Abstract
Both synaptic strength and spine size vary from spine to spine, but are strongly correlated. This gradation is regulated by activity and may underlie information storage. Ca2+-calmodulin-dependent kinase II (CaMKII) is critically involved in the regulation of synaptic strength and spine size. The high amount of the kinase in the postsynaptic density has suggested that the kinase has a structural role at synapses. We demonstrated previously that the bound amount of CaMKIIα in spines persistently increases after induction of long-term potentiation, prompting the hypothesis that this amount may correlate with synaptic strength. To test this hypothesis we combined two recently developed methods, two-photon uncaging of glutamate for determining the EPSC of individual spines (uEPSC) and quantitative microscopy for measuring bound CaMKIIα in the same spines. We found that under basal conditions the relative bound amount of CaMKIIα varied over a 10-fold range and positively correlated with the uEPSC. Both the bound amount of CaMKIIα in spines and uEPSC also positively correlated with spine size. Interestingly, the bound CaMKIIα fraction (bound/total CaMKIIα in spines) remained remarkably constant across all spines. The results are consistent with the hypothesis that bound CaMKII serves as a structural organizer of postsynaptic molecules and thereby may be involved in maintaining spine size and synaptic strength.
Keywords: dendritic spine, synaptic plasticity, CaMKII, slice culture, fluorescence microscopy, LTP, long-term potentiation, imaging, uncaging
Introduction
Activity-dependent plasticity of both spine size and synaptic strength is involved in a number of physiological processes including learning and memory (Segal, 2005). Spine size and synaptic strength are strongly positively correlated under basal conditions and coordinately modulated by activity. How this correlation is maintained is not known. Ca2+/CaM-dependent kinase II (CaMKII) has been implicated in both activity-dependent synaptic strengthening (Lisman et al., 2002) and spine morphogenesis (Fink et al., 2003; Jourdain et al., 2003; Matsuzaki et al., 2004; Narayanan et al., 2006). In dendritic spines, CaMKII is associated with the postsynaptic density (PSD) (Kennedy, 1998; Schulman, 2004) and with the spine cytoskeleton (Shen et al., 1998; Fink et al., 2003; Okamoto et al., 2004). CaMKII is the most abundant protein in the PSD, exceeding in amount even the main structural PSD protein, PSD-95 (Cheng et al., 2006). This large amount of the kinase has suggested that CaMKII has a structural role at synapses in addition to its enzymatic role (Erondu and Kennedy, 1985). Indeed, CaMKII holoenzymes are capable of self-association and can bind to multiple spine proteins, including NMDA receptors (NMDARs) in the PSD and F-actin in the spine head (Kennedy, 1998; Colbran, 2004; Schulman, 2004; Merrill et al., 2005). Notably, binding to the NMDAR is required for regulating synaptic strength (Barria and Malinow, 2005) and binding to F-actin has been implicated in controlling spine morphogenesis (Fink et al., 2003; Narayanan et al., 2006).
CaMKII enrichment at synapses is regulated by synaptic activity (Meyer and Shen, 2000; Colbran, 2004; Schulman, 2004; Merrill et al., 2005). Numerous studies have shown that strong synaptic activation results in an increase in binding of the kinase to NMDAR (Colbran, 2004; Schulman, 2004; Merrill et al., 2005). Using fluorescent imaging in living cells, we demonstrated previously that the amount of bound CaMKIIα in spines persistently increases after induction of LTP (Otmakhov et al., 2004). We also observed a substantial amount of bound CaMKIIα in many spines under basal conditions, indicating that these basal levels may be a result of previous LTP-like processes that increased synaptic strength. If this notion is correct, the amount of bound CaMKIIα in individual spines should positively correlate with spine synaptic strength. To directly test this prediction, we used a combination of two-photon glutamate uncaging, whole-cell recording and confocal imaging of living neurons.
Materials and Methods
Method of slice culture.
Hippocampal slice cultures were prepared at postnatal day 6 (P6)–P10 as described by Otmakhov et al. (2004).
Transfection.
After 5 to 10 d in vitro, slice cultures were bolistically cotransfected with plasmids containing monomeric green fluorescent protein (mGFP)-CaMKIIα (wild type) and monomeric red fluorescent protein (mRFP) cDNAs. In some experiments CaMKIIα (wild type) tagged with photoactivatable GFP (PAGFP) and mRFP(Cherry) were coexpressed.
