Abstract
Several areas of the macaque brain are known to be related to the reward during the performance of saccadic eye-movement tasks. Neurons in the supplementary eye field (SEF) have been reported to be involved in the prediction and detection of a reward. We describe a group of neurons in the SEF that became active during the period of reward delivery after saccades toward a specific direction, but showed weaker activity in other directions, although the same amount of reward was given in each direction. Moreover, this directional reward activity was modulated by the reward size. Our results demonstrate that the SEF cells may reflect both reward amount and target positions toward which a movement was rewarded, and suggest that they may play an important role in providing information about the value of each saccade according to the spatial target location.
Keywords: saccadic eye movement, reward amount, directional bias, motivation, monkey, neural activity
Introduction
For the successful performance of a task, reward is an essential element of a trial. Thus, the reward nature of behavior has interested researchers for many years (Tolman, 1932). Many areas of the brain contribute to the reward process during voluntary movements, such as the basal ganglia (Hollerman and Schultz, 1998; Kawagoe et al., 1998; Schultz et al., 1998), prefrontal cortex (Watanabe, 1996; Leon and Shadlen, 1999), cingulate motor area (Shima and Tanji, 1998), and parietal cortex (Platt and Glimcher, 1999). More specifically, several areas of the macaque brain underlying saccadic eye movements, including the caudate, frontal eye field (FEF), and supplementary eye field (SEF), are known to signal rewards during performance of saccadic eye-movement tasks. The caudate provides information about both reward size and the target positions toward which a movement was rewarded (rewarded target position) (Takikawa et al., 2002; Kawagoe et al., 2004; Lau and Glimcher, 2005; Ding and Hikosaka, 2006), whereas the FEF provides only information on the rewarded target position, in particular (Coe et al., 2002; Roesch and Olson, 2003; Ding and Hikosaka, 2006). Although the SEF plays an important role in predicting and detecting rewards (Amador et al., 2000; Stuphorn et al., 2000), it is unknown whether activity in the SEF actually reflects rewarded target position or reward amount.
To address this question, we trained two monkeys in a saccadic eye-movement task, in which each monkey had to make a total of eight center-out saccades to each of eight targets located at 0° (right), 45°, 90° (up), 135°, 180° (left), 225°, 270° (down), and 315°. Rewards of varying size were delivered after each successful saccade. Single-cell recordings were performed in the SEFs while the monkeys performed the task.
Materials and Methods
Subjects.
Animals were cared for in accordance with the Guiding Principles for the Care and Use of Animals, approved by the Council of the Physiological Society of Japan. All experiments were approved by the Committee for Animal Experimentation of Juntendo University School of Medicine.
We used two Japanese monkeys (Macaca fuscata), monkey SU (6.7 kg) and monkey SO (7.2 kg). The experiments were performed while the monkey's head was fixed and its eye movements were recorded. For this purpose, a head holder, an eye coil, and a recording chamber were implanted surgically (Lu et al., 1998).
Task procedures.
The monkeys were trained to perform a visually guided saccadic eye movement task in which each monkey made 18° saccades to each of eight targets located at 0° (right), 45°, 90° (up), 135°, 180° (left), 225°, 270° (down), and 315° from a central fixation point. A trial began with presentation of the fixation point, on which the monkey had to fixate for 500 ms (fixation period). After the fixation period, a target was presented randomly at one of the eight target locations. The animal continued to fixate for 500–800 ms, until the fixation point disappeared (“go” signal, delay period). After the go signal, the monkey made a saccade to the target (saccade period) and held on the target for 200 ms (target hold period). The saccade period was the duration from leaving the fixation point to acquiring the target (“target in”). The target-hold period was followed by water reward delivery. The reward was delivered in two different manners: (1) the monkey received a constant amount of reward (valve opening time of 60 ms) at the end of each successful trial, through a block consisting of 80 trials; (2) small (40 ms) and big (80 ms) rewards were delivered after 16 trials alternately, through a block consisting of 160 trials. Once the animals performed consistently, with >85% accuracy at the end of the training period, we began to record the activity of single cells extracellularly using a tungsten microelectrode (diameter, 0.25 mm; length, 80 mm; impedance, 0.4–1.0 MΩ; measured at 1 kHz; Frederic Haer, Bowdoinham, ME). Action potentials were isolated using a cluster cutting technique (off-line sorter; Plexon, Dallas, TX). Eye movements were recorded using a search coil method (MEL-20U; Enzanshi-Kogyo, Tokyo, Japan) (Robinson, 1963).
