Abstract
Neurotrophins and their cognate receptors might serve as feedback regulators for the efficacy of synaptic transmission. We analyzed mice overexpressing TrkC (TgNTRK3) for synaptic plasticity and the expression of glutamate receptor subunits. Animals were conditioned using a trace [conditioned stimulus (CS), tone; unconditioned stimulus (US), shock] paradigm. A single electrical pulse presented to the Schaffer collateral–commissural pathway during the CS–US interval evoked a monosynaptic field EPSP (fEPSP) at ipsilateral CA1 pyramidal cells. In wild types, fEPSP slopes increased across conditioning sessions and decreased during extinction, being linearly related to learning evolution. In contrast, fEPSPs in TgNTRK3 animals reached extremely high values, not accompanied with a proportionate increase in their learning curves. Long-term potentiation evoked in conscious TgNTRK3 was also significantly longer lasting than in wild-type mice. These functional alterations were accompanied by significant changes in NR1 and NR2B NMDA receptor subunits, with no modification of NR1Ser 896 or NR1Ser 897 phosphorylation. No changes of AMPA and kainate subunits were detected. Results indicate that the NT-3/TrkC cascade could regulate synaptic transmission and plasticity through modulation of glutamatergic transmission at the CA3–CA1 synapse.
Keywords: long-term potentiation, neurotrophins, NMDA, NT-3, TrkC, eyeblink conditioning
Introduction
Neurotrophins are proteins that control the survival and differentiation of many types of neurons during development (Blum and Konnerth, 2005; Chao et al., 2006). There is increasing evidence indicating that neurotrophins are also involved in activity-dependent neuronal plasticity in the CNS (Thoenen, 1995; Bonhoeffer, 1996; Lu, 2004; Blum and Konnerth, 2005; Agassandian et al., 2006; Shimazu et al., 2006). Brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) mRNAs are highly expressed in the hippocampus and neocortex (Ernfors et al., 1990; Tokuyama et al., 1999; Sugiyama et al., 2003), together with their cognate receptors tyrosine receptor kinase B (TrkB) and TrkC (Kokaia et al., 1993; Barbacid, 1994; Fryer et al., 1996; Roskoden et al., 1999; Xu et al., 2002), making likely NT-3-induced synaptic enhancement via activation of TrkC receptors.
The release of BDNF and NT-3 is regulated by neuronal activity (Lessmann, 1998; Gomez-Pinilla et al., 2002; Yamada et al., 2002). Interestingly, high-frequency stimulation (HFS), a paradigm used to induce long-term potentiation (LTP) in the hippocampus, can induce both BDNF and NT-3 mRNA expression in different hippocampal areas (Korte et al., 1998; Kovalchuk et al., 2002; Gartner et al., 2006). BDNF and NT-3 can acutely enhance glutamatergic synaptic transmission in diverse hippocampal and neocortical preparations via activation of TrkB and TrkC receptors (Gottschalk et al., 1999; Messaoudi et al., 2002; Tyler and Pozzo-Miller, 2003; Tyler et al., 2006). Nevertheless, the cellular mechanisms and molecular targets of the neurotrophin-mediated synaptic modulation remain to be elucidated.
We have tested the hypothesis that in vivo overexpression of the TrkC receptor could produce an increase in survival and/or neuronal induction or promotion in the hippocampus, with some putative consequences for learning and synaptic plasticity in adult mice. To this aim, we have used transgenic mice overexpressing the full-length NT-3 receptor, TrkC, in the CNS (Dierssen et al., 2006; Sahún et al., 2007). The main objective of this study was to explore the possibility that TrkC influences both LTP induction in the hippocampus and associative learning. Mice were prepared for the chronic recording of the electromyographic (EMG) activity of the orbicularis oculi muscle and the electrical stimulation of the ipsilateral supraorbitary nerve, and for the classical conditioning of eyeblink responses using a trace paradigm. To this end, a tone (20 ms) was presented as a conditioned stimulus (CS), followed 500 ms from its end by an electrical shock applied to the supraorbitary nerve as an unconditioned stimulus (US). Evoked eyelid conditioned responses (CRs) were recorded and quantified following procedures described previously (Domínguez-del-Toro et al., 2004; Porras-García et al., 2005; Gruart et al., 2006). To follow the putative changes in the evoked extracellular field EPSPs (fEPSPs) at the CA3–CA1 synapse across conditioning (Gruart et al., 2006), a single pulse was presented at the Schaffer collateral/commissural pathway 300 ms after each CS presentation. LTP of the CA3–CA1 was also achieved in conscious animals by HFS of Schaffer collaterals.
Materials and Methods
Subjects.
Experiments were performed in TgNTRK3 mice (Dierssen et al., 2002, 2006; Sahún et el., 2007) and wild-type littermates, grown on a hybrid B6/SJL background and generated and maintained by the Genomic Regulation Center (Universidad Pompeu Fabra, Barcelona, Spain). Animals were 4 ± 2 months old at the moment of surgery, weighing 31 ± 3 g (range, 24–38 g). Before experimental manipulations, animals were housed in separate cages (n = 10 per cage). Mice were kept on a 12 h light/dark cycle with constant ambient temperature (21 ± 1°C) and humidity (55 ± 7%). Food and water were available ad libitum. Electrophysiological and behavioral studies were performed in accordance with the guidelines of the European Union Council (86/609/EU) and Spanish regulations (BOE 67/8509-12, 1988) for the use of laboratory animals in chronic experiments and approved by the local Ethical Committee. For classical conditioning, ≥10 animals per group were used. Double-pulse and LTP studies were performed on ≥10 additional animals per experimental group.
Quantification of hippocampal cell populations.
Six TgNTRK3 and seven wild-type mice were used. Mice were anesthetized with isofluorane and then perfused intracardially with 100 ml of 0.1 m PBS, pH 7.4, followed by 100 ml of chilled 4% paraformaldehyde (Sigma, St. Louis, MO). The brains were postfixed in the same fixative overnight, rinsed, and then cryoprotected for 48 h in 30% sucrose–PBS at 4°C. Coronal sections (50 μm) were incubated with anti-NeuN (4G2; 1:1000; Abcam, Cambridge, UK) or anti-glial fibrillary acidic protein (GFAP) (1:2000; Dako, High Wycombe, UK) antibodies overnight at 4°C (1:8000; Sigma) followed by incubation with a biotinylated secondary rabbit antibody and Streptavidin-HRP (Dako). Peroxidase activity was visualized with 0.05% diaminobenzidine and 0.05% hydrogen peroxide. Sections were counterstained with the Nissl technique. After completion of the staining, sections were dehydrated with increasing concentrations of ethanol, mounted on silanized slides, and coverslipped with DPX (distyrene, tricresyl phosphate, and xylene; BDH Laboratory Supplies, Dorset, UK). Stereological estimates of total number of anti-NeuN and anti-GFAP-positive neurons in coronal sections (50 μm) through the hippocampus (bregma, −1.2 to −3.4 mm), according to the stereotaxic coordinates adopted from a mouse brain atlas (Paxinos and Franklin, 2001), were obtained with the aid of the CAST-GRID software package adapted to an Olympus BX51 microscope (Olympus, Ballerup, Denmark). Estimation of the volume (Vref) of these regions was performed using the Cavalieri method, and the optical dissector method was used to estimate neuronal density (Nv). Fifteen dissector probes of 713 μm2 (Sdis) with a thickness (Hdis) of 20 μm [Vdis = Sdis × Hdis = 14260 μm3; guard zone, 3 μm to the surface of section] were analyzed per section, using a 40× objective to count neuronal nuclei in sampling probes. The total number of neurons was estimated using the following formula: Nneu = Nv × V. The coefficient of error, CE = SEM/mean, was calculated to evaluate the precision of the estimates (Gundersen and Jensen, 1987). Sampling was optimized to produce a CE under the observed biological variability (West and Gundersen, 1990). To estimate the volumetric shrinkage factor (SV), the thickness before and after processing was analyzed using the computer-driven z-axis of the microscope. This analysis revealed an average thickness shrinkage factor of ∼0.86, which was similar in wild-type and TgNTRK3 mice.
