Abstract
In this study, we address why metabotropic and ionotropic cholinergic signaling pathways are used to facilitate motor behaviors. We demonstrate that a Gαq-coupled muscarinic acetylcholine receptor (mAChR) signaling pathway enhances nicotinic acetylcholine receptor (nAChR) signaling to facilitate the insertion of the Caenorhabditis elegans male copulatory spicules into the hermaphrodite during mating. Previous studies showed that ACh (acetylcholine) activates nAChRs on the spicule protractor muscles to induce the attached spicules to extend from the tail. Using the mAChR agonist Oxo M (oxotremorine M), we identified a GAR-3(mAChR)-Gαq pathway that promotes protractor muscle contraction by upregulating nAChR signaling before mating. GAR-3(mAChR) is expressed in the protractor muscles and in the spicule-associated SPC and PCB cholinergic neurons. However, ablation of these neurons or impairing cholinergic transmission reduces drug-induced spicule protraction, suggesting that drug-stimulated neurons directly activate muscle contraction. Behavioral analysis of gar-3 mutants indicates that, in wild-type males, GAR-3(mAChR) expression in the SPC and PCB neurons is required for the male to sustain rhythmic spicule muscle contractions during attempts to breach the vulva. We propose that the GAR-3(mAChR)/Gαq pathway sensitizes the spicule neurons and muscles before and during mating so that the male can respond to hermaphrodite vulva efficiently.
Keywords: cholinergic signaling, gar-3, mAChR, muscle contraction, nAChR, spontaneous synaptic transmission
Introduction
Acetylcholine (ACh) can stimulate excitable cells by binding two types of receptors, nicotinic acetylcholine receptors (nAChRs) and muscarinic acetylcholine receptors (mAChRs). nAChRs are ligand-gated cation channels that consist of five subunits and were first identified by their sensitivity to the agonist nicotine (Langley, 1907; Chang et al., 1977; Hucho et al., 1978; Conti-Tronconi and Raftery, 1982). mAChRs are seven-transmembrane G-protein-coupled receptors, which were identified by their sensitivity to muscarine (Dale, 1914; Ewins, 1914; Kubo et al., 1986; Lanzafame et al., 2003). Many behavioral circuits coexpress nAChRs and mAChRs (Miller et al., 1992; Raizen et al., 1995; Weeks et al., 1997; Kim et al., 2001; Bany et al., 2003; Steger and Avery, 2004). Using agonists and antagonists for different receptor types, effects of nicotinic and muscarinic receptors signaling have been described in organismal behaviors such as early visual processing, mice memory of conditioned fear, and human memory (Erskine et al., 2004; Feiro and Gould, 2005; Ellis et al., 2006). However, determining how different cholinergic signaling events regulate vertebrate behaviors is difficult because of the complexity of the vertebrate nervous system. In this study, we use Caenorhabditis elegans male spicule protraction behavior as a model to understand how in vivo nicotinic and muscarinic signaling pathways regulate a simple behavioral circuit.
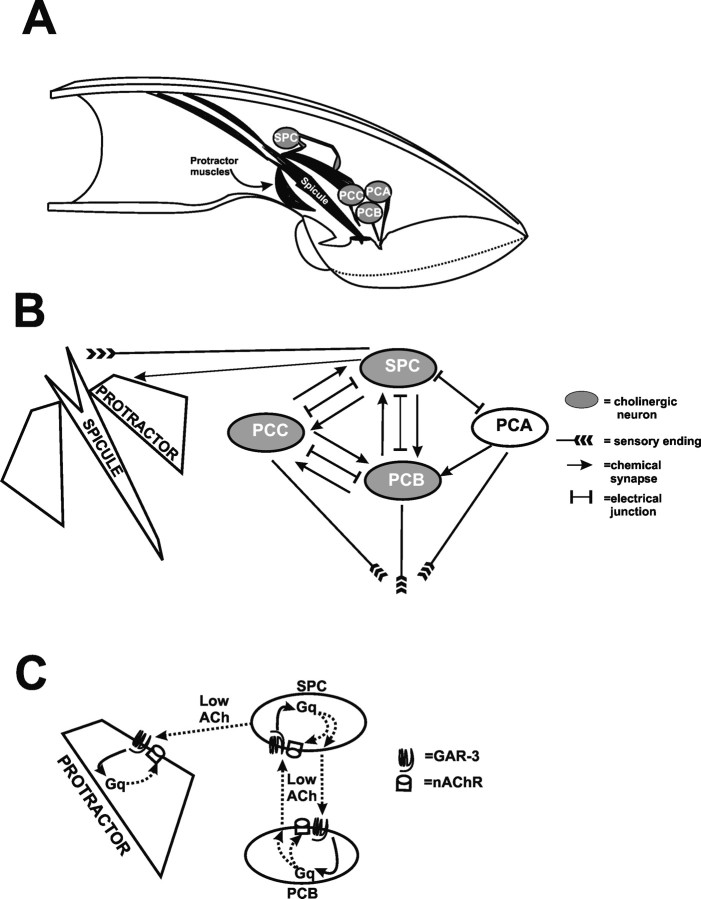
During mating, males rhythmically protract a pair of copulatory spicules from their tails; the spicule tips repetitively tap against the hermaphrodite vulval slit. When the vulva is partially penetrated, rhythmic spicule movements cease, and the spicules fully insert and remain inserted until ejaculation is completed (Garcia et al., 2001). Two protractor muscles control the motions of each spicule (Fig. 1A). The integration of vulva location and spicule insertion is controlled by the bilateral PCA, PCB, and PCC neurons, located posterior to the cloacal opening. These postcloacal sensilla neurons sense the vulva and initiate spicule insertion behavior. On vulval contact, the PCB and PCC neurons release ACh that stimulates rhythmic protractor muscle contractions. When the spicules partially penetrate the vulva, the cholinergic SPC neurons, whose proprioceptive sensory processes are attached to the spicule base, directly activate sustained protractor contraction (Garcia et al., 2001).
Figure 1.
Anatomy, circuit diagram, and model of GAR-3(mAChR)-mediated cell interactions. A, Cutaway representation of muscles and neurons used in spicule protraction behavior. B, Partial circuit diagram of connections between the SPC neuron, protractor muscles, and the p.c.s. neurons [adapted from Sulston et al. (1980) and S. W. Emmons, D. H. Hall, and M. Xu, personal communication] (www.wormatlas.org). The SPC and p.c.s. neurons make more extensive connections to other cells not covered in this report. C, Before mating, activated GAR-3(mAChR)/Gαq on the SPC and PCB neurons promotes low levels of acetylcholine release. The low-level release of acetylcholine also indirectly results in an increased sensitivity of postsynaptic nAChR signaling potential on the spicule protractor muscle cells and possibly also in the SPC and PCB neurons.
Protractor contractions can be induced by the nicotinic ACh agonists levamisole and nicotine. Levamisole-activated receptors induce muscle contraction by mobilizing intracellular calcium through the unc-68-encoded ryanodine receptors. In contrast, nicotine-activated receptors transmit their signals by mobilizing intracellular and extracellular calcium through UNC-68(ryanodine receptor) and through egl-19-encoded voltage-gated calcium channels, respectively. Interestingly, the heterotrimeric G-protein Gαq, and phospholipase C² are required for levamisole-induced spicule protraction, suggesting that nAChR signaling interacts with a Gαq-coupled pathway (Garcia et al., 2001; Gower et al., 2005). Because ACh regulates the spicule protraction circuit, the Gαq pathway might be coupled to an mAChR.
To understand how Gαq integrates with nAChRs signaling, we investigated how the mAChR GAR-3 promotes spicule muscle contraction. We found that the cholinergic neurons, which control protractor muscle contractions, express GAR-3(mAChR). During copulation, the spicule protraction circuit requires GAR-3(mAChR) to sustain rhythmic muscle contractions during conditions when the vulva is difficult to breach.
Materials and Methods
C. elegans strains
C. elegans strains were grown at 20°C on nematode growth media (NGM) plates seeded with the bacterial strain OP50 (Brenner, 1974). Pharmacological and behavioral assays were conducted at room temperature (21–23°C).
All males used in this study contain him-5(e1490) on linkage group V (LGV) (Horvitz et al., 1979). Additional alleles used were as follows: unc-38(sy576) (Garcia et al., 2001), egl-30(ad805) (Brundage et al., 1996), egl-30(tg26gf) (Doi and Iwasaki, 2002), unc-29(e1072) (Lewis et al., 1980) on LGI; pha-1(e2123) (Schnabel and Schnabel, 1990), unc-64(e246) (Brenner, 1974) on LGIII; unc-17(e245) (Brenner, 1974), egl-8(n488) (Miller et al., 1999) on LGV. gar-3(gk305) on LGV was generated by the C. elegans Gene Knockout Consortium.
The sy576 mutation in unc-38 creates a C-to-T missense mutation that causes the sequence ttgttcccgtttgat to change to tggttcctgtttgat. The proline-to-leucine change occurs in the dicysteine loop of the protein.
Pharmacology
Levamisole (LEV) was purchased from ICN Biomedicals (Aurora, OH) and oxotremorine M (Oxo M) was from Sigma-Aldrich (St. Louis, MO). These agonists were prepared in distilled water. LEV was kept frozen at −20°C as a 1 mm stock solution. Oxo M was kept at 4°C as a 1 m stock solution. These solutions were diluted in distilled water as needed. Approximately 400 μl of diluted drug was added to a Pyrex three-well titer dish, and ∼5–10 virgin males were put into the drug. For LEV, we observed the males for 5 min with a Leica (Wetzlar, Germany) MZ 7.5 stereomicroscope. A male was considered resistant to the drug if he did not protract his spicules within the observation window. For Oxo M, we observed the males for 10 min, because males responded less rapidly to this drug. A male was considered to have protracted his spicules if he extended them for ≥5 s. Fresh drug was used after ∼30 virgin males were assayed. The EC50 value for LEV sensitivity was determined using the GraphPad Prism (version 4.03) software. The Oxo M EC90 value for wild-type males was calculated using SPSS 11 software. Statistical analyses were done using GraphPad Prism (version 4.03) and GraphPad InStat (version 3.06) software.