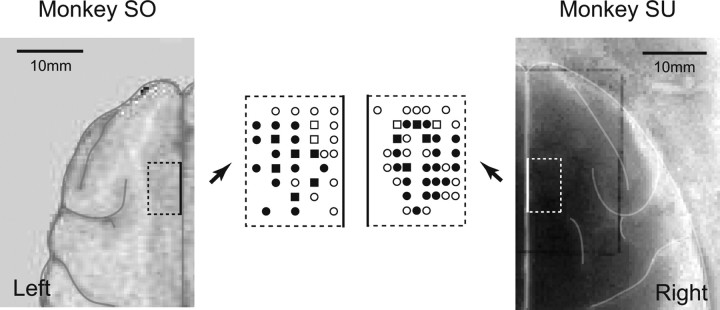
We established that the site of recording was in the SEF, using criteria based on preoperative anatomic magnetic resonance imaging (AIRIS, 0.3 T; Hitachi, Tokyo, Japan) and intracortical microstimulation (50 negative pulses of 0.2 ms duration at 330 Hz with currents <50 μA) (Schlag and Schlag-Rey, 1987) (Fig. 1).
Figure 1.
Recording and stimulation sites (dotted lines) of SEF neurons for monkeys SO and SU. Dotted lines are drawn relative to brain anatomy, constructed from the MRI image. The recordings were centered ∼5 mm to the right or left of the midline for each monkey. Views of the recording and stimulation sites are enlarged. Squares show sites of microstimulation for evoking saccades (threshold ≤50 μA) and recording, circles show sites of recording, and closed symbols indicate the existence of reward-related cells.
Data analysis.
We examined whether a cell's discharge rate was significantly higher for anyone of the five task periods (fixation, delay, saccade, target hold, reward period) than for the prefixation control period (t test, p < 0.05). If so, the neuron was defined as a “task-related cell.” In this study, we focused on the neuronal responses during saccade and reward periods because larger numbers of task-related cells were seen in the saccade and reward periods (supplemental Table 1, available at www.jneurosci.org as supplemental material). The task-related activity of the saccade and reward periods was classified according to the following six types of neuron, depending on the degree of specificity for saccade, reward, and spatial biases.
S-cell
We examined whether a cell's discharge rate was significantly higher for the saccade period (go signal to target-in) than for the control period (200 ms). If so, the cell was defined as a “saccade-related cell” (S-cell or S-activity; t test, p < 0.05).
R-cell
We examined whether a cell's discharge rate was significantly higher for the reward period (300 ms post reward onset) than for the control period. If so, the cell was defined as a “reward-related cell” (R-cell or R-activity; t test, p < 0.05).
SD-cell
We examined whether an S-cell's discharge rate differed significantly among the eight directions. If so, the cell was defined as a “directionally biased S-cell” (SD-cell; ANOVA, p < 0.05).
RD-cell
We examined whether an R-cell's discharge rate differed significantly among the eight directions. If so, the cell was defined as a “directionally biased R-cell” (RD-cell; ANOVA, p < 0.05).
