Abstract
Amblyopia is a neurodevelopmental disorder of the visual system, as a result of discordant visual experience during infancy or early childhood. Because amblyopia is typically defined as monocularly reduced visual acuity accompanied by one or more known amblyogenic factors, it is often assumed that the fellow eye is normal and sufficient for tasks like reading and eye-hand coordination. Recent scientific evidence of ocular motor, visual, and visuomotor deficits that are present with fellow eye monocular viewing and with binocular viewing calls this assumption into question. This clinical update reviews the research that has revealed fellow ocular motor and visual deficits and the effect that these deficits have on an amblyopic child’s visuomotor and visuocognitive skills. We need to understand how to prevent and rehabilitate the effects of amblyopia not only on the nonpreferred eye but also on the fellow eye.
Keywords: Amblyopia, Fellow eye, Ocular motor, Motion perception, Motor skills
Amblyopia is a neurodevelopmental disorder of the visual system, as a result of discordant visual experience during infancy or early childhood. The most common causes of amblyopia are strabismus and anisometropia. It is widely appreciated that stereoacuity is disrupted in amblyopia and that interocular suppression plays a central role in causing amblyopia. Yet, because amblyopia is typically defined as monocularly reduced visual acuity accompanied by one or more known amblyogenic factors, it is often assumed that the fellow eye is normal and sufficient for tasks like reading and eye-hand coordination. Recent scientific evidence of ocular motor, visual, and visuomotor deficits that are present with fellow eye monocular viewing and with binocular viewing that calls this assumption into question are reviewed here.
Ocular Motor Function
Binocular vision relies on balanced visual input from the two eyes, as well as the ability of the eyes to make conjugate (saccades) and disconjugate (vergence) eye movements to fixate a target and maintain gaze. When binocular vision is disrupted early in life by strabismus, anisometropia, or amblyopia, a host of ocular motor deficits can occur, including fixation instability and abnormal saccades. In general, impaired ocular motor function is more pronounced when viewing with the non-preferred amblyopic eye, and when the visual acuity or stereoacuity deficit is severe.1–4 Yet, ocular motor deficits are also present when viewing with the fellow eye, even in nonamblyopic individuals with strabismus.5–7 Reduced visual acuity is present only in the amblyopic group, while both amblyopic and nonamblyopic individuals with strabismus or anisometropia have experienced binocularly discordant visual input during the critical period of visual maturation, suggesting that the fellow eye deficit is a consequence of discordant binocular experience, not the visual acuity deficit. Studies examining fellow eye deficits in amblyopic and nonamblyopic individuals with strabismus or anisometropia will be reviewed here.
Fixation Instability
During normal fixation, the eyes constantly make small, involuntary movements such as microsaccades, slow drifts, and tremors that aid in maintaining fixation and that prevent image fading.8–11 Remarkably, binocularity in the normal visual system is not affected by these eye movements. When these involuntary eye movements become excessive, instability occurs in the ability to maintain fixation and visual function is disrupted. Fixation instability is a common consequence of strabismus, anisometropia, and amblyopia. Instability associated with these pediatric eye conditions is typically composed of abnormal fixational saccades, ocular drift, and fusion maldevelopment nystagmus.1,4,8–10,16 Although amblyopia is associated with larger fixation instability, amblyopia is not a necessary condition for instability as it is also found in nonamblyopic strabismus.1–6,13,17
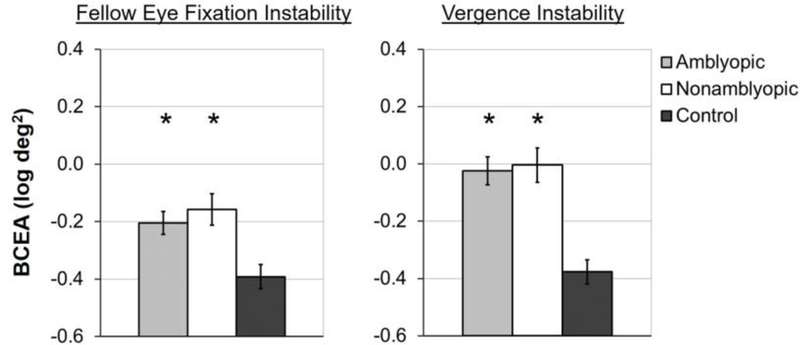
Research to date has typically focused on fixation instability under monocular viewing conditions with inconsistent results. Some studies have reported fellow eye stability with the amblyopic eye occluded.2,3,16,17 Other studies report increased fellow eye instability compared with controls.4,6,13,18,19 However, smaller angles of deviation and preserved binocular function may lessen instability.13 Unpublished monocular viewing data from the cohort described by Kelly et al20 also show amblyopic and nonamblyopic children with strabismus and/or anisometropia have larger fellow eye instability than controls (Figure 1).
Figure 1.
Unpublished monocular viewing data from the cohort described in Kelly et al19 showing larger mean fellow eye instability for 85 amblyopic children (light gray bars) and 55 nonamblyopic children with strabismus or anisometropia (white bars), compared with 43 normal control children (dark grey bars). Error bars represent ±SE.
*significantly different than controls (p<0.01).
Only a handful of studies have examined the fellow eye under binocular viewing conditions in strabismic and amblyopic adults, with inconsistent results. Gonzalez et al17 found that the fellow eye was relatively stable in adults with strabismus and/or anisometropia compared with controls. In contrast, Ciuffreda et al5 found fellow eye instability in amblyopic adults with strabismus; however, instability was absent in a small group of three amblyopic adults without strabismus.