Behavioral assays
Assay for spontaneous spicule protraction (Prc) phenotype.
L4 males were separated from hermaphrodites and put onto a fresh NGM agar plate containing Escherichia coli OP50. Approximately 20 h later, adult virgin males were scored for spontaneous spicule protraction. Males with spicules partially or fully protracted were scored positive for the Prc phenotype.
Observation of behaviors on Oxo M plates.
A total of 0.5 ml of 100 mm Oxo M was applied on top of NGM plates containing an E. coli OP50 lawn. When the drug had soaked into the agar, 5–10 virgin adult males were placed onto the bacterial OP50 lawn. Behaviors were then observed with a Zeiss (Oberkochen, Germany) Stemi SV 11 microscope.
Mating observation.
Wild-type L4 males, gar-3(gk305) L4 males and unc-64(e246) L4 hermaphrodites were picked onto separate plates 14 h before observation. To make the bacterial lawn used for mating observations, we grew OP50 in LB at 37°C overnight without aeration, concentrated 1 ml of culture via centrifugation, and resuspended the bacterial pellet in 20 μl of LB. We then placed ∼10 μl of the concentrated bacteria onto the center of the NGM agar plate. After the excess liquid soaked into the agar, we placed 15–30 young adult unc-64(e246) hermaphrodites and one virgin adult male onto the lawn. Male mating behavior was observed with a Zeiss Stemi SV 11 microscope.
We recorded how long the male spent performing each step of mating behavior using a computer and a time recording Visual Basic Macro written in Microsoft Excel:
Sub Macrotimerecorder()
Dim row As Integer
Dim InputVal As String
InputVal = “ ”
row = 1
Sheets(“Sheet1”).Activate
Columns(“A:B”).Select
Selection.ClearContents
Do While (InputVal <> “E”)
InputVal = InputBox(“Enter S, 1, 2, or E”)
If InputVal = “e” Then
InputVal = “E”
End If
If InputVal = “s” Then
InputVal = “S”
End If
Cells(row, 1).Value = InputVal
Cells(row, 2).Formula = “ = NOW()”
Cells(row, 2).Select
Selection.Copy
Cells(row, 2).Select
Selection.PasteSpecial Paste: = xlValues, Operation: = xlNone, SkipBlanks:= False, Transpose: = False
Application.CutCopyMode = False
row = row + 1
Loop
End Sub
We measured the duration and number of times a male: backed along the length of hermaphrodite searching for the vulva, located and rhythmically prod the vulva with his spicules, moved off the vulva or fully inserted his spicules, ejaculated, retracted his spicules, and moved off the hermaphrodite. After the male finished mating, we removed both the male and his hermaphrodite mate. A fresh virgin male was used for each observation.
Laser ablation
Standard protocol was used to laser ablate neurons in the male tail (Bargmann and Avery, 1995). Laser ablation was conducted using a Spectra-Physics (Mountain View, CA) VSL-337ND-S Nitrogen Laser attached to an Olympus (Center Valley, PA) BX51 microscope. Mid-L4 (the stage in L4 during tail spike retraction) males were operated on pads containing 4 mm NaN3. To rule out any effect the anesthetic pads might have on behavior, control males were also placed on similar pads for a comparable time. We found that the anesthetic pad did not affect Oxo M sensitivity in the control males. SPC and PCB neurons were identified by cell position and killed at mid-L4 stage. After the operation, males were put onto a fresh NGM plate with OP50 lawn overnight.
Expression pattern of unc-38 and gar-3
The transgenic line xuEx[Punc-38::YFP + lin-15(+)]; lin-15(n765ts) was a gift from Dr. Shawn Xu (Life Sciences Institute, University of Michigan). Plasmids containing the unc-38 promoter::YFP construct (100 ng/μl) and the lin-15 gene (40 ng/μl) were coinjected into lin-15(n765ts) hermaphrodites. The unc-38 promoter::YFP plasmid is a transcriptional fusion that contains YFP expressed from 1.1 kb DNA upstream of the first ATG of unc-38.
To analyze the gar-3 expression pattern driven by the promoter Pgar-3A as reported by Steger and Avery (2004), we amplified 6 kb of DNA upstream of gar-3 ATG, using primers ATTB1gar-3Aup and ATTB2gar-3Adwn (Table 1). The primers contain the Invitrogen Gateway attB sites, thus allowing the PCR product to be recombined into the entry vector pDG15 (Reiner et al., 2006), using BP clonase (Invitrogen, Carlsbad, CA). The resulting plasmid was called pLR56. Using LR clonase (Invitrogen), pLR56 was then recombined with pGW322YFP (Reiner et al., 2006) to generate pLR59. pGW322YFP is a pBR322-based vector that contains the Gateway Vector Conversion Reading frame Cassette C.1 (Invitrogen), located upstream of the yellow fluorescent protein (YFP) gene. pLR59 (50 ng/μl) was coinjected with pBX1 (100 ng/μl) (Granato et al., 1994) and pUC18 (50 ng/μl) into pha-1(e2123); him-5 (e1490) to generate the transgenic line pha-1(e2123); him-5 (e1490); rgEx93.
Table 1.
Primers used in this study
| Primer name | Primer sequence |
|---|---|
| ATTB1gar-3Aup | GGGGACAAGTTTGTACAAAAAAGCAGGCTCATAAGCATCATGAGCAACAT |
| ATTB2gar-3Adwn | GGGGACCACTTTGTACAAGAAAGCTGGGTCGATTAATAAATGTGCAGGAG |
| Gar3upstrmRv | CATAAGCATCATGAGCAACATCTCCACTTCTCGTGAGC |
| Dwngar3B | GATTAATAAATGTGCAGGAGGAGTAATAATGGTGTATGT |
| ATTB1GAR-3strt | GGGGACAAGTTTGTACAAAAAAGCAGGCTATGCAGTCCTCTTCGTTGGGGAATGCTGATGATCCTCGAT |
| ATTB2Gar-3end | GGGGACCACTTTGTACAAGAAAGCTGGGTCTAGTTGCGTCGGACATATCCCTGATTCATTGTGGGAC |
| ATTB1gar-3Bup | GGGGACAAGTTTGTACAAAAAAGCAGGCTGGTTGTTGTCACAGATTGTCT |
| ATTB2gar-3Bdwn | GGGGACCACTTTGTACAAGAAAGCTGGGTCCCTCTCGTCTGTGGTGATCC |
| Gar3xbaF | GGGGTCTAGAATGCAGTCCTCTTCGTTGGGGAATGCTG |
| Gar3avrR | GGGGCCTAGGGTTGCGTCGGACATATCCCTGATTCATTG |
To test whether the sequence Pgar-3B drove gar-3 expression, 3.5 kb DNA upstream of the gar-3 isoform Y40H4A.1a.1 5′ untranslated region (UTR) was amplified using primers Gar3upstrmRv and Dwngar3B (Table 1). The Pgar-3B promoter was blunt-end cloned into the SmaI site of the YFP-containing plasmid pSX322 (Reiner et al., 2006) to generate pYL1. pYL1 (50 ng/μl), pBX1 (100 ng/μl), and pUC18 (50 ng/μl) were injected into pha-1(e2123); him-5 (e1490), to generate pha-1(e2123); him-5 (e1490); rgEx15. Images were recorded using an Olympus BX51 research microscope and an Olympus FV1000 confocal microscope.
Rescue using genomic gar-3 gene
Rescue using gar-3 genomic sequence driven by the hsp16–2 promoter.
To drive gar-3 expression using the heat shock promoter hsp16–2, the Gateway Conversion Reading frame Cassette C.1 (Invitrogen) was cloned into the EcoRV site of the heat shock promoter vector pPD49.78 (courtesy of A. Fire, Stanford University School of Medicine, Stanford, CA) (Addgene plasmid 1447; Addgene, Cambridge, MA) to generate the Gateway destination vector pTG14. Primers ATTB1GAR-3strt and ATTB2Gar-3end (Table 1) were used to amplify 4.9 kb of gar-3 genomic DNA and flank the DNA with attB sites. Using BP clonase, the PCR product was recombined into pDG15 to generate pYL3. gar-3 was then recombined from pYL3 into pTG14, using LR clonase, to generate pYL4. An injection mixture containing pYL4 (25 ng/μl), pBX1 (100 ng/μl) and pUC18 (75 ng/μl) was injected into pha-1(e2123; him-5 (e1490) gar-3(gk305). Three lines were obtained; all transgenic lines were rescued after heat shock. One of these lines, pha-1(e2123); him-5 (e1490) gar-3(gk305); rgEx65 was further analyzed.
Rescue using gar-3 genomic sequence driven by gar-3 endogenous promoters.
A 4.9 kb genomic gar-3-containing sequence from the ATG to last asparagine codon was PCR amplified via primers Gar3xbaF and Gar3avrR (Table 1). To fuse the C-terminal end of GAR-3 to YFP, the PCR product was cut with XbaI and AvrII, and then cloned into the XbaI site of the YFP-containing plasmid pSX322 to generate pYL5. To add different promoters in front of gar-3::YFP, pYL5 was cut with XbaI and ligated to the Gateway Vector Conversion Reading frame Cassette C.1 (Invitrogen) to generate the Gateway destination vector pYL6. To generate Pgar-3A::gar-3::YFP, the gar-3 promoter Pgar-3A contained on pLR56 was recombined into pYL6, using LR clonase, to generate pYL8. To construct Pgar-3B::gar-3::YFP, the 3.5 kb gar-3 promoter Pgar-3B was PCR amplified via primers ATTB1gar-3Bup and ATTB2gar-3Bdwn (Table 1), and recombined into plasmid pDG15, using BP clonase, to generate the entry clone pLR57. pLR57 was then recombined with pYL6, using LR clonase, to generate pYL9.
