Abstract
It is currently hypothesized that adenosine is involved in the induction of sleep after prolonged wakefulness. This effect is partially reversed by the application of caffeine, which is a nonselective blocker of adenosine receptors. Here, we report that the most abundant and highly concentrated A1 subtype of cerebral adenosine receptors is upregulated after 24 h of sleep deprivation. We used the highly selective A1 adenosine receptor (A1AR) radioligand [18F]CPFPX ([18F]8-cyclopentyl-3-(3-fluoropropyl)-1-propylxanthine) and quantitative positron emission tomography to assess cerebral A1ARs before and after sleep deprivation in 12 healthy volunteers and a control group (n = 10) with regular sleep. In sleep deprived subjects, we found an increase of the apparent equilibrium total distribution volume in a region-specific pattern in all examined brain regions with a maximum increase in the orbitofrontal cortex (15.3%; p = 0.014). There were no changes in the control group with regular sleep. This is the first molecular imaging study that provides in vivo evidence for an A1AR upregulation in cortical and subcortical brain regions after prolonged wakefulness, indicating that A1AR expression is contributing to the homeostatic sleep regulation.
Keywords: imaging, adenosine A1 receptor, positron emission tomography, [18F]CPFPX, sleep deprivation, human
Introduction
There are numerous reports indicating that endogenous adenosine is a candidate for the homeostatic sleep factor theory inducting sleep after prolonged wakefulness (for review, see Basheer et al., 2004). Evidence supporting the role of adenosine derives from the wakefulness inducing effects of caffeine, which are mediated through the blockade of cerebral adenosine receptors. Continuous monitoring of adenosine levels during a sleep-wake cycle of freely moving cats showed that adenosine accumulates during prolonged wakefulness (6 h) in cats in the basal forebrain and, to a lower degree, in the cortex (Porkka-Heiskanen et al., 1997). Subsequent recovery-sleep restores the adenosine concentrations to baseline levels. These experiments have been repeated in rats with similar results (Basheer et al., 1999; Murillo-Rodriguez et al., 2004). An experimental elevation of the adenosine concentration in the cholinergic zones of the basal forebrain (Portas et al., 1997) or by inhibiting equilibrative nucleoside transporters (Porkka-Heiskanen et al., 2000) mimicked the electroencephalographic and behavioral effects of sleep deprivation.
Whereas adenosine is widely accepted as a sleep factor, the mediating subtype of adenosine receptors is highly debated. The A1 subtype has the widest distribution in the CNS with particularly high concentrations in cortex, hippocampus, striatum, and thalamus (Fredholm, 1995). Direct application of a selective A1 adenosine receptor (A1AR) agonist increases the propensity to sleep in rats (Benington et al., 1995; Schwierin et al., 1996) and correspondingly a selective A1AR antagonist decreases sleep propensity in cats (Strecker et al., 2000). Furthermore, 3 and 6 h of sleep deprivation increased A1AR mRNA in the basal forebrain (Basheer et al., 2001). Consequently, microdialysis perfusion of A1AR antisense oligonucleotides, which inhibit the translation of the A1AR mRNA, significantly decreased non-rapid eye movement (REM) sleep and increased wakefulness in rats (Thakkar et al., 2003). However, A1AR knock-out mice did not show different reactions to sleep deprivation than their wild-type littermates (Stenberg et al., 2003). Similarly it has been reported for the adenosine A2A receptor (A2AAR), that subarachnoidal infusion of a selective agonist (Satoh et al., 1999) promoted deep sleep.
Data on sleep-related changes in the human adenosine system are rare because neither adenosine nor its receptors are easily accessible in vivo in humans. A recent microdialysis study in epileptic patients revealed no increase in adenosine concentration in amygdala during 40 h of sleep deprivation (Zeitzer et al., 2006).
We have previously proposed and evaluated a method to quantify cerebral A1ARs with receptor positron emission tomography (PET) and the radioligand [18F]8-cyclopentyl-3-(3-fluoropropyl)-1-propylxanthine ([18F]CPFPX) (Holschbach et al., 2002; Bauer et al., 2003; Meyer et al., 2004, 2005), which now allows to assess changes of A1ARs in vivo. [18F]CPFPX is a highly affine (dissociation constant, KD = 1.26 nmol/L to cloned human A1AR) and selective (KD = 940 nmol/L for A2AAR) compound, which shows a rapid brain uptake (time to peak within 5 min). The aim of the present study was to investigate whether prolonged wakefulness alters A1AR availability in the human brain. For this purpose, volunteers were examined with PET on 2 subsequent days. One group of subjects was deprived of sleep for 24 h, another group had regular 8 h per night sleep between the two scans and served as a control.
Materials and Methods
Subjects.