Lastly, vergence instability (i.e., variability in ocular alignment over time) has been assessed. During fixation, ocular alignment varies due to microsaccades and drift. While microsaccades are typically conjugate, the horizontal component of drift has less conjugacy, and sometimes has a “wave-like” appearance with alternating periods of convergence and divergence.8 Vergence instability has been reported while viewing with two eyes in adults with large angle exotropia, suggesting an association of suppression with disconjugate eye movements.6 Recently, Kelly et al20 reported increased fixation instability and vergence instability, regardless of current amblyopia, in strabismic and anisometropic children compared with controls (Figure 2). Fellow eye instability was not associated with amblyopic eye visual acuity or stereoacuity. However, vergence instability was related to worse stereoacuity and to a larger suppression scotoma as measured by Worth 4-dot at 7 different distances21,22 (Figure 2). When interocular suppression was artificially reduced via contrast-rebalancing, fellow eye instability was significantly improved in strabismic amblyopes.22
Figure 2.
Mean fellow eye instability and vergence instability during binocular viewing for 98 amblyopic children (light gray bars), 62 nonamblyopic children with strabismus or anisometropia (white bars), and 46 normal control children (dark grey bars). Amblyopic and nonamblyopic children exhibited larger fixation and vergence instability compared to controls. Error bars represent ± SE. *significantly different than controls (p<0.01).
The presence of ocular motor deficits during binocular viewing indicates a fellow eye impairment since the amblyopic eye is likely suppressed, with the fixating fellow eye driving vision. Fellow eye instability during monocular and binocular viewing suggests that discordant binocular visual experience, even in the absence of amblyopia, interferes with the development of ocular motor control. Instability points to a common neural mechanism that controls the stability of both the fellow and amblyopic eye. Further, the presence of vergence instability in amblyopia may limit the potential for recovery of binocular vision.
Abnormal Saccades
Saccades are small, high velocity conjugate eye movements that direct the fovea onto a target of interest. The distinction between saccades and fixational microsaccades is somewhat artificial. Fixational eye movements include microsaccades, which are small involuntary saccades that cannot be differentiated from saccades according to their magnitude or any physical characteristic.23 Given the fixation instability of the fellow eye in strabismic and amblyopic individuals, we might also expect abnormalities in fellow eye saccades.
A limited number of studies exist that compare fellow eye saccades with the amblyopic eye occluded to controls, as most compare amblyopic to fellow eye viewing. While one study reported similar fellow eye saccadic latencies as controls,24 another found latencies to be delayed and more variable.7 During binocular viewing, saccadic latency is also delayed and more variable in amblyopic adults.7,25 Further, the typical pattern of faster saccades during binocular viewing compared with monocular viewing (i.e., binocular advantage) is absent in amblyopic adults.25,26 Lastly, binocular coordination is impaired such that saccades are disconjugate.27,28
Abnormal saccades in amblyopic individuals relative to controls can be exacerbated by more severe amblyopia and by a lack of stereopsis.24,25–27 Latencies of saccades are longer in visually normal children compared with adults, suggesting an immaturity of the eye movement system during development and susceptibility to dysfunction as a result of early abnormal visual experience.29 The presence of abnormal fellow eye saccades in amblyopia points to a disruption in ocular motor control, and provides further evidence that binocular discordant input impacts the development of visual pathways associated with the fellow eye.
Motion Perception
Although the subnormal or nil stereoacuity that typically accompanies amblyopia is the most studied binocular outcome measure for amblyopia treatment, measurement of stereoacuity does not allow the effects of discordant binocular visual experience on the amblyopic and fellow eyes to be examined separately. Minor monocular fellow eye deficits have been reported for several aspects of visual function, and stronger fellow eye deficits have been reported for motion perception tasks that rely on binocular cortical regions.30 In non-human primates, motion-sensitive brain regions such as the middle temporal visual area (MT) contain neurons that respond to motion presented to either eye.31–34 The human homolog (MT+ or V5) is activated by several types of motion stimuli that require integration of local motion signals.35,36 Early discordant visual experience may disrupt the development of this brain region and lead to impaired motion processing in the fellow eye as well as the amblyopic eye.
Impaired monocular motion processing has been demonstrated using dynamic random-dot patterns composed of signal dots that move coherently in one direction mixed with noise dots that move randomly. Performance is measured by determining the smallest percentage of signal dots necessary to accurately determine the coherent motion direction (global motion task;37 Figure 3 left; e-supplement video) or the orientation of a shape created by signal dots moving in opposite directions inside and outside the shape (motion-defined form task;38 Figure 3 right; e-supplement video).
Figure 3.
Left: global motion stimuli with 100% coherence (top) and 75% coherence (bottom). Right: motion-defined form stimuli with 100% coherence (top) and 75% coherence (bottom). Arrows were added to illustrate the direction of motion of each dot. The dotted-line boxes were added to illustrate the target shape. Neither the arrows nor the boxes were present in the actual stimulus displays.
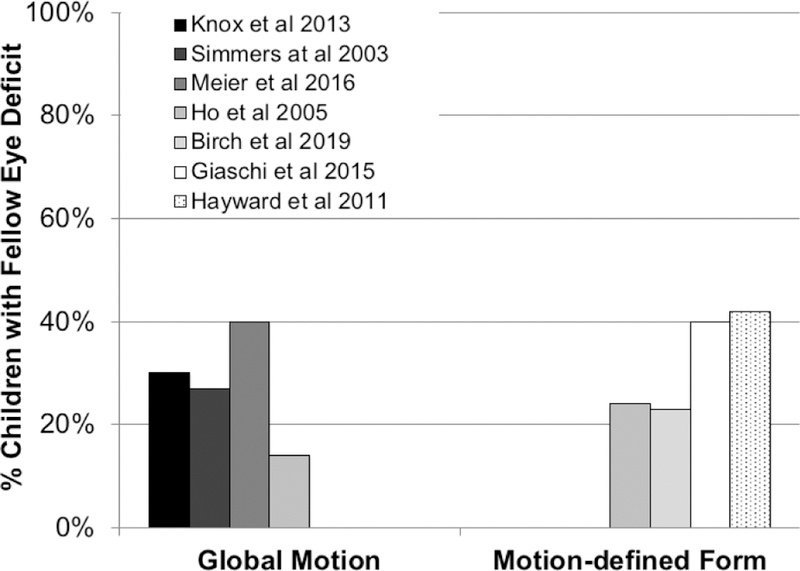
Fellow eye global motion deficits have been reported in several studies of amblyopic children and adults,39–41 but there also are several studies that report few or no fellow eye deficits.42,43 (Figure 4) The discrepancies in results of various global motion studies appear to depend on the specific spatiotemporal parameters of the visual stimuli, requiring prior identification of the parameters that revealed an amblyopic eye deficit in order to observe the fellow eye deficit.41
Figure 4.
