Abstract
Infant maternal separation, a paradigm of early life stress in rodents, elicits long-lasting changes in gene expression that persist into adulthood. In BALB/c mice, an inbred strain with spontaneously elevated anxiety and stress reactivity, infant maternal separation led to increased depression-like behavioral responses to adult stress and robustly increased editing of serotonin 2C receptor pre-mRNA. Chronic fluoxetine treatment of adult BALB/c mice exposed to early life stress affected neither their behavioral responses to stress nor their basal 5-HT2C pre-mRNA editing phenotype. However, when fluoxetine was administered during adolescence, depression-like behavioral responses to stress were significantly diminished in these mice, and their basal and stress-induced 5-HT2C pre-mRNA editing phenotypes were significantly lower. Moreover, when BALB/c mice exposed to early life stress were raised in an enriched postweaning environment, their depression-like behavioral responses to adult stress were also significantly diminished. However, their 5-HT2C pre-mRNA editing phenotype remained unaltered. Hence, the similar behavioral effects of enrichment and fluoxetine treatment during adolescence were not accompanied by similar changes in 5-HT2C pre-mRNA editing. Enriched and nonenriched BALB/c mice exposed to early life stress also exhibited significantly increased expression of mRNA and protein encoding the Gαq subunit of G-protein that couples to 5-HT2A/2C receptors. In contrast, Gαq expression levels were significantly lower in fluoxetine-treated mice. These findings suggest that compensatory changes in Gαq expression occur in mice with persistently altered 5-HT2C pre-mRNA editing and provide an explanation for the dissociation between 5-HT2C receptor editing phenotypes and behavioral stress responses.
Keywords: early life stress, serotonin 2C receptor, RNA editing, G-protein, antidepressant drugs, environmental enrichment
Introduction
In subjects with a genetic predisposition, early life trauma is a prominent risk factor for adult-onset depression. Adverse early life events lead to abnormalities in several brain functions that persist into adulthood and are often also resistant to adult treatment with antidepressant drugs (Caspi et al., 2003; Penza et al., 2003; Nemeroff et al., 2003; Kendler et al., 2004; Holmes et al., 2005).
Genetically distinct strains of mice that exhibit substantial differences in anxiety, stress reactivity, brain tissue levels of serotonin, and responsiveness to antidepressant treatment are suitable animal models for studies on the role of genetic and environmental factors in determining the impact of early life stress on the development of adult depression-like behavior. For example, in contrast to the resilient strain C57BL/6, BALB/c mice have lower forebrain serotonin levels (Zhang et al., 2004), exhibit spontaneously elevated anxiety and stress reactivity (Belzung and Griebel, 2001; Tang et al., 2002), and show increased responsiveness to chronic, but not acute, antidepressant treatment (Dulawa et al., 2004). They also differ in their basal and adult stress-induced expression of serotonin 2C (5-HT2C) receptor mRNA isoforms (Englander et al., 2005) that result from pre-mRNA editing via base modification of adenosine to inosine (Burns et al., 1997). In 5-HT2C pre-mRNA, five exonic adenosines (named A, B, C', C, and D editing sites) can be converted to inosine to alter the coding potential of three triplet codons, and distinctly edited isoforms encode receptors with decreased constitutive activity and decreased agonist-stimulated activation of Gαq protein (Niswender et al., 1999; Wang et al., 2000). Because 5-HT2C receptors are thought to play a role in the regulation of mood (Roth et al., 1998), it is of interest to note that significantly increased editing of 5-HT2C pre-mRNA was found in the prefrontal cortex of suicide victims with a history of major depression (Gurevich et al., 2002a), a disease in which a genetic predisposition and early life trauma are significant components of the underlying psychopathology (Caspi et al., 2003).
To test the effect of adverse early life events on the expression of the adult 5-HT2C pre-mRNA editing phenotype, we exposed BALB/c to infant maternal separation (IMS), a powerful paradigm of early life stress in rodents that elicits adult depression-like behaviors (Plotsky and Meaney, 1993). We found that adult mice exposed to IMS exhibit increased passive behavioral responses to stress that are resistant to antidepressant treatment with fluoxetine during adulthood. They also exhibit increased 5-HT2C pre-mRNA editing and increased Gαq expression in forebrain neocortex. In addition, although adolescent fluoxetine treatment and environmental enrichment both diminished behavioral responses to adult stress in these mice, only fluoxetine treatment altered the 5-HT2C pre-mRNA editing phenotype and significantly lowered the increased expression of Gαq that resulted from early life stress. Our findings suggest that, in mice exposed to early life stress, increased 5-HT2C pre-mRNA editing that leads to mRNA isoforms encoding receptors with reduced sensitivity to serotonin elicits either a compensatory increase in Gαq expression or, alternatively, that increased Gαq activation increases 5-HT2C pre-mRNA editing.
Materials and Methods
Animals.
All experiments involving animals were approved by the Institutional Animal Care and Use Committee of Columbia University. BALB/cJ mice were housed in a facility with a 12 h light/dark cycle with lights on at 6:00 A.M. and were given food and water ad libitum.
IMS.
Maternal separation of pups from their dam was conducted daily between 1:00 and 4:00 P.M., starting at postnatal day 2 (P2) and terminating at P15 (IMS animals). All dams were first-time mothers, and only litters of six to eight animals were used in this study. First, the dam was removed from the home cage and placed into a clean cage. Then, pups were collected and placed into another clean cage. After the 180 min separation, pups and dams were returned to their home cage. Control animals were standard facility-reared offspring of first-time mothers (SFR animals). For both SFR and IMS animals, the first cage change occurred 2 d after birth of pups, and cages were then changed every fourth day. SFR animals did not receive extra handling, and none of the SFR and IMS pups were culled and sexed after birth to keep handling of all animals to a minimum.
For all experiments on IMS and SFR mice, a total of 12 litters per group were used. At the time of weaning at P28, animals were randomly selected from different litters so that three to four animals per cage were derived from three to four different litters. These cages were then assigned to one of three different treatment conditions in which mice were either raised to adulthood without postweaning or adult treatment, treated with fluoxetine between P60 and P88, or treated with fluoxetine between P32 and P61.