All procedures were approved by the Ethics Committee of the Medical Faculty of the University of Duesseldorf, Germany, and the German Federal Office for Radiation Protection. Twenty-two healthy male volunteers participated after giving written informed consent. Volunteers were screened for the following exclusion criteria: history of neurological and psychiatric diseases, sleep disorders, shift work, night work, head injury, and alcohol or substance abuse. All subjects were nonsmokers and not on any current or chronic medication. Caffeine intake was not allowed for at least 36 h before PET scanning. All subjects underwent two [18F]CPFPX PET studies at the same time of day on consecutive days under identical conditions. Time of injection of the radioligand was between 10:00 A.M. and 2:00 P.M. in all subjects. The plasma metabolite analyses failed in one of the measurements of two subjects. Therefore, the data of these two subjects could only be analyzed using a reference region model. Thus, a total 22 subjects (control group, n = 10; sleep-deprivation group, n = 12) could be analyzed by the reference region analysis while 20 subjects (control group, n = 8) could also be analyzed using plasma data.
Before and after each PET scan, the subjects were asked to give a rating of their actual sleepiness on the Stanford Sleepiness Scale (SSS). The results were then averaged per scan.
Sleep-deprived subjects were monitored by staff members throughout the time between both scans, who supported them during the night to stay awake. During the 120 min period of PET data acquisition, the subjects were requested to stay awake and keep the eyes open. Via video monitoring system, it was assured that no subjects closed their eyes longer than usual. In this case, the subjects were addressed not to sleep and open their eyes again.
Detailed results about nine subjects of the control group will be published in a separate report addressing the test–retest stability of A1AR quantification using [18F]CPFPX.
[18F]CPFPX PET was performed as described previously (Meyer et al., 2005) using a bolus/infusion schedule with a slightly different Kbol value (denotes the amount of bolus equaling an infusion of a certain length) of 55 min. The scan duration was prolonged from 90 to 120 min. Three subjects (two of the sleep deprivation and one of the control group) were scanned according to the original 90 min protocol (Kbol = 48 min).
PET acquisition and blood sampling.
PET measurements were performed in three-dimensional mode on a Siemens (Knoxville, TN) ECAT EXACT HR+ scanner. Emission scans were started with the injection of 259 ± 4.3 MBq (specific activity, 105 ± 79 GBq/μmol).
Arterialized venous blood samples were collected at 1, 5, and 10 min and in 10 min intervals thereafter. It has been validated previously for [18F]CPFPX bolus/infusion experiments that during equilibrium, venous and arterial concentrations equilibrate and venous blood sampling can substitute arterial (Meyer et al., 2005). Radioactivity determinations in whole blood and plasma, and plasma metabolite analyses and assessment of the fraction of free [18F]CPFPX in plasma (denoted by f1) were performed as described previously (Meyer et al., 2005). Plasma caffeine levels were assessed by HPLC.
Image analysis.
Segmentation, realignment, normalization, and coregistration of PET and the individual magnetic resonance image (MRI) were done with SPM2 (Statistical Parametric Mapping, Wellcome Department of Cognitive Neurology, London, UK). Regions of interest (ROIs) were defined by freehand drawing of polygonal ROI on the individual MRI according to anatomical landmarks on transversal planes using the software PMOD (version 2.5; PMOD Group, Zurich, Switzerland). Within these ROIs, only PET voxels, which were classified as gray matter in the MRI, were included into time–activity curve (TAC) generation. This image-analysis procedure is adopted from the method proposed by Abi-Dargham et al. (2000). TACs were corrected for the contribution of intracerebral blood volume to the regional activity assuming a fractional blood volume of 5%.
The cholinergic basal forebrain and the nucleus basalis of Meynert are of particular interest for comparing the results of this study to animal experiments. The anatomical definition was done as proposed by Herholz et al. (2004), referring to landmarks [anterior commissure (ac), third ventricle, optic tract] identifiable on the MRI by positioning rectangular ROIs (7 × 3 mm) on three coronal planes on the right and left hemisphere. The localization was 2 mm caudal to ac on the ac–posterior commissure line, ventral to the ac and dorsolateral to the optic tract, at the base of the forebrain.
Outcome parameters.
