Abstract
Apolipoprotein E (apoE)-containing lipoproteins (LPs) are secreted by glia and play important roles in lipid homeostasis in the CNS. Glia-derived LPs also promote synaptogenesis and stimulate axon growth of CNS neurons. Here, we provide evidence that glia-derived LPs protect CNS neurons from apoptosis by a receptor-mediated signaling pathway. The protective effect was greater for apolipoprotein E3 than for apolipoprotein E4, the expression of which is a risk factor for Alzheimer's disease. The anti-apoptotic effect of LPs required the association of apolipoprotein E with lipids but did not require cholesterol. Apoptosis was not prevented by lipids alone or by apoA1- or apoJ-containing lipoproteins. The prevention of neuronal apoptosis was initiated after the binding of LPs to the low-density lipoprotein receptor-related protein (LRP), a multifunctional receptor of the low-density lipoprotein receptor family. We showed that inhibition of LRP activation, by treatment of neurons with receptor-associated protein or anti-LRP antibodies, or by LRP gene-silencing experiments, reduced the protective effect of LPs. Furthermore, another LRP ligand, α2-macroglobulin, also protected the neurons from apoptosis. After binding to LRP, LPs initiate a signaling pathway that involves activation of protein kinase Cδ and inactivation of glycogen synthase kinase-3β. These findings indicate the potential for using glial lipoproteins or an activator of the LRP signaling pathway for treatment for neurodegenerative disorders such as Alzheimer's disease.
Keywords: apolipoprotein E, apoptosis, glia, lipoproteins, low-density lipoprotein receptor-related protein, glycogen synthase kinase-3β
Introduction
Cholesterol is highly enriched in the brain compared with peripheral tissues, and almost all cholesterol in the brain is synthesized in situ in the CNS (Dietschy and Turley, 2004). Although the delivery of plasma lipoproteins from the circulation to the CNS is precluded by the blood–brain barrier, the CNS contains a distinct population of high-density lipoproteins that are secreted by glia and contain apolipoprotein E (apoE) as their major apolipoprotein (Han, 2004). ApoE is thought to play an important role in the CNS, because apoE synthesis increases dramatically after injury of either the CNS or peripheral nervous system (Ignatius et al., 1986; Mahley, 1988). In addition, neurodegeneration is increased by apoE deficiency during aging (Masliah et al., 1995). Furthermore, the ε4 allele of apoE is the strongest known genetic risk factor for development of late-onset Alzheimer's disease (Strittmatter et al., 1993). Based on these and other observations, apoE has been proposed to contribute to the repair and/or protection of neurons from injury, although the mechanisms involved are unknown. ApoE is a ligand for receptors of the low-density lipoprotein receptor (LDLR) superfamily, several of which act both as endocytic receptors (Brown and Goldstein, 1976) and signaling receptors (Herz and Bock, 2002). Some of these receptors, including the multifunctional LDLR-related protein (LRP), are expressed in neurons in the CNS (Zhuo et al., 2000).
In addition to providing nutrient support for neurons, glial cells are thought to deliver apoE-containing lipoproteins (LPs) to neurons and to play an active role in synaptogenesis (Mauch et al., 2001) and axon growth (Hayashi et al., 2004). A loss of neurons by apoptosis is characteristic of many neurodegenerative disorders such as Alzheimer's disease, cerebral ischemia, and Niemann–Pick type C disease. In this study, we provide the first evidence that glial LPs protect CNS neurons from apoptosis and that the protection is mediated by an LRP-mediated signaling pathway in which protein kinase Cδ (PKCδ) is activated and the proapoptotic kinase glycogen synthase kinase-3β (GSK3β) (Linseman et al., 2004) is inactivated.
Materials and Methods
Materials.
A rabbit polyclonal antibody (R2629) directed against human LRP was generously provided by Dr. D. K. Strickland (University of Maryland School of Medicine, Baltimore MD) (Kounnas et al., 1993). Rabbit polyclonal antibodies directed against the rat LDLR were provided by Dr. G. C. Ness (University of South Florida, Tampa, FL). A cDNA construct encoding human receptor-associated protein (RAP) as a glutathione S-transferase (GST) fusion protein was a gift from Dr. Z. Yao (University of Ottawa Heart Institute, Ottawa, Ontario, Canada). cDNAs encoding human apoE3 and apoE4, as glutathione S-transferase fusion proteins, were generously provided by Dr. C. L. Wellington (University of British Columbia, Vancouver, British Columbia, Canada). Recombinant apoE3 and apoE4 were purified using a GSTrap FF column and Precision Protease (GE Healthcare, Uppsala, Sweden). Recombinant human apoE3 (used for the experiments depicted in Fig. 2 A) was purchased from Panvera (Madison, WI). RAP was purified using a GSTrap FF column, thrombin, and a HiTrap Benzamidine FF column (GE Healthcare). Human apoA1 was prepared from human plasma (Choi et al., 2003) and was provided by Dr. G. A. Francis (University of Alberta, Edmonton, Alberta, Canada). A colony of ApoE −/− mice was established at the University of Alberta from mice obtained from The Jackson Laboratory (Bar Harbor, ME). α2-Macroglobulin was purchased from Sigma (St. Louis, MO).
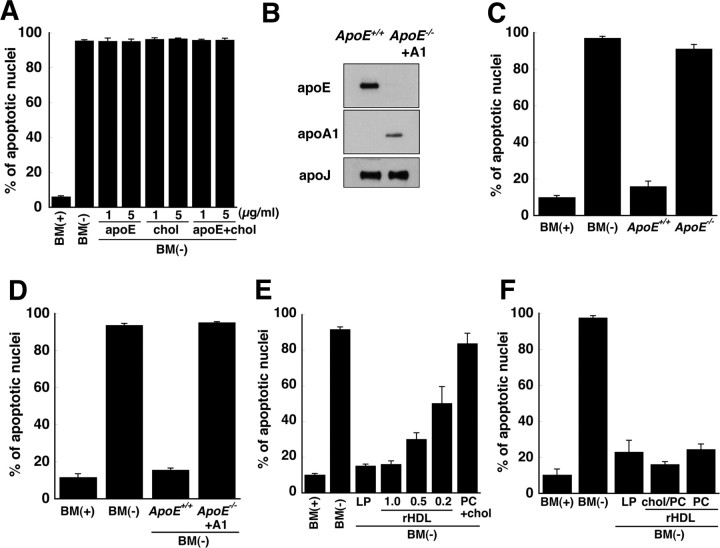
Figure 2.
ApoE-containing lipoproteins, but not apoE alone, cholesterol alone, or apoA1/apoJ-containing lipoproteins, prevent apoptosis of RGCs. A, Recombinant human apoE3 and/or cholesterol (5 and 1 μg/ml, respectively) were added to BM(−) and incubated with RGCs for 24 h. Control cultures were given BM(−) or BM(+). The number of apoptotic neurons as a percentage of all neurons was determined by Hoechst staining. B, Immunoblot of apoE, apoA1, and apoJ in lipoproteins isolated by ultracentrifugation from cultures of ApoE +/+ and ApoE −/− glia. ApoA1-containing lipoproteins (+A1) were generated by addition of human apoA1 to glia isolated from ApoE −/− mice. C, Apoptosis was assessed in RGCs incubated in BM(−) supplemented with lipoproteins (120 μg of protein/ml) isolated from glial cultures from ApoE +/+ and ApoE −/− mice. D, BM(−) was supplemented with apoE-containing lipoproteins (ApoE +/+) or apoA1-containing lipoproteins (ApoE −/− + A1) so that the concentration of cholesterol in the medium was 1 μg/ml. E, rHDLs that contained apoE, phosphatidylcholine (PC), and cholesterol (molar ratio, 1:100:10) were prepared. BM(−) was supplemented with rHDLs so that the amount of apoE was the same as (1.0), 50% (0.5), or 20% (0.2) of that in glial LPs. Liposomes lacking apoE, but containing the same amount of phosphatidylcholine and cholesterol as in rHDLs (PC + chol), did not prevent apoptosis. F, RGCs were incubated in BM(−) for 24 h with rHDLs that contained the same amount of apoE as in LPs and either contained (chol/PC) or lacked (PC) cholesterol. For all experiments, fragmented/shrunken nuclei were detected by Hoechst staining and designated as apoptotic cells. Data are means ± SE from three or four independent experiments. chol, Cholesterol.
Isolation and culture of retinal ganglion cells.
