Abstract
Although it is commonly appreciated that spaced training is superior to massed training in memory formation, the molecular mechanisms underlying this feature of memory are largely unknown. We previously described the selective benefit of multiple spaced (vs massed) training trials in the induction of long-term memory (LTM) for sensitization in Aplysia californica. We now report that LTM can be induced with only two spaced training trials [tail shocks (TSs)] when the second TS is administered 45 min after the first. In contrast, spacing intervals of 15 and 60 min are ineffective. This surprisingly narrow permissive training window for two-trial LTM is accompanied by an equally narrow window of transient mitogen-activated protein kinase (MAPK) activation, a necessary signaling molecule for LTM induction, at 45 min after a single TS. Thus, the transient recruitment of MAPK following a single TS may provide a narrow molecular window for two-trial LTM formation.
Keywords: LTM, patterning, spacing interval, rest interval, lag effect, ERK
Introduction
More than a century ago, the psychologist Herman Ebbinghaus (1885) first experimentally described the benefit of intervening rest periods during repeated study in long-term memory (LTM) retention. Subsequent studies in humans and animals have confirmed that this “spacing effect” is a general learning principle for LTM induction (Tully et al., 1994; Gerber et al., 1998; Donovan and Radosevich, 1999; Genoux et al., 2002; Sutton et al., 2002; Cepeda et al., 2006). However, despite its virtually universal nature, little is known about how the spacing effect is implemented at the cellular and molecular level.
We have begun to explore this question in the marine mollusk Aplysia californica, which has a simple, well characterized nervous system that can support both associative and nonassociative forms of learning (Pinsker et al., 1970; Walters et al., 1979; Susswein and Schwarz, 1983; Brembs et al., 2002). Memory expressed in this system has similar mechanistic requirements to that described in vertebrates, including a dependence of long-lasting forms of memory and synaptic plasticity on activation of signaling kinases such as mitogen-activated protein kinase (MAPK) that give rise to nuclear signaling and cAMP response element-binding protein (CREB)-mediated transcription (Silva et al., 1998; Kandel, 2001; Sharma and Carew, 2004; Sweatt, 2004).
We showed previously that the induction of LTM for sensitization of a defensive reflex, the tail-elicited siphon withdrawal reflex (T-SWR), is highly sensitive to the pattern and number of training stimuli [tail shocks (TSs)]: with a spacing interval of 15 min, LTM requires at least four TSs (Sutton et al., 2002). Surprisingly, here we report that LTM can be induced by only two spaced TSs, when the second TS is administered within a narrow temporal window. We also found that a single TS induces a transient activation of MAPK that is restricted to the permissive window for two-trial LTM. Because MAPK is a necessary signaling cascade for LTM induction (Sharma et al., 2003b), its activation may provide a molecular mechanism for the restricted temporal window we observe as a defining feature of two-trial LTM.
Materials and Methods
Behavioral procedures.
Adult Aplysia californica (200–300 g) obtained through Marinus Scientific (Long Beach, CA) were housed and fed individually within a tank of circulating artificial seawater (Instant Ocean; Aquarium Systems) maintained at ∼16°C.
Five days before the start of training, animals were anesthetized by cooling, and the parapodia surrounding the siphon were removed. Baseline T-SWR duration was measured beginning immediately after a test stimulus (0.5 s water jet, Teledyne Water Pik, Fort Collins, CO) to the tail midline, ∼1 cm above the posterior tip, until the first signs of relaxation of the posterior portion of the siphon. In all experiments, the baseline T-SWR for each animal was established by the average of three pretests [intertest interval (ITI) of 15 min]. Animals were randomly assigned to experimental and control groups. Twenty minutes after the last pretest, the experimental group received sensitization training consisting of electrical shocks (1.5 s, 80 mA, alternating current) applied through a handheld electrode to the tail midline. Posttests were always taken by an observer blind to the training history of the animals. Sensitization memory at 24 and 48 h was calculated by the average response of three posttests (ITI of 15 min) and was expressed for each animal as the percentage change from its mean baseline response. Untrained (no shock) controls received matched testing but no training.
Analysis of MAPK activation.
The activation of MAPK in tail sensory neuron (SN) cell bodies was measured from trained and control animals. All animals received a single pretest 20 min before training. Tail SN clusters were collected at 15, 45, or 60 min after a single 1.5 s, 80 mA TS. SN clusters from control animals were collected at 65 min after the pretest. Because TSs were applied to the tail midline, both left and right tail SN clusters were collected. To harvest tail SN cell bodies, we used methods described previously (Sharma et al., 2003b). Isolated SN cell body clusters were immediately transferred into 20 μl of lysis buffer (Sharma et al., 2003b). Samples were mixed vigorously and stored at −70°C until analysis.
