Abstract
Central respiratory chemoreception is the mechanism by which the CNS maintains physiologically appropriate pH and PCO2 via control of breathing. A prominent hypothesis holds that neural substrates for this process are distributed widely in the respiratory network, especially because many neurons that make up this network are chemosensitive in vitro. We and others have proposed that TASK channels (TASK-1, K2P3.1 and/or TASK-3, K2P9.1) may serve as molecular sensors for central chemoreception because they are highly expressed in multiple neuronal populations in the respiratory pathway and contribute to their pH sensitivity in vitro. To test this hypothesis, we examined the chemosensitivity of two prime candidate chemoreceptor neurons in vitro and tested ventilatory responses to CO2 using TASK channel knock-out mice. The pH sensitivity of serotonergic raphe neurons was abolished in TASK channel knock-outs. In contrast, pH sensitivity of neurons in the mouse retrotrapezoid nucleus (RTN) was fully maintained in a TASK null background, and pharmacological evidence indicated that a K+ channel with properties distinct from TASK channels contributes to the pH sensitivity of rat RTN neurons. Furthermore, the ventilatory response to CO2 was completely retained in single or double TASK knock-out mice. These data rule out a strict requirement for TASK channels or raphe neurons in central respiratory chemosensation. Furthermore, they indicate that a non-TASK K+ current contributes to chemosensitivity of RTN neurons, which are profoundly pH-sensitive and capable of driving respiratory output in response to local pH changes in vivo.
Keywords: background potassium channel, KCNK, raphe, RTN, pH signaling, brain slice, plethysmography
Introduction
Breathing serves two essential functions; it provides the means to obtain O2 and eliminate CO2, and it rapidly and dynamically regulates acid-base balance. The importance of this latter function is underscored by the existence of an exquisitely sensitive homeostatic mechanism – so-called central respiratory chemoreception – by which the rate and depth of breathing are controlled by brainstem CO2/pH (Nattie, 1999; Feldman et al., 2003; Putnam et al., 2004; Nattie and Li, 2006). Despite concerted investigation, the molecular basis for respiratory chemoreception has yet to be determined, and the neuronal basis remains unresolved. A number of pH-sensitive ion channels have been suggested as candidate sensor molecules, and likewise, diverse cell types have been advanced as candidate neuronal substrates (Nattie, 1999; Putnam et al., 2004; Richerson, 2004; Guyenet et al., 2005b; Jiang et al., 2005; Nattie and Li, 2006). However, to date, it has not been possible to unambiguously demonstrate that targeted cellular or molecular ablation of any of these candidates results in deficits in the CO2/pH drive for breathing.
In previous work, we and others have proposed that the tandem pore-domain K+ channels TASK-1 and TASK-3 (K2P3.1 and K2P9.1) serve as molecular substrates for central chemoreception (Bayliss et al., 2001; Washburn et al., 2002, 2003; Feldman et al., 2003; Lesage, 2003; Talley et al., 2003; Mulkey et al., 2004; Putnam et al., 2004). These channels contribute to background K+ currents and are highly sensitive to physiological shifts in pH (Lesage, 2003; Talley et al., 2003). We showed that a number of different neuron types in the respiratory network express TASK channels that can impart intrinsic chemosensitivity to cells in which they are expressed (Sirois et al., 2000; Talley et al., 2000; Washburn et al., 2002, 2003).
In separate work, we have amassed substantial evidence from in vivo experiments that implicates a subpopulation of chemosensitive neurons within the RTN as prime cellular substrates for central respiratory chemoreception (Mulkey et al., 2004; Weston et al., 2004; Guyenet et al., 2005a,b; Stornetta et al., 2006). This area of the rostroventrolateral medulla has long been implicated in central respiratory chemosensitivity (Nattie, 1999; Feldman et al., 2003; Putnam et al., 2004; Nattie and Li, 2006). The neurons we identified are robustly chemosensitive, even in the absence of excitatory synaptic transmission, and they selectively innervate components of the central respiratory pattern generator (Mulkey et al., 2004; Rosin et al., 2006; Takakura et al., 2006). In vitro, we identified a corresponding subset of chemosensitive RTN neurons; furthermore, we showed that a pH-sensitive and K+-selective background current contributes to this chemosensitivity, suggesting that it could be carried by TASK channels (Mulkey et al., 2004; Weston et al., 2004; Guyenet et al., 2005a,b; Stornetta et al., 2006).
Here, we used knock-out mice to test the importance of TASK channels to pH sensitivity in putative chemosensors and to whole animal ventilatory responses to CO2. Our data indicate that TASK channels do not mediate the pH-sensitive background K+ current in RTN neurons. In contrast, we find that the pH sensitivity of serotonergic raphe neurons is eliminated in TASK channel knock-out mice. Because ventilatory responses to CO2 are fully retained in these mice, our results demonstrate that pH sensing by raphe neurons or TASK channels is not required for respiratory chemosensitivity.
Materials and Methods
Knock-out mice.
We targeted TASK-1 and TASK-3 gene loci for homologous recombination in mouse embryonic stem cells, flanking the second exon of each 2-exon gene with loxP sites (Fig. 1). A bacterial artificial chromosome (BAC) library of mouse genomic DNA (CITB Mouse BAC, catalog #96040H; Invitrogen, Carlsbad, CA) was probed with rat cDNA for TASK-1 (GenBank accession number AF031384) and TASK-3 (GenBank accession number AF192366). Positive clones were verified by Southern analysis; restriction fragment sizes matched fragments from mouse genomic DNA, and also matched sizes predicted from the mouse genome sequence (GenBank accession numbers: TASK-1, NC_000071; TASK-3, NC_000081). To generate targeting constructs (Fig. 1A,D), restriction fragments (TASK-1: BglII/XbaI, 9 kb; TASK-3: BamHI/HindIII, 5.9kb) from the respective BAC clones were ligated into pBluescript. In the case of TASK-1, a diphtheria toxin gene was added at the 5′ end of the targeting construct (derived from pKODT, GenBank accession number AF090452, gift from John Lye, University of Virginia) to select against random incorporation. For TASK-3, an extra 1.6 kb segment at the 5′ end was generated by PCR, fully sequenced, and ligated upstream of a unique NdeI site in the initial construct to generate a longer 5′ arm for targeting. We added a neomycin resistance cassette (excised from plasmid 4369G9, gift from Richard Palmiter, University of Washington), which contained an upstream loxP site and two yeast frt sites flanking an SV40 promoter-driven neo gene. This cassette allows for subsequent excision of the neo gene using the yeast Flp recombinase, but because the presence of the neo cassette did not affect TASK-1 or TASK-3 mRNA levels (data not shown), the neo gene was not removed. The cassette was inserted into a double NdeI site in TASK-1 (removing 118 nt) and a single BglII site in TASK-3, both upstream of exon II. The downstream loxP sites were generated using PCR fragments containing the full loxP sequence plus flanking sequence from each of the respective genes. These PCR products were used to prime Quickchange site-directed mutagenesis (Stratagene) to generate the final mutated gene. For TASK-1 the loxP sequence was placed over a unique PmeI site; for TASK-3 it was placed over a unique MluI site. Both constructs were sequenced through the full coding region, as well as at the 5′ and 3′ ends, and in the regions flanking the neo cassette and the downstream loxP sites.
Figure 1.