Primary cultures of rat retinal ganglion cells (RGCs) were prepared from 2-d-old Sprague Dawley rats according to the procedure of Barres et al. (1988) with minor modifications. The RGCs were suspended in basal medium containing “trophic additives” [defined herein as forskolin (10 μm; Sigma), brain-derived neurotrophic factor (50 ng/ml; PeproTech, Rocky Hill, NJ), ciliary neurotrophic factor (50 ng/ml; PeproTech), basic fibroblast growth factor (PeproTech), and B27 supplement (Invitrogen, Carlsbad, CA)] (Barres et al., 1988). Basal medium contained glutamine (1 mm), insulin (5 μg/ml), N-acetylcysteine (60 μg/ml), progesterone (62 ng/ml), putrescine (16 μg/ml), sodium selenite (40 ng/ml), bovine serum albumin (0.1 mg/ml), triiodothyronine (40 ng/ml), transferrin (0.1 mg/ml), and sodium pyruvate (1 mm) in Neurobasal medium (Invitrogen). RGCs were plated at a density of 5000 cells per well on 96-well culture plates coated with poly-d-lysine (Sigma) and laminin (Sigma) and were cultured for 6–14 d before the start of experiments.
Culture of cortical glial cells.
Glial cells were isolated from the cerebral cortex of 1-d-old rats (Gong et al., 2002) and were maintained in DMEM containing 10% fetal bovine serum. The cell preparation was highly enriched (>90%) in astrocytes (Hayashi et al., 2004).
Isolation of lipoproteins from glia-conditioned medium.
Glial cells were washed three times with PBS. Basal medium (the same as used for culture of RGCs but lacking brain-derived neurotrophic factor, ciliary neurotrophic factor, fibroblast growth factor, forskolin, and B27 supplement) was added for 3 d. Glial cell-conditioned medium was collected and then centrifuged at room temperature for 10 min at 1000 × g. The supernatant, designated glia-conditioned medium, was subjected to ultracentrifugation (Hayashi et al., 2004) on discontinuous sucrose gradients consisting of sucrose solutions of densities 1.30, 1.20, 1.10, and 1.006 g/ml in a Beckman SW40-Ti (Beckman Coulter, Fullerton, CA) rotor at 4°C for 48 h at 160,000 × g. Twelve fractions were collected sequentially from the top of the tube and immunoblotted for apolipoproteins as described below. Fractions of densities 1.07–1.12 g/ml were combined and concentrated on an Amicon (Beverly, MA) Ultra filter (100 kDa molecular weight cutoff; Millipore, Bedford, MA). Cholesterol was extracted from the fractions twice with hexane/isopropanol (3:2, v/v), the solvents were evaporated, and then bis(trimethylsilyl)trifluoroacetamide was added for 20 min at 50°C to generate the trimethylsilyl derivative of cholesterol for analysis by gas–liquid chromatography (Hayashi et al., 2004).
Immunoblotting.
For immunoblotting of signaling molecules, RGCs were harvested on ice in sample buffer containing 62.5 mm Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, and 5% β-mercaptoethanol, 9 h after the induction of apoptosis. For immunoblotting of apolipoproteins, lipoprotein fractions were mixed with an equal volume of twice-concentrated sample buffer. Samples were boiled for 5 min, and proteins were separated by 0.1% SDS-PAGE and then transferred to polyvinylidene difluoride membranes at 65 V for 2 h at 4°C. The membranes were incubated with 5% nonfat milk or 5% bovine serum albumin in 10 mm Tris-HCl, pH 7.4, containing 0.9% NaCl and 0.1% Tween 20 for 1 h at room temperature. The membranes were then incubated overnight at 4°C with primary antibodies in 10 mm Tris, pH 7.4, containing 0.9% NaCl, 0.1% Tween 20, and 5% bovine serum albumin, and subsequently incubated with horseradish peroxidase-conjugated mouse anti-goat IgG (dilution, 1:5000; Pierce Biotechnology, Rockford, IL), horseradish peroxidase-conjugated goat anti-mouse IgG (dilution, 1:2000; Pierce Biotechnology), or horseradish peroxidase-conjugated goat anti-rabbit IgG (dilution, 1:2000; Pierce Biotechnology) for 1 h at room temperature. Immunoreactive proteins were detected by enhanced chemiluminescence (GE Healthcare), Super Signal West Dura (Pierce Biotechnology), or West Femto (Pierce Biotechnology). The following antibodies were used: rabbit anti-rat LDLR (dilution, 1:1000), mouse anti-human lipoprotein receptor 11 (LR11; dilution, 1:1000; BD Biosciences, San Jose, CA), goat anti-human apoE receptor 2 (apoER2; dilution, 1:400; Santa Cruz Biotechnology, Santa Cruz, CA), goat anti-human LRP (dilution, 1:1000; Santa Cruz Biotechnology), rabbit anti-human apoA1 (dilution, 1:10,000; Merck Chemicals, Darmstadt, Germany), goat anti-human apoE (dilution, 1:5000; Biodesign, Saco, ME), goat anti-human apoJ (dilution, 1:2500; Rockland Immunochemicals, Gilbertsville, PA), rabbit anti-human GSK3β and phospho(Ser-9)-GSK3β (dilution, 1:1000; Cell Signaling Technology, Danvers, MA), mouse anti-Drosophila GSK3 and phospho(Tyr279/Tyr216)-GSK3 (dilution, 1:500; Millipore), rabbit anti-mouse signal transducer and activator of transcription 3 (STAT3) and phospho(Tyr-705)-STAT3 (dilution, 1:1000; Cell Signaling Technology), rabbit anti-mouse Akt and phospho(Ser-473)-Akt (dilution, 1:1000; Cell Signaling Technology), rabbit anti-human p38 mitogen-activated protein kinase (MAPK) and phospho(Thr-180/Tyr-182)-p38 MAPK (dilution, 1:1000; Cell Signaling Technology), mouse anti-rat p44/42 MAPK and phospho(Thr-202/Tyr-204)-p44/42 MAPK (dilution, 1:1000; Cell Signaling Technology), rabbit anti-human c-Jun N-terminal protein kinase (JNK) and phospho(Thr-183/Tyr-185)-JNK (dilution, 1:1000; Cell Signaling Technology), and mouse anti-β-actin (dilution, 1:10,000; Sigma).
Induction of apoptosis in RGCs and incubation of RGCs with LPs.
Apoptosis was induced in RGCs by withdrawal of trophic additives that support RGC survival (brain-derived neurotrophic factor, ciliary neurotrophic factor, basic fibroblast growth factor, forskolin, and B27 supplement) from the culture medium. RGCs were washed three times with 150 μl/well basal medium lacking these trophic additives [BM(−)] and then incubated with 100 μl of BM(−) for 2 h at 37°C. In some experiments, as indicated, BM(−) was supplemented with protein kinase inhibitors, antibodies, or RAP. The culture medium was then changed to medium (100 μl/well) that contained the indicated supplements, such as LPs and/or methylamine-activated α2-macroglobulin (100 nm). α2-Macroglobulin was activated by treatment with 100 mm methylamine for 1 h at room temperature (Bacskai et al., 2000).
Detection of apoptotic RGCs with Hoechst dye or annexin V.
For detection of apoptosis, RGCs were stained with Hoechst 33258. The neurons were washed twice with 100 μl of PBS per well and then fixed by incubation for 15 min with 4% paraformaldehyde. Cells were washed with 100 μl of PBS per well, incubated with Hoechst dye (1 μg/ml; Invitrogen) for 15 min at room temperature, and then washed twice with 100 μl/well PBS. Fluorescence was detected using a Leica DM IRE2 fluorescence microscope (Leica Microsystems, Bannockburn, IL). Stained nuclei of RGCs were counted on a monitor connected to the microscope. Neurons containing fragmented or shrunken nuclei were scored as apoptotic. Data indicate the number of apoptotic neurons as a percentage of total number of neurons. For detection of apoptosis with annexin V–fluorescein and propidium iodide, instructions from the manufacturer (Roche Diagnostics, Penzberg, Germany) were followed, and images were viewed with a Leica DM IRE2 fluorescence microscope (Leica Microsystems). Annexin V binds phosphatidylserine that becomes exposed on the outer leaflet of the plasma membrane during the early stages of apoptosis. Propidium iodide is a membrane-impermeable reagent that binds DNA and stains nuclei of necrotic cells and cells in the end stages of apoptosis. Normal living cells do not stain with either reagent, whereas cells in the early stages of apoptosis stain with annexin V–fluorescein but not propidium iodide, and cells in the late stages of apoptosis, necrotic cells, and dead cells stain with both annexin V and propidium iodide.
LRP-1 small interfering RNA transfection.