Western blot analysis.
Animals (n = 4–5 per genotype) were deeply anesthetized using a CO2 chamber. The hippocampus was dissected out, rapidly frozen, and stored at −80°C until processing. Total protein homogenates were obtained and loaded in SDS-PAGE. Then, proteins were transferred to Immobilon-P membranes (Millipore, Billerica, MA), incubated for 1 h with 5% BSA and 5% fat-free dry milk in Tris-buffered saline containing 0.1% Tween 20, and incubated overnight at 4°C with primary antibodies against NR1 (1:500), NR2A (1:1000), and NR2B (1:1000; Chemicon International, Temecula, CA). Antibodies against GluR1 (1:4000), GluR2/3 (1:2000), GluR4 (1:1250), GluR5 (1:2000), GluR6/7 (1:2000), phosphor-NR1Ser 896, or phosphor-NR1Ser 897 were also used (Upstate Biotechnology, Lake Placid, NY). Next, membranes were incubated with horseradish peroxidase-conjugated anti-rabbit antibody (1:3000; Promega, Madison, WI). To standardize total protein content in each lane, membranes were incubated with α-tubulin (1:100,000; Sigma, St. Louis, MO) primary antibodies, followed by incubation with horseradish peroxidase-conjugated anti-mouse antibody (1:3000; Promega). Finally, the reaction was visualized with the ECL Western blotting analysis system (Meridian Bioscience, Cincinnati, OH). Western blot replicates were scanned and quantified using the Phoretix 1D Gel Analysis (Phoretix International, Durham, NC).
NT-3 ELISA.
Samples (n = 6 per genotype) were analyzed using an NT-3 ELISA kit according to the recommendations of the manufacturer (Promega). In brief, hippocampus and cortex were lysed in 1 ml of lysis buffer (137 mm NaCl, 20 mm Tris, 1% octylphenoxpolyethoxyethanol, 10% glycerol, 10 mm NAF, 2 mm sodium vanadate, and proteinase inhibitor mixture tablets). A volume of 25 μl of the sample was diluted in 75 μl of sample buffer and incubated on a plate coated with NT-3 antibody. Standard curves of pure NT-3 protein, provided in the kit, were used to quantify the production of NT-3 in pg/mg total protein.
Surgery for chronic experiments.
Animals were anesthetized with 0.8–3% halothane (AstraZeneca, Madrid, Spain) delivered via a home-made mask. Halothane was administered from a calibrated Fluotec 5 (Fluotec-Ohmeda, Tewksbury, MA) vaporizer at a flow rate of 1–4 L/min oxygen. Animals were implanted with bipolar stimulating electrodes on the left supraorbitary nerve and with bipolar recording electrodes in the ipsilateral orbicularis oculi muscle (see Fig. 2A). Electrodes were made of 50 μm, Teflon-coated, annealed stainless steel wire (A-M Systems, Carlsborg, WA). Electrode tips were bared of the isolating cover for ≈0.5 mm and bent as a hook to facilitate a stable insertion in the upper eyelid. In addition, animals were implanted with bipolar stimulating electrodes aimed at the right (contralateral) Schaffer collateral-commissural pathway of the dorsal hippocampus (2 mm lateral and 1.5 mm posterior to bregma; depth from brain surface, 1.0–1.5 mm) (Paxinos and Franklin, 2001) and with two recording electrodes aimed at the ipsilateral stratum radiatum underneath the CA1 area (1.2 mm lateral and 2.2 mm posterior to bregma; depth from brain surface, 1.0–1.5 mm). These electrodes were made of 50 μm, Teflon-coated tungsten wire (Advent Research Materials, Eynsham, UK). The final position of hippocampal electrodes was determined under recording and stimulating procedures, as explained below. A bare silver wire (0.1 mm) was affixed to the skull as a ground. The eight wires were connected to two four-pin sockets (RS Amidata, Madrid, Spain). The ground wire was also connected to the recording system with a single wire. Sockets were fixed to the skull with the help of two small screws and dental cement (Domínguez-del-Toro et al., 2004; Gruart et al., 2006).
Figure 2.
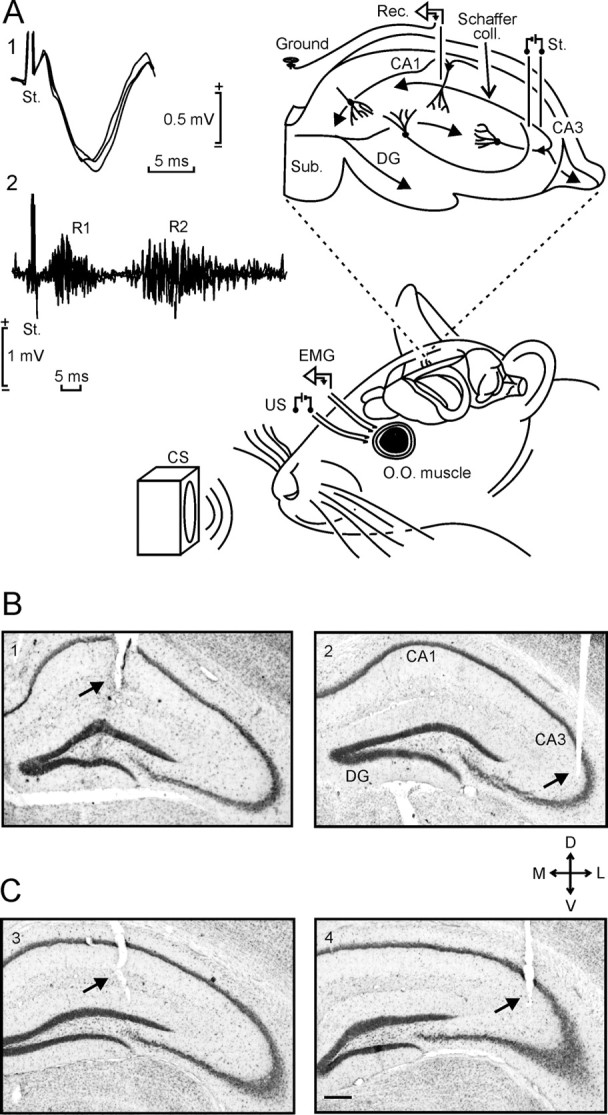
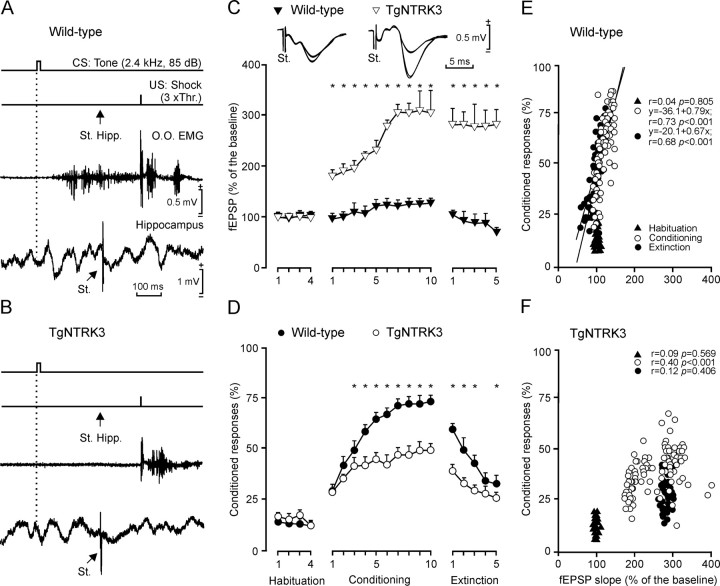
Experimental design for classical conditioning and fEPSP recording. A, Electrodes to record the EMG activity of the left orbicularis oculi (O.O.) muscle were implanted in the upper eyelid. For classical conditioning of eyelid responses, we used a tone (20 ms, 2.4 kHz, 85 dB) as a CS, delivered from a loudspeaker located 30 cm in front of the animal's head. Bipolar stimulating electrodes were implanted on the supraorbitary nerve for US presentation. fEPSPs were recorded (Rec.) at the stratum radiatum of the hippocampal CA1 area after electrical stimulation (St.) of the Schaffer collateral (coll.)/commissural pathway (right hippocampus). Representative examples (3 superimposed traces) of fEPSPs (1) and EMG (2) activities are also illustrated. Sub., Subiculum. B, C, Photomicrographs illustrating the location of recording (CA1; 1, 3) and stimulating (CA3; 2, 4) sites in wild-type (B) and TgNTRK3 (C) mice. Scale bar, 200 μm. D, Dorsal; L, lateral; M, medial; V, ventral; DG, dentate gyrus.