Although receptor density is the parameter of interest, the maximal number of receptors available for binding (Bmax′) cannot be determined in a single PET study. Therefore, outcome parameters which are directly proportional to Bmax′ have been proposed. For this report, two outcome measures with specific advantages and disadvantages for this setting have been chosen: equilibrium total distribution volume (DVt′) and binding potential (BP2). DVt′ is composed of the specific distribution volume (DVs, equal to Bmax′/KD) and the distribution volume of free and nonspecifically bound ligand (DVf+ns). In the case of [18F]CPFPX, the relatively low fraction of specific binding (∼2/3 of DVt′) decreases the sensitivity for possible changes in DVt′. Another shortcoming is the dependence on error-prone individual plasma input functions, which increases variability. These drawbacks can be avoided by using an approach independent on blood sampling by using a reference region input as for BP2. Herein, the ratio of DVs/DVf+ns − 1 is also related to Bmax′. The cerebellar cortex is used as reference region for [18F]CPFPX PET (Meyer et al., 2007). Although the cerebellar cortex is the region in the human brain with the lowest A1AR density, one third of the DVt′ is displaceable with unlabeled CPFPX (Meyer et al., 2006). Therefore, changes in BP2 deserve careful consideration because of their dependency on DVs changes in the reference region. BP2 was determined by Logan's noninvasive graphical analysis (Logan et al., 1996). Under equilibrium conditions (time span from 50 to 100 min), DVt′ can be calculated by the ratio of TAC to plasma activity (Cp): DVt′ = f1 DVt = TAC/Cp. The annex prime (′) was used to indicate that the DVt′ is not corrected for the free fraction of ligand in plasma (f1). As discussed previously (Meyer et al., 2005), f1 was not included into the analysis because it introduces a substantial error. [The f1 value (2.01 ± 0.58%) correlated with DVt (e.g., temporal cortex, r2 = 0.63), but not with DVt′ (r2 = 0.24).]
Statistical analyses.
Average values are given as mean ± SD. Statistical significance was assessed with an ANOVA for repeated measures. If the day by group interaction indicated a significant effect (p < 0.05), post hoc Tukey–Kramer tests were used for pairwise comparisons.
Results
Group characteristics
The sleep-deprivation (n = 12) and the control (n = 8) groups were matched with regard to age (26.7 ± 2.5 and 27.6 ± 1.3 years, respectively; p = 0.34; Student's t test), gender (all male), and constitution (body mass index, 24.3 ± 2.5 and 24.8 ± 3.72 kg/m2, respectively; p = 0.68). Average chronic caffeine consumption (in 0.15 L cups of coffee) was ∼1.4 ± 1.4 and 2.2 ± 1.6 cups/d, respectively (p = 0.48). Sleep durations before the first and second scan of the control (8.3 ± 1 and 8.18 ± 0.5 h, respectively) and the first scan of the sleep-deprivation group (7.95 ± 0.6 h) did not differ significantly. Likewise, the time spent awake before these three scans (237.2 ± 74.2, 229.5 ± 84.0, and 246.1 ± 65.9 min, respectively) was not statistically different. The groups and conditions were not significantly different with regard to injected activity or mass of injected CPFPX, specific activity, f1 value, or mean rates of change of the parent compound in plasma.
Imaging quantification
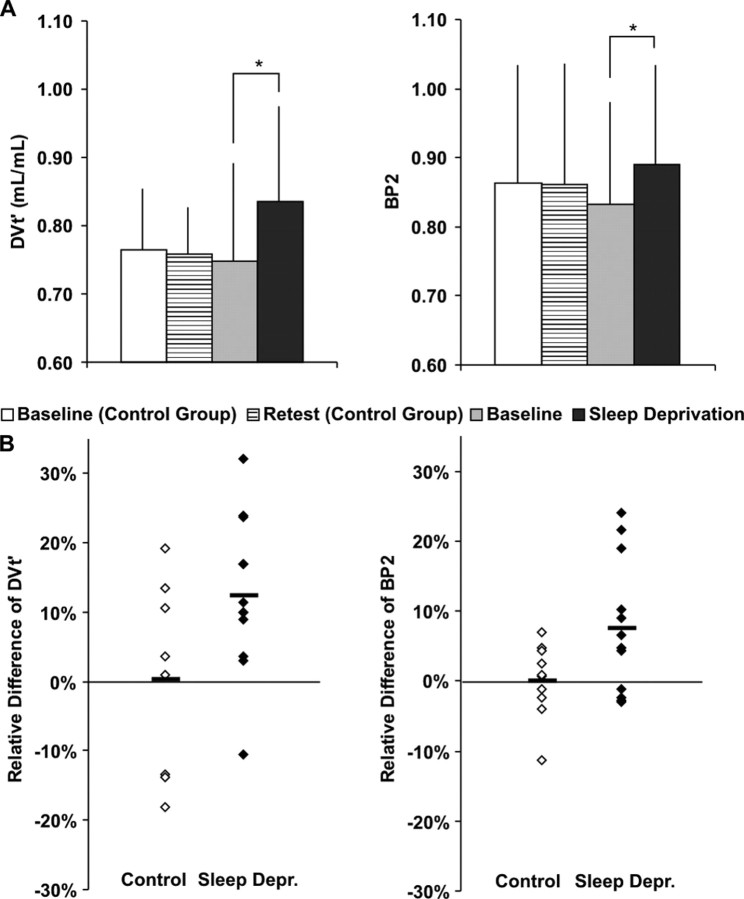
No between-group differences in DVt′ and BP2 were observed between controls and experimental group in the examined regions at the first day. Figure 1 displays the average DVt′ and BP2 values per group and condition for the temporal cortex as a representative ROI. The A1AR availability of the second day of the control group was not significantly different from the first scans of both groups, although the average BP2 values of the control group were slightly higher than the average BP2 values of the sleep-deprivation group. The regional DVt′ and BP2 values and the results of the test for a significant interaction of day by group by repeated measures ANOVA are presented in Table 1. In case of significant interactions, pairwise comparison of groups and conditions were subsequently performed. This post hoc comparison revealed only significant differences between the first and second scan of the sleep-deprivation group. Therefore, only the results of these comparisons are displayed. Figure 1 shows the distribution of the relative difference between both days [(day 2 − day 1)/day 1] of both groups for the temporal cortex. As can be seen from this scatterplot, the test–retest variability is more prominent for DVt′ than for BP2. The mean DVt′ values were elevated ∼12.5% and the mean BP2 values ∼7.5% after sleep deprivation in the temporal cortex.