Control small interfering RNA (siRNA; AllStars Negative siRNA–fluorescein) and two siRNAs (designated LRP #1 and LRP #2, catalog numbers SI01630055 and SI01630062, respectively) specific for LRP-1 were purchased from Qiagen (Mississauga, Ontario, Canada). RGCs that had been cultured for 3–4 d were transfected with 20 pmol of siRNA (control or LRP-1) using the GeneSilencer siRNA Transfection Reagent (Gene Therapy Systems, San Diego, CA) according to the manufacturer's instructions. In brief, siRNA and GeneSilencer were separately diluted in OptiMEM (Invitrogen) and then mixed and incubated for 10 min at room temperature. The mixture was incubated with RGCs for 3 d. Transfected RGCs were collected for immunoblotting or apoptosis experiments, which were performed as described above.
Reconstituted apoE-containing high-density lipoproteins.
Recombinant human apoE3 and apoE4 were prepared as described previously (Mabile et al., 2003; Hirsch-Reinshagen et al., 2004) and reconstituted into high-density lipoproteins (rHDLs) according to Li et al. (2002) and Thuahnai et al. (2001). The molar ratio of 1-palmitoyl-2-oleoyl-glycerophosphocholine:cholesterol:apoE in the reconstituted particles was either 100:10:1 or 100:0:1, as indicated. Briefly, 2.71 mg of 1-palmitoyl-2-oleoyl-glycerophosphocholine (Sigma) with or without 0.14 mg of cholesterol (Sigma) in chloroform was placed in a glass tube, and the solvent was evaporated under a stream of nitrogen. Tris-HCl (400 μl; 10 mm), pH 7.4, containing 0.9% NaCl, was added, and the suspension was incubated for 1 h on ice with vortexing for 30 s every 15 min. Sodium cholate (final concentration, 3 mg/ml) was added, and the mixture was incubated for 2 h on ice, by which time the solution became translucent. Recombinant apoE3 or apoE4 (1 mg) was added, and the mixture was incubated for 1 h on ice. To this solution, we added 100 mg of Bio-Beads (Bio-Rad, Hercules, CA) that had been equilibrated according to manufacturer's instructions in 10 mm Tris-HCl, pH 7.4, containing 0.9% NaCl. The mixture was gently mixed by rotation for 3 h at 4°C. The solution containing the beads was removed with a syringe, filtered using a 0.45 μm sterile filter, and subjected to sucrose density gradient centrifugation as described above for isolation of glia-derived LPs. ApoE-containing fractions (density, 1.07–1.21 g/ml) were collected and combined.
Results
ApoE-containing lipoproteins protect neurons from apoptosis
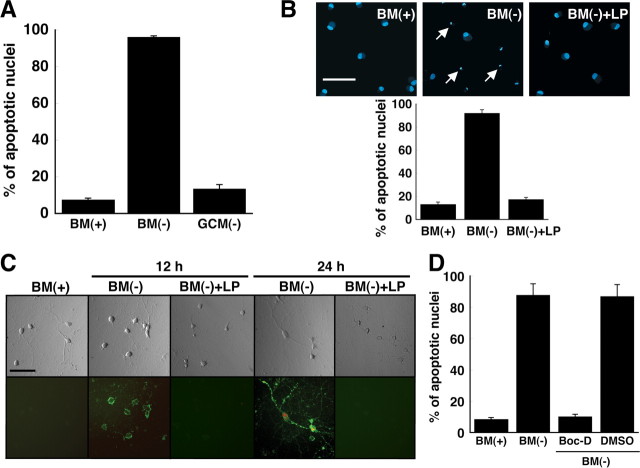
Rat RGCs were chosen for the studies because they are CNS neurons that can be cultured with a high degree of purity. Apoptosis of RGCs was induced by withdrawal of growth supplements (brain-derived neurotrophic factor, ciliary neurotrophic factor, fibroblast growth factor, B27 supplement, and forskolin) from the culture medium. These additives support RGC survival, and we term them collectively trophic additives. Because each of these components contributes to neuronal survival, we investigated neuronal survival after simultaneous elimination of all of these additives from the medium. Twenty-four hours after removal of the trophic additives, Hoechst staining revealed that >90% of the neurons contained shrunken or fragmented nuclei, which are markers of apoptosis (Fig. 1 A,B). Withdrawal of each trophic additive individually from the culture medium did not result in significant numbers of apoptotic nuclei after 24 h (data not shown). Exposure of phosphatidylserine on the surface of the neurons (another marker of apoptosis) 12 h after withdrawal of trophic additives was monitored by the binding of annexin V–fluorescein in the absence of propidium iodide staining (Fig. 1 C). In addition, the broad-spectrum caspase inhibitor Boc-D fmk prevented neuronal death (Fig. 1 D), implying that withdrawal of trophic additives from RGC culture medium induced caspase-dependent apoptosis.
Figure 1.
ApoE-containing glial lipoproteins prevent neuronal apoptosis induced by withdrawal of trophic additives. A, B, Fragmented/shrunken nuclei were detected by Hoechst staining of RGCs after incubation for 24 h in BM(+)] or BM(−). Glia-conditioned medium without trophic additives [GCM(−)] or basal medium without trophic additives but with apoE-containing LPs was added to RGCs. The final cholesterol concentration of LPs in the media was 1 μg/ml. The number of apoptotic neurons was quantified as a percentage of the total number of neurons. Data are means ± SE from five independent experiments. B, Typical images of Hoechst-stained RGCs are shown. Typical apoptotic nuclei are indicated by arrows. C, Shown are phase-contrast (top) and fluorescence (bottom) images of RGCs stained with annexin V–fluorescein (green) and propidium iodide (red) after incubation for 12 and 24 h under the same conditions as for B. Representative images are shown from one of three independent experiments with similar results. Scale bars: B, C, 50 μm. D, Apoptosis induced by withdrawal of trophic additives is inhibited by a broad-spectrum caspase inhibitor. RGCs were incubated for 24 h in BM(+) or BM(−). The medium added to some cultures contained the broad-spectrum caspase inhibitor Boc-D fmk (Boc-D) (100 μm) dissolved in dimethylsulfoxide. Medium provided to control cells (DMSO) contained an equivalent amount (2 μl/ml) of dimethylsulfoxide without inhibitor. Apoptotic neurons were detected by Hoechst staining. Data are means ± SE from four independent experiments.
Medium conditioned by primary cultures of rat cortical glia (>90% astrocytes) for 3 d was supplied to RGCs for 24 h in the absence of trophic additives. The glia-conditioned medium prevented apoptosis of the neurons (Fig. 1 A). Because apoE and lipoprotein receptors have been implicated in signaling pathways in the brain (Herz and Bock, 2002), we determined whether glia-derived LPs present in conditioned medium were active in preventing apoptosis. Glial LPs were isolated from conditioned medium by ultracentrifugation and supplied in basal medium to RGCs at concentrations of cholesterol and apoE equivalent to those in glia-conditioned medium (∼1 μg/ml cholesterol and 3–5 μg/ml apoE) (Hayashi et al., 2004). Figure 1, B and C, shows that LPs secreted by glia prevented apoptosis of RGCs. Neurons that were given LPs did not stain with annexin V–fluorescein or propidium iodide after 24 h and were, therefore, not apoptotic. However, the majority of RGCs cultured in basal medium lacking trophic additives were annexin V and propidium iodide positive after 24 h (Fig. 1 C), indicating that these neurons were in the late stages of apoptosis.
The addition of recombinant human apoE alone, cholesterol alone, or a mixture of cholesterol and recombinant apoE3 in amounts equivalent to those in glia-conditioned medium, failed to prevent neuronal apoptosis (Fig. 2 A). To determine whether or not apoE was required for protection against apoptosis, lipoproteins that contained or lacked apoE were isolated from the conditioned medium of glia cultured from ApoE +/+ and ApoE −/− mice, respectively (Fig. 2 B). The lipoproteins (120 μg of protein/ml) were supplied to RGCs in basal medium, and apoptosis was assessed. Lipoproteins isolated from ApoE −/− glia failed to prevent apoptosis of the RGCs, whereas lipoproteins secreted by ApoE +/+ glia protected the neurons from apoptosis (Fig. 2 C). Because the conditioned medium from ApoE −/− glia contained only a small amount of cholesterol compared with that in ApoE +/+ glial conditioned medium, we wished to determine whether the failure of lipoproteins from ApoE −/− glia to protect against apoptosis was caused by a deficiency of cholesterol in the lipoprotein particles. We therefore generated lipoproteins lacking apoE but containing apoA1 by incubation of primary cultures of cortical glia from ApoE −/− mice with human apoA1. The apoA1-containing lipoproteins contained the same amount of cholesterol (1 μg/ml) and were of the same density as the apoE-containing lipoproteins but contained apoA1 instead of apoE (Fig. 2 B). The addition of apoA1-containing lipoproteins to RGCs failed to protect the neurons from apoptosis (Fig. 2 D). Because the apoJ content of the apoE- and apoA1-containing lipoprotein fractions was similar (Fig. 2 B), the apoJ-containing lipoproteins from ApoE +/+ and ApoE −/− glia could not have imparted the protective effect. These observations demonstrate that apoE is required for preventing apoptosis of the RGCs and that the protective effect of apoE requires its association with lipid.
