Abstract
Intraventricular hemorrhage (IVH) in preterm infants results in reduced proliferation and maturation of oligodendrocyte progenitor cells (OPCs), and survivors exhibit reduced myelination and neurological deficits. Wnt signaling regulates OPC maturation and myelination in a context dependent manner. Herein, we hypothesized that the occurrence of IVH would downregulate Wnt signaling, and that activating Wnt signaling by GSK-3β inhibition or Wnt3A recombinant human protein (rh-Wnt3A) treatment might promote maturation of OPCs, myelination of the white matter, and neurological recovery in premature rabbits with IVH. These hypotheses were tested in autopsy samples from preterm infants and in a rabbit model of IVH. Induction of IVH reduced expressions of activated β-catenin, TCF-4, and Axin2 transcription factors in preterm newborns. Both AR-A014418 (ARA) and Wnt-3A treatment activated Wnt signaling. GSK-3β inhibition by intramuscular ARA treatment accelerated maturation of OPCs, myelination, and neurological recovery in preterm rabbits with IVH compared to vehicle controls. In contrast, intracerebroventricular rh-Wnt3A treatment failed to enhance myelination and neurological function in rabbits with IVH. ARA treatment reduced microglia infiltration and IL1β expression in rabbits with IVH relative to controls, whereas Wnt3A treatment elevated TNFα, IL1β, and IL6 expression without affecting microglia density. GSK-3β inhibition downregulated, while rh-Wnt3A treatment upregulated Notch signaling; and none of the two treatments affected the Sonic-Hedgehog pathway. The administration of ARA or rh-Wnt3A did not affect gliosis. The data suggest that GSK-3β inhibition promoted myelination by suppressing inflammation and Notch signaling; and Wnt3A treatment failed to enhance myelination because of its pro-inflammatory activity and synergy with Notch signaling. GSK-3β inhibitors might improve the neurological outcome of preterm infants with IVH.
Keywords: Wnt3A, GSK-3β, Oligodendrocyte precursor cell, Myelination, Inflammation, Notch
INTRODUCTION
Intraventricular hemorrhage (IVH) remains the most common neurological complication of prematurity (Ballabh, 2010). Premature infants with IVH suffer from neurological consequences, including cerebral palsy, cognitive deficits, and intellectual disability (Ballabh, 2010). IVH inhibits proliferation and maturation of oligodendrocyte progenitor cells (OPCs), and thereby reduces myelination of the periventricular white matter in preterm newborns (Dummula et al., 2011). Currently, no therapeutic or preventive strategy exists to minimize white matter injury in premature infants with IVH. Wnt/β-catenin signaling has been linked with both neurodevelopmental and neurodegenerative disorders (Moon et al., 2004). Importantly, it regulates oligodendrogensis and myelination, however the underlying mechanisms are complex, context dependent, and much debated (Guo et al., 2015). Herein, we asked whether IVH would affect Wnt signaling and if so, whether activation of Wnt/β-catenin signaling pathway would enhance generation and maturation of oligodendrocyte progenitor cells (OPCs), and myelination in premature newborns with IVH.
The activation of Wnt/β-catenin pathway involves the binding of extracellular Wnt ligands to transmembrane fizzled and LRP5/6 receptors (Moon et al., 2004). This results in the dissociation of this destruction complex and consequent elevation in cytoplasmic β-catenin and its translocation in the nucleus to induce Wnt-target genes through TCF4 activation (TCF7L2). The intracellular β-catenin destruction complex consists of Axin, adenomatous polyposis coli (APC), glycogen synthase kinase 3β (GSK3β), and casein kinase 1. Indeed, Wnt signaling is regulated at multiple levels; and GSK-3β inhibition has been employed in a number studies to activate Wnt-β-catenin signaling pathways (Moon et al., 2004; Rockenstein et al., 2007).
