Abstract
Conditioned stimuli are important guides for behavioral actions. This experiment determined the role of the dorsal striatum in conditioned-stimulus modulation of instrumental responding using the pavlovian-instrumental transfer (PIT) paradigm. Rats received pavlovian training wherein two different auditory stimuli predicted the delivery of two food rewards. Next, rats were trained to perform two instrumental actions earning the same two rewards. Finally, the impact of pavlovian stimuli on instrumental performance was assessed in extinction: the stimuli were periodically presented while rats were free to perform the lever-press response. Before testing, medial or lateral dorsal striatum was infused with saline or bacolfen/muscimol, to temporarily inactivate the region. Under saline, outcome-selective PIT was observed: presentation of a stimulus paired with the same outcome as the instrumental action elevated responding, whereas presentation of a stimulus paired with a different outcome did not. Inactivation of the dorsolateral striatum dramatically reduced this effect. Inactivation of the dorsomedial striatum left intact the ability of reward-related stimuli to invigorate responding; however, the selectivity of the stimulus effect was lost (i.e., both stimuli excited responding). These results indicate that subregions of the dorsal striatum play distinct roles in stimulus modulation of instrumental performance with the lateral region being vital for reward-related stimuli to excite responding and the medial region being involved in the integration of stimulus-reward associations with specific response-outcome associations to produce selective responding. These findings identify new roles for the dorsal striatum in mediating the incentive effects of reward-predictive stimuli on behavioral actions made to obtain reward.
Keywords: pavlovian conditioning, instrumental learning, S–R learning, habit, goal-directed, pavlovian instrumental transfer, rat
Introduction
Environmental stimuli carry important predictive information about the availability of required commodities or potential dangers and, therefore, powerfully influence the initiation or vigor of behavioral responses instrumental in gaining access to desired outcomes. Knowledge of the neural mechanisms controlling stimulus influences on response selection and performance is important for understanding decision-making processes in general, and for providing insight into maladaptive cases, such as the control of drug-seeking behaviors by stimuli. Indeed, drug-paired stimuli are thought to play an important role in eliciting craving, supporting compulsive drug use, or precipitating relapse (O'Brien et al. 1998; Volkow et al. 2006), and may play an increasing role in directing performance as it becomes more automatic or habitual in nature (Everitt and Robbins, 2005; Yin and Knowlton, 2006).
The pavlovian-instrumental transfer (PIT) paradigm examines the modulatory role of reward cues on performance of independently acquired reward-seeking behaviors. In this paradigm, subjects receive both pavlovian training wherein discrete stimuli are paired with reward delivery, and instrumental training wherein performance of a particular response earns reward. In the test phase, the impact of the previously trained stimuli on instrumental performance is assessed. Results generally show that presentation of reward-predictive stimuli elevates instrumental responses directed toward that same reward (Corbit and Balleine, 2005). This outcome-selective PIT must involve the integration of multiple associations including those between response and outcome, and stimulus and outcome, as well as an evaluation of the congruence of the information carried in these associations. As such, it is not surprising that multiple neural structures contribute to the generation of PIT effects (Corbit and Balleine, 2005; Corbit et al., 2001; Hall et al., 2001; Murschall and Hauber, 2006).
Previous findings have emphasized a role for the dorsal striatum in the acquisition of stimulus-reward (Aosaki et al. 1994) and stimulus–response associations (Jog et al., 1999; Brasted and Wise, 2004; Yin et al., 2004; Barnes et al., 2005) and in stimulus-guided performance (Kawagoe et al., 1998; Tremblay et al., 1998; Adams et al., 2001; Hassani et al., 2001; Cromwell and Schultz, 2003; Samejima et al., 2005; Bailey and Mair, 2006). These findings suggest that conditioned stimuli may direct instrumental behavior through dorsal striatal circuitry. In all of these situations, animals learn the relationships among stimuli, responses, and rewards in the same sessions. In contrast, in PIT, the conditioned stimuli have never been experienced during the instrumental conditioning sessions and, thus, at test, subjects are responding to a novel situation. The aim of the current study was to test the hypothesis that the dorsal striatum is critically involved in the ability of pavlovian conditioned stimuli to trigger or invigorate instrumental responding, using the PIT procedure.