Figure 1.
Adenosine receptor DVt′ and BP2 in temporal cortex of 10 control (baseline and retest condition) and 12 sleep-deprived subjects (baseline and after sleep deprivation). A, Absolute changes [significant interaction of day by group (p = 0.04 and p = 0.04 for DVt′ and BP2, respectively), ANOVA for repeated measures, followed by Tukey–Kramer test (*) (p = 0.02 for DVt′; p = 0.03 for BP2)]. Error bars depict SD. B, Scatterplot of relative changes [(day 2 − day 1)/day 1]. Horizontal bars show the mean.
Table 1.
Regional A1AR DVt′ (in milliliter/milliliter) and BP2 in control and sleep-deprived subjects
| Region | Control |
Sleep deprivation |
ANOVA | Pairwise | ||
|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 1 | Day 2 | p | adjusted p | |
| DVt′ | ||||||
| Frontal ctx | 0.80 ± 0.09 | 0.78 ± 0.08 | 0.77 ± 0.16 | 0.87 ± 0.15 | 0.041 | 0.033 |
| Orbitofrontal ctx | 0.75 ± 0.08 | 0.75 ± 0.06 | 0.72 ± 0.13 | 0.82 ± 0.13 | 0.047 | 0.014 |
| Cingulate gyrus | 0.74 ± 0.09 | 0.75 ± 0.08 | 0.73 ± 0.15 | 0.80 ± 0.15 | 0.118 | - |
| Parietal ctx | 0.78 ± 0.09 | 0.77 ± 0.08 | 0.77 ± 0.16 | 0.85 ± 0.15 | 0.074 | - |
| Occipital ctx | 0.80 ± 0.11 | 0.78 ± 0.09 | 0.80 ± 0.16 | 0.89 ± 0.16 | 0.035 | 0.036 |
| Temporal ctx | 0.76 ± 0.09 | 0.76 ± 0.07 | 0.75 ± 0.14 | 0.84 ± 0.14 | 0.041 | 0.021 |
| Thalamus | 0.77 ± 0.09 | 0.78 ± 0.07 | 0.78 ± 0.16 | 0.86 ± 0.16 | 0.157 | - |
| Striatum | 0.78 ± 0.11 | 0.77 ± 0.07 | 0.79 ± 0.17 | 0.89 ± 0.17 | 0.050 | 0.037 |
| Cerebellum | 0.41 ± 0.06 | 0.40 ± 0.02 | 0.40 ± 0.08 | 0.44 ± 0.08 | 0.127 | - |
| Nucleus basalis | 0.60 ± 0.07 | 0.63 ± 0.10 | 0.63 ± 0.10 | 0.67 ± 0.14 | 0.477 | - |
| BP2 | ||||||
| Frontal ctx | 0.96 ± 0.17 | 0.94 ± 0.18 | 0.91 ± 0.15 | 0.97 ± 0.14 | 0.023 | 0.073 |
| Orbitofrontal ctx | 0.83 ± 0.16 | 0.84 ± 0.13 | 0.77 ± 0.15 | 0.85 ± 0.12 | 0.086 | - |
| Cingulate gyrus | 0.83 ± 0.16 | 0.83 ± 0.16 | 0.78 ± 0.12 | 0.82 ± 0.14 | 0.179 | - |
| Parietal ctx | 0.95 ± 0.15 | 0.92 ± 0.18 | 0.89 ± 0.14 | 0.93 ± 0.13 | 0.038 | 0.203 |
| Occipital ctx | 0.98 ± 0.16 | 0.94 ± 0.20 | 0.97 ± 0.14 | 1.03 ± 0.13 | 0.008 | 0.088 |
| Temporal ctx | 0.86 ± 0.16 | 0.86 ± 0.14 | 0.83 ± 0.12 | 0.89 ± 0.12 | 0.042 | 0.028 |
| Thalamus | 0.89 ± 0.11 | 0.91 ± 0.16 | 0.90 ± 0.15 | 0.95 ± 0.12 | 0.622 | - |
| Striatum | 0.93 ± 0.15 | 0.91 ± 0.12 | 0.96 ± 0.18 | 1.03 ± 0.18 | 0.033 | 0.064 |
| Nucleus basalis | 0.17 ± 0.22 | 0.11 ± 0.17 | 0.13 ± 0.10 | 0.14 ± 0.17 | 0.872 | - |
Values are mean ± SD. ANOVA p, Probability value of a repeated-measures ANOVA for the interaction of the effects day by group; Pairwise adjusted p, multiple-comparison-adjusted probability value of day 1 versus day 2 of the sleep-deprivation group according to the method of Tukey–Kramer; pairwise p is the result of a paired t test of this contrast. ctx, Cortex.