To confirm that the apoE component of LPs was responsible for the protection of RGCs against apoptosis, recombinant apoE was generated and reconstituted into rHDLs that contained phospholipids and cholesterol (molar ratio of apoE:1-palmitoyl-2-oleoyl-glycerophosphocholine:cholesterol of 1:100:10). When these rHDLs were supplied to RGCs, so that the amount of apoE was equivalent to that in glia-derived LPs, apoptosis was prevented (Fig. 2 E). In contrast, liposomes that had the same lipid composition but lacked apoE did not protect the neurons from apoptosis (Fig. 2 E). To determine whether the protection against apoptosis was mediated by cholesterol in the rHDLs, we prepared rHDLs that contained 1-palmitoyl-2-oleoyl-glycerophosphocholine and apoE (molar ratio of 100:1) but did not contain cholesterol. The rHDLs that lacked cholesterol were as effective in preventing apoptosis as were the rHDLs that contained cholesterol (Fig. 2 F). These observations demonstrate that the anti-apoptotic effect of glial lipoproteins requires apoE that is associated with lipid but that cholesterol is not required.
Protection from apoptosis is mediated by LRP
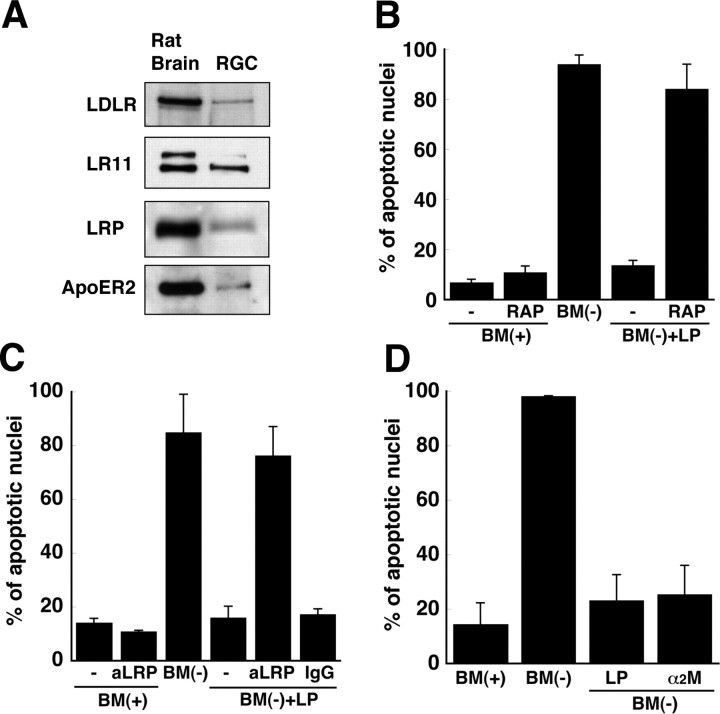
Previous studies have demonstrated that apoE in association with lipids, but not lipid-free apoE, is a ligand for receptors of the LDLR family and that lipid-free apoE and lipid-associated apoE have different affinities for different members of the LDLR family (Innerarity et al., 1979; Ruiz et al., 2005). We therefore determined whether the protective effect of LPs was mediated by a member of this receptor superfamily. The following receptors of the LDLR family were detected in RGCs by immunoblotting: LDLR, LR11, LRP, and apoER2 (Fig. 3 A).
Figure 3.
LRP mediates the protective effect of apoE-containing lipoproteins. A, Receptors of the low-density lipoprotein receptor family were detected by immunoblotting of 20 μg of protein from lysates of RGCs. Rat brain proteins (20 μg) were used as a positive control. Data are from one experiment representative of three experiments with similar results. B–D, RGCs were incubated for 24 h with BM(+) or BM(−) in the presence of apoE-containing LPs and/or 300 nm RAP (B), 10 μg/ml anti-LRP antibody (aLRP) (C), or 10 μg/ml nonspecific IgG (C), after a 2 h pretreatment with RAP, antibodies, or 100 nm α2-macroglobulin (α2M) (D). The percentage of cells containing apoptotic nuclei was determined by Hoechst staining. Data are means ± SE of at least three independent experiments.
To determine whether one of these receptors mediated the protection against apoptosis, we incubated RGCs with RAP, which is known to compete with ligands for binding to receptors of the LDLR superfamily (Innerarity et al., 1979). RAP itself did not induce apoptosis in RGCs, but the ability of LPs to protect RGCs from apoptosis was eliminated by RAP (Fig. 3 B). To determine whether LRP was involved in protecting the RGCs from apoptosis, we blocked the binding of LPs to LRP by adding a polyclonal antibody directed against LRP (Kounnas et al., 1992). The antibody blocked the protective effect of LPs but did not by itself cause apoptosis (Fig. 3 C). These observations suggest that LRP is involved in the LP-mediated protection of RGCs from apoptosis. LRP has multiple ligands, including α2-macroglobulin (Herz and Strickland, 2001). Thus, we incubated RGCs with α2-macroglobulin (100 nm). α2-Macroglobulin prevented apoptosis of the RGCs as effectively as did LPs (Fig. 3 D).
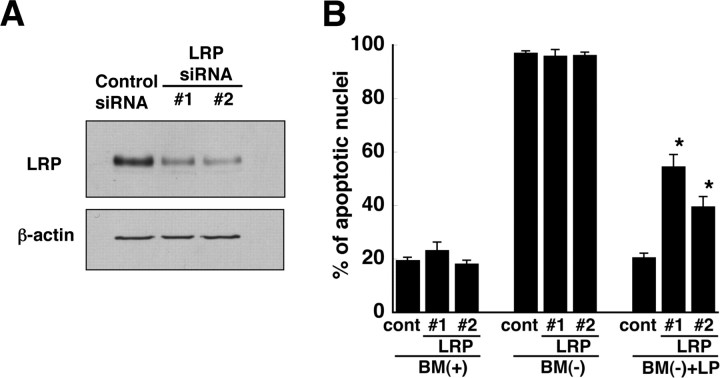
As additional evidence that LRP was responsible for mediating the anti-apoptotic effect of LPs, we reduced the expression of LRP-1 in RGCs using RNA silencing. RGCs were transfected with either a nonspecific, control siRNA or with two different siRNAs specific for LRP-1. To determine whether the specific LRP-1 siRNAs (LRP #1 and LRP #2) reduced the expression of LRP-1 in RGCs, we performed immunoblotting experiments with anti-LRP-1 antibodies. Figure 4 A shows that in RGCs transfected with the LRP-1-specific siRNAs, the amount of LRP-1 protein was markedly less than in RGCs transfected with control siRNA. To determine whether the decreased expression of LRP-1 reduced the ability of LPs to protect RGCs from apoptosis, we assessed apoptosis in the siRNA-transfected neurons. Neither the LRP-1-specific siRNAs nor the control siRNA induced apoptosis in RGCs cultured in the presence of trophic additives (Fig. 4 B). As expected, essentially all of the RGCs cultured without LPs in the absence of trophic additives were apoptotic. In contrast, for RGCs transfected with LRP-1-specific siRNAs and cultured in the absence of trophic additives and in the presence of LPs, up to 60% of the neurons were apoptotic, whereas only ∼20% of RGCs transfected with control siRNA were apoptotic (Fig. 4 B). Thus, decreased expression of LRP-1 reduced the ability of LPs to protect the neurons from apoptosis. The data in Figures 3 and 4 demonstrate, therefore, that glial LPs prevent apoptosis via a mechanism that involves LRP-1.
Figure 4.