Materials and Methods
Subjects and apparatus.
Sixteen naive male Long–Evans rats (Harlan, Indianapolis, IN) weighing ∼350 g were singly housed and had ad libitum access to food and water in the home cage. All procedures were approved by the Institutional Animal Care and Use Committee of the Ernest Gallo Clinic and Research Center. Training and testing took place in Med Associates (East Fairfield, VT) operant chambers described previously (Corbit and Janak, 2007).
Pavlovian training.
Two auditory stimuli [white noise (N) and clicker (C)] served as conditioned stimuli (CSs). One of these was paired with delivery of a 5% sucrose solution (weight/volume) whereas the other stimulus was paired with a 5% polycose solution (with 0.9% NaCl; w/v), counterbalanced across subjects. Six 2 min presentations of each stimulus were given in each session in random order interspersed with intertrial intervals averaging 5 min in duration. During each stimulus, 0.1 ml of the appropriate outcome (sucrose or polycose) was delivered on a random time 30 s schedule. Rats received 10 sessions, 75 min in length. The number of magazine entries during each stimulus and in a 2 min prestimulus interval was measured.
Instrumental training.
Rats were next trained to respond on the two levers to self-administer sucrose or polycose (counterbalanced), with each lever-response trained independently on alternating days in 60 min sessions. For the first two sessions per outcome, responding was reinforced on a continuous reinforcement schedule, followed by two additional sessions per outcome under a random ratio 2 (RR2) schedule.
Surgery.
Rats were assigned to the lateral or medial group in an attempt to equate baseline instrumental response rates for the two groups, with eight subjects per group. Stereotaxic surgery was conducted under isoflorane anesthesia to implant 26 gauge guide cannulas (Plastics One, Roanoke, VA) targeted at either the dorsolateral striatum [DLS; anteroposterior (AP) +1.2 mm, mediolateral (ML) ±3.4 mm, dorsoventral (DV) 1.0 mm, coordinates relative to bregma, and dura for DV] or dorsomedial striatum (DMS; AP +1.2 mm, ML ±1.5 mm, DV −1.4 mm). The tips of the guide cannulas were positioned 3 mm dorsal to the intended infusion site and anchored with machine screws and dental acrylic.
Retraining.
Ten days after surgery, rats received one session of training under the RR2 schedule for each outcome, and then were shifted to a RR4 schedule for an additional three sessions per outcome. Rats had one additional session of pavlovian training before testing.
Pavlovian-instrumental transfer test.
Subjects received two pairs of extinction tests. During each test, one lever was available and each stimulus was presented twice interspersed with intervals of no stimulus (Ø). The 22 min test contained eight 2 min bins (two white noise trials and two clicker trials alternated with four Ø trials in the following order: N, C, C, N). Each stimulus bin was separated from the subsequent baseline (Ø) bin by 1 min.
Infusions.
For each pair of transfer tests, half of the animals from each group received infusions of a combination of the GABAB receptor agonist, baclofen, and the GABAA receptor agonist, muscimol (BAC/MUS; 1.0/0.1 mm; Sigma, St Louis, MO), or saline vehicle via infusion cannulas (33 gauge; Plastics One) extending 3 mm below the guide cannula tip (0.3 μl per min/total volume of 0.3 μl delivered per hemisphere) 10 min before test. Infusions took place over 1 min and the cannulas were left in place for an additional 2 min to allow for diffusion.
Control tests.