An injection mixture containing pYL8 (20 ng/μl), pBX1 (100 ng/μl), and pUC18 (80 ng/μl) was injected into pha-1(e2123); him-5 (e1490) gar-3(gk305). Three transgenic lines were obtained, and the line that contained rgEx90 [Pgar-3A::gar-3::YFP] was further analyzed. An injection mixture containing pYL9 (50 ng/μl), pBX1 (100 ng/μl), and pUC18 (50 ng/μl) was also injected into pha-1(e2123); him-5 (e1490) gar-3(gk305). The him-5 (e1490) gar-3(gk305); pha-1(e2123); rgEx92 [Pgar-3B::gar-3::YFP] transgenic line expressed brightly fluorescing GAR-3::YFP. When assayed with 50 mm Oxo M, this line was rescued for Oxo M sensitivity (see Table 3). However, after three to four generations, YFP expression became faint and disappeared eventually. Reducing pYL9 concentration to 10 and 1 ng/μl in the injection mixture did not solve this problem. We speculated that the reduced YFP expression might be attributable to silencing of repetitive transgene arrays. Thus, we used PvuII-digested C. elegans genomic DNA (50 ng/μl) as the carrier DNA to coinject with pYL9 (50 ng/μl) and pBX1 (100 ng/μl). Fifteen lines were obtained and YFP expression in all of them was stable. The line him-5 (e1490) gar-3(gk305); pha-1(e2123); rgEx107 was further analyzed. Interestingly, when pYL8 and pYL9 were coinjected at 30 ng/μl, expression from both constructs was stable, even when PvuII-digested C. elegans genomic DNA was not used as a carrier. Presumably, some sequence in pYL8 can reduce the silencing of pYL9.
Table 3.
Efficiency of oxotremorine M-induced spicule protraction
| Genotype | Males protracted in 50 mmOxo M | n | p valuea |
|---|---|---|---|
| Wild type | 86 | 72 | |
| unc-64(lf) | 43 | 30 | <0.0001 |
| unc-17(lf) | 33 | 30 | <0.0001 |
| egl-30(lf) | 7 | 30 | <0.0001 |
| egl-8(lf) | 65 | 40 | 0.015 |
| unc-38(lf) unc-29(lf) | 58 | 31 | 0.0037 |
| gar-3(lf) | 4 | 50 | <0.0001 |
| SPC-ablated wild type | 11 | 19 | <0.0001 |
| PCB-ablated wild type | 30 | 20 | <0.0001 |
| SPC- and PCB-ablated wild type | 28 | 67 | <0.0001 |
| gar-3(lf)b | 3 | 34 | |
| Wild typeb | 63 | 87 | <0.001 |
| gar-3(lf); rgEx90(Pgar-3A::gar-3 (+))b | 10 | 20 | |
| gar-3(lf); rgEx92(Pgar-3B::gar-3 (+))b | 62.5 | 32 | <0.0001 |
| gar-3(lf); rgEx99(Pgar-3A+Pgar-3B::gar-3 (+))b | 89 | 38 | <0.0001 |
| gar-3(lf); rgEx94(Punc-103 E::gar-3(+))b | 92 | 13 | <0.0001 |
| gar-3(lf); rgEx95(Punc-103 F::gar-3 (+))b | 97 | 29 | <0.0001 |
| gar-3(lf); rgEx65(Phsp-16–2::gar-3 (+) − heat shockb | 9 | 32 | |
| + heat shock | 100 | 22 | <0.0001 |
aValues of p were calculated using Fisher's exact test. Nontransgenic males were compared with nonoperated wild type. Males that contained pha-1(e2123) were compared to pha-1(e2123);gar-3(lf).
bMales contained pha-1(e2123).
Rescue using tissue specifically expressed gar-3 gene.
Entry clones pLR21 and pLR28 contain the 5.4 kb unc-103 promoter Punc-103E and the 5 kb unc-103 promoter Punc-103F, respectively (Reiner et al., 2006). The unc-103 promoters were recombined from pLR21 and pLR28 into the Gateway destination vector pYL6 to generate pYL10 and pYL11. Fifty nanograms per microliter of both plasmids were injected separately with pBX1 (100 ng/μl) and pUC18 (50 ng/μl) to generate the extrachromosomal arrays rgEx94 (containing Punc-103E::gar-3::YFP) and rgEx95(containing Punc-103F ::gar-3::YFP).
Results
EGL-30 Gαq subunit and synaptic transmission are required for ACh-induced spicule protraction
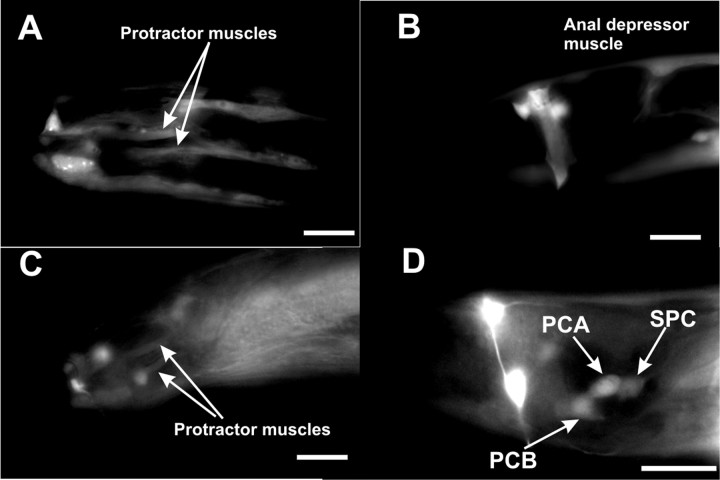
The nAChR agonist LEV can induce spicule protractor muscle contraction (Fig. 2) through activating nAChRs composed of the UNC-38 and UNC-29 subunits (Fleming et al., 1997; Garcia et al., 2001). Other LEV-sensitive nAChR subunits such as UNC-63 (Culetto et al., 2004) and LEV-1 (Fleming et al., 1997) are not essential for LEV-induced spicule protraction (L. R. Garcia, unpublished observation). The SPC cholinergic neurons directly innervate the spicule muscles (Sulston et al., 1980) and are not essential for LEV to induce protractor contraction (Garcia et al., 2001). However, it is possible that the physiological dose–response to LEV might be attributable to the drug activating these neurons in addition to the muscles. To determine whether LEV-sensitive receptors could act in both muscles and neurons that control spicule insertion, we used a construct containing YFP expressed from the unc-38 promoter to identify UNC-38(nAChR)-expressing cells in the male tail. We found that UNC-38(nAChR) expresses in body wall and sex muscles, including the protractor muscles, but not in any of the neurons that are associated with spicule function (Fig. 3A,B). Thus, we conclude that LEV directly induces spicule protraction only through nAChRs on the protractor muscles. This is consistent with the published result that muscle-expressed UNC-38(nAChR) can restore LEV-induced spicule protraction to unc-38 mutants (Garcia et al., 2001).
Figure 2.
Levamisole-induced spicule protraction requires GAR-3(mAChR)/Gαq signaling and ACh synaptic transmission. The numbers on the vertical axis represent the percentage of males that protract their spicules at each concentration of LEV. The numbers above or within the bars are the actual percentage of males that protracted in the drug. The numbers of males assayed at each concentration are listed below the bars.
Figure 3.
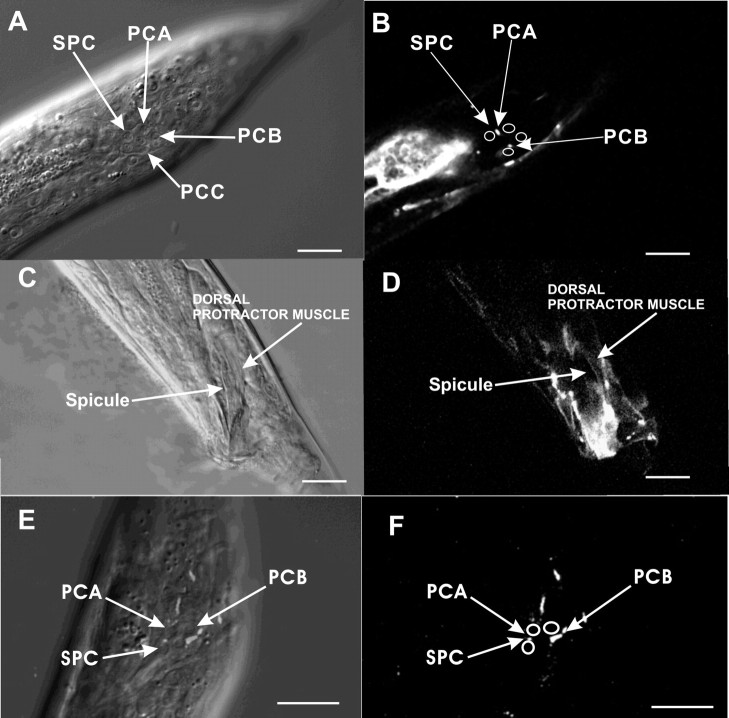
Male tail expression of unc-38 and gar-3 promoters. Fluorescence images of the right lateral tail region of an adult male expressing the unc-38 promoter::YFP construct (A), early L4 male expressing the unc-38 promoter::YFP construct (B), and adult (C) and late L4 (D) males expressing the Pgar-3B::YFP construct pYL1. Scale bar, 10 μm.