Electrophysiology.
Whole-cell recordings were performed as described previously (Otmakhov et al., 2004). Slices were continuously superfused (1 ml/min) with artificial CSF (ACSF) containing the following (in mm): 124 NaCl, 2.5 KCl, 4 CaCl2, 4 MgCl2, 1.25, NaH2PO4, 26 NaHCO3, 0.001 TTX, 0.05 picrotoxin, 0.1 d,l-APV, balanced with 95% O2 and 5% CO2, pH 7.4. Electrical signals were amplified (Axopatch 1D), low-pass filtered at 1 kHz, and digitized at 10 kHz. Recordings were performed at room temperature (22–24°C). The pipette solution contained (in mm) 120 Cs-methanesulfonate, 20 CsCl, 10 HEPES, 4 MgATP, 0.3 Na3GTP, 0.2 EGTA, and 10 phosphocreatine, pH, 7.3, osmolarity, 320 mOsm. In some experiments, red fluorescent dye Alexa 594 was added to the pipette solution to facilitate cell volume measurements. Whole-cell currents were measured in voltage-clamp mode at a holding potential of −65 mV. Series and input resistance were monitored after each sequence of 50 uncaging events.
Imaging, uncaging, and photoactivation were performed using a commercial Leica (Wetzlar, Germany) SP2MP upright confocal microscope equipped with a 60× (1.1 numerical aperture, water immersion) objective, visible light (Ar/Kr) lasers and a femtosecond pulse infrared laser (Chameleon; Coherent, Santa Clara, CA). mGFP (or PAGFP) and mRFP were imaged in confocal mode sequentially with 488 and 543 nm excitation and 500–550 and 580–680 nm emission respectively. Normally, stacks of 9–20 images with 0.5 μm focal steps were collected of dendritic segment containing 5–50 spines. Only spines that clearly extended out from dendrite horizontally were taken into account in this study. Caged glutamate (MNI-glutamate; Tocris, Ellisville, MO) was included in the recirculating bath ACSF (total volume 3 ml) at a final concentration 8 mm. The Leica system was upgraded with a fast mechanical shutter, fast photodiodes and custom software written in Igor Pro. Uncaging was performed by a 1–2 ms pulse of the IR laser (720 nm, 4–6 mW) focused at a single pixel location (Matsuzaki et al., 2001). The uncaging locations were scanned semi randomly (0.4 Hz) within a region of interest (ROI) that normally included three or more spines appearing at the same focal plan. The peaks of uEPSCs were then mapped to corresponding locations on the imaged structure. The lateral spatial sampling interval for uncaging was 0.3 μm and the pixel size for imaging was 0.093 μm. For photoactivation, dendritic segments were scanned once with a 720 nm IR laser through all the Z planes used for imaging. After photoactivation, confocal imaging of the dendrite was repeated at 2, 5, 10, and 30 min. Integral fluorescence of activated PAGFP in the three best focal planes was used as a relative value for the bound amount (BA) of PAGFP-CaMKIIα.
Estimation of relative bound amount and bound fraction GFP-CaMKIIα in spines.
Measurements of fluorescence values in spines and dendritic shafts and calculations of relative total, bound amount, and bound fraction in spines were performed as described previously (Otmakhov et al., 2004) (see also supplemental Fig. 1, available at www.jneurosci.org as supplemental material). The relative spine volume was estimated as proportional to the integral red fluorescence within the spine ROI (Holtmaat et al., 2005). To visualize the location of bound CaMKII, a similar approach was used, but the BA was calculated for each pixel within the spine at the three best focal planes and then a projection image was constructed (see Fig. 1).
Figure 1.