Reduction of LRP-1 in RGCs by siRNA. A, After 3 d in culture, RGCs were transfected with 20 pmol of control siRNA or two different LRP-1 siRNAs (LRP #1 and LRP #2). RGCs were harvested 3 d after transfection, and proteins were separated by 0.1% SDS-PAGE. LRP was detected by immunoblotting. Immunoblotting of β-actin was used as a loading control. Data are from one experiment that is representative of three experiments with similar results. B, RGCs were transfected with control siRNA (cont) or two different siRNAs (LRP #1 and #2) that were specific for LRP-1. After 3 d, the neurons were incubated for 24 h with BM(+), BM(−), or BM(−) in the presence of apoE-containing lipoproteins [BM(−) + LP]. The percentage of cells containing apoptotic nuclei was determined by Hoechst staining. Data are means ± SE of four independent experiments. *p < 0.0001 for control versus LRP #1; *p = 0.0003 for control versus LRP #2. Statistical analysis was performed using one-way ANOVA followed by Bonferroni's multiple comparison.
Binding of LP to LRP initiates a signaling pathway
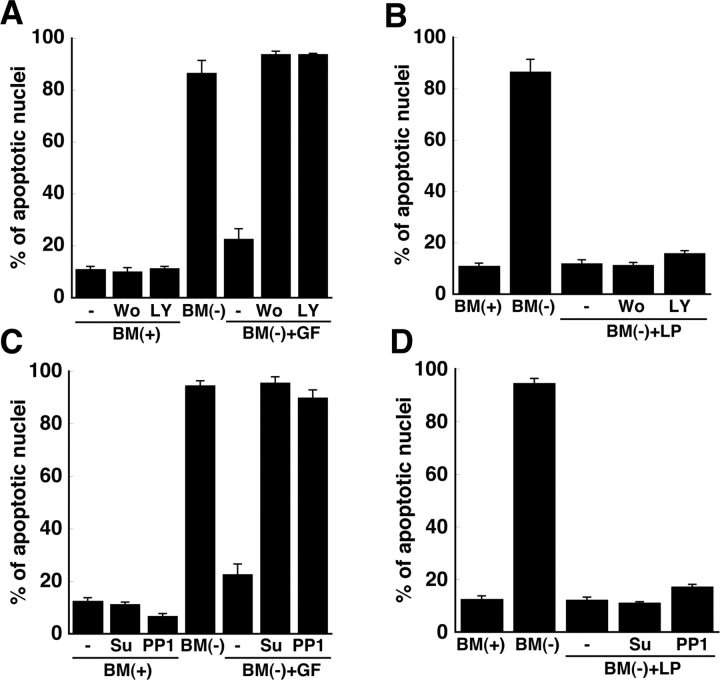
Because LRP can initiate signaling cascades in the CNS (Herz and Bock, 2002), our observations suggested that the apoE-mediated protection against apoptosis occurred via a signaling pathway that was induced after binding of lipid-associated apoE to LRP. To define this putative signaling pathway, several kinases known to participate in pathways of survival and apoptosis in neurons were examined. Src family kinases and phosphatidylinositol-3-kinase mediate survival pathways in neurons induced by neurotrophic factors such as brain-derived neurotrophic factor and ciliary neurotrophic factor. We therefore initially tested the efficacy of inhibitors of phosphatidylinositol-3-kinase [wortmannin and 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002)] and inhibitors of Src family kinases [protein phosphatase 1 (PP1) and Su6656] in RGCs in which survival is supported by these neurotrophic factors. Although the inhibitors blocked the protective effect of the neurotrophic factors (Fig. 5 A,C), these agents did not block the protective effect of glial LPs (Fig. 5 B,D), implying that these kinases do not contribute to the LP-mediated protective pathway.
Figure 5.
Phosphatidylinositol-3-kinase and Src family kinases do not contribute to the anti-apoptotic effect of apoE-containing lipoproteins. Retinal ganglion cells were incubated in BM(+), BM(−), or BM(−) plus apoE-containing lipoproteins [BM(−) + LP] for 24 h. Apoptosis was assessed by Hoechst staining. The medium added to some neuronal cultures contained brain-derived neurotrophic factor (50 ng/ml), ciliary neurotrophic factor (50 ng/ml), and forskolin (10 μm; +GF; A, C), and/or the phosphatidylinositol-3-kinase inhibitors wortmannin (Wo; 100 nm) or LY294002 (LY; 50 μm; A, B) or Src kinase inhibitors PP1 (10 μm) or Su6656 (Su; 10 μm; C, D). Data are means ± SE of three independent experiments.
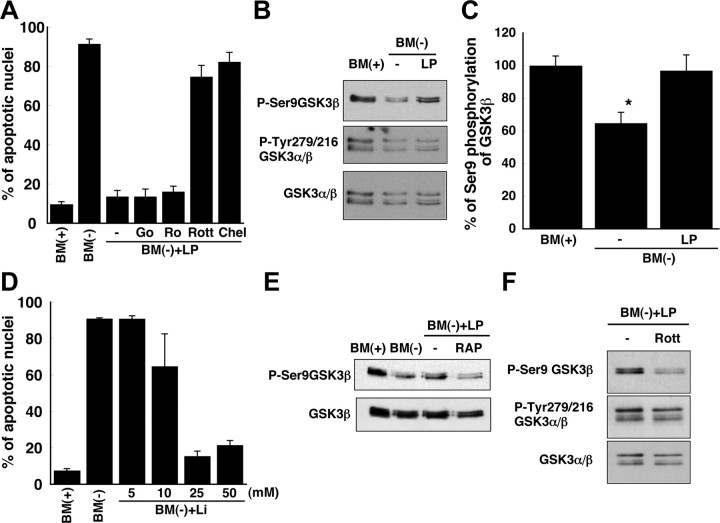
We also investigated whether or not the anti-apoptotic pathway involved PKC by determining whether LPs prevented neuronal apoptosis in the presence of inhibitors of PKC. 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole (Gö6976) and 2-[8-[(dimethylamino)methyl]-6,7,8,9-tetrahydropyrido(1,2-a)indol-3-yl]-3-(1-methylindol-3-yl)maleimide (Ro32-0432) were used as inhibitors of PKCα, PKCβ, and PKCγ, whereas chelerythrine was used as an inhibitor of PKCα, PKCβ, PKCγ, and PKCδ, and rottlerin was used as an inhibitor of PKCδ (Wilkinson et al., 1993; Bright and Mochly-Rosen, 2005). Chelerythrine and rottlerin abrogated the protective effect of LPs, whereas Gö6976 and Ro32-0432 did not (Fig. 6 A). Importantly, chelerythrine and rottlerin did not reduce RGC survival in basal culture medium (data not shown). These data indicate that PKCδ protects RGCs from apoptosis via a pathway that is initiated by LPs and LRP-1.
Figure 6.
PKCδ and GSK3β contribute to the anti-apoptotic effect of apoE-containing lipoproteins. A, RGCs were incubated with inhibitors of PKC: Gö6976 (Go; 20 nm), Ro32-0432 (Ro; 50 nm), rottlerin (Rott; 2 μm), and chelerythrine (Chel; 1 μm). BM(−) was supplemented with apoE-containing LPs and PKC inhibitors and then supplied to RGCs for 24 h after a 2 h pretreatment of the cells with PKC inhibitors. Apoptosis was assessed by Hoechst staining. Data are means ± SE from three independent experiments. B, Immunoblotting of GSK3α/β with antibodies directed against GSK3β phosphorylated at Ser-9 (P-Ser9GSK3β), GSK3α/β phosphorylated at Tyr-279/216 (P-Tyr279/216 GSK3α/β), or total GSK3α/β protein, from the same experiment. RGCs were incubated in BM(+) or BM(−) with or without apoE-containing lipoproteins. Data are from one experiment representative of six independent experiments with similar results. C, Quantitation of phosphorylation at Ser-9 of GSK3β from immunoblotting experiments shown in B as a percentage of the phosphorylation in the presence of trophic additives. Data are means ± SE from six independent experiments. *p = 0.016 for BM(+) versus BM(−); p = 0.029 for BM(−) versus BM(−) plus LP. Statistical analysis was performed using one-way ANOVA followed by Bonferroni's multiple comparison. D, Apoptosis was assessed by Hoechst staining of neurons incubated for 24 h in BM(+) or BM(−) and the indicated concentrations of lithium chloride (Li), an inhibitor of GSK3. Data are means ± SE from three independent experiments. E, RGCs were incubated as in B, but in the presence or absence of RAP, and then immunoblotted for total GSK3β protein and P-Ser9GSK3β. Data are representative of four independent experiments with similar results. F, Immunoblotting of GSK3α/β, GSK3β phosphorylated at Ser-9, and GSK3α/β phosphorylated at Tyr-279/216 in neurons incubated as in B in BM(−) plus LP in the absence or presence of rottlerin. Data are representative of three independent experiments with similar results.