Animals received an infusion of either BAC/MUS (1.0/0.1 mm; 0.3 μl) or saline (order counterbalanced) 10 min before the beginning of an instrumental or pavlovain test session. For the instrumental test, lever pressing delivered the reward appropriate for the available lever as in training. For the pavlovian test, entries to the magazine were measured during stimulus intervals and prestimulus intervals of equal length in rewarded sessions identical to the original pavlovian training sessions.
Histology.
Coronal sections (50 μm) of formalin-fixed tissue were sliced, mounted, and stained with thionin, to allow verification of injector placement and assessment of any extraneous damage.
Data Analysis.
Data were analyzed using repeated-measures ANOVA. Paired t tests were used to further assess significant main effects and interactions. Preliminary ANOVAs indicated no effect either of stimulus or of outcome type for both pavlovian and instrumental training (F values < 1); therefore, the data were collapsed across those factors.
Results
Histology
Figure 1 displays the placement of the injector tips; based on these placements, all subjects were included in the behavioral analysis.
Figure 1.
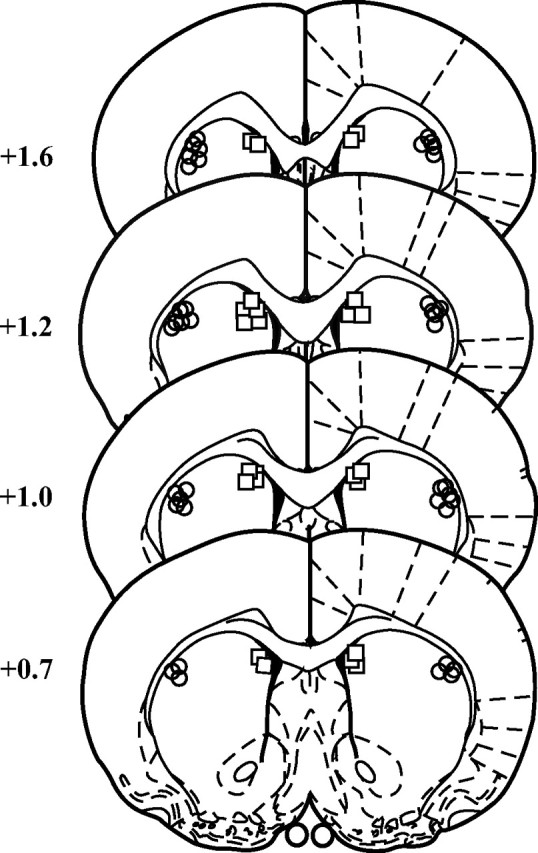
Schematic representation of the injection cannulas placements in coronal sections (Paxinos and Watson, 1998). The location of the injector tips is represented by circles for the DLS group and by squares for the DMS group. Numbers indicate the distance anterior to bregma in millimeters.
Pavlovian training
On the final training day rats assigned to the lateral group made an average of 85 (±16.6 SEM) entries to the magazine during the stimuli and 11 (±2.9) during the prestimulus interval, and rats assigned to the medial group made an average of 94 (±9.9) entries during the stimuli and 14 (±3.7) during the prestimulus interval. ANOVA revealed no effect of group (F(1,14) = 0.26; p > 0.05), but there was a main effect of interval (F(1,14) = 84.4; p < 0.01), confirming that the rats made more entries during the stimuli than in the prestimulus interval. Furthermore, there was no interaction between these factors (F < 1).
Instrumental training
On the final day of training, rats in the lateral group made an average of 102 (±17.2) lever presses whereas rats in the medial group made an average of 173 (±35.2) lever presses. This numeric difference failed to reach significance (F(1,14) = 3.37; p > 0.05).