The prevailing notion was that activation of Wnt/β-catenin signaling inhibits OPC differentiation and contributes to myelination failure. Accordingly, administration of Wnt antagonist (Shimizu et al., 2005) and inactivation of Wnt signaling in mouse model increases OPC generation (Langseth et al., 2010; Ye et al., 2009); and stabilization of axin2 by tankyrase inhibition, or downregulation of β-catenin enhances OPC maturation and myelination (Fancy et al., 2011). Conditional ablation of APC from oligodendroglial lineage inhibits maturation of OPCs through both β catenin dependent and independent mechanisms (Lang et al., 2013). However, these studies have been challenged by recent reports, which show that activation of canonical Wnt signaling in culture and in vivo experiments promotes oligodendrogenesis (Azim and Butt, 2011; Ortega et al., 2013). Likewise, Wnt signaling activation by GSK3 β inhibition increases OPC specification and differentiation in the dorsal domain of the subventricular zone (Azim et al., 2017; Azim and Butt, 2011; Azim et al., 2014). Hence, a refined concept has been proposed that Wnt signaling plays distinct roles in OPC proliferation, maturation, and myelination in a context dependent manner.
Glycogen synthase kinases are serine/threonine kinases which play crucial roles in both neurogenesis as well as gliogenesis (Hur and Zhou, 2010). Glycogen synthase kinases-3β (GSK-3 β) is a dynamic enzyme which controls various signaling pathways regulating oligodendrogenesis, including TGFβ, Shh, Notch, and Wnt pathways (Hur and Zhou, 2010). In addition, it participates in signaling pathways that control innate immune response, including pro-inflammatory cytokine and interleukin production (Jope et al., 2007). Importantly, pharmacological inhibition of Gsk3-β improves myelination in both in vivo and in vitro experimental models of demyelination (Azim and Butt, 2011), and suppresses inflammation as well as confers neuroprotection in Alzheimer’s disease (Rockenstein et al., 2007; Sereno et al., 2009). Based on these considerations, we hypothesized that occurrence of IVH would affect Wnt/β-catenin signaling pathways, and that activation of Wnt signaling by either Wnt3A recombinant protein or GSK-3β inhibition might promote OPC maturation, myelination, and neurological recovery in preterm newborns with IVH. We also postulated that GSK-3β inhibition might reduce IVH-induced inflammation and influence Notch and Shh signaling pathways, thereby contributing to restoration of myelination and clinical recovery.
To test these hypotheses, we analyzed autopsy samples from premature infants and employed preterm rabbit model of IVH. We demonstrated that IVH down-regulated Wnt/β-catenin signaling, and the activation of Wnt/β-catenin pathway by GSK-3β inhibition, but not rh-Wnt3A treatment, enhanced maturation of OPCs and myelination in preterm rabbits with IVH. Moreover, GSK-3β inhibition ameliorates inflammation and downregulates Notch signaling, whereas rh-Wnt3A treatment potentiates Notch signaling and inflammation. The study highlights the pro-myelinating effect of GSK-3β inhibitor as opposed to lack of impact of rh-Wnt3A protein on myelination in rabbits with IVH.
MATERIALS AND METHODS
Animals:
This study was performed after approval from the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine, Bronx, NY. We employed a preterm rabbit model of glycerol-induced IVH that has been extensively validated in our previous studies (Chua et al., 2009; Vinukonda et al., 2010; Vose et al., 2013). Intraperitoneal glycerol results in IVH by causing intravascular dehydration, an increase in serum osmolality, consequent decline in intracranial pressure and rupture of fragile vessels in the ganglionic eminence (Ballabh et al., 2007; Georgiadis et al., 2008). We purchased Timed-pregnant New Zealand rabbits from Charles River Laboratories, Inc. (Wilmington, MA). C-section was performed to deliver the preterm pups at 29 days of gestational age (full-term=32 days). Newborn pups were reared in an infant incubator at a temperature of 35oC. We used rabbit milk replacer (Wombaroo, Glen Osmond, Australia) to gavage-feed the pups in a volume of ~2 ml every 12 h (100ml/kg/day) for the first 2 days, and feeds were advanced to 125,150, 200, 250 and 280 ml/kg at postnatal days 3, 5, 7, 10 and 14, respectively. To induce IVH, we treated rabbit pups of either sex with 50% glycerol (6.5 gm/kg) intraperitoneally at 4 h of age. We evaluated the severity of IVH by measuring ventricular volume (length, breadth & depth in coronal & sagittal views) on head ultrasound at 24 h age using an Acuson X700 (Siemens) ultrasound machine. Pups with IVH were classified as moderate (70–150 mm3) and severe (151–250mm3) IVH, based on ventricular volume (Fig. 1A). A ventricular volume <70 mm3 indicated either an absence of IVH or presence of small or microscopic hemorrhage. The rabbit pups with moderate and severe IVH were assigned to either treatment or control group, so that the severity of IVH was balanced between the comparison groups.