Postweaning enrichment.
In additional experiments, IMS and SFR animals were weaned at P28 and then raised in an enriched environment. A major component of this enrichment was cross-housing of BALB/c mice with the stress-resistant C57BL/6 mice. These experiments were motivated by a pilot study by Holmes et al. (2005), which showed that cross-housing juvenile mice of two inbred strains that differ markedly in their emotionality (AJ and C57BL/6 mice) reversed the heightened anxiety-like phenotype of AJ mice in the free exploration tests but left the behavior of C57BL/6 cross-housed with AJ mice unaltered. Hence, in our experiments, IMS BALB/c mice were group housed with age- and sex-matched C57BL/6 mice postweaning (two BALB/c and two C57BL/6 mice per cage). Their cages were also supplemented with two established components of environmental enrichments, namely igloos and cotton swabs that stimulate nest-building activities. Compared with IMS BALB/c mice raised in a nonenriched environment, BALB/c mice cross-housed with C57BL/6 mice were far better groomed, and their body weights at P60 were 20% higher.
Modified forced swim test.
A modified version of the forced swim test (FST) was used, which, after re-exposure to the FST, elicits a state of “learned helplessness” that is sensitive to chronic, but not acute or subacute, antidepressant treatment (Dulawa et al., 2004). Briefly, mice were placed into plastic buckets (23 cm deep and 19 cm in diameter) filled with 25°C water and videotaped for 6 min to monitor the number of passive episodes and their duration (in seconds) as well as the time spent actively swimming. On the following day, mice were re-exposed to the FST for 6 min, and their behavior was recorded as described above. Twenty-four hours after the second FST, mice were killed by rapid decapitation, their brains were removed, and forebrain neocortical tissue was dissected.
Drug treatment.
For chronic fluoxetine treatments, mice received the drug dissolved in drinking water (10 mg/ml) as described previously (Gurevich et al., 2002a). The amount of fluoxetine intake was monitored via daily measurements of water consumption, which revealed an average intake of 16 mg/kg/d fluoxetine for adult SFR and IMS mice. Mice treated with fluoxetine between P32 and P61 consumed an average of 7.5 mg/kg/d in the first week of treatment, 10 mg/kg/d in the second week, 13.5 mg/kg/d in the third week, and 16 mg/kg/d in the fourth week. The latter dose has been shown previously to lead to serum levels of fluoxetine in BALB/c mice that are equivalent to therapeutic doses used in humans (Dulawa et al., 2004). Mice receiving either adult or adolescent fluoxetine were housed in at least four cages so that animals could be randomly selected from different cages for measurements of basal 5-HT2C pre-mRNA editing or behavioral testing followed by measurements of 5-HT2C pre-mRNA editing responses.
RNA extraction, real-time reverse transcription-PCR, and nucleotide sequencing.
Forebrain neocortical tissue was dissected using the mesodiencephalic junction as the anatomic landmark for the caudal border of the forebrain. RNA was extracted using guanidine/cesium chloride ultracentrifugation. Ten micrograms of total RNA were used for first-strand cDNA synthesis using murine moloney leukemia virus reverse transcriptase (USB, Cleveland, OH). Real-time PCRs were performed using the iQ5 Real-Time PCR Detection System (Bio-Rad, Hercules, CA). All reactions were run in triplicate and contained Sybr green (Bio-Rad), which fluoresces when intercalated with DNA. PCR amplification of 5-HT2C cDNA was specified by the primer pair 5′-TATTGTGCCCCGTCTGG-3′/5′-GAGCACGCAGGTAGTATT-3′. PCR products were gel purified and cloned into the plasmid vector pCRII (Invitrogen, Carlsbad, CA). Single bacterial transformations, plated onto 150 mm dishes, yielded >300 recombinant colonies from which 48 were randomly picked for plasmid DNA extraction and nucleotide sequencing using Sanger's dideoxy chain termination method.
Additional primers were designed to amplify 300 nt fragments near the N terminus of the Gαq subunit of G-protein (5′-ACTCTGGA-GTCCATCATG-3′/5′-TGTATGGGATCTTGAGCG-3′), the GABAA receptor α1 subunit (5′-CATTCTGAGCACACTGTC-3′/5′-AAGGTT-GTTTAGCCGGAG-3′), and the early growth response gene 3 (egr-3) (5′-ATGACCGGCAAACTCGCC-3′/5-GGCACTCATGAGGCTAAT-3′). In all real-time PCR experiments, measurements were made of the number of cycles required to reach the threshold fluorescence intensity [cycle threshold (Ct)]. Ct values for each reaction were subtracted from Ct values for SFR animals that served as a baseline, and the result was referred to as ΔCt. Fold changes in gene expression were calculated as 2ΔCt to reflect the fact that, under optimal conditions, the amount of PCR product doubles with each amplification cycle. Results were then normalized to those obtained for amplifications of the same cDNA samples using primers designed against β-actin, which acts as an internal standard, and averaged for each treatment group.
Immunoblotting.
Protein was extracted in a buffer containing 250 mm sodium chloride, 25 mm Tris-HCl, pH 7.5, 5 mm EDTA, pH 8, 1% Triton X-100, and protease inhibitors. Total protein (10 μg) was analyzed by Western blotting using rabbit polyclonal antibodies directed against Gαq/11α (1:1000; Chemicon, Temecula, CA) and β-actin (1:1000; Cell Signaling Technology, Berkeley, MA). Bound antigen was visualized using a peroxidase-conjugated anti-rabbit IgG secondary antibody (KPL, Gaithersburg, MD) in conjunction with enhanced chemiluminescence (ECL) (GE Healthcare, Piscataway, NJ), and optical densities of ECL signals on autoradiograms were then analyzed using NIH Image Analysis software.