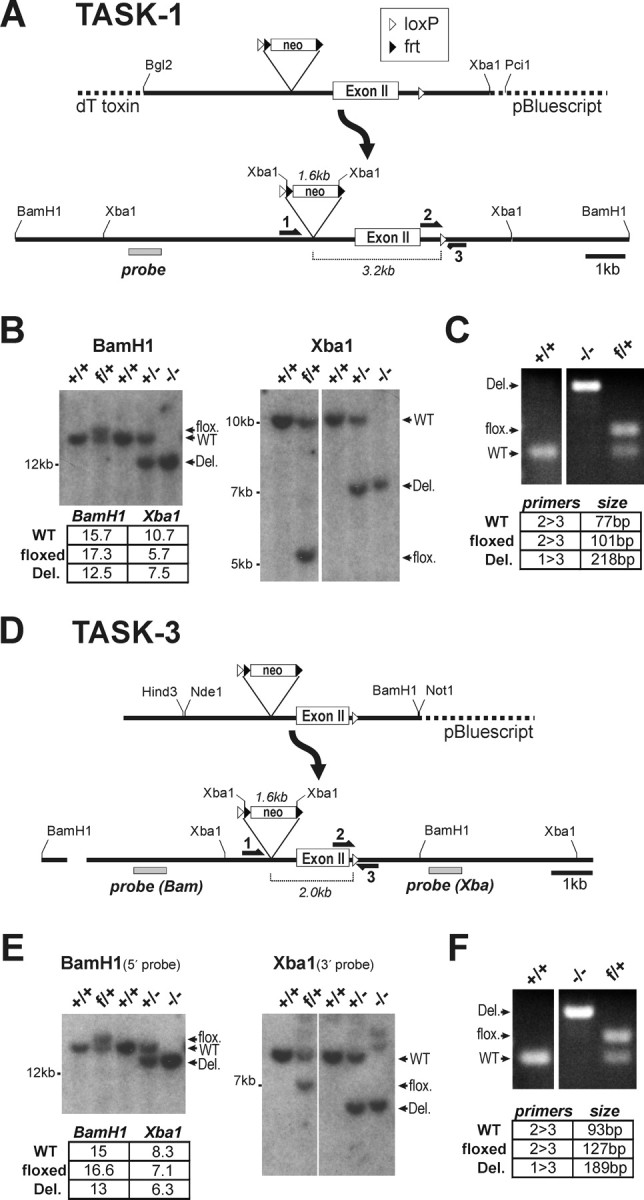
Generation of TASK-1 and TASK-3 knock-out mice. Targeting constructs for TASK-1 (A) and TASK-3 (D) were transfected into ES cells to generate “floxed” alleles containing loxP sites flanking exon II of the respective genes. Initial crosses between germ-line chimeric animals and C57BL/6 mice yielded progeny with heterozygous floxed (f/+) TASK alleles. These animals were crossed with a deleter Hs-Cre1 mouse strain to generate heterozygous (+/−) offspring, and those animals were intercrossed to produce mice homozygous for the deleted alleles (−/−). Tail DNA was assayed by Southern blot analysis (B, E) using the indicated probes and by multiplex PCR (C, F) across the downstream loxP site using the indicated primers. The probed restriction fragments and PCR products matched the predicted sizes indicated in the panels. WT, Wild type.
R1 ES cell culture was performed in the UVA Mouse Transgenic Core facility according to procedures developed for this line (Nagy and Vintersten, 2006). Linearized plasmids (TASK-1: PciI, TASK-3: NotI) were transfected by electroporation (Matise et al., 2003) and DNA samples from G418-resistant colonies were assayed by PCR using a 5′ primer external to the targeted sequence and a 3′ primer internal to the neo cassette. Positive colonies were characterized by Southern analysis using probes corresponding to the 5′ and 3′ sequence flanking the targeted regions, and corresponding to the coding regions, to ensure integrity of targeted and flanking sequences (data not shown). Blastocyst injection was performed by the UVA Mouse Transgenic Core facility. Chimeric animals were identified by coat color, and crossed to C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME). Tail DNA was assayed by PCR to select progeny bearing the “floxed” allele, which were then crossed to a deleter strain that expresses cre recombinase at the two-cell stage of development (HS-cre1, obtained from Scott Zeitlin at University of Virginia) (Dietrich et al., 2000). PCR primers were designed for a multiplex reaction across the downstream loxP site or spanning the deleted region (5′-3′, TASK-1: GAAGCCCCTGCAGGCAAC, GCTCAGGCTGGGGCTTTTG, GGTCTGACTCTGCTTGGC, TASK-3: GACCTAACTCCTCTCTTCTTCC, CAACACACCTGCACACAGAAG, GCACCCCAAAATGCTTCAGC) (Fig. 1C,F). The Cre recombinase gene was assayed in a separate PCR using primers from the Cre coding region (CTGCCACGACCAAGTGACAGC, CTTCTCTACACCTGCGGTGCT). Tail DNA was probed by Southern blotting, which resulted in hybridized fragments of the appropriate sizes for the wild-type, floxed, and knock-out alleles of the two TASK channel genes (Fig. 1B,E).
Brain slices.
The preparation of brain slices has been described previously (Mulkey et al., 2004). Briefly, neonatal rat (Sprague Dawley) or mouse pups (7–12 d postnatal) were anesthetized (ketamine/xylazine), decapitated and transverse slices (300 μm) were prepared from brainstem (RTN and medullary raphe) and midbrain (dorsal raphe) using a microslicer (DSK 1500E, Dosaka, Japan) in ice-cold sucrose (260 mm)-substituted Ringer's solution (Pineda and Aghajanian, 1997) containing kynurenate (1 mm). Slices were incubated for 30 min at 37°C and then at room temperature in normal Ringer's solution (in mm): 130 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose; substituted and normal Ringer's solutions were bubbled with 95% O2/5% CO2.
Electrophysiology.
Recordings were obtained from slices in a chamber on a fixed-stage microscope (Zeiss Axioskop FS); slices were perfused continuously (∼2 ml/min) with a bath solution composed of (mm): 140 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, 10 glucose; the pH of the bath solution was adjusted between 6.9 and 7.5 by addition of HCl or NaOH, and tetrodotoxin (TTX, 0.5 μm) was added where indicated.
The RTN was identified by its location below the caudal end of the facial motor nucleus and individual neurons were visualized using Nomarski optics. We targeted neurons with fusiform somas and long axis parallel to the ventral medullary surface, typically within 100 μm of the surface. Neurons in the dorsal and caudal medullary raphe were identified visually by their location ventral to the aqueduct or along the midline, respectively, and characterized electrophysiologically by their response to serotonin [5-hydroxytryptamine (5-HT)] (Bayliss et al., 1997). The serotonergic phenotype of raphe cells was confirmed using immunohistochemistry (see below).
Recordings were obtained at room temperature using pClamp 9.0 to drive an Axopatch 200B amplifier via a Digidata 1322A analog-to-digital converter (all from Molecular Devices, Union City, CA). Patch electrodes had a DC resistance of 3–6 MΩ when filled with internal solution containing (mm): 120 KCH3SO3, 4 NaCl, 1 MgCl2, 0.5 CaCl2, 10 HEPES, 10 EGTA, 3 Mg-ATP, 0.3 GTP-Tris, 0.2% biocytin, pH 7.2; electrode tips were coated with Sylgard 184 (Dow Corning). Firing rate histograms were generated by integrating action potential discharge in 10-s bins and plotted using Spike 5.0 software. Under voltage clamp, cells were held at −60 mV and holding current, input conductance and I–V relationships were determined using voltage steps (Δ 10 mV) between −40 and −120 mV. Series resistance was typically <20 MΩ and was compensated by 65–70%. A liquid junction potential of 10 mV was corrected off-line.
Histochemistry
In situ hybridization was performed on mouse brain sections (10–30 μm) using either [33P]-labeled or digoxigenin-labeled cRNA probes, essentially as described (Berg et al., 2007); mouse TASK-1 and TASK-3 (in pcDNA3) were linearized with SpeI and StuI, respectively, to yield templates that encompass only the second (i.e., the deleted) exon of each gene. We detected tryptophan-hydroxylase (TrpH) immunoreactivity in biocytin-filled neurons by incubating either the whole slice or re-cut horizontal sections (50 μm) with a mouse monoclonal antibody (1:1000, Sigma-Aldrich, St. Louis, MO) followed by Alexa 488 goat anti-mouse IgG3 (1:200; Invitrogen) and avidin Cy3 (1:200, Jackson ImmunoResearch, West Grove, PA), as described (Bayliss et al., 1997).
