Abstract
Cochlear ganglion neurons communicate sound information from cochlear hair cells to auditory brainstem neurons through precisely wired circuits. Understanding auditory circuit assembly is a significant challenge because of the small size of the otic vesicle and difficulties labeling and imaging embryonic neurons. We used genetic fate mapping in the mouse to visualize the morphologies of individual cochlear ganglion neurons throughout development, from their origin in the Neurogenin1-positive neurogenic domain in the otic vesicle to the formation of connections with targets in the cochlea and in the cochlear nucleus. We found that auditory neurons with different patterns of connectivity arise from discrete populations of Neurogenin1-positive precursors that make stereotyped wiring decisions depending on when and where they are born. Auditory precursors are segregated from vestibular precursors early in neurogenesis. Within this population, cochlear ganglion neurons with type I and type II morphologies are apparent before birth and develop within common pools of precursors. The peripheral projections are initially complex and branched and then become simple and straight after reaching the edge of the sensory epithelium. Subsequently, a small number of projections attain obvious type II morphologies, beginning at embryonic day 16.5 (E16.5), when hair cells begin to differentiate. Centrally, cochlear ganglion axons are topographically organized in the auditory brainstem as early as E15.5, when the cochlear nucleus is still immature. These findings suggest that Neurogenin1 precursors possess intrinsic programs of differentiation that direct early auditory circuit assembly events before the maturation of presynaptic and postsynaptic target cells.
Keywords: cochlear ganglion, spiral ganglion, type I, type II, tonotopy, circuit assembly
Introduction
Cochlear ganglion neurons mediate the perception of sound by transmitting information from cochlear hair cells, out the auditory nerve, and into the cochlear nucleus (Rubel and Fritzsch, 2002). Vestibular circuits develop and function in parallel in the inner ear to detect balance (Maklad and Fritzsch, 2003). Auditory circuit assembly begins with the segregation of cochlear and vestibular ganglion precursors (Fekete and Wu, 2002). Subsequently, auditory neurons establish specific connections that are organized by the type and optimal sound frequency of the hair cells that they contact. Type I cochlear ganglion neurons innervate inner hair cells, the primary receptor cells in the cochlea, whereas type II neurons innervate outer hair cells, which serve an amplifying function (Berglund and Ryugo, 1987). Hair cells and neurons are arranged according to frequency along the apical-basal axis of the cochlea. This organization is preserved in the cochlear nucleus, with low-frequency information communicated to ventral regions and high-frequency information to dorsal regions (Rubel et al., 2004).
Cochlear and vestibular ganglion neurons are born together in a common domain of the otic vesicle that expresses the transcription factor Neurogenin1 (Ngn1), which directs cochlear-vestibular ganglion (CVG) formation in the mouse (Ma et al., 1998). Subsequently, the CVG is split into a medial cochlear division and a lateral vestibular division (Lawoko-Kerali et al., 2004a). Cochlear ganglion neurons extend peripheral and central processes shortly after becoming postmitotic, but it is not known how cues from the presynaptic and postsynaptic cells influence the final wiring diagram. Peripheral processes are thought to reach hair cells neonatally, and a period of refinement has been inferred from the morphologies of Golgi-stained neurons at this stage (Perkins and Morest, 1975; Echteler, 1992; Bruce et al., 1997). Although central processes are topographically organized by birth, how and when this map arises remains unclear (Rubel et al., 2004).
Efforts to understand auditory circuit assembly have been hampered by the small size of the inner ear, its complex morphology, and the paucity of markers for following specific populations of neurons and their connections. Traditional labeling methods have been useful but suffer from a number of drawbacks. Injection of axonal tracers (Ginzberg and Morest, 1983; Berglund and Ryugo, 1987; Brown et al., 1988) requires laborious surgeries and cannot be used to study early stages of development. Moreover, methods such as dye labeling (Bruce et al., 1997) do not permit reproducible targeting of specific populations of neurons, leading to large variability in the number and types of neurons labeled in each experiment. Hence, few descriptions of cochlear ganglion development have been confirmed by multiple methods, raising the concern that transient or rare aspects of development have been missed.
We generated a Ngn1-CreER T2 transgenic mouse line and used it to genetically label Ngn1-positive precursors in the inner ear. By tracking the specification and wiring of cochlear ganglion neurons throughout development, we found that peripheral and central guidance decisions occur in a stereotyped manner in the embryo, suggesting that early auditory circuit assembly events are controlled intrinsically.
Materials and Methods
Production of the Ngn1-CreERT2 BAC transgenic mouse.
A universal ET recombination template to make CreER T2 transgenics (pED006) was created by combining the CreER T2 coding sequence from the pCreER T2 plasmid (Feil et al., 1997), the polyadenylation signal (pA) of the human GCSF gene (NM_000759), and the flippase recognition target (FRT)-flanked dual-promotered Neo cassette from PGK-neo-FRT (Genebridges, Dresden, Germany). The Ngn1-containing BAC, pBACe3.6:RP23–457E22, was obtained from Children's Hospital Oakland Research Institute (Oakland, CA). pED006 was used as a template for PCR with the following primers: forward, GTACGGACAGTAAGTGCGCTTCGAAGGCCGACCTCCAAACCTCCTGTCCGTCTGTCGGTCCTGCACACTGCAAGATGTCCAATTTACTGACCGTACACC, and reverse, CTTCAGCCAGTTCCCCATCTATTATTGCTCTTAGACTGGGGGAGGAAGAAAGTATTGATGTTGCCTTACAAAGGCCTAGGGGTAACCGAAGTTCCTATAC. This product was then used to replace the single Ngn1 exon with CreER T2 by ET recombination as described previously (Lee et al., 2001). After ET recombination, the FRT-Neo was excised by the arabinose-inducible Flpe of the EL250 cell. Ultimately, the BAC had its entire Ngn1 coding sequence replaced by CreERT2-pA such that the final nucleotide of the Ngn1 promoter is followed by the ATG of CreER T2 and the final nucleotide of the Cre construct is followed by the first nucleotide of the Ngn1 3′ UTR. The fidelity of the Ngn1-CreER T2 BAC was confirmed by bidirectional sequencing.
A 115 kb NotI digestion fragment containing the Ngn1-CreER T2 construct was separated by FIGE, excised, and electroluted as described previously (Strong et al., 1997). The BAC fragment was concentrated and buffer exchanged in Centricon-100 columns (Millipore, Billerica, MA). The BAC fragment was diluted to 1 ng/ul in injection buffer (10 mm Tris-HCl, pH 7.5, 0.1 mm EDTA, 30 mm spermine, 70 mm spermidine, 100 mm NaCl) (http://www.med.umich.edu/tamc/BACDNA.html) and injected into the pronuclei of C57BL/6 blastocysts. Founders were identified by PCR.
Animals.
All mice were maintained on the CD1 background. For most experiments, mice carrying the Ngn1-CreER T2 transgene and one of the reporter transgenes were crossed to wild-type CD1 females (Charles River Laboratory, Wilmington, MA). Mice were PCR genotyped using the following primers: 7024 (upstream to Ngn1), AGCCCATTCACTCCCTGAG, and 7007 (in Cre), ATCAACGTTTTCTTTTCGGA. This primer pair amplifies a 527 bp product that spans the junction between the Ngn1 locus and the CreER T2 coding sequence. Z/AP and Z/EG mice (The Jackson Laboratory, Bar Harbor, ME) were genotyped by staining for β-galactosidase or placental alkaline phosphatase (PLAP) activity. R26R embryos were PCR genotyped as described previously (Zambrowicz et al., 1997). Plugs were assumed to occur at midnight, so that the day of plug was called E0.5. Animals were maintained as approved by the Institutional Animal Care and Use Committee at Harvard Medical School.
Tamoxifen regime.