Results
Effect of early life stress on adult behavioral responses to the forced swim test
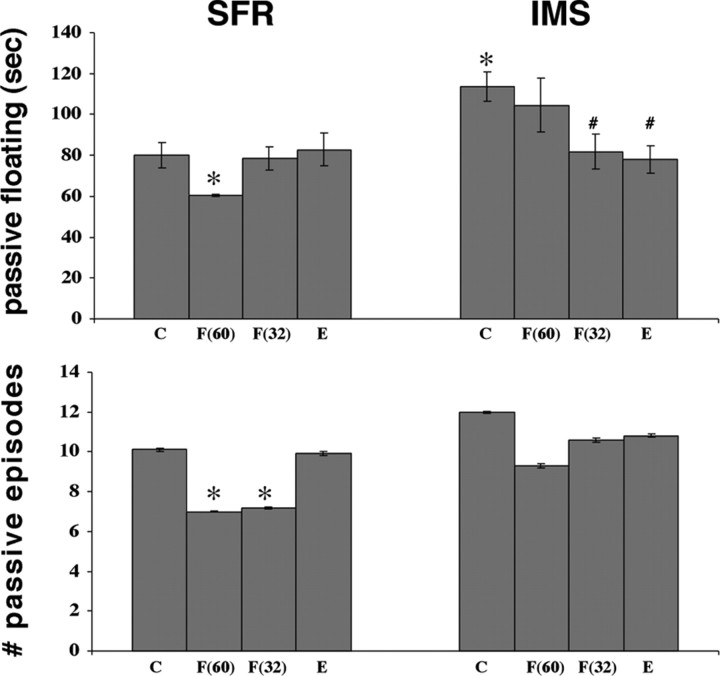
In the first series of experiments, we compared the behavioral responses of adult SFR and IMS mice to the FST (see Materials and Methods). As shown in Figure 1, IMS mice spent significantly more time passively floating compared with SFR controls. Moreover, SFR mice treated chronically with fluoxetine (16 mg/kg/d) between P60 and P88 exhibited significantly decreased passive floating time and significantly decreased numbers of passive episodes in the FST compared with nontreated SFR mice. In IMS mice, however, the same treatment with fluoxetine did not significantly alter the behavioral responses to the FST (Fig. 1). Thus, early life stress increases passive behavioral responses to adult FST exposure, and this behavioral phenotype is unaffected by adult fluoxetine treatment.
Figure 1.
Effect of early life stress (IMS) on behavioral responses to the forced swim test. Total time spent passively floating and number of passive episodes recorded during the second FST exposure of adult nontreated SFR and IMS mice (labeled C), SFR and IMS mice treated with fluoxetine between P60 and P88 [labeled F(60)] or between P32 and P61 [labeled F(32)], or exposed to postweaning environmental enrichment (E). Data represent means ± SEM of determinations made from 10 to 16 animals per group. Each group was composed of an equal number of male and female mice. Within each group, no significant differences were found in measurements obtained from male and female mice. A one-way ANOVA revealed significant differences (F(7,73) = 3.719; p = 0.0017 for passive floating time, and F(7,73) = 5.28; p < 0.0001 for the number of passive episodes) that were resolved post hoc (Tukey–Kramer multiple comparisons) as indicated. *p < 0.05 compared with SFR controls, and #p < 0.03 compared with IMS controls.
In additional experiments, SFR and IMS mice were treated with fluoxetine during adolescence (i.e., between P32 and P61), and their behavioral responses to the FST (measured at P61) are shown in Figure 1. In SFR animals, adolescent fluoxetine treatment, like adult fluoxetine treatment, significantly decreased the number of passive episodes, but their total time spent passively floating was not significantly different from corresponding measures obtained from nontreated SFR mice. In IMS mice, however, adolescent fluoxetine treatment led to significantly decreased passive floating time compared with nontreated IMS mice, and these behavioral measures no longer differed from nontreated SFR mice and SFR mice treated with fluoxetine between P32 and P61. In contrast to SFR mice, however, in fluoxetine-treated IMS mice, the total number of passive episodes remained unaltered, suggesting that fluoxetine differentially modulates the behavioral strategies (i.e., reducing the number of passive episodes vs reducing the duration of passive episodes) of SFR and IMS mice to ultimately reduce overall passive behavior.
Additional groups of SFR and IMS mice were raised in an enriched environment (see Materials and Methods). This treatment did not alter the behavioral response of SFR mice to the FST (Fig. 1). In IMS mice, however, this enrichment resulted in significantly decreased time spent passively floating compared with corresponding measures obtained from IMS mice raised in a nonenriched environment, and the behavioral responses of these mice to the FST differed neither from IMS mice treated with fluoxetine during adolescence nor from all groups of SFR mice (Fig. 1). Thus, adolescent fluoxetine treatment and environmental enrichment during adolescence are equally effective in diminishing the elevated passive behavioral responses of IMS mice to the FST, and both treatments reduced the duration of passive episodes but not the total number of these episodes.
Differential effects of postnatal enrichment and fluoxetine on forebrain neocortical 5-HT2C pre-mRNA editing and Gαq mRNA and protein expression
We have shown previously that normally raised adult BALB/c mice express predominantly nonedited and AB sites-edited forebrain neocortical 5-HT2C mRNA that encode receptors with the highest sensitivity to serotonin (Englander et al., 2005). We also found that exposure to the FST increased 5-HT2C pre-mRNA editing in these mice and that this effect was blocked by chronic fluoxetine treatment during adulthood (Englander et al., 2005). These results pointed to a significant role of stress in modulating 5-HT2C pre-mRNA editing phenotypes and suggested the possibility that different 5-HT2C pre-mRNA editing phenotypes detected in the forebrain neocortex reflect different behavioral/emotive states of the animal. To test this, we examined whether early life stress alters basal and stress-induced 5-HT2C pre-mRNA editing phenotypes of adult IMS mice and whether the similar behavioral effects of adolescent fluoxetine and postweaning enrichment described in Figure 1 are also accompanied by corresponding changes in 5-HT2C pre-mRNA editing.