% of children with amblyopia who had a deficit in perception of global motion direction (left) or motion-defined form orientation (right) when viewing with their fellow eye, compared with controls. Thresholds for both tasks were measured by the minimum percentage of coherently moving signal dots required for accurate discrimination.
Up to 40% of amblyopic children have been reported to have a fellow eye deficit in perceiving motion-defined form.43–45 Unlike global motion, fellow eye motion-defined form deficits are reported consistently across studies of amblyopic children and adults, despite some variations in visual stimuli. (Figure 4) Fellow eye motion-defined form deficits have been reported to be resistant to rehabilitation by patching,44 but were responsive to binocular amblyopia treatment. 46
Reading
Reading is a vision-reliant ability fundamental to academic achievement. Saccades during reading allow us to move forward and regress backward through text. Fixations, or pauses, occur during reading as decoding of phonemes occurs. Increased fixation duration or abnormal saccades could result in slower reading that, in turn, could be detrimental to academic performance and learning. Most studies of reading by amblyopic children have been conducted with monocular viewing, comparing amblyopic eye versus fellow eye reading performance to determine whether the treated amblyopic eye provides a useful “spare eye” in the event that the fellow eye can no longer be used for reading.47–49 More recently, monocular reading speed for the fellow eye has been studied; the fellow eye has decreased reading speed in strabismic and anisometropic amblyopia relative to monocular reading in visually normal children.48,50,51 Slower monocular reading with the fellow eye is associated with an abnormally increased number of saccades.51
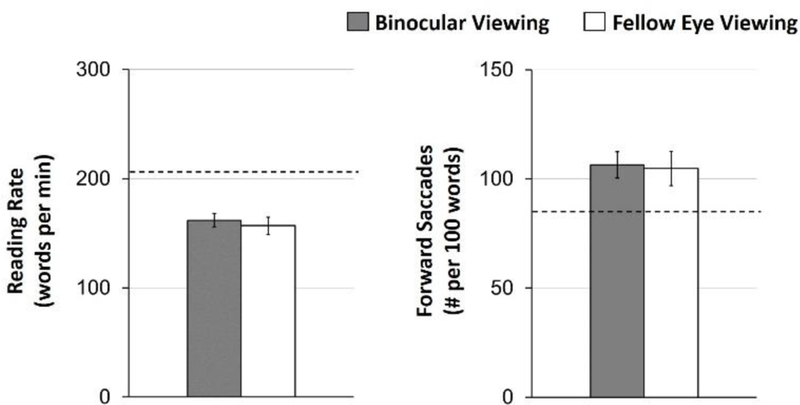
With binocular viewing by amblyopic children and adults, there are several reports of slow reading of sentences and words, and slow reading of paragraphs of text projected on a distant screen.48–50 More recently, natural, binocular silent reading of age-appropriate print paragraphs at habitual reading distance has been assessed in amblyopic children.52,53Amblyopic children with strabismus or anisometropia read slowly compared with controls and nonamblyopic strabismic children.52,53 Unlike the earlier studies, this more recent work clearly identified that amblyopia alone was sufficient to cause slow reading; reading performance was not related to severity of amblyopia, nor to diagnosis (strabismic, anisometropic). Further, data from Kelly et al51 reported similar reading rate and number of forward saccades for binocular reading versus fellow eye reading in amblyopic children, suggesting that rather than binocular inhibition (i.e., amblyopic eye interference during binocular reading), slow reading is due to a fellow eye deficit even when reading with two eyes (Figure 5).
Figure 5.
Mean reading rate (words per minute) and number of forward saccades (per 100 words) assessed with the Readalyzer ® during binocular viewing (grey bars) and fellow eye viewing (white bars) for 49 amblyopic children No differences were found (ps≥0.50). Error bars represent ± SE. The dashed line represents previously published control data for binocular reading.52
Importantly, reading comprehension did not differ significantly between amblyopic children and controls, nor did fixation duration or number of regressive eye movements.52–54 Taken together, these findings suggest that amblyopic children did not read slowly because they had dyslexia or a learning disability. In fact, amblyopic children show low (<5%) prevalence for specific reading disability on the Wide Range Achievement Test II (WRAT II), similar to control children.55 Slow reading likely results from the fixation instability associated with amblyopia and binocular dysfunction and the resultant vergence instability.19 A larger number of forward saccades occurs (per 100 words) in amblyopic children during reading compared with nonamblyopic strabismic children and controls.52 Moreover, there is a strong relationship between slow reading and fixation instability of the fellow eye in children with anisometropic amblyopia.53
Because perceived scholastic competence is a key determinant of self-esteem in school-aged children, slow reading may be expected to influence a child’s self-perception. A recent study reported that school-age children with amblyopia had significantly lower scores than control children for self-perception of scholastic competence.56 Moreover, their lower self-perception of scholastic competence was associated with a slower reading speed. 56
Visually-Guided Motor Skills
Coordination between eye movements and hand movements is essential in object manipulation. Normal binocular vision during childhood provides important sensory input for optimal development of eye-hand coordination. Reduced visual acuity, impaired depth perception, and abnormal ocular motor function typically found with binocularly discordant visual experience may have significant effects on the development of visually-guided motor skills.