Tamoxifen (T5648; Sigma-Aldrich, St. Louis, MO) was diluted to 10 mg/ml in sunflower oil. All doses were adjusted for maternal bodyweight, so that a 1 mg dose of tamoxifen means that 1 mg per 40 g of bodyweight was provided. For most experiments, pregnant dams received 1 mg/40 g bodyweight by gavage. β-Estrodiol (E8875; Sigma-Aldrich) at 1/1000th concentration to the tamoxifen was included in most gavage experiments to help alleviate the anti-estrogen effects of the tamoxifen treatments.
Whole-mount in situ hybridizations.
Whole-mount in situ hybridizations were performed as described previously (http://genetics.med.harvard.edu/∼cepko/protocol/ctlab/ish.ct.htm). DIG-labeled probes were made for Cre (plasmid from Susan Dymecki) and Ngn1 (Gray et al., 2004).
Histochemical staining.
For all staining methods, tissues were fixed in 4% paraformaldehyde in PBS. To detect β-galactosidase activity, whole embryos were fixed for 2 h, washed in PBS, and incubated overnight at 37°C in X-gal staining solution (1 mg/ml X-gal, 0.1 m phosphate buffer (pH 7.3), 2 mm MgCl2, 0.02% NP-40, 0.1% sodium deoxycholate, 5 mm potassium ferrocyanide [K4Fe(CN)6], and 5 mm potassium ferricyanide [K3Fe(CN)6]). The following day, stained specimens were washed, fixed for at least 2 h, and cleared in 80% glycerol. For cryosectioning, embryos were fixed as above, sunk in a gradient of sucrose (10, 20, 30%), and frozen in Neg -50 (PerkinElmer, Waltham, MA). Sections (14 μm) were collected and dried for 1 h at room temperature. After a PBS wash, the slides were incubated in X-gal staining solution overnight. The following day, the slides were washed with PBS, postfixed, and counterstained with Nuclear Fast Red (Vector Laboratories, Burlingame, CA) for at least 10 min, followed by methanol dehydration and xylenes. Slides were mounted with Cytoseal (Richard-Allan Scientific, Kalamazoo, MI).
For detection of alkaline phosphatase activity, tissues were fixed overnight at 4°C. Endogenous alkaline phosphatase activity was heat inactivated by incubation of the whole head or embryo at 72°C for 1 h and 15 min. Tissues were incubated for up to 2 d in a dilute AP staining solution (0.33 mg/ml nitroblue–tetrazolium–chloride, 0.033 mg/ml 5-bromo-4-chlor-indolyl-phosphate, 100 mm Tris-HCl, pH 9.5, 100 mm NaCl, 50 mm MgCl2), then methanol dehydrated to reduce background, rehydrated to PBS, postfixed, and cleared in 80% glycerol. For sectioning, brains were embedded in 7.5% agarose, and 75–100 μm coronal sections were collected using a vibrating microtome. Floating sections were incubated in AP staining solution for up to 4 d at room temperature, washed with PBS, postfixed, and dried onto slides. The tissue was then destained in 100% methanol overnight, rehydrated, and coverslipped.
Quantification of type I/type II neurons.
To classify cells in a postnatal day 0 (P0) cochlea, only small clusters with distinct borders were analyzed. These criteria allowed us to be more confident that we were studying descendents from a single Ngn1 precursor, rather than descendents from multiple Ngn1-precursors that might have expanded to create two overlapping clusters. Hence, clusters were defined as containing 12 or fewer cells contained within a single quadrant of the cochlea that spanned <90°. In addition, the distance between the two farthest spaced cells in a cluster was required to be less than the distance to the next labeled cell. Clusters were only analyzed if both the cell body and peripheral ending of every neuron were clearly labeled, ensuring that only spiral ganglion neurons were evaluated. To classify a cell as a type II neuron, its process needed to extend beyond the level of the inner hair cells and turn toward the base. At E16.5, only cells in the base and middle of the cochlea were counted, because they had developed enough to demonstrate these features. Tamoxifen was given on embryonic day 9.5 (E9.5) for these experiments.
Quantification of tontopic projections.
Sections (75 μm) through the cochlear nucleus were cut in the transverse plane on a vibratome and stained for PLAP activity. At least three sections were examined per animal, with the absolute number varying because of differences in section angle. Photographs of each section were taken, and the anlage of the cochlear nucleus was outlined. The borders were defined by DIC imaging, which revealed obvious cell packing differences, and guided by distinct anatomical landmarks (the entry of the eighth nerve, the rhombic lip, the fourth ventricle, and the trigeminal tract). This region was then divided into four equal quadrants. The labeling in the dorsomedial and ventrolateral quadrants was rated as present or absent in each section along the rostrocaudal axis.
Immunolabeling and confocal imaging.
Ngn1-CreERT2; Z/EG pups were killed by CO2, and the skin and skull cap were dissected away before fixation in 4% paraformaldehyde in PBS for 4 h at room temperature. Floating sections (100 μm) were collected as described above. Sections were blocked for 1 h in 4% normal goat serum with 0.25% Triton X-100 in PBS and incubated overnight at 4°C in 1:1000 rabbit anti-green fluorescent protein (GFP) serum in block (A-6455; Invitrogen, Carlsbad, CA). Slices were washed with PBS with 0.25% Triton X-100 and incubated for 2 h at room temperature with 1:2000 Alexa 488-conjugated donkey anti-rabbit (A-21206; Invitrogen). After thorough washing, the slices were mounted onto slides and coverslipped with Vectashield mounting medium (Vector Laboratories). Images were taken with a Zeiss (Oberkochen, Germany) LCM500 confocal microscope or with a Nikon (Tokyo, Japan) E800 compound microscope.
Results
Cre is present and active in Ngn1-positive cells of the Ngn1-CreERT2 transgenic mouse
To trace the lineage of cochlear ganglion neurons with different patterns of connectivity, we took advantage of the fact that Ngn1 is produced by all inner ear neural precursors (Ma et al., 1998, 2000; Raft et al., 2004). We generated a Ngn1-CreERT2 BAC transgenic mouse line that transcribes the gene for tamoxifen-inducible Cre recombinase (CreERT2) in Ngn1-positive cells (Fig. 1 a,b). Cre activity was monitored in crosses to the Rosa26 Reporter (R26R) mouse line (Zambrowicz et al., 1997; Soriano, 1999). In these mice, CreERT2 is active only in the presence of the synthetic ligand tamoxifen (Fig. 1 c). Induction of Cre activity at E9.5 led to β-galactosidase activity in Ngn1-positive cells, including the dorsal root ganglia and dorsal spinal cord neurons. Because of the high levels of β-galactosidase, there was also staining of projections from labeled neurons (Fig. 1 c), but there was no evidence of widespread Cre activity outside of the normal Ngn1-expression domain. Persistent activation of Cre from E8.5 to E12.5 induced β-galactosidase activity in all predicted regions of the nervous system, with prominent labeling in the cortex, hippocampus, thalamus, and hypothalamus (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Visualization of neuronal morphologies revealed that Ngn1 descendents acquire diverse fates (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). The pattern of Cre expression in transgenic embryos combined with the absence of unexpected sites of Cre activity suggest that the Ngn1-CreER T2 line faithfully reports the behavior of Ngn1-positive cells in the nervous system.
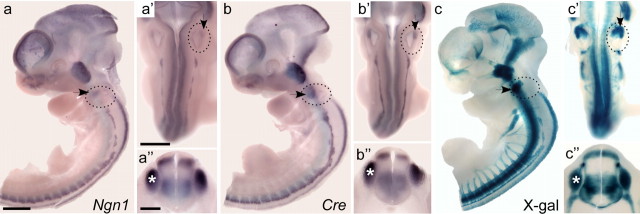
Figure 1.
