Abstract
Increasing evidence supports the idea of a beneficial effect of cannabinoid compounds for the treatment of multiple sclerosis (MS). However, most experimental data come from animal models of MS. We investigated the status of cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase (FAAH) enzyme in brain tissue samples obtained from MS patients. Areas of demyelination were identified and classified as active, chronic, and inactive plaques. CB1 and CB2 receptors and FAAH densities and cellular sites of expression were examined using immunohistochemistry and immunofluorescence. In MS samples, cannabinoid CB1 receptors were expressed by cortical neurons, oligodendrocytes, and also oligodendrocyte precursor cells, demonstrated using double immunofluorescence with antibodies against the CB1 receptor with antibodies against type 2 microtubule-associated protein, myelin basic protein, and the platelet-derived growth factor receptor-α, respectively. CB1 receptors were also present in macrophages and infiltrated T-lymphocytes. Conversely, CB2 receptors were present in T-lymphocytes, astrocytes, and perivascular and reactive microglia (major histocompatibility complex class-II positive) in MS plaques. Specifically, CB2-positive microglial cells were evenly distributed within active plaques but were located in the periphery of chronic active plaques. FAAH expression was restricted to neurons and hypertrophic astrocytes. As seen for other neuroinflammatory conditions, selective glial expression of cannabinoid CB1 and CB2 receptors and FAAH enzyme is induced in MS, thus supporting a role for the endocannabinoid system in the pathogenesis and/or evolution of this disease.
Keywords: cannabinoid receptors, multiple sclerosis, immunohistochemistry, neuroinflammation, FAAH, glia
Introduction
Cannabis sativa preparations have been used for >4000 years for recreational and medicinal purposes, but the mechanistic characterization of some of its constituent chemicals, the “cannabinoids,” is much more recent (Mechoulam et al., 1994; Porter and Felder, 2001). To date, two cannabinoid receptors have been cloned, the CB1 and the CB2 receptors (Matsuda et al., 1990; Munro et al., 1993). In addition, several endocannabinoids have been identified, most important among them are N-arachidonoylethanolamine [anandamide (AEA)] and 2-arachidonoylglycerol (Mechoulam et al., 1998). These endocannabinoids are synthesized and released on demand and, after release, are removed from their sites of action by cellular uptake processes. The endocannabinoids are metabolized intracellularly by fatty acid amide hydrolase (FAAH) and monoglyceride lipase, among other enzymes (Bisogno et al., 2005).
Currently, the endocannabinoid system (ECS) is a target for the treatment of several diseases, including multiple sclerosis (MS) (Pryce and Baker, 2005). Clinical evidence confirms the therapeutic potential of cannabinoids in the treatment of symptoms of MS. A randomized, placebo-controlled trial in which patients with both stable MS and muscle spasticity were treated with cannabis extract or Δ9-tetrahydrocannabinol (Δ9-THC) for 15 weeks found that the drug treatments did not reduce the spasticity using objective measures, although patients reported improvements (Zajicek et al., 2003). Patients who continued Δ9-THC treatment for up to 12 months did exhibit a small reduction in objective measures of spasticity (Zajicek et al., 2005). Patients also reported a reduction in pain together with improvements in mobility (Zajicek et al., 2005). Another clinical trial using the oromucosal spray Sativex (a combination of Δ9-THC and cannabidiol) to treat central neuropathic pain syndromes attributable to MS showed that this preparation produced a significant reduction in pain and sleep disturbances (Rog et al., 2005).
Results from studies in animal models support the hypothesis that activation of the ECS can relieve certain signs of disease of MS. For example, Δ9-THC administration delayed the onset of the disease and remarkably reduced CNS inflammation in experimental autoimmune encephalomyelitis (EAE) (Lyman et al., 1989). In addition, administration of synthetic cannabinoids ameliorated the tremor and spasticity in mice with chronic relapsing EAE, through a CB1-mediated mechanism (Baker et al., 2000). Cannabinoid receptor agonists also improved neurological deficits in the Theiler's murine encephalitis virus model as a result of both a reduction in CNS inflammation and extensive remyelination (Arevalo-Martin et al., 2003).
Interestingly, Eljaschewitsch et al. (2006) have reported recently that AEA levels are increased in human active MS lesions. To our knowledge, only one immunohistochemical study of cannabinoid receptors in human MS samples has been published (Yiangou et al., 2006). This study, performed using spinal cord, found strong CB2 immunoreactivity in microglia/macrophages in white matter areas in MS sections, usually within or at the edge of plaque areas (Yiangou et al., 2006). These results confirm previous reports that CB2 receptors are expressed by CNS glial cells and in healthy human brains (Nunez et al., 2004) and that CB2 receptor expression is upregulated by neuroinflammation (Benito et al., 2003, 2005).
Materials and Methods
Tissues.
Postmortem brain tissues from MS donors (n = 6; age range, 54–72 years) and controls with no background of neuropsychiatric disease (n = 2; 60 and 76 years) were provided by the UK Multiple Sclerosis Tissue Bank (Table 1). Cortical and periventricular brain samples were fixed in Formalin, embedded in paraffin, and cut into 4-μm-thick sections for the immunohistochemical study.