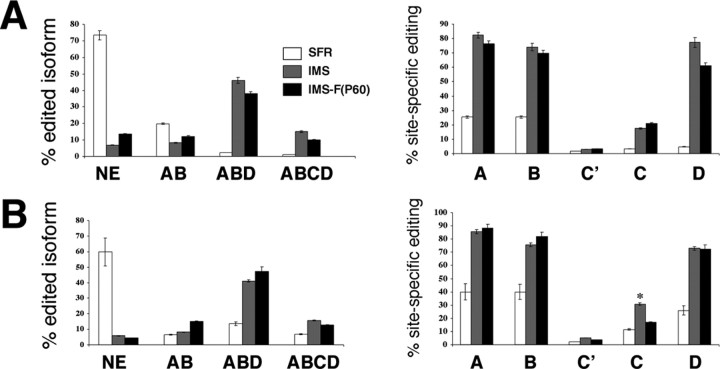
As shown in Figure 2A, compared with SFR mice, adult IMS-BALB/c mice exhibited a profoundly different basal 5-HT2C pre-mRNA editing phenotype. In fact, these mice exhibited significantly increased editing at the A, B, C, and D sites (p < 0.001) that led to decreased expression of nonedited and AB sites-edited 5-HT2C mRNA (p < 0.001 and p < 0.05, respectively) and increased expression of ABD- and ABCD-edited mRNA isoforms (p < 0.01) (Fig. 2A). This basal editing phenotype was unaltered in IMS mice treated chronically with fluoxetine in adulthood. Hence, in contrast to SFR mice that express predominantly 5-HT2C mRNA isoforms that encode receptors with the highest sensitivity to serotonin, >60% of edited 5-HT2C mRNA expressed in IMS mice encode receptors with reduced function regardless of whether they received fluoxetine in adulthood.
Figure 2.
Comparison of basal and FST-induced 5-HT2C pre-mRNA editing between SFR mice, drug-naive IMS mice, and IMS mice treated with fluoxetine between P60 and P88 [IMS-F(P60)]. A, Basal editing phenotype. B, Editing phenotype after exposure to the FST. The percentages of the four major edited isoforms (that, in all animals, represent >70% of all sequences) are shown on the left, and the percentages of site-specific editing (calculated for all sequences) are shown on the right. Data represent means ± SEM of determinations made from 48 sequences per animal and five animals per group (totaling 1440 sequences of 30 animals). A one-way ANOVA (F(14, 60) = 18.41; p < 0.0001) revealed that IMS mice exhibited significantly increased editing at the A, B, C, and D sites (post hoc Tukey–Kramer multiple comparisons test; p < 0.001), decreased expression of nonedited and AB sites-edited 5-HT2C mRNA (p < 0.001 and p < 0.05, respectively), and increased expression of ABD- and ABCD-edited mRNA isoforms (p < 0.01) compared with SFR mice. Measurements obtained after FST exposure (ANOVA; F(9, 55) = 33.6; p < 0.0001) also revealed that IMS-FST mice exhibited significantly higher editing at A, B, C, and D sites [p < 0.001 (A, B, and D sites); p < 0.02 (C site)], lower expression of nonedited mRNA (p < 0.001), and significantly higher expression of ABCD-edited (p < 0.05) and ABD-edited (p < 0.01) mRNA (B) compared with SFR mice. Fluoxetine treatment of IMS mice only led to lower C site editing when compared with IMS controls. *p < 0.05 compared with SFR and IMS-F(P60) mice. NE, Nonedited.
Figure 2B compares the editing phenotypes of adult SFR and IMS mice that were exposed to the FST. Although SFR BALB/c mice exhibited increased editing of 5-HT2C pre-mRNA in response to the FST as described previously (Englander et al., 2005), the editing phenotype of IMS-BALB/c mice exposed to the FST remained significantly different when compared with SFR-FST mice (i.e., they still exhibited significantly higher editing at A, B, C, and D sites) [p < 0.001 (A, B, and D sites); p < 0.02 (C site)], lower expression of nonedited mRNA (p < 0.001), and significantly higher expression of ABCD-edited (p < 0.05) and ABD-edited (p < 0.01) mRNA (Fig. 2B).
A statistical comparison between basal (Fig. 2A) and FST-induced (Fig. 2B) 5-HT2C pre-mRNA editing also revealed that, although the percentages of major edited 5-HT2C mRNA isoforms expressed in IMS-FST mice with and without adult fluoxetine treatment did not differ from the corresponding basal editing phenotypes, C site editing (calculated for all sequences) was significantly (p < 0.05) higher in IMS mice exposed to the FST but unaltered in IMS mice treated with fluoxetine [IMS (basal), 17.5 ± 1.5%; IMS/fluoxetine (basal), 20.96 ± 2.2%; IMS-FST, 30.8 ± 3.3%; IMS/fluoxetine/FST, 21.7 ± 2.7%]. In IMS-FST mice, increased C site editing was primarily attributable to the expression of the A(B)C-edited mRNA isoform, which represented ∼8% of their edited mRNA isoforms but was never detected at baseline or after adult fluoxetine treatment. Thus, adult stress exposure of IMS BALB/c mice further increased C site editing to increase the pool of mRNAs encoding receptors with reduced function, and this effect was blocked by adult fluoxetine treatment.