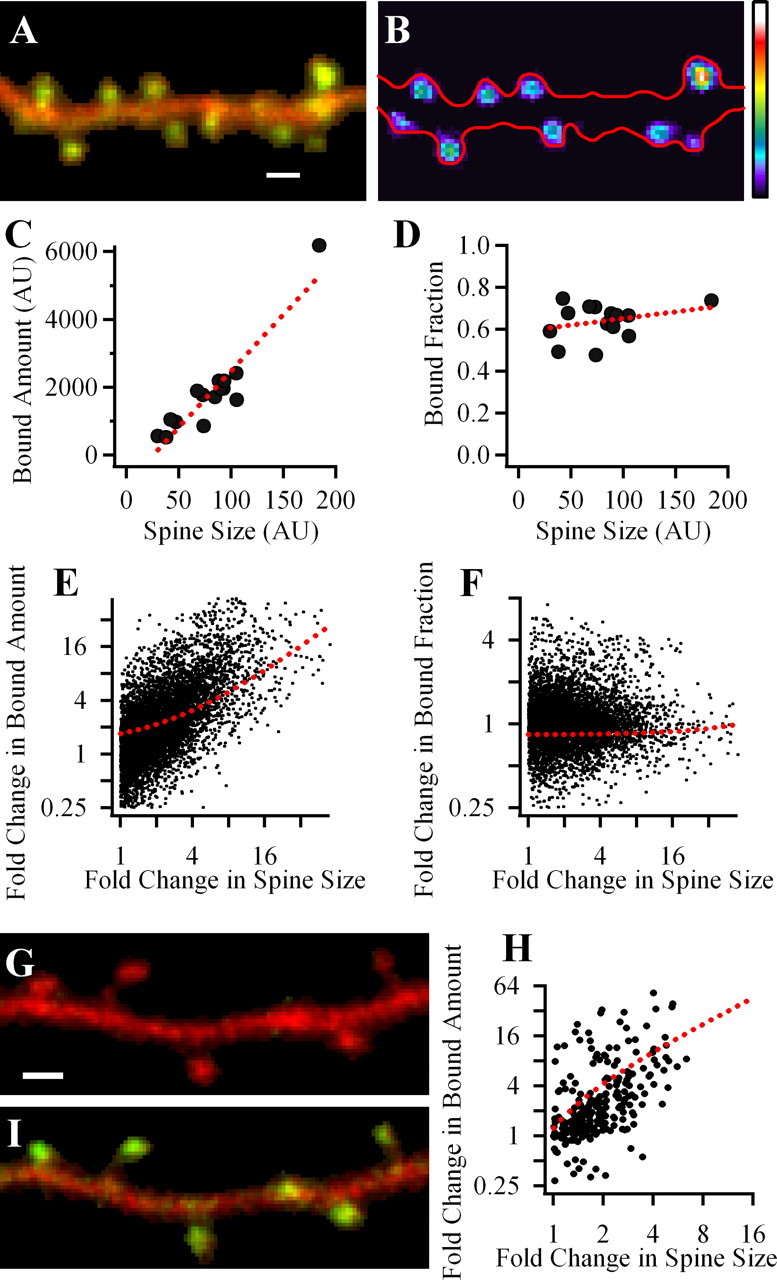
Bound amount of GFP-CaMKIIα strongly correlates with spine volume but bound fraction does not. A, Merged image of a dendritic segment of neuron expressing mGFP-CaMKIIα and infused with Alexa 594 dye. B, A map of the bound mGFP-CaMKIIα in spines in pseudocolor coding; warmer colors correspond to larger bound amount. Red outline shows the dendrite morphology. BA was calculated only in clearly identified spines. Scale, 1 μm. C, D, Plots of data obtained from the dendrite shown in A and B. Each symbol shows the value for an individual spine. Dashed line, Linear fit of data. E, F, Summary data for the correlations of spine BA or BF of GFP-CaMKIIα and spine volume. Each data point is a result of comparison of two spines from the same dendrite. E, Fold change in BA versus fold change in spine size. F, Fold change in BF versus fold change in spine size. G, H, Measuring BA using PAGFP-CaMKIIα. Merged image of a dendritic segment of neuron expressing PAGFP-CaMKIIα and mRFP(Cherry) before (G) and 2 min after (I) the photoactivation of PAGFP. H, Summary graph, showing fold change in spine size versus fold change in BA of PAGFP-CaMKIIα, reveals strong correlation between these parameters.