Quantitative real-time PCR.
Expression of the two TASK genes was assayed in control and TASK knock-out mice using quantitative real-time PCR (qRT-PCR). Mice were rapidly decapitated and brainstems were removed and either used immediately or frozen in liquid nitrogen and stored at −80°C. RNA extraction was performed using Trizol reagent (Molecular Research Center, Cincinnati, OH) or the RNeasy kit (Qiagen, Valencia, CA), and reverse transcription and qRT-PCR used iScript and IQ SYBR Green Supermix kits (both from Bio-Rad, Hercules, CA), according to manufacturer's instruction. Primers were specific for exon 2 of TASK-1 (5′-3′: AGGACGAGAAGCGTGATG, CAGCACCTCGGCATAGAC) and TASK-3 GGAGGGAGAAGTTGCGGAGATTC, CGTGGTGCCTCTTGCGTCTC); cyclophilin served as an internal standard (GGCTCTTGAAATGGACCCTTC, CAGCCAATGCTTGATCATATTCTT). Samples were in run in triplicate (or quadruplicate) on an ICycler (Bio-Rad, Hercules, CA) using the following conditions: 95°C, 3 min; 95°C, 10 s, 65°C, 10 s, 72°C, 25 s (40 cycles) that were optimized in preliminary experiments to yield ≥97% efficiency. The identity of PCR products was verified in initial experiments by agarose gel electrophoresis (which yielded amplicons of appropriate size) and in all experiments by melt curve analysis (which yielded a single peak at appropriate Tm). We analyzed qRT-PCR data by using a modification of the so-called ΔΔCt normalization procedure to obtain TASK subunit mRNA levels for each genotype, relative to cyclophilin (Pfaffl, 2001).
Behavioral analysis.
To assay coordination and gross motor function of the mice, we used the rotarod and wire hang test, essentially as described (Crawley, 2000). Mice were placed on a rod rotating at constant acceleration and the length of time the animal was able to stay on the rod was recorded, up to 500 s (ENV-576M; Med Associates, St. Albans, VT). To allow for acclimation to the apparatus and to assess any genotype-dependent training effects, we administered five trials for each animal (with at least 20 min rest between trials). For the wire hang test, mice were suspended from a wire mesh and the length of time they clung to the wire was recorded, up to 60 s.
A tail flick assay was performed to assess basal sensitivity to painful stimuli (Crawley, 2000). Animals were lightly restrained in a commercially available apparatus (Columbus Instruments, Columbus, OH) while a radiant heat source was directed onto the tail; the time to remove the tail from the heat source was recorded. We used low- and high-intensity stimuli to provide response times in wild-type animals of ∼8–10 s and ∼3–4 s, respectively. Cutoff times for low- and high-intensity heat stimuli were set at 20 and 10 s, respectively, to avoid tissue damage.
Whole animal plethysmography.
Ventilatory responsiveness to CO2 was assessed by whole animal plethysmography in unrestrained adult control and TASK knock-out mice. The averaged ages (range: 4–8 mos.) and weights (range: 24–31 g) of TASK knock-out mice used in these studies were not significantly different from those of control littermates (by one-way ANOVA). Animals were placed individually into Plexiglas chambers (∼1000 cm3) and allowed 30 min to acclimate. Inspiration and expiration were detected using a pressure transducer calibrated before and after each experiment by injecting 1 ml of air (Buxco Max II; Buxco Electronics). The pressure signal was amplified, digitized and recorded using IOX software (EMKA Technologies). Tidal volume (measured in ml, normalized to body weight) and respiratory frequency (breaths/min) were recorded on a breath-to-breath basis and analyzed from periods of relative quiescence; the product of tidal volume and frequency is minute ventilation (ml/min/g). The ventilatory response to graded hypercapnia (3, 5 and 10% CO2) was measured under hyperoxic conditions (balance O2). Multiple determinations of CO2 effects on ventilation were obtained from each animal (at least 2, usually 4–6 over 2–3 d); data from multiple trials were averaged and those values were treated as a single data point for subsequent statistical analysis.
Data analysis.
All data are expressed as mean ± SE. Statistical tests included paired Student's t test, one-way ANOVA and Newman–Keuls multiple-comparison test or repeated measures ANOVA, as indicated, with a significance level of p < 0.05.
Results
We developed TASK channel knock-out mice to test the hypothesis that TASK-1 and/or TASK-3 subunits contribute to central respiratory chemoreception. We examined pH sensitivity of two principal candidate populations of chemoreceptor neurons [serotonergic neurons of the raphe nuclei (Richerson, 2004) and chemosensitive neurons of the RTN (Mulkey et al., 2004; Guyenet et al., 2005b)] and we determined whole animal ventilatory responses to CO2 in TASK knock-out mice.
TASK channel knock-out mice
We generated conventional single and double TASK subunit knock-out mice by gene targeting and Cre-mediated excision of the second exon of TASK channel genes (see Materials and Methods) (Fig. 1); removal of this exon disrupts the first pore domain and removes the M2, M3 and M4 transmembrane domains, the second pore loop and the entire cytoplasmic C terminus. For these studies, mice were examined on a mixed genetic background, using littermates with intact TASK genes as controls.
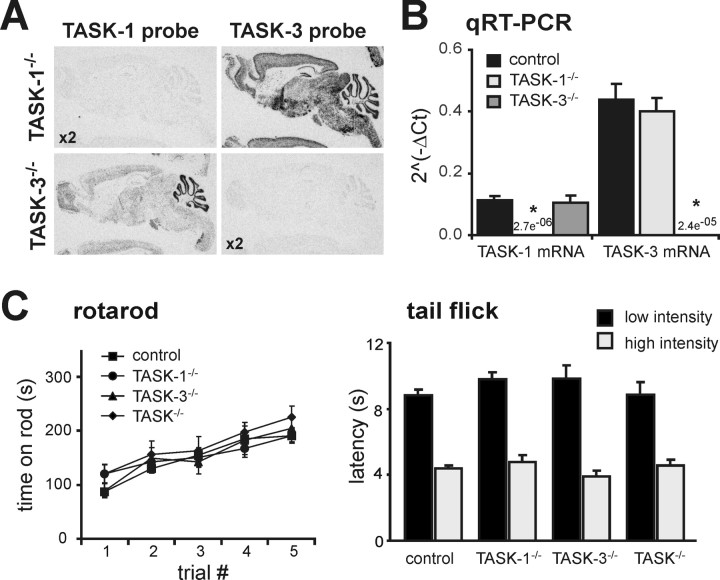
As depicted in images from in situ hybridization of mouse brain sagittal sections using exon 2-specific probes (Fig. 2A), there was no evidence for expression of the cognate transcript from TASK-1−/− or TASK-3−/− knock-out mice, even at twice the probe concentration; in addition, the brain distribution of the alternate TASK transcript in each knock-out was the same as noted previously for rat and wild type mouse (Talley et al., 2001; Bayliss et al., 2003). We also assayed TASK-1 and TASK-3 mRNA levels by qRT-PCR in brainstem samples from control and TASK knock-out mice; as evident in Figure 2B, the relevant TASK transcript was undetectable and there was no compensatory upregulation of the alternate TASK subunit mRNA in either of the TASK knock-outs. Similar to our results, another group has reported that expression of the remaining TASK subunit is not upregulated in separate lines of single TASK-1−/− and TASK-3−/− knock-out mice (Aller et al., 2005; Brickley et al., 2007); in addition, that group reported no change in expression of a broad panel of K2P channels in their TASK knock-out mouse lines (Aller et al., 2005; Brickley et al., 2007).
Figure 2.