Table 1.
Summary of brain samples used for immunohistochemistry and histopathological studies
| Patients | Age (years)/sex | Diagnosis | Postmortem interval (h) | Number of blocks examined | Number of MS lesions | Lesional activity |
||
|---|---|---|---|---|---|---|---|---|
| Active | Chronic | Inactive | ||||||
| MS96 | 58/F | SPMS | 36 | 5 | 7 | 4 | 3 | |
| MS110 | 54/F | SPMS | 18 | 5 | 33 | 15 | 9 | 9 |
| MS123 | 63/M | SPMS | 12 | 5 | 9 | 6 | 1 | 2 |
| MS132 | 72/F | SPMS | 23 | 5 | 4 | 1 | 3 | |
| MS152 | 55/F | SPMS | 36 | 5 | 2 | 1 | 1 | |
| MS177 | 60/F | SPMS | 24 | 5 | 11 | 4 | 6 | 1 |
| C17 | 76/F | Normal | 74 | 2 | ND | ND | ND | ND |
| C18 | 60/F | Normal | 44 | 2 | ND | ND | ND | ND |
| Total | 34 | 66 | 31 | 22 | 13 | |||
F, Female; M, male; MS multiple sclerosis; SPMS, secondary progressive multiple sclerosis; C, control; ND, nondetectable.
aLesional activity was determined by histological examination and divided into three categories: active, chronic, and inactive as described in Materials and Methods.
Classification of MS plaques.
To identify regions of demyelination in MS tissue samples, Luxol fast blue staining was performed. Lesions were classified according to Trapp et al. (1999) by performing immunohistochemistry of the D-region-related–human leukocyte-associated antigen (HLA-DR), a member of the class II of the major histocompatibility complex (MHC-II). We defined three main categories of demyelinated lesions or “plaques” based on the distribution and density of inflammatory cells and activated microglia (MHC-II positive). “Active” plaques exhibit abundant and evenly distributed HLA-DR-positive cells. Cells within the lesioned area are mostly large, round, and lipid-laden macrophages. These lesions are thought to be relatively recent (2–3 months) (Trapp et al., 1999). If phagocytes contain myelin protein debris, the plaques are considered even more recent (2–3 weeks). Conversely, “chronic” plaques are characterized by an enrichment of HLA-DR-positive, lipid-laden macrophages at the border of the lesion. Finally, “inactive” lesions contain very few HLA-DR-positive cells.
Immunohistochemistry.
The protocol used was as described previously (Tsou et al., 1998; Benito et al., 2003, 2005), with slight modifications. Tissue sections were deparaffinized and washed extensively in 50 mm potassium–PBS (KPBS). To obtain more efficient immunostaining, samples were subjected to an antigen retrieval procedure (Shi et al., 2001). Briefly, tissue sections were placed into a stainless-steel pressure cooker containing Antigen Retrieval Solution (DakoCytomation, Glostrup, Denmark). After heating under pressure for 2 min, samples were removed and washed extensively in KPBS. Then, endogenous peroxidase was blocked by 30 min incubation at room temperature in peroxidase-blocking solution (DakoCytomation). After several washes with KPBS, tissues were incubated with primary antibody (Table 2) overnight at 4°C. Antibodies were diluted in KPBS containing 1% BSA (Sigma, St. Louis, MO) and 1% Triton X-100 (Sigma). After the incubation, sections were washed in KPBS, followed by incubation with biotinylated goat anti-rabbit antibody (1:200) (for polyclonal antibodies) or biotinylated horse anti-mouse antibody (1:200) (for monoclonal antibodies) for 1 h at room temperature. Avidin–biotin complex (Vector Elite; Vector Laboratories, Burlingame, CA) and a diaminobenzidine substrate–chromogen system (DakoCytomation) were used to obtain a visible reaction product. Controls for the immunohistochemistry included preabsorption and coincubation of the antibodies with the corresponding immunogenic proteins (when available) as described in previous studies (Benito et al., 2003, 2005). Sections were dehydrated, sealed, and coverslipped. A Nikon (Tokyo, Japan) Eclipse 90i microscope and DXM1200F camera were used for the observations and photography of the slides, respectively.
Table 2.
Antibodies used in the study
| Antibody | Target | Dilution (IHC) | Dilution (IF) | Class | Manufacturer |
|---|---|---|---|---|---|
| HLA-DR | Microglia/macrophages (MHC-II) | 1:200 | 1:100 | Monoclonal | DakoCytomation |
| CD68 | Macrophages | 1:100 | Monoclonal | DakoCytomation | |
| CD3 | T-lymphocytes | 1:50 | 1:50 | Monoclonal | DakoCytomation |
| GFAP | Astrocytes | 1:200 | Monoclonal | Sigma | |
| MAP-2 | Neurons | 1:200 | Monoclonal | Calbiochem (La Jolla, CA) | |
| PDGFR-α | Oligodendrocyte precursor cells | 1:100 | Monoclonal | BD PharMingen (San Diego, CA) | |
| MBP | Adult oligodendrocytes/myelin debris | 1:500 | Monoclonal | Sternberger Monoclonals (Lutherville, MD) | |
| CB1 | Residues 1–99 of N-terminal of human CB1 protein | 1:300 | 1:100 | Polyclonal | Affinity BioReagents (Golden, CO) |
| CB2 | Residues 1–33 of N-terminal of human CB2 protein | 1:300 | 1:100 | Polyclonal | Affinity BioReagents |
| FAAH | Residues 561–579 of C-terminal of rat FAAH protein | 1:50 | 1:50 | Polyclonal | C. J. Hillard |
IHC, Immunohistochemistry, IF immunofluorescence.