Recording and stimulation procedures.
The EMG activity of the orbicularis oculi muscle was recorded with Grass P511 differential amplifiers at a bandwidth of 0.1 Hz to 10 kHz (Grass-Telefactor, West Warwick, RI). fEPSP recordings were also made with Grass P511 differential amplifiers through a high-impedance probe (2 × 1012 Ω, 10 pF). Electrodes were implanted in the CA1 area after the field potential depth profile evoked by paired (20–50 ms of interval) pulses presented at the ipsilateral Schaffer collateral pathway. Synaptic field potentials in the CA1 area were evoked during habituation, conditioning, and extinction sessions by a single 100 μs, square, biphasic (negative–positive) pulse applied to Schaffer collaterals 300 ms after CS presentation. Stimulus intensities ranged from 50 to 350 μA. For each animal, the stimulus intensity was set well below the threshold for evoking a population spike, usually 30–40% of the intensity necessary for evoking a maximum fEPSP response (Gureviciene et al., 2004; Gruart et al., 2006). An additional criterion for selecting stimulus intensity was that a second stimulus, presented 20–50 ms after a conditioning pulse, evoked a larger (>20%) synaptic field potential than the first (Bliss and Gardner-Medwin, 1973). For LTP induction, each animal was presented with five 200 Hz, 100 ms trains of pulses at a rate of 1/s. This protocol was presented 6 times in total, at intervals of 1 min. The 100 μs, square, biphasic pulses used to evoke LTP were applied at the same intensity used for the single pulse presented after CS presentation. Additional details of this chronic preparation have been published previously (Gruart et al., 2006).
Classical conditioning.
For recordings, two animals at a time were placed in separate small (5 × 5 × 10 cm) plastic chambers located inside a larger Faraday box (30 × 30 × 20 cm). Classical conditioning was achieved using a trace paradigm (see Fig. 3A,B). For this, a tone (20 ms, 2.4 kHz, 85 dB) was presented as a CS. The US consisted of a 500 μs, 3 times threshold, square, cathodal pulse applied to the supraorbitary branch of the trigeminal nerve. The US started 500 ms after the end of the CS. A total of four habituation, 10 conditioning, and five extinction sessions were performed for each animal. A conditioning session consisted of 60 CS–US presentations and lasted ≈30 min. For a proper analysis of the CR, the CS was presented alone in 10% of the cases. CS–US presentations were separated at random by 30 ± 5 s. For habituation and extinction sessions, only the CS was presented, also for 60 times per session, at intervals of 30 ± 5 s. As criteria, we considered a CR the presence, during the CS–US period, of EMG activity lasting >10 ms and initiated >50 ms after CS onset. In addition, the integrated EMG activity recorded during the CS–US interval had to be at least 2.5 times greater than the averaged activity recorded immediately before CS presentation (Porras-García et al., 2005).
Figure 3.
Learning curves and evolution of the synaptic field potential for wild-type and TgNTRK3 groups. A, At the top is a schematic representation of the conditioning paradigm illustrating CS and US stimuli and the moment at which a single pulse (100 μs, square, biphasic) was presented to Schaffer collaterals (St. Hipp.). An example of an EMG record from the orbicularis oculi (O.O.) muscle, obtained from the eighth conditioning session, is illustrated, as well as an extracellular record of hippocampal activity from the same animal, session, and trial. Note the fEPSP evoked by the single pulse presented to Schaffer collaterals. B, A similar set of records collected from a TgNTRK3 mouse during the eighth conditioning session. Note the presence of a noticeable fEPSP but the absence of an eyelid CR. C, At the top are illustrated superimposed (n = 4) extracellular fEPSP traces collected from the wild-type and TgNTRK3 groups during the second habituation (n = 2) and the ninth conditioning (n = 2) sessions. Evolution of the fEPSP slope in wild-type (black triangles) and TgNTRK3 (white triangles) groups, expressed as the change (in percentage) with respect to mean values collected during the four habituation sessions. Differences between wild-type and TgNTRK3 groups were statistically significant for all of the conditioning and extinction sessions (*p < 0.001; F(18,162) = 149). D, Evolution of the percentage (in percentage) of CRs during the successive sessions for wild-type (black circles) and TgNTRK3 (white circles) groups. Mean percentage values are followed by ±SEM. Differences between wild-type and TgNTRK3 groups were statistically significant from the third to the 10th conditioning sessions and for the first-third and fifth extinction sessions (*p < 0.01; F(18,162) = 8.6). E, F, Quantitative analysis of the relationships between the percentage of CRs and fEPSP slopes for the wild-type (E) and TgNTRK3 (F) groups during habituation (black triangles), conditioning (white circles), and extinction (black circles) sessions. Each point represents the mean value collected from a single animal during the corresponding session. Regression lines, and their corresponding equations, are included only for coefficients of correlation, r > 0.6. The corresponding values for p and r, for each regression analysis, are always indicated. Hipp., Hippocampus; St., stimulation; Thr., threshold.
Histology.
At the end of the behavioral studies, mice were deeply reanesthetized (sodium pentobarbital, 50 mg/kg), and perfused transcardially with saline and 4% phosphate-buffered paraformaldehyde. Selected sections (50 μm) including the dorsal hippocampus were mounted on gelatinized glass slides and stained using the Nissl technique with 0.1% cresyl violet, to determine the location of stimulating and recording electrodes (see Fig. 2B,C).
Data collection and analysis.