Cre is expressed in the Ngn1 expression domain in Ngn1-CreER T2 transgenic mice. a, b, In Ngn1-CreER T2 transgenic mouse embryos, Cre is expressed in the same pattern as Ngn1, with expression in the anteroventral otic vesicle (arrows), the spinal cord, and the dorsal root ganglia (drg) (asterisks; a″, b”). Lateral (a, b), dorsal (a′, b′), and transverse (a″, b”) views of E10.5 Ngn1-CreER T2 embryos hybridized with probes to Ngn1 (a) or Cre (b). c, After tamoxifen treatment, Cre-mediated recombination occurs in sites of Ngn1 expression, including the cochlear-vestibular ganglion (arrows), spinal cord, and drg (asterisk; c″). Lateral (c), dorsal (c′), and transverse (c”) views of a Ngn1-CreERT2; R26R embryo that was given a single dose of tamoxifen on E9.5 and collected and stained for β-galactosidase activity at E10.5. Blue staining indicates cells that have undergone Cre-mediated recombination. Because of the high amount of precipitated X-gal product, projections in the trigeminal, glossopharyngeal, and vagus cranial nerves, dorsal root, ventral root, and spinal cord are also labeled. Scale bars: a, a′, 500 μm; a″, 100 μm.
Like Ngn1, CreERT2 protein is produced in the neurogenic domain of the otic vesicle and in delaminating neural precursors (Fig. 2 a). No activity was detected in the absence of tamoxifen (Fig. 2 b). However, daily tamoxifen treatments labeled Ngn1 descendents in the vestibular and cochlear ganglia, the utricle, and the saccule, with occasional cells found in nonsensory regions of the cochlea and lateral crista (Fig. 2 c). No cells in the auditory sensory epithelium were labeled. These findings are consistent with reports that the organ of Corti, the lateral crista, and the anterior crista arise at the border of Tbx1 and Ngn1 (Raft et al., 2004), whereas the utricle, saccule, and cochlear-vestibular ganglion develop from within the Ngn1 expression domain (Satoh and Fekete, 2005). In addition, the presence of both Ngn1-derived hair cells and Ngn1-derived neurons in the utricle and saccule is consistent with the reciprocal regulation of sensory fates by Ngn1 and Math1 in the vestibular maculae (Raft et al., 2007), although our studies did not reveal a similar relationship in the organ of Corti.
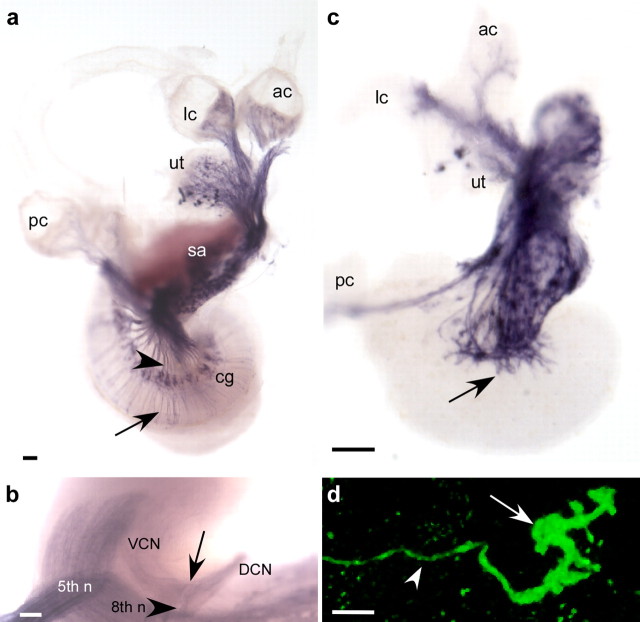
Figure 2.
Ngn1 descendents populate the utricle, saccule, and cochlear-vestibular ganglion. a, In the Ngn1-CreER T2 ear, Cre protein is produced in the neurogenic region of the otic vesicle (boundaries indicated by arrows) as well as in delaminating neuroblasts (asterisks). Transverse section through an E10.5 Ngn1-CreER T2 head stained with antibodies to Cre (red) and NeuroD (green). The dorsal boundary of the otic vesicle is indicated by the dashed line. b, c, When no tamoxifen was administered (b), few labeled cells were present in the embryo. In contrast, robust tamoxifen-induced recombination in the utricle (ut), saccule (sa), vestibular ganglion (vg), and cochlear ganglion (cg) indicates that these cell populations are derived from Ngn1-positive precursors (c). However, structures hypothesized to originate along the border of the Ngn1-positive domain of the otocyst, including the lateral crista (lc) and cochlea (asterisk), have only scattered cells labeled (arrowheads). Sections through the inner ears of E14.5 Ngn1-CreERT2; R26R embryos were stained with X-gal and counterstained with Nuclear Fast Red. Sections were cut transverse to the ear, as shown in the inset. Embryos received either no tamoxifen (b) or daily 1 mg doses from E8.5 to E12.5 (c), with tamoxifen (tam) treatments indicated by green arrowheads and the day they were killed (sac) by red arrowheads. nt, Neural tube; hb, hindbrain. Scale bars: a, 50 μm; b, 100 μm.
Early segregation of auditory and vestibular precursors
Having confirmed that Cre activity can be used to track the development of neurons in the cochlear-vestibular ganglion, we investigated the origin of cochlear ganglion neurons, which develop together with vestibular ganglion neurons in a common neurogenic domain. Neurogenesis persists from E8 to E12 in the otic vesicle. Previous birth-dating studies revealed that vestibular ganglion neurons are born before cochlear ganglion neurons, but these could not resolve whether both cell types descend from a common progenitor (Ruben, 1967). One possibility is that the neurogenic domain is subdivided into vestibular and auditory regions. Alternatively, this domain may contain progenitors that first produce vestibular ganglion neurons and then gradually acquire the ability to produce cochlear ganglion neurons, either because of intrinsic changes or because of altered conditions in the environment encountered after delamination. If vestibular and cochlear ganglion precursors are generated from a common Ngn1-positive population, then activation of Cre at the earliest stages should result in labeling in both ganglia. In contrast, we found that tamoxifen treatment on E8.5 caused strong labeling in the vestibular ganglion and little labeling in the cochlear ganglion (Fig. 3 a). Later Ngn1 descendents, which were labeled by E12.5 tamoxifen treatments, became cochlear ganglion neurons, with very few becoming vestibular ganglion neurons (Fig. 3 c). Treatments at intermediate time points labeled both populations, and E13.5 treatments induced little to no labeling in either ganglion (Fig. 3 b) (data not shown). Thus, early Ngn1-positive cells contribute almost exclusively to the vestibular ganglion, whereas the late Ngn1-expression domain is dedicated to the production of cochlear ganglion neurons.
Figure 3.
Vestibular and cochlear ganglion neurons are generated during two overlapping waves of neurogenesis. a–c, Early Cre activation (a) labeled many vestibular ganglion neurons (vg), but few cochlear ganglion neurons (cg), indicating that auditory neurons derive from an independent population of Ngn1-positive precursors. Both ganglia were labeled strongly when tamoxifen (tam) was provided on E10.5 (b), whereas tamoxifen treatment on E12.5 labeled predominantly the cochlear ganglion and very few cells in the vestibular ganglion (c). Transverse sections through the inner ear of E14.5 Ngn1-CreERT2; R26R embryos stained for β-galactosidase activity and counterstained with Nuclear Fast Red. The time of tamoxifen treatment (tam) is indicated by green arrowheads and the day they were killed (sac) by red arrowheads. Cochlear ducts are indicated by asterisks. Scale bar, 100 μm.
Type I and type II neurons may arise from common Ngn1-positive progenitors
Within the auditory lineage, cochlear ganglion neurons are further subdivided into type I and type II neurons. Type I cochlear ganglion neurons send radial, unbranched peripheral processes that innervate one or two inner hair cells. Type II neurons have thinner peripheral processes that make a stereotyped turn toward the base of the cochlea and form synapses with multiple outer hair cells. To visualize cochlear ganglion neuron morphology, Ngn1-CreERT2 was crossed to indicator strains that express either enhanced GFP (Novak et al., 2000) or the axonal marker PLAP (Lobe et al., 1999) after exposure to Cre recombinase. This allowed us to follow labeled neurons from their connections with hair cells in the cochlea, out the eighth nerve, and into the auditory brainstem (Fig. 4 a,b). Unlike other methods, we were able to visualize neurons at any stage of development, as early as E12.5, before the expression of the earliest hair cell markers in the embryonic organ of Corti (Fig. 4 c) (Woods et al., 2004). Individual endbulbs of Held could be identified in older animals (Fig. 4 d). Using this approach, we imaged peripheral projections from E12.5 to P0 and asked when type I and type II morphologies are acquired.