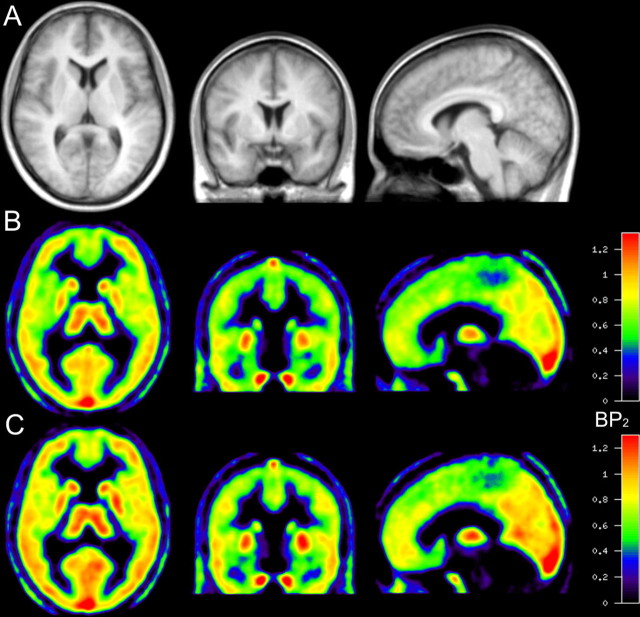
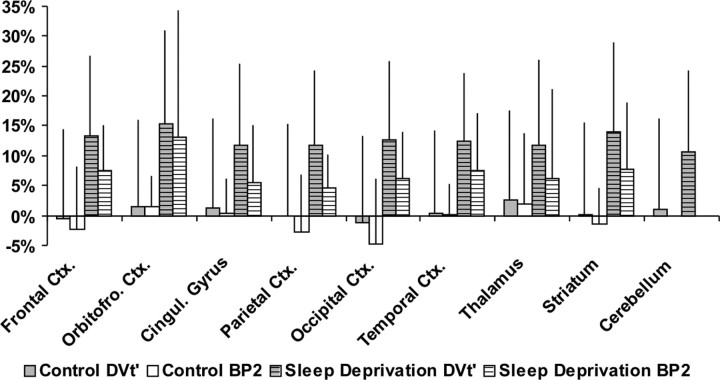
Mean parametric images of BP2 are depicted in Figure 2. The increase in cortical binding is already appreciable in various regions by visual inspection. The corresponding average relative differences of the examined ROIs (except basal forebrain) are presented in Figure 3. The average regional change in the control group ranged between a decrease of −4.8% (BP2) and −1.2% (DVt′) (occipital cortex), and an increase of 2.0% (BP2) and 2.5% (DVt′) (thalamus). In contrast, in the sleep-deprivation group the average regional change of DVt′ and BP2 ranged from 10.6% (cerebellum) to 15.3% (orbitofrontal cortex) and 4.7% (parietal cortex) to 13.2% (orbitofrontal cortex), respectively. The most pronounced difference was found in the orbitofrontal cortex (DVt′, 15.3%; BP2, 13.2%). The average DVt′ increase was lowest in the cerebellum (10.6%), which is the cerebral region with the smallest amount of A1AR in the human brain. The temporal cortex is the only region in the analysis of BP2, which was significantly different after correction for multiple comparisons, although several other regions like the frontal and occipital cortex and striatum showed a trend toward significance (p < 0.1).
Figure 2.
Average images of the sleep-deprivation group (n = 12) after spatial normalization. A, MRIs. B, Parametric image of BP2 before sleep deprivation. C, Image after sleep deprivation.
Figure 3.
Average relative changes [(day 2 − day 1)/day1] of adenosine receptor DVt′ and BP2 in various brain regions for the control and the sleep-deprivation group. Error bars depict SD.