Results
Bound amount of GFP-CaMKIIα strongly correlates with spine volume, but bound fraction does not
Coexpressing mGFP-CaMKIIα with the soluble fluorescent protein, mRFP allowed us to calculate the relative BA and bound fraction (BF) (see Materials and Methods) of CaMKII in individual spines as well as the relative spine volume. Because of the limitation of our methods, we were restricted to quantitative comparison of spines on the same dendritic segment. We first tested whether the BA and BF of CaMKII in spines correlates with spine size. On the basis of our previous results (Otmakhov et al., 2004), we expected that both parameters would be correlated with spine size under basal conditions. Figure 1 shows an example of a dendritic segment from a neuron in hippocampal slice culture 5 d after transfection. The greenish-yellow spines compared with reddish dendrites on the green/red merged image (Fig. 1A) indicate that there is an accumulation of CaMKII in most spines. This is also evident from the calculated image of the BA in each spine (Fig. 1B). Comparing the BA of mGFP-CaMKIIα and the volumes of these spines shows a strong correlation (Fig. 1C) [Pearson's coefficient (Pr) = 0.93; n = 15]. Surprisingly the bound fractions for these spines were very similar for spines of all sizes (Fig. 1D) (Pr = 0.29).
The average data for 788 spines in 17 different experiments are shown in Figure 1, E and F. Each pair of spines on a dendritic branch was compared and the fold change in one parameter was plotted against the fold change of a second parameter. On average there was a strong positive correlation between the BA of mGFP-CaMKIIα and spine volume for a given branch (Pr = 0.82 ± 0.02; p < 0.001), but the BF of CaMKII was invariant of spines size (Pr = 0.01 ± 0.05; p > 0.05). The average BF of mGFP-CaMKIIα in these experiments was 0.53 ± 0.12 (SD) of the total amount of the kinase in spines.
We next sought to check the correlation of BA with spine size by an independent method. We took advantage of the properties of PAGFP, which undergoes a persistent increase in the ability to fluoresce after a short period of illumination with UV light. Because PAGFP is practically undetectable before photoactivation, to identify transfected cells, we coexpressed PAGFP-CaMKIIα together with mRFP (Cherry) (Fig. 1G). After performing photoactivation in a small dendritic segment with spines, all soluble fluorescent molecules should diffuse out of these spines within seconds (Gray et al., 2006) allowing imaging of the remaining bound pool of PAGFP-CaMKII (Fig. 1G,I). Figure 1H shows summary results obtained an average of 6 min after the photoactivation (3 cells, 7 branches, 62 spines). The data reveal a strong correlation of spine BA of CaMKIIα and spine size (Pr = 0.84 ± 0.06; p < 0.001), confirming this correlation by an independent method.
Bound amount of GFP-CaMKIIα of individual spines positively correlates with postsynaptic spine strength
We then combined two-photon uncaging of caged glutamate and whole-cell recordings from cells coexpressing mGFP-CaMKIIα and mRFP to measure the relative synaptic strength of individual spines and correlate this with the relative amount of bound CaMKII in the same spines.
To do this, we first mapped uEPSCs onto the spine structure. Consistent with previous findings (Matsuzaki et al., 2001), maps of uncaging currents for individual spines showed that regions of maximal uEPSCs were normally smaller than the spine and were usually localized at spine heads at their focal planes (Fig. 2). This is expected for large spines, given that the postsynaptic density, where glutamate receptors are concentrated, occupies only a fraction of the spine surface (Harris and Stevens, 1989). Similar, but normally weaker “hot spots” of uEPSCs could sometimes be found on dendrites perhaps because of responses of vertically oriented spines in these areas.
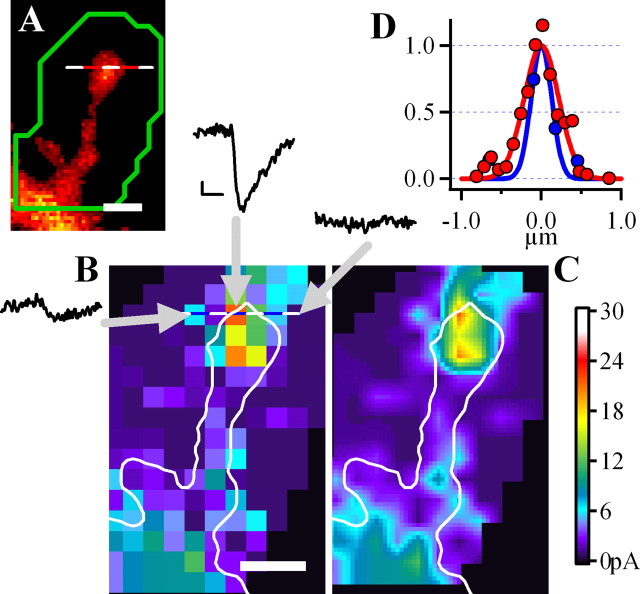
Figure 2.