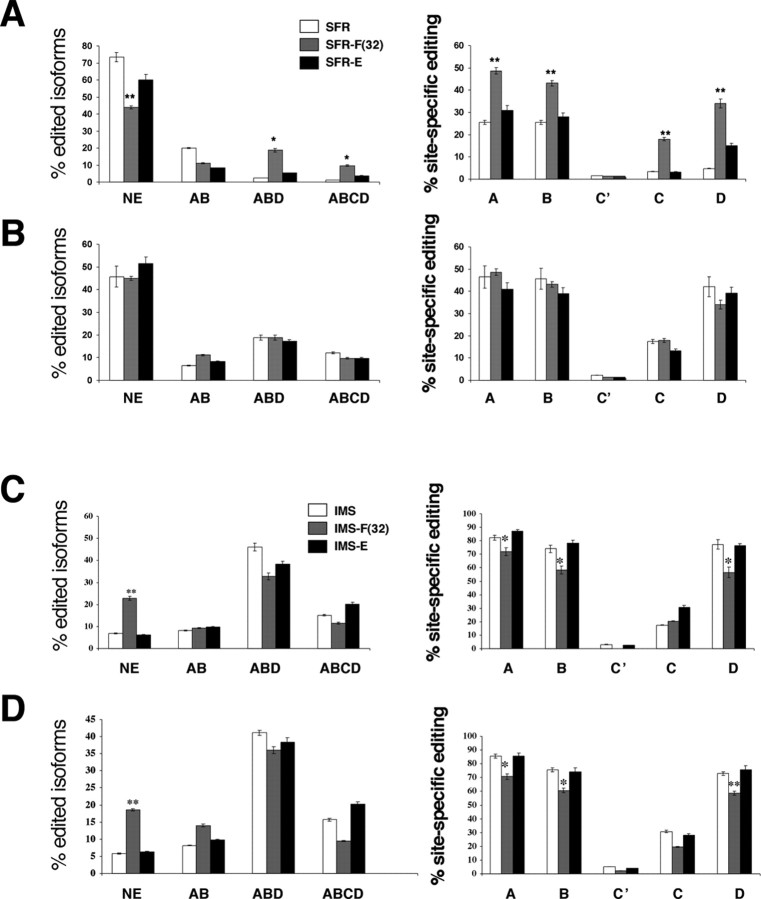
Finally, although both adolescent fluoxetine and postweaning enrichment exerted similar behavioral effects in the FST, 5-HT2C pre-mRNA editing phenotypes were not equally modulated by these treatments (Fig. 3). In SFR mice exposed to postweaning enrichment, neither the basal editing phenotype nor the editing phenotype after FST exposure differed from corresponding measures of SFR controls (Fig. 3A,B). In contrast, adolescent fluoxetine treatment of SFR mice led to an approximately twofold increase in basal A, B, C, and D site editing (ANOVA; F(7,24) = 23.93, p < 0.0001; post hoc, p < 0.01 for all four editing sites) resulting in decreased expression of nonedited mRNA and increased expression of ABD and ABCD sites-edited mRNA (ANOVA; F(11,32) = 57.84; p < 0.0001; post hoc, p < 0.001 for the nonedited isoform, p < 0.05 for ABD- and ABCD-edited isoforms) (Fig. 3A). The editing phenotype remained unaltered after FST exposure. In fact, after FST exposure, editing phenotypes of mice exposed to postweaning enrichment or adolescent fluoxetine did not differ from FST-exposed SFR controls (Fig. 3B).
Figure 3.
Effect of adolescent fluoxetine [F(32)] and postweaning enrichment on 5-HT2C pre-mRNA editing in SFR and IMS BALB/c mice. Basal (A) and FST-induced (B) editing phenotypes of SFR mice. Basal (C) and FST-induced (D) editing phenotype of IMS mice. In SFR mice, fluoxetine treatment resulted in significantly increased basal editing, and this editing was unaltered after FST exposure. **p < 0.01; *p < 0.05 compared with SFR controls. Enrichment had no effect on basal editing and did not protect from increased editing in response to FST exposure. In IMS mice, enrichment affected neither basal nor FST-induced editing phenotypes, but fluoxetine decreased basal editing of A, B, and D sites (leading to increased nonedited mRNA) and protected from increased editing responses to the FST. **p < 0.001; *p < 0.01 compared with IMS controls. Data represent means ± SEM of determinations made from 48 sequences per animal and five animals per group. NE, Nonedited.
The basal 5-HT2C pre-mRNA editing phenotype of IMS mice raised in the enriched environment was also indistinguishable from the basal editing phenotype determined for nontreated IMS mice (Fig. 3C). The basal editing phenotype of IMS mice treated with fluoxetine during adolescence, however, differed significantly from IMS controls (ANOVA; F(11,56) = 35.96; p < 0.0001 for editing combinations, and F(13, 68) = 127.98, p < 0.0001 for editing sites). Post hoc Tukey–Kramer multiple comparisons revealed that fluoxetine-treated IMS mice exhibited significantly increased expression of nonedited 5-HT2C mRNA (p < 0.001), and editing of the A, B, and D sites was significantly decreased (p < 0.01) compared with nontreated IMS mice and IMS mice raised in the enriched environment (Fig. 3C). Thus, in contrast to the effect of adolescent fluoxetine in SFR mice with low basal editing (where fluoxetine leads to increased basal editing), the same treatment decreased basal editing in IMS mice with high basal editing.
Within the groups of FST-exposed IMS mice, differences in the percentages of major edited isoforms revealed by ANOVA (F(11,60) = 36.27; p < 0.0001) were resolved post hoc only for FST-exposed mice treated with fluoxetine between P32 and P61 (Fig. 3D). These mice exhibited significantly increased expression of the nonedited isoform (p < 0.01 compared with nontreated IMS mice, and p < 0.05 compared with IMS mice raised in the enriched environment). In addition, significant differences in the percentages of site-specific editing (Fig. 3D) (ANOVA; F(14, 75) = 140.63; p < 0.0001) were found only for fluoxetine-treated mice that had lower A and B (p < 0.05) as well as D site editing (p < 0.01). Finally, no significant differences were found between basal (Fig. 3C) and FST-induced editing phenotypes of fluoxetine-treated IMS (Fig. 3D), indicating that adolescent fluoxetine treatment not only decreased basal 5-HT2C pre-mRNA editing but also blocked increased editing responses to adult stress.
A comparison between results obtained from the groups of SFR (Fig. 3A,B) and IMS (Fig. 3C,D) mice exposed to either postweaning enrichment or adolescent fluoxetine revealed that, regardless of treatment, editing of 5-HT2C pre-mRNA editing was always significantly higher in IMS mice. For example, FST-exposed IMS mice treated with fluoxetine or subjected to postweaning enrichment showed significantly higher A, B, and D site editing compared with corresponding groups of SFR mice (ANOVA; F(15, 44) = 31.040, p < 0.0001; post hoc, p < 0.05 for A, B, and D site editing of fluoxetine-treated mice and p < 0.001 for A, B, and D site editing of mice raised in the enriched environment).