Immunofluorescence.
To identify specific cell populations, we performed colocalization studies with immunofluorescence together with specific markers for macrophages, microglia, astrocytes, neurons, T-lymphocytes, and oligodendrocytes (Table 2). After the antigen retrieval procedure, tissue sections were washed with Tris-buffered saline (TBS) before overnight incubation at 4°C with the monoclonal antibodies used for identification of the cell types (Table 2), followed by incubation with an Alexa 546 anti-mouse antibody conjugate (Invitrogen, Carlsbad, CA) at 37°C for 2 h, rendering red fluorescence. Afterward, the primary antibodies for CB1, CB2, or FAAH were incubated overnight at 4°C after extensive washes in TBS, followed by incubation with Alexa 488 anti-rabbit antibody conjugate (Invitrogen), rendering green fluorescence. FAAH was visualized by incubation with biotinylated anti-rabbit secondary antibody, followed by streptavidin–Alexa 488 conjugate (Invitrogen), as described previously (Nunez et al., 2004). To quench endogenous autofluorescence, tissue sections were treated with 1% Sudan Black in 70% ethanol for 5 min and differentiated with 70% ethanol (Schnell et al., 1999). Sections were mounted onto glass slides with aqueous solution (Vectashield; Vector Laboratories). A Nikon Eclipse 90i microscope and DXM1200F camera were used for the observations and photography of the slides, respectively.
Results
Characteristics of MS plaques status and cellular composition
No areas of demyelination were observed in control samples. In control cases, CB1 and FAAH expression was limited to neuronal elements of the cortex, whereas CB2 receptors were almost undetectable (data not shown). Conversely, a total of 66 plaques were identified in the samples from patients with MS, as described in Table 1. Using HLA-DR-positive cell density and distribution in the plaques as our criteria (Fig. 1), as described in Materials and Methods, 31 of the plaques were classified as “active,” 22 as “chronic,” and 13 as “inactive.” A semiquantitative analysis of the expression of CB1, CB2, and FAAH proteins was performed (Table 3). The number of positive cells per section was counted and grouped according to cell subtypes based on the expression of phenotypic markers: neurons [microtubule-associated protein 2-positive (MAP-2+)], astrocytes [glial fibrillary acidic protein-positive (GFAP+)], oligodendrocyte precursor cells (OPCs) [identified as platelet-derived growth factor receptor-α-positive (PDGFR-α+)], adult oligodendrocytes [myelin basic protein-positive (MBP+)], microglia [HLA-positive (HLA+)], macrophages (CD68+), and T-lymphocytes (CD3+). CB1 and CB2 immunoreactivities were observed in active and chronic MS plaques, whereas FAAH was only evident in active plaques. Inactive plaques were devoid of immunostaining for these markers, in concordance with the extremely low cellularity of these pathological structures (Table 3).
Figure 1.
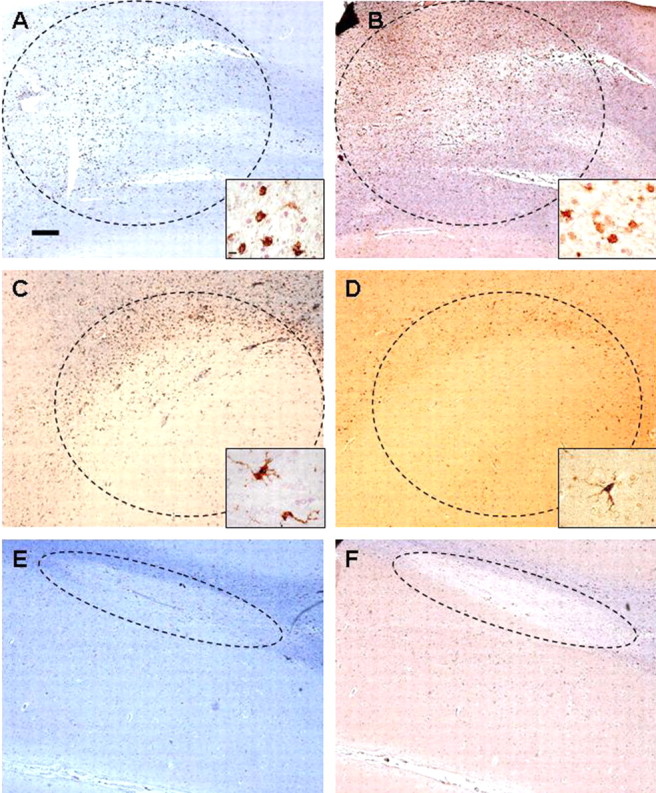
Distribution of CB2-positive cells (right column) compared with that of HLA-DR-positive cells (left column) in the three different types of MS plaques (delineated with dashed lines). A–F, The morphology and distribution of CB2-positive cells in an active (B) and chronic (D) plaques were nearly identical to those of HLA-DR-positive microglia (see insets; A, C). No CB2-positive cells were evident in inactive plaques (E, F). Luxol fast blue staining (blue) combined with immunostaining (brown). Scale bar: A–F, 500 μm; insets, 20 μm.