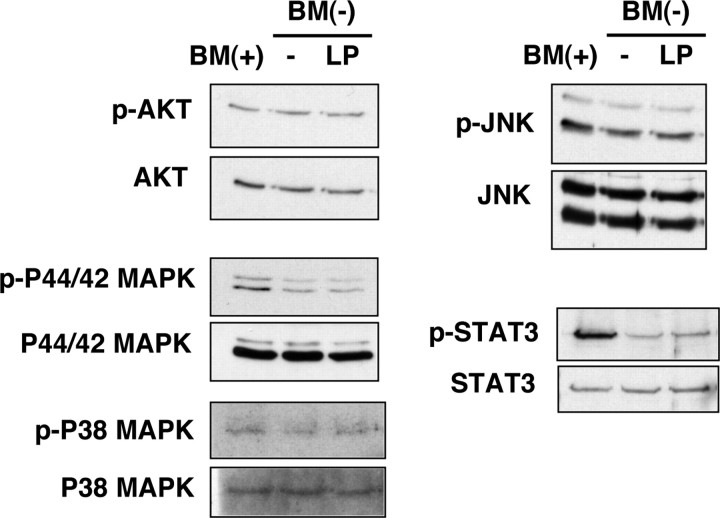
Alterations in the phosphorylation state of several other signaling proteins (p44/42 MAPK, p38 MAPK, STAT3, Akt, and JNK) that have been reported to participate in anti-apoptotic or proapoptotic pathways in neurons (Segal and Greenberg, 1996; Yuan and Yankner, 2000) were examined by immunoblotting (Fig. 7). No significant differences in phosphorylation levels of these proteins were detected in the presence or absence of LPs, suggesting that these kinases do not contribute to the anti-apoptotic effect of LPs. In contrast, the phosphorylation of Ser-9 of GSK3β, a proapoptotic kinase that is inactivated by phosphorylation at Ser-9 (Linseman et al., 2004), was decreased under apoptotic conditions and was restored when RGCs were incubated with LPs (Fig. 6 B,C). The phosphorylation of GSK3α at Tyr-279 and GSK3β at Tyr-216 was not significantly changed by the presence of the lipoproteins (Fig. 6 B). In support of a role of GSK in the LP-mediated increased survival of neurons, lithium, an inhibitor of GSK (Noble et al., 2005), also prevented the apoptosis in a dose-dependent manner (Fig. 6 D). These data suggest that GSK3β contributes to the apoptosis caused by withdrawal of trophic additives from the RGCs and that LPs mediate their protective effect via a pathway that involves GSK3β and PKCδ. Consistent with this idea, the phosphorylation of Ser-9 of GSK3β was reduced by RAP (Fig. 6 E), a protein that blocks the binding of LPs to LRP (Fig. 3 B). Moreover, the PKCδ inhibitor rottlerin prevented the increased phosphorylation of Ser-9 of GSK3β that was induced by LPs, whereas the phosphorylation of GSK3α/β at Tyr-216/279 was unchanged (Fig. 6 F). Rottlerin did not alter the phosphorylation of Ser-9 of GSK3β in basal culture medium (data not shown).
Figure 7.
Phosphorylation of signaling molecules in RGCs in response to apoE-containing lipoproteins. Apoptosis was initiated in RGCs by withdrawal of trophic additives [BM(−)] from basal medium [BM(+)]. RGCs were incubated for 9 h in the presence (LP) or absence (−) of apoE-containing lipoproteins and then harvested and analyzed by immunoblotting using antibodies directed against phosphorylated (p) Akt (phosphorylated on Ser-473), P44/42 MAPK (phosphorylated on Thr-202/Tyr-204), P38 MAP kinase (phosphorylated on Thr-180/Tyr-182), STAT3 (phosphorylated on Tyr-705), and JNK (phosphorylated on Thr-183/Tyr-185). Total amounts of the proteins were detected by immunoblotting. Data are from one experiment representative of three experiments with similar results.
ApoE3 increases survival of RGCs to a greater extent than does apoE4
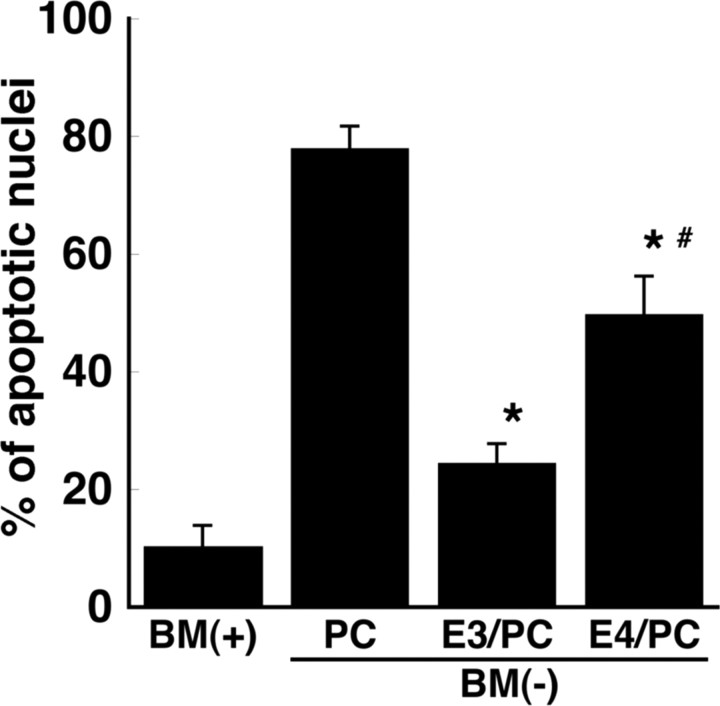
In view of many reports that apoE4 is detrimental for neuronal function, we compared the efficacy of lipoproteins containing apoE3 or apoE4 in the protection of RGCs against apoptosis. Recombinant human apoE3 and apoE4 were reconstituted into rHDLs that contained phosphatidylcholine (1-palmitoyl-2-oleoyl-glycerophosphocholine) (molar ratio of 1:100). Liposomes containing phosphatidylcholine alone did not protect the RGCs from apoptosis induced by withdrawal of trophic additives (Fig. 8). However, rHDLs that contained apoE3 reduced the number of apoptotic neurons from 78.0 to 24.5%, whereas with apoE4-containing rHDLs, 49.8% of the RGCs were apoptotic (Fig. 8). Thus, rHDLs that contain apoE3 are more protective against apoptosis than are rHDLs that contain apoE4.
Figure 8.
ApoE4-containing rHDLs are less protective against apoptosis than are apoE3-containing rHDLs. rHDLs containing recombinant human apoE3 or apoE4 were prepared with phosphatidylcholine (PC) (molar ratio, 1:100). The apoE3- (E3/PC) or apoE4 (E4/PC)-containing rHDLs were added to BM(−) and incubated with retinal ganglion cells for 24 h. Control cultures were given BM(+). Liposomes that lacked apoE but contained the same amount of PC as in rHDLs (+PC) were used as a negative control. The number of apoptotic neurons was determined by Hoechst staining as a percentage of the total number of neurons. Data are means ± SE from five independent experiments. *p < 0.005 for BM(−) plus PC versus BM(−) plus E3/PC and BM(−) plus E4/PC. # p = 0.0057 for E3/PC versus E4/PC. Statistical analysis was performed using one-way ANOVA followed by Bonferroni's multiple comparison.
Discussion
Our studies reveal a novel function of LRP-1 that is induced in CNS neurons by glia-derived LPs: protection against apoptosis. Neuronal apoptosis is a characteristic feature of many neurodegenerative disorders, such as Alzheimer's disease and Parkinson's disease. Moreover, the levels of neurotrophic factors, such as brain-derived neurotrophic factor, in the brains of individuals with these diseases have been reported to be decreased (Siegel and Chauhan, 2000; Murer et al., 2001). Our study demonstrates that RGCs are protected from apoptosis induced by removal of trophic additives (including brain-derived neurotrophic factor and ciliary neurotrophic factor) via a signaling pathway that is initiated after binding of LPs to LRP, a receptor of the LDLR family. This signaling pathway involves GSK3β downstream of PKCδ (Fig. 9). LRP has been reported to contribute to calcium signaling (Bacskai et al., 2000), long-term potentiation (Zhuo et al., 2000), neurite outgrowth (Holtzman et al., 1995), brain development (Herz and Chen, 2006), and Alzheimer's disease (Harris-White and Frautschy, 2005). The multifunctional receptor LRP is expressed in RGCs, the CNS neurons that we studied. ApoE is a high-affinity ligand for this receptor and shows higher affinity for LRP when the apoE is associated with lipids (Ruiz et al., 2005). Our studies demonstrate that apoE in association with lipids, but not lipid-free apoE, protects neurons from apoptosis.