Figure 4.
Visualization of developing and adult cochlear ganglion neurons using Ngn1-CreER T2. a, Cochlear ganglion neurons (cg) are labeled along their entire length, including peripheral (arrow) and central (arrowhead) processes. Vestibular projections are also labeled and can be seen innervating the anterior (ac), lateral (lc), and posterior (pc) cristae, as well as the utricle (ut) and saccule (sa). PLAP-stained inner ear dissected from a Ngn1-CreERT2; Z/AP animal that received 1 mg of tamoxifen on E10.5 and was collected on P0. b, The eighth nerve (arrowhead) can be followed as it enters the auditory brainstem and bifurcates to form ascending branch to the ventral cochlear nucleus (VCN) and descending branch toward the dorsal cochlear nucleus (DCN). Staining was also prominent in the trigeminal nerve (5th n). Lateral view of an E14.5 PLAP stained hindbrain dissected from a Ngn1-CreERT2; Z/AP animal that received tamoxifen daily from E9.5 to E12.5. c, The beginning of auditory circuit assembly can be visualized in E12.5 ears. At this stage, cochlear ganglion (cg) neurons have begun to extend peripheral processes toward the developing organ of Corti (arrow). d, Within the cochlear nucleus, individual cochlear ganglion axons (arrowhead) were traced down to the level of the endbulb of Held (arrow). A confocal image of a single GFP-positive axon from a P14 Ngn1-CreERT2; Z/EG animal that received 0.1 mg of tamoxifen on E11.5. Scale bars: a, c, 100 μm; b, 250 μm; d, 10 μm.
First, we took advantage of the dose sensitivity of CreERT2 to fate map isolated Ngn1-positive precursors. We applied the same logic that was used to define clones of cells labeled by CreER-induced methods in the cerebellum (Zong et al., 2005). Because cortical development is well characterized, we first tested this principle by inducing sparse labeling in the cerebral cortex, where ventricular zone precursors generate radial clones of cells. As expected, treatment with low levels of tamoxifen induced labeling in columns of cells, suggesting that each cluster of labeled cells consists of a clone of Ngn1 descendents (Fig. 5 a–c). Similarly, low-level tamoxifen treatments led to distinct groups of labeled neurons in the cochlea (Fig. 5 d), suggesting that each isolated cluster observed at P0 reflects the expansion of a single cell that expressed Ngn1 at the time of tamoxifen treatment. Within these clusters, we were able to identify type I and type II cochlear ganglion neurons in all 79 cochleas examined at P0, with no neurons displaying ambiguous morphologies. Of 172 neurons that could be visualized from the cell body to the end of the peripheral projection, 13 had obvious type II morphologies (8%). Because the proportion of labeled type II neurons is the same as what was determined by other methods (Romand and Romand, 1987), it is likely that recombination occurs reliably in Ngn1-positive type II precursors.
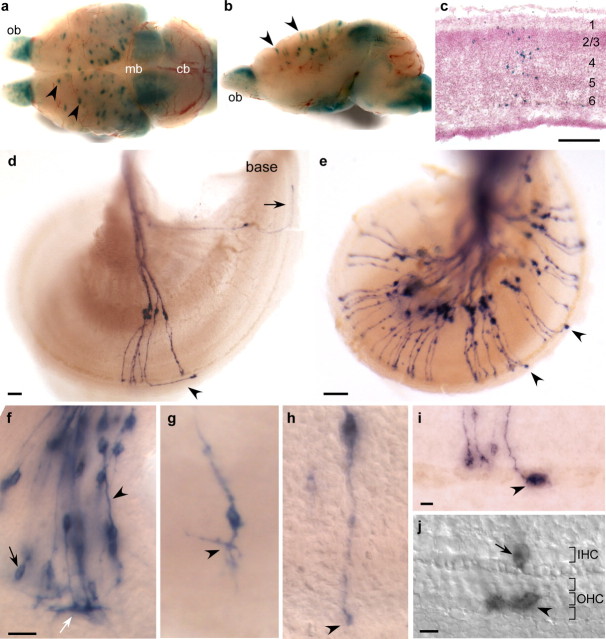
Figure 5.
Type I and type II cochlear ganglion neurons arise in common clusters and attain distinct morphologies by E16.5. a–c, A single low dose of tamoxifen caused recombination in isolated clusters of labeled cells throughout the cortex (a; arrowheads). Each cluster appeared to span all six layers of the cortex (b, arrowheads, c), a common feature of radial clones. The distribution and composition of cell clusters suggest that each cluster consists of clones of cells descended from a single Ngn1-positive progenitor. Broad labeling was also present in the olfactory bulbs (ob) and midbrain (mb). Dorsal (a) and medial (b) views of the same brain, and coronal sections (c) of a different brain, all from Ngn1-CreERT2; R26R animals given 1 mg of tamoxifen on E10.5 and stained for β-galactosidase activity on P0. cb, Cerebellum. d, Clusters of cochlear ganglion neurons contained both type I and type II neurons. This isolated group of seven labeled neurons, which includes six with type I endings and one with a type II ending (arrowhead), was most likely derived from a single Cre-positive precursor. The nearest neighboring labeled neuron, a lone type II (arrow), is a quarter of a turn away. P0 cochlea dissected from a Ngn1-CreERT2; Z/AP animal given 0.5 mg tamoxifen on E9.5 and stained for PLAP activity. The base of the cochlea is indicated. e–j, By inducing sparse labeling in Ngn1-CreERT2; Z/AP ears, it was possible to examine neuronal morphology at the single-cell level as early as E12.5. Although some cells had not yet begun to extend processes (f, black arrow), others were clearly bipolar and had central projections that already extended into the eighth nerve (f, arrowhead). Some peripheral projections had complex endings (f, white arrow), suggesting that they were growing along a boundary and exploring their environment. E12.5 Ngn1-CreERT2; Z/AP embryo that received 0.1 mg of tamoxifen daily from E9.5 to E11.5 and was stained for PLAP activity. g, h, A gradient of neuronal development could be inferred by comparing the short, branched morphology of immature neurons in the apex (g) to the long, straight morphology of more mature neurons in the base (h). E15.5 cochleas dissected from Ngn1-CreERT2; Z/AP animals given 0.5 mg of tamoxifen on E9.5 and stained for PLAP activity. Arrowheads indicate the peripheral endings. g represents a Z-projection image of a flat-mounted cochlea. e, i, j, Distinct morphologies were first apparent by E16.5. e, A whole-mounted PLAP-stained E16.5 cochlea shows two clusters of neurons that contain immature type II neurons (arrowheads). Within each cluster, one neuron projects to the outer hair cells and has turned toward the base of the cochlea, the two characteristics of type II neurons. The prominent endings of the immature type II neurons are apparent. i, A higher magnification image of a third example of a E16.5 cluster containing a single immature type II neuron. j, In a DIC image, a large immature type II ending is seen projecting to the outer hair cells (OHC), whereas the type I ending halts its projections at the inner hair cell (IHC) row. E16.5 cochleas dissected from Ngn1-CreERT2; Z/AP animals given 0.5 mg of tamoxifen on E9.5 and stained for PLAP activity. Scale bars: c–e, 100 μm; f–h, 25 μm; i, j, 10 μm.
To determine whether type I and type II ganglion neurons might arise from a common progenitor, we characterized the distribution of these two cell types in 33 clusters that were defined by strict criteria to increase the probability of studying descendents from single Ngn1-positive precursors (see Materials and Methods). Twenty-two clusters (67%) contained only type I neurons, whereas 11 clusters (33%) contained both type I and type II neurons. Type II-containing clusters nearly always had a single type II ganglion neuron, with an average of 1.2 ± 0.40 type II neurons per cluster, regardless of the size of the cluster. Two clusters had two type II neurons, and no cluster had more than two. For example, we identified one cluster of seven labeled cells where six were type I ganglion neurons and one was a type II ganglion neuron (Fig. 5 d). Based on the sparseness of labeling, it is most likely that these seven neurons derived from a single Ngn1-positive progenitor that underwent several cell divisions, although it is formally possible that multiple independent progenitors activated Cre and eventually produced neighboring neurons.