Characterization of TASK channel knock-out mice. A, In situ hybridization using [33P]-labeled cRNA probes specific for exon 2 of either TASK-1 (left) or TASK-3 (right) in sagittal sections of brains from TASK-1−/− (top) and TASK-3−/− (bottom) mice; note the absence of hybridization for the cognate gene in each knock-out, despite using twice the probe concentration (x2). B, TASK-1 and TASK-3 mRNA levels were assayed by qRT-PCR in brainstem samples from control (n = 22) and TASK knock-out (n = 11 each) mice (*p < 0.0001 vs control by ANOVA). C, D, Control and TASK knock-out mice were examined for their ability to run on an accelerating rotating rod (C; n ≥ 8 per group) and to remove their tails from a radiant heat source, at either low or high intensity (D; n ≥ 7 per group). None of the TASK knock-out mice lines showed any obvious gross sensorimotor deficits.
Homozygous single TASK-1−/− and TASK-3−/− knock-out mice and doubly deleted TASK-1−/−:TASK-3−/− mice (hereafter called TASK−/− mice) were viable and presented with no obvious health problems. We used a rotarod and wire hang test, along with a tail flick assay, to characterize basic sensorimotor function in the knock-outs (Crawley, 2000). TASK knock-out mice were not different from littermate controls in their ability to maintain balance on a rotationally accelerating rod (Fig. 2C) and they showed no deficit in their ability to cling to a wire mesh for a test period of 60 s (mean values ≥55 s for all groups, n ≥10 per group; data not shown). In addition, as shown in Figure 2D, TASK knock-out mice removed their tails from a radiant heat source, at either low or high intensity, as quickly as did their control littermate counterparts. These observations, although not comprehensive, indicate no obvious neurological abnormalities in TASK channel knock-out mice, even those deleted for both TASK-1 and TASK-3. Although this outcome seems surprising given the widespread expression of TASK channel subunits in mouse brain, these results are in good agreement with previous reports on different lines of TASK subunit knock-out mice (Aller et al., 2005; Linden et al., 2006; Brickley et al., 2007), although that group noted a slight decrement in performance of TASK-1 knock-outs on the rotarod (Aller et al., 2005) that our data do not reproduce.
TASK channels are required for pH sensitivity of serotonergic raphe neurons
To enrich for serotonergic neurons within the sample of recorded raphe neurons, we took advantage of their characteristic response to 5-HT (i.e., decreased excitability attributable to activation of an inwardly rectifying K+ current) (Bayliss et al., 1997). In addition, recorded dorsal raphe neurons were filled with biocytin and labeled with an antibody for tryptophan hydroxylase (TrpH), the rate limiting enzyme in serotonin synthesis (Fig. 3A) (Bayliss et al., 1997). We recovered 78% of the 5-HT-responsive raphe neurons filled during recording (n = 45/58) and verified TrpH immunoreactivity (ir) in 84% of those recovered (n = 38/45). There were no differences in properties (basal firing rate or holding current/conductance) of identified serotonergic raphe neurons and those 5-HT-responsive neurons that were not recovered or for which immunostaining was equivocal, and therefore these data were combined. In single TASK-1−/− and TASK-3−/− knock-out mice (data not shown) and in doubly deleted TASK−/− mice, the relevant TASK channel transcripts were not present in raphe neurons (Fig. 3B).
Figure 3.
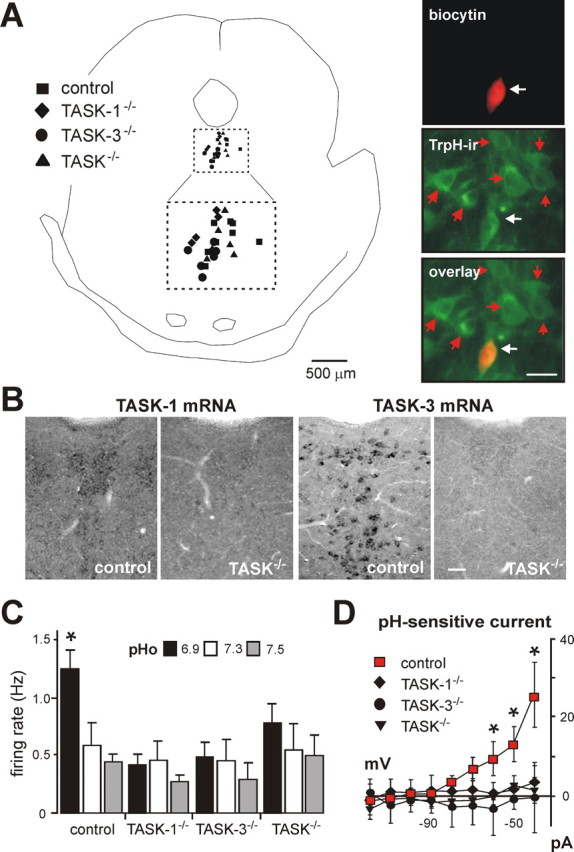
TASK channels confer pH sensitivity to serotonergic raphe neurons. A, Left, Composite map shows location of dorsal raphe neurons from each genotype recovered after recording: ■, control; ♦, TASK-1−/−; •, TASK-3−/−; ▴, TASK−/− (double knock-outs). The area encompassed by dashed lines is an enlargement of the corresponding square defining the dorsal raphe region. Right, pH-sensitive raphe neuron filled with biocytin and immunostained with an antibody for tryptophan hydroxylase (TrpH-ir); the overlay image confirms the serotonergic phenotype of the recorded raphe neuron (white arrow), which was located in a cluster of other serotonergic neurons (red arrows). B, Non-isotopic in situ hybridization for TASK-1 and TASK-3 in dorsal raphe of control and TASK−/− double knock-out mice. C, Averaged firing rate at the indicated extracellular pH of raphe neurons from control (n = 11), TASK-1−/− (n = 7), TASK-3−/− (n = 6), and TASK−/− (n = 7) mice; deletion of TASK subunits, singly or in combination, eliminated pH sensitivity. D, Averaged I–V relationships reveal a prominent weakly rectifying pH-sensitive K+ current in raphe neurons from control mice (■; n = 14); this current was absent in raphe neurons from TASK-1−/− (♦; n = 7), TASK-3−/− (•; n = 5), or TASK−/− (▴; n = 4) mice (*p < 0.05, two-way RM-ANOVA). Scale bars, 50 μm.
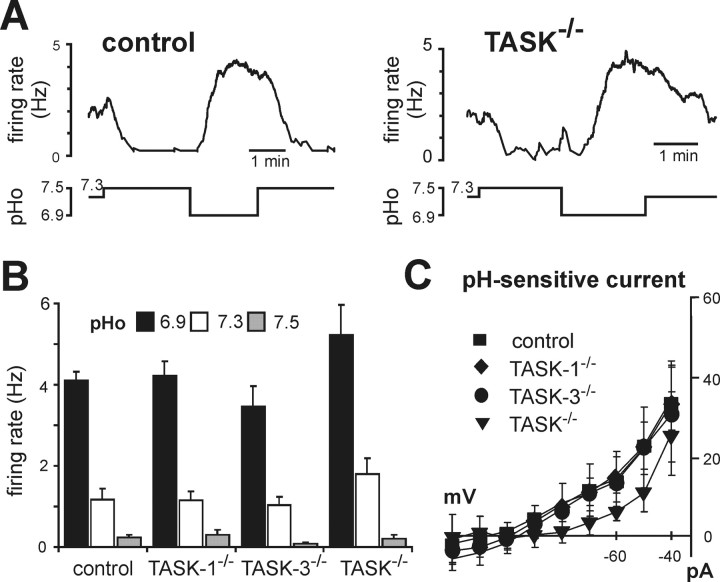
The pH sensitivity in dorsal raphe neurons from control mice was similar to that observed previously in serotonergic raphe neurons cultured from rat brainstem (Richerson, 2004), with firing rate decreasing from 1.2 ± 0.3 Hz to 0.5 ± 0.2 Hz over a pH range from pH 6.9 to pH 7.5 (n = 11) (Fig. 3C). In contrast, pH-dependent changes in discharge were eliminated in raphe neurons from single and double TASK knock-out mice (Fig. 3C). Warming the preparation to 34°C did not reveal a latent pH sensitivity in raphe neurons from TASK knock-out animals (n = 15, data not shown), although it increased basal activity of control raphe neurons by ∼1 Hz (at pH 7.3) and enhanced the dynamic range of pH sensitivity from 0.8 ± 0.1 Hz to 1.6 ± 0.1 Hz (at pH 7.5 and pH 6.9, Q10 = 2).