EMG and extracellular hippocampal activity, and 1 V rectangular pulses corresponding to CS and US presentations, were stored digitally on a computer through an analog/digital converter (CED 1401 Plus; Cambridge Electronic Design, Cambridge, England), at a sampling frequency of 11–22 kHz and an amplitude resolution of 12 bits. Data were analyzed off-line for quantification of CRs and fEPSP slope with the help of commercial (Spike 2 and SIGAVG from Cambridge Electronic Design) and home-made (Porras-García et al., 2005; Gruart et al., 2006) representation programs. Computed results were processed for statistical analysis using the SPSS (Chicago, IL) for Windows package. Unless otherwise indicated, data are represented as the mean ± SEM. Acquired data were analyzed using a two-way ANOVA test, with session, time, or interstimulus interval as repeated measure. Contrast analysis was added to study additional significant differences. Regression analysis was used to test the relationship between the fEPSP slope and the percentage of CRs. The Student's t test was used to analyze the data from Western blot and ELISA experiments.
Results
Increase in the number of hippocampal neurons in TgNTRK3
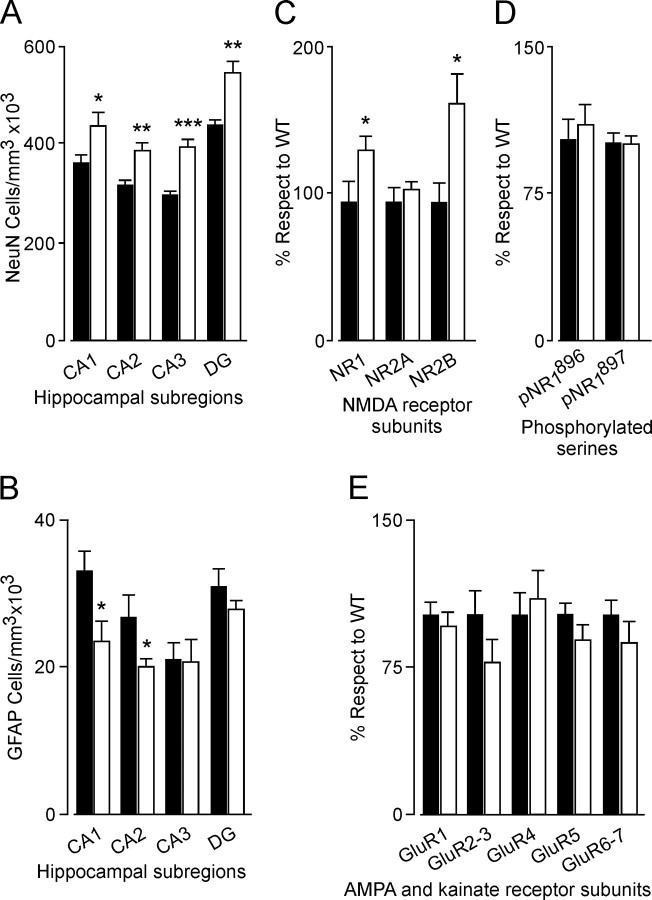
Brain sections from mice with overexpression of the TrkC receptor gene exhibited normal cytoarchitectonics when compared with those from age-matched wild-type littermates. For instance, the typical layering of the hippocampal formation was preserved in TgNTRK3 mice. The total volume of the hippocampus was not significantly different between genotypes. Nevertheless, we observed that, in sections stained with the Nissl technique (0.1% cresyl violet), cell density and numbers in TgNTRK3 mice showed a tendency to increase that reached its maximum statistical significance in the CA3 region. As shown in Figure 1A, the mean density of NeuN-positive neurons in CA1 (20.4%; p < 0.05; F(1,14) = 4.791), CA2 (21.2%; p < 0.01; F(1,12) = 11.574), CA3 (32.2%; p < 0.0001; F(1,14) = 27.77), and dentate gyrus (23.8%; p < 0.01; F(1,12) = 14.979) was significantly increased in TgNTRK3 mice.
Figure 1.
Stereological analyses and expression of NMDA and non-NMDA receptor subunits in the hippocampus of TgNTRK3 mice. A, A significant increase in NeuN-positive cell density in CA1, CA2, CA3, and dentate gyrus (DG) was observed in TgNTRK3 mice. B, The density of GFAP-positive cells was reduced in CA1 and CA2 regions of TgNTRK3 mice. C–E, Basal hippocampal expression of NMDA and AMPA receptor subunits and phosphorylated serines NR1896 and NR1897 from wild-type and TgNTRK3 mice was analyzed by Western blot. C, Graphs show that TgNTRK3 mice express higher levels of NR1 and NR2B subunits. In contrast, basal levels of NR2A (C), phosphorylated serines NR1896 and NR1897 (D), and GluR1, GluR2–3, GluR4, GluR5, and GluR6–7 subunits (E) were not modified in TgNTRK3 mice with respect to wild types. Results are expressed as a percentage of values collected from TgNTRK3 (white bars) with respect to wild-type (WT) mice (black bars). Data are expressed as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
When GFAP-positive cells were evaluated (Fig. 1B), the mean density was significantly reduced in CA1 (−28.9%; p < 0.05; F(1,12) = 6.018) and CA2 (−25.4%; p < 0.05; F(1,12) = 4.799) regions. No differences were observed in the CA3 region (p = 0.974; F(1,12) = 0.001) or in the dentate gyrus (−10.0%; p = 0.275; F(1,12) = 1.309) between genotypes.
Differential regulation of NMDA and non-NMDA receptor subunits in TgNTRK3 mice
Western blot analysis was performed to study the effect of the overexpression of TrkC in the regulation of NMDA and non-NMDA receptor subunits. Interestingly, only NR1 and NR2B were increased (TgNTRK3, 135.5 ± 7.3; wild-type, 100.0 ± 14.2 and TgNTRK3, 167.5 ± 18.4; wild-type, 100.0 ± 15.9, respectively); NR2A protein levels were not modified in TgNTRK3 mice with respect to wild-type animals (Fig. 1C). However, the phosphorylation of NR1Ser 896 or NR1Ser 897 was not affected (Fig. 1D). Changes in the protein levels of AMPA (GluR1, GluR2–3, and GluR4) and kainate (GluR5 and GluR6/7) subunits were not detected in TgNTRK3 mice (Fig. 1E).
Levels of NT-3 protein in hippocampus and cortex of TgNTRK3 mice
ELISA of the NT-3 protein was performed to study the effect of overexpression of TrkC in its regulation. Values for the cortex were TgNTRK3, 72.9 ± 1.9, and wild-type, 71.3 ± 2.9 pg/mg protein; p = 0.30887. Values for hippocampus were TgNTRK3, 84.9 ± 1.5, and wild-type, 109.4 ± 19.8 pg/mg protein; p = 0.12838.
EMG and fEPSP recordings in alert behaving mice
Electrodes implanted in the upper eyelid did not disturb its normal kinematics and allowed the generation of spontaneous and reflexively evoked blinks. As illustrated in Figure 2A, 2, the electrical stimulation (2 times threshold) of the supraorbitary branch of the trigeminal nerve evoked (in both wild-type and TgNTRK3 mice) an early EMG activation of the orbicularis oculi muscle at a latency of ≈5 ms, followed by a second EMG activation, with a latency from the stimulus of 15–20 ms. These successive muscle activations correspond to the R1 and R2 components described previously in humans (Kugelberg, 1952) and other species of mammals (Gruart et al., 2000), including mice (Domínguez-del-Toro et al., 2004; Gruart et al., 2006).