MAPK activation within each sample was determined by methods described previously (Sharma et al., 2003b). Briefly, 20 μl of each lysed SN cluster was loaded onto 4–12% Tris-BCA gels (NuPAGE; Invitrogen, Carlsbad, CA), electrophoretically separated, and then transferred onto nitrocellulose membranes. Primary (anti-phospho p44/p42 MAPK and phospho-independent p44/p42 MAPK) and secondary (anti-rabbit IgG HRP-linked) antibodies (Cell Signaling Technology, Beverly, MA) were used to assess MAPK activation within each sample. This was done by (1) immunodetection of first the phosphorylated MAPK (P-MAPK) fraction, (2) stripping the membrane, and (3) reprobing with a phospho-independent antibody to measure the total MAPK (T-MAPK) fraction. For comparison of MAPK activation between samples loaded onto different gels, a single lane on each gel was loaded with a common 10 μl sample of Aplysia CNS (the pleural-pedal ganglia from an untrained animal lysed in 300 μl of lysis buffer). MAPK activation in trained or control animals was then measured by first normalizing the P-MAPK and T-MAPK exposures to that of the common CNS sample and then generating a normalized P-MAPK/T-MAPK ratio (activation ratio) for each sample. Data are presented as the means ± SEM of normalized MAPK activation ratios.
Data analysis.
Parametric statistics were used for all analyses. When appropriate, we first performed an ANOVA, followed by planned within- and between-group comparisons. Within-group comparisons were performed using t tests on difference scores between T-SWR in the test condition compared with the mean baseline response. Between-group comparisons were made using t tests for independent means. All reported probabilities reflect two-tailed analyses (α = 0.05) unless otherwise indicated.
Results
Spaced but not massed training trials induce LTM
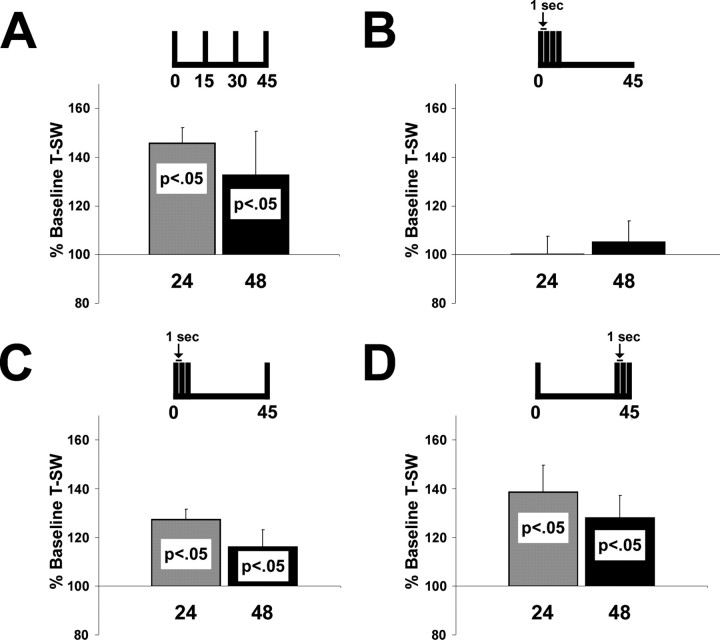
Previously, we showed that four or five TSs induced LTM for sensitization when they were administered in a spaced training session [interstimulus interval (ISI) of 15 min] but not when trials were massed (ISI of 1 s) (Sutton et al., 2002). We replicated this basic spacing effect. We found that four TSs induced 24 h LTM within the T-SWR after spaced training (ISI of 15 min; mean ± SEM, 146 ± 6%; n = 14; p < 0.01) (Fig. 1A) but not massed training (ISI of 1 s; 100 ± 7%; n = 6; NS) (Fig. 1B). We also extended our previous observations by showing that LTM persisted at least 48 h after spaced training (133 ± 18%; p < 0.05, one-tail analysis). These data confirm that the pattern of training trials is a critical feature in the induction of LTM for sensitization.
Figure 1.