To establish that the pH sensitivity in mouse raphe neurons depends on a TASK-like conductance, we used voltage clamp recordings to test for anesthetic- and pH-sensitive leak K+ currents in neurons from wild-type and knock-out animals. The pH-sensitive current was derived by subtraction of I–V curves obtained during extracellular acidification (pH 6.9) from those during bath alkalization (pH 7.5); as shown in Figure 3D, the pH-sensitive current in raphe neurons from control mice rectified weakly and reversed near the expected EK (approximately −94 mV). In control raphe neurons, halothane increased outward current and conductance (by 12.3 ± 1.9 pA and 0.4 ± 0.1 nS, n = 10). Halothane also enhanced the effect of pH on holding current and conductance; in a subset of cells tested before and during exposure to halothane (n = 10), we found the pH-sensitive current and conductance were 6.9 ± 0.9 pA and 0.13 ± 0.01 nS under control conditions and 9.7 ± 1.3 pA and 0.32 ± 0.1 nS in halothane (p < 0.05, paired t test). These data from control mice are consistent with our previous report of anesthetic- and pH-sensitive TASK-like currents in rat serotonergic dorsal raphe neurons (Washburn et al., 2002). Accordingly, there was no measurable effect of changes in bath pH on membrane current in serotonergic raphe neurons from single TASK-1−/− or TASK-3−/− knock-outs, or from TASK−/− double knock-out animals. This is evident in the averaged I–V relationships of pH-sensitive currents obtained in cells from TASK subunit knock-out mice (Fig. 3D). Moreover, halothane was without effect on raphe neurons from TASK knock-out mice and effects of pH on holding current and conductance were not enhanced in the presence of halothane (data not shown). Thus, these data indicate that the mild pH sensitivity of serotonergic dorsal raphe neurons is attributable primarily to TASK channels.
We also examined effects of changing bath pH on firing rate and membrane currents in caudal raphe neurons from control and TASK−/− doubly deleted mice, specifically focusing on cells of raphe obscurus and raphe pallidus. As in the dorsal raphe, we found that firing activity in 5-HT-responsive caudal raphe neurons increased by 0.5 ± 0.1 Hz (n = 5) in response to bath acidification from pH 7.5–6.9 whereas TASK−/− mice were completely unresponsive to these changes in pH (Δ Hz: 0.06 ± 0.3 Hz, n = 5). Likewise, changing bath pH from 7.5–6.9 had no effect on holding current or conductance at −60 mV in caudal raphe neurons from TASK−/− mice (IpH was <1 pA and GpH was <0.02 nS, n = 5).
TASK channels are not the molecular substrate of RTN chemosensitivity
To examine chemosensitivity and pH-sensitive conductances in RTN neurons of TASK channel knock-outs, we identified this population of neurons in neonatal mice and compared them with their rat counterparts; these data are provided in supplemental data (available at www.jneurosci.org as supplemental material). In short, by using anatomical techniques, loose patch recording and single-cell RT-PCR, we found a population of glutamatergic, Phox2b-expressing RTN neurons in neonatal rat and mouse that correspond to the respiratory-related RTN chemosensitive neurons identified in adult rat in vivo (Mulkey et al., 2004; Weston et al., 2004; Stornetta et al., 2006).
As depicted in Figure 4A, we readily obtained pH-sensitive neurons in the RTN of TASK channel knock-out mice; the proportion of pH-sensitive neurons was similar across genotypes (28–30% of neurons tested were pH sensitive, n = 121). In addition, we found no difference in effects of pH on firing activity in chemosensitive RTN neurons from control mice or from any of the TASK knock-out lines (Fig. 4B). Moreover, under voltage clamp, we uncovered a pH-sensitive background K+ current in chemosensitive RTN neurons that was not different in cells from control mice and TASK knock-outs (Fig. 4C). These data from knock-out animals indicate that TASK channels do not underlie the pH-sensitive K+ current in RTN neurons of the mouse.
Figure 4.
RTN neurons from TASK channel knock-out mice retain pH sensitivity and express a pH-sensitive K+ current like their wild-type counterparts. A, Effects on firing rate of changing bath pH in representative RTN neurons from control (left) and TASK−/− (right) mice. B, Averaged firing rate at the indicated extracellular pH of RTN neurons from control (n = 8), TASK-1−/− (n = 8), TASK-3−/− (n = 9), and TASK−/− (n = 5) mice; there was no difference in pH sensitivity of RTN neurons from control and TASK knock-out mice. C, Averaged I–V relationships of pH-sensitive currents expressed by RTN neurons in control and TASK knock-out mice (n = 7, 7, 9, and 5; p = 0.3, by two-way RM-ANOVA).
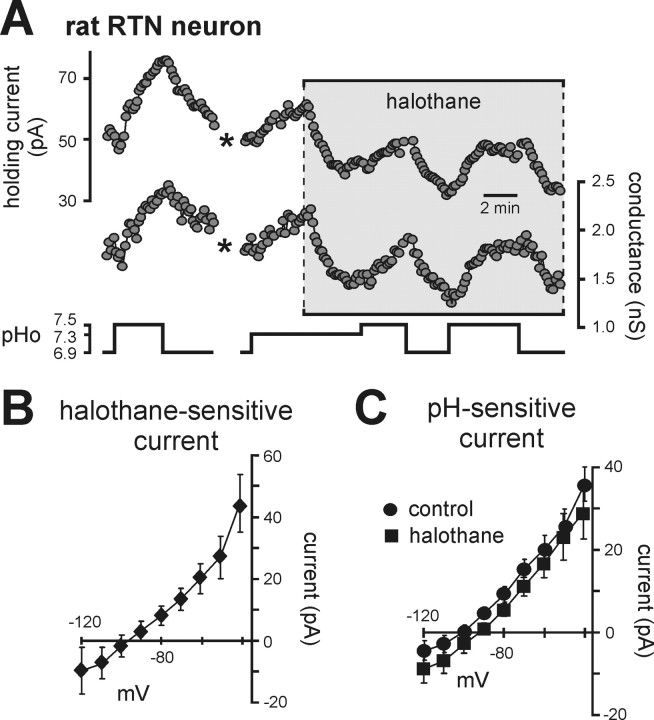
We also tested for TASK channel contributions to pH sensitivity in rat RTN neurons by taking advantage of the known modulation of TASK channels by inhalational anesthetics (Patel and Honore, 2001; Talley and Bayliss, 2002). In contrast to the activation by halothane expected for TASK-like channels, we found that halothane actually decreased outward current and conductance in RTN neurons (Fig. 5A); averaged I–V of the halothane-sensitive current suggest that it was mediated by inhibition of a background K+ current (Fig. 5B). In addition, the effects of changing bath pH on holding current and conductance were unaffected by halothane, and averaged I–V relationships of pH-sensitive currents were essentially identical under control conditions and in the presence of halothane (Fig. 5C). Thus rat RTN chemosensitive neurons express both a halothane-inhibited background K+ current and a proton-inhibited background K+ current – but those currents are distinct from each other. Importantly, because neither current was TASK-like, and the chemosensitivity of RTN neurons was retained in TASK knock-out mice, the data indicate that neither TASK-1 nor TASK-3 contribute to the pH sensitivity of RTN chemoreceptors.
Figure 5.