No significant changes in the outcome parameters were found in the nucleus basalis of Meynert. There was a weak tendency for an increase of DVt′ (and BP2) after sleep deprivation of 6.3% (7.7%) for this ROI.
Looking at the relative change of sleepiness, the rating scale used (SSS) revealed a weak correlation between the increase in sleepiness and the change in DVt′ (r = 0.303; p = 0.34) but no correlation in case of BP2 (r = −0.004; p = 0.99).
Discussion
This study demonstrates that a single night of total sleep deprivation leads to a significant increase of A1AR availability in the human brain. This effect was observed regardless of the applied quantification method. The present findings point to an upregulation of A1ARs by prolonged wakefulness because the reported outcome measures DVt′ and BP2 are linearly related to the maximum concentration of available A1ARs. This so far unknown mechanism could sustain long-term sleep-inducing effects of adenosine.
Both groups did not differ in chronic caffeine consumption, which was moderate in both groups. The withdrawal of caffeine before the study, to eliminate caffeine from plasma, had apparently no ongoing effects between 36 and 60 h after caffeine restriction on the A1AR availability. There is no difference of DVt′ and BP2 detectable in the control group between both days. The sleep durations of the subjects reported here were based on standardized self-reportings. If the self-reporting were somehow inaccurate, this could increase the variation of the results, but not affect the proposition per se.
Most of the subjects had only moderate difficulties to stay awake during the scanning and had rarely to be asked to keep their eyes open. One subject, however, had severe problems to maintain wakefulness during the scan after sleep deprivation and fell asleep for approximately one-third of the scanning period. It was not excluded from the study, because it is rather unlikely that 40 min of fragmented sleep could restore the effects of overnight sleep deprivation. When DVt′ data are evaluated without this subject, the results of the repeated-measures ANOVA show additional significant increases to those reported in Table 1 in the parietal cortex and cerebellum. For BP2, the frontal, temporal, and occipital cortex as well as striatum still show the same magnitude of increase, but changes in the temporal cortex are no longer significant (p = 0.075).
These results in humans are in line with previous evidence from animal experiments. Basheer et al. (2001) showed that the A1AR mRNA levels in rats that were exposed to 6 h of sleep deprivation increased in the basal forebrain (78%), but not in the cingulate gyrus. Nevertheless, this increase did not lead to similar alterations in A1AR density in the same samples measured with [3H]DPCPX ([3H]-8-cyclopentyl-1,3-dipropylxanthine) autoradiography. Yanik and Radulovacki (1987) detected a significant increase of A1AR after 48 h of REM sleep deprivation in the cortex (15%) and striatum (23%) of rats with autoradiography. More recent findings in rats kept awake for 12 and 24 h showed an upregulation of A1AR density after this prolonged wakefulness using [3H]CPFPX autoradiography. This increased density was found in the basal forebrain but not in the cingulate cortex (McCarley et al., 2005). However, other cortical areas showed an increase in a range of ∼10% (our unpublished observation).
In several animal experiments, adenosine accumulated during prolonged wakefulness (Porkka-Heiskanen et al., 1997, 2000; Basheer et al., 1999). This raises the question why the consequence of elevated levels of agonists is an upregulation and not a downregulation as commonly observed in G-protein-coupled receptors (Bohm et al., 1997). There is evidence that receptor density regulation is varying according to the time of agonist stimulation. In the early phase (minutes to hours) receptors are internalized, but after long-term stimulation (>2 h) receptor mRNA increases, which consequently leads to increased receptor densities (Souaze, 2001). The physiological relevance could be to maintain responsiveness to stimuli and/or to adapt cellular responses to external stimuli.
An endogenous displacement of the radioligand caused by elevated adenosine concentrations, as found in animal experiments during sleep deprivation, could have mitigated the observed effects in this study. Given this fact, the underlying receptor upregulation would be even larger. At present, however, there is no experimental evidence that [18F]CPFPX is displaceable in vivo by endogenous adenosine.
As stated previously, there is evidence that adenosine modulates cholinergic cell clusters in the basal forebrain (Ch4 or basal nucleus of Meynert). This target region is characterized by both a small volume and a low regional A1AR density so that the given scanner resolution and the low DVs′/DVf+ns′ ratio impede precise quantification by [18F]CPFPX PET. The observed tendency of A1AR increase is therefore probably related to technical and physiological limitations.
There are numerous theories on a potential function of sleep, which point to a role in activity-dependent synaptic reorganization (for review, see Benington, 2000). It has, for instance, been proposed that sleep is directly linked to synaptic homeostasis and regulation of synaptic weight (Tononi and Cirelli, 2006). According to this hypothesis, the function of sleep is to downscale synaptic strength to a baseline level that is physiologically stable. Sleep deprivation would thus impair the recalibration of synaptic strength resulting in elevated levels of synaptic formations and, thus, synaptic receptors compared with the postsleep condition, which would also be in line with our observations.