In summary, in both SFR and IMS mice, postweaning environmental enrichment did not affect basal or FST-induced 5-HT2C pre-mRNA editing phenotypes. Adolescent fluoxetine treatment, however, had opposite effects on the basal 5-HT2C pre-mRNA editing phenotypes of SFR (increased editing) and IMS (decreased editing). Nevertheless, regardless of these treatments, 5-HT2C pre-mRNA editing was always significantly higher in IMS compared with SFR mice.
Results of real-time reverse transcription (RT)-PCR experiments revealed nearly identical cytoplasmic 5-HT2C mRNA levels in SFR mice, nontreated IMS mice, and IMS mice treated with fluoxetine between P60 and P88 or between P32 and P61 (threshold cycles were 19.4 ± 0.6, 19.2 ± 1.1, 19.0 ± 0.9, and 19.2 ± 0.8, respectively). Hence, the changes in editing described above occurred in the absence of changes in cytoplasmic 5-HT2C mRNA levels. However, an initial cDNA microarray screen pointed to differences in levels of mRNA encoding the Gαq subunit of G-protein in these samples. Gαq couples to 5-HT2A/2C receptors, and increased editing of 5-HT2C receptors decreases this coupling efficiency (Niswender et al., 1999; Wang et al., 2000). Results of independent real-time RT-PCR experiments (Table 1) revealed that, compared with nontreated SFR mice, Gαq mRNA levels were neither significantly altered in fluoxetine-treated SFR mice nor in SFR mice exposed to postweaning enrichment. In nontreated IMS mice, however, Gαq mRNA was significantly (approximately twofold) increased (p < 0.001) compared with SFR controls, and IMS mice raised in the enriched environment also exhibited increased levels of Gαq mRNA, although this increase did not reach significance. In contrast, IMS mice treated with fluoxetine between P60 and P88 exhibited significantly decreased Gαq mRNA levels compared with SFR controls (∼2.5-fold; p < 0.05), and this decrease was even larger in IMS mice treated with fluoxetine between P32 and P61 (approximately fourfold; p < 0.01).
Table 1.
Effect of chronic fluoxetine and environmental enrichment on forebrain neocortical gene expression in SFR and IMS micea
| Gαq | egr-3 | GABAA α1 | |
|---|---|---|---|
| SFR-F(P60) | 0.87 ± 0.16 | 0.57 ± 0.12 | 0.74 ± 0.12 |
| SFR-F(P28) | 1.35 ± 0.21 | 2.24 ± 0.43*** | 1.20 ± 0.16 |
| SFR-E | 1.26 ± 0.16 | 1.08 ± 0.15 | 1.54 ± 0.07** |
| IMS | 2.43 ± 0.42*** | 0.82 ± 0.29 | 1.11 ± 0.31 |
| IMS-F(P60) | 0.38 ± 0.12* | 0.55 ± 0.13 | 0.96 ± 0.14 |
| IMS-F(P28) | 0.24 ± 0.07** | 0.22 ± 0.06** | 0.53 ± 0.07** |
| IMS-E | 1.34 ± 0.17 | 0.67 ± 0.25 | 0.91 ± 0.21 |
aData are expressed as fold-change relative to SFR controls and represent mean ± SEM of determinations made from five to eight animals per group. ***p < 0.001; **p < 0.01; *p < 0.05 (ANOVA; post hoc Tukey–Kramer multiple comparisons).
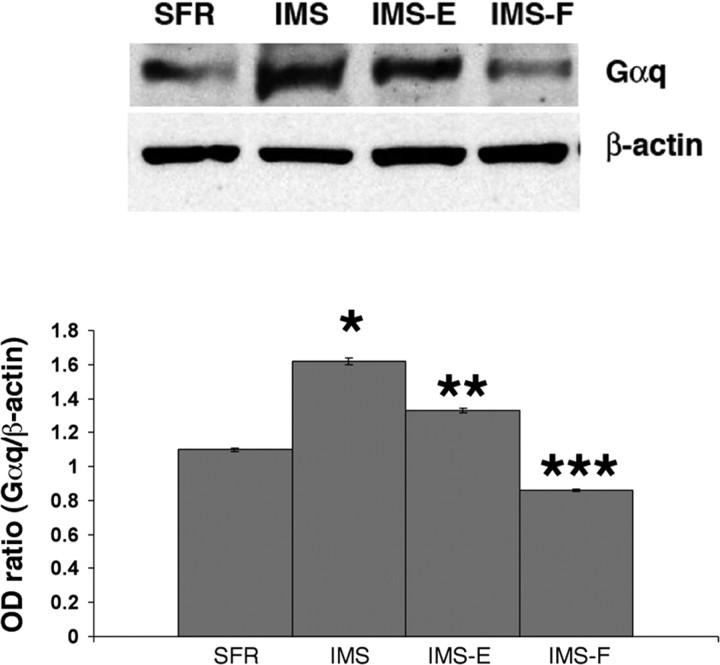
The microarray screen revealed additional genes with altered expression in IMS mice treated with fluoxetine, and real-time RT-PCR experiments confirmed that mRNA encoding the transcription factor egr-3 was significantly increased in SFR mice treated with fluoxetine between P32 and P61 (p < 0.001) but decreased in IMS mice that received the same treatment (p < 0.02) (Table 1). Furthermore, IMS mice treated with fluoxetine between P32 and P61 exhibited significantly decreased expression of mRNA encoding the α1 subunit of the GABAA receptor (p < 0.01), but SFR mice raised in the enriched environment exhibited increased expression of this receptor subunit (p < 0.01). Hence, early life stress, postweaning environmental enrichment, and fluoxetine treatment exert differential effects on forebrain neocortical gene expression in SFR and IMS mice (Table 1). The changes (increased or decreased expression) in Gαq, egr-3, or GABAA α1 mRNA expression differ for each treatment group. Thus, the different Gαq expression levels measured in this study are unlikely to be a result of general differences in RNA stability and/or turnover between the different groups of mice. Moreover, the differences in Gαq mRNA levels shown in Table 1 could also be confirmed with measurements of forebrain neocortical Gαq protein levels (Fig. 4). IMS BALB/c mice expressed 63 ± 12.9% more Gαq protein compared with SFR controls, and the Gαq protein levels in IMS mice raised in the enriched environment were 33 ± 5.7% higher. In fluoxetine-treated IMS mice, however, Gαq protein levels were 25 ± 5.1% lower compared with SFR controls, and more than twofold lower compared with nontreated IMS mice.