A single spaced TS before or after massed training induces LTM. A, B, Four spaced TSs (ISI of 15 min) induce 24 and 48 h LTM (A), whereas massed TSs (ISI of 1 s) do not (B). C, D, Three massed TSs contribute to LTM induction when a single spaced TS is administered either 45 min after (C) or 45 min before (D) the massed TSs. Training patterns are illustrated above their respective datasets. In this and subsequent figures, data are expressed as mean ± SEM.
A single training trial spaced before or after massed training is sufficient to induce LTM
Massed and spaced training differ in two ways: (1) net training duration and (2) rest intervals between trials. As an additional component of the overall experiment (Fig. 1), we asked whether massed training failed to induce LTM because it lacked rest intervals between trials. To explore this question, we spaced the last TS of a four TS training pattern from three massed TSs (ISI of 1 s). The fourth TS was spaced 45 min after the three massed TSs, making the net training duration the same as the four TS spaced (ISI of 15 min) training (Fig. 1C). Surprisingly, this training pattern was sufficient to induce significant LTM, reflected by a within-group comparison of responses at both 24 h (n = 9; 128 ± 4%; p < 0.01) and 48 h (116 ± 7%; p < 0.05) after training. The induced LTM was also not significantly different from that induced by four spaced TSs (Fig. 1A). Thus, although the majority of the training (three TSs) was in a massed pattern, the spacing of a single TS was sufficient to promote LTM induction.
In a final component of the experiment, we asked whether the reverse training pattern would result in the induction of LTM. We trained animals with a single TS, followed 45 min later by three massed TSs (Fig. 1D). A within-group analysis showed that this pattern also induced significant LTM at 24 h (n = 9; 139 ± 7%; p < 0.01) and 48 h (128 ± 9%; p < 0.05) after training (Fig. 1D). The LTM was not significantly different in either duration or intensity from that of animals trained with (1) three massed TSs followed by a single spaced TS (Fig. 1C), as well as (2) four spaced TSs (Fig. 1A). These data show that the spacing of a single TS 45 min before or after massed training is sufficient to induce LTM.
Two spaced trials can induce LTM
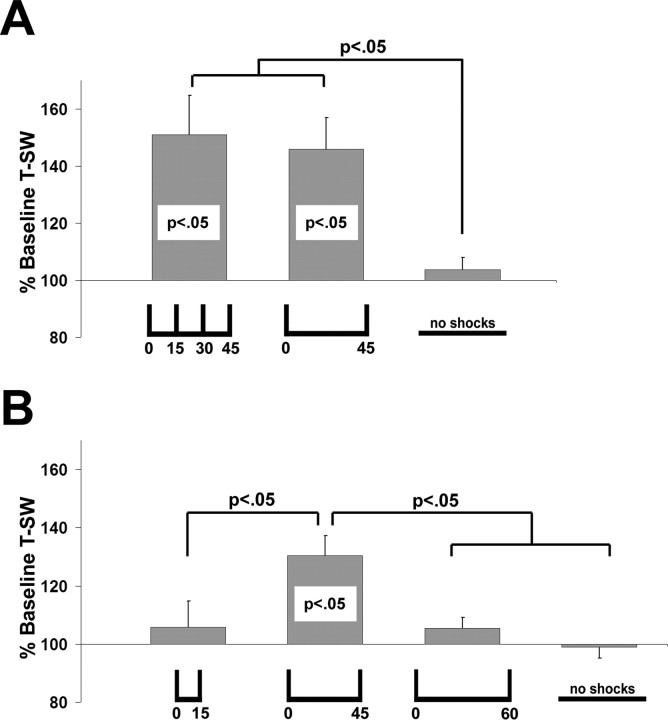
The finding that LTM is induced regardless of whether three massed TSs precede or follow a spaced TS suggests that massed training might be encoded as a single TS. If this is the case, then two single TSs, spaced by 45 min, should also be sufficient for the induction of LTM. Consistent with this prediction, animals trained with two spaced TSs (ISI of 45 min) demonstrated significant LTM (n = 11; 146 ± 11%; p < 0.01) (Fig. 2A) 24 h after training. Two-trial LTM was not significantly different from LTM induced by four spaced TSs (ISI of 15 min) (n = 13; 151 ± 14% at 24 h), and both trained groups were significantly different from untrained control animals (n = 6; 104 ± 4% at 24 h; p < 0.05 in each case). Moreover, both two spaced TSs (ISI of 45 min) and four spaced TSs (ISI of 15 min) induced significant 48 h LTM (127 ± 7%, p < 0.01 and 121 ± 6%, p < 0.01, respectively; data not shown). Thus, LTM can be induced by training with only two TSs.
Figure 2.