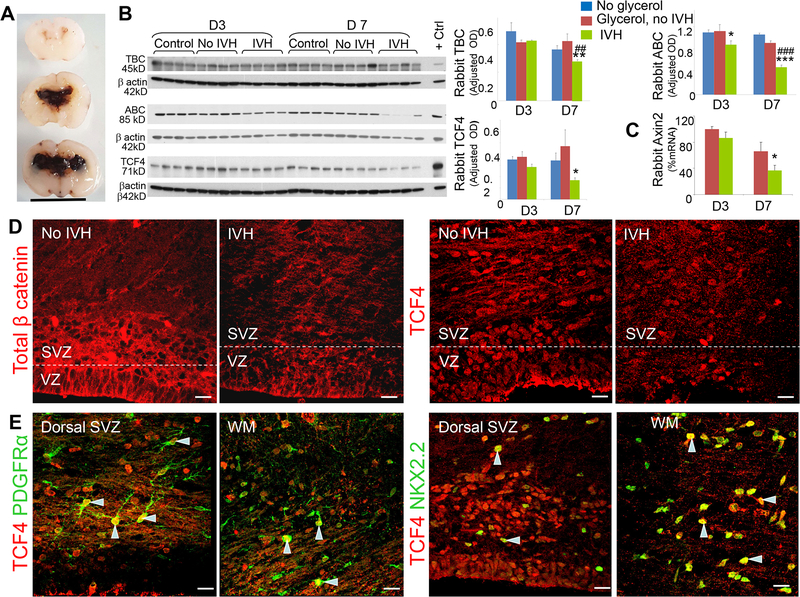
Fig.1: IVH reduced active β-catenin (ABC) and TCF4 in preterm rabbits.
A) Coronal brain slice from the fronto-parietal lobe of E29 rabbit pups which show slit like ventricles in pup without IVH (upper panel) and moderate to severe IVH resulting in fusion of the lateral ventricles (middle and lower panel). Scale bar, 1 cm. B) Representative Western blot analyses for total β-catenin (TBC), activated β-catenin (ABC) and TCF4 on brain homogenates of preterm rabbits with and without IVH at D3 and D7. The bar charts are mean ± s.e.m. (n = 5 each). Values were normalized to β actin levels. TBC levels were reduced in pups with IVH compared to both glycerol-treated and untreated controls without IVH at D7. ABC was reduced in rabbits with IVH compared to both glycerol and no glycerol controls without IVH at D7. ABC was also reduced in pups with IVH compared to glycerol treated controls without IVH at D3. TCF4 was also reduced in rabbits with IVH compared to controls without IVH at D7. C) mRNA expressions of Axin2 was reduced in rabbits with IVH compared to controls without IVH at D7. D) Representative immunofluorescence of cryosections from E29 rabbit pups with and without at D7 (as indicated) labeled with β-catenin and TCF4 specific antibodies. Note reduced expression of β-catenin and TCF4 in the VZ and SVZ of dorsal telencephalon of rabbits with IVH compared to controls without IVH. E) Cryosections were stained with TCF4 in combination with PDGFRα or Nkx2.2 specific antibodies. TCF4 immunoreactivity co-localized with PDGFRα extensively in the SVZ of dorsal telencephalon (arrowhead). TCF4 signals also overlapped with Nkx2.2 reactivity (arrowhead). Scale bar, 20 μm. *P<0.05, **P<0.01, ***P<0.001 glycerol treated no IVH controls vs. pups with IVH. ##P<0.01, ### P<0.001 pups not treated with glycerol vs. pups with IVH. VZ, ventricular zone; SVZ, subventricular zone.
AR-A014418 and Wnt3A recombinant human protein treatment:
Rabbit pups with IVH were sequentially treated with either intramuscular vehicle (DMSO) or AR-A014418 (10 μl, 20 mg/kg) twice a day for seven days, starting at day 1. The severity of IVH, measured by ultrasound, was similar between the comparison groups—vehicle treated pups with IVH and AR-A014418-treated pups with IVH. In another set of the experiment, rabbit pups with moderate-to-severe IVH were sequentially treated with intracerebroventricular (ICV) rh-Wnt3A (500 ng in 5 μl saline) or ICV saline at postnatal days 1, 3, and 6. The dose of IM AR-A014418 and ICV Wnt3A was determined based on the previous studies (Kalinichev and Dawson, 2011; Lee et al., 2016).