What consequences might the observed increase of cerebral A1AR density have? First, it might be an alternative way to enhance adenosine functions besides an increase of adenosine itself. Modulating sleep by varying concentrations of adenosine alone would be highly dynamic. In contrast, longer-lasting changes of receptor density could modulate the level of adenosine efficacy in a stable and robust manner.
Second, the regional distribution of adenosine receptors is an important aspect regarding the local control of sleep–wake organization. Another important finding is that the increase of A1AR density occurs all over the brain, which is consistent with a global effect, linked to basic cell functions. In man, the cerebral A1AR shows highest densities in the thalamus and the neocortex, both of which are important structures in the induction and maintenance of slow-wave sleep.
In conclusion, this study provides in vivo evidence of higher levels of A1AR availability in the human brain after 24 h of prolonged wakefulness as demonstrated with [18F]CPFPX PET. Our data suggest that the A1 subtype of adenosine receptors could be addressed as a potential sleep factor besides the already well established role of adenosine itself.
Footnotes
This work was supported by grants from the Hermann von Helmholtz-Gemeinschaft Deutscher Forschungszentren, the Deutsche Forschungsgemeinschaft, and the German Ministry of Education and Research (Brain Imaging Center West). We thank Stefan Stüsgen, Jürgen Burhenne, and Walter E. Haefeli for the determination of plasma caffeine levels. Marlene Vögeling, Lutz Tellmann, Elisabeth Theelen, Suzanne Schaden, Hans Herzog, and Markus Lang are gratefully acknowledged for excellent technical assistance, and Silke Grafmüller, Bettina Palm, and Erika Wabbals for the synthesis of [18F]CPFPX. We thank Dr. R. McCarley for valuable comments on this manuscript.
References
- Abi-Dargham A, Martinez D, Mawlawi O, Simpson N, Hwang DR, Slifstein M, Anjilvel S, Pidcock J, Guo NN, Lombardo I, Mann JJ, Van Heertum R, Foged C, Halldin C, Laruelle M. Measurement of striatal and extrastriatal dopamine D1 receptor binding potential with [11C]NNC 112 in humans: validation and reproducibility. J Cereb Blood Flow Metab. 2000;20:225–243. doi: 10.1097/00004647-200002000-00003. [DOI] [PubMed] [Google Scholar]
- Basheer R, Porkka-Heiskanen T, Stenberg D, McCarley RW. Adenosine and behavioral state control: adenosine increases c-Fos protein and AP1 binding in basal forebrain of rats. Brain Res Mol Brain Res. 1999;73:1–10. doi: 10.1016/s0169-328x(99)00219-3. [DOI] [PubMed] [Google Scholar]
- Basheer R, Halldner L, Alanko L, McCarley RW, Fredholm BB, Porkka-Heiskanen T. Opposite changes in adenosine A1 and A2A receptor mRNA in the rat following sleep deprivation. NeuroReport. 2001;12:1577–1580. doi: 10.1097/00001756-200106130-00013. [DOI] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Bauer A, Holschbach MH, Meyer PT, Boy C, Herzog H, Olsson RA, Coenen HH, Zilles K. In vivo imaging of adenosine A1 receptors in the human brain with [18F]CPFPX and positron emission tomography. NeuroImage. 2003;19:1760–1769. doi: 10.1016/s1053-8119(03)00241-6. [DOI] [PubMed] [Google Scholar]
- Benington JH. Sleep homeostasis and the function of sleep. Sleep. 2000;23:959–966. [PubMed] [Google Scholar]
- Benington JH, Kodali SK, Heller HC. Stimulation of A1 adenosine receptors mimics the electroencephalographic effects of sleep deprivation. Brain Res. 1995;692:79–85. doi: 10.1016/0006-8993(95)00590-m. [DOI] [PubMed] [Google Scholar]
- Bohm SK, Grady EF, Bunnett NW. Regulatory mechanisms that modulate signalling by G-protein-coupled receptors. Biochem J. 1997;322:1–18. doi: 10.1042/bj3220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB. Purinoceptors in the nervous system. Pharmacol Toxicol. 1995;76:228–239. doi: 10.1111/j.1600-0773.1995.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Herholz K, Weisenbach S, Zundorf G, Lenz O, Schroder H, Bauer B, Kalbe E, Heiss WD. In vivo study of acetylcholine esterase in basal forebrain, amygdala, and cortex in mild to moderate Alzheimer disease. NeuroImage. 2004;21:136–143. doi: 10.1016/j.neuroimage.2003.09.042. [DOI] [PubMed] [Google Scholar]