The pH-sensitive current expressed by rat RTN chemoreceptors is not sensitive to halothane. A, Traces of holding current and conductance in a rat RTN neuron (Vh of −60 mV) during changes in bath pH under control conditions and during exposure to halothane (3%). Note that halothane decreased current and conductance, an effect opposite to that expected for TASK channels, and that effects of pH were similar in magnitude under control conditions and in the presence of halothane. (*, trace blanked and corrected for artifact in recording). B, The I–V relationship of the halothane-sensitive current shows a weakly rectifying profile with a reversal near EK, suggesting inhibition of a background K+ current. C, I–V relationships of pH-sensitive currents under control conditions and in halothane were not different, indicating that TASK channels do not contribute to the pH-sensitive current in rat RTN neurons.
Central respiratory chemosensitivity is retained in TASK knock-out mice
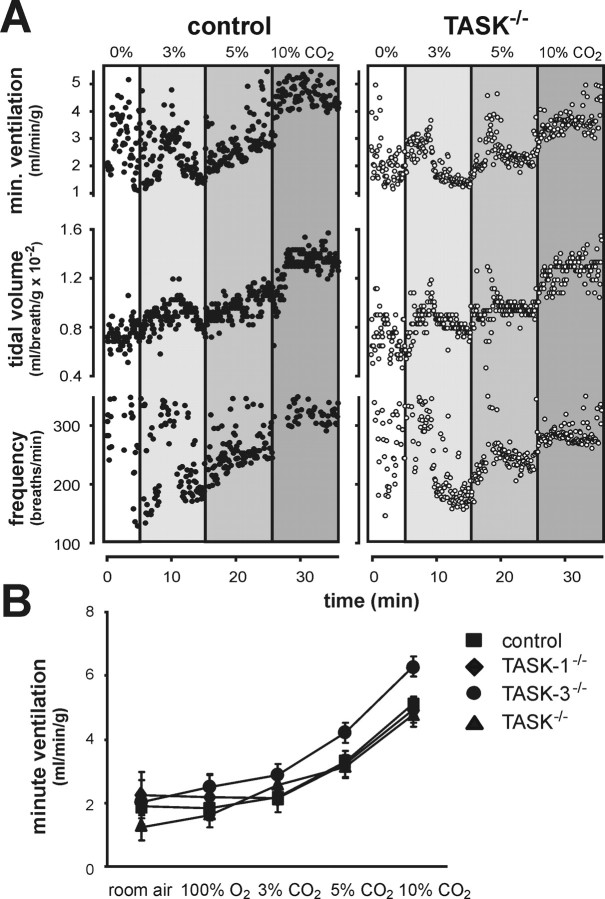
The availability of TASK channel knock-out mice in which pH sensitivity is retained in RTN neurons but essentially eliminated from serotonergic raphe neurons provides a unique opportunity to examine the role of TASK channels and the relative contributions of these two groups of candidate respiratory chemoreceptor neurons to central respiratory chemosensitivity. Using whole animal plethysmography, we measured the hypercapnic ventilatory response of TASK knock-out animals; we exposed animals to elevated inspired CO2 in a hyperoxic gas mixture to minimize inputs from peripheral chemoreceptors and focus on central chemoreceptor drive (Fig. 6). Regardless of genotype, all animals responded to hypercapnia with an increase in minute ventilation (i.e., the product of respiratory frequency and tidal volume). The increase in ventilation observed with elevated inspired CO2 was similar to that measured in studies of other wild type mouse strains (Tankersley et al., 1994), and there was no significant difference in CO2 sensitivity between control mice and any of the TASK knock-out animals. These results indicate that TASK channels are not essential for normal central respiratory chemosensitivity.
Figure 6.
TASK channels are not the molecular substrate of central chemosensitivity. Whole animal ventilatory response to CO2 was measured in control animals and TASK knock-out mice by plethysmography. A, Exemplar records of respiratory frequency, tidal volume (normalized to body weight), and minute ventilation (product of tidal volume and respiratory frequency) from control and TASK−/− mice during exposure to incrementing inspired CO2. B, Averaged values of minute ventilation with each of the indicated inspired gas concentrations; there were no significant differences in CO2 sensitivity between control and TASK knock-out animals (p = 0.21, by two-way RM-ANOVA, n ≥ 5 per group).
Discussion
We used TASK subunit knock-out mice to test whether TASK-1 and TASK-3, two KCNK family background K+ channels with intrinsic pH sensitivity in the physiological range, contribute to chemosensitivity of putative central respiratory chemoreceptor neurons and to the ventilatory response to hyperoxic hypercapnia. In brainstem slices from control mice, we found a halothane- and pH-sensitive K+ current in serotonergic raphe neurons that was absent in cells from TASK-1−/−, TASK-3−/− or TASK−/− double knock-out mice, and we showed that ablating TASK channel genes disrupted pH-dependent firing in those neurons. In contrast, the effects of pH on cell firing and on pH-sensitive background K+ currents were fully preserved in chemosensitive RTN neurons from knock-out mice in which the genes for TASK-1, TASK-3 or both were deleted. Despite the absence of pH sensitivity in raphe neurons, the ventilatory response to CO2 was intact in TASK knock-out mice. Therefore, our experiments effectively dissociate pH sensitivity of raphe neurons from central respiratory chemosensitivity. Furthermore, they indicate that the extensive expression of pH-sensitive TASK channels within various neuronal elements throughout the respiratory control system is not required for normal ventilatory responses to hypercapnia.
pH sensitivity of serotonergic raphe neurons is mediated by TASK channels but is not required for central respiratory chemosensitivity
Serotonergic neurons in the brainstem have been championed as central respiratory chemoreceptors (Richerson, 2004), primarily based on the findings that local acidification of medullary raphe stimulates breathing during sleep (Feldman et al., 2003; Richerson, 2004) and that midbrain and medullary serotonergic neurons in slices and/or culture are stimulated by acidification (Washburn et al., 2002; Richerson, 2004). Our experiments using knock-out mice indicate that pH sensitivity of serotonergic raphe neurons derives predominantly from TASK channels; pH-dependent changes in firing rate were eliminated in either dorsal or caudal raphe neurons from TASK knock-out mice. We found that the pH-sensitive K+ current in raphe cells was abolished in mice with deletions in either TASK subunit alone, as well as in the TASK−/− double knock-outs, suggesting that native TASK channels are likely heterodimeric. The reasons for this preferred conformation are not clear because homodimeric TASK channels are obtained in heterologous expression systems (Patel and Honore, 2001; Lesage, 2003; Talley et al., 2003).
Although we found that TASK channels account for the pH sensitivity of two different groups of serotonergic neurons (i.e., in dorsal and caudal raphe) that have been proposed as central chemoreceptor neurons (Richerson, 2004), the possibility still remains that some unidentified subpopulation of raphe neurons relies on a different, non-TASK channel for its pH sensitivity. However, our results are consistent with other data indicating that adult serotonergic neurons are insensitive (Mulkey et al., 2004) or, at best, weakly sensitive to hypercapnic challenge in vivo (Veasey et al., 1995), despite reliable observation of pH sensitivity in neonatal serotonergic neurons in vitro (Washburn et al., 2002; Richerson, 2004). In any case, whereas our data from TASK knock-out mice indicate that pH sensing by two major groups of dorsal and caudal raphe neurons is not essential for respiratory responses to hyperoxic hypercapnia, they still leave open the likely possibility that serotonergic neurons contribute to integrated ventilatory responses in ways that are not dependent on cell-intrinsic pH sensitivity (e.g., by modulating excitability in neurons of the respiratory control system, including chemosensory cells).