A, B, A narrow temporal window for LTM induction. Two TSs (ISI of 45 min) induce LTM comparable to that induced by four TSs (ISI of 15 min) (A), whereas two TSs spaced 15 or 60 min apart do not (B).
Two-trial LTM induction has a narrow permissive window
The finding that only two TSs can induce LTM was particularly surprising given our previous work that showed that training with two TSs, spaced by either 15 or 60 min, was not sufficient to induce LTM (Sutton et al., 2002). In the context of our present findings, these previous data suggest the existence of a relatively narrow temporal window after an initial TS, during which the administration of a second TS can induce LTM. To directly examine this possibility, we assessed LTM induction in animals that received two-trial training at the permissive interval (45 min; n = 18), the nonpermissive intervals of 15 min (n = 8) and 60 min (n = 9), or in untrained controls (n = 9) (Fig. 2B). An initial ANOVA revealed a significant difference in 24 h LTM induction (F(3,40) = 4.91; p < 0.01). Subsequent planned comparisons revealed that LTM only occurred with two TSs spaced by 45 min (131 ± 7%; p < 0.01) but not in animals receiving two TSs spaced by either 15 or 60 min (106 ± 9 and 106 ± 4%, respectively; NS) or in untrained controls (99 ± 4%; NS) (Fig. 2B). Moreover, this two-trial LTM (ISI of 45 min) was significantly different from all other groups (p < 0.05 in all cases), which were not significantly different from each other. Together, the data in Figure 2B delimit a surprisingly narrow training interval (45 min but not 15 or 60 min) for two-trial induction of LTM. Data from all behavioral experiments are summarized schematically in Figure 3.
Figure 3.
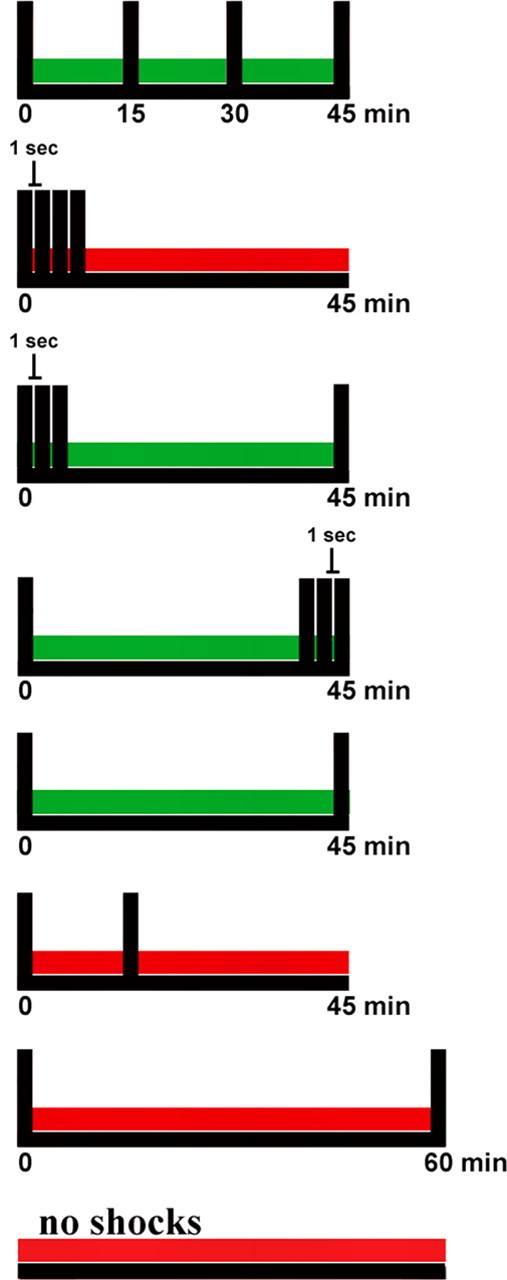
Schematic summary of permissive and nonpermissive training patterns for LTM induction. Green horizontal bars represent permissive training patterns for LTM induction, and red horizontal bars represent nonpermissive patterns.