SDT-cell
We examined whether there was a directional tuning effect in an SD-cell. The mean firing rate during the saccade period (f) was fitted by a cosine function with a lower limit of zero: f = max(0,αcos((θ − d)π/180) + β), (−45 < θ ≤ 315), where α, θ, d, and β denote the amplitude of modulation, the direction of the target (in degrees), the preferred direction, and the baseline activity, respectively. The function had three parameters to be estimated (α, d, β) that are illustrated in Figure 1 (amplitude, preferred direction, mean). If the correlation between the actual firing rate in the saccade period and the cosine model prediction (Pearson's correlation coefficient) was significant, the cell was defined as an SD-cell with directional tuning (SDT-cell; p < 0.05) (Georgopoulos et al., 1982; Shibuya et al., 2007).
RDT-cell
The same technique was applied to RD-cells. If the correlation between the actual firing rate in the reward period and the cosine model prediction was significant, the RD-cell was defined as an RD-cell with directional tuning (RDT-cell; p < 0.05)
Results
Directional tuning of reward-related activity
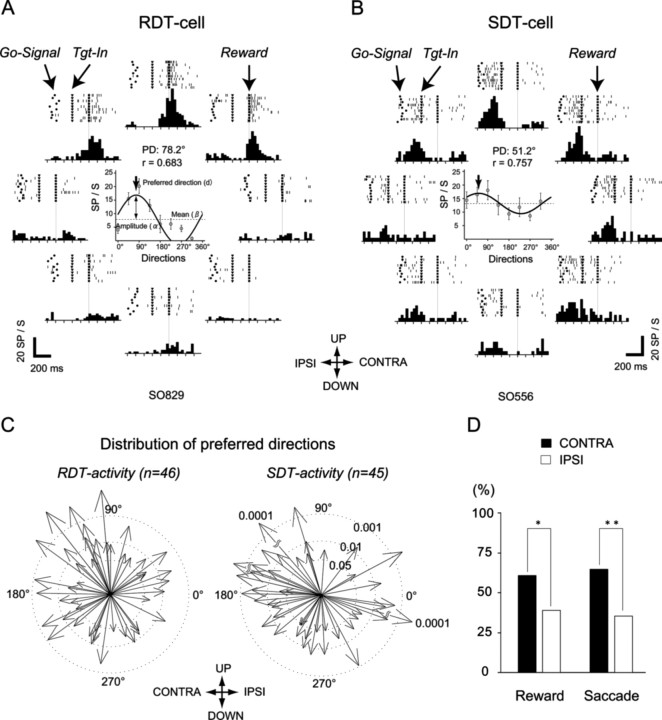
The SEF was identified by its location (dorsomedial frontal cortex, 1–4 mm from midline, slightly anterior to the level of the frontal eye field) and the results of intracortical microstimulation (saccades, not body movements, evoked with currents of <50 μA) (Schlag and Schlag-Rey, 1987) (Fig. 1). Among 181 cells recorded from the SEFs, there were 42, 37, 81, 54, and 78 cells that showed significantly higher activity during fixation, delay, saccade, target hold, and reward periods, respectively (supplemental Table 1, available at www.jneurosci.org as supplemental material). We focused on saccade- and reward-related cells (S- and R-cells) in this study. We found that among 81 S-cells and 78 R-cells, more than half showed directionally biased activity (SD-cells, 67%, n = 54; RD-cell, 72%, n = 56). Moreover, most SD- and RD-cells showed significant directional tuning effects. We refer to these cells as SDT-cells (83%; n = 45) (Fig. 2B) and RDT-cells (82%; n = 46) (Fig. 2A). Twenty-one of them were both members of the SDT- and RDT-cell groups.
Figure 2.