- Holschbach MH, Olsson RA, Bier D, Wutz W, Sihver W, Schuller M, Palm B, Coenen HH. Synthesis and evaluation of no-carrier-added 8-cyclopentyl-3-(3-[(18)F]fluoropropyl)-1-propylxanthine ([(18)F]CPFPX): a potent and selective A(1)-adenosine receptor antagonist for in vivo imaging. J Med Chem. 2002;45:5150–5156. doi: 10.1021/jm020905i. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Bauer A, Ramesh V, Basheer R. Sleep deprivation-induced upregulation of A1 receptor protein in rodent cholinergic basal forebrain. Soc Neurosci Abstr. 2005;31:63–8. [Google Scholar]
- Meyer PT, Bier D, Holschbach MH, Boy C, Olsson RA, Coenen HH, Zilles K, Bauer A. Quantification of cerebral A1 adenosine receptors in humans using [18F]CPFPX and PET. J Cereb Blood Flow Metab. 2004;24:323–333. doi: 10.1097/01.WCB.0000110531.48786.9D. [DOI] [PubMed] [Google Scholar]
- Meyer PT, Elmenhorst D, Bier D, Holschbach MH, Matusch A, Coenen HH, Zilles K, Bauer A. Quantification of cerebral A1 adenosine receptors in humans using [18F]CPFPX and PET: an equilibrium approach. NeuroImage. 2005;24:1192–1204. doi: 10.1016/j.neuroimage.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Meyer PT, Elmenhorst D, Matusch A, Winz O, Zilles K, Bauer A. A1 adenosine receptor PET using [18F]CPFPX: displacement studies in humans. NeuroImage. 2006;32:1100–1105. doi: 10.1016/j.neuroimage.2006.04.202. [DOI] [PubMed] [Google Scholar]
- Meyer PT, Elmenhorst D, Boy C, Winz O, Matusch A, Zilles K, Bauer A. Effect of aging on cerebral A(1) adenosine receptors: a [(18)F]CPFPX PET study in humans. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2006.08.005. in press. [DOI] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Blanco-Centurion C, Gerashchenko D, Salin-Pascual RJ, Shiromani PJ. The diurnal rhythm of adenosine levels in the basal forebrain of young and old rats. Neuroscience. 2004;123:361–370. doi: 10.1016/j.neuroscience.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Portas CM, Thakkar M, Rainnie DG, Greene RW, McCarley RW. Role of adenosine in behavioral state modulation: a microdialysis study in the freely moving cat. Neuroscience. 1997;79:225–235. doi: 10.1016/s0306-4522(96)00640-9. [DOI] [PubMed] [Google Scholar]
- Satoh S, Matsumura H, Koike N, Tokunaga Y, Maeda T, Hayaishi O. Region-dependent difference in the sleep-promoting potency of an adenosine A2A receptor agonist. Eur J Neurosci. 1999;11:1587–1597. doi: 10.1046/j.1460-9568.1999.00569.x. [DOI] [PubMed] [Google Scholar]
- Schwierin B, Borbely AA, Tobler I. Effects of N6-cyclopentyladenosine and caffeine on sleep regulation in the rat. Eur J Pharmacol. 1996;300:163–171. doi: 10.1016/0014-2999(96)00021-0. [DOI] [PubMed] [Google Scholar]
- Souaze F. Maintaining cell sensitivity to G-protein coupled receptor agonists: neurotensin and the role of receptor gene activation. J Neuroendocrinol. 2001;13:473–479. doi: 10.1046/j.1365-2826.2001.00658.x. [DOI] [PubMed] [Google Scholar]
- Stenberg D, Litonius E, Halldner L, Johansson B, Fredholm BB, Porkka-Heiskanen T. Sleep and its homeostatic regulation in mice lacking the adenosine A1 receptor. J Sleep Res. 2003;12:283–290. doi: 10.1046/j.0962-1105.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- Strecker RE, Morairty S, Thakkar MM, Porkka-Heiskanen T, Basheer R, Dauphin LJ, Rainnie DG, Portas CM, Greene RW, McCarley RW. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav Brain Res. 2000;115:183–204. doi: 10.1016/s0166-4328(00)00258-8. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. J Neurosci. 2003;23:4278–4287. doi: 10.1523/JNEUROSCI.23-10-04278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Yanik G, Radulovacki M. REM sleep deprivation up-regulates adenosine A1 receptors. Brain Res. 1987;402:362–364. doi: 10.1016/0006-8993(87)90046-1. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Morales-Villagran A, Maidment NT, Behnke EJ, Ackerson LC, Lopez-Rodriguez F, Fried I, Engel J, Wilson CL. Extracellular adenosine in the human brain during sleep and sleep deprivation: an in vivo microdialysis study. Sleep. 2006;29:455–461. doi: 10.1093/sleep/29.4.455. [DOI] [PubMed] [Google Scholar]