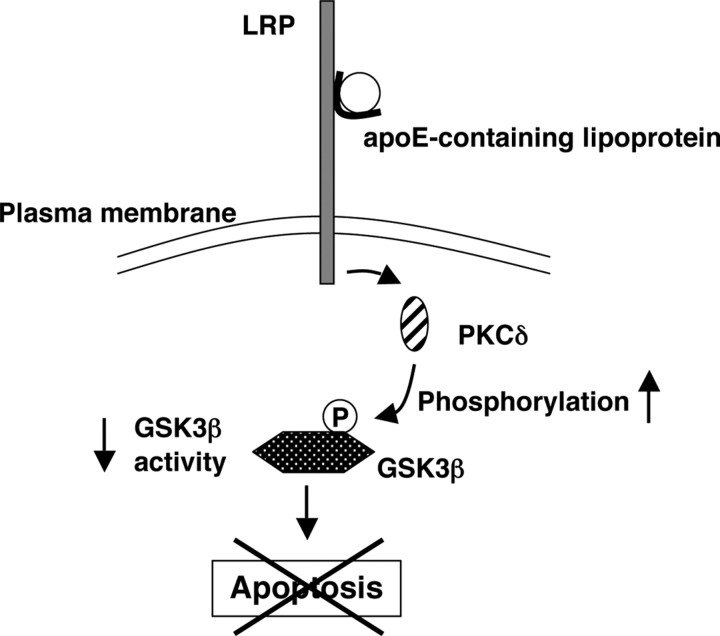
Figure 9.
Proposed anti-apoptotic signaling pathway induced in RGCs by apoE-containing lipoproteins. When apoptosis is induced in RGCs by removal of trophic additives, apoE-containing lipoproteins secreted by glia protect the RGCs from apoptosis. After binding of apoE-containing lipoproteins to LRP on the cell surface, activation of PKCδ, either directly or indirectly, increases the phosphorylation (P) of GSK3β, thereby inactivating GSK3β and protecting the RGCs from apoptosis.
Although cholesterol is regarded as an important molecule for neuronal function [e.g., cholesterol associated with apoE promotes synaptogenesis (Mauch et al., 2001), and inhibition of intracellular cholesterol trafficking causes neuronal death (Huang et al., 2006)], the anti-apoptotic effect of LPs that we observed is not mediated by cholesterol. We found that neither cholesterol alone nor cholesterol with recombinant lipid-free apoE protected the neurons from apoptosis. Furthermore, glial lipoproteins from ApoE −/− mice did not rescue the neurons from apoptosis, nor did apoA1- or apoJ-containing lipoproteins that contained the same amount of cholesterol as LPs. However, rHDLs that contained apoE and phosphatidylcholine without any cholesterol protected the RGCs from apoptosis to the same extent as did the LPs. These observations demonstrate that the protective effect of LPs does not require cholesterol. In experiments in which we used the inhibitory protein RAP, as well as in experiments with anti-LRP antibodies and LRP-1 siRNA, we identified LRP-1 as the receptor that mediates the protective effect of LPs.
We investigated the neuronal signaling pathway that is initiated when LPs bind to LRP. Our data show that phosphorylation of Ser-9 of GSK3β is decreased during apoptosis and is restored by addition of LPs, suggesting that these lipoproteins protect neurons from apoptosis by inactivation of GSK3β caused by direct or indirect phosphorylation at Ser-9 by PKCδ (Fig. 9). Phosphatidylinositol-3-kinase and Src family tyrosine kinases are known to play important roles in cellular survival, growth, differentiation and proliferation (Bondy and Cheng, 2004; Kuo et al., 2005). However, although inhibition of these kinases blocked the survival effects of brain-derived neurotrophic factor and ciliary neurotrophic factor in RGCs, none of these inhibitors eliminated the protective effect of LPs. Nor did MAP kinases (P44/42 and P38) appear to contribute to the protective effect of LPs, because the phosphorylation state of these kinases was not altered by LPs. Phosphorylation of the cytosolic domain of LRP has been reported to be increased by PKCα (Ranganathan et al., 2004). However, agents that inhibited only PKCδ, not PKCα, PKCβ, or PKCγ, blocked the anti-apoptotic effect of LPs in RGCs, implicating PKCδ in the signaling pathway. GSK3β is proapoptotic in neurons (Linseman et al., 2004), and its activity is decreased by phosphorylation at Ser-9 (Cross et al., 1995). Consistent with our observations, inactivation of GSK3β has been shown previously to prevent neuronal apoptosis (Mora et al., 2001). Furthermore, a GSK3 inhibitor has been shown to reduce tauopathy (Noble et al., 2005) and Aβ production (Sun et al., 2002; Su et al., 2004), which are pathological hallmarks of Alzheimer's disease. Because the binding of LPs to LRP inactivates GSK3β in CNS neurons, it is possible that this pathway is a potential target for treatment of Alzheimer's disease.
ApoE appears to play important roles in the nervous system. For example, apoE synthesis in glia is dramatically increased in response to nerve injury in the CNS and peripheral nervous system (Ignatius et al., 1986; Petegnief et al., 2001). CNS neurons are more susceptible to ischemic injury in ApoE −/− mice than in ApoE +/+ mice (Sheng et al., 1999). ApoE deficiency in mice also reduces the stability of the neuronal cytoskeleton during the aging process (Masliah et al., 1995). In addition, persistent nerve degeneration has been observed in injured brains of ApoE −/− but not ApoE +/+ mice (Fagan et al., 1998), suggesting that apoE plays a role in the repair and/or protection of neurons.
The mechanism by which apoE is involved in Alzheimer's disease is not clear (Goedert and Spillantini, 2006). In a mouse model of Alzheimer's disease, the presence of apoE3 decreased Aβ levels and deposition (DeMattos, 2004; DeMattos et al., 2004). We now report that LPs secreted by glia contribute to the survival of CNS neurons by a novel mechanism that requires LRP-1. Furthermore, our data show that lipoproteins that contain apoE3 protect RGCs from apoptosis, whereas lipoproteins that contain apoE4 are significantly less protective. Our observations might provide at least a partial explanation for why inheritance of the ε4, rather than the ε3, allele markedly increases the risk of developing late-onset Alzheimer's disease.
Footnotes
This work was supported by a grant from the Canadian Institutes for Health Research. D.E.V. is a scientist of the Alberta Heritage Foundation for Medical Research and holder of the Canada Research Chair in Molecular and Cell Biology of Lipids. We thank Russell Watts, Priscilla Gao, and Audric Moses for excellent technical assistance.