The EC50 concentration of LEV that caused 50% of males to protract their spicules was 422 nm. In a previous report, mutations that reduce signaling in excitable cells were assayed for their effects on LEV-induced spicule protraction. One of the genes found to be involved was egl-30(Gαq) (Garcia et al., 2001). This was interesting because activated heterotrimeric G-proteins are known to transmit signals from metabotropic but not ionotropic receptors. We revisited this observation and confirmed that the LEV dose–response requires the egl-30-encoded Gαq protein. The strong loss-of-function (lf>) allele egl-30(ad805) results in a 13-fold increase in the EC50 (Fig. 2). This suggests that, in the wild type, LEV-activated muscle nAChRs require Gαq function. Because Gαq is expressed broadly in excitable cells in C. elegans and has been shown to regulate neuronal synaptic transmission (Lackner et al., 1999; Bastiani et al., 2003), we asked whether synaptic transmission is also required for nAChR-induced muscle contraction. To test this, we assayed the sensitivity of unc-64 and cha-1 mutant males to LEV. unc-64(e246) is a loss-of-function allele of the gene that encodes syntaxin (Ogawa et al., 1998), a plasma membrane receptor for intracellular neurotransmitter vesicles. cha-1(p1152) is a loss-of-function allele of the gene that encodes the choline acetyltransferase enzyme (Alfonso et al., 1994). When treated with 1 μm LEV, 79% of wild-type males (n = 85) protracted their spicules, whereas only 5% of unc-64(lf) (n = 20; p < 0.0001) and cha-1(lf) males (n = 20; p < 0.0001) responded to the drug (Fig. 2). This indicates that cholinergic synaptic transmission is required for efficient LEV-induced spicule muscle contraction. This was surprising considering that activating muscle receptors via LEV should bypass the requirement for endogenous ACh secretion. To test further whether endogenous ACh transmission was required, we ablated the cholinergic SPC cholinergic motor neurons in wild-type males. In 1 μm LEV, only 22.2% of SPC-ablated males protracted their spicules (n = 18; p = 0.028), compared with 60% of control males (n = 25), demonstrating that synaptic transmission between neurons and muscles contributes to LEV sensitivity.
mAChRs are required in EGL-30(Gαq) regulation of spicule protraction
Because EGL-30(Gαq) was required to facilitate agonist-induced spicule muscle contraction, we used the gain-of-function (gf) allele egl-30(tg26gf) to test whether activated EGL-30(Gαq), by itself, can also induce spicule protraction (Doi and Iwasaki, 2002; Gruninger et al., 2006). In these worms, 76% of males protracted their spicules spontaneously (Prc phenotype) (Table 2).
Table 2.
Suppression of the egl-30(tg26)-induced Prc phenotype
| Genotype | Males protracted spontaneously | n | p value |
|---|---|---|---|
| egl-30(tg26gf) | 76 | 97 | |
| Wild type | 7 | 100 | <0.0001 |
| egl-30(tg26gf); unc-64(e246) | 22 | 275 | <0.0001 |
| egl-30(tg26gf); unc-17(e245) | 15 | 27 | <0.0001 |
Values of p were calculated using Fisher's exact test, relative to egl-30(tg26gf).
To test whether activated EGL-30(Gαq)-induced spicule protraction requires cholinergic synaptic transmission, we made double mutants of egl-30(gf) with unc-64(lf) and unc-17(e245). unc-17(e245) is a loss-of-function allele that affects the synaptic vesicle acetylcholine transporter gene (Alfonso et al., 1993). We found that double mutants containing egl-30(gf) and cha-1(lf) were too sick to propagate, whereas egl-30(gf); unc-17(lf) animals were healthier. For egl-30(gf); unc-17(lf) and egl-30(gf); unc-64(lf) males, the Prc phenotype was lower than the level displayed by egl-30(gf) single mutants (Table 2). This indicated that EGL-30(Gαq)-stimulated protractor muscle contraction requires cholinergic synaptic transmission.
EGL-30(Gαq) is expressed in many excitable cells in C. elegans, and it affects multiple behaviors through different cellular circuits (Brundage et al., 1996; Bastiani et al., 2003; Matsuki et al., 2006). To study the effects of activated Gαq more precisely, we first needed to identify a compound that activates Gαq signaling in the spicule protraction circuit. Because Gαq can be activated by mAChRs (Caulfield, 1993), we asked whether Gαq is coupled to a mAChR that can induce spicule muscle contraction. One muscarinic agonist that has been reported to stimulate Gαq in C. elegans is arecoline; it has been shown to modulate C. elegans pharyngeal pumping behavior through an EGL-30(Gαq)-dependent mechanism (Brundage et al., 1996). This drug can also induce male spicule protraction; however, in contrast to pharyngeal pumping, loss-of-function mutations in egl-30 do not reduce arecoline-induced spicule protraction (Garcia et al., 2001). Thus, this drug cannot be used to study how Gαq regulates male spicule behaviors.
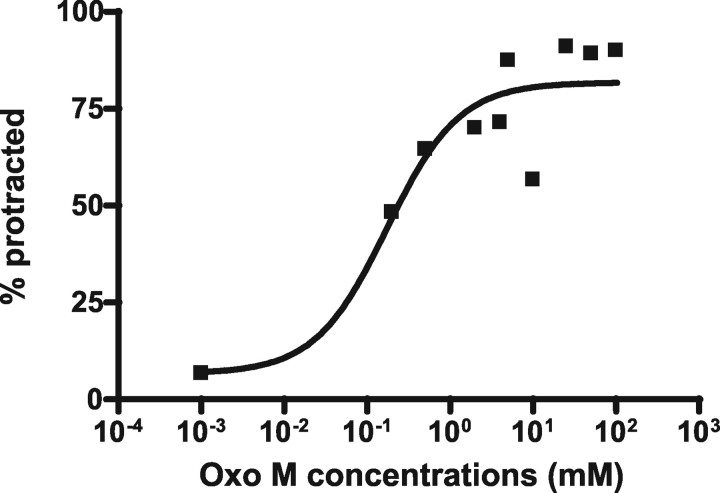
Next, we looked at the nonselective mAChR agonist Oxo M. In vertebrate cell cultures, Oxo M can activate all muscarinic receptors and downstream effector G-proteins such as Gq, Go/i, and Gs (Freedman et al., 1988; Kaneda et al., 1993; Tayebati et al., 1999; Mistry et al., 2005). In contrast to arecoline, we found that males protracted their spicules in an EGL-30(Gαq)-dependent manner when bathed in Oxo M. Approximately 90% wild-type males (n = 72) protracted their spicules in 50 mm Oxo M (EC90) (Fig. 4, Table 3), whereas only 7% of males (n = 30) containing the egl-30(ad805) loss-of-function mutation protracted their spicules at this concentration (Table 3).
Figure 4.
Dose–response of wild-type males to oxotremorine M. The numbers on the x-axis represent the concentrations of Oxo M at which the wild-type males were treated. The numbers on the y-axis represent the percentage of males that protracted their spicules within 10 min. The black squares represent the percentage of males that protracted their spicules. Approximately 30 males were assayed for each drug concentration.
Because LEV-induced protraction partially required Gαq and cholinergic synaptic transmission, we tested whether Oxo M/Gαq-promoted spicule protraction also required UNC-38 and UNC-29 LEV-sensitive nAChRs and cholinergic synaptic transmission. Compared with 86% (n = 72) of wild-type males protracting their spicules in 50 mm Oxo M, we found that 58% (n = 30; p = 0.0037) of unc-38(lf) unc-29(lf) double mutant males, 43% of unc-64(lf) males (n = 30; p < 0.0001), and 33% of unc-17(lf) males (n = 30; p < 0.0001) responded to the drug (Table 3). The participation of nAChRs and cholinergic synaptic transmission in Gαq-promoted spicule muscle contraction suggests that an intact behavioral circuit of neurons and muscles is required for normal dose–response to both muscarinic and nicotinic agonists.
Oxo M not only causes spicule protraction, but also induces other male behaviors. Within 5–10 min of being placed on NGM agar plates containing 5 mm Oxo M, 100% of wild-type males (n = 20) will display behaviors that normally are seen during mating with hermaphrodites. Their tails become hypersensitive to touch, causing the males to initiate backward movements and use their tails to scan themselves or other males at high frequencies. Additionally, males will produce spontaneous dorsal or ventral tail curling and display spicule protraction behavior. Surprisingly, hermaphrodites placed on 5 mm Oxo M plates do not display any obvious abnormal behavior (for a video presentation of Oxo M-induced behaviors, see supplemental OxoMovie, available at www.jneurosci.org as supplemental material). This phenotype suggests that, in contrast to hermaphrodite behaviors, neuronal circuits and muscles associated with male mating behavior are more sensitive to Oxo M, maybe because of different neuronal wiring and/or molecular properties of the different excitable cells.
mAChR GAR-3 activates Gαq in regulating spicule protraction behavior
The C. elegans genome encodes three mAChRs, GAR-1, GAR-2, and GAR-3 (Hwang et al., 1999; Lee et al., 1999, 2000). All show high similarity in amino acid sequences to the known mammalian mAChRs M1, M2, M3, M4, and M5, which are coupled to different G-proteins, (e.g., M1, M3, and M5 activate Gq/11, whereas M2 and M4 activate Gi/o). In contrast to mammalian mAChRs, pharmacological studies using cultured cells showed that of the three C. elegans mAChRs, only GAR-3 is activated by oxotremorine, a derivative of oxotremorine M (Hwang et al., 1999; Lee et al., 1999, 2000; Park et al., 2000, 2006). In Chinese hamster ovary cell culture, GAR-3(mAChR) has been shown to activate PLC, which is a downstream effector of Gαq signaling (Park et al., 2003). Loss-of-function gar-3 mutants are grossly normal in behaviors. However, in studies conducted by Steger and Avery (2004), mutant worms containing a deletion in the gar-3 gene showed increased pharyngeal pumping rates and increased resistance to the effects of arecoline-induced Gαq signaling relative to control animals.