Figure 4.
Immunoblot analysis of Gαq protein expression in IMS BALB/c mice exposed to either enriched environment or fluoxetine. Ten micrograms of total protein, extracted from forebrain neocortex of adult SFR controls, nontreated IMS mice (IMS), IMS mice raised in the enriched environment (IMS-E), and IMS mice treated with fluoxetine between P60 and P88 (IMS-F), were loaded onto each lane. Optical densities of ECL signals on autoradiograms were measured using NIH Image software. Differences in the expression of Gαq immunoreactivity relative to the expression of β-actin were determined from measurements obtained from five animals per group. Statistical differences (ANOVA; F(3,13) = 18.33; p < 0.0001) were resolved post hoc using Tukey–Kramer multiple comparisons tests. *p < 0.01 compared with SFR; **p < 0.01 compared with IMS; ***p < 0.001 compared with IMS.
Discussion
The present study shows that, in the inbred strain BALB/c, early life stress increased expression of mRNA encoding 5-HT2C receptor isoforms with reduced sensitivity to serotonin in adulthood. This is achieved posttranscriptionally by RNA editing. Moreover, early life stress and genetic vulnerability act synergistically to produce persistent changes in 5-HT2C pre-mRNA editing that are more severe compared with those previously reported to result from adult stress exposure alone (Englander et al., 2005).
Early life stress of BALB/c mice also altered the effectiveness of chronic fluoxetine treatment in ameliorating behavioral and 5-HT2C pre-mRNA editing responses to adult stress. Consistent with the results of Dulawa et al. (2004), chronic fluoxetine treatment of adult SFR mice significantly decreased their passive behavioral response to the FST, and chronic adolescent fluoxetine treatment of these mice exerted a similar, albeit somewhat weaker effect (Fig. 1). In IMS BALB/c mice, however, chronic fluoxetine administered during adulthood did not significantly alter the behavioral response to the FST, and only fluoxetine administered during adolescence significantly decreased their passive behavioral responses to the FST. In this regard, it is of interest to note that reduced responsiveness to antidepressant treatment has also been reported for adult human subjects with a history of adverse early life events (Nemeroff et al., 2003). Moreover, in the present study, SFR and IMS BALB/c mice exhibited substantial differences in the modulation of 5-HT2C pre-mRNA editing phenotypes in response to stress and adult fluoxetine. Compared with SFR mice, IMS mice exhibited a robust increase in basal 5-HT2C pre-mRNA editing in the adult forebrain neocortex. In fact, although SFR mice exposed to the FST exhibited increased 5-HT2C pre-mRNA editing, in FST-exposed IMS mice, editing of 5-HT2C pre-mRNA was still significantly higher.
We have shown previously that adult fluoxetine treatment blocked the increased editing responses to adult FST exposure in normally raised BALB/c mice (Englander et al., 2005). The present study revealed, however, that although adult fluoxetine treatment of IMS BALB/c mice also blocked the increase in C site editing that occurred in response to FST exposure of nontreated IMS mice, it did not alter the abnormally increased basal 5-HT2C pre-mRNA editing phenotype. However, adolescent fluoxetine treatment of IMS mice diminished the magnitude of their increased basal 5-HT2C pre-mRNA and also blocked 5-HT2C pre-mRNA editing responses to adult stress. This suggests that fluoxetine can modulate the 5-HT2C pre-mRNA editing phenotype of IMS mice only when the drug is administered before the establishment of the adult editing phenotype.
Our data also show that, although postweaning environmental enrichment and adolescent fluoxetine treatment ameliorated the heightened passive behavioral responses of IMS BALB/c mice to the FST to a similar extent, only adolescent fluoxetine treatment led to decreased editing of 5-HT2C pre-mRNA in these mice. Moreover, in SFR mice, postweaning enrichment affected neither the behavioral responses to the FST nor the 5-HT2C pre-mRNA editing phenotype, and fluoxetine decreased passive behavioral responses to the FST but increased 5-HT2C pre-mRNA editing in these mice. These differential effects of enrichment and adolescent fluoxetine on the behavioral responses to stress and corresponding 5-HT2C pre-mRNA editing phenotypes in SFR and IMS BALB/c mice illustrate clearly that 5-HT2C receptor editing per se cannot be a major modulator of the behavioral (affective) state of the animal. Hence, it is unlikely that the increased 5-HT2C pre-mRNA editing previously reported for depressed suicide victims (Gurevich et al., 2002a) is the cause of depressed mood. Rather, our data suggest the possibility that the extent of alteration in 5-HT2C receptor function predicted to result from significantly altered 5-HT2C pre-mRNA editing is ultimately determined by the extent to which changes in Gαq expression levels can compensate for such changes in editing. In fact, the present study shows that IMS mice with increased editing of 5-HT2C pre-mRNA also exhibited increased Gαq expression. Because 5-HT2C receptor stimulation activates Gαq-stimulated signal transduction pathways, and because 5-HT2C pre-mRNA editing is increased in response to sustained stimulation of 5-HT2C receptors to encode receptors with decreased G-protein-coupling efficiencies (Gurevich et al., 2002b), these findings suggest that a persistent increase in editing elicits a compensatory increase in Gαq expression to maintain homeostatic signaling through 5-HT2C receptors. Indeed, we show here that vehicle-treated IMS mice or IMS mice raised in the enriched environment exhibit the highest Gαq expression levels and highest basal and FST-induced 5-HT2C pre-mRNA editing. IMS mice treated with fluoxetine during adulthood exhibit an approximately twofold decrease in Gαq mRNA expression compared with SFR controls, and these mice do not show an increase in editing in response to adult stress. IMS mice treated with fluoxetine during adolescence exhibit an approximately fourfold decrease in Gαq expression and also exhibit significantly decreased basal 5-HT2C pre-mRNA editing. Thus, in our study, a reversal of the increased basal 5-HT2C pre-mRNA editing in IMS mice was detected only in those mice that exhibited the largest decrease in Gαq expression levels.