A limited number of studies have investigated fine motor ability in children and adults with strabismus and/or anisomteropia during fellow eye viewing. These studies show deficits in the speed and accuracy of manual dexterity tasks.57–61 One study found that strabismic and anisometropic children ages 5–11 years spent almost double the amount of time in the final approach to objects during a reach-to-grasp task compared with controls, and that they made 1.5 to 3 times more errors.57 These findings were echoed in another study, but only for younger children (5–6 years old) and not with older children (7–9 years old).58
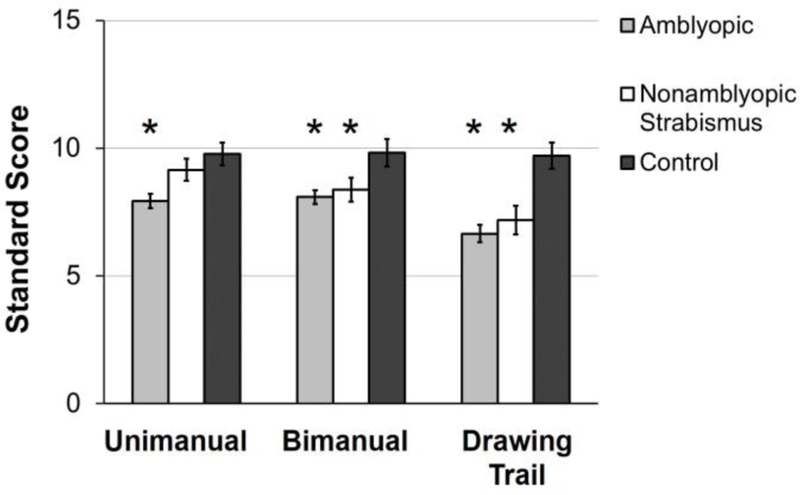
Similar to fellow eye viewing, adults and children with amblyopia due to strabismus and/or anisometropia are slow to reach and grasp objects, and to place pegs in holes and thread beads under binocular viewing conditions.59,60,62,63 Kelly et al64 have also reported deficits in manual dexterity tasks (unimanual, bimanual, drawing trail) of the Movement Assessment Battery for Children (MABC-2) in amblyopic and nonamblyopic children age 3–12 years treated for strabismus and/or anisometropia. (Figure 6) In a recent publication, Kelly et al65 reported that school-age children with strabismus and/or amblyopia required 28% more time than controls to transfer answers from a test booklet to a multiple-choice answer sheet, which could translate into poorer academic performance. Fine motor skills improved with binocular, contrast re-balanced game treatment,66 and with improved stereo acuity in a small cohort of children who patched.58 Motor skills as an amblyopia treatment outcome measure may be beneficial.
Figure 6.
Mean standard scores for manual dexterity tasks completed during binocular viewing for 129 amblyopic children (light gray bars), 47 nonamblyopic children with strabismus (white bars), and 40 normal control children (dark grey bars). Amblyopic children had lower scores for all three tasks compared to controls. Nonamblyopic children with strabismus had lower scores on the bimanual and drawing trail tasks compared with controls. Error bars represent ± SE.
*significantly different than controls (p<0.01).
Impaired kinematics appears to be behind the fine motor deficits found in amblyopic children and adults, both for fellow eye viewing and for binocular viewing. Reach planning and execution are slower, and the precision of reaching and grasping is reduced, although compensatory strategies may improve performance with age.26,57,58,60,67,68 During reaching, amblyopic adults have reduced peak acceleration and prolonged acceleration indicating their ability to use vision to plan movements is impaired.69 Amblyopic children also show longer movement times, lower reach velocity, and a longer duration of contact prior to lifting an object.60 Temporal eye-hand coordination in amblyopic adults is associated with increased corrective saccades, a compensatory strategy to increase reach accuracy; particularly among adults with nil stereoacuity.70,71 Visuomotor deficits appear more closely associated with stereo deficits than with the severity of visual acuity deficit.59,61,68 This is supported by the finding of fine motor deficits in nonamblyopic individuals with deficient stereoacuity or strabismus,61 and of normal reach-related saccades with monocular in normal controls.26,62
In turn, impaired motor skills may affect self-perception of scholastic and social competence. Using the Self-Perception Scale for Children, Birch et al56 recently reported that children with amblyopia had significantly lower scores than control children for social and athletic competence domains. Lower self-perception of social and athletic competence was associated with worse aiming and catching performance on a standardized test of motor skills, the Movement ABC-2.
What Causes Fellow Eye Deficits in Amblyopia
Persistence of fellow eye deficits even when the amblyopic eye is occluded for monocular testing suggests that fellow eye deficits cannot be attributed to inhibition/suppression by the amblyopic eye. While binocular inhibition, i.e., seeing better with the fellow eye than with both eyes, is related to slow reading in age-related macular degeneration,72 binocular reading speed does not differ from fellow eye reading speed in amblyopic children (i.e., no binocular inhibition).51
Instead, fellow eye deficits are likely a result of binocular dysfunction. In this context, a valuable model of severe disruption of binocularity is early monocular enucleation, since only the one eye remains. The other eye cannot contribute to visual performance nor can it suppress or otherwise interfere with the visual performance of the remaining eye. Adults who experience enucleation before 5 years of age due to retinoblastoma have many of the same motion perception deficits as found in the fellow eyes of amblyopic individuals, including speed discrimination, motion-defined form perception, and the perception of motion in depth.73–77 Further, enucleated adults display a different pattern of ocular motor function compared to binocularly intact controls; asymmetrical optokinetic nystagmus (OKN) favoring nasalward motion78 but have normal saccades and stable fixation.79,80 The complete deafferentation of visual input from one eye in enucleation results in the removal of binocular interactions and competition during visual development, and thus may be different than the binocularly discordant information present in strabismic and anisometropic amblyopia.