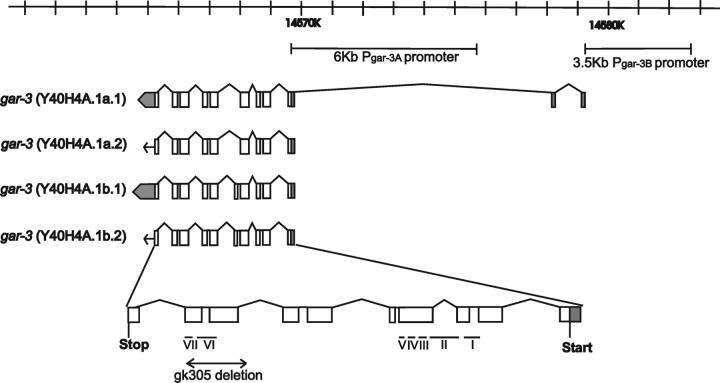
To determine whether GAR-3(mAChR) receptors are used in Gαq/Oxo M-induced spicule protraction, we obtained the deletion allele gar-3(gk305) from the C. elegans Gene Knockout Consortium. This deletion removes two exons and generates a premature stop before the translation of the last exon (http://www.wormbase.org; stable release WS160) (Fig. 5). When treated with 50 mm Oxo M (EC90), 4% of gar-3(lf) males protracted their spicules (Table 3), suggesting that Oxo M activates EGL-30(Gαq) to promote spicule protraction through GAR-3(mAChR). Additionally, when put on NGM agar plates containing 5 mm Oxo M, no gar-3(lf) males (n = 20) displayed agonist-induced mating behavior, suggesting that GAR-3(mAChR) is essential for Oxo M sensitivity. In addition to Oxo M resistance, the spicule protraction circuit in gar-3(lf) males also has reduced sensitivity for LEV. Compared with wild-type males, the LEV EC50 concentration of gar-3(lf) males was six times greater than wild-type males. Although this is not as severe as a mutation in egl-30, this suggests that some of the signaling potential of EGL-30(Gαq) is activated by GAR-3(mAChR).
Figure 5.
Locations of the gar-3 promoters and the gk305 deletion. The genomic position of gar-3 is indicated on top; the scale bar is in kilobases. Two promoters used in this study, Pgar-3A and Pgar-3B, are also depicted. Four transcriptional isoforms of gar-3 are also shown [adapted from Hwang et al. (1999) and http://www.wormbase.org, stable release WS160]. The open boxes are exons, the gray boxes are UTRs, and the lines are introns; the arrows and arrows merged with gray boxes depict the translation directions. The deletion in the gar-3(lf) allele is indicated by a double arrowed line, and the positions of putative transmembrane domains (I–VII) are indicated with horizontal lines [adapted from Park et al. (2003)].
Development of neurons and muscles that are used for spicule insertion occurs during the L3 and L4 stage of larval development. To rule out the possibility that gar-3(lf) causes Oxo M resistance by disrupting neuronal or muscular development, we expressed the gar-3 transcript in adult animals using the hsp16–2 heat shock promoter (Stringham et al., 1992). We heat-shocked transgenic virgin adult gar-3(lf);rgEx65 males at 32°C for 3 h to induce GAR-3(mAChR) expression, and found that 100% of the males (n = 22) can protract their spicules in 50 mm Oxo M, compared with 9% (n = 32) of control males (not heat shocked) (Table 3). Because we induced gene expression after the period of neural and muscular development, restored Oxo M sensitivity after heat shock induction argues that GAR-3(mAChR) is involved in promoting behavior.
Activated GAR-3(mAChR)/Gαq-induced spicule protraction partially requires phospholipase Cβ
Activated Gαq has been shown to generate diacylglycerol and mobilize intracellular calcium through the inositol 1,4,5-trisphosphate (IP3) receptor. These downstream effectors might directly activate synaptic transmission or muscle contraction (Zhou et al., 1994; An et al., 2002; Hubbard and Hepler, 2006). Gαq-activated IP3 and diacylglycerol are generated by phospholipase Cβ (PLCβ) (Makhlouf and Murthy, 1997; Singer et al., 1997). In C. elegans, PLCβ is encoded by egl-8 (Lackner et al., 1999). Together with itr-1, which encodes the IP3 receptor, egl-8 was demonstrated to be required for LEV-induced spicule protraction (Gower et al., 2005). To determine whether PLCβ transduces the GAR-3(mAChR) signal in response to 50 mm Oxo M stimulation, we used egl-8(n488) (Miller et al., 1999), an allele of PLCβ that deletes exons 10 and 11 and disrupts the reading frame before Y catalytic domain. We found that 65% (n = 40) of egl-8(lf) males protracted their spicules in the drug, compared with 86% (n = 72) of wild-type males (p = 0.015) (Table 3). This result suggests that PLCβ signaling contributes partially to GAR-3(mAChR)-induced spicule protraction. However, greater than one-half of egl-8 deletion mutant males responded to Oxo M, compared with 7% of egl-30(lf) males. Thus, GAR-3(mAChR)/Gαq signaling promotes spicule protraction through other pathways in addition to IP3-diacylglycerol (DAG) signaling. This is not surprising because other Gαq-regulated behaviors in C. elegans such as locomotion and egg-laying behavior have also been suggested to be regulated by a putative EGL-8(PLCβ)-independent pathway (Miller et al., 1999; Bastiani et al., 2003; Charlie et al., 2006).
GAR-3(mAChR) is expressed in a cellular circuit that regulates male mating behavior
To determine where GAR-3(mAChR)/Gαq functions to regulate spicule protraction, we looked at the expression pattern of the gar-3 gene in C. elegans males. Steger and Avery (2004) used a construct of GFP (green fluorescent protein) fused to 6 kb of DNA upstream of the gar-3 start codon. They found that GAR-3(mAChR) is expressed in pharyngeal muscle and the I3 neuron, and other extrapharyngeal neurons. We also used the same 6 kb sequence, which we termed promoter Pgar-3A, to drive YFP expression (pLR59). In addition to the expression pattern reported by Steger and Avery, we found YFP expression in body wall muscles, male diagonal muscles, and one neuron in the male ray 8 (data not shown). However, none of these muscles and neurons controls spicule protraction behavior directly. cDNA expressed sequence tag data listed on www.wormbase.org report an alternative 5′ UTR ∼10 kb upstream of gar-3 start codon (Hwang et al., 1999) (http://www.wormbase.org; stable release WS160). To test whether sequences upstream of this UTR can drive GAR-3(mAChR) expression in cells that regulate spicule protraction, we fused 3.5 kb of sequence (Pgar-3B) upstream of the gar-3 isoform Y40H4A.1a.1 to the YFP gene (pYL1). We found that this construct is expressed in the SPC motor neurons, PCB and PCA postcloacal sensilla (p.c.s.) neurons, male spicule protractor muscles and anal depressor muscle, VD and DD ventral cord neurons, some tail and nerve ring neurons, and body wall muscles (Fig. 3C,D). Among these cells, the SPC, PCB, and PCA neurons and the spicule protractor muscles regulate spicule insertion behavior (Garcia et al., 2001). In hermaphrodites, YFP expression is in the same set of cells, except those specific to males.
GAR-3(mAChR) regulates spicule protraction through neurons and muscles
To determine which cells require GAR-3(mAChR), we fused YFP to the last codon of the gar-3 gene, and cloned the translational construct downstream of the Pgar-3A promoter and the Pgar-3B promoter. We then injected these constructs into gar-3(lf) worms. Fusion proteins expressed from the promoter constructs were found on the membranes and processes (Fig. 6A,B). We found that only gar-3(lf) males that expressed gar-3 from the Pgar-3B promoter restored the Oxo M sensitivity (Table 3), indicating that spicule protraction behavior might be regulated by gar-3 expressed from the SPC, PCA, and PCB neurons, and the spicule protractor muscles.
Figure 6.
Native and heterologous expression of the gar-3::YFP translational fusion protein. A, B, DIC (A) and fluorescence (B) images of the left lateral tail region of a mid L4 male expressing both Pgar-3A::gar-3::YFP (pYL8) and Pgar-3B::gar-3::YFP (pYL9). Nuclei of the neurons are labeled with arrows. In B, positions of these neurons are indicated with circles, and localizations of GAR-3::YFP on the cell membranes are denoted by arrows. C, D, DIC (C) and fluorescence (D) images of left lateral tail region of an adult male expressing Punc-103 E::gar-3::YFP (pYL10). E, F, Merged DIC and fluorescence (E) and fluorescence (F) images of left lateral tail region of a mid L4 male expressing the Punc-103 F ::gar-3::YFP (pYL11). In E, nuclei of neurons are labeled with arrows. GAR-3::YFP localization on cell membranes can be seen as white puncta. In F, neuronal positions are indicated with circles, and localizations of GAR-3::YFP on the cell membranes are denoted by arrows. Scale bar, 10 μm.
The cholinergic SPC neurons directly innervate the spicule protractor muscles (Sulston et al., 1980), and the cholinergic PCB neurons might activate these muscles indirectly via their chemical and electrical connections to the SPC neurons (S. W. Emmons, D. H. Hall, and M. Xu, personal communication) (www.wormatlas.org). To determine whether GAR-3(mAChR) functions in presynaptic or postsynaptic cells, we expressed the gar-3::YFP construct from the Punc-103E promoter (rgEx94) and Punc-103F promoter (rgEx95) (Gruninger et al., 2006; Reiner et al., 2006). The Punc-103E promoter expresses in the protractor muscles, but not in the neurons that innervate them (Fig. 6C,D), whereas the Punc-103F promoter expresses in the SPC, PCA, and PCB neurons (Fig. 6E,F). When tested in 50 mm Oxo M, 92% of gar-3(lf) males (n = 13) that expressed GAR-3(mAChR) in sex muscles, and 97% of males (n = 29) that expressed GAR-3(mAChR) in spicule-associated neurons protracted their spicules in the drug (Table 3). This demonstrates that, when overexpressed, GAR-3(mAChR) can act in both neurons and muscles.