Reward- and saccade-related activity with a directional tuning effect. A, Reward-related activity, shown in raster and histogram format, is aligned to reward onset. The filled dots, filled triangles, and filled squares indicate the Go-signal, Target-in (end of the saccade), and reward onset, respectively. The cell was consistently active during the reward periods after upward saccades and less strongly after left- and right-upward saccades. The center graph indicates the fitted sinusoidal curve of the neuronal activities across eight directions. PD, Preferred direction; r, a correlation coefficient to test the association between the actual firing rate of the reward period and the cosine model prediction. B, Saccade-related activity. The cell was consistently active during the upward saccades and right-up saccade, and less strongly after left-up and rightward saccades. Other conventions are as in A. C, Spatial distribution of the preferred directions of rewarded-target-position-specific activity and saccade-direction-specific activity of SEF cells. Vectors indicate the preferred directions of the reward-related activities (left) or saccade-related activities (right). Three broken circles of different diameters indicate the three levels of significance of correlation between the actual firing rate of the reward (left) or saccade (right) periods and the sine model prediction. D, Comparison between the number of preferred directions contralateral and ipsilateral to recorded SEF. *p < 0.05; **p < 0.01.
Figure 2A shows a sample RDT-cell. There was enhancement of the neural discharge in the upward direction only during a reward period. The discharge became weaker in the unpreferred directions. Statistically, the spike rate of neuronal activity during the reward period (300 ms postreward onset) differed significantly across the eight directions (ANOVA, p = 0.001). Correlation between the spike rate of the reward period and the cosine model prediction was significant (r = 0.68; p = 0.006). The preferred direction of neuronal activity was 78 degrees. However, for an SDT-cell (Fig. 2B), there was enhancement of the neural discharge in the up and right-upward directions only during a saccade period. The discharge became weaker in the unpreferred directions. Statistically, the spike rate of the neuronal activity during the saccade period (from go signal to target in) differed significantly across the eight directions (ANOVA, p = 0.008). Correlation between the spike rate of the saccade period and the cosine model prediction was significant (r = 0.76; p = 0.001). The preferred direction of neuronal activity was 51°.
The distributions of the preferred spatial directions of the SDT and RDT activities are shown in Figure 2, C and D. The preferred directions of SDT and RDT activities were both significantly biased toward the side contralateral to the side of the recording (Fig. 2D, binomial test, reward, p = 0.03; saccade, p = 0.004), although the preferred directions were distributed all around. Overall, reward-related cells (R-cell, 43%; n = 78) were as common as saccade-related cells (S-cell, 45%; n = 81). Most of the R-cells showed directionally biased activity (RD-cells, 72%; n = 56). Moreover, most of the RD-cells showed neural activity with directional cosine tuning (RDT-cells, 82%; n = 46). Thus, these results demonstrate rewarded target position selectivity in the SEF neurons.
In 21 neurons that were both members of RDT and SDT cell classes, the preferred direction during the reward period correlated significantly with the preferred direction during the saccade (r = 0.63; p = 0.0021).
Reward-amount-dependent activity in SEF
We further investigated whether there was reward size selectivity in 84 SEF neurons, by delivering constant rewards, and then small and large rewards. Many of the 84 neurons showed significantly higher activity during fixation (n = 19; 23%), delay (n = 21; 25%), saccade (n = 33; 39%), target hold (n = 27; 32%), and reward (n = 37; 44%) periods than in the control period (supplemental Table 1, available at www.jneurosci.org as supplemental material). The ratio of neurons with significant activation was comparatively large during the saccade and reward periods, although such neurons were found in all task periods.