Table 3.
Relative abundance of CB1 and CB2 cannabinoid receptors and FAAH in specific cell subpopulations in active and chronic MS lesions
| Active lesions |
Chronic lesions |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HLA-DR | CD68 | GFAP | PDGFR-α | MBP | CD3 | MAP-2 | HLA-DR | CD68 | GFAP | PDGFR-α | MBP | CD3 | MAP-2 | |
| CB1 | ++ | ++ | ND | + | + | ++ | ++ | ND | ND | ND | ND | ND | ++ | ++ |
| CB2 | ++++ | ++++ | + | ND | ND | ++ | ND | ++++ | ND | ND | ND | ND | ++ | ND |
| FAAH | ND | ND | +++ | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
Data shown are the number of positive cells for two markers in at least three sections per case. +, <10 cells/tissue section; ++, 10–25 cells/tissue section; +++, 25–50 cells/tissue section; ++++, >50 cells/tissue section; ND, nondetectable.
CB2 receptors in MS
CB2 receptor immunoreactivity was restricted to cells located within active plaques (Fig. 1A,B) and cells located in the periphery of chronic lesions (Fig. 1C,D). Inactive plaques exhibited no staining for the CB2 receptor (Fig. 1E,F). The CB2-positive cells were morphologically similar and showed identical distribution to microglial HLA-DR-positive cells in the plaques (Fig. 1). This coincidence was confirmed by double immunostaining with HLA-DR (Fig. 2A–C). In addition, macrophages (CD68+) were positive for CB2 receptor immunoreactivity (Fig. 2D–F). Importantly, a fraction of the CB2 positive-macrophages also contained MBP (Fig. 2G–I), indicating recent phagocytic activity and suggesting that CB2 receptor expression in plaque-associated macrophages is an early event in plaque evolution. GFAP fluorescence immunostaining also revealed that CB2-positive astrocytes were present in white matter areas (Fig. 2J–L). Finally, perivascular T-lymphocytes exhibited intense CB2 receptor immunoreactivity (Fig. 2M–O). No CB2 receptor was observed in cortical neuronal elements.
Figure 2.
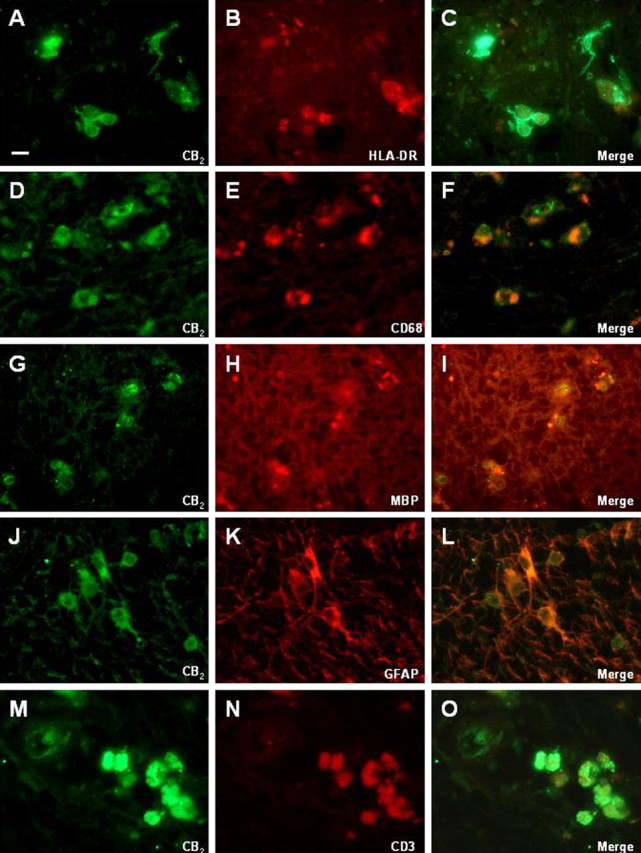
Double-immunofluorescence assays of CB2-positive cells (left column) and phenotypic markers (middle columns). A–O, CB2 was observed in HLA-DR-positive microglia (A–C), CD68-positive macrophages (D–F), MBP-containing macrophages (G–I), astrocytes (GFAP positive; J–L) and T-lymphocytes (M–O). Scale bar: 20 μm.