Peripheral projections acquire type II morphologies between E15.5 and E16.5
The presence of type I and type II cochlear ganglion neurons in common clusters does not provide sufficient information to conclude whether each subtype of neuron arises because of an asymmetric cell division or whether neuronal identity is determined instead by interactions with the environment. Golgi stains of cochleas from early postnatal kittens, rats, and gerbils suggest that cochlear ganglion neurons initially extend projections toward outer hair cells, followed by selective pruning so that type I ganglion neurons eventually innervate only inner hair cells (Perkins and Morest, 1975; Echteler, 1992). Because we observed clear type I and type II morphologies already by birth, we examined earlier stages to ask how the peripheral projections are established in the mouse.
We captured the initial stages of peripheral neurite extension by examining neuronal morphology at three embryonic stages. At E12.5, early postmitotic neurons were already bipolar. Although the central projections were simple and unbranched, the distal endings of the peripheral projections were complex and tangled and extended laterally along the border of the nascent spiral lamina (Figs. 4 c, 5 f). To follow the subsequent development of peripheral processes, we analyzed E15.5 cochleas. At E15.5, most hair cell precursors are immature and cannot be distinguished from the surrounding support cell precursors (Woods et al., 2004). However, in the base, inner hair cells have begun to differentiate, as evidenced by expression of the hair cell marker Myo7a and enhanced expression of the differentiation factor Math1 (Chen and Segil, 1999; Woods et al., 2004). Outer hair cells remain unrecognizable at this stage, even in the base. The gradient of development makes it possible to infer a sequence of guidance events by comparing morphologies in the base and apex of the E15.5 cochlea.
We visualized 82 isolated neurons distributed along the length of the cochlea at E15.5 (n = 15 cochleas). Rows of hair cells could not yet be identified by DIC imaging. Two distinct neuronal morphologies were apparent. Twenty-eight cells had thick peripheral projections with many branches that were close to the cell body, similar to the morphology of the E12.5 neurons (Fig. 5 g). The remaining neurons (n = 54) had single radial projections that ended at the edge of the spiral lamina (Fig. 5 h). Most neurons in the apex had branched endings (n = 15 of 20), whereas nearly every neuron in the base (n = 29 of 30) had unbranched projections. Both types of projections were present in the middle of the cochlea (n = 12 branched and 20 unbranched), suggesting that immature neurons extend initially complex projections that become refined after reaching the border between the spiral lamina and the developing organ of Corti.
By E16.5, cochlear ganglion processes had begun to penetrate the developing sensory epithelium in the base of the cochlea (Fig. 5 e,i,j), which contained a single row of differentiating Myo7a-positive inner hair cells and three rows of differentiating outer hair cells in the most mature regions (Fig. 5 j) (data not shown). We examined 605 stained neurons in the middle and base of the cochlea (n = 30 cochleas), because apical neurons still had branched and immature morphologies. Most processes terminated together at the level of the immature inner hair cells, similar to those seen at E15.5 (Fig. 5 j). However, 20 neurons (3.3%) had projections that grew into the rows of developing outer hair cells and turned toward the base, the two characteristic features of the type II morphology (Fig. 5 e,i,j). In contrast to observations made in other species (Perkins and Morest, 1975; Echteler, 1992), we did not observe any neurons that projected to both inner and outer hair cell rows. By qualitatively comparing where peripheral processes are relative to the spiral lamina in fixed cochleas at E15 and E16, we observed that most processes line up at the edge of the developing sensory epithelium at E15 (Figs. 5 h, 7 c) but that type II neurons do not extend projections into the outer hair cells until E16 and at this stage, only in the base (Fig. 5 e,i). Hence, we infer that cochlear ganglion neurons extend peripheral projections toward a cue in the sensory epithelium and then extend projections rapidly and specifically toward either immature inner or outer hair cells, but not to both. Based on the absence of neurons with intermediate morphologies and the presence of a small number of neurons with type II-like projections extending toward the vicinity of the outer hair cells at E16.5, we conclude that the fates of type I and type II ganglion neurons are determined embryonically and are independent of interactions with fully differentiated hair cells.
Figure 7.
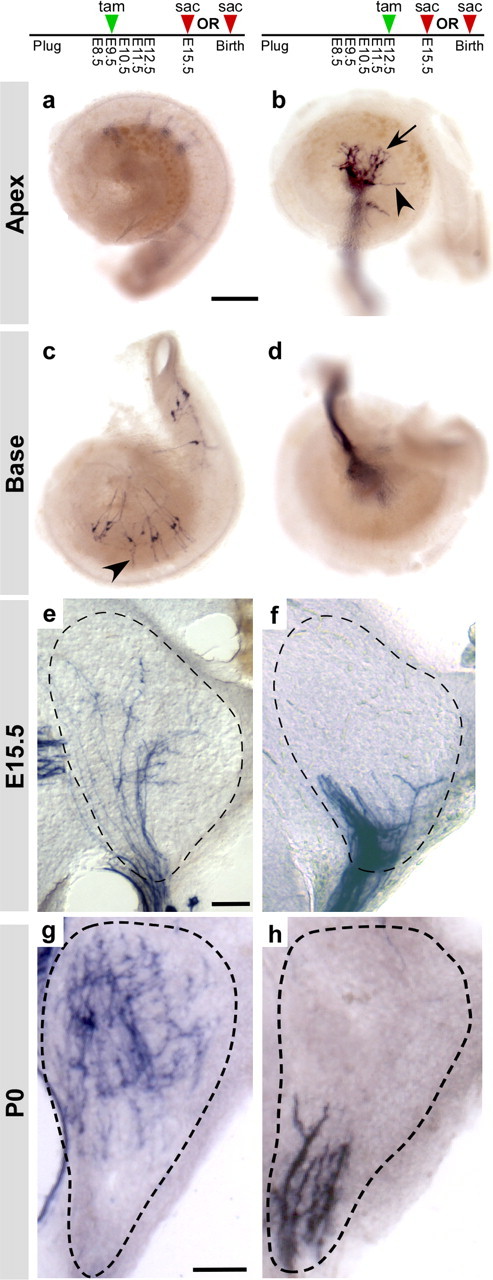
Early tonotopic organization of auditory circuits. a–d, As seen at P0, early tamoxifen (tam) treatments labeled E15.5 neurons in the base but not in the apex of the cochlea (a, c), whereas later treatments labeled neurons in the apex but not in the base (b, d). Cochlear ganglion neurons are at an early stage of circuit assembly, particularly in the apex where processes formed a tangled network close to the cell bodies (arrow, b), with only occasional extensions (arrowheads, b, c) toward the region where hair cells will eventually develop. Ngn1-CreERT2; Z/AP animals were treated with 0.5 mg of tamoxifen on E9.5 (a, c) or on E12.5 (b, d). Cochleas were collected (sac) at E15.5 and stained for PLAP activity. e–h, Although the cochlear nucleus anlage is not cellularly or morphologically mature at E15.5, cochlear ganglion projections were tonotopically organized, with endings from high-frequency neurons restricted to the dorsal half of the nuclei (e) and endings from low-frequency neurons located ventrally (f). This tonotopic organization was similar to what was observed at P0 (g, h). Ngn1-CreERT2; Z/AP animals were given 0.5 mg of tamoxifen on E9.5 (e, g) or E12.5 (f, h) and collected either at E15.5 (e, f) or P0 (g, h). Brains were sectioned coronally; the cochlear nuclei are outlined with dashed lines. For P0, only the posterior ventral cochlear nucleus (PVCN) is shown. Scale bars: a, 250 μm; e, g, 50 μm.