MAPK activation maps onto the narrow permissive window for two-trial LTM induction
One way to consider the data in Figure 3 is that an initial training trial sets in motion molecular events that can be encountered, within a narrow temporal window, by a second training trial to initiate downstream signaling sufficient for LTM. To begin to explore this possibility, we asked which molecular pathways might be recruited in a manner that could explain the permissive temporal window at 45 min (Figs. 2B, 3). Because signaling through MAPK is a necessary requirement for LTM induction in Aplysia, we focused on its activation. We found that MAPK was transiently activated in the input pathway of the T-SWR, the tail SNs, after a single TS. MAPK activation was assessed in SN cell body clusters isolated from animals at the permissive training interval (45 min), as well as at both nonpermissive intervals (15 and 60 min), and from untrained control animals (Fig. 4). An ANOVA revealed a significant difference in MAPK activation at the various time points after the TS (F(3,28) = 4.37; p < 0.05). Subsequent between-group comparisons with untrained controls (n = 8; 1.3 ± 0.2) revealed significant transient MAPK activation only at the permissive 45 min window (n = 9; 2.3 ± 0.2; p < 0.01). MAPK activation in SNs isolated 15 min (n = 8; 1.6 ± 0.2) and 60 min (n = 7; 1.3 ± 0.2) after a single TS were not significantly different from untrained controls nor from each other. Moreover, MAPK activation at 45 min was significantly greater (p < 0.05) than activation levels just 15 min later (60 min). These data show that a single TS establishes transient activation of MAPK in tail SNs at 45 min, during the same temporal window that is permissive for the induction of two-trial LTM.
Figure 4.
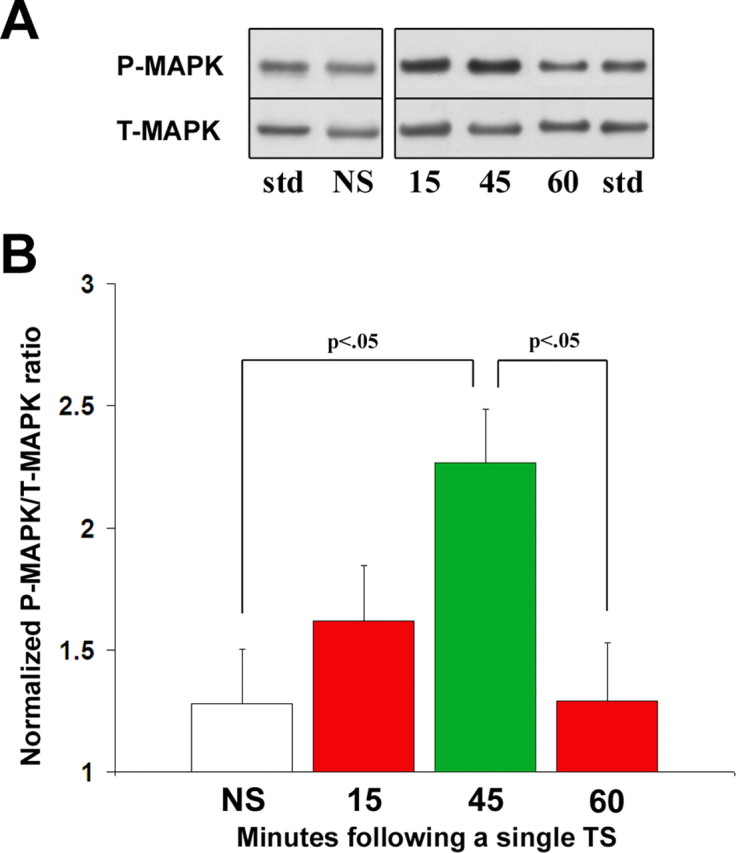
MAPK is transiently activated in tail SNs 45 min following a single TS. A, Representative immunoblots of membranes probed for both P-MAPK and T-MAPK fractions in tail SNs from trained animals. std, A common CNS standard used to normalize MAPK activation from different samples. B, Histograms reflect mean ± SEM normalized MAPK activation ratios from tail SNs isolated (1) during nonpermissive intervals for LTM induction (15 and 60 min after a single TS; red), (2) during a permissive training interval (45 min; green), or (3) from untrained controls (white).
Discussion
The formation of lasting memories is extremely sensitive to the spacing of training trials (Donovan and Radosevich, 1999; Cepeda et al., 2006). Thus, a fundamental question in the mechanistic analysis of memory centers on the question of the molecular substrates of pattern sensitivity. As a first step in this analysis, we have identified a simple training pattern for the induction of LTM for sensitization in Aplysia. We find that induction of two-trial LTM requires that the second of two TSs be delivered within a narrow temporal window following the initial TS. Moreover, we discovered that transient recruitment of activated MAPK, a necessary signaling event for LTM in Aplysia (Sharma et al., 2003b) and in other systems (Sweatt, 2004), occurs in tail SNs selectively during the permissive temporal window for LTM induction. Thus, MAPK, through its transient activation by the initial training trial, appears to define a molecular feature that can account for the temporal window for two-trial LTM induction.