In gar-3(lf) males, the ability of muscle-expressed GAR-3(mAChR) to rescue Oxo M sensitivity suggested that the drug can bypass upstream GAR-3(mAChR)-expressing neurons and act directly on the muscle. However, this contradicts the previous result that globally lowering synaptic transmission, via unc-64 and unc-17 mutations, reduced Oxo M sensitivity. To readdress whether GAR-3(mAChR)/Gαq regulation of muscle contraction requires neuron function, we ablated the gar-3-expressing SPC and PCB neurons individually, and in combination, and asked whether loss of these neurons impairs sensitivity to Oxo M. Eleven percent of the SPC-ablated males (n = 19; p < 0.0001), 30% of PCB-ablated males (n = 20; p < 0.0001), and 28% of double-ablated males (n = 67; p < 0.0001 vs intact males; p = 0.14 vs SPC-ablated males) responded to the agonist (Table 3). These data indicate that, in the absence of SPC and PCB, endogenous levels of muscular GAR-3(mAChR) are not sufficient to mediate Oxo M-promoted spicule protraction. However, when GAR-3(mAChR) is artificially overexpressed in the spicule muscles via the Punc-103E promoter, upstream cholinergic synaptic transmission is not required. Ablation of SPC and PCB neurons in gar-3(lf); rgEx94 males resulted in ∼88% (n = 8) of transgenic males protracting their spicules in the drug.
Loss of GAR-3 function results in subtle defect during mating
Our molecular, mutational, and pharmacological studies suggested that GAR-3(mAChR)/Gαq functions in cholinergic neurons to promote muscarinic and nicotinic agonist-induced spicule muscle contraction. Although these assays were informative, they did not address why the circuit uses GAR-3(mAChR) for behavior. To address this, we observed the mating behavior of gar-3(lf) males. gar-3(lf) males showed no overt behavioral defects in locomotion or mating behavior; they backed along the hermaphrodite cuticle, they turned at the ends of the hermaphrodite and located the hermaphrodite vulva with the same efficiency as wild-type males (data not shown).
Previous work showed that, on contact with the vulva, the postcloacal sensilla neurons stimulate periodic protractor muscle contractions (Garcia et al., 2001). Because GAR-3(mAChR) is expressed in the protractor muscles and postcloacal sensilla neurons, we asked whether lack of GAR-3(mAChR) changes the frequency of muscle contractions. We found that when gar-3(lf) males attempted to breach the vulva with their spicules, the protractor muscles contracted repetitively at the same frequency as wild type (wild type: 5.8 ± 0.7 contractions/s; gar-3: 6.2 ± 1.2 contractions/s; p = 0.6, independent-sample t test; n = 5 males for each genotype). This suggests that the spicule protraction circuit does not require Gαq-coupled mAChR signaling for regulating the muscle contraction–relaxation cycle.
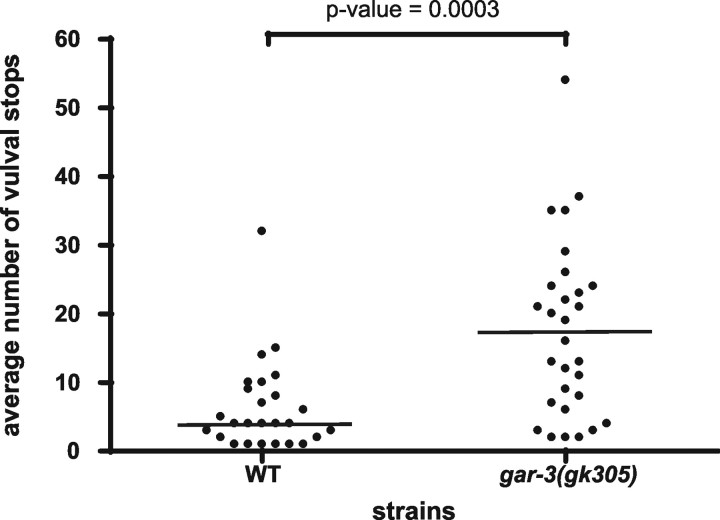
However, we found that if the hermaphrodite vulva was not breached instantaneously, gar-3(lf) males moved off the vulva rather than sustaining their attempts to penetrate the vulva. The vulva of a young adult hermaphrodite (∼24 h) is difficult for males to breach (Garcia et al., 2001). In a 10 min assay, we counted the number of times a male located and prodded the vulva of a young hermaphrodite with his spicules until he completely inserted. We found that wild-type males moved on and off the vulva on average 6.3 times (n = 26), whereas gar-3(lf) males (n = 29) moved on and off the vulva on average 17.5 times (p < 0.001) during their spicule insertion attempts (Fig. 7). This suggests that, when the task of breaching the vulva is prolonged, GAR-3(mAChR) integrates the functions of the postcloacal sensilla neurons, SPC and the protractor muscles to maintain spicule insertion attempts and vulva location behavior.
Figure 7.
gar-3(lf) males use more spicule insertion attempts to achieve vulva penetration. The spots represent the total number of vulval stops (insertion attempts) used by individual males. Twenty-six wild-type males and 29 gar-3(lf) males were assayed. The horizontal bar indicates the sample median. The p value was calculated using the Mann–Whitney test.
Discussion
GAR-3(mAChR)/Gαq signaling sensitizes the spicule protraction circuit
In this study, we use C. elegans male spicule protraction behavior as a model to address how nicotinic and G-protein-coupled muscarinic acetylcholine signaling regulate a specific motor behavior. Partial electron microscopy reconstruction of the male tail reveals that the spicule protractor muscles are directly innervated by the cholinergic SPC motor neurons (Sulston et al., 1980). Additionally, the SPC motor neurons innervate and receive innervation from both the cholinergic PCB and PCC p.c.s. neurons (Fig. 1B) (S.W. Emmons, D.H. Hall and M. Xu, personal communication; www.wormatlas.org). Based on data presented in this study, we suggest that, before copulation, mAChR/Gαq signaling in presynaptic cells regulates the sensitivity of postsynaptic cells to ACh, particularly in the spicule muscles, but potentially also in the GAR-3(mAChR)-expressing SPC and PCB neurons because of their interconnectivities (Fig. 1C).
In virgin males, the wild-type dose–response to LEV requires the protractor muscles to be preexposed or concurrently exposed to ACh secreted from the spicule-associated neurons. Synaptic transmission from the SPC, PCB, and PCC neurons is not strictly essential for drug-induced muscle contraction because animals that lack these neurons will protract their spicules at high drug concentrations such as 1 mm (Garcia et al., 2001). These observations suggest that the protractor muscles are capable of expressing functional UNC-38 and UNC-29 nAChR receptors in the absence of the SPC neurons; however, either the number of receptors or their signaling capacity must be partially regulated by the synaptic partners of the protractor muscles.
In wild-type males, the behavioral outputs of the postcloacal sensilla and SPC neurons are evident during mating behavior (Liu and Sternberg, 1995); however, virgin males used in this study were never exposed to hermaphrodites before drug exposure. So in virgins, when do postsynaptic cells in the spicule circuit become exposed to secreted acetylcholine? We favor the idea that, before and between periods of copulation, the neurons in the spicule circuit release small amounts of neurotransmitters that are partially regulated by the GAR-3(mAChR)/Gαq pathway.
Spontaneous neurotransmitter release has been observed at the neuromuscular junctions in other organisms, such as frogs, flies, and rodents, and was first observed in the frog neuromuscular junction. In absence of stimulation, presynaptic cells spontaneously secrete ACh at a low frequency to activate ACh receptors on postsynaptic cells, causing miniature membrane depolarization (Fatt and Katz, 1952). In C. elegans, the miniature postsynaptic potential has been measured at the body wall muscle neuromuscular junction. Gαq signaling in the ventral cord motor neurons, and GABA and nicotinic ACh receptors on body wall muscles were found to contribute to the miniature postsynaptic current (Richmond and Jorgensen, 1999). Here, we hypothesize that this general phenomenon of background levels of ACh release also occurs in the spicule circuit; however, it is partly regulated by the GAR-3 mAChR. Others have previously shown that spontaneous neurotransmitter release can regulate the clustering of receptors on postsynaptic cells (Saitoe et al., 2001). In the spicule protraction circuit, a similar mechanism might be occurring. GAR-3(mAChR)/Gαq might define the cholinergic sensitivity of the postsynaptic cells either by regulating the number or clustering of nAChRs, activating a facilitator of nAChR signaling or by inhibiting mechanisms that reduce cell excitability.
GAR-3(mAChR)/Gαq stimulation of spicule protraction uses EGL-8 PLCβ dependent and independent pathways
In the classic Gαq signal transduction pathway, Gαq-activated PLCβ hydrolyzes PIP2 (phosphatidylinositol 4,5-bisphosphate) into IP3 and diacylglycerol (DAG). IP3 binds to the IP3 receptor to induce calcium mobilization from intracellular stores, whereas DAG activates phorbol ester binding proteins such as protein kinase C. Gower and colleagues have previously shown PLCβ to be widely expressed in the male nervous system and sex muscles, whereas the IP3 receptor was found to be expressed in the sex muscles. RNA interference against PLCβ and IP3 receptor transcripts reduced LEV-induced spicule protraction, demonstrating that these two downstream components regulate the output of the spicule protraction circuit (Gower et al., 2005). However, although we found that induction of spicule protraction through activated GAR-3(mAChR) is strongly dependent on Gαq, it partly required PLCβ function. This is supported by our result that the PLCβ deletion allele egl-8(n488) lowers, but does not eliminate male Oxo M sensitivity. GAR-3(mAChR) has been demonstrated to act with calcium channels to regulate pharyngeal muscle contractions. PLCβ is not expressed in the pharynx, but defects in EGL-8(PLCβ) can indirectly affect how pharyngeal GAR-3(mAChR) regulates pharyngeal muscle contractions (Steger and Avery, 2004). Similar to pharyngeal pumping behavior, defects in EGL-8(PLCβ) might compromise some spicule function that is not directly related to GAR-3(mAChR) signaling. The strong requirement for Gαq, but not PLCβ suggests that Gαq directly activates other downstream effectors, or that Gβγ molecules, which specifically couple to Gαq and GAR-3(mAChR), also signal to promote spicule protraction.