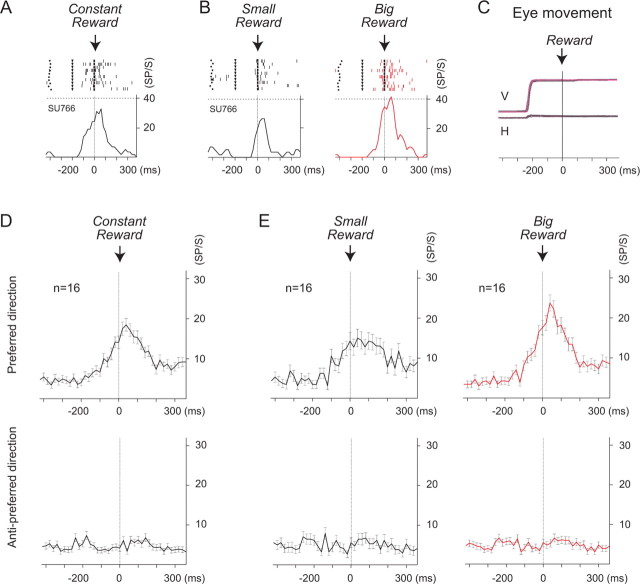
In contrast, among the 84 SEF cells, cells with activity that changed with the size of reward were most commonly found in the reward period (n = 25; 30%), but rarely in other task periods (fixation, 4%, n = 3; delay, 10%, n = 8; saccade, 8%, n = 7; target hold, 12%, n = 10) (supplemental Table 1, available at www.jneurosci.org as supplemental material). We called cells with reward size modulation during the reward period “reward amount cells.” Most of these reward amount cells (n = 22; 88%) showed significantly higher activity during the delivery of the big reward (big-reward cells) than for the smaller reward (t test, p < 0.05). In contrast, few cells (n = 3; 12%) showed significantly higher activity during the delivery of the small reward (t test, p < 0.05). Among 22 big-reward cells, 16 displayed directionally biased activity with a directional tuning effect. An example of a big-reward cell in the preferred direction is shown in Figure 3, A and B. The cells became active during the reward delivery period when the same reward was consistently given after each successful trial (Fig. 3A). The same cells showed significantly higher spike activity during the big reward trials than during the small reward trials (Figs. 3B) (p = 0.006). The mean neural discharges of the big reward-specific cells (n = 16) in the preferred direction and in the anti-preferred direction are shown in Figure 3, D and E. The mean activities in the preferred direction (top) (n = 16) were analogous to the examples shown in Figure 3, A and B.
Figure 3.
Reward size modulation in SEF cells. The activity in the preferred direction, shown in raster and histogram format, is aligned with reward onset. A, When the monkey received the same amount of reward at the end of each trial through a block (see Materials and Methods), the cell was consistently active during the reward period. B, When small and big rewards were delivered after 16 trials, alternately, through a block (see Materials and Methods), the same cell as in A was consistently active during the big-reward trials. The activity became weaker during the small-reward trials. C, Saccadic eye movements: V, vertical; H, horizontal. Black, Eye-movement traces during the small-reward trials; red, eye movement traces during the big-reward trials. D, E, The neuronal activity of a total of 16 reward-size-related cells are shown on average. Top, Neuronal activity in the preferred direction. Bottom, The neuronal activity in the nonpreferred direction.
Although we found no significant difference in the saccade parameters between the big- and small-reward trials, including saccade latency and saccade speed (Fig. 3C), trial success rates changed. The trial success rate was the ratio of 160 successful trials in a block to the total number of trials that the monkey performed within the block. The trial success rate was greater in the large-reward trials than in the small-reward trials (95% versus 81%). The increased error rate (14%), which was primarily caused by abandoning trials in which the fixations were cut short, may reflect lowered motivation during the small-reward trials.
In summary, among 37 R-cells, 25 (68%) showed reward-size selectivity. This result demonstrated reward-size selectivity in the SEF. In addition, many reward-size-related cells also showed spatial-location specificity (64%; n = 16). Thus, together with the finding of a total of 46 rewarded-target-position-specific cells (RDT cells), our results suggest that SEF cells show both reward-size selectivity and rewarded-target-position selectivity. These two dynamics of reward can be present in a single SEF cell.