Electrical stimulation of Schaffer collaterals in behaving mice evoked a large negative wave when recorded at the stratum radiatum (i.e., on the apical dendrites of the CA1 pyramidal cells) with a latency of 3.5–4 ms (Fig. 2A, 1). The proper location of stimulating and recording electrodes in the hippocampus is illustrated in Figure 2, B and C. On occasions, waves recorded in the CA1 area presented a positive shape, corresponding to a more dorsal location of the recording electrode, i.e., near the pyramidal cell layer (Schwartzkroin, 1986). It has been shown previously (Gruart et al., 2006) that the slope of fEPSPs evoked by the stimulation of Schaffer collaterals (60 times per day) is stable (<10% variation) across the maximum period of time used here for classical conditioning (19 d), indicating that electrical stimulation per se, at this low rate, does not modify fEPSP profiles across time. A spectral analysis of extracellular hippocampal recordings collected from wild-type and TgNTRK3 mice indicates no significant differences in their power spectra (for sample records, see Fig. 3A,B) (p ≥ 0.1, χ2-distributed test) and the absence of abnormal EEG activities (i.e., large spikes).
Classical conditioning of eyelid responses
In each experimental group, 10 successful animals were classically conditioned using a trace (CS, tone; US, shock) paradigm (Fig. 3A,B). The time interval between the end of the CS and the beginning of the US was 500 ms. As illustrated in Figure 3D, the percentage of CRs increased significantly across conditioning sessions (p < 0.001; F(18,162) = 81.8) for both wild-type and TgNTRK3 mice, with a profile similar to previous descriptions in mice, using the same trace conditioning procedure (Takatsuki et al., 2003; Domínguez-del-Toro et al., 2004). However, the learning curves for the wild-type group showed larger values (≥60% of CRs by the seventh conditioning session) than those for TgNTRK3 animals (< 50% of CRs by the seventh session). In fact, TgNTRK3 mice were unable to reach the asymptotic values that wild types acquired by the 10th conditioning session. The percentage of CRs presented by the wild-type group was significantly different from that of the TgNTRK3 group from the third to the 10th conditioning session, and for the first to third and fifth extinction sessions (p < 0.01; F(18,162) = 8.68). In summary, wild types reached acquisition levels significantly greater than those reached by TgNTRK3 mice.
Evolution of CA3–CA1 fEPSP across classical conditioning
The experimental design used here included the presentation of a single electrical pulse to the Schaffer collateral/commissural pathway 300 ms after CS onset. As illustrated in Figure 3, the electrical stimulation of Schaffer collaterals during the CS–US interval evoked an fEPSP in the recording (CA1) area in both wild-type (Fig. 3A) and TgNTRK3 (Fig. 3B) groups. It should be indicated that although the stimuli presented to Schaffer collaterals disrupted the regular theta rhythm recorded in the CA1 area, the rhythm reappeared in phase shortly (100–200 ms) afterward. As illustrated in Figure 3C, fEPSPs evoked in the two experimental groups by the electrical stimulation of Schaffer collaterals increased progressively in slope (taking the slope of fEPSPs collected during the four habituation sessions as 100%) across conditioning, to ∼125% during the 5th through 10th sessions for the wild-type group (p < 0.01; F(18,162) = 220). Usually, the increase in fEPSP slopes for a given session took place after several (>20) CS–US pairs of stimuli were presented (>10 min from session start). Similar results have been reported recently for wild-type mice using the same trace conditioning and Schaffer collateral stimulation procedures (Gruart et al., 2006). In contrast, TgNTRK3 animals presented significantly larger values for fEPSP slopes than did wild types (p < 0.001; F(18,162) = 148). Thus, fEPSPs evoked in TgNTRK3 animals across conditioning were >200% higher than baseline records by the fourth conditioning session and ≈300% from the 7th to the 10th sessions.
As expected (Gruart et al., 2006), during extinction, the fEPSP slope decreased to <80% of baseline values (by the fifth session) for the wild-type group. In contrast, the slope of fEPSPs recorded from TgNTRK3 animals during extinction did not return to baseline but remained at values (≈280%) similar to those reached during the 10th conditioning session; i.e., they showed no extinction of the increased fEPSP values acquired during conditioning sessions.
As illustrated in Figure 3E and demonstrated previously in wild-type C57BL/6 mice (Gruart et al., 2006), the slope of fEPSPs evoked by Schaffer collateral stimulation at the CA3–CA1 synapse in wild types was linearly related (r ≥ 0.68; p < 0.001) to the percentage of CRs across conditioning (slope, 0.79) and extinction (slope, 0.67) sessions but not during habituation. In contrast, TgNTRK3 mice presented very low coefficients of correlation (r ≤ 0.4) between fEPSP slopes and the percentage of CRs for conditioning (r = 0.4; p < 0.001) and extinction (r = 0.12; p = 0.406) sessions (Fig. 3F). The fact that the regression lines for acquisition and extinction sessions presented similar slopes suggests that the activity-dependent plasticity at the CA3–CA1 synapse functions as a continuum for both acquisition and extinction processes, at least for wild-type mice (Gruart et al., 2006). In summary, wild-type mice presented significant relationships between fEPSP slope and the percentage of CRs during conditioning and extinction but not during habituation sessions (Fig. 3E), whereas TgNTRK3 mice did not demonstrate any significant relationship between the two parameters for habituation, conditioning, or extinction sessions (Fig. 3F).
Paired-pulse facilitation in wild-type and TgNTRK3 mice
The facilitation evoked by the presentation of a pair of pulses is a typical presynaptic short-term plastic property of some excitatory synapses, including the hippocampal CA3–CA1, and has been related to the process of neurotransmitter release (Zucker, 1989). It is to be expected that at this type of synapse, wild-type animals present a larger fEPSP response to the second (with respect to the first) stimulus at short intervals (<100 ms). In this regard, the increased density of CA3 and CA1 pyramidal neurons observed in TgNTRK3 mice (Fig. 1A) could modify the facilitation process. As illustrated in Figure 4A, wild-type mice presented a significant (p < 0.01; F(5,45) = 55.1) increase of the response to the second pulse at short (20–100 ms) time intervals. TgNTRK3 mice also presented a paired-pulse facilitation at the same intervals, but with a nonsignificant decrease in the amount of facilitation, when compared with the wild-type group (p = 0.45). Thus, the overexpression of pyramidal cells at the CA3 and CA1 layers did not significantly modify the paired-pulse facilitation characteristic of the CA3–CA1 synapse.
Figure 4.