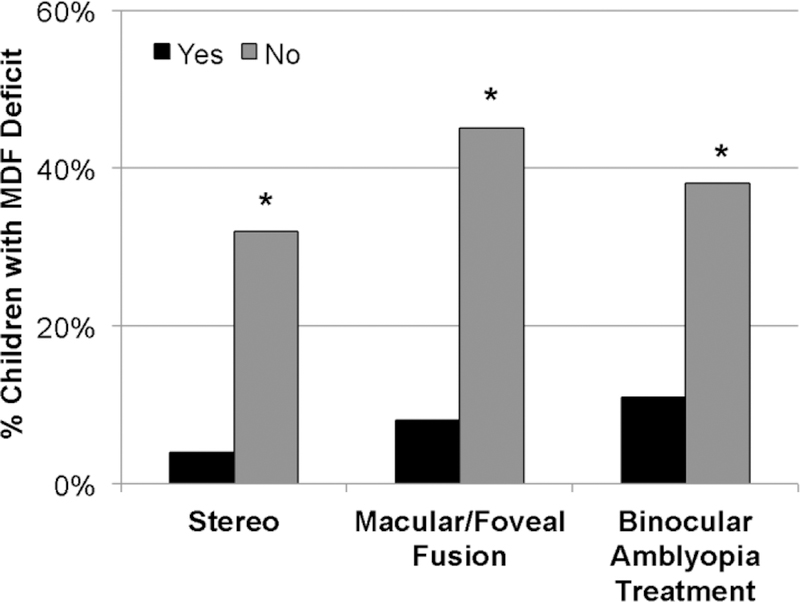
Additional support for the central role of binocular dysfunction in fellow eye deficits comes from our recent research on motion-defined form perception. Amblyopic children who have had evidence of abnormal binocularity, including nil stereo or only peripheral fusion, were significantly more likely to have a fellow eye deficit (Figure 7).
Figure 7.
% of amblyopic children who have a fellow eye deficit for motion-defined form perception in subgroups with randot stereoacuity of 1.6–2.9 log arcsecs (Yes) vs nil stereoacuity (No), with macular/foveal fusion by Worth 4-dot test (Yes) vs peripheral/no fusion, and with prior binocular amblyopia treatment (Yes) vs no prior binocular treatment (No).
Fellow Eye Deficits and Amblyopia Treatment
Fellow eye deficits prompt us to reconsider whether amblyopia is adequately characterized as a monocular visual acuity deficit, and whether treatment by occlusion of the fellow eye, or occlusion coupled with monocular perceptual learning is optimal. Fellow eye motion-defined form perception deficits have been reported to be resistant to rehabilitation by patching44 but responsive to binocular amblyopia treatment.46 (Figure 7). More effective treatments for amblyopia may target suppression, by requiring integration of information between the two eyes to play dichoptic games or watch dichoptic videos. Like occlusion therapy, binocular treatment results reported to date are generally positive for young children 81–87 but less effective for older children due to poor compliance and/or intractable residual amblyopia.88–90
Conclusion
For many years, the fellow eye was considered to be normal and study after study assessed the child’s ability to see, read, or perform visuomotor tasks using the amblyopic eye to determine whether it was a useful spare eye. The overarching goal was to evaluate whether the monetary and personal cost of years of occlusion therapy were worth the benefit of a few lines gain in visual acuity. Now the paradigm has shifted; we have discovered that the fellow eye also has visual deficits and that these deficits affect an amblyopic child’s visuomotor and visuocognitive skills. We need to understand how to prevent and rehabilitate the effects of amblyopia not only on the nonpreferred eye but also on the fellow eye.
Supplementary Material
Acknowledgments
Supported in part by a grant from the National Eye Institute EY022313
Footnotes
None of the authors have a financial interest in the material included in this Clinical Update.
References
- 1.Birch EE, Subramanian V, Weakley DR. Fixation instability in anisometropic children with reduced stereopsis. J AAPOS 2013;17:287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subramanian V, Jost RM, Birch EE. A quantitative study of fixation stability in amblyopia. Invest Ophthalmol Vis Sci 2013;54:1998–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung ST, Kumar G, Li RW, Levi DM. Characteristics of fixational eye movements in amblyopia: Limitations on fixation stability and acuity? Vision Res 2015;114:87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaikh AG, Otero-Millan J, Kumar P, Ghasia FF. Abnormal fixational eye movements in amblyopia. PLoS One 2016;11:e0149953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciuffreda KJ, Kenyon RV, Stark L. Fixational eye movements in amblyopia and strabismus. J Am Optom Assoc 1979;50:1251–1258. [PubMed] [Google Scholar]
- 6.Economides JR, Adams DL, Horton JC. Variability of ocular deviation in strabismus. JAMA Ophthalmol 2016;134:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perdziak M, Witkowska DK, Gryncewicz W, Ober JK. Not only amblyopic but also dominant eye in subjects with strabismus show increased saccadic latency. J Vis 2016;16:12. [DOI] [PubMed] [Google Scholar]
- 8.Otero-Millan J, Macknik SL, Martinez-Conde S. Fixational eye movements and binocular vision. Front Integr Neurosci 2014;8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez-Conde S Fixational eye movements in normal and pathological vision. Prog Brain Res 2006;154:151–176. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Conde S, Otero-Millan J, Macknik SL. The impact of microsaccades on vision: towards a unified theory of saccadic function. Nat Rev Neurosci 2013;14:83–96. [DOI] [PubMed] [Google Scholar]
- 11.Rolfs M Microsaccades: small steps on a long way. Vision Res 2009;49:2415–2441. [DOI] [PubMed] [Google Scholar]
- 12.Ciuffreda KJ, Kenyon RV, Stark L. Increased drift in amblyopic eyes. Br J Ophthalmol 1980;64:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghasia FF, Otero-Millan J, Shaikh AG. Abnormal fixational eye movements in strabismus. Br J Ophthalmol 2018;102:253–259. [DOI] [PubMed] [Google Scholar]
- 14.Tychsen L Causing and curing infantile esotropia in primates: the role of decorrelated binocular input. Trans Am Ophthalmol Soc 2007;105:564–593. [PMC free article] [PubMed] [Google Scholar]
- 15.Tychsen L, Richards M, Wong A, Foeller P, Bradley D, Burkhalter A. The neural mechanism for Latent (fusion maldevelopment) nystagmus. J Neuro-ophthalmol 2010;30:276–283. [DOI] [PubMed] [Google Scholar]