Discussion
Our data demonstrate that many neurons in SEF exhibited reward-related activity, associated with not only the reward amount but also the target positions toward which a movement was rewarded. The results suggest that SEF cells provided information about both reward size and rewarded target position. Previous studies have shown that neurons in monkey SEFs change their behavior flexibly depending on many kinds of task-specific demands, including object-centered coding of saccades (Olson and Gettner, 1995), arbitrary visuo-oculomotor association (Chen and Wise, 1995, 1996, 1997), anti-saccades (Schlag-Rey et al., 1997), performance monitoring (Stuphorn et al., 2000), anticipatory pursuit (Missal and Heinen, 2004), predicting the correct saccade (Amador et al., 2004), planning of saccades in response to pattern and spatial cues (Olson et al., 2000), remembered spatial sequence (Histed and Miller, 2006), saccade collision (Park et al., 2006), saccade sequence (Lu et al., 2002), and countermanding saccades (Stuphorn and Schall, 2006). Given such diverse capabilities of SEF neurons, rewarded target position and reward-amount selectivity in supplementary eye field may not be surprising. However, to our knowledge, no study has reported this.
Our findings may be relevant to several remarkable studies in primate frontal lobes underlying eye movements, including the SEF, FEF, and caudate, all of which are associated with rewards. In the SEF, many neurons are clearly related to reward, predicting and detecting reward events (Amador et al., 2000). Our data show that a number of cells display reward-related activity during the reward period. This result may correspond with the detecting activity in their study. That is, such reward-detecting cells may play an important role in presenting the value of each saccade, which is perhaps essential for motivating the behavior. In the FEF, neurons show strong associations with rewarded target position, but not reward size (Ding and Hikosaka, 2006), whereas clear selectivity for both reward size and rewarded target position was seen in the caudate neurons (Lau and Glimcher, 2005; Ding and Hikosaka, 2006).
The interesting finding in our study is the target/saccade position effect. Although the caudate and FEF cells have been shown to be involved in providing information about reward size and rewarded target position (Ding and Hikosaka, 2006), they used a paradigm in which only one of two movements was rewarded. In contrast, in our study, saccades in all eight directions were rewarded. Thus, this paradigm in which eight target/saccade positions were rewarded enabled us to better understand the target/saccade position effect in the SEF. It is clear from our data that there were some SEF cells that became active during the period of reward delivery in a specific target direction, but weaker activity was seen for other directions, although the same amount of reward was given for each direction. This suggests that the SEF cells may reflect target positions toward which a movement was rewarded and they perhaps encode the relationship between particular movement and reward.
Another important difference with other reward-related effects described previously is the timing and nature of the reward effect. The activity in FEF and caudate responded to target onsets or other events that led up to the reward (Ding and Hikosaka, 2006). Reward modulated the gain in these sensorimotor representations before the reward delivery. In our present study, however, only a smaller number of cells (20%; n = 37) showed task related activity in the delay period (target onset to Go-signal) than in the reward period (43%; n = 73). Furthermore, only a few cells showed the reward amount related activity in the delay (10%; n = 8), saccade (8%; n = 7) and target-hold (12%; n = 10) periods, before the reward delivery (supplemental Table 1, available at www.jneurosci.org as supplemental material). Thus, unlike the study by Ding and Hikosaka (2006), we found only a weak reward amount effect during the prereward periods. In the study Ding and Hikosaka (2006), the target was only presented at two positions (left or right). In a given block of trials, one position was associated with big reward (for example, right) and the other position was associated with small reward (left). In other words, in a single block, the monkeys always received the big reward when the target was presented at right position. Therefore, the target position was actually a cue to instruct the reward amount. In contrast, in our study, target position was not associated with the reward amount. There was no instruction of reward amount until monkey received the reward. This probably led to the absence of the modulation of prereward activity.
At this point, we do not know what will occur in the SEF cells if we use a task in which the target position is associated with reward size. Yet, in Amador et al.'s study, they found that the SEF cells were related to both reward expectation/prediction and detection when the constant reward was supplied. But the number of predicting cells (n = 23, 35%) was found to be smaller than that (n = 31; 48%) of detecting (Amador et al., 2000). Their results were actually consistent with our data that 28 (28%) and 78 (43%) cells displayed higher activity before and after the reward onset when the reward was supplied constantly (supplemental Table 1, available at www.jneurosci.org as supplemental material). Thus, our data together with the data from Amador et al.'s study has shown a clear tendency that reward is explicitly represented by the SEF cells. That does not seem to be the case for the caudate and FEF cells, which seem to represent reward implicitly.