Human subjects:
The Research Administration of Albert Einstein College of Medicine, Bronx, NY approved the use of autopsy brain samples from premature infants for the present study. The postmortem samples included forebrain tissue samples harvested from premature infants with and without IVH of 23–27 gestational weeks (gw). These infants were of ≤5 days of postnatal age (Table 1), and the postmortem materials were obtained within 18 h of their demise. We excluded premature neonates with hypoxic-ischemic encephalopathy, meningitis, culture proven sepsis, major brain or spinal cord malformation, and chromosomal defects. We included 6 preterm infants from each group--IVH and no IVH. The wall of the cerebral hemisphere in premature infants consists of ventricular zone (VZ), subventricular zone (SVZ), intermediate zone, cortical plate, and marginal zone, as described by the Boulder Committee (Bystron et al., 2008). In the present manuscript, we used the term intermediate-zone embryonic white matter interchangeably with white matter, ganglionic eminence with germinal matrix, and cerebral cortex with cortical plate.
Table 1:
Characteristics of human infants with and without IVH
| Post-conceptional age (Weeks) | Sex | Birth weight (Kg) | IVH / No IVH | Cause of death |
|---|---|---|---|---|
| 26 | Male | 0.810 | IVH grade 3 | Clinical sepsis |
| 23 | Male | 0.57 | IVH grade 2 | Clinical sepsis |
| 23 | Female | 0.58 | IVH grade 3 | Respiratory failure |
| 24 | Male | 0.64 | IVH grade 4 | Pulmonary hemorrhage |
| 24 | Male | 0.6 | IVH grade 4 | Clinical sepsis |
| 23 | Female | 0.52 | IVH grade 3 | Respiratory failure |
| 23 | Female | 0.53 | no IVH | Respiratory failure |
| 25 | Female | 0.71 | no IVH | Metabolic acidosis, respiratory failure |
| 23 | Male | 0. 45 | no IVH | RDS, Respiratory failure |
| 24 | Male | 0.61 | no IVH | Clinical sepsis |
| 25 | Male | 0.73 | no IVH | Metabolic acidosis, resp. failure |
| 24 | Female | 0.56 | no IVH | Respiratory failure |
Rabbit tissue collection and processing:
We processed the tissues as described before (Ballabh et al., 2007). Briefly, the brain slices were immersed into 4% paraformaldehyde in phosphate buffered saline (PBS; 0.1 M, pH 7.4) overnight and then were cryoprotected by keeping them into 15% sucrose in 0.1 M PBS buffer for 24 hours followed by 30% sucrose for the next 24 hours. We then froze the tissue slices after embedding them into an optimum cutting temperature compound (Sakura, Japan). Frozen coronal blocks were cut on a cryostat into coronal sections of 18 μm thickness. For Western blot analyses, a 1–2 mm thick coronal slice was harvested at the level of the mid septal nucleus and snap-frozen on dry ice.
Human tissue collection and processing:
We processed the human tissues as before (Ballabh et al., 2007). Coronal slices of 3–4 mm thickness were taken at the level of head of caudate nucleus from the fronto-parietal lobe. The coronal blocks consisted of cortical plate, embryonic white matter, and ganglionic eminence. The samples were immersion-fixed into 4% paraformaldehyde in PBS for 12–18 h and were then cryoprotected by keeping them into a 15% sucrose solution in PBS, followed by 30% sucrose in PBS. The tissues were then frozen after embedding them into optimum cutting temperature compound (Sakura, Japan). Frozen coronal blocks were cut into sections of 18 μm thickness. For Western blot analyses, pieces of tissues were directly harvested from the cortex, white matter, and ganglionic eminence into Eppendorf tubes and were snap frozen on dry ice.