Central projections are topographically organized by E15.5
Finally, we asked whether central projections are also organized before maturation of their postsynaptic targets. The emergence of tonotopic maps in the mouse embryo has not been well studied, likely because of the difficulty of labeling subsets of ganglion neurons in the embryonic cochlea (Rubel and Fritzsch, 2002; Rubel et al., 2004). Cochlear ganglion neurons are born first in the base and last in the apex (Ruben, 1967). This apical-basal gradient of neurogenesis allowed us to reproducibly label cochlear ganglion neurons that respond to different sound frequencies by providing single doses of tamoxifen on E9.5, E10.5, E11.5, or E12.5 (Fig. 6 a–d). Quantification of the total number of neurons and their location along the length of the cochlea revealed a clear progression of development from the base to the apex (Fig. 6 e–h). E9.5 tamoxifen treatments labeled large clusters of neurons in the middle and base of the cochlea, with most cochleas containing no labeled cells in apical regions (Fig. 6 e). Similarly, administration of tamoxifen at E10.5 induced labeling in the basal half of the cochlea, extending into the hook (Fig. 6 f). The proportion of stained neurons shifted away from the base and toward the middle of the cochlea after E11.5 treatments (Fig. 6 g), and most apical neurons were labeled after E11.5 and E12.5 (Fig. 6 g,h). Although most E9.5 cochleas had labeling restricted to the basal half, some cochleas exhibited broader labeling, most likely reflecting recombination in highly proliferative progenitors at early stages of neurogenesis (Fig. 6 e). This distribution is consistent with previous reports but is broader after early tamoxifen treatments, because progenitors were permanently labeled in this experiment while in classic birth-dating studies, the mitotic label was diluted after multiple cell divisions (Ruben, 1967). Similar results were seen in E15.5 cochleas (Fig. 7 a–d) (data not shown).
Figure 6.
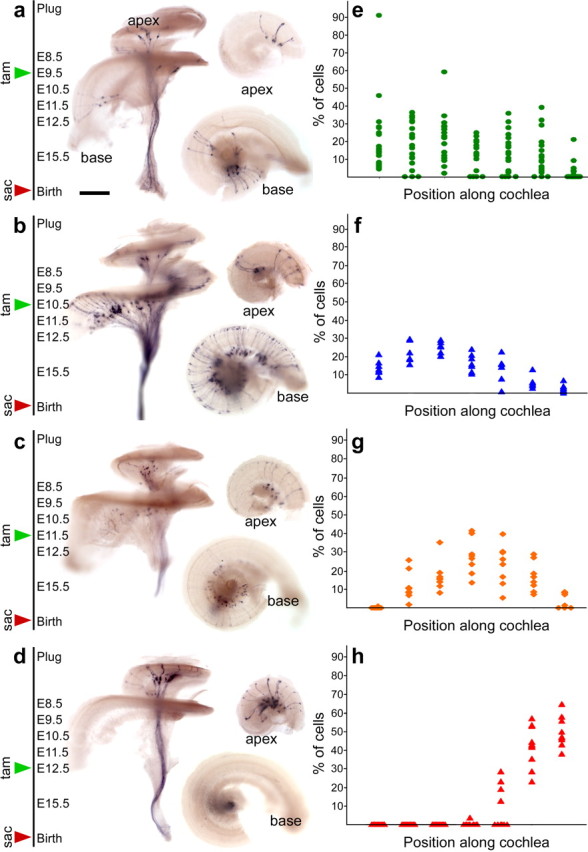
The orderly production of neurons from the base to the apex can be used to label populations of neurons that carry different sound frequency information. a–e, Consistent with the base to apex gradient of development in the cochlea, later Cre activation labeled progressively more apical neurons. Activation of Cre activity on E9.5 sparsely labeled neurons in the mid-basal region (a, e). One day later, labeling was extensive throughout the base and in the middle of the cochlea, with little in the apex (b, f). Tamoxifen treatment on E11.5 predominantly labeled neurons in the middle turn (c, g), whereas treatment on E12.5 exclusively labeled neurons in the apex (d, h). To quantify the results, each cochlea was divided into seven equal regions from base to apex (x-axis), and the percentage of labeled neurons in each region (y-axis) was determined. This information is plotted as a scatterplot of the percentage labeling in each segment per cochlea (e–h). E9.5, n = 1246 neurons from 19 ears; E10.5, n = 914 neurons from 7 ears; E11.5, n = 558 neurons from 8 ears; and E12.5, n = 171 neurons from 10 ears. P0 cochleas dissected from Ngn1-CreERT2; Z/AP animals that were given 0.5 mg of tamoxifen (tam) at the times indicated, killed at birth (sac), and stained for PLAP activity. Each cochlea was cut in half and imaged from the basal surface to best visualize the distribution of labeled neurons in the apex and base (right panels). Scale bar, 500 μm.
Taking advantage of the ability to label neurons reliably in the base or apex of the embryonic cochlea, we examined the distribution of apical and basal projections at E15.5. At this stage, hair cells have just begun to differentiate in the base, and no hair cells are functionally mature (Ruben, 1967; Geleoc and Holt, 2003). Similarly, ganglion neurons are at an early stage of circuit assembly, particularly in the apex (Figs. 5 f, 7 a–d). In the brainstem, the anlage of the cochlear nucleus has just become apparent and neurogenesis is ongoing (Pierce, 1967; Martin and Rickets, 1981; Ivanova and Yuasa, 1998). Cochleas were stained to confirm that labeling was restricted to the mid-base or apex. Then, sections through the cochlear nucleus were stained to determine the location of central projections. Despite the immature state of the cochlear nucleus anlage, projections from the middle and base of the cochlea were concentrated in the dorsomedial quadrant (n = 6 cochlear nuclei) (Fig. 7 e), whereas projections from the apex were bundled together along the ventral edge, predominantly in the ventrolateral quadrant (n = 18 cochlear nuclei) (Fig. 7 f). The apparent segregation of projections into dorsal and ventral domains was similar to what was observed at P0 (Fig. 7 g,h).
We quantified this result by analyzing the distribution of apical projections, because basal neurons were never labeled after E12.5 tamoxifen treatments (Fig. 6 e). Sections along the length of the cochlear nucleus anlage were scored for the presence of labeling in the dorsomedial quadrant, where basal neurons project (Fig. 7 e), or in the ventrolateral quadrant, where apical projections are present (Fig. 7 f). We observed that all cochlear nuclei examined (n = 18) had prominent staining in the ventrolateral quadrant but not in the dorsomedial quadrant along the entire rostrocaudal extent. In 15 cochlear nuclei, apical projections were fully confined ventrolaterally, with no labeling in the dorsomedial quadrant. The remaining cochlear nuclei (n = 3) had a few labeled projection in the dorsomedial quadrant in a small number of sections (2 of 14 sections, 1 of 8 sections, 2 of 9 sections). Because this staining had a sporadic appearance and was not present along the entire rostrocaudal extent of the nucleus, it was most likely caused by cellular debris or variations in section angle. However, we cannot rule out rare errors by low-frequency neurons. Nevertheless, this analysis indicates that apical projections were restricted to the topographically correct domain of the cochlear nucleus, although it was not possible to assess the coarseness of this map. Thus, cochlear ganglion axons are topographically organized in the developing cochlear nucleus, before the establishment of contacts with hair cells in the periphery.
Discussion
We used genetic fate mapping to track the progressive specification of cochlear ganglion neurons within the neurogenic domain of the otic vesicle. By following the fates of specific subsets of Ngn1-positive progenitors throughout development, including early embryonic stages previously inaccessible to detailed analysis, we revealed a remarkably stereotyped series of fate and wiring decisions, beginning with the early segregation of auditory and vestibular lineages and followed by the growth of peripheral and central projections directly toward their targets, but before the differentiation of presynaptic and postsynaptic cells. Our method permitted a more comprehensive and systematic analysis of auditory circuit assembly than was previously possible and revealed new insights that suggest that some accepted principles of auditory circuit assembly need to be revisited.