Effect of inactivation of the dorsolateral striatum on pavlovian-instrumental transfer
As seen in Figure 2A, presentation of the stimulus paired with the same outcome as the available lever enhanced responding relative to the prestimulus interval in subjects treated with saline. This effect was selective; elevation of responding during the same stimulus was greater than that during the different stimulus. Inactivation of the DLS greatly attenuated this transfer effect. The ANOVA revealed a significant excitatory effect of stimulus presentation, such that stimulus presentation elevated responding relative to baseline (F(2,14) = 10.8; p < 0.01). Additionally, there was a significant effect of inactivation (F(1,7) = 7.2; p < 0.05), as well as a significant interaction between these factors (F(2,14) = 6.4; p < 0.05). Post hoc analyses show that after saline, responding was elevated during the Same stimulus compared either to the prestimulus interval (t(7) = 4.1; p < 0.01) or to the Different stimulus (t(7) = 2.7; p < 0.05). After BAC/MUS infusion, presentation of the Same stimulus elevated responding relative to baseline (t(7) = 2.6; p < 0.05), but not relative to the Different stimulus (t(7) = 1.2; p > 0.05). The clearest evidence for a reduction in PIT comes from the observation that the impact of the Same stimulus was greatly reduced after BAC/MUS infusion relative to the saline condition (t(7)3.2; p < 0.01). Presentation of the Different stimulus produced a numerically small but significant increase in responding after saline (t(7) = 2.6; p < 0.05), but not inactivation (t(7)1.5; p > 0.05). Finally, there was no difference between saline and BAC/MUS treatments in responding during the Different stimulus or during the baseline period (Different, t(7) = 1.1, p > 0.05; prestimulus, t(7) = 1.1, p > 0.05), suggesting that DLS inactivation selectively reduced the impact of stimulus presentation without producing a general response impairment.
Figure 2.
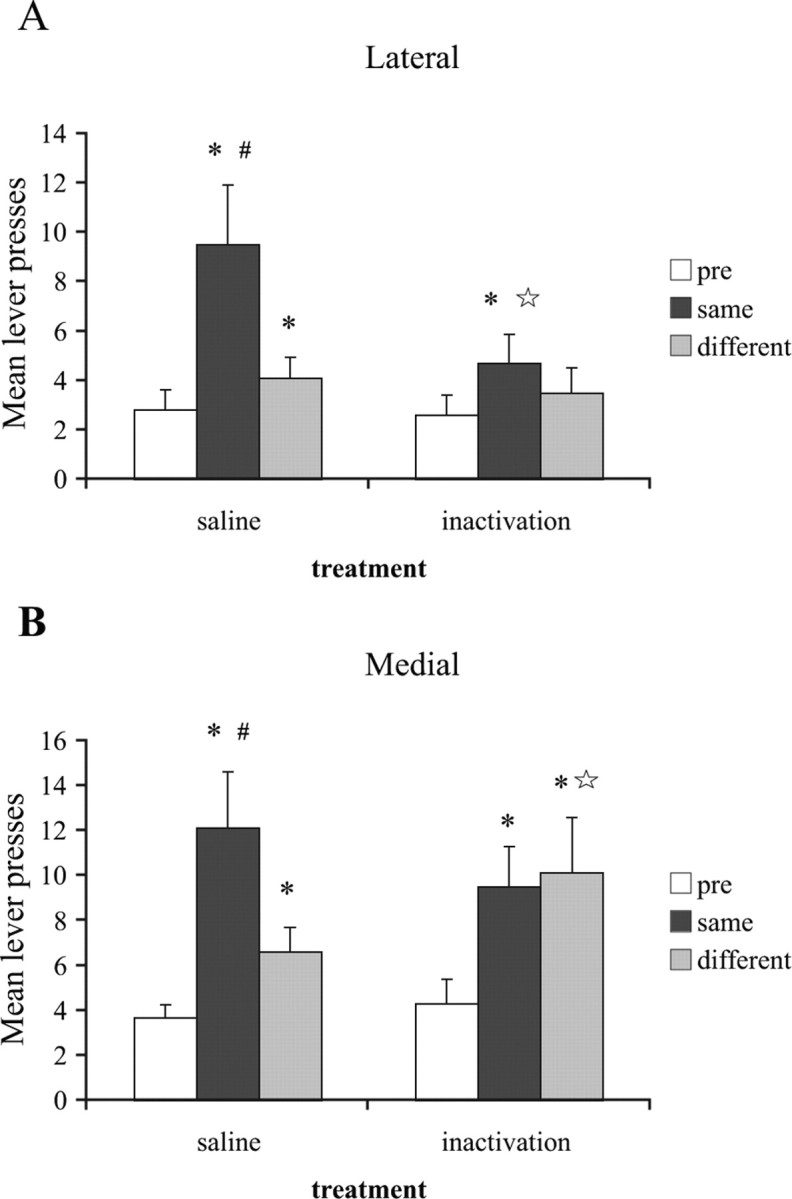
Effects of inactivation of the DLS and the DMS on lever-press responding during a pavlovian stimulus within the pavlovian-instrumental transfer procedure. For all figures, “pre” represents the baseline instrumental responding in the absence of any stimuli, “same” represents responding during presentation of the stimulus paired with the same outcome as the available lever, and “different” represents responding during the stimulus that was paired with the outcome earned by the alternate, currently unavailable lever. A, Mean (±SEM) lever press responding after inactivation of the DLS. After saline infusions, responding was elevated during the Same and Different stimuli relative to baseline and the impact of the same stimulus was greater than that of the Different stimulus. After BAC/MUS infusion, the enhancement of responding by presentation of the Same stimulus was greatly reduced. B, Mean (±SEM) lever press responding after inactivation of the DMS. After saline infusions, responding was elevated during the Same and Different stimuli relative to baseline and the impact of the Same stimulus was greater than that of the Different stimulus. After BAC/MUS infusion, responding was elevated during both the Same and Different stimuli compared with baseline and responding during these stimuli did not differ. n = 8 per group. Please refer to the text for complete statistical analyses. *Difference between a stimulus and baseline (p < 0.05); #difference between the Same and Different stimuli; ☆difference between saline and inactivation conditions (p < 0.05).
Effect of inactivation of the dorsomedial striatum on pavlovian-instrumental transfer
In contrast to the effects of inactivation of the DLS, inactivation of the medial region of this structure left the excitatory impact of stimulus presentation intact, but the selectivity of this effect was eliminated (Fig. 2B). ANOVA revealed a significant effect of stimulus presentation (F(2,14) = 7.7; p < 0.01) and a significant stimulus by inactivation interaction (F(2,14) = 5.2; p < 0.05), but no main effect of inactivation (F(1,7) = 0.2; p > 0.05). Post hoc analyses confirm that presentation of the stimulus paired with the same outcome as the available lever elevated performance in both the saline (t(7) = 3.3; p < 0.05) and BAC/MUS (t(7) = 3.0; p < 0.05) conditions, and the excitatory impact of the Same stimulus was equivalent after these infusions (t(7) = 1.5; p > 0.05). Importantly, although responding during the Same stimulus was higher than responding during the Different stimulus after saline (t(7) = 2.6; p < 0.05), there was no difference in the excitatory impact of the Same and Different stimuli after BAC/MUS infusion (t(7) = 0.3; p > 0.05). Under saline, presentation of the Different stimulus produced a small but significant increase in responding relative to baseline (t(7) = 2.7; p < 0.05). After BAC/MUS infusion, presentation of the Different stimulus again elevated responding relative to baseline (t(7) = 3.0; p < 0.05) and produced an effect that was similar to that seen with the Same stimulus (t(7) = 0.3; p > 0.05). The impact of the Different stimulus showed a marginal increase under the inactivation condition relative to saline (t(7) = 2.3; p = 0.05), suggesting that inactivation of the DMS acted to disinhibit the impact of this stimulus. Finally, baseline responding was not affected by MUS/BAC infusion (t(7) = 0.9; p > 0.05).
Effect of inactivation on reinforced instrumental responding
There was no difference in reinforced instrumental responding under saline compared with BAC/MUS treatment in DLS-infused (t(7) = 0.2; p > 0.05), or DMS-infused (t(7) = 0.004; p > 0.05) subjects (Table 1), suggesting that the impairment observed in the transfer tests does not reflect a general deficit in instrumental performance.