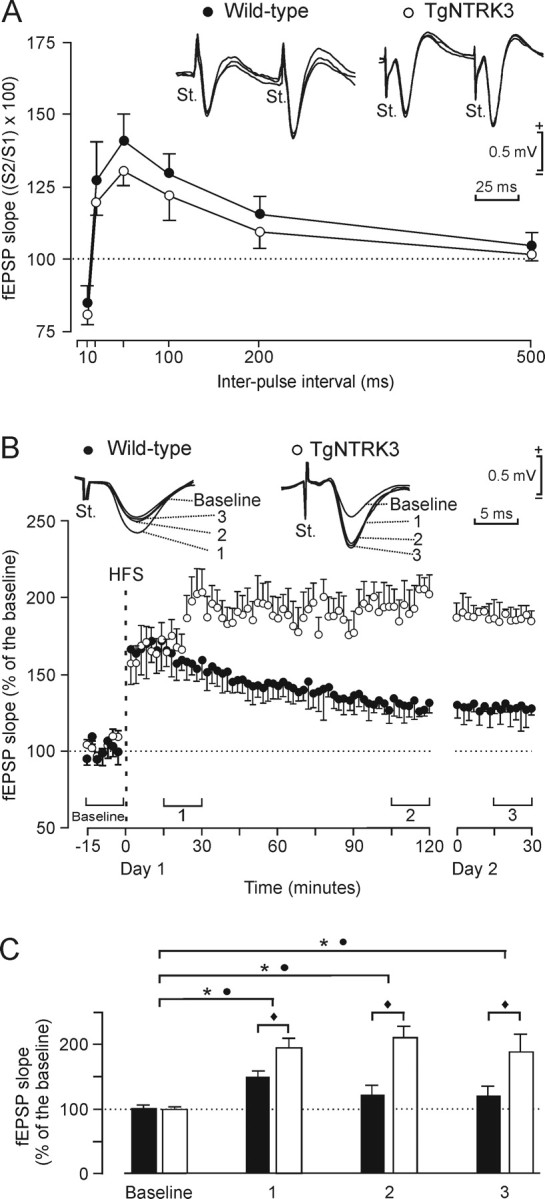
Paired-pulse facilitation and LTP induction of fEPSPs recorded in the CA1 area after stimulation of Schaffer collaterals. A, At the top are three superimposed extracellular fEPSP paired traces collected from the wild-type and TgNTRK3 groups at interpulse intervals of 50 ms. The data shown are mean ± SEM slopes of the second fEPSP expressed as a percentage from the corresponding value of the first fEPSP, for the six (10, 20, 50, 100, 200, and 500 ms) selected interstimulus intervals. Wild-type mice presented a paired-pulse facilitation similar to that obtained from TgNTRK3 animals at intervals of 20 and 50 ms. B, At the top are examples (4 superimposed traces) of fEPSPs collected from selected animals of each experimental group before and after HFS of Schaffer collaterals. The bottom graphs illustrate the time course of LTP evoked in the CA1 area (fEPSP mean ± SEM) after HFS for wild-type (black circles) and TgNTRK3 (white circles) mice. The HFS was presented after 15 min of control recordings, at the time marked by the dashed line. The fEPSP is given as a percentage of the baseline (100%) slope. Illustrated data were collected up to 2 h after HFS during the first day (Day 1) and for 30 min 24 h later (Day 2). C, The two groups presented a significant increase (ANOVA, two-tailed) in fEPSP slope after HFS when compared with baseline records (* for wild-type and ● for transgenic, p < 0.01; F(1,9) = 183). Nevertheless, values collected from the TgNTRK3 group were significantly (♦p < 0.01; F(1,9) = 142) larger than those collected from wild-type mice at the indicated times. Data included in each histogram were collected for the intervals (Baseline, 1, 2, and 3) indicated in B.
Comparison of LTP evoked in alert behaving wild-type and TgNTRK3 mice
For the LTP study, and to obtain a baseline, animals (n = 10 for each group) were stimulated every 20 s for 15 min at Schaffer collaterals. The stimulus consisted of a single 100 μs, square, biphasic pulse presented 12 times/min. Pulse intensity (50–350 μA) was set at 30–40% of the amount necessary to evoke a maximum fEPSP response (Gruart et al., 2006). For LTP induction, each animal was presented with an HFS consisting of five trains (200 Hz, 100 ms) of pulses at a rate of 1/s. This protocol was presented six times in total, at intervals of 1 min. To avoid evoking a population spike and/or unwanted EEG seizures, the stimulus intensity for HFS was set at the same amount used for generating the baseline record. After HFS, the same single stimulus used to generate baseline records was presented at the initial rate (12/min) for another 120 min (Fig. 4B). An additional set of records (30 min) were performed 24 h after HFS (Fig. 4B). With this protocol, the two experimental groups presented a significant LTP (p < 0.01; F(1,9) = 183) up to 24 h after HFS, but with some interesting differences between them. Thus, when quantified at 15–30 min after HFS (Fig. 4C, 1), the LTP response presented by TgNTRK3 mice was larger (≈195% of baseline values) than that presented by the wild-type group (≈150%; p < 0.01; F(1,9) = 142). When quantified at 105–120 min after HFS, LTP values from the TgNTRK3 (≈210% larger than baseline values) and wild-type (≈120%) groups were also (p < 0.01) different (Fig. 4C, 2). At the end of the recording time (24 h after HFS), LTP values collected from TgNTRK3 mice were still significantly (p < 0.01) larger than those from controls (Fig. 4C, 3).
Discussion
NT-3, a member of the neurotrophin family, has been suggested as a possible molecular mediator of synaptic plasticity and behavior (Poo, 2001; Segal, 2003). NT-3 and its cognate receptor TrkC are widely and similarly distributed in the brain. NT-3 is especially rich in the hippocampus, an important area for memory and plasticity (Hofer et al., 1990; Kokaia et al., 1993; Fryer et al., 1996; Roskoden et al., 1999). The present results provide evidence for a direct, causal role for the NT-3–TrkC cascade in early and late LTP maintenance, as well as in the physiological potentiation of fEPSP potentials evoked at the CA3–CA1 synapses during the acquisition of an associative, trace learning task. In this regard, several reports have demonstrated that acute application of exogenous BDNF, or NT-3, can potentiate synaptic transmission in rat hippocampal cultures and slices (Gottschalk et al., 1999; Tyler and Pozzo-Miller, 2003; Tyler et al., 2006), a fact confirmed during acute intrahippocampal infusion of BDNF (Messaoudi et al., 2002).
Interestingly, overexpression of TrkC reduces the efficiency of conditioned learning, an effect that has previously been observed after LTP “saturation” induced experimentally in behaving mice (Gruart et al., 2006). It is thus possible that TrkC overexpression enhances hippocampal synaptic activity and LTP, which then occludes learning. Alternatively, the straightforward interpretation that the measured LTP is involved in the given behavioral task may not be warranted. In fact, apparent dissociation between hippocampal LTP and gene activation, effects of amygdala stimulation on hippocampal subregions, or spatial learning has been reported recently (Richter-Levin et al., 1998; Gilbert et al., 2000; Vouimba and Richter-Levin, 2005). Although the present experiments suggest that neurotrophins can participate in synaptic plasticity, work with mice heterozygous for a null mutation in the NT-3 gene showed that the lack of endogenous NT-3 does not influence LTP (Elmer et al., 1997).
NT-3 has been implicated in the regulation of synaptic transmission and plasticity (Schuman, 1999; Lessmann et al., 2003; Lim et al., 2003; Shimazu et al., 2006). For example, LTP induction leads to increases in NT-3 mRNA levels in the CA1 region of the hippocampus (Patterson et al., 1992), and several reports have demonstrated that acute application of exogenous NT-3 can alter synaptic transmission in rat hippocampal cultures and slices (Kang and Schuman, 1995; Gottschalk et al., 1999; Tyler and Pozzo-Miller, 2003; Tyler et al., 2006) Although these experiments indirectly suggest that NT-3 can participate in synaptic plasticity, work with NT-3 knock-out mice has suggested that neuronal NT-3 does not play an essential role in normal synaptic transmission and in some forms of plasticity in the mouse hippocampus (Ma et al., 1999; Xu et al., 2002). However, our results demonstrate that overexpression of TrkC levels produces facilitation of LTP without changes in NT-3, thus ruling out an indirect effect of NT-3 on TrkB receptor.