CB1 receptors in MS
As expected, the CB1 receptor was abundantly expressed in cortical neurons and, specifically, in large pyramidal cells of both control and MS samples (data not shown). Additionally, CB1 receptor immunoreactivity was also detected in neurons located in white matter areas (Fig. 3A,B), as revealed by double-immunofluorescence staining with MAP-2 (Fig. 3D–F). This staining was more evident in demyelinated areas, probably attributable to decreased interference from axonal staining. Intense staining for the CB1 receptor was also observed in non-neuronal cell types. Remarkably, adult oligodendrocytes and OPCs in active plaques, identified with the specific markers MBP and PDGFR-α, were also immunoreactive for the CB1 receptor (Fig. 3G–I,J–L). Importantly, not all active lesions contained CB1-positive adult oligodendrocytes or OPCs. In addition, double-labeling experiments with CD68 demonstrated abundant CB1-positive macrophages within active plaques (Fig. 3C,M–O). Finally, perivascular T-lymphocytes also exhibited staining for CB1 receptors (Fig. 3P–R).
Figure 3.
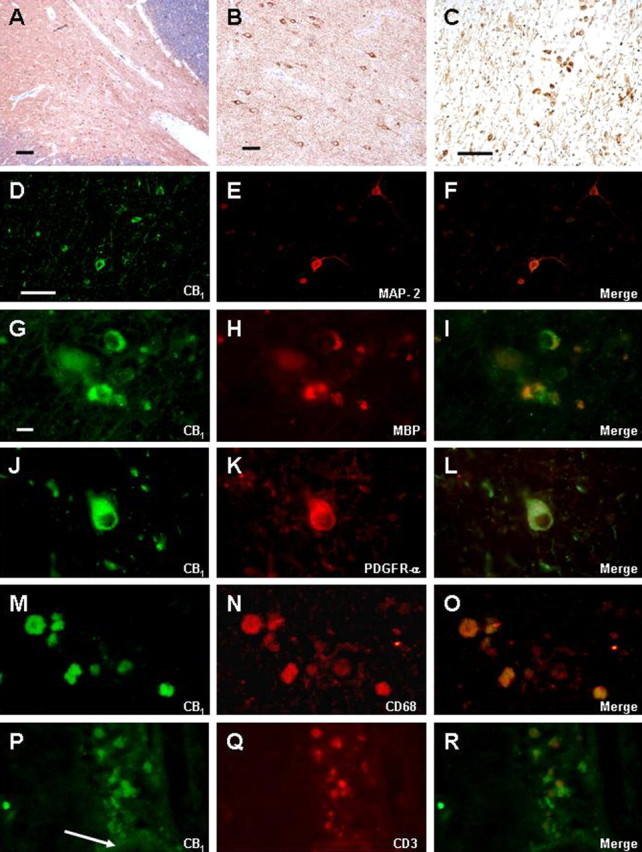
CB1 distribution in MS samples. A, B, D, Subcortical white matter cells exhibited CB1 immunoreactivity (A, B); CB1-positive cells (D) were identified as neurons by double staining with MAP-2 (E). G–I, Adult oligodendrocytes exhibited cytoplasmic staining for CB1. J, K, Scarce OPCs in active plaques were positive for CB1 (J) and PDGFR-α (K). Round-shaped macrophages (CD68 positive; N) also showed CB1 immunostaining (C, M). Perivascular T-lymphocytes (Q; see vessel wall, arrow in P) expressed CB1 receptors (P). Scale bars: A, 500 μm; B, 200 μm; C–F, 100 μm; G–R, 20 μm.
FAAH in MS
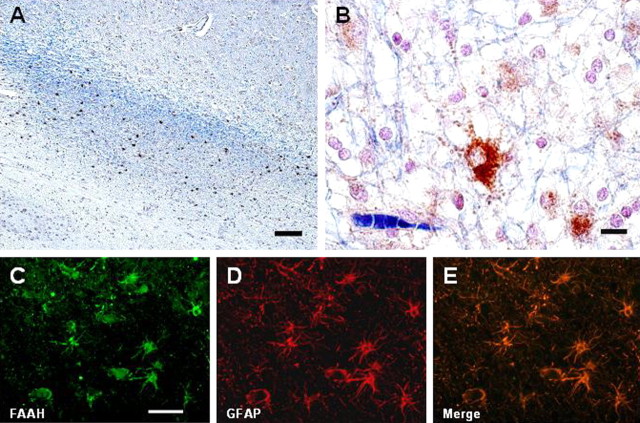
FAAH expression was observed in cortical neurons in both control and MS samples (data not shown). Contrary to the pattern of CB1 receptor expression, FAAH was not detected in subcortical white matter neurons. In MS tissue samples, FAAH expression was evident only in plaques of the active type (Fig. 4A). These FAAH-positive cells exhibited strong immunoreactivity in cell bodies, whereas processes were weakly immunoreactive (Fig. 4B). High-magnification pictures revealed an astrocyte-like morphology (Fig. 4B) that was confirmed by double staining with GFAP (Fig. 4C–E).
Figure 4.
FAAH is expressed by hypertrophic astrocytes in active MS plaques. A–E, FAAH-positive cells were abundant in active plaques (A) and exhibited morphological (B) and phenotypic (GFAP positive; C–E) characteristics of astrocytes. Scale bars: A, 500 μm; B, 20 μm; C–E, 100 μm.