- 16.Shi XF, Xu LM, Li Y, Wang T, Zhao KX, Sabel BA. Fixational saccadic eye movements are altered in anisometropic amblyopia. Restor Neurol Neurosci 2012;30:445–462. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez EG, Wong AM, Niechwiej-Szwedo E, Tarita-Nistor L, Steinbach MJ. Eye position stability in amblyopia and in normal binocular vision. Invest Ophthalmol Vis Sci 2012;53:5386–5394. [DOI] [PubMed] [Google Scholar]
- 18.Bedell HE, Flom MC. Bilateral oculomotor abnormalities in strabismic amblyopes: evidence for a common central mechanism. Doc Ophthalmol 1985;59:309–321. [DOI] [PubMed] [Google Scholar]
- 19.Schor C, Hallmark W. Slow control of eye position in strabismic amblyopia. Invest Ophthalmol Vis Sci 1978;17:577–581. [PubMed] [Google Scholar]
- 20.Kelly KR, Cheng-Patel CS, Jost RM, Wang YZ, Birch EE. Fixation instability during binocular viewing in anisometropic and strabismic children. Exp Eye Res 2018. July 10 10.1016/j.exer.2018.07.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 21.Rosenbaum AL, Santiago AP. Clinical strabismus management : principles and surgical techniques Philadelphia: Saunders; 1999. [Google Scholar]
- 22.Raveendran RN, Babu RJ, Hess RF, Bobier WR. Transient improvements in fixational stability in strabismic amblyopes following bifoveal fixation and reduced interocular suppression. Ophthalmic Physiol Opt 2014;34:214–225. [DOI] [PubMed] [Google Scholar]
- 23.Otero-Millan J, Troncoso XG, Macknik SL, Serrano-Pedraza I, Martinez-Conde S. Saccades and microsaccades during visual fixation, exploration, and search: foundations for a common saccadic generator. J Vis 2008;8:21 21–18. [DOI] [PubMed] [Google Scholar]
- 24.McKee SP, Levi DM, Schor CM, Movshon JA. Saccadic latency in amblyopia. J Vis 2016;16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niechwiej-Szwedo E, Goltz HC, Chandrakumar M, Hirji ZA, Wong AM. Effects of anisometropic amblyopia on visuomotor behavior, I: saccadic eye movements. Invest Ophthalmol Vis Sci 2010;51:6348–6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niechwiej-Szwedo E, Chandrakumar M, Goltz HC, Wong AM. Effects of strabismic amblyopia and strabismus without amblyopia on visuomotor behavior, I: saccadic eye movements. Invest Ophthalmol Vis Sci 2012;53:7458–7468. [DOI] [PubMed] [Google Scholar]
- 27.Kapoula Z, Bucci MP, Eggert T, Garraud L. Impairment of the binocular coordination of saccades in strabismus. Vision Res 1997;37:2757–2766. [DOI] [PubMed] [Google Scholar]
- 28.Maxwell GF, Lemij HG, Collewijn H. Conjugacy of saccades in deep amblyopia. Invest Ophthalmol Vis Sci 1995;36:2514–2522. [PubMed] [Google Scholar]
- 29.Yang Q, Bucci MP, Kapoula Z. The latency of saccades, vergence, and combined eye movements in children and in adults. Invest Ophthalmol Vis Sci 2002;43:2939–2949. [PubMed] [Google Scholar]
- 30.Meier K, Giaschi D. Unilateral amblyopia affects two eyes: fellow eye deficits in amblyopia. Invest Ophthalmol Vis Sci 2017;58:1779–1800. [DOI] [PubMed] [Google Scholar]
- 31.Kiorpes L, Walton PJ, O’Keefe LP, Movshon JA, Lisberger SG. Effects of early-onset artificial strabismus on pursuit eye movements and on neuronal responses in area MT of macaque monkeys. J Neurosci 1996;16:6537–6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maunsell JH, van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci 1983;3:2563–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maunsell JH, Van Essen DC. Functional properties of neurons in middle temporal visual area of the macaque monkey. II. Binocular interactions and sensitivity to binocular disparity. J Neurophysiol 1983;49:1148–1167. [DOI] [PubMed] [Google Scholar]
- 34.Maunsell JH, Van Essen DC. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J Neurophysiol 1983;49:1127–1147. [DOI] [PubMed] [Google Scholar]
- 35.Meier K, Partanen M, Giaschi D. Neural Correlates of Speed-Tuned Motion Perception in Healthy Adults. Perception 2018;47:660–683. [DOI] [PubMed] [Google Scholar]
- 36.Braddick OJ, O’Brien JM, Wattam-Bell J, Atkinson J, Turner R. Form and motion coherence activate independent, but not dorsal/ventral segregated, networks in the human brain. Curr Biol 2000;10:731–734. [DOI] [PubMed] [Google Scholar]
- 37.Watamaniuk SN, Sekuler R, Williams DW. Direction perception in complex dynamic displays: the integration of direction information. Vision Res 1989;29:47–59. [DOI] [PubMed] [Google Scholar]
- 38.Regan D, Hong XH. Visual acuity for optotypes made visible by relative motion. Optom Vis Sci 1990;67:49–55. [DOI] [PubMed] [Google Scholar]
- 39.Simmers AJ, Ledgeway T, Hess RF, McGraw PV. Deficits to global motion processing in human amblyopia. Vision Res 2003;43:729–738. [DOI] [PubMed] [Google Scholar]
- 40.Knox PJ, Ledgeway T, Simmers AJ. The effects of spatial offset, temporal offset and image speed on sensitivity to global motion in human amblyopia. Vision Res 2013;86:59–65. [DOI] [PubMed] [Google Scholar]
- 41.Meier K, Sum B, Giaschi D. Global motion perception in children with amblyopia as a function of spatial and temporal stimulus parameters. Vision Res 2016;127:18–27. [DOI] [PubMed] [Google Scholar]