Measuring the postsynaptic strength of a single spine using glutamate uncaging. A, A spine from a neuron filled with Alexa 594 dye with ROI for uncaging shown in green; scale, 1 μm. B, The map of uncaging locations within the ROI indicated in A. Three examples of uEPSCs are from locations indicated by arrows. Calibration: 5pA, 5 ms. Different uncaging locations yielded uEPSCs ranging from negligible to 25 pA. C, The same map but smoothed by interpolation. D, The spatial distribution of fluorescence along the dotted line in A (red) showing that the spine diameter as estimated by full width at half maximum (FWHM) is ∼0.5 μm. Blue line, The distribution of the uncaging response, which is slightly narrower (FWHM, 0.4 μm) than the diameter of the spine.
Figure 3 shows a result of one experiment in which measurements of the bound amount of mGFP-CaMKIIα and uncaging mapping were performed for several spines on a small dendritic segment. Z-stacks of green and red images were taken from this dendritic segment to calculate relative BA and BF of spine mGFP-CaMKIIα. The mapping was performed only in regions containing spines in the same focal plane. Analysis shows that spines with larger amounts of bound mGFP-CaMKIIα had bigger maximal uEPSCs (Fig. 3D) (Pr = 0.88; n = 8). We also confirm previous findings (Matsuzaki et al., 2001), that spines with stronger postsynaptic responses were larger in size, because maximal uEPSCs of individual spines positively correlates with spine volume (Pr = 0.97). As described above, in these uncaging experiments we also observed that the BA, but not BF of spine mGFP-CaMKIIα positively correlates with spine volume (BA, Pr = 0.88; BF, Pr = −0.19). More examples of experiments of this kind are shown in the supplemental Fig. 2 (available at www.jneurosci.org as supplemental material).
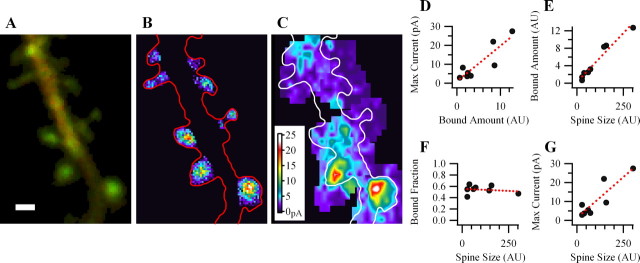
Figure 3.
At individual spines, the maximal uEPSC positively correlates with the relative bound amount of GFP-CaMKIIα. A, An overlay of green and red fluorescence from a dendrite expressing GFP-CaMKIIα (green) and mRFP (red). B, Calculated locations of relative bound GFP-CaMKIIα amount. C, uEPSCs maps for spines of the same dendrite. D–G, Plots showing dependence of maximal postsynaptic uEPSC on the amount of bound CaMKII and dependence of CaMKII bound amount, bound fraction and uEPSC on spine volume for the spines shown in A. Scale bar, 1 μm.
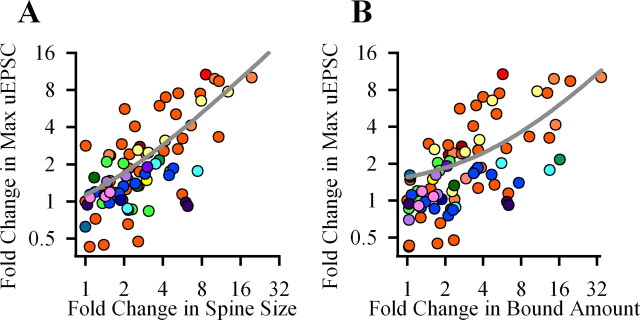
Figure 4 shows summary data for the above experiments. Calculations of the mean coefficient of correlations demonstrates that maximal uEPSC strongly correlates with both BA of spine mGFP-CaMKIIα and spine volume [Pr for uEPSC over spine size (Fig. 4A), 0.75 ± 0.013, p < 0.001; Pr for uEPSC over bound amount (B), 0.86 ± 0.06, p < 0.001; 55 spines, 17 experiments].