Discussion
MS is an inflammatory, demyelinating disease of the CNS and is a major cause of neurological disability among young adults (Noseworthy et al., 2000). The characteristic symptoms of this disease (such as painful muscle spasms, tremor, ataxia, weakness, or paralysis) are thought to be the result of both newly formed CNS lesions and expansion of old lesions. Neuropathology of MS includes axonal degeneration, oligodendrocyte loss, and subsequent induction of areas of demyelination. Lymphocytes and monocytes infiltrate the white matter surrounding the blood vessels, destroying myelin but usually sparing axons. Finally, cells of monocytic origin are responsible for myelin removal by phagocytosis and contribute to the inflammatory process (for review, see Noseworthy et al., 2000).
The ECS is a putative target for the development of new therapeutic tools for the treatment of MS. Despite relevant preclinical data obtained in the past few years, the status of the ECS in human MS remains unstudied. To that end, we performed an extensive immunohistochemical analysis of the changes in cannabinoid CB1 and CB2 receptors and in the FAAH enzyme in tissue samples from MS donors. Our results show that, as with other human neuroinflammatory diseases, profound alterations in the expression profile of these elements of the ECS occur in the brains of MS patients compared with normal patients. The current data support the hypothesis suggested previously that a shift in the ECS from predominantly neuronal to glial occurs in neuroinflammation (Pazos et al., 2005).
Neuronal expression was only evident for CB1 and FAAH, mostly on pyramidal neurons. CB1 receptors, but not FAAH, were also detected in subcortical white matter neurons, as revealed by double-immunofluorescent labeling with MAP-2. This observation suggests a regional segregation in the pattern of CB1 and FAAH expression. CB1 receptors located on glutamatergic neurons could exert a direct neuroprotective effect by dampening the characteristic excitotoxic insult that is triggered in neuroinflammatory conditions, as well as by modulating the activity of ion channels (Mechoulam et al., 2002).
Interestingly, CB1 receptors were also present in adult oligodendrocytes and OPCs located within MS plaques. These cells are known to play a critical role in the remyelination process that takes place during the course of the disease (Levine et al., 2001). It is important to note that we reported previously that both CB1 and CB2 receptors are expressed by different rat oligodendrocyte subpopulations in vivo and in vitro (Molina-Holgado et al., 2002). Previous studies have shown that cannabinoids act through both receptor-dependent and -independent mechanisms to promote oligodendrocyte survival via a phosphatidylinositol 3-kinase/Akt-dependent mechanism and thereby enhance axonal remyelination in an animal model of MS (Molina-Holgado et al., 2002; Arevalo-Martin et al., 2003).
Our data indicate that both CB1 and CB2 receptors are present in microglia/macrophages located in MS plaques. These cells are thought to participate in inflammatory processes and in the removal of myelin and debris from damaged cells (Bruck et al., 1996). The coexistence of CB2 with intracellular MBP suggests that the CB2-positive subset of macrophages were recently phagocytic and are part of active plaques. Because immunohistochemical staining of MS lesions with anti-myelin antibodies to estimate lesion age is widely accepted (Bruck et al., 1995; Noseworthy et al., 2000; Chang et al., 2002; van der Goes et al., 2005), these data provide evidence that the induction of CB2 receptor expression in plaque-associated macrophages could be an early event in the maturation of MS plaques. Although little is known regarding the effects of the cannabinoids on myelin phagocytosis, it has been shown previously that activation of CB1 and CB2 receptors decreases the production of proinflammatory cytokines in macrophages, indicating that an anti-inflammatory mechanism could potentiate the neuroprotection induced by cannabinoids (Mestre et al., 2005; Ortega-Gutierrez et al., 2005a). As reviewed by Croxford and Yamamura (2005), several characteristics of macrophages, such as migration, presentation of peptide antigens, or phagocytosis of foreign particles, are significantly influenced by cannabinoids.
The highest levels of CB2 receptor immunoreactivity were detected in microglia, as observed previously by Yiangou et al. (2006) in spinal cord sections from MS donors and in other human diseases with a neuroinflammatory component (Benito et al., 2003, 2005). In addition, Maresz et al. (2005) have shown previously that CB2 expression is upregulated in microglial cells in autoimmune-induced inflammation of the CNS in mice. Our results show that the distribution of these CB2-positive cells correlates with that of MHC-II-positive cells, whose localization and abundance are used as defining markers of plaque subtype (Trapp et al., 1999). Thus, MHC-II-positive cells are present abundantly throughout the entire extension of acutely active plaques, although these cells are restricted to the periphery of chronically active plaques. The similarity between CB2-positive cells and microglia and the colocalization of CB2 receptors with HLA-DR leads us to postulate the presence of CB2 receptors as a novel diagnostic marker for the identification of MS plaques of the active type.
CB2 expression was also detected in white matter astrocytes. To our knowledge, this is the first observation of an astrocytic expression of these receptors in situ in the human CNS and contrast with previous data obtained in other pathologies such as Alzheimer's disease (Benito et al., 2003; Ramirez et al., 2005). Little is known about the role that CB2 receptors play in astrocytes, although previous data suggest that they may modulate the production of proinflammatory molecules in vitro (Ortega-Gutierrez et al., 2005b; Sheng et al., 2005).