Massed training can interact with a single spaced trial to induce LTM
We found that the spacing of a single TS, either 45 min before or after three massed TSs, was sufficient for LTM induction. The study of massed training in other systems (Muzzio et al., 1999; Huang and Farley, 2001; Genoux et al., 2002) has suggested that shorter training intervals preferentially result in increases in phosphatase activity, which can disrupt memory formation. Although a similar mechanism may also exist in Aplysia (Sharma et al., 2003a), our behavioral data suggest that, in addition to the potential recruitment of inhibitory events, massed training is also able to interact positively with events recruited by an additional TS. The ability to enable the formation of LTM by coupling massed training with a single spaced TS suggests that the massed training may be encoded as a single training event. Consistent with this possibility are recent observations by Marinesco and colleagues (Marinesco and Carew, 2002; Marinesco et al., 2004b) who examined serotonin (5-HT), a critical neuromodulator in Aplysia. After a single sensitizing stimulus, serotonergic cells increase their firing rates and release 5-HT at tail SN cell bodies and synapses (Marinesco and Carew, 2002; Marinesco et al., 2004a). The increased release of 5-HT is detectable for ∼40 s, and the increased firing of serotonergic cells persists for several minutes. Thus, massed training, which occurs within seconds, may result in a single 5-HT release event, thereby being coded as a single sensitizing event.
Two-trial LTM induction
The discovery of two-trial LTM was surprising. Previous work showed that LTM formation required at least four spaced trials (Sutton et al., 2002). Our evidence for two-trial LTM induction, however, shows that only two trials are required when events established by the initial training trial interact with subsequent training during a specific temporal window to induce LTM. In our current work, we demonstrated the existence of such a permissive temporal window for the second trial (Fig. 2B). This permissive window is delimited by two bracketing nonpermissive intervals at 15 and 60 min after an initial TS. These data now raise the important question of the functional significance of this restricted window for LTM induction.
Potential mechanisms contributing to effective spacing intervals for LTM induction
What cellular and molecular properties of neurons in the T-SWR support a 45 min spacing interval for two-trial LTM? Studies of the patterning requirements for the induction of long-term facilitation (LTF) of the tail SN–motor neuron (MN) synapse parallel those for sensitization memory in the T-SWR (Mauelshagen et al., 1996, 1998). For example, four spaced (ISI of 15 min) pulses of exogenous 5-HT are required to induce LTF at tail SN–MN synapses (Mauelshagen et al., 1996). If 5-HT release within the CNS is a critical signaling event initiated by TS, an important experimental question is whether two spaced 5-HT pulses (ISI of 45 min) can induce LTF or whether additional 5-HT signaling (provided by additional pulses) is required.
The analysis of repeated trial learning in Aplysia has identified several critical molecular requirements for LTM induction downstream of 5-HT. These include the signaling kinases protein kinase A (PKA), MAPK, the transcription factor CREB1, and CRE-mediated transcription (Kandel, 2001). Importantly, these signaling pathways also participate in memory induction in mammalian systems (Silva et al., 1998; Sweatt, 2004). In the present paper, we have described transient MAPK activation after a single trial in temporal register with the permissive window for LTM induction. These results support the following conclusions: (1) the narrow temporal window for LTM induction during two-trial training is paralleled at the molecular level by the narrow temporal activation of at least one critical signaling molecule, MAPK; (2) MAPK activation in the SNs may play a critical role in defining the spacing intervals for permissive training; and (3) transient MAPK activation following a single TS is not sufficient for LTM induction (because a single TS does not induce LTM). Rather, MAPK activity must interact with subsequent training to induce LTM. We showed previously that sustained (1–3 h) MAPK activation is induced following repeated TSs and is necessary for LTM induction (Sharma et al., 2003b). Thus, it will now be instructive to assess the impact of a second TS on the induction of sustained MAPK activation following permissive and nonpermissive training intervals. Importantly, LTM induced by two-trial training may not be mechanistically identical to that induced following repeated (at least four TSs) training. Consistent with this possibility, LTM induction typically recruits overt structural changes in the CNS but can sometimes occur in the absence of such changes (Wainwright et al., 2002). This general point is also reflected at the synaptic level where LTF at the SN–MN synapse can have different underlying molecular mechanisms (Casadio et al., 1999; Sherff and Carew, 2004; Hu et al., 2007).