In C. elegans, unc-103 encodes an ether-a-go-go-related (erg) K+ channel (Reiner et al., 2006). When deleted, mutant males display spontaneous spicule protraction and other male mating behaviors in the absence of mating cues (Garcia and Sternberg, 2003). UNC-103(ERG K+ channel) and GAR-3(mAChR) are coexpressed in the protractor muscles, SPC and PCB neurons. The phenotype of unc-103 deletion males [unc-103(0)] is similar to the behavior of wild-type males that are treated with Oxo M, suggesting that UNC-103(ERG K+ channel) and GAR-3(mAChR) oppositely regulate the functions of cells in the spicule protraction circuit. Before mating behavior, it is possible that one role of UNC-103 K+ channels is to keep the low levels of ACh secretion from the SPC and PCB neurons from evoking membrane depolarization and muscle contraction. This is consistent with the observations that cha-1 and egl-30 mutations and SPC ablations can reduce unc-103(0)-induced spicule protraction (Garcia and Sternberg, 2003). However, mutation in gar-3 does not suppress unc-103(0)-induced spicule protraction (Y. Liu, unpublished observation), suggesting that more than one type of Gαq-coupled receptor regulate the spicule circuit.
During copulation, we observed that gar-3(lf) mutants have difficulty in maintaining their position over the vulva during spicule insertion attempts. During normal mating behavior, the p.c.s. neurons PCA, PCB, and PCC and the hook sensillum neurons HOA and HOB sense the vulva and attenuate backward locomotion (Liu and Sternberg, 1995; Barr and Sternberg, 1999). Simultaneously, ACh from the PCB and PCC neurons induces the spicule protractor muscles to undergo high-frequency contractions, which cause the spicule tips to prod the vulva rhythmically. gar-3(lf) mutants can locate the vulva with wild-type efficiency, indicating that the sensory signaling machinery of the p.c.s. neurons is functional. However, the failure of gar-3(lf) males to maintain their position during spicule insertion behavior suggests that downstream events are perturbed. The coupling of vulva location with spicule insertion might be facilitated via the reciprocal innervations of the cholinergic SPC motor neurons with the cholinergic p.c.s. neurons PCB and PCC. The signals that activate the p.c.s. neurons to attenuate backward locomotion might come from two sources. One source certainly comes from the PCB and PCC sensory endings, which senses the vulva lips. The other source might come during high frequency spicule insertion attempts from the cholinergic SPC motor neurons, which might reinforce signals that come from the p.c.s. sensory endings. nAChRs composed of UNC-38 subunits are not expressed in the SPC, PCC, or PCB neurons; however, the C. elegans genome encodes 20 other nAChR α-subunits that might facilitate cholinergic communication between the neurons in the spicule protraction circuit (Jones and Sattelle, 2003). The behavioral defect in gar-3(lf) male mating might be attributable to an intrinsic decrease in nAChR signaling potential in the SPC, PCB, and spicule protractor muscles before mating, or to an additional decrease in mAChR signaling between these cells during copulation.
Footnotes
Some of the nematode strains were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health (NIH) Center for Research Resources. Y.L., B.L., and L.R.G. were supported by NIH Grant GM70431 and by the Searle Scholars Program. We thank T. Gruninger, R. Lints, and D. Gualberto for technical assistance and reading of this manuscript.
References
- Alfonso A, Grundahl K, Duerr JS, Han HP, Rand JB. The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science. 1993;261:617–619. doi: 10.1126/science.8342028. [DOI] [PubMed] [Google Scholar]
- Alfonso A, Grundahl K, McManus JR, Rand JB. Cloning and characterization of the choline acetyltransferase structural gene (cha-1) from C. elegans. J Neurosci. 1994;14:2290–2300. doi: 10.1523/JNEUROSCI.14-04-02290.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JY, Yun HS, Lee YP, Yang SJ, Shim JO, Jeong JH, Shin CY, Kim JH, Kim DS, Sohn UD. The intracellular pathway of the acetylcholine-induced contraction in cat detrusor muscle cells. Br J Pharmacol. 2002;137:1001–1010. doi: 10.1038/sj.bjp.0704954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bany IA, Dong MQ, Koelle MR. Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior. J Neurosci. 2003;23:8060–8069. doi: 10.1523/JNEUROSCI.23-22-08060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI, Avery L. Laser killing of cells in Caenorhabditis elegans. Methods Cell Biol. 1995;48:225–250. doi: 10.1016/s0091-679x(08)61390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr M, Sternberg PW. A polycystic kidney-disease gene homologue required for mating behaviour in C. elegans. Nature. 1999;401:386–389. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- Bastiani CA, Gharib S, Simon MI, Sternberg PW. Caenorhabditis elegans Galphaq regulates egg-laying behavior via a PLCbeta-independent and serotonin-dependent signaling pathway and likely functions both in the nervous system and in muscle. Genetics. 2003;165:1805–1822. doi: 10.1093/genetics/165.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundage L, Avery L, Katz A, Kim UJ, Mendel JE, Sternberg PW, Simon MI. Mutations in a C. elegans Gqalpha gene disrupt movement, egg laying, and viability. Neuron. 1996;16:999–1009. doi: 10.1016/s0896-6273(00)80123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield MP. Muscarinic receptors—characterization, coupling and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- Chang R, Potter L, Smith D. Postsynaptic membranes in the electric tissue of Narcine. IV. Isolation and characterization of the nicotinic receptor protein. Tissue Cell. 1977;9:623–644. doi: 10.1016/0040-8166(77)90031-3. [DOI] [PubMed] [Google Scholar]
- Charlie N, Schade M, Thomure A, Miller K. Presynaptic UNC-31(CAPS) is required to activate the Gαs Pathway of the Caenorhabditis elegans synaptic signaling network. Genetics. 2006;172:943–961. doi: 10.1534/genetics.105.049577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Tronconi BM, Raftery MA. The nicotinic cholinergic receptor: correlation of molecular structure with functional properties. Annu Rev Biochem. 1982;51:491–530. doi: 10.1146/annurev.bi.51.070182.002423. [DOI] [PubMed] [Google Scholar]
- Culetto E, Baylis HA, Richmond JE, Jones AK, Fleming JT, Squire MD, Lewis JA, Sattelle DB. The Caenorhabditis elegans unc-643 gene encodes a levamisole-sensitive nicotinic acetylcholine receptor alpha subunit. J Biol Chem. 2004;279:42476–42483. doi: 10.1074/jbc.M404370200. [DOI] [PubMed] [Google Scholar]
- Dale HH. The action of certain esters and ethers of choline, and their relation to muscarine. J. Pharmacol Exp Ther. 1914;6:147–190. [Google Scholar]
- Doi M, Iwasaki K. Regulation of retrograde signaling at neuromuscular junctions by the novel C2 domain protein AEX-1. Neuron. 2002;33:249–259. doi: 10.1016/s0896-6273(01)00587-6. [DOI] [PubMed] [Google Scholar]
- Ellis JR, Ellis KA, Bartholomeusz CF, Harrison BJ, Wesnes KA, Erskine FF, Vitetta L, Nathan PJ. Muscarinic and nicotinic receptors synergistically modulate working memory and attention in humans. Int J Neuropsychopharmacol. 2006;9:175–189. doi: 10.1017/S1461145705005407. [DOI] [PubMed] [Google Scholar]
- Erskine FF, Ellis JR, Ellis KA, Stuber E, Hogan K, Miller V, Moore E, Bartholomeusz C, Harrison BJ, Lee B, Phan KL, Liley D, Nathan PJ. Evidence for synergistic modulation of early information processing by nicotinic and muscarinic receptors in humans. Hum Psychopharmacol. 2004;19:503–509. doi: 10.1002/hup.613. [DOI] [PubMed] [Google Scholar]
- Ewins AJ. Acetylcholine, a new active principle of ergot. Biochem J. 1914;8:44–49. doi: 10.1042/bj0080044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J Physiol (Lond) 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- Feiro O, Gould TJ. The interactive effects of nicotinic and muscarinic cholinergic receptor inhibition on fear conditioning in young and aged C57BL/6 mice. Pharmacol Biochem Behav. 2005;80:251–262. doi: 10.1016/j.pbb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Fleming JT, Squire MD, Barnes TM, Tornoe C, Matsuda K, Ahnn J, Fire A, Sulston JE, Barnard EA, Sattelle DB, Lewis JA. Caenorhabditis elegans levamisole resistance genes lev-1, unc-29, and unc-38 encode functional nicotinic acetylcholine receptor subunits. J Neurosci. 1997;17:5843–5857. doi: 10.1523/JNEUROSCI.17-15-05843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman SB, Harley EA, Iversen LL. Relative affinities of drugs acting at cholinoceptors in displacing agonist and antagonist radioligands: the NMS/Oxo-M ratio as an index of efficacy at cortical muscarinic receptors. Br J Pharmacol. 1988;93:437–445. doi: 10.1111/j.1476-5381.1988.tb11451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia LR, Sternberg PW. Caenorhabditis elegans UNC-103 ERG-like potassium channel regulates contractile behaviors of sex muscles in males before and during mating. J Neurosci. 2003;23:2696–2705. doi: 10.1523/JNEUROSCI.23-07-02696.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia LR, Mehta P, Sternberg PW. Regulation of distinct muscle behaviors controls the C. elegans male's copulatory spicules during mating. Cell. 2001;107:777–788. doi: 10.1016/s0092-8674(01)00600-6. [DOI] [PubMed] [Google Scholar]