Immunohistochemistry (IHC):
Immunohistochemical staining was performed as described before (Ballabh et al., 2007). The primary antibodies used in experiments included: Rabbit polyclonal GSK 3β (Cell signaling Inc; catalog # 9315), rabbit monoclonal β-catenin (Cell signaling; catalog# 8480), mouse monoclonal activated β-catenin (Millipore, catalog # 05–665) , mouse monoclonal TCF4 (Catalog# H00006925-M03, Clone 1G4, Novus Biologicals), mouse polyclonal Ki67 (catalog #M7240, DAKO), goat polyclonal Olig2 (catalog #AF-2418, R&D, Minneapolis, MN), mouse monoclonal GFAP (catalog #G6171, Sigma-Aldrich, St. Louis, MO), mouse monoclonal myelin basic protein (MBP; catalog #ab62631, Abcam, Cambridge, MA), goat polyclonal PDGFRα (catalog #AR307, R & D, Minneapolis, MN), mouse monoclonal myelin associated glycoprotein (MAG; catalog #AB89780, Abcam, Cambridge, MA), mouse Nkx2.2 (Developmental studies Hybridoma Bank, University of Iowa), goat Iba1 (catalog# 5076, Abcam), rabbit monoclonal Axin2 (Catalog #2141, Cell Signaling Inc, Danvers, MA) and sheep polyclonal NICD (catalog # AF3647, R&D, Minneapolis, MN). Secondary antibodies used were Cy-3 conjugate donkey anti-mouse, Cy-3 conjugate donkey anti-goat, and FITC conjugate donkey anti-rat (Jackson Immunoresearch, West Grove, PA). Briefly, we hydrated the fixed sections in 0.1M PBS, blocked the sections with normal donkey serum in PBS with 0.01% Triton-X (PBST), and incubated them with the primary antibodies diluted in PBS at 4°C overnight. After several washes in PBS, the sections were incubated with secondary antibody diluted in 2% normal donkey serum in PBS at room temperature for 60 minutes. Finally, after washing in PBS, sections were mounted with SlowFade Light Antifade reagent (Molecular Probes, Invitrogen, CA) and were visualized under a Confocal microscope (Nikon Instruments, Japan). Stereology was performed using a fluorescent microscope (Axioskop 2 plus, Carl Zeiss Inc) with motorized specimen stage for automated sampling (ASI, Eugene, OR), CCD color video camera (Microfire; Optronics, Goleta, CA) and stereology software (Stereologer, SRC, Baltimore, MD).
Fluorescent in situ detection of DNA fragmentation (TUNEL).
We performed TUNEL staining on fixed brain sections as described before (Dummula et al., 2011). For TUNEL staining, tissue sections of 14 μm thickness were air dried on slides, hydrated in 0.01 M PBS, and permeabilized for 5 min in 1:1 ethanol:acetic acid. An ApopTag-fluorescein in situ DNA fragmentation detection kit (catalog #S7110; Millipore) was used to visualize TUNEL-labeled nuclei.
Quantification of oligodendrocyte progenitors and microglia:
Proliferation and maturation of OPCs were assessed in the corona radiata and corpus callosum of pups without IVH and pups with IVH treated with vehicle or AR-A014418. Cycling OPCs were identified by double-labeling the coronal sections with PDGFRα and Ki67 antibodies, while maturation of OPCs was evaluated by double-labeling the sections with Olig2 and Nkx2.2 antibodies. Four coronal sections were obtained at the level of the mid septal nucleus (five 20 μm sections collected at 100 μm interval). Quantification was performed by a blinded investigator in a random, unbiased fashion using a confocal microscope with a 60x lens (Nikon Instruments, Japan). Cells were counted in ~25 images (5 images × 4–5 sections) for each brain region for every parameter for each pup (n=5 pups per group). Iba1+ microglia and Tunnel+ cells were quantified in the corona radiata and ganglionic eminence in a similar manner.
Stereological assessment of myelin and astrocytes in the white matter:
We quantified a number of stereological parameters using a computerized software system (Stereologer, Stereology Resource Center, Chester, MD). Briefly, 30 μm thick coronal sections were cut on a cryostat with a section sampling interval of 90 μm to achieve 6 sections or more at the level of mid-septal nucleus. The sections were double-labeled with myelin basic protein (MBP) antibody and DAPI (nuclear stain) and quantified as follows. The reference spaces (corona radiata and corpus callosum) were outlined on the coronal section under 5x objective. The volume of the outlined area (reference space) was quantified using a point counting probe (frame 25 μm × 25 μm; guard zone 2 μm, inter-frame interval = 300 μm). The total volume fraction (load) of myelin stained by MBP antibody through a defined reference space was measured using the object area fraction probe under 60x oil lens. For the area fraction probe (frame 25 μm ×25 μm; guard zone 2 μm, interframe interval 400 μm), the user clicked on the grid points that overlapped the myelin fibers in sections labeled with MBP. The area fraction of myelination was quantified as the ratio of product of the area per point and number of points hitting reference area the over the product of the area per point and number of points hitting the sampled area [a(point)• ∑Psamp], as reported previously. A coefficient of error (CE) less than 0.10 was considered acceptable. To assess gliosis, we quantified total volume fraction of astrocyte cell body and glial fibers in a similar manner as for myelin (Mouton et al., 2009).