Common learning mechanisms
MAPK is critically involved in the induction of long-lasting forms of both synaptic plasticity and memory in vertebrate systems (Sweatt, 2004). Spaced depolarizing stimuli, but not massed stimuli, recruit sustained phosphorylation of MAPK in hippocampal dendrites to support stable changes in dendrite structure (Wu et al., 2001). MAPK is also maximally recruited by an optimal ISI for LTP induction (10 min) but not ISIs of 20 s and 30 min (Ajay and Bhalla, 2004). The mechanisms underlying regulation of MAPK activation also appear to be highly conserved. For example, Sindreu et al. (2007) recently found that MAPK activation after contextual fear conditioning in mice depends on calcium-stimulated adenylyl cyclase activity. In Aplysia, cAMP-dependent signaling is also necessary both for MAPK activation and for its translocation to the nucleus (Martin et al., 1997). In light of these findings, it will be of considerable interest to examine cAMP levels in the SNs during two-trial training. Besides MAPK, PKA (Woo et al., 2003), protein phosphatase-1 (Genoux et al., 2002), and CREB (Josselyn et al., 2001) have also all been implicated as potential pattern recognition molecules in vertebrates. Importantly, the depletion of two CREB isoforms changes both the appropriate spacing interval and number of trials required for LTM induction in mice (Kogan et al., 1997).
In summary, the well established benefit of spaced (over massed) training in memory formation in a wide range of animals, as well as the identification of common, pattern-sensitive signaling molecules in many species, supports the notion that elucidation of the mechanisms that specify permissive spacing intervals for LTM induction in Aplysia can also critically inform how these conserved learning mechanisms define appropriate spacing intervals in more complex systems.
Footnotes
This work was supported by National Institutes of Health Grant 2R01MH041083-19 and National Science Foundation Grant IOB-0444762 (T.J.C.). We thank Dan Berlau, Kasia Berlau, Marcelo Wood, and the Carew laboratory for helpful discussions.
References
- Ajay SM, Bhalla US. A role for ERKII in synaptic pattern selectivity on the time-scale of minutes. Eur J Neurosci. 2004;20:2671–2680. doi: 10.1111/j.1460-9568.2004.03725.x. [DOI] [PubMed] [Google Scholar]
- Brembs B, Lorenzetti FD, Reyes FD, Baxter DA, Byrne JH. Operant reward learning in Aplysia: neuronal correlates and mechanisms. Science. 2002;296:1706–1709. doi: 10.1126/science.1069434. [DOI] [PubMed] [Google Scholar]
- Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99:221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Pashler H, Vul E, Wixted JT, Rohrer D. Distributed practice in verbal recall tasks: a review and quantitative synthesis. Psychol Bull. 2006;132:354–380. doi: 10.1037/0033-2909.132.3.354. [DOI] [PubMed] [Google Scholar]
- Donovan JJ, Radosevich DJ. A meta-analytic review of the distribution of practice effect: now you see it, now you don't. J Applied Psychol. 1999;84:795–805. [Google Scholar]
- Ebbinghaus HE. Memory: a contribution to experimental psychology. New York: Dover; 1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418:970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- Gerber B, Wustenberg D, Schutz A, Menzel R. Temporal determinants of olfactory long-term retention in honeybee classical conditioning: nonmonotonous effects of the training trial interval. Neurobiol Learn Mem. 1998;69:71–78. doi: 10.1006/nlme.1997.3801. [DOI] [PubMed] [Google Scholar]
- Hu JY, Chen Y, Schacher S. Protein kinase C regulates local synthesis and secretion of a neuropeptide required for activity-dependent long-term synaptic plasticity. J Neurosci. 2007;27:8927–8939. doi: 10.1523/JNEUROSCI.2322-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Farley J. PP1 inhibitors depolarize Hermissenda photoreceptors and reduce K+ currents. J Neurophysiol. 2001;86:1297–1311. doi: 10.1152/jn.2001.86.3.1297. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Shi C, Carlezon WA, Jr, Neve RL, Nestler EJ, Davis M. Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J Neurosci. 2001;21:2404–2412. doi: 10.1523/JNEUROSCI.21-07-02404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Blendy JA, Coblentz J, Marowitz Z, Schutz G, Silva AJ. Spaced training induces normal long-term memory in CREB mutant mice. Curr Biol. 1997;7:1–11. doi: 10.1016/s0960-9822(06)00022-4. [DOI] [PubMed] [Google Scholar]