The present results clearly implicate TrkC in the enhancement of synaptic transmission in the hippocampus and suggest the involvement of a glutamatergic mechanism. At the molecular level, neither the presynaptic nor the postsynaptic modulation of glutamatergic synapses by neurotrophins is well understood. Electrophysiological data have shown that NT-3 induces enhancement of glutamatergic synapses mediated by increasing both the efficacy of glutamate release from the presynaptic neuron and the neurotrophin-dependent postsynaptic enhancement of NMDA (but not AMPA) receptor responsiveness (Lessmann, 1998; Oestreicher et al., 2000). Our results confirm and extend these previous observations, indicating that overexpression of TrkC leads to significant changes in the level of hippocampal expression of NMDA receptor subunits, but not AMPA receptors, an effect that can be considered to serve as the “set point” for the control of synaptic plasticity. Thus, these results suggest that the modulation of the synaptic efficacy at these junctions in TgNTRK3 occurs through a postsynaptic effect. It has been proposed that neurotrophins acutely affect phosphorylation of NMDA-type glutamate receptors, which could account for the postsynaptic effects. However, no changes in phosphorylation were observed in TgNTRK3 mice. Neurotrophins can also affect intraneuronal Ca2+ levels and influence molecular components of the transmitter release machinery, which could underlie presynaptic modifications that could also account for the observed effects.
The present study documents the presence of morphological modifications in the hippocampal formation of mice with overexpression of the neurotrophin TrkC receptor. TrkC mRNA is expressed both in the pyramidal layers of the hippocampus and in the granule layer of the dentate gyrus, but apparently more prominently in the latter (Hassink et al., 1999; Huang and Reichardt, 2001), and it has been suggested that TrkC promotes the survival of specific neuronal populations in the CNS, such as catecholaminergic neurons (Dierssen et al., 2006; Sahún et al., 2007). Moreover, heterozygous TrkC mice have altered neuronal numbers in the hippocampal formation, suggesting that reduced levels of TrkC receptors reduce neuronal survival in this region (von Bohlen und Halbach et al., 2003). These developmental effects may also participate in the enhanced LTP observed in adult TgNTRK3 mice.
Together, the present results suggest that NT-3 might operate as a locally released feedback modulator of synaptic transmission through an effect on glutamatergic transmission, leading to a facilitation of LTP in conditions of overexpression of its cognate receptor TrkC. This could be a cellular correlate for certain aspects of information processing in the mammalian brain during the acquisition of new motor and/or cognitive abilities, because learning is significantly affected in TgNTRK3 mice.
Footnotes
This work supported by Spanish Ministerio de Educación y Ciencia Grants BFU2005-01024, BFU2005-02512, GEN2003-20651-Co6-03, and SAF2004-02808, as well as Generalitat de Catalunya Grant DURSI-SGR0500008 and Spanish Ministry of Health Grant CIBER-CB06/07/0089. We thank María Esteban for her technical assistance and Roger Churchill for his editorial help.
References
- Agassandian K, Gedney M, Cassell MD. Neurotrophic factors in the central nucleus of amygdala may be organized to provide substrates for associative learning. Brain Res. 2006;1076:78–86. doi: 10.1016/j.brainres.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Gardner-Medwin AR. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaesthetized rabbit following stimulation of the perforant path. J Physiol (Lond) 1973;232:357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum R, Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: mechanisms and functions. Physiology (Bethesda) 2005;20:70–78. doi: 10.1152/physiol.00042.2004. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer T. Neurotrophins and activity-dependent development of the neocortex. Curr Opin Neurobiol. 1996;6:119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci (Lond) 2006;110:167–173. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- Dierssen M, Fotaki V, Martinez de Lagran M, Gratacos M, Arbones M, Fillat C, Estivill X. Neurobehavioral development of two mouse lines commonly used in transgenic studies. Pharmacol Biochem Behav. 2002;73:19–25. doi: 10.1016/s0091-3057(02)00792-x. [DOI] [PubMed] [Google Scholar]
- Dierssen M, Gratacos M, Sahun I, Martin M, Gallego X, Amador-Arjona A, Martinez de Lagran M, Murtra P, Marti E, Pujana MA, Ferrer I, Dalfo E, Martinez-Cue C, Florez J, Torres-Peraza JF, Alberch J, Maldonado R, Fillat C, Estivill X. Transgenic mice overexpressing the full-length neurotrophin receptor TrkC exhibit increased catecholaminergic neuron density in specific brain areas and increased anxiety-like behavior and panic reaction. Neurobiol Dis. 2006;24:403–418. doi: 10.1016/j.nbd.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Domínguez-del-Toro E, Rodriguez-Moreno A, Porras-Garcia E, Sanchez-Campusano R, Blanchard V, Laville M, Bohme GA, Benavides J, Delgado-Garcia JM. An in vitro and in vivo study of early deficits in associative learning in transgenic mice that over-express a mutant form of human APP associated with Alzheimer's disease. Eur J Neurosci. 2004;20:1945–1952. doi: 10.1111/j.1460-9568.2004.03643.x. [DOI] [PubMed] [Google Scholar]
- Elmer E, Kokaia M, Ernfors P, Ferencz I, Kokaia Z, Lindvall O. Suppressed kindling epileptogenesis and perturbed BDNF and TrkB gene regulation in NT-3 mutant mice. Exp Neurol. 1997;145:93–103. doi: 10.1006/exnr.1997.6478. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Wetmore C, Olson L, Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990;5:511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- Fryer RH, Kaplan DR, Feinstein SC, Radeke MJ, Grayson DR, Kromer LF. Developmental and mature expression of full-length and truncated TrkB receptors in the rat forebrain. J Comp Neurol. 1996;374:21–40. doi: 10.1002/(SICI)1096-9861(19961007)374:1<21::AID-CNE2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Gartner A, Polnau DG, Staiger V, Sciarretta C, Minichiello L, Thoenen H, Bonhoeffer T, Korte M. Hippocampal long-term potentiation is supported by presynaptic and postsynaptic tyrosine receptor kinase B-mediated phospholipase Cgamma signaling. J Neurosci. 2006;26:3496–3504. doi: 10.1523/JNEUROSCI.3792-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert ME, Mundy WR, Crofton KM. Spatial learning and long-term potentiation in the dentate gyrus of the hippocampus in animals developmentally exposed to Aroclor 1254. Toxicol Sci. 2000;57:102–111. doi: 10.1093/toxsci/57.1.102. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Gottschalk WA, Jiang H, Tartaglia N, Feng L, Figurov A, Lu B. Signaling mechanisms mediating BDNF modulation of synaptic plasticity in the hippocampus. Learn Mem. 1999;6:243–256. [PMC free article] [PubMed] [Google Scholar]
- Gruart A, Schreurs BG, del Toro ED, Delgado-García JM. Kinetic and frequency-domain properties of reflex and conditioned eyelid responses in the rabbit. J Neurophysiol. 2000;83:836–852. doi: 10.1152/jn.2000.83.2.836. [DOI] [PubMed] [Google Scholar]