T-cells are known to participate in the pathogenesis of MS (Frohman et al., 2006). In particular, myelin-specific T-lymphocytes are thought to be directly involved in the demyelinating process and to cause inflammation. We have shown previously that cannabinoids, acting through both CB1 and CB2 receptors, decrease CD4+ infiltration into the spinal cord in an animal model of MS (Arevalo-Martin et al., 2003). As suggested previously, this could account for the anti-inflammatory action of cannabinoids. The present data corroborate previous findings of the expression of both types of cannabinoid receptors in CNS-infiltrated T-lymphocytes (Benito et al., 2005), particularly in perivascular fields. These data are highly suggestive of a possible role of the ECS in MS-linked, T-cell-mediated neuroinflammation.
Expression of the endocannabinoid-degrading enzyme FAAH was increased in astrocytes within MS plaques compared with control brain. This seems to be a strikingly constant feature of FAAH, because reactive astrocytes exhibit increased expression of this enzyme in Alzheimer's disease samples (Benito et al., 2003) as do perivascular astrocytes and astrocytic processes reaching cellular infiltrates in an animal model of encephalitis (Benito et al., 2005). Importantly, other arachidonic acid-related enzymes, such as cyclooxygenase-2 and phospholipase-A2, are also known to be selectively upregulated in astrocytes after inflammatory stimuli (Sun et al., 2005). As suggested previously, FAAH inhibition could have beneficial effects during inflammation attributable to both decreased local production of arachidonic acid and enhanced endogenous cannabinoid tone (Benito et al., 2003; Karanian et al., 2005).
Although our study does not include a quantitative approach attributable to the low number of cases and tissue availability, the presence of different elements of the ECS in selective, specific, cell types provide a neuroanatomical rationale for the effects of cannabinoids on MS symptoms and progression in humans. Possible targets of ECS that could provide benefit for the treatment of MS have focused only on CB1 activation so far. Our present results allow us to postulate that other elements of the ECS such as the CB2 receptors and FAAH are potential therapeutic targets for the treatment of MS in humans.
Footnotes
This work was supported by Ministerio de Educación y Ciencia Grant SAF 2004-00237 and Comunidad Autónoma de Madrid Grant S-SAL/0261/2006. Tissue samples were supplied by the United Kingdom Multiple Sclerosis Tissue Bank, funded by the Multiple Sclerosis Society of Great Britain and Northern Ireland, registered charity 207495. We thank Dr. J. A. Martínez-Orgado for helpful discussion. The administrative support of Julia Molina and the technical contribution of Montserrat Díaz-Meco, Ana Belén Rebolledo, and Carolina Herranz are gratefully acknowledged.
References
- Arevalo-Martin A, Vela JM, Molina-Holgado E, Borrell J, Guaza C. Therapeutic action of cannabinoids in a murine model of multiple sclerosis. J Neurosci. 2003;23:2511–2516. doi: 10.1523/JNEUROSCI.23-07-02511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, Layward L. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature. 2000;404:84–87. doi: 10.1038/35003583. [DOI] [PubMed] [Google Scholar]
- Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, Romero J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer's disease brains. J Neurosci. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C, Kim WK, Chavarria I, Hillard CJ, Mackie K, Tolon RM, Williams K, Romero J. A glial endogenous cannabinoid system is upregulated in the brains of macaques with simian immunodeficiency virus-induced encephalitis. J Neurosci. 2005;25:2530–2536. doi: 10.1523/JNEUROSCI.3923-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Ligresti A, Di Marzo V. The endocannabinoid signalling system: biochemical aspects. Pharmacol Biochem Behav. 2005;81:224–238. doi: 10.1016/j.pbb.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Bruck W, Porada P, Poser S, Rieckmann P, Hanefeld F, Kretzschmar HA, Lassmann H. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol. 1995;38:788–796. doi: 10.1002/ana.410380514. [DOI] [PubMed] [Google Scholar]
- Bruck W, Sommermeier N, Bergmann M, Zettl U, Goebel HH, Kretzschmar HA, Lassmann H. Macrophages in multiple sclerosis. Immunobiology. 1996;195:588–600. doi: 10.1016/S0171-2985(96)80024-6. [DOI] [PubMed] [Google Scholar]
- Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- Croxford JL, Yamamura T. Cannabinoids and the immune system: potential for the treatment of inflammatory diseases? J Neuroimmunol. 2005;166:3–18. doi: 10.1016/j.jneuroim.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, Hoertnagl H, Raine CS, Schneider-Stock R, Nitsch R, Ullrich O. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49:67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis–the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Karanian DA, Brown QB, Makriyannis A, Kosten TA, Bahr BA. Dual modulation of endocannabinoid transport and fatty acid amide hydrolase protects against excitotoxicity. J Neurosci. 2005;25:7813–7820. doi: 10.1523/JNEUROSCI.2347-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- Lyman WD, Sonett JR, Brosnan CF, Elkin R, Bornstein MB. Delta 9-tetrahydrocannabinol: a novel treatment for experimental autoimmune encephalomyelitis. J Neuroimmunol. 1989;23:73–81. doi: 10.1016/0165-5728(89)90075-1. [DOI] [PubMed] [Google Scholar]
- Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95:437–445. doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Hanus L, Martin BR. Search for endogenous ligands of the cannabinoid receptor. Biochem Pharmacol. 1994;48:1537–1544. doi: 10.1016/0006-2952(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur J Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Panikashvili D, Shohami E. Cannabinoids and brain injury: therapeutic implications. Trends Mol Med. 2002;8:58–61. doi: 10.1016/s1471-4914(02)02276-1. [DOI] [PubMed] [Google Scholar]
- Mestre L, Correa F, Arevalo-Martin A, Molina-Holgado E, Valenti M, Ortar G, Di Marzo V, Guaza C. Pharmacological modulation of the endocannabinoid system in a viral model of multiple sclerosis. J Neurochem. 2005;92:1327–1339. doi: 10.1111/j.1471-4159.2004.02979.x. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E, Vela JM, Arevalo-Martin A, Almazan G, Molina-Holgado F, Borrell J, Guaza C. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J Neurosci. 2002;22:9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- Nunez E, Benito C, Pazos MR, Barbachano A, Fajardo O, Gonzalez S, Tolon RM, Romero J. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study. Synapse. 2004;53:208–213. doi: 10.1002/syn.20050. [DOI] [PubMed] [Google Scholar]
- Ortega-Gutierrez S, Molina-Holgado E, Arevalo-Martin A, Correa F, Viso A, Lopez-Rodriguez ML, Di Marzo V, Guaza C. Activation of the endocannabinoid system as therapeutic approach in a murine model of multiple sclerosis. FASEB J. 2005a;19:1338–1340. doi: 10.1096/fj.04-2464fje. [DOI] [PubMed] [Google Scholar]
- Ortega-Gutierrez S, Molina-Holgado E, Guaza C. Effect of anandamide uptake inhibition in the production of nitric oxide and in the release of cytokines in astrocyte cultures. Glia. 2005b;52:163–168. doi: 10.1002/glia.20229. [DOI] [PubMed] [Google Scholar]
- Pazos MR, Nunez E, Benito C, Tolon RM, Romero J. Functional neuroanatomy of the endocannabinoid system. Pharmacol Biochem Behav. 2005;81:239–247. doi: 10.1016/j.pbb.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Porter AC, Felder CC. The endocannabinoid nervous system: unique opportunities for therapeutic intervention. Pharmacol Ther. 2001;90:45–60. doi: 10.1016/s0163-7258(01)00130-9. [DOI] [PubMed] [Google Scholar]
- Pryce G, Baker D. Emerging properties of cannabinoid medicines in management of multiple sclerosis. Trends Neurosci. 2005;28:272–276. doi: 10.1016/j.tins.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, De Ceballos ML. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65:812–819. doi: 10.1212/01.wnl.0000176753.45410.8b. [DOI] [PubMed] [Google Scholar]
- Schnell SA, Staines WA, Wessendorf MW. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem. 1999;47:719–730. doi: 10.1177/002215549904700601. [DOI] [PubMed] [Google Scholar]
- Sheng WS, Hu S, Min X, Cabral GA, Lokensgard JR, Peterson PK. Synthetic cannabinoid WIN55,212-2 inhibits generation of inflammatory mediators by IL-1beta-stimulated human astrocytes. Glia. 2005;49:211–219. doi: 10.1002/glia.20108. [DOI] [PubMed] [Google Scholar]
- Shi SR, Cote RJ, Taylor CR. Antigen retrieval techniques: current perspectives. J Histochem Cytochem. 2001;49:931–937. doi: 10.1177/002215540104900801. [DOI] [PubMed] [Google Scholar]
- Sun GY, Xu J, Jensen MD, Yu S, Wood WG, Gonzalez FA, Simonyi A, Sun AY, Weisman GA. Phospholipase A2 in astrocytes: responses to oxidative stress, inflammation, and G protein-coupled receptor agonists. Mol Neurobiol. 2005;31:27–41. doi: 10.1385/MN:31:1-3:027. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Bo L, Mork S, Chang A. Pathogenesis of tissue injury in MS lesions. J Neuroimmunol. 1999;98:49–56. doi: 10.1016/s0165-5728(99)00081-8. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- van der Goes A, Boorsma W, Hoekstra K, Montagne L, de Groot CJ, Dijkstra CD. Determination of the sequential degradation of myelin proteins by macrophages. J Neuroimmunol. 2005;161:12–20. doi: 10.1016/j.jneuroim.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, Banati RR, Anand P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajicek J, Fox P, Sanders H, Wright D, Vickery J, Nunn A, Thompson A. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentre randomised placebo-controlled trial. Lancet. 2003;362:1517–1526. doi: 10.1016/S0140-6736(03)14738-1. [DOI] [PubMed] [Google Scholar]
- Zajicek JP, Sanders HP, Wright DE, Vickery PJ, Ingram WM, Reilly SM, Nunn AJ, Teare LJ, Fox PJ, Thompson AJ. Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry. 2005;76:1664–1669. doi: 10.1136/jnnp.2005.070136. [DOI] [PMC free article] [PubMed] [Google Scholar]