- 42.Ho C, Paul P, Asirvatham A, Cavanagh P, Cline R, Giaschi D. Abnormal spatial selection and tracking in children with amblyopia. Vision Res 2006;46:3274–3283. [DOI] [PubMed] [Google Scholar]
- 43.Ho CS, Giaschi DE, Boden C, Dougherty R, Cline R, Lyons C. Deficient motion perception in the fellow eye of amblyopic children. Vision Res 2005;45:1615–1627. [DOI] [PubMed] [Google Scholar]
- 44.Giaschi D, Chapman C, Meier K, Narasimhan S, Regan D. The effect of occlusion therapy on motion perception deficits in amblyopia. Vision Res 2015;114:122–134. [DOI] [PubMed] [Google Scholar]
- 45.Hayward J, Truong G, Partanen M, Giaschi D. Effects of speed, age, and amblyopia on the perception of motion-defined form. Vision Res 2011;51:2216–2223. [DOI] [PubMed] [Google Scholar]
- 46.Birch E, Jost R, Wang Y-Z, Kelly K, Giaschi D. Impaired fellow eye motion perception and abnormal binocular function in amblyopia. Invest Ophthalmol Vis Sci 2019; in press. [DOI] [PMC free article] [PubMed]
- 47.Repka MX, Kraker RT, Beck RW, et al. Monocular oral reading performance after amblyopia treatment in children. Am J Ophthalmol 2008;146:942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stifter E, Burggasser G, Hirmann E, Thaler A, Radner W. Monocular and binocular reading performance in children with microstrabismic amblyopia. Br J Ophthalmol 2005;89:1324–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stifter E, Burggasser G, Hirmann E, Thaler A, Radner W. Evaluating reading acuity and speed in children with microstrabismic amblyopia using a standardized reading chart system. Graefes Arch Clin Exp Ophthalmol 2005;243:1228–1235. [DOI] [PubMed] [Google Scholar]
- 50.Kanonidou E, Proudlock FA, Gottlob I. Reading strategies in mild to moderate strabismic amblyopia: an eye movement investigation. Invest Ophthalmol Vis Sci 2010;51:3502–3508. [DOI] [PubMed] [Google Scholar]
- 51.Kelly KR, Jost RM, De La Cruz B, Hunter JS, Dao L, Beauchamp CL, Leffler JN, Luu B, Birch EE. What causes slow binocular reading in amblyopic children? American Association for Pediatric Ophthalmology & Strabismus; 2019; San Diego. [Google Scholar]
- 52.Kelly KR, Jost RM, De La Cruz A, Birch EE. Amblyopic children read more slowly than controls under natural, binocular reading conditions. J AAPOS 2015;19:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly KR, Jost RM, De La Cruz A, et al. Slow reading in children with anisometropic amblyopia is associated with fixation instability and increased saccades. J AAPOS 2017;21:447–451 e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Birch EE, Kelly KR. Pediatric ophthalmology and childhood reading difficulties: Amblyopia and slow reading. J AAPOS 2017;21:442–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koklanis K, Georgievski Z, Brassington K, Bretherton L. The prevalence of specific reading disability in an amblyopic population. A preliminary report. Binocul Vis Strabismus Q 2006;21:27–32. [PubMed] [Google Scholar]
- 56.Birch EE, Castaneda YS, Cheng-Patel CS, et al. Self-perception of school-aged children with amblyopia and its association with reading speed and motor skills. JAMA Ophthalmol 2018;137:167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suttle CM, Melmoth DR, Finlay AL, Sloper JJ, Grant S. Eye-hand coordination skills in children with and without amblyopia. Invest Ophthalmol Vis Sci 2011;52:1851–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grant S, Suttle C, Melmoth DR, Conway ML, Sloper JJ. Age- and stereovision-dependent eye-hand coordination deficits in children with amblyopia and abnormal binocularity. Invest Ophthalmol Vis Sci 2014;55:5687–57015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Connor AR, Birch EE, Anderson S, Draper H. Relationship between binocular vision, visual acuity, and fine motor skills. Optom Vis Sci 2010;87:942–947. [DOI] [PubMed] [Google Scholar]
- 60.Buckley JG, Pacey IE, Panesar GK, Scally A, Barrett BT. Prehension of a Flanked Target in Individuals With Amblyopia. Invest Ophthalmol Vis Sci 2015;56:7568–7580. [DOI] [PubMed] [Google Scholar]
- 61.O’Connor AR, Birch EE, Anderson S, Draper H, Group FR. The functional significance of stereopsis. Invest Ophthalmol Vis Sci 2010;51:2019–2023. [DOI] [PubMed] [Google Scholar]
- 62.Grant S, Moseley MJ. Amblyopia and real-world visuomotor tasks. Strabismus 2011;19:119–128. [DOI] [PubMed] [Google Scholar]
- 63.Webber AL, Wood JM, Gole GA, Brown B. The effect of amblyopia on fine motor skills in children. Invest Ophthalmol Vis Sci 2008;49:594–603. [DOI] [PubMed] [Google Scholar]
- 64.Kelly KR, Morale SE, Felius J, Jost RM, Birch EE. Amblyopia disrupts the development of eye-hand coordination. Paper presented at: AAPOS 2016; Vancouver, BC. [Google Scholar]
- 65.Kelly KR, Jost RM, De La Cruz A, Birch EE. Multiple-choice answer form completion time in children with amblyopia and strabismus. JAMA Ophthalmol 2018;136:938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Webber AL, Wood JM, Thompson B. Fine motor skills of children with amblyopia improve following binocular treatment. Invest Ophthalmol Vis Sci 2016;57:4713–4720. [DOI] [PubMed] [Google Scholar]