- Gruart A, Munoz MD, Delgado-García JM. Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. J Neurosci. 2006;26:1077–1087. doi: 10.1523/JNEUROSCI.2834-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gureviciene I, Ikonen S, Gurevicius K, Sarkaki A, van Groen T, Pussinen R, Ylinen A, Tanila H. Normal induction but accelerated decay of LTP in APP + PS1 transgenic mice. Neurobiol Dis. 2004;15:188–195. doi: 10.1016/j.nbd.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Hassink GC, van Esseveldt KE, Dijkhuizen PA, Verhaagen J, Boer GJ. Ontogeny of neurotrophin receptor trkC expression in the rat forebrain and anterior hypothalamus with emphasis on the suprachiasmatic nucleus. Neuroscience. 1999;92:705–712. doi: 10.1016/s0306-4522(99)00007-x. [DOI] [PubMed] [Google Scholar]
- Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kokaia Z, Bengzon J, Metsis M, Kokaia M, Persson H, Lindvall O. Coexpression of neurotrophins and their receptors in neurons of the central nervous system. Proc Natl Acad Sci USA. 1993;90:6711–6715. doi: 10.1073/pnas.90.14.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Kang H, Bonhoeffer T, Schuman E. A role for BDNF in the late-phase of hippocampal long-term potentiation. Neuropharmacology. 1998;37:553–559. doi: 10.1016/s0028-3908(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A. Postsynaptic induction of BDNF-mediated long-term potentiation. Science. 2002;295:1729–1734. doi: 10.1126/science.1067766. [DOI] [PubMed] [Google Scholar]
- Kugelberg E. Facial reflexes. Brain. 1952;75:385–396. doi: 10.1093/brain/75.3.385. [DOI] [PubMed] [Google Scholar]
- Lessmann V. Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS. Gen Pharmacol. 1998;31:667–674. doi: 10.1016/s0306-3623(98)00190-6. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Lim KC, Lim ST, Federoff HJ. Neurotrophin secretory pathways and synaptic plasticity. Neurobiol Aging. 2003;24:1135–1145. doi: 10.1016/j.neurobiolaging.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Lu B. Acute and long-term synaptic modulation by neurotrophins. Prog Brain Res. 2004;146:137–150. doi: 10.1016/s0079-6123(03)46010-x. [DOI] [PubMed] [Google Scholar]
- Ma L, Reis G, Parada LF, Schuman EM. Neuronal NT-3 is not required for synaptic transmission or long-term potentiation in area CA1 of the adult rat hippocampus. Learn Mem. 1999;6:267–275. [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Ying SW, Kanhema T, Croll SD, Bramham CR. Brain-derived neurotrophic factor triggers transcription-dependent, late phase long-term potentiation in vivo. J Neurosci. 2002;22:7453–7461. doi: 10.1523/JNEUROSCI.22-17-07453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreicher E, Knipper M, Arnold A, Zenner HP, Felix D. Neurotrophin 3 potentiates glutamatergic responses of IHC afferents in the cochlea in vivo. Eur J Neurosci. 2000;12:1584–1590. doi: 10.1046/j.1460-9568.2000.00049.x. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. London: Academic; 2001. The mouse brain in stereotaxic coordinates. [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Porras-García E, Cendelin J, Dominguez-del-Toro E, Vozeh F, Delgado-García JM. Purkinje cell loss affects differentially the execution, acquisition and prepulse inhibition of skeletal and facial motor responses in Lurcher mice. Eur J Neurosci. 2005;21:979–988. doi: 10.1111/j.1460-9568.2005.03940.x. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G, Canevari L, Bliss TV. Spatial training and high-frequency stimulation engage a common pathway to enhance glutamate release in the hippocampus. Learn Mem. 1998;4:445–450. doi: 10.1101/lm.4.6.445. [DOI] [PubMed] [Google Scholar]
- Roskoden T, Heese K, Otten U, Schwegler H. Modulation of mRNA expression of the neurotrophins of the nerve-growth-factor family and their receptors in the septum and hippocampus of rats after transient postnatal thyroxine treatment. II. Effects on p75 and trk receptor expression. Exp Brain Res. 1999;127:307–313. doi: 10.1007/s002210050800. [DOI] [PubMed] [Google Scholar]
- Sahún I, Gallego X, Gratacòs M, Murtra P, Trullás R, Maldonado R, Estivill X, Dierssen M. Differential responses to anxiogenic drugs in a mouse model of panic disorder as revealed by fos immunocytochemistry in specific areas of the fear circuitry. Amino Acids. 2007 doi: 10.1007/s00726-006-0464-1. in press. [DOI] [PubMed] [Google Scholar]
- Schuman EM. Neurotrophin regulation of synaptic transmission. Curr Opin Neurobiol. 1999;9:105–109. doi: 10.1016/s0959-4388(99)80013-0. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA. Regulation of excitability in hippocampal neurons. In: Isacson RL, Pribram KH, editors. The hippocampus. New York: Plenum; 1986. pp. 113–136. [Google Scholar]
- Segal RA. Selectivity in neurotrophin signaling: theme and variations. Annu Rev Neurosci. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- Shimazu K, Zhao M, Sakata K, Akbarian S, Bates B, Jaenisch R, Lu B. NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis. Learn Mem. 2006;13:307–315. doi: 10.1101/lm.76006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama N, Kanba S, Arita J. Temporal changes in the expression of brain-derived neurotrophic factor mRNA in the ventromedial nucleus of the hypothalamus of the developing rat brain. Brain Res Mol Brain Res. 2003;115:69–77. doi: 10.1016/s0169-328x(03)00184-0. [DOI] [PubMed] [Google Scholar]
- Takatsuki K, Kawahara S, Kotani S, Fukunaga S, Mori H, Mishina M, Kirino Y. The hippocampus plays an important role in eyeblink conditioning with a short trace interval in glutamate receptor subunit delta 2 mutant mice. J Neurosci. 2003;23:17–22. doi: 10.1523/JNEUROSCI.23-01-00017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- Tokuyama W, Hashimoto T, Li YX, Okuno H, Miyashita Y. Quantification of neurotrophin-3 mRNA in the rat hippocampal subregions using the RT-PCR-based coamplification method. Brain Res Brain Res Protoc. 1999;4:407–414. doi: 10.1016/s1385-299x(99)00046-x. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller L. Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J Physiol (Lond) 2003;553:497–509. doi: 10.1113/jphysiol.2003.052639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Zhang XL, Hartman K, Winterer J, Muller W, Stanton PK, Pozzo-Miller L. BDNF increases release probability and the size of a rapidly recycling vesicle pool within rat hippocampal excitatory synapses. J Physiol (Lond) 2006;574:787–803. doi: 10.1113/jphysiol.2006.111310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Minichiello L, Unsicker K. Haploinsufficiency in trkB and/or trkC neurotrophin receptors causes structural alterations in the aged hippocampus and amygdala. Eur J Neurosci. 2003;18:2319–2325. doi: 10.1046/j.1460-9568.2003.02953.x. [DOI] [PubMed] [Google Scholar]
- Vouimba RM, Richter-Levin G. Physiological dissociation in hippocampal subregions in response to amygdala stimulation. Cereb Cortex. 2005;15:1815–1821. doi: 10.1093/cercor/bhi058. [DOI] [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- Xu B, Michalski B, Racine RJ, Fahnestock M. Continuous infusion of neurotrophin-3 triggers sprouting, decreases the levels of TrkA and TrkC, and inhibits epileptogenesis and activity-dependent axonal growth in adult rats. Neuroscience. 2002;115:1295–1308. doi: 10.1016/s0306-4522(02)00384-6. [DOI] [PubMed] [Google Scholar]
- Yamada MK, Nakanishi K, Ohba S, Nakamura T, Ikegaya Y, Nishiyama N, Matsuki N. Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci. 2002;22:7580–7585. doi: 10.1523/JNEUROSCI.22-17-07580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]