Table 1.
Instrumental control session
| Infusion |
||
|---|---|---|
| saline | inactivation | |
| DLS | 225 (64) | 211 (60) |
| DMS | 427 (74) | 428 (78) |
Mean (± SEM) lever presses in reinforced instrumental sessions following infusion of saline or BAC/MUS into the DLS or the MLS. There were no significant treatment effects.
Effect of inactivation on reinforced pavlovian responding
Pavlovian performance of the DLS group was similar after either saline or BAC/MUS infusion (Table 2), with a significant effect of stimulus (prestimulus vs stimulus, F(1,7) = 30.7, p < 0.01), but no effect of treatment and no interaction (F values < 1). Pavlovian performance by the DMS group also was not impaired by BAC/MUS infusion: there was a significant effect of stimulus (F(1,7) = 174.3; p < 0.01), but no effect of treatment (F(2,14) = 2.4; p > 0.05) and no interaction (F < 1). These findings demonstrate that inactivation of the striatum does not interfere with the rats' ability to detect or respond to the stimuli in the situation in which they were trained, pointing rather to an inability of the stimuli to impact an independently trained instrumental response.
Table 2.
Pavlovian control session
| Infusion |
||
|---|---|---|
| saline | inactivation | |
| DLS | ||
| pre-CS | 10 (5) | 15 (6) |
| CS | 140 (45) | 143 (46) |
| DMS | ||
| pre-CS | 18 (7) | 50 (25) |
| CS | 225 (71) | 263 (22) |
Mean (± SEM) magazine entries in reinforced Pavlovian sessions following infusion of saline or BAC/MUS into the DLS or the MLS. There were no significant treatment effects.
Discussion
These data demonstrate that neural activity within the dorsal striatum is importantly involved in stimulus modulation of goal-seeking behaviors, and that the lateral and medial regions of this structure control different aspects of the ability of a stimulus to enhance instrumental performance. Specifically, the DLS is required for the incentive effects of pavlovian stimuli to alter responding, whereas the DMS is required for the incentive effects of those stimuli to be outcome-selective. These findings reveal new roles for the dorsal striatum in the control of goal-seeking behavior by pavlovian conditioned stimuli.
We found that pharmacological inactivation of the DLS before testing greatly attenuated the excitatory effect of a conditioned stimulus on instrumental responding. This effect is not explained by a basic deficit in instrumental or pavlovian performance as inactivation failed to affect performance in control tests. These data show that activity within the DLS is necessary for pavlovian reward-related stimuli to modulate, in this case enhance, goal-directed actions. Our results add to previous findings indicating a role for the DLS in cocaine-seeking supported by response-contingent cue presentation (Vanderschuren et al., 2005). Hence, the DLS may be critical for reward-seeking triggered by cues that both precede (the present work) and follow (Vanderschuren et al. 2005) the reward-seeking motor response. Our results also indicate that the remaining functional striatal circuits, including the DMS and the ventral striatum, cannot compensate for the absence of the DLS, supporting a critical role for this subregion in the behavioral effects of pavlovian stimuli on reward-seeking.