TASK channels do not underlie the pH-sensitive background K+ current in RTN neurons
Functionally identified RTN chemoreceptors express a pH-sensitive background K+ current that is activated by alkalization and inhibited by acidification and that contributes to intrinsic pH sensitivity of RTN neurons (Mulkey et al., 2004). Although TASK-1 and/or TASK-3 are renowned for generating pH-sensitive neuronal background K+ currents (Lesage, 2003; Talley et al., 2003), our studies indicate that those channels do not underlie the native pH-sensitive current in RTN neurons. We found that halothane, a volatile anesthetic that activates TASK channels (Patel and Honore, 2001; Talley and Bayliss, 2002), had no effect on the pH-sensitive K+ current in rat RTN neurons. Moreover, we were able to identify a corresponding group of pH-sensitive RTN neurons in mice in which manipulations of bath pH evoked changes in firing rate and a background K+ current that were not different in control and TASK knock-out mice. Thus, the pH sensitivity of RTN neurons appears to be mediated by a distinct non-TASK pH-sensitive background K+ current.
A number of alternative pH-sensitive K+ channels have been suggested as candidates for mediating respiratory chemoreception (for review, see Putnam et al., 2004). For example, several pH-sensitive Kir channels are expressed in brainstem neurons. However, there is no evidence for inward rectification in the I–V of the pH-sensitive K+ current in RTN neurons. Large conductance KCa channels are also pH-sensitive and widely expressed (Putnam et al., 2004) but we found that charybdotoxin (100 nm, bath) had no effect on pH-dependent modulation of firing activity in RTN cells (D. K. Mulkey and D. A. Bayliss, unpublished observations). Finally, several members of the Kv channel superfamily are pH-sensitive (Putnam et al., 2004), and it is possible that a constitutive “window” current mediated by Kv subunits could contribute to the background pH-sensitive K+ current in RTN neurons. In this respect, those Kv channels would have to be relatively insensitive to TEA, because we found only a small decrement in pH sensitivity of RTN neurons when the perfusate included up to 40 mm TEA (Mulkey and Bayliss, unpublished observations).
RTN chemoreceptors
A major impediment to detailed cellular and molecular analysis of central respiratory chemosensitivity has been uncertainty regarding the identity of brainstem chemosensitive neurons that mediate this function. Historically, the region of the RTN in the rostroventrolateral medulla has been implicated in central chemoreception (Nattie, 1999; Feldman et al., 2003; Putnam et al., 2004; Nattie and Li, 2006), and our recent in vivo experiments have uncovered a population of pH-sensitive RTN neurons that represent excellent candidate neuronal substrates; those cells are glutamatergic (Mulkey et al., 2004; Weston et al., 2004; Guyenet et al., 2005a,b; Stornetta et al., 2006), and they express Phox2b (Stornetta et al., 2006), a transcription factor mutated in patients with congenital central hypoventilation syndrome in which chemical drive for breathing is selectively blunted (Amiel et al., 2003; Weese-Mayer et al., 2005a,b). In the present work, we used single cell RT-PCR to demonstrate that pH-sensitive RTN neurons recorded in vitro represent the cellular correlate of the glutamatergic and Phox2b-expressing chemoreceptors characterized in vivo (Mulkey et al., 2004; Weston et al., 2004; Guyenet et al., 2005a,b; Stornetta et al., 2006). Importantly, this identification allows direct experimental tests of the ionic basis for pH sensitivity in RTN chemoreceptor neurons in reduced preparations, and our characterization of phenotypic markers for these cells in mice will allow additional genetic exploration of the cellular and molecular bases for chemical control of breathing.
TASK channel contributions to respiration
Expression of TASK channel transcripts is widespread throughout the CNS (Karschin et al., 2001; Talley et al., 2001; Vega-Saenz et al., 2001), and it is now clear that TASK-like background K+ currents contribute to regulation of excitability in numerous cell types (Millar et al., 2000; Sirois et al., 2000; Han et al., 2003; Meuth et al., 2003, 2006; Berg et al., 2004; Aller et al., 2005; Taverna et al., 2005; Burdakov et al., 2006; Torborg et al., 2006; Berg and Bayliss, 2007; Brickley et al., 2007), including many central respiratory-related neurons (Talley et al., 2000; Washburn et al., 2002, 2003). In addition, it has been proposed that TASK channels may be involved in regulation of respiration by peripheral chemoreceptors (e.g., via hypoxic inhibition of TASK-like channels in carotid body glomus cells) (Buckler, 2007). In light of this, it is perhaps surprising that TASK knock-out mice, including the double TASK−/− mice reported here for the first time, present such an unremarkable respiratory and neurological phenotype (Aller et al., 2005; Linden et al., 2006; Meuth et al., 2006; Brickley et al., 2007). Indeed, we also tested peripheral chemosensory responses of these mice and found a significant hyperventilation in TASK−/− double knock-out mice exposed to 10% O2 (n = 5, p < 0.0005 by two-way RM-ANOVA) that was not different from that in control mice (n = 6, p = 0.64). It is important to point out that we have not investigated any cellular or molecular mechanisms associated with this response, and therefore we do not know, for example, whether TASK−/− knock-out mice express a distinct (non-TASK) hypoxia-sensitive current in carotid body glomus cells that might account for their maintained peripheral chemoreception.
It is also noteworthy that although serotonergic raphe neurons in TASK knock-out mice did not express TASK currents and were unresponsive to changes in pH, they nevertheless displayed relatively normal baseline firing properties, consistent with a homeostatic regulation of cell excitability; the relevant compensatory mechanism(s) remain to be identified. In this respect, it is unlikely that upregulation of other background K2P channels account generally for the seemingly normal phenotype in TASK knock-out mice because there is no obvious increase in expression or altered distribution of other K2P subunits (Aller et al., 2005; Brickley et al., 2007) (E. M. Talley and D. A. Bayliss, unpublished microarray data). However, analysis of these animals has not yet been exhaustive, and it seems likely that specific roles for these well modulated TASK channels will be discovered as these mice are presented with additional physiological challenges.
Footnotes
This work was supported by National Institutes of Health Grants F32 HL80890 (D.K.M.), HL74011 (P.G.G.), and NS33583 (D.A.B.), by the Summer Research Internship Program at the University of Virginia (A.R.S.), and by a Parker B. Francis Fellowship from the Francis Families Foundation (E.M.T.). We thank the following for reagents and/or conceptual support: Drs. Timothy Bender, Scott Zeitlin, Sonia Pearson-White, and Richard Palmiter. We are grateful to Taylor Herbert, Rowena Crittenden, and Maggie Ober for technical support in making targeting constructs, performing ES cell culture, and blastocyst injections; and to Jules Manger, Shaofang Shu, Jamie Keller-Robinson, Katherine Hopper, and Steve Rekant for help with basic characterization of the knock-outs.