References
- Bacskai BJ, Xia MQ, Strickland DK, Hyman BT. The endocytic receptor protein LRP also mediates neuronal calcium signaling via N-methyl-d-aspartate receptors. Proc Natl Acad Sci USA. 2000;97:11551–11556. doi: 10.1073/pnas.200238297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Silverstein BE, Corey DP, Chun LLY. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol. 2004;490:25–31. doi: 10.1016/j.ejphar.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Bright R, Mochly-Rosen D. The role of protein kinase C in cerebral ischemic and reperfusion injury. Stroke. 2005;36:2781–2790. doi: 10.1161/01.STR.0000189996.71237.f7. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Receptor-mediated control of cholesterol metabolism. Science. 1976;191:150–154. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- Choi HY, Karten B, Chan T, Vance JE, Greer WL, Heidenreich RA, Garver WS, Francis GA. Impaired ABCA1-dependent lipid efflux and hypoalphalipoproteinemia in human Niemann-Pick type C disease. J Biol Chem. 2003;278:32569–32577. doi: 10.1074/jbc.M304553200. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- DeMattos RB. Apolipoprotein E dose-dependent modulation of beta-amyloid deposition in a transgenic mouse model of Alzheimer's disease. J Mol Neurosci. 2004;23:255–262. doi: 10.1385/JMN:23:3:255. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Cirrito JR, Parsadanian M, May PC, O'Dell MA, Taylor JW, Harmony JA, Aronow BJ, Bales KR, Paul SM, Holtzman DM. ApoE and clusterin cooperatively suppress Abeta levels and deposition. Evidence that apoE regulates extracellular Abeta metabolism in vivo. Neuron. 2004;41:193–202. doi: 10.1016/s0896-6273(03)00850-x. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Murphy BA, Patel SN, Kilbridge JF, Mobley WC, Bu G, Holtzman DM. Evidence for normal aging of the septo-hippocampal cholinergic system in apoE (−/−) mice but impaired clearance of axonal degeneration products following injury. Exp Neurol. 1998;151:314–325. doi: 10.1006/exnr.1998.6818. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG. A century of Alzheimer's disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- Gong JS, Kobayashi M, Hayashi H, Zou K, Sawamura N, Fujita SC, Yanagisawa K, Michikawa M. Apolipoprotein E (ApoE) isoform-dependent lipid release from astrocytes prepared from human ApoE3 and ApoE4 knock-in mice. J Biol Chem. 2002;277:29919–29926. doi: 10.1074/jbc.M203934200. [DOI] [PubMed] [Google Scholar]
- Han X. The role of apolipoprotein E in lipid metabolism in the central nervous system. Cell Mol Life Sci. 2004;61:1896–1906. doi: 10.1007/s00018-004-4009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-White ME, Frautschy SA. Low density lipoprotein receptor-related proteins (LRPs), Alzheimer's and cognition. Curr Drug Targets CNS Neurol Disord. 2005;4:469–480. doi: 10.2174/156800705774322102. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Campenot RB, Vance DE, Vance JE. Glial cell lipoproteins stimulate axon growth of central nervous system neurons in compartmented cultures. J Biol Chem. 2004;279:14009–14015. doi: 10.1074/jbc.M313828200. [DOI] [PubMed] [Google Scholar]
- Herz J, Bock HH. Lipoprotein receptors in the nervous system. Annu Rev Biochem. 2002;71:405–434. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7:850–859. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch-Reinshagen V, Zhou S, Burgess BL, Bernier L, McIsaac SA, Chan JY, Tansley GH, Cohn JS, Hayden MR, Wellington CL. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J Biol Chem. 2004;279:41197–41207. doi: 10.1074/jbc.M407962200. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Pitas RE, Kilbridge J, Nathan B, Mahley RW, Bu G, Schwartz AL. Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc Natl Acad Sci USA. 1995;92:9480–9484. doi: 10.1073/pnas.92.21.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Hou Q, Cheung NS, Li QT. Neuronal cell death caused by inhibition of intracellular cholesterol trafficking is caspase dependent and associated with activation of the mitochondrial apoptosis pathway. J Neurochem. 2006;97:280–291. doi: 10.1111/j.1471-4159.2006.03733.x. [DOI] [PubMed] [Google Scholar]
- Ignatius MJ, Gebicke-Harter PJ, Skene JH, Schilling JW, Weisgraber KH, Mahley RW, Shooter EM. Expression of apolipoprotein E during nerve degeneration and regeneration. Proc Natl Acad Sci USA. 1986;83:1125–1129. doi: 10.1073/pnas.83.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innerarity TL, Pitas RE, Mahley RW. Binding of arginine-rich (E) apoprotein after recombination with phospholipid vesicles to the low density lipoprotein receptors of fibroblasts. J Biol Chem. 1979;254:4186–4190. [PubMed] [Google Scholar]
- Kounnas MZ, Argraves WS, Strickland DK. The 39-kDa receptor-associated protein interacts with two members of the low density lipoprotein receptor family, alpha 2-macroglobulin receptor and glycoprotein 330. J Biol Chem. 1992;267:21162–21166. [PubMed] [Google Scholar]
- Kounnas MZ, Chappell DA, Strickland DK, Argraves WS. Glycoprotein 330, a member of the low density lipoprotein receptor family, binds lipoprotein lipase in vitro. J Biol Chem. 1993;268:14176–14181. [PubMed] [Google Scholar]
- Kuo G, Arnaud L, Kronstad-O'Brien P, Cooper JA. Absence of Fyn and Src causes a reeler-like phenotype. J Neurosci. 2005;25:8578–8586. doi: 10.1523/JNEUROSCI.1656-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Kan HY, Lavrentiadou S, Krieger M, Zannis V. Reconstituted discoidal ApoE-phospholipid particles are ligands for the scavenger receptor BI. The amino-terminal 1–165 domain of ApoE suffices for receptor binding. J Biol Chem. 2002;277:21149–21157. doi: 10.1074/jbc.M200658200. [DOI] [PubMed] [Google Scholar]
- Linseman DA, Butts BD, Precht TA, Phelps RA, Le SS, Laessig TA, Bouchard RJ, Florez-McClure ML, Heidenreich KA. Glycogen synthase kinase-3β phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci. 2004;24:9993–10002. doi: 10.1523/JNEUROSCI.2057-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabile L, Lefebvre C, Lavigne J, Boulet L, Davignon J, Lussier-Cacan S, Bernier L. Secreted apolipoprotein E reduces macrophage-mediated LDL oxidation in an isoform-dependent way. J Cell Biochem. 2003;90:766–776. doi: 10.1002/jcb.10697. [DOI] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Ge N, Alford M, Veinbergs I, Roses AD. Neurodegeneration in the central nervous system of apo E-deficient mice. Exp Neurol. 1995;136:107–122. doi: 10.1006/exnr.1995.1088. [DOI] [PubMed] [Google Scholar]
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- Mora A, Sabio G, Gonzalez-Polo RA, Cuenda A, Alessi DR, Alonso JC, Fuentes JM, Soler G, Centeno F. Lithium inhibits caspase 3 activation and dephosphorylation of PKB and GSK3 induced by K+ deprivation in cerebellar granule cells. J Neurochem. 2001;78:199–206. doi: 10.1046/j.1471-4159.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, LaFrancois J, Feinstein B, Burns M, Krishnamurthy P, Wen Y, Bhat R, Lewis J, Dickson D, Duff K. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci USA. 2005;102:6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petegnief V, Saura J, de Gregorio-Rocasolano N, Paul SM. Neuronal injury-induced expression and release of apolipoprotein E in mixed neuron/glia co-cultures: nuclear factor kappaB inhibitors reduce basal and lesion-induced secretion of apolipoprotein E. Neuroscience. 2001;104:223–234. doi: 10.1016/s0306-4522(01)00046-x. [DOI] [PubMed] [Google Scholar]
- Ranganathan S, Liu CX, Migliorini MM, Von Arnim CA, Peltan ID, Mikhailenko I, Hyman BT, Strickland DK. Serine and threonine phosphorylation of the low density lipoprotein receptor-related protein by protein kinase Calpha regulates endocytosis and association with adaptor molecules. J Biol Chem. 2004;279:40536–40544. doi: 10.1074/jbc.M407592200. [DOI] [PubMed] [Google Scholar]
- Ruiz J, Kouiavskaia D, Migliorini M, Robinson S, Saenko EL, Gorlatova N, Li D, Lawrence D, Hyman BT, Weisgraber KH, Strickland DK. The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. J Lipid Res. 2005;46:1721–1731. doi: 10.1194/jlr.M500114-JLR200. [DOI] [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- Sheng H, Laskowitz DT, Mackensen GB, Kudo M, Pearlstein RD, Warner DS. Apolipoprotein E deficiency worsens outcome from global cerebral ischemia in the mouse. Stroke. 1999;30:1118–1124. doi: 10.1161/01.str.30.5.1118. [DOI] [PubMed] [Google Scholar]
- Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer's and Parkinson's disease brain. Brain Res Brain Res Rev. 2000;33:199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer's disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Ryder J, Li B, Wu X, Fox N, Solenberg P, Brune K, Paul S, Zhou Y, Liu F, Ni B. Lithium, a common drug for bipolar disorder treatment, regulates amyloid-beta precursor protein processing. Biochemistry. 2004;43:6899–6908. doi: 10.1021/bi035627j. [DOI] [PubMed] [Google Scholar]
- Sun X, Sato S, Murayama O, Murayama M, Park JM, Yamaguchi H, Takashima A. Lithium inhibits amyloid secretion in COS7 cells transfected with amyloid precursor protein C100. Neurosci Lett. 2002;321:61–64. doi: 10.1016/s0304-3940(01)02583-6. [DOI] [PubMed] [Google Scholar]
- Thuahnai ST, Lund-Katz S, Williams DL, Phillips MC. Scavenger receptor class B, type I-mediated uptake of various lipids into cells. Influence of the nature of the donor particle interaction with the receptor. J Biol Chem. 2001;276:43801–43808. doi: 10.1074/jbc.M106695200. [DOI] [PubMed] [Google Scholar]
- Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J. 1993;294:335–337. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Holtzman DM, Li Y, Osaka H, DeMaro J, Jacquin M, Bu G. Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation. J Neurosci. 2000;20:542–549. doi: 10.1523/JNEUROSCI.20-02-00542.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]