In contrast, when the DMS was inactivated, presentation of reward-related stimuli retained the ability to enhance instrumental responding but, interestingly, this effect was nonselective. Hence, when the DMS was inactivated before testing, both stimuli had a similar excitatory effect on performance of a given response. Thus, stimuli can still arouse or activate responding when the DMS is temporarily inactivated; however, the lack of selectivity of these effects suggests that DMS inactivation prevents the integration of information about specific response–outcome (R–O) relationships with the specific excitatory information carried by the stimuli. A deficit in using specific R–O associations would be consistent with the previous observation that lesions or inactivation of the DMS impair selective sensitivity to either outcome devaluation or contingency degradation (Yin et al., 2005). However, it is important to note that these deficits were observed only for placements within the DMS caudal to those used in the current study. The reason for this is not immediately clear. Whereas cortical projections to the striatum show a mediolateral topographic organization, these afferents project to extended longitudinal regions of the striatum and so inputs from cortical regions involved in R–O learning are widely overlapping across these regions (McGeorge and Faull, 1989). Alternatively, the lack of selectivity could be explained if the anterior DMS targeted in the current study is important for processing information about the specific sensory properties of different stimuli, or stimulus–outcome (S–O) associations, and ultimately the ability of these sensory events to impact performance. The basolateral amygdala, a region previously demonstrated to be critical for outcome-specific transfer effects (Corbit and Balleine, 2005) is reported to project broadly through the rat striatum excluding only the dorsolateral quadrant of this structure (Kelley et al., 1982). Functional loss of this pathway could account for the observed lack of stimulus selectivity while the excitatory impact of the stimuli remained intact.
Our findings suggest that different circuits within the DS control the cuing versus activating components of stimulus effects on responding (Corbit and Balleine, 2005), with the DMS mediating the specific cuing effects, and the DLS playing a more fundamental, perhaps permissive, role in allowing excitatory stimuli to impact response output. In considering the differences between the effects of inactivating the DMS versus the DLS, the question arises as to how stimulus-reward associations access the associative structure that controls response output more generally. That is, although DMS inactivation eliminates the selectivity of the transfer effect, stimulus presentation still activates performance, suggesting that at least the excitatory information associated with the stimulus retains access to behavioral output even if the sensory-specific properties do not. In contrast, the lack of transfer observed after DLS inactivation could mean that this structure is a critical part of the pathway through which excitatory stimuli activate performance by increasing appetitive arousal generally, in which case, many stimulus-related behaviors should be expected to be affected by the inactivation. The lack of an effect of DLS inactivation on magazine entries in the pavlovian control test suggests that the ability of the stimuli to activate at least some behaviors remains intact. As such, the role of the DLS may be more specific relating in particular to the integration of excitatory information carried by the stimuli with the neural control of particular responses. This finding is consistent with previous demonstrations that the DLS contributes to performance of only some classes of learned behavior (Han et al. 1997). In our view, it is likely that some or all of the excitatory effects of the conditioned stimuli observed here depend on dopaminergic inputs from the substantia nigra pars compacta (SNc). Indeed, it has been demonstrated previously that even unilateral lesions of the SNc are sufficient to attenuate PIT (El-Amamy and Holland, 2007). These topographically organized inputs (Beckstead et al., 1979) may provide the neural basis for enhanced instrumental output by the dorsal striatum congruent with previous ideas regarding incentive salience attribution to conditioned stimuli and their consequent behavior-activating properties (Berridge, 2007; Robbins and Everitt, 2007). Together, the differences observed after inactivation of the lateral versus medial dorsal striatum likely relate to the information processed by the different cortical and subcortical networks projecting to these regions.
It is important to note that PIT generates a novel situation; before testing, the rats have never had the opportunity to lever-press in the presence of the stimuli. Therefore, the facilitatory effects likely reflect the integration of previously acquired S–O and R–O associations, as an S–R association, at least between the pavlovian stimuli and the lever response, has not had an opportunity to develop. Hence, these results suggest a larger function for the dorsal striatum, especially its lateral aspect, than current proposed roles in S–R or habit learning. This is consistent with the prevalence throughout the striatum of phasic neural responses to reward and reward-predictive stimuli, and the modulation of response-related neural activity by reward, in electrophysiological studies (Schultz, 2000). In particular, electrophysiological studies find that the striatum, including its dorsal regions, encodes attributes of rewards, a finding in contrast to the view that DLS-mediated behavior, and by extension, habitual behavior, is relatively insensitive to momentary changes in reward value. For example, neural activity within the dorsal striatum reflects the expectation of particular rewards that may differ in magnitude, probability, or identity (Kawagoe et al., 1998; Hassani et al., 2001; Lauwereyns et al., 2002; Cromwell and Schultz, 2003; Brasted and Wise, 2004). Indeed, in the primate, neural activity across the medial-lateral extent of the striatum reflects the influence of reward expectation on response encoding (Samejima et al., 2005). Together, these findings suggest that the role of the dorsal striatum in stimulus-directed responding is more complex than simply mediating the formation and/or implementation of S–R learning and that this region may also contribute to some aspects of goal-directed performance.