Figure 4.
Summary data for the correlation of postsynaptic uEPSC with spine bound amount of GFP-CaMKIIα or spine volume. Each data point is a result of comparison of two spines from the same dendrite. A, B, Each graph shows a fold change in one parameter versus fold change of another parameters: uEPSC versus spine volume (A) or versus bound amount of CaMKII (B). In A and B, data for each dendrite is color coded. Gray lines, Linear fits.
Discussion
The molecular determinants of synaptic strength are not understood. Newly developed methods have allowed us to compare the molecular content of CaMKII with functional postsynaptic strength of the same spine. We have tested the hypothesis that the postsynaptic strength is related to the CaMKII content of the spine. Our main finding is that spine strength positively correlates with the BA of CaMKII. Remarkably, the BF is the same even in spines that vary in sizes by an order of magnitude. We also find that the spine volume correlates with the BA of CaMKII. These correlative properties observed under basal conditions are consistent with the previous findings that spine strength, volume and BA of CaMKII all increase after LTP induction (Matsuzaki et al., 2004; Otmakhov et al., 2004). However, we were surprised to find that the bound fraction was independent of spine size because our previous data demonstrated that the BF also significantly increased after LTP induction. This finding implies that the change in the BF after LTP is only transient and returns to the prepotentiation level within some period of time after LTP induction (see below) (supplemental material, available at www.jneurosci.org).
CaMKII content and spine size
One of the main postsynaptic binding partners of CaMKII at the synapse is the NMDA receptor (Colbran, 2004; Schulman, 2004; Merrill et al., 2005). Labeling studies indicate that the number of NMDARs at individual synapses weakly but significantly correlates with synapse size (Racca et al., 2000), suggesting that CaMKII binding to NMDAR could contribute to the correlation we observe, although kinase self-association or binding to other PSD targets may also be a participating factor.
In our experiments, we measured GFP-CaMKIIα associated not only with the PSD, but all bound kinase in the spine head. The F-actin cytoskeleton mesh, which is believed to be involved in control of spine shape and size (Tada and Sheng, 2006), is another pool for anchored CaMKII. CaMKIIα associates with spine F-actin indirectly although CaMKIIβ or α-actinin (Shen et al., 1998; Fink et al., 2003; Okamoto et al., 2004; Robison et al., 2005). Accordingly, actin depolymerizing agents dramatically reduce the amount of CaMKIIα found at spines (Allison et al., 2000; Asrican et al., 2006). Importantly, the amount of F-actin in spines increases after LTP induction (Okamoto et al., 2004; Lin et al., 2005) providing more targets for CaMKII binding. This binding is likely to contribute to the increase in the BA of CaMKII after LTP and for the correlation of the BA with spine size reported in this study.
CaMKII and spine morphogenesis
The role of CaMKII in activity-dependent spine morphogenesis is just beginning to be elucidated. Several studies have demonstrated that CaMKII activity is necessary for the increase in spine size after LTP induction (Matsuzaki et al., 2004) and may also affect spine density after other forms of neuronal activity (Rongo and Kaplan, 1999; Fink et al., 2003). Moreover, direct infusion of catalytically active CaMKII alone is sufficient to induce appearance of new spines (Jourdain et al., 2003). Previous reports suggest that CaMKII may play a structural role in controlling spine size. For example, CaMKIIβ directly binds to actin filaments and presumably serves as a linker that bundles actin fibers in vitro and stabilizes F-actin in living cells (Narayanan et al., 2006). Notably, this nonenzymatic property of CaMKIIβ was suggested to be an important requirement for its involvement in the regulation of spine morphogenesis (Fink et al., 2003; Narayanan et al., 2006).