Finally, although SFR mice also exhibit increased editing in response to either stress or fluoxetine, the changes in editing were not accompanied by significant changes in Gαq expression levels. This finding indicates that possible compensatory responses mediated at the level of Gαq expression occur in only mice with persistently altered 5-HT2C pre-mRNA editing phenotypes (IMS mice) and not in mice that exhibit increased editing responses to acute stress exposure or fluoxetine treatment (i.e., SFR mice exhibiting physiologically normal editing responses). Nevertheless, the a priori assumption that alterations in 5-HT2C pre-mRNA editing in vivo lead to those alterations in 5-HT2C receptor function that are predicted from the pharmacological properties of cloned receptor isoforms expressed in cultured cells is challenged by our finding that persistently altered editing can occur along with altered Gαq expression.
An alternative explanation for the present finding of altered Gαq expression in IMS BALB/c mice could be that Gαq activation itself plays a role in modulating 5-HT2C pre-mRNA editing. Although studies on the regulation of nuclear pre-mRNA editing are complicated by the fact that the mechanisms by which adenosine deaminases acting on RNA (ADAR) catalyze the deamination of adenosines are essentially unknown (Bass, 2002), it is relevant to note that Gαq activates phospholipase C, which in turn generates inositol trisphosphate that can be further phosphorylated to inositol hexakisphosphate (IP6). Interestingly, the crystal structure of one of the editing enzymes that act on 5-HT2C pre-mRNA, ADAR2, revealed that IP6 is buried within the enzyme core where it is necessary and essential for proper protein folding and catalytic activity (Macbeth et al., 2005). Nevertheless, despite previous speculation that signaling through 5-HT2C receptors may play a role in editing of 5-HT2C pre-mRNA (Gurevich et al., 2002b; Macbeth et al., 2005), a mechanistic link between receptor-mediated, Gαq-stimulated activation of phospholipase C and 5-HT2C pre-mRNA editing remains to be established.
In summary, in the stress-susceptible strain BALB/c, early life stress significantly altered the adult 5-HT2C pre-mRNA phenotype and rendered behavioral responses to adult stress resistant to adult fluoxetine treatment. Although postweaning enrichment and adolescent fluoxetine treatment exert significant behavioral effects in an adult stress paradigm, their effects on 5-HT2C pre-mRNA editing and Gαq expression differ. The finding that significant changes in 5-HT2C pre-mRNA editing phenotypes of IMS mice occur together with possibly compensatory changes in Gαq expression could explain why changes in behavioral phenotypes do not correlate with corresponding changes in 5-HT2C pre-mRNA editing phenotypes.
Footnotes
This work was supported by National Institutes of Health Grants MH061906 and MH062185.
References
- Bass BL. RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem. 2002;71:817–846. doi: 10.1146/annurev.biochem.71.110601.135501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviors in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Burns C, Chu H, Rueter S, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Dulawa S, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacol. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Englander MT, Dulawa S, Bhansali P, Schmauss C. How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. J Neurosci. 2005;25:648–651. doi: 10.1523/JNEUROSCI.3895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich I, Tamir H, Arango V, Dwork A, Mann JJ, Schmauss C. Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron. 2002a;43:349–356. doi: 10.1016/s0896-6273(02)00660-8. [DOI] [PubMed] [Google Scholar]
- Gurevich I, Englander MT, Adlersberg M, Siegal N, Schmauss C. Modulation of serotonin 2C receptor editing by sustained changes in serotonergic neurotransmission. J Neurosci. 2002b;22:10529–10532. doi: 10.1523/JNEUROSCI.22-24-10529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, le Guisquet AM, Vogel E, Millstein RA, Lerman S, Belzung C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neurosci Behav Rev. 2005;29:1335–1346. doi: 10.1016/j.neubiorev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk factors for major depression in women. Psychol Med. 2004;34:1475–1482. doi: 10.1017/s003329170400265x. [DOI] [PubMed] [Google Scholar]
- Macbeth MR, Schubert HL, VanDemark AP, Lingam AT, Hill CP, Bass BL. Inositol hexakisphosphate is bound in the ADAR2 core and required for RNA editing. Science. 2005;309:1534–1539. doi: 10.1126/science.1113150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP, Weiss PM, Dunner DL, Rothbaum BO, Kornstein S, Keitner G, Keller MB. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci USA. 2003;100:14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender C, Copeland SC, Herrick-Davis K, Emeson RB, Sanders-Bush E. RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. J Biol Chem. 1999;274:9472–9478. doi: 10.1074/jbc.274.14.9472. [DOI] [PubMed] [Google Scholar]
- Penza KM, Heim C, Nemeroff CB. Neurobiological effects of childhood abuse: implications for the pathophysiology of depression and anxiety. Arch Womens Mental Health. 2003;6:15–22. doi: 10.1007/s00737-002-0159-x. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult brain. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Roth BL, Willins DL, Kristiansen K, Kroetze WK. 5-hydroxytrypta-mine2-family receptors (5-hydroxytryptamine2A, 5-hydroxytryptamine2B, 5-hydroxytryptamine2C): where structure meets function. Pharmacol Ther. 1998;79:231–257. doi: 10.1016/s0163-7258(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Tang X, Orchard SM, Sanford LD. Home cage activity and behavioral performance in inbred and hybrid mice. Behav Brain Res. 2002;136:555–569. doi: 10.1016/s0166-4328(02)00228-0. [DOI] [PubMed] [Google Scholar]
- Wang Q, O'Brien P, Chen C-X, Cho D-SC, Murray JM, Nishikura K. Altered G protein-coupling functions of RNA editing isoform and splicing variant serotonin 2C receptors. J Neurochem. 2000;74:1290–1300. doi: 10.1046/j.1471-4159.2000.741290.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Beaulieu J-M, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]