Footnotes
This work was supported by funds from the state of California for medical research on alcohol and substance abuse through the University of California, San Francisco.
References
- Adams S, Kesner RP, Ragozzino ME. Role of the medial and lateral caudate-putamen in mediating an auditory conditional response association. Neurobiol Learn Mem. 2001;76:106–116. doi: 10.1006/nlme.2000.3989. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Graybiel AM, Kimura M. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 1994;265:412–415. doi: 10.1126/science.8023166. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Mair RG. The role of striatum in initiation and execution of learned action sequences in rats. J Neurosci. 2006;26:1016–1025. doi: 10.1523/JNEUROSCI.3883-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJ. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Brasted PJ, Wise SP. Comparison of learning-related neuronal activity in the dorsal premotor cortex and striatum. Eur J Neurosci. 2004;19:721–740. doi: 10.1111/j.0953-816x.2003.03181.x. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Balleine BW. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J Neurosci. 2005;25:962–970. doi: 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH. Ethanol-associated cues produce general pavlovian-instrumental transfer. Alcohol Clin Exp Res. 2007;31:766–774. doi: 10.1111/j.1530-0277.2007.00359.x. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J Neurosci. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell HC, Schultz W. Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J Neurophysiol. 2003;89:2823–2838. doi: 10.1152/jn.01014.2002. [DOI] [PubMed] [Google Scholar]
- El-Amamy H, Holland PC. Dissociable effects of disconnecting amygdala central nucleus from the ventral tegmental area or substantia nigra on learned orienting and incentive motivation. Eur J Neurosci. 2007;25:1557–1567. doi: 10.1111/j.1460-9568.2007.05402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating pavlovian influences on instrumental behaviour. Eur J Neurosci. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- Han JS, McMahan RW, Holland PC, Gallagher M. The role of an amygdalo-striatal pathway in associative learning. J Neurosci. 1997;17:3913–3919. doi: 10.1523/JNEUROSCI.17-10-03913.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, Cromwell HC, Schultz W. Influence of expectation of different rewards on behavior-related neuronal activity in the striatum. J Neurophysiol. 2001;85:2477–2489. doi: 10.1152/jn.2001.85.6.2477. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nat Neurosci. 1998;1:411–416. doi: 10.1038/1625. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJ. The amygdaostriatal projection in the rat -An anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Lauwereyns J, Watanabe K, Coe B, Hikosaka O. A neural correlate of response bias in monkey caudate nucleus. Nature. 2002;418:413–417. doi: 10.1038/nature00892. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Murschall A, Hauber W. Inactivation of the ventral tegmental area abolished the general excitatory influence of pavlovian cues on instrumental performance. Learn Mem. 2006;13:123–126. doi: 10.1101/lm.127106. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 2. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. A role for mesencephalic dopamine in activation: commentary on Berridge (2006) Psychopharmacology (Berl) 2007;191:433–437. doi: 10.1007/s00213-006-0528-7. [DOI] [PubMed] [Google Scholar]
- Samejima K, Ueda Y, Doya K, Kimura M. Representation of action-specific reward values in the striatum. Science. 2005;310:1337–1340. doi: 10.1126/science.1115270. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Hollerman JR, Schultz W. Modifications of reward expectation-related neuronal activity during learning in primate striatum. J Neurophysiol. 1998;80:964–977. doi: 10.1152/jn.1998.80.2.964. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]