One might ask whether the higher neuronal activity during the large reward trials could simply have been caused by the longer time taken by the monkey to consume the water reward. We think that this is unlikely for several reasons. First, inspection of the monkeys during recordings revealed no association between the activity of these neurons and movements of the mouth. Second, the monkeys received water rewards for all eight directions, but reward neurons showed higher activity in their preferred directions and lower activity in the antipreferred direction when monkeys performed either large- or small-reward trials (Figs. 3D,E). Finally, others have reported that reward-related activity in the SEF could be dissociated from activity related to mouth movements (Amador et al., 2000).
In conclusion, SEF may be involved in reinforcement in performing or learning the correct saccadic eye movement, by providing spatially relevant reward mechanisms and information on reward size.
Footnotes
This work was supported by Grants-in-Aid for Scientific Research on Priority Areas, system study on higher-order brain functions (17022033) to S.K., and Scientific Research (18500247) to X.L. from the Japanese Ministry of Education, Culture, Sports, Science and Technology. We are grateful to Hidetoshi Takada and Haruyo Kimizuka for technical assistance. We thank Yoriko Takikawa and Reiko Kawagoe for their helpful comments.
References
- Amador N, Schlag-Rey M, Schlag J. Reward-predicting and reward-detecting neuronal activity in the primate supplementary eye field. J Neurophysiol. 2000;84:2166–2170. doi: 10.1152/jn.2000.84.4.2166. [DOI] [PubMed] [Google Scholar]
- Amador N, Schlag-Rey M, Schlag J. Primate antisaccade. II. Supplementary eye field neuronal activity predicts correct performance. J Neurophysiol. 2004;91:1672–1689. doi: 10.1152/jn.00138.2003. [DOI] [PubMed] [Google Scholar]
- Chen LL, Wise SP. Supplementary eye field contrasted with the frontal eye field during acquisition of conditional oculomotor associations. J Neurophysiol. 1995;73:1122–1134. doi: 10.1152/jn.1995.73.3.1122. [DOI] [PubMed] [Google Scholar]
- Chen LL, Wise SP. Evolution of directional preferences in the supplementary eye field during acquisition of conditional oculomotor associations. J Neurosci. 1996;16:3067–3081. doi: 10.1523/JNEUROSCI.16-09-03067.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Wise SP. Conditional oculomotor learning: population vectors in the supplementary eye field. J Neurophysiol. 1997;78:1166–1169. doi: 10.1152/jn.1997.78.2.1166. [DOI] [PubMed] [Google Scholar]
- Coe B, Tomihara K, Matsuzawa M, Hikosaka O. Visual and anticipatory bias in three cortical eye fields of the monkey during an adaptive decision-making task. J Neurosci. 2002;22:5081–5090. doi: 10.1523/JNEUROSCI.22-12-05081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Hikosaka O. Comparison of reward modulation in the frontal eye field and caudate of the macaque. J Neurosci. 2006;26:6695–6703. doi: 10.1523/JNEUROSCI.0836-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci. 1982;2:1527–1537. doi: 10.1523/JNEUROSCI.02-11-01527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Histed MH, Miller EK. Microstimulation of frontal cortex can reorder a remembered spatial sequence. PLoS Biol. 2006;4:e134. doi: 10.1371/journal.pbio.0040134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nat Neurosci. 1998;1:411–416. doi: 10.1038/1625. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. Reward-predicting activity of dopamine and caudate neurons—a possible mechanism of motivational control of saccadic eye movement. J Neurophysiol. 2004;91:1013–1024. doi: 10.1152/jn.00721.2003. [DOI] [PubMed] [Google Scholar]