- Marinesco S, Carew TJ. Serotonin release evoked by tail nerve stimulation in the CNS of Aplysia: characterization and relationship to heterosynaptic plasticity. J Neurosci. 2002;22:2299–2312. doi: 10.1523/JNEUROSCI.22-06-02299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinesco S, Kolkman KE, Carew TJ. Serotonergic modulation in Aplysia. I. Distributed serotonergic network persistently activated by sensitizing stimuli. J Neurophysiol. 2004a;92:2468–2486. doi: 10.1152/jn.00209.2004. [DOI] [PubMed] [Google Scholar]
- Marinesco S, Wickremasinghe N, Kolkman KE, Carew TJ. Serotonergic modulation in Aplysia. II. Cellular and behavioral consequences of increased serotonergic tone . J Neurophysiol. 2004b;92:2487–2496. doi: 10.1152/jn.00210.2004. [DOI] [PubMed] [Google Scholar]
- Martin KC, Michael D, Rose JC, Barad M, Casadio A, Zhu H, Kandel ER. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- Mauelshagen J, Parker GR, Carew TJ. Dynamics of induction and expression of long-term synaptic facilitation in Aplysia. J Neurosci. 1996;16:7099–7108. doi: 10.1523/JNEUROSCI.16-22-07099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauelshagen J, Sherff CM, Carew TJ. Differential induction of long-term synaptic facilitation by spaced and massed applications of serotonin at sensory neuron synapses of Aplysia californica. Learn Mem. 1998;5:246–256. [PMC free article] [PubMed] [Google Scholar]
- Muzzio IA, Ramirez RR, Talk AC, Matzel LD. Interactive contributions of intracellular calcium and protein phosphatases to massed-trials learning deficits in Hermissenda. Behav Neurosci. 1999;113:103–117. doi: 10.1037//0735-7044.113.1.103. [DOI] [PubMed] [Google Scholar]
- Pinsker H, Kupfermann I, Castellucci V, Kandel E. Habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167:1740–1742. doi: 10.1126/science.167.3926.1740. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Bagnall MW, Sutton MA, Carew TJ. Inhibition of calcineurin facilitates the induction of memory for sensitization in Aplysia: requirement of mitogen-activated protein kinase. Proc Natl Acad Sci USA. 2003a;100:4861–4866. doi: 10.1073/pnas.0830994100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Sherff CM, Shobe J, Bagnall MW, Sutton MA, Carew TJ. Differential role of mitogen-activated protein kinase in three distinct phases of memory for sensitization in Aplysia. J Neurosci. 2003b;23:3899–3907. doi: 10.1523/JNEUROSCI.23-09-03899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Carew TJ. The roles of MAPK cascades in synaptic plasticity and memory in Aplysia: facilitatory effects and inhibitory constraints. Learn Mem. 2004;11:373–378. doi: 10.1101/lm.81104. [DOI] [PubMed] [Google Scholar]
- Sherff CM, Carew TJ. Parallel somatic and synaptic processing in the induction of intermediate-term and long-term synaptic facilitation in Aplysia. Proc Natl Acad Sci USA. 2004;101:7463–7468. doi: 10.1073/pnas.0402163101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Sindreu CB, Scheiner ZS, Storm DR. Ca2+-stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron. 2007;53:79–89. doi: 10.1016/j.neuron.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susswein AJ, Schwarz M. A learned change of response to inedible food in Aplysia. Behav Neural Biol. 1983;39:1–6. doi: 10.1016/s0163-1047(83)90535-6. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ide J, Masters SE, Carew TJ. Interaction between amount and pattern of training in the induction of intermediate- and long-term memory for sensitization in Aplysia. Learn Mem. 2002;9:29–40. doi: 10.1101/lm.44802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Wainwright ML, Zhang H, Byrne JH, Cleary LJ. Localized neuronal outgrowth induced by long-term sensitization training in Aplysia. J Neurosci. 2002;22:4132–4141. doi: 10.1523/JNEUROSCI.22-10-04132.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ET, Carew TJ, Kandel ER. Classical conditioning in Aplysia californica. Proc Natl Acad Sci USA. 1979;76:6675–6679. doi: 10.1073/pnas.76.12.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NH, Duffy SN, Abel T, Nguyen PV. Temporal spacing of synaptic stimulation critically modulates the dependence of LTP on cyclic AMP-dependent protein kinase. Hippocampus. 2003;13:293–300. doi: 10.1002/hipo.10086. [DOI] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nat Neurosci. 2001;4:151–158. doi: 10.1038/83976. [DOI] [PubMed] [Google Scholar]