We found that auditory and vestibular lineages are segregated early in development, with the neurogenic domain of the E8.5 otic vesicle dedicated to the production of vestibular ganglion neurons. This finding is consistent with retroviral fate mapping studies in the chick that revealed the presence of a small number of common precursors for auditory and vestibular neurons but showed that the vast majority of clones are restricted to one system (Satoh and Fekete, 2005). Thus, any common precursor must be present before the otic cup stage and before the onset of Ngn1 expression. Our fate mapping results reveal that Ngn1-positive cells are not bipotential precursors. If they were, then early treatments should have labeled both the precursors for vestibular ganglion neurons born at E8 and the cochlear ganglion neurons born after E8. Instead, our results infer a change in the potential of cells in the neurogenic domain beginning at E9.5, when auditory neurons are first produced. One possibility is that signals from the environment induce expression of a transcription factor in a restricted region of the neurogenic domain, which gains the ability to generate cochlear ganglion neurons. GATA3 has been suggested to serve this function because of its restricted expression at E10.5, although the key event might also be the downregulation of NeuroD (Lawoko-Kerali et al., 2004a,b). Because of other ear defects, the specific functions of GATA3 and NeuroD during auditory neuron specification remain unclear (Liu et al., 2000; Karis et al., 2001; Kim et al., 2001). Nevertheless, our data suggest that a still earlier acting pathway acts upstream of GATA3 and NeuroD to define a proauditory domain. This result has intriguing implications for the evolution of the auditory system, which arose after the vestibular system, with the cochlea viewed as an outgrowth of the saccule (Fritzsch et al., 2002). The ability to produce auditory neurons within what was originally a field of vestibular progenitors was therefore an essential step in evolution of the auditory system.
Once specified, cochlear ganglion neurons exhibit stereotyped guidance behaviors. For example, in the cochlea, neurons first extend branched neurites that resolve into simple projections lined up at the edge of the spiral lamina by E15.5. By E16.5, a small number of neurons in the base have begun to acquire type II morphologies, defined by the growth of a peripheral process that extends beyond the inner hair cells and turns toward the base. Just 2 d later, cells with obvious type I and type II morphologies are present in the correct proportions, suggesting that peripheral projections grow into the organ of Corti rapidly and with no prolonged period of exploration. Although we cannot rule out the presence of immature neurons that briefly innervate both inner and outer hair cells, such a population must be small or exist transiently, because at E16.5, a minority of neurons extended projections toward outer hair cells, even in heavily labeled cochleas. Growth into the cochlear epithelium is highly directed and rapid, because we did not see any neurons with intermediate morphologies either at E15.5 or E16.5, but cells with obvious type II morphologies were apparent by E16.5. These results conflict with the widely accepted notion that cochlear ganglion neurons initially innervate both inner and outer hair cells (Perkins and Morest, 1975; Echteler, 1992; Huang et al., 2007). It is possible that pruning of the final arbor does occur, but is not a major determinant of the type I/type II fate decision, because other aspects of these distinct morphologies are already apparent. For example, there may be a period of neurite extension concurrent with the beginning of synaptogenesis, because previous studies only investigated neonatal and postnatal stages. Alternatively, the few neurons that were seen previously to innervate both inner and outer hair cells may represent a minor population that is unusually susceptible to Golgi impregnation. Indeed, previous studies reported the presence of these neurons only in the apex (Perkins and Morest, 1975; Ginzberg and Morest, 1983; Echteler, 1992), whereas our studies focused on the mid-region and base region of the cochlea. Finally, pruning may be more prominent in species other than the mouse.
The tonotopic organization of cochlear ganglion axons in the E15.5 brainstem is especially interesting in that the cochlear nucleus is quite immature in two respects at the stage. First, cells of the cochlear nucleus continue to divide, and the nucleus will increase in size considerably over the next week (Pierce, 1967; Martin and Rickets, 1981; Ivanova and Yuasa, 1998). Second, shortly after this stage, the hindbrain flexes, so that the dorsal cochlear nucleus moves from a fully caudal position to come to rest on top of the ventral cochlear nucleus (Farago et al., 2006). The tonotopic map may therefore arise in two distinct phases, with axons first growing into the correct general region of the cochlear nucleus and then waiting until nucleus morphogenesis is complete before selecting their final synaptic targets, perhaps in an activity-dependent manner. Our results are in agreement with studies in the embryonic chicken, where dye labeling revealed gross topographic segregation of axons shortly after arriving in nucleus magnocellularis (Molea and Rubel, 2003) and in the newborn cat, where tonotopic projections are well organized at birth (Leake et al., 2002). However, a subsequent period of activity-dependent refinement is likely to occur, because tonotopic maps are mildly degraded in deafened animals (Leake et al., 2006).
Although it is tempting to speculate that tonotopic maps arise passively with early arriving axons simply projecting to the dorsal-most regions of the cochlear nucleus, a more likely model is that molecular differences in axons from different regions of the cochlea guide topographic map formation. Indeed, cochlear ganglion neurons express different Trk receptors depending on their position along the axis of the cochlea (Farinas et al., 2001). In the visual system, retinal axons also project topographically according to birth order. However, axons project to the correct region of the optic tectum even when forced to grow in last instead of first, disproving the “first-come first-served” model (Holt, 1984). Unless significantly different principles are used in the auditory system, the most straightforward interpretation is that Ngn1-positive precursors generate cochlear ganglion neurons that are biased at the molecular level to extend processes toward specific regions of the cochlear nucleus, for example by expressing different levels of an Eph receptor (Siddiqui and Cramer, 2005). Thus, instead of producing a spatial gradient of Eph receptor, cochlear ganglion neurons might instead produce a temporal gradient of Eph receptor with different levels determined by the birth order of the neuron.
Many regions of the nervous system develop from progenitor populations that gradually change their properties over time, as we have observed in the inner ear. This phenomenon is best understood in the fly nervous system, where neuroblasts are generated in a predictable order that correlates both with changes in transcription factor expression patterns and with their final connectivity (Lee et al., 1999). In addition, similar to our observations of embryonic cochlear ganglion neurons, visualization of individual dendritic arbors revealed that olfactory neurons extend projections to stereotyped regions of the glomerulus before the presynaptic cells have differentiated (Jefferis et al., 2001). Accumulating evidence suggests that hard-wired mechanisms operate widely in the vertebrate nervous system. For example, cortical neurons are generated in a characteristic inside-out manner, and isolated cortical progenitor cells recapitulate the in vivo order of events, generating specific subsets of neurons at precise times in their lineage and gradually becoming more restricted in their ability to produce early-born cell types (Shen et al., 2006). Similarly, Ngn1-positive progenitors seem to undergo a progressive restriction in fate that guides the assembly of auditory circuits, with early born neurons ending up exclusively in vestibular circuits and cochlear neurons produced in a predictable order that correlates with the emergence of tonotopic maps at early stages. It is important to note that the final fate of a cell is also sculpted by its interactions with the environment. Hence, whereas intrinsic biases may play an important role, additional mechanisms are likely to exist for correcting errors and responding to potential traumas in the embryo. In the future, we will test the prediction that Ngn1-positive precursors have distinct gene expression profiles over time and that these programs influence the final wiring decisions of individual neurons. Ultimately, this information will allow us to engineer specific changes in auditory circuits that can then be evaluated using electrophysiology and behavioral testing.
Footnotes
This work was supported by the Mathers Charitable Foundation, the Alfred P. Sloan Foundation, and the Medical Foundation (L.V.G.); National Institutes of Health Grants 5 F31 DC007775-02 (E.J.K.) and T32 MH020017 (E.J.K., J.L.A.); and Stuart H. Q. and Victoria Quan Fellowships (E.J.K., J.L.A.). Pronuclear injections were performed by the Gene Manipulation Facility of the Mental Retardation and Development Disabilities Research Center at Children's Hospital Boston (Boston, MA) (Grant NIHP30-HD 18655). We thank Andrew Tucker for technical assistance, Kelvin Kwan for advice on ET recombination, Margaret Thompson and Hong Ye for help with pronuclear injections, Nina Lu for teaching us how to administer tamoxifen, and Steven Raft for providing Ngn1-CreERT2; Z/EG tissue. We are indebted to Marge Livingstone for her suggestion to administer tamoxifen together with estradiol to promote litter survival.
