. [DOI] [PubMed] [Google Scholar]
- Honkanen RE, Zwiller J, Moore RE, Daily SL, Khatra BS, Dukelow M, Boynton AL. Characterization of microcystin-LR, a potent inhibitor of type 1 and type 2A protein phosphatases. J Biol Chem. 1990;265:19401–19404. [PubMed] [Google Scholar]
- Kindler S, Mohr E, Rehbein M, Richter D. Extrasomatic targeting of MAP2, vasopressin and oxytocin mRNAs in mammalian neurons. Results Probl Cell Differ. 2001;34:83–104. doi: 10.1007/978-3-540-40025-7_6. [DOI] [PubMed] [Google Scholar]
- Kins S, Crameri A, Evans DR, Hemmings BA, Nitsch RM, Gotz J. Reduced protein phosphatase 2A activity induces hyperphosphorylation and altered compartmentalization of tau in transgenic mice. J Biol Chem. 2001;276:38193–38200. doi: 10.1074/jbc.M102621200. [DOI] [PubMed] [Google Scholar]
- Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, Fischer U. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet. 2001;10:329–338. doi: 10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- Lechward K, Awotunde OS, Swiatek W, Muszynska G. Protein phosphatase 2A: variety of forms and diversity of functions. Acta Biochim Pol. 2001;48:921–933. [PubMed] [Google Scholar]
- Lu R, Wang H, Liang Z, Ku L, O'Donnell WT, Li W, Warren ST, Feng Y. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci USA. 2004;101:15201–15206. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P, Davis JB. The role of monoamine metabolism in oxidative glutamate toxicity. J Neurosci. 1996;16:6394–6401. doi: 10.1523/JNEUROSCI.16-20-06394.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Yang L, Arora A, Choe ES, Zhang G, Liu Z, Fibuch EE, Wang JQ. Role of protein phosphatase 2A in mGluR5-regulated MEK/ERK phosphorylation in neurons. J Biol Chem. 2005;280:12602–12610. doi: 10.1074/jbc.M411709200. [DOI] [PubMed] [Google Scholar]
- Mazroui R, Huot ME, Tremblay S, Filion C, Labelle Y, Khandjian EW. Trapping of messenger RNA by Fragile X mental retardation protein into cytoplasmic granules induces translation repression. Hum Mol Genet. 2002;11:3007–3017. doi: 10.1093/hmg/11.24.3007. [DOI] [PubMed] [Google Scholar]
- McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, Sehgal A, Siwicki KK, Dockendorff TC, Nguyen HT, McDonald TV, Jongens TA. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Morimoto BH, Koshland DE., Jr Excitatory amino acid uptake and N-methyl-D-aspartate-mediated secretion in a neural cell line. Proc Natl Acad Sci USA. 1990;87:3518–3521. doi: 10.1073/pnas.87.9.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muddashetty RS, Kelic S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J Neurosci. 2007;27:5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namboodiripad AN, Jennings ML. Permeability characteristics of erythrocyte membrane to okadaic acid and calyculin A. Am J Physiol. 1996;270:C449–C456. doi: 10.1152/ajpcell.1996.270.2.C449. [DOI] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Desai BN, Hardwick JS, Schreiber SL. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycinassociated protein. Proc Natl Acad Sci USA. 1999;96:4438–4442. doi: 10.1073/pnas.96.8.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CR, Thompson VL, Tate WP, Abraham WC. Metabotropic glutamate receptors trigger homosynaptic protein synthesis to prolong long-term potentiation. J Neurosci. 2000;20:969–976. doi: 10.1523/JNEUROSCI.20-03-00969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routtenberg A, Rekart JL. Post-translational protein modification as the substrate for long-lasting memory. Trends Neurosci. 2005;28:12–19. doi: 10.1016/j.tins.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Sagara Y, Schubert D. The activation of metabotropic glutamate receptors protects nerve cells from oxidative stress. J Neurosci. 1998;18:6662–6671. doi: 10.1523/JNEUROSCI.18-17-06662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer C, Bardoni B, Mandel JL, Ehresmann B, Ehresmann C, Moine H. The fragile X mental retardation protein binds specifically to its mRNA via a purine quartet motif. EMBO J. 2001;20:4803–4813. doi: 10.1093/emboj/20.17.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani G, Fraser CE, Darnell JC, Darnell RB. Fragile X mental retardation protein is associated with translating polyribosomes in neuronal cells. J Neurosci. 2004;24:7272–7276. doi: 10.1523/JNEUROSCI.2306-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler A, Winograd C, Sayegh J, Cheever A, Patton E, Zhang X, Clarke S, Ceman S. Identification and characterization of the methyl arginines in the fragile X mental retardation protein Fmrp. Hum Mol Genet. 2006;15:87–96. doi: 10.1093/hmg/ddi429. [DOI] [PubMed] [Google Scholar]
- Thelin WR, Kesimer M, Tarran R, Kreda SM, Grubb BR, Sheehan JK, Stutts MJ, Milgram SL. The cystic fibrosis transmembrane conductance regulator is regulated by a direct interaction with the protein phosphatase 2A. J Biol Chem. 2005;280:41512–41520. doi: 10.1074/jbc.M507308200. [DOI] [PubMed] [Google Scholar]
- Todd PK, Mack KJ, Malter JS. The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95. Proc Natl Acad Sci USA. 2003;100:14374–14378. doi: 10.1073/pnas.2336265100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderklish PW, Edelman GM. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci USA. 2002;99:1639–1644. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kanegan MJ, Adams DG, Wadzinski BE, Strack S. Distinct protein phosphatase 2A heterotrimers modulate growth factor signaling to extracellular signal-regulated kinases and Akt. J Biol Chem. 2005;280:36029–36036. doi: 10.1074/jbc.M506986200. [DOI] [PubMed] [Google Scholar]
- Weiler IJ, Spangler CC, Klintsova AY, Grossman AW, Kim SH, Bertaina-Anglade V, Khaliq H, de Vries FE, Lambers FA, Hatia F, Base CK, Greenough WT. Fragile X mental retardation protein is necessary for neurotransmitter-activated protein translation at synapses. Proc Natl Acad Sci USA. 2004;101:17504–17509. doi: 10.1073/pnas.0407533101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder DG, Sweatt JD. Roles of serine/threonine phosphatases in hippocampal synaptic plasticity. Nat Rev Neurosci. 2001;2:461–474. doi: 10.1038/35081514. [DOI] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major fragile X syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, Speese SD, Rubin GM, Broadie K. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107:591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]