- Lau B, Glimcher PW. Caudate neurons encode both saccade and reward information in a free-choice task. Soc Neurosci Abstr. 2005;31:400–14. [Google Scholar]
- Leon MI, Shadlen MN. Effect of expected reward magnitude on the response of neurons in the dorsolateral prefrontal cortex of the macaque. Neuron. 1999;24:415–425. doi: 10.1016/s0896-6273(00)80854-5. [DOI] [PubMed] [Google Scholar]
- Lu X, Hikosaka O, Miyachi S. Role of monkey cerebellar nuclei in skill for sequential movement. J Neurophysiol. 1998;79:2245–2254. doi: 10.1152/jn.1998.79.5.2245. [DOI] [PubMed] [Google Scholar]
- Lu X, Matsuzawa M, Hikosaka O. A neural correlate of oculomotor sequences in supplementary eye field. Neuron. 2002;34:317–325. doi: 10.1016/s0896-6273(02)00657-8. [DOI] [PubMed] [Google Scholar]
- Missal M, Heinen SJ. Supplementary eye fields stimulation facilitates anticipatory pursuit. J Neurophysiol. 2004;92:1257–1262. doi: 10.1152/jn.01255.2003. [DOI] [PubMed] [Google Scholar]
- Olson CR, Gettner SN. Object-centered direction selectivity in the macaque supplementary eye field. Science. 1995;269:985–988. doi: 10.1126/science.7638625. [DOI] [PubMed] [Google Scholar]
- Olson CR, Gettner SN, Ventura V, Carta R, Kass RE. Neuronal activity in macaque supplementary eye field during planning of saccades in response to pattern and spatial cues. J Neurophysiol. 2000;84:1369–1384. doi: 10.1152/jn.2000.84.3.1369. [DOI] [PubMed] [Google Scholar]
- Park J, Schlag-Rey M, Schlag J. Frames of reference for saccadic command tested by saccade collision in the supplementary eye field. J Neurophysiol. 2006;95:159–170. doi: 10.1152/jn.00268.2005. [DOI] [PubMed] [Google Scholar]
- Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400:233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Impact of expected reward on neuronal activity in prefrontal cortex, frontal and supplementary eye fields and premotor cortex. J Neurophysiol. 2003;90:1766–1789. doi: 10.1152/jn.00019.2003. [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M. Evidence for a supplementary eye field. J Neurophysiol. 1987;57:179–200. doi: 10.1152/jn.1987.57.1.179. [DOI] [PubMed] [Google Scholar]
- Schlag-Rey M, Amador N, Sanchez H, Schlag J. Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature. 1997;390:398–401. doi: 10.1038/37114. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward prediction in primate basal ganglia and frontal cortex. Neuropharmacology. 1998;37:421–429. doi: 10.1016/s0028-3908(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Shibuya S, Takahashi T, Kitazawa S. Effects of visual stimuli on temporal order judgments of unimanual finger stimuli. Exp Brain Res. 2007;179:709–721. doi: 10.1007/s00221-006-0829-4. [DOI] [PubMed] [Google Scholar]
- Shima K, Tanji J. Role for cingulate motor area cells in voluntary movement selection based on reward. Science. 1998;282:1335–1338. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci. 2006;9:925–931. doi: 10.1038/nn1714. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature. 2000;408:857–860. doi: 10.1038/35048576. [DOI] [PubMed] [Google Scholar]
- Takikawa Y, Kawagoe R, Hikosaka O. Reward-dependent spatial selectivity of anticipatory activity in monkey caudate neurons. J Neurophysiol. 2002;87:508–515. doi: 10.1152/jn.00288.2001. [DOI] [PubMed] [Google Scholar]
- Tolman EC. New York: Century; 1932. Purposive behavior in animals and men. [Google Scholar]
- Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]