References
- Berglund AM, Ryugo DK. Hair cell innervation by spiral ganglion neurons in the mouse. J Comp Neurol. 1987;255:560–570. doi: 10.1002/cne.902550408. [DOI] [PubMed] [Google Scholar]
- Brown MC, Berglund AM, Kiang NY, Ryugo DK. Central trajectories of type II spiral ganglion neurons. J Comp Neurol. 1988;278:581–590. doi: 10.1002/cne.902780409. [DOI] [PubMed] [Google Scholar]
- Bruce LL, Kingsley J, Nichols DH, Fritzsch B. The development of vestibulocochlear efferents and cochlear afferents in mice. Int J Dev Neurosci. 1997;15:671–692. doi: 10.1016/s0736-5748(96)00120-7. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Echteler SM. Developmental segregation in the afferent projections to mammalian auditory hair cells. Proc Natl Acad Sci USA. 1992;89:6324–6327. doi: 10.1073/pnas.89.14.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farago AF, Awatramani RB, Dymecki SM. Assembly of the brainstem cochlear nuclear complex is revealed by intersectional and subtractive genetic fate maps. Neuron. 2006;50:205–218. doi: 10.1016/j.neuron.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Wu DK. Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol. 2002;12:35–42. doi: 10.1016/s0959-4388(02)00287-8. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Jones K, Farinas I, Maklad A, Lee J, Reichardt LF. Development and evolution of inner ear sensory epithelia and their innervation. J Neurobiol. 2002;53:143–156. doi: 10.1002/neu.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Hansen LA. The molecular basis of neurosensory cell formation in ear development: a blueprint for hair cell and sensory neuron regeneration? BioEssays. 2006;28:1181–1193. doi: 10.1002/bies.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleoc GS, Holt JR. Developmental acquisition of sensory transduction in hair cells of the mouse inner ear. Nat Neurosci. 2003;6:1019–1020. doi: 10.1038/nn1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzberg RD, Morest DK. A study of cochlear innervation in the young cat with the Golgi method. Hear Res. 1983;10:227–246. doi: 10.1016/0378-5955(83)90056-4. [DOI] [PubMed] [Google Scholar]
- Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk DI, Tsung EF, Cai Z, Alberta JA, Cheng LP, Liu Y, Stenman JM, Valerius MT, Billings N, Kim HA, Greenberg ME, McMahon AP, Rowitch DH, et al. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Holt CE. Does timing of axon outgrowth influence initial retinotectal topography in Xenopus? J Neurosci. 1984;4:1130–1152. doi: 10.1523/JNEUROSCI.04-04-01130.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LC, Thorne PR, Housley GD, Montgomery JM. Spatiotemporal definition of neurite outgrowth, refinement and retraction in the developing mouse cochlea. Development. 2007;134:2925–2933. doi: 10.1242/dev.001925. [DOI] [PubMed] [Google Scholar]
- Ivanova A, Yuasa S. Neuronal migration and differentiation in the development of the mouse dorsal cochlear nucleus. Dev Neurosci. 1998;20:495–511. doi: 10.1159/000017350. [DOI] [PubMed] [Google Scholar]
- Jefferis GS, Marin EC, Stocker RF, Luo L. Target neuron prespecification in the olfactory map of Drosophila . Nature. 2001;414:204–208. doi: 10.1038/35102574. [DOI] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawoko-Kerali G, Rivolta MN, Lawlor P, Cacciabue-Rivolta DI, Langton-Hewer C, van Doorninck JH, Holley MC. GATA3 and NeuroD distinguish auditory and vestibular neurons during development of the mammalian inner ear. Mech Dev. 2004a;121:287–299. doi: 10.1016/j.mod.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Lawoko-Kerali G, Milo M, Davies D, Halsall A, Helyer R, Johnson CM, Rivolta MN, Tones MA, Holley MC. Ventral otic cell lines as developmental models of auditory epithelial and neural precursors. Dev Dyn. 2004b;231:801–814. doi: 10.1002/dvdy.20187. [DOI] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT. Postnatal refinement of auditory nerve projections to the cochlear nucleus in cats. J Comp Neurol. 2002;448:6–27. doi: 10.1002/cne.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Chair L, Snyder RL. Neonatal deafness results in degraded topographic specificity of auditory nerve projections to the cochlear nucleus in cats. J Comp Neurol. 2006;497:13–31. doi: 10.1002/cne.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- Lee T, Lee A, Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development. 1999;126:4065–4076. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai MJ. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev. 2000;14:2839–2854. doi: 10.1101/gad.840500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double reporter for cre-mediated recombination. Dev Biol. 1999;208:281–292. doi: 10.1006/dbio.1999.9209. [DOI] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. Development of vestibular afferent projections into the hindbrain and their central targets. Brain Res Bull. 2003;60:497–510. doi: 10.1016/s0361-9230(03)00054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MR, Rickets C. Histogenesis of the cochlear nucleus of the mouse. J Comp Neurol. 1981;197:169–184. doi: 10.1002/cne.901970113. [DOI] [PubMed] [Google Scholar]
- Molea D, Rubel EW. Timing and topography of nucleus magnocellularis innervation by the cochlear ganglion. J Comp Neurol. 2003;466:577–591. doi: 10.1002/cne.10896. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Perkins RE, Morest DK. A study of cochlear innervation patterns in cats and rats with the Golgi method and Nomarkski optics. J Comp Neurol. 1975;163:129–158. doi: 10.1002/cne.901630202. [DOI] [PubMed] [Google Scholar]
- Pierce ET. Histogenesis of the dorsal and ventral cochlear nuclei in the mouse. An autoradiographic study. J Comp Neurol. 1967;131:27–54. doi: 10.1002/cne.901310104. [DOI] [PubMed] [Google Scholar]
- Raft S, Nowotschin S, Liao J, Morrow BE. Suppression of neural fate and control of inner ear morphogenesis by Tbx1. Development. 2004;131:1801–1812. doi: 10.1242/dev.01067. [DOI] [PubMed] [Google Scholar]
- Raft S, Koundakjian EJ, Quinones H, Jayasena CS, Goodrich LV, Johnson JE, Segil N, Groves A. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134:4405–4415. doi: 10.1242/dev.009118. [DOI] [PubMed] [Google Scholar]
- Romand MR, Romand R. The ultrastructure of spiral ganglion cells in the mouse. Acta Otolaryngol. 1987;104:29–39. doi: 10.3109/00016488709109044. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Parks TN, Zirpel L. Assembling, connecting and maintaining the cochlear nucleus. In: Parks TN, Rubel EW, Fay RR, Popper AN, editors. Plasticity of the auditory system. New York: Springer; 2004. [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;220:221–244. [PubMed] [Google Scholar]
- Satoh T, Fekete DM. Clonal analysis of the relationships between mechanosensory cells and the neurons that innervate them in the chicken ear. Development. 2005;132:1687–1697. doi: 10.1242/dev.01730. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE, Temple S. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- Siddiqui SA, Cramer KS. Differential expression of Eph receptors and ephrins in the cochlear ganglion and eighth cranial nerve of the chick embryo. J Comp Neurol. 2005;482:309–319. doi: 10.1002/cne.20396. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Strong SJ, Ohta Y, Litman GW, Amemiya CT. Marked improvement of PAC and BAC cloning is achieved using electroelution of pulsed-field gel-separated partial digests of genomic DNA. Nucleic Acids Res. 1997;25:3959–3961. doi: 10.1093/nar/25.19.3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7:1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci USA. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, Espinosa JS, Su HH, Muzumdar MD, Luo L. Mosaic analysis with double markers in mice. Cell. 2005;121:479–492. doi: 10.1016/j.cell.2005.02.012. [DOI] [PubMed] [Google Scholar]