- 67.Grant S, Conway ML. Reach-to-precision grasp deficits in amblyopia: Effects of object contrast and low visibility. Vision Res 2015;114:100–110. [DOI] [PubMed] [Google Scholar]
- 68.Grant S, Melmoth DR, Morgan MJ, Finlay AL. Prehension deficits in amblyopia. Invest Ophthalmol Vis Sci 2007;48:1139–1148. [DOI] [PubMed] [Google Scholar]
- 69.Niechwiej-Szwedo E, Goltz HC, Chandrakumar M, Hirji Z, Crawford JD, Wong AM. Effects of anisometropic amblyopia on visuomotor behavior, part 2: visually guided reaching. Invest Ophthalmol Vis Sci 2011;52:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niechwiej-Szwedo E, Goltz HC, Chandrakumar M, Hirji Z, Wong AM. Effects of anisometropic amblyopia on visuomotor behavior, III: Temporal eye-hand coordination during reaching. Invest Ophthalmol Vis Sci 2011;52:5853–5861. [DOI] [PubMed] [Google Scholar]
- 71.Niechwiej-Szwedo E, Goltz HC, Chandrakumar M, Wong AM. Effects of strabismic amblyopia and strabismus without amblyopia on visuomotor behavior: III. Temporal eye-hand coordination during reaching. Invest Ophthalmol Vis Sci 2014;55:7831–7838. [DOI] [PubMed] [Google Scholar]
- 72.Tarita-Nistor L, Brent MH, Markowitz SN, Steinbach MJ, Gonzalez EG. Maximum reading speed and binocular summation in patients with central vision loss. Can J Ophthalmol 2013;48:443–449. [DOI] [PubMed] [Google Scholar]
- 73.Gonzalez EG, Steeves JK, Kraft SP, Gallie BL, Steinbach MJ. Foveal and eccentric acuity in one-eyed observers. Behav Brain Res 2002;128:71–80. [DOI] [PubMed] [Google Scholar]
- 74.Kelly KR, McKetton L, Schneider KA, Gallie BL, Steeves JK. Altered anterior visual system development following early monocular enucleation. Neuroimage Clin 2014;4:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nicholas JJ, Heywood CA, Cowey A. Contrast sensitivity in one-eyed subjects. Vision Res 1996;36:175–180. [DOI] [PubMed] [Google Scholar]
- 76.Reed MJ, Steeves JK, Steinbach MJ. A comparison of contrast letter thresholds in unilateral eye enucleated subjects and binocular and monocular control subjects. Vision Res 1997;37:2465–2469. [DOI] [PubMed] [Google Scholar]
- 77.Steeves JK, Wilkinson F, Gonzalez EG, Wilson HR, Steinbach MJ. Global shape discrimination at reduced contrast in enucleated observers. Vision Res 2004;44:943–949. [DOI] [PubMed] [Google Scholar]
- 78.Reed MJ, Steinbach MJ, Anstis SM, Gallie B, Smith D, Kraft S. The development of optokinetic nystagmus in strabismic and monocularly enucleated subjects. Behav Brain Res 1991;46:31–42. [DOI] [PubMed] [Google Scholar]
- 79.Gonzalez EG, Lillakas L, Lam A, Gallie BL, Steinbach MJ. Horizontal saccade dynamics after childhood monocular enucleation. Invest Ophthalmol Vis Sci 2013;54:6463–6471. [DOI] [PubMed] [Google Scholar]
- 80.Day S Vision development in the monocular individual: implications for the mechanisms of normal binocular vision development and the treatment of infantile esotropia. Trans Am Ophthalmol Soc 1995;93:523–581. [PMC free article] [PubMed] [Google Scholar]
- 81.Birch EE, Li SL, Jost RM, et al. Binocular iPad treatment for amblyopia in preschool children. J AAPOS 2015;19:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Holmes JM, Manh VM, Lazar EL, et al. Effect of a binocular iPad game vs part-time patching in children aged 5 to 12 years with amblyopia: a randomized clinical trial. JAMA Ophthalmol 2016;134:1391–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kelly KR, Jost RM, Dao L, Beauchamp CL, Leffler JN, Birch EE. binocular iPad game vs patching for treatment of amblyopia in children: a randomized clinical trial. JAMA Ophthalmol 2016;134:1402–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kelly KR, Jost RM, Wang YZ, et al. Improved binocular outcomes following binocular treatment for childhood amblyopia. Invest Ophthalmol Vis Sci 2018;59:1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Birch EE, Jost RM, De La Cruz A, et al. Bincular amblyopia treatment with contrast re-balanced movies. J AAPOS 2019; May 16 10.1016/j.jaapos.2019.02.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 86.Li SL, Reynaud A, Hess RF, et al. Dichoptic movie viewing treats childhood amblyopia. J AAPOS 2015;19:401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Knox PJ, Simmers AJ, Gray LS, Cleary M. An exploratory study: prolonged periods of binocular stimulation can provide an effective treatment for childhood amblyopia. Invest Ophthalmol Vis Sci 2012;53:817–824. [DOI] [PubMed] [Google Scholar]
- 88.Gao TY, Guo CX, Babu RJ, et al. Effectiveness of a binocular video game vs placebo video game for improving visual functions in older children, teenagers, and adults with amblyopia: a randomized clinical trial. JAMA Ophthalmol 2018;136:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Manh VM, Holmes JM, Lazar EL, et al. A randomized trial of a binocular iPad game versus part-time patching in children aged 13 to 16 years with amblyopia. Am J Ophthalmol 2018;186:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pediatric Eye Disease Investigator G, Holmes JM, Manny RE, et al. A randomized trial of binocular Dig Rush game treatment for amblyopia in children aged 7 to 12 years. Ophthalmology 2019;126(3):456–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.