CaMKII content and postsynaptic strength
The number of AMPA receptors (AMPARs) in the synapse strongly correlates with the size of the PSD, which itself correlates with spine size (Harris and Stevens, 1989; Nusser et al., 1998). Moreover, the functional synaptic strength (measured by uncaging of glutamate at synapses) also strongly correlates with spine size under basal conditions, and spine size increases after LTP induction (Matsuzaki et al., 2001, 2004). How this correlation occurs is not known, although previous data demonstrated that fusion of postsynaptic vesicles containing AMPA receptors (and possibly other structural elements of the PSD) near the synapse provides the extra membrane and signaling molecules for both the synapse and spine enlargement (Park et al., 2006). The NMDAR is one of the main binding targets of CaMKII at the PSD (Colbran, 2004; Schulman, 2004; Merrill et al., 2005), and LTP induction requires this binding (Barria and Malinow, 2005). Because a single CaMKII subunit can bind multiple proteins (Robison et al., 2005) it can serve as structural organizer of PSD signaling molecules, playing a purely structural role in maintaining synaptic strength. Specifically, CaMKII could be involved in the organization of a structural link at the PSD (Lisman et al., 2002) and spine cytoskeleton (Narayanan et al., 2006) necessary to stabilize AMPARs at the synapse. Consistent with this notion, recent data directly demonstrated that an inhibitor that interferes with CaMKII binding to NR2B subunit (Vest et al., 2007) significantly suppresses basal synaptic transmission (Sanhueza et al., 2007).
BF of CaMKII is independent of spine size
We found that the BF of the kinase did not correlate with spine size and spine strength. This was unexpected, because our previous study showed that the BF increased for at least 60 min after LTP induction. We, therefore, expected that the BF would also correlate with spine size and strength. Our findings here suggest that the observed increase in BF after LTP induction is only transient. One possible scenario is that LTP produces an accumulation of CaMKII at spines because of binding to the PSD and enlarged F-actin cytoskeleton. An enhanced density of the kinase at the PSD (Otmakhov et al., 2004) and kinase self-association (Hudmon et al., 2005) may account for the excessive amount of bound CaMKII responsible for the increased BF. Slowly this excessive amount may leave the spine, bringing the BF back to the initial prepotentiated level while the BA of CaMKII remains elevated proportionally with the increased PSD (Ostroff et al., 2002) and F-actin mesh (Lin et al., 2005).
Conclusion
We have combined two newly developed techniques, two-photon uncaging of glutamate for monitoring postsynaptic strength and quantitative fluorescent microscopy to measure the relative BA of one of the most abundant synaptic molecules, CaMKIIα. We found that this amount positively correlates with synaptic strength and spine size. This finding is consistent with the hypothesis that CaMKII may work as a synaptic organizing molecule in maintaining structure and function.
Footnotes
This work was supported by National Institutes of Health Grant R01 NS-27337. We thank P. De Koninck, R. Tsien, G. Patterson, J. Lippincott-Schwartz, and K. Svoboda for kind gifts of DNA constructs.
References
- Allison DW, Chervin AS, Gelfand VI, Craig AM. Postsynaptic scaffolds of excitatory and inhibitory synapses in hippocampal neurons: maintenance of core components independent of actin filaments and microtubules. J Neurosci. 2000;20:4545–4554. doi: 10.1523/JNEUROSCI.20-12-04545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asrican B, De Koninck P, Lisman J, Otmakhov N. Measuring CaMKII binding to different postsynaptic targets in live hippocampal neurons. Soc Neurosci Abstr. 2006;32:633–638. [Google Scholar]
- Barria A, Malinow R. NMDA Receptor Subunit Composition Controls Synaptic Plasticity by Regulating Binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Cheng D, Hoogenraad CC, Rush J, Ramm E, Schlager MA, Duong DM, Xu P, Wijayawardana SR, Hanfelt J, Nakagawa T, Sheng M, Peng J. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol Cell Proteomics. 2006;5:1158–1170. doi: 10.1074/mcp.D500009-MCP200. [DOI] [PubMed] [Google Scholar]