References
- Aller MI, Veale EL, Linden AM, Sandu C, Schwaninger M, Evans LJ, Korpi ER, Mathie A, Wisden W, Brickley SG. Modifying the subunit composition of TASK channels alters the modulation of a leak conductance in cerebellar granule neurons. J Neurosci. 2005;25:11455–11467. doi: 10.1523/JNEUROSCI.3153-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiel J, Laudier B, Attie-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. doi: 10.1038/ng1130. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Li YW, Talley EM. Effects of serotonin on caudal raphe neurons: activation of an inwardly rectifying potassium conductance. J Neurophysiol. 1997;77:1349–1361. doi: 10.1152/jn.1997.77.3.1349. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Talley EM, Sirois JE, Lei Q. TASK-1 is a highly modulated pH-sensitive “leak” K+ channel expressed in brainstem respiratory neurons. Respir Physiol. 2001;129:159–174. doi: 10.1016/s0034-5687(01)00288-2. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Sirois JE, Talley EM. The TASK family: two-pore domain background K+ channels. Mol Interv. 2003;3:205–219. doi: 10.1124/mi.3.4.205. [DOI] [PubMed] [Google Scholar]
- Berg AP, Bayliss DA. Striatal cholinergic interneurons express a receptor-insensitive homomeric TASK-3-like background K+ current. J Neurophysiol. 2007;97:1546–1552. doi: 10.1152/jn.01090.2006. [DOI] [PubMed] [Google Scholar]
- Berg AP, Talley EM, Manger JP, Bayliss DA. Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J Neurosci. 2004;24:6693–6702. doi: 10.1523/JNEUROSCI.1408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AP, Sen N, Bayliss DA. TrpC3/C7 and Slo2.1 are molecular targets for metabotropic glutamate receptor signaling in rat striatal cholinergic interneurons. J Neurosci. 2007;27:8845–8856. doi: 10.1523/JNEUROSCI.0551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Aller MI, Sandu C, Veale EL, Alder FG, Sambi H, Mathie A, Wisden W. TASK-3 two-pore domain potassium channels enable sustained high-frequency firing in cerebellar granule neurons. J Neurosci. 2007;27:9329–9340. doi: 10.1523/JNEUROSCI.1427-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ. TASK-like potassium channels and oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2007;157:55–64. doi: 10.1016/j.resp.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O'Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Crawley JN. What's wrong with my mouse. New York: Wiley; 2000. [Google Scholar]
- Dietrich P, Dragatsis I, Xuan S, Zeitlin S, Efstratiadis A. Conditional mutagenesis in mice with heat shock promoter-driven cre transgenes. Mamm Genome. 2000;11:196–205. doi: 10.1007/s003350010037. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci. 2005a;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol. 2005b;90:247–253. doi: 10.1113/expphysiol.2004.029637. [DOI] [PubMed] [Google Scholar]
- Han J, Gnatenco C, Sladek CD, Kim D. Background and tandem-pore potassium channels in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol (Lond) 2003;546:625–639. doi: 10.1113/jphysiol.2002.032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Rojas A, Wang R, Wang X. CO2 central chemosensitivity: why are there so many sensing molecules? Respir Physiol Neurobiol. 2005;145:115–126. doi: 10.1016/j.resp.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Karschin C, Wischmeyer E, Preisig-Muller R, Rajan S, Derst C, Grzeschik KH, Daut J, Karschin A. Expression pattern in brain of TASK-1, TASK-3, and a tandem pore domain K+ channel subunit, TASK-5, associated with the central auditory nervous system. Mol Cell Neurosci. 2001;18:632–648. doi: 10.1006/mcne.2001.1045. [DOI] [PubMed] [Google Scholar]
- Lesage F. Pharmacology of neuronal background potassium channels. Neuropharmacology. 2003;44:1–7. doi: 10.1016/s0028-3908(02)00339-8. [DOI] [PubMed] [Google Scholar]
- Linden AM, Aller MI, Leppa E, Vekovischeva O, Aitta-Aho T, Veale EL, Mathie A, Rosenberg P, Wisden W, Korpi ER. The in vivo contributions of TASK-1-containing channels to the actions of inhalation anesthetics, the α2 adrenergic sedative dexmedetomidine, and cannabinoid agonists. J Pharmacol Exp Ther. 2006;317:615–626. doi: 10.1124/jpet.105.098525. [DOI] [PubMed] [Google Scholar]
- Matise MP, Auerbach W, Joyner AL. Production of targeted embryonic stem cell clones. In: Joyner AL, editor. Gene targeting: a practical approach. New York: Oxford UP; 2003. pp. 101–132. [Google Scholar]
- Meuth SG, Budde T, Kanyshkova T, Broicher T, Munsch T, Pape HC. Contribution of TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 channels to the control of activity modes in thalamocortical neurons. J Neurosci. 2003;23:6460–6469. doi: 10.1523/JNEUROSCI.23-16-06460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuth SG, Aller MI, Munsch T, Schuhmacher T, Seidenbecher T, Meuth P, Kleinschnitz C, Pape HC, Wiendl H, Wisden W, Budde T. The contribution of TWIK-related acid-sensitive K+-containing channels to the function of dorsal lateral geniculate thalamocortical relay neurons. Mol Pharmacol. 2006;69:1468–1476. doi: 10.1124/mol.105.020594. [DOI] [PubMed] [Google Scholar]
- Millar JA, Barratt L, Southan AP, Page KM, Fyffe RE, Robertson B, Mathie A. A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc Natl Acad Sci USA. 2000;97:3614–3618. doi: 10.1073/pnas.050012597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nagy A, Vintersten K. Murine embryonic stem cells. Methods Enzymol. 2006;418:3–21. doi: 10.1016/S0076-6879(06)18001-5. [DOI] [PubMed] [Google Scholar]
- Nattie E. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol. 1999;59:299–331. doi: 10.1016/s0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception 2005: a brief review. Auton Neurosci. 2006:126–127. 332–338. doi: 10.1016/j.autneu.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001;24:339–346. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience. 1997;77:723–743. doi: 10.1016/s0306-4522(96)00485-x. [DOI] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Sirois JE, Lei Q, Talley EM, Lynch C, III, Bayliss DA. The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. J Neurosci. 2000;20:6347–6354. doi: 10.1523/JNEUROSCI.20-17-06347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol (Lond) 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Bayliss DA. Modulation of TASK-1 (Kcnk3) and TASK-3 (Kcnk9) potassium channels: volatile anesthetics and neurotransmitters share a molecular site of action. J Biol Chem. 2002;277:17733–17742. doi: 10.1074/jbc.M200502200. [DOI] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Sirois JE, Lei Q, Bayliss DA. Two-pore-domain (KCNK) potassium channels: dynamic roles in neuronal function. Neuroscientist. 2003;9:46–56. doi: 10.1177/1073858402239590. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Fitzgerald RS, Kleeberger SR. Differential control of ventilation among inbred strains of mice. Am J Physiol Regul Integr Comp Physiol. 1994;36:R1371–R1377. doi: 10.1152/ajpregu.1994.267.5.R1371. [DOI] [PubMed] [Google Scholar]
- Taverna S, Tkatch T, Metz AE, Martina M. Differential expression of TASK channels between horizontal interneurons and pyramidal cells of rat hippocampus. J Neurosci. 2005;25:9162–9170. doi: 10.1523/JNEUROSCI.2454-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torborg CL, Berg AP, Jeffries BW, Bayliss DA, McBain CJ. TASK-like conductances are present within hippocampal CA1 stratum oriens interneuron subpopulations. J Neurosci. 2006;26:7362–7367. doi: 10.1523/JNEUROSCI.1257-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Saenz de Miera E, Lau DH, Zhadina M, Pountney D, Coetzee WA, Rudy B. KT3.2 and KT3.3, two novel human two-pore K+ channels closely related to TASK-1. J Neurophysiol. 2001;86:130–142. doi: 10.1152/jn.2001.86.1.130. [DOI] [PubMed] [Google Scholar]
- Washburn CP, Sirois JE, Talley EM, Guyenet PG, Bayliss DA. Serotonergic raphe neurons express TASK channel transcripts and a TASK-like pH- and halothane-sensitive K+ conductance. J Neurosci. 2002;22:1256–1265. doi: 10.1523/JNEUROSCI.22-04-01256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn CP, Bayliss DA, Guyenet PG. Cardiorespiratory neurons of the rat ventrolateral medulla contain TASK-1 and TASK-3 channel mRNA. Respir Physiol Neurobiol. 2003;138:19–35. doi: 10.1016/s1569-9048(03)00185-x. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Marazita ML. In pursuit (and discovery) of a genetic basis for congenital central hypoventilation syndrome. Respir Physiol Neurobiol. 2005a;149:73–82. doi: 10.1016/j.resp.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Zhou L. Adult identified with congenital central hypoventilation syndrome–mutation in PHOX2b gene and late-onset CHS. Am J Respir Crit Care Med. 2005b;171:88. doi: 10.1164/ajrccm.171.1.950. [DOI] [PubMed] [Google Scholar]
- Weston MC, Stornetta RL, Guyenet PG. Glutamatergic neuronal projections from the marginal layer of the rostral ventral medulla to the respiratory centers in rats. J Comp Neurol. 2004;473:73–85. doi: 10.1002/cne.20076. [DOI] [PubMed] [Google Scholar]