- Gower NJ, Walker DS, Baylis HA. Inositol 1,4,5-trisphosphate signaling regulates mating behavior in Caenorhabditis elegans males. Mol Biol Cell. 2005;16:3978–3986. doi: 10.1091/mbc.E05-02-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato M, Schnabel H, Schnabel R. pha-1, a selectable marker for gene transfer in C. elegans. Nucleic Acids Res. 1994;22:1762–1763. doi: 10.1093/nar/22.9.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruninger TR, Gualberto DG, LeBoeuf B, Garcia LR. Integration of male mating and feeding behaviors in Caenorhabditis elegans. J Neurosci. 2006;26:169–179. doi: 10.1523/JNEUROSCI.3364-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz HR, Brenner S, Hodgkin J, Herman RK. A uniform genetic nomenclature for the nematode Caenorhabditis elegans. Mol Gen Genet. 1979;175:129–133. doi: 10.1007/BF00425528. [DOI] [PubMed] [Google Scholar]
- Hubbard KB, Hepler JR. Cell signalling diversity of the Gqalpha family of heterotrimeric G proteins. Cell Signal. 2006;18:135–150. doi: 10.1016/j.cellsig.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Hucho F, Bandini G, Suarez-Isla B. The acetylcholine receptor as part of a protein complex in receptor-enriched membrane fragments from Torpedo californica electric tissue. Eur J Biochem. 1978;83:335–340. doi: 10.1111/j.1432-1033.1978.tb12099.x. [DOI] [PubMed] [Google Scholar]
- Hwang JM, Chang DJ, Kim US, Lee YS, Park YS, Kaang BK, Cho NJ. Cloning and functional characterization of a Caenorhabditis elegans muscarinic acetylcholine receptor. Receptors Channels. 1999;6:415–424. [PubMed] [Google Scholar]
- Jones A, Sattelle D. Functional genomics of the nicotinic acetylcholine receptor gene family of the nematode Caenorhabditis elegans. BioEssays. 2003;26:39–49. doi: 10.1002/bies.10377. [DOI] [PubMed] [Google Scholar]
- Kaneda T, Kitamura Y, Nomura Y. Presence of m3 subtype muscarinic acetylcholine receptors and receptor-mediated increases in the cytoplasmic concentration of Ca2+ in Jurkat, a human leukemic helper T lymphocyte line. Mol Pharmacol. 1993;43:356–364. [PubMed] [Google Scholar]
- Kim J, Poole DS, Waggoner LE, Kempf A, Ramirez DS, Treschow PA, Schafer WR. Genes affecting the activity of nicotinic receptors involved in Caenorhabditis elegans egg-laying behavior. Genetics. 2001;157:1599–1610. doi: 10.1093/genetics/157.4.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Fukuda K, Mikami A, Maeda A, Takahashi H, Mishina M, Haga T, Haga K, Ichiyama A, Kangawa K, Kojima M, Matsuo H, Hirose T, Numa S. Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature. 1986;323:411–416. doi: 10.1038/323411a0. [DOI] [PubMed] [Google Scholar]
- Lackner MR, Nurrish SJ, Kaplan JM. Facilitation of synaptic transmission by EGL-30 Gqalpha and EGL-8 PLCbeta: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron. 1999;24:335–346. doi: 10.1016/s0896-6273(00)80848-x. [DOI] [PubMed] [Google Scholar]
- Langley J. On the contraction of muscle chiefly in relation to the presence of receptive substances. J Physiol (Lond) 1907;36:347–384. doi: 10.1113/jphysiol.1907.sp001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzafame AA, Christopoulos A, Mitchelson F. Cellular signaling mechanisms for muscarinic acetylcholine receptors. Receptors Channels. 2003;9:241–260. [PubMed] [Google Scholar]
- Lee YS, Park YS, Chang DJ, Hwang JM, Min CK, Kaang BK, Cho NJ. Cloning and expression of a G protein-linked acetylcholine receptor from Caenorhabditis elegans. J Neurochem. 1999;72:58–65. doi: 10.1046/j.1471-4159.1999.0720058.x. [DOI] [PubMed] [Google Scholar]
- Lee YS, Park YS, Nam S, Suh SJ, Lee J, Kaang BK, Cho NJ. Characterization of GAR-2, a novel G protein-linked acetylcholine receptor from Caenorhabditis elegans. J Neurochem. 2000;75:1800–1809. doi: 10.1046/j.1471-4159.2000.0751800.x. [DOI] [PubMed] [Google Scholar]
- Lewis JA, Wu CH, Berg H, Levine JH. The genetics of levamisole resistance in the nematode Caenorhabditis elegans. Genetics. 1980;95:905–928. doi: 10.1093/genetics/95.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KS, Sternberg PW. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron. 1995;14:79–89. doi: 10.1016/0896-6273(95)90242-2. [DOI] [PubMed] [Google Scholar]
- Makhlouf GM, Murthy KS. Signal transduction in gastrointestinal smooth muscle. Cell Signal. 1997;9:269–276. doi: 10.1016/s0898-6568(96)00180-5. [DOI] [PubMed] [Google Scholar]
- Matsuki M, Kunitomo H, Iino Y. Goalpha regulates olfactory adaptation by antagonizing Gqalpha-DAG signaling in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2006;103:1112–1117. doi: 10.1073/pnas.0506954103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KG, Emerson MD, Rand JB. Goalpha and diacylglycerol kinase negatively regulate the Gqalpha pathway in C. elegans. Neuron. 1999;24:323–333. doi: 10.1016/s0896-6273(00)80847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Vu ET, Krasne FB. Cholinergic transmission at the first synapse of the circuit mediating the crayfish lateral giant escape reaction. J Neurophysiol. 1992;68:2174–2184. doi: 10.1152/jn.1992.68.6.2174. [DOI] [PubMed] [Google Scholar]
- Mistry R, Dowling MR, Challiss RA. An investigation of whether agonist-selective receptor conformations occur with respect to M2 and M4 muscarinic acetylcholine receptor signalling via Gi/o and Gs proteins. Br J Pharmacol. 2005;144:566–575. doi: 10.1038/sj.bjp.0706090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, Harada S, Sassa T, Yamamoto H, Hosono R. Functional properties of the unc-64 gene encoding a Caenorhabditis elegans syntaxin. J Biol Chem. 1998;273:2192–2198. doi: 10.1074/jbc.273.4.2192. [DOI] [PubMed] [Google Scholar]
- Park YS, Lee YS, Cho NJ, Kaang BK. Alternative splicing of gar-1, a Caenorhabditis elegans G-protein-linked acetylcholine receptor gene. Biochem Biophys Res Commun. 2000;268:354–358. doi: 10.1006/bbrc.2000.2108. [DOI] [PubMed] [Google Scholar]
- Park YS, Kim S, Shin Y, Choi B, Cho NJ. Alternative splicing of the muscarinic acetylcholine receptor GAR-3 in Caenorhabditis elegans. Biochem Biophys Res Commun. 2003;308:961–965. doi: 10.1016/s0006-291x(03)01508-0. [DOI] [PubMed] [Google Scholar]
- Park YS, Cho TJ, Cho NJ. Stimulation of cyclic AMP production by the Caenorhabditis elegans muscarinic acetylcholine receptor GAR-3 in Chinese hamster ovary cells. Arch Biochem Biophys. 2006;450:203–207. doi: 10.1016/j.abb.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Raizen DM, Lee RY, Avery L. Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics. 1995;141:1365–1382. doi: 10.1093/genetics/141.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner DJ, Weinshenker D, Tian H, Thomas JH, Nishiwaki K, Miwa J, Gruninger T, Leboeuf B, Garcia LR. Behavioral genetics of Caenorhabditis elegans unc-103-encoded erg-like k+ channel. J Neurogenet. 2006;20:41–66. doi: 10.1080/01677060600788826. [DOI] [PubMed] [Google Scholar]
- Richmond JE, Jorgensen EM. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat Neurosci. 1999;2:791–797. doi: 10.1038/12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoe M, Schwarz TL, Umbach JA, Gundersen CB, Kidokoro Y. Absence of junctional glutamate receptor clusters in Drosophila mutants lacking spontaneous transmitter release. Science. 2001;293:514–517. doi: 10.1126/science.1061270. [DOI] [PubMed] [Google Scholar]
- Schnabel R, Schnabel H. Early determination in the C. elegans embryo: a gene, cib-1, required to specify a set of stem-cell-like blastomeres. Development. 1990;108:107–119. doi: 10.1242/dev.108.Supplement.107. [DOI] [PubMed] [Google Scholar]
- Singer WD, Brown HA, Sternweis PC. Regulation of eukaryotic phosphatidylinositol-specific phospholipase C and phospholipase D. Annu Rev Biochem. 1997;66:475–509. doi: 10.1146/annurev.biochem.66.1.475. [DOI] [PubMed] [Google Scholar]
- Steger KA, Avery L. The GAR-3 muscarinic receptor cooperates with calcium signals to regulate muscle contraction in the Caenorhabditis elegans pharynx. Genetics. 2004;167:633–643. doi: 10.1534/genetics.103.020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringham EG, Dixon DK, Jones D, Candido EP. Temporal and spatial expression patterns of the small heat shock (hsp16) genes in transgenic Caenorhabditis elegans. Mol Biol Cell. 1992;3:221–233. doi: 10.1091/mbc.3.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Albertson DG, Thomson JN. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Dev Biol. 1980;78:542–576. doi: 10.1016/0012-1606(80)90352-8. [DOI] [PubMed] [Google Scholar]
- Tayebati SK, Piergentili A, Natale D, Amenta F. Evaluation of an agonist index: affinity ratio for compounds active on muscarinic cholinergic M2 receptors. J Auton Pharmacol. 1999;19:77–84. doi: 10.1046/j.1365-2680.1999.00118.x. [DOI] [PubMed] [Google Scholar]
- Weeks JC, Jacobs GA, Pierce JT, Sandstrom DJ, Streichert LC, Trimmer BA, Wiel DE, Wood ER. Neural mechanisms of behavioral plasticity: metamorphosis and learning in Manduca sexta. Brain Behav Evol. 1997;50(Suppl 1):69–80. doi: 10.1159/000113356. [DOI] [PubMed] [Google Scholar]
- Zhou CJ, Akhtar RA, Abdel-Latif AA. Identification of phosphoinositide-specific phospholipase C-beta 1 and GTP-binding protein, Gq alpha, in bovine iris sphincter membranes: characteristics of the phospholipase and its coupling to cholinergic muscarinic receptors. Exp Eye Res. 1994;59:377–384. doi: 10.1006/exer.1994.1121. [DOI] [PubMed] [Google Scholar]