- Colbran RJ. Targeting of calcium/calmodulin-dependent protein kinase II. Biochem J. 2004;378:1–16. doi: 10.1042/BJ20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erondu NE, Kennedy MB. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci. 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink CC, Bayer KU, Myers JW, Ferrell JE, Jr, Schulman H, Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39:283–297. doi: 10.1016/s0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- Gray NW, Weimer RM, Bureau I, Svoboda K. Rapid redistribution of synaptic PSD-95 in the neocortex in vivo. PLoS Biol. 2006;4:e370. doi: 10.1371/journal.pbio.0040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1989;9:2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Lebel E, Roy H, Sik A, Schulman H, Waxham MN, De Koninck P. A mechanism for Ca2+/calmodulin-dependent protein kinase II clustering at synaptic and nonsynaptic sites based on self-association. J Neurosci. 2005;25:6971–6983. doi: 10.1523/JNEUROSCI.4698-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Fukunaga K, Muller D. Calcium/calmodulin-dependent protein kinase II contributes to activity-dependent filopodia growth and spine formation. J Neurosci. 2003;23:10645–10649. doi: 10.1523/JNEUROSCI.23-33-10645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MB. Signal transduction molecules at the glutamatergic postsynaptic membrane. Brain Res Brain Res Rev. 1998;26:243–257. doi: 10.1016/s0165-0173(97)00043-x. [DOI] [PubMed] [Google Scholar]
- Lin B, Kramar EA, Bi X, Brucher FA, Gall CM, Lynch G. Theta stimulation polymerizes actin in dendritic spines of hippocampus. J Neurosci. 2005;25:2062–2069. doi: 10.1523/JNEUROSCI.4283-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioral memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill MA, Chen Y, Strack S, Hell JW. Activity-driven postsynaptic translocation of CaMKII. Trends Pharmacol Sci. 2005;26:645–653. doi: 10.1016/j.tips.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Meyer T, Shen K. In and out of the postsynaptic region: signalling proteins on the move. Trends Cell Biol. 2000;10:238–244. doi: 10.1016/s0962-8924(00)01764-5. [DOI] [PubMed] [Google Scholar]
- Narayanan R, Okamoto K, Moroto K, Hayashi Y. CaMKII is an actin bundling protein that stabilizes the dendritic spine cytoskeleton. Soc Neurosci Abstr. 2006;32:134–134. [Google Scholar]
- Nusser Z, Lujan R, Laube G, Roberts JD, Molnar E, Somogyi P. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron. 1998;21:545–559. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- Ostroff LE, Fiala JC, Allwardt B, Harris KM. Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron. 2002;35:535–545. doi: 10.1016/s0896-6273(02)00785-7. [DOI] [PubMed] [Google Scholar]
- Otmakhov N, Tao-Cheng JH, Carpenter S, Asrican B, Dosemeci A, Reese TS, Lisman J. Persistent accumulation of calcium/calmodulin-dependent protein kinase II in dendritic spines after induction of NMDA receptor-dependent chemical long-term potentiation. J Neurosci. 2004;24:9324–9331. doi: 10.1523/JNEUROSCI.2350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racca C, Stephenson FA, Streit P, Roberts JD, Somogyi P. NMDA receptor content of synapses in stratum radiatum of the hippocampal CA1 area. J Neurosci. 2000;20:2512–2522. doi: 10.1523/JNEUROSCI.20-07-02512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ, Bass MA, Jiao Y, Macmillan LB, Carmody LC, Bartlett RK, Colbran RJ. Multivalent interactions of calcium/calmodulin-dependent protein kinase II with the postsynaptic density proteins NR2B, densin-180 and alpha -actinin-2. J Biol Chem. 2005;280:35329–35336. doi: 10.1074/jbc.M502191200. [DOI] [PubMed] [Google Scholar]
- Rongo C, Kaplan JM. CaMKII regulates the density of central glutamatergic synapses in vivo. Nature. 1999;402:195–199. doi: 10.1038/46065. [DOI] [PubMed] [Google Scholar]
- Sanhueza M, McIntyre CC, Lisman JE. Reversal of synaptic memory by Ca2+/calmodulin-dependent protein kinase II inhibitor. J Neurosci. 2007;27:5190–5199. doi: 10.1523/JNEUROSCI.5049-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman Activity-dependent regulation of calcium/calmodulin-dependent protein kinase II localization. J Neurosci. 2004;24:8399–8403. doi: 10.1523/JNEUROSCI.3606-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. Dendritic spines and long-term plasticity. Nat Rev Neurosci. 2005;6:277–284. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Subramanian K, Meyer T. CaMKIIbeta functions as an F-actin targeting module that localizes CaMKIIalpha/beta heterooligomers to dendritic spines. Neuron. 1998;21:593–606. doi: 10.1016/s0896-6273(00)80569-3. [DOI] [PubMed] [Google Scholar]
- Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16:95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Vest RS, Davies KD, O'Leary H, Port JD, Bayer KU. Dual mechanism of a natural CaMKII inhibitor. Mol Biol Cell. 2007;18:5024–5033. doi: 10.1091/mbc.E07-02-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]