Western blot analyses:
We homogenized the frozen brain tissue in a sample buffer (3% SDS, 10% glycerol and 62.5 mM Tris-HCl) using a mechanical homogenizer and then sonicated the lysate before centrifugation. The supernatant protein concentration was measured using a BCA protein assay kit (Pierce Kit #23227, Thermo Scientific, Rockford, IL) and dilutions of BSA were used to create a standard curve. After boiling the samples in Laemmli buffer (catalog #161–0737, Bio-Rad, CA), total protein samples were separated by SDS-PAGE (Vinukonda et al., 2010). Equal amounts of protein (10–20 μg) were loaded onto 4–15% or 4–20% gradient precast gels (Bio-Rad, CA), based on the molecular weight of the target protein. Separated proteins were transferred onto polyvinylidene difluoride (PVDF) membrane by electro-transfer. Membranes were then incubated overnight with primary antibodies. We detected target proteins with chemiluminescence ECL system (GE Healthcare) by using secondary antibodies conjugated with horseradish peroxidase (Jackson Immunore- search, West Grove, PA). We stripped the membrane using a stripping buffer (2.5% SDS, 0.7% 2-mercaptoethanol, 62.5 mM Tris-HCl, pH 6.8) and then incubated with β-actin antibody (catalog #A5316, Sigma, St. Louis, MO) followed by secondary antibody and detection with chemiluminescence ECL system. As described previously (Vinukonda et al., 2010), the blots from each experiment were densitometrically analyzed using Image J, and optical density (OD) values for each protein of interest were normalized to those of β-actin. The antibodies used for Western blot analyses were the same as for immunohistochemistry.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR):
Gene expression was quantified by real time PCR, as described previously (Ballabh et al., 2007). Briefly, total RNA was isolated using a RNeasy Mini kit (catalog #74104, Qiagen) from a coronal brain slice taken at the level of the mid-septal nucleus. cDNA was synthesized using Superscript II RT enzyme (catalog # 05081955001, Roche, Indianapolis, IN) followed by a real-time quantitation using an ABI Prism 7900HT detection system. TaqMan probes were bought from Life technologies. Their assay IDs were as follows: GAPDH (Oc03823402_g1), TNFα (Oc03397716_g1), IL1β (Oc03823250_s1), CNTF (Oc03397817_m1), LIF (Hs01055668_m1), IL-6 (Oc04097053_m1), Hes 5 (Mm00439311_g1), Hes 1 (APH49WG), GSk3b(Hs01047719_m1).
Electron microscopy:
We processed brains (d14) from the glycerol treated rabbit pups without IVH, pups with IVH, and the AR-A014418-treated pups with IVH (n = 3– 4 each). We took coronal slices (2 mm thickness) from freshly harvested rabbit pup brain using a brain slicer matrix and then dissected corona radiata and corpus callosum in a Petri dish under a Stereo discovery microscope (Carl Zeiss). The tissue samples from the white matter were fixed into 2.5% glutaraldehyde overnight. The tissue samples were then washed in 0.1 M sodium cacodylate buffer, pH 7.4, postfixed in buffered osmium tetroxide for 1–2 h, stained en bloc with 1% uranyl acetate, dehydrated in graded ethanol solutions, and then embedded in epoxy resin. We placed sections of 60–90 nm thicknesses onto 200 mesh grids, stained with uranyl acetate and lead citrate, and then examined with a Techni 12 electron microscope at 80 Kv. For each brain, we acquired 12–20 digital images using a 16 megapixel Advanced Microscopy Techniques camera. Electron micrographs were assessed for myelinated axons per unit area; and the g-ratio (ratio of axonal diameter with myelin sheath to the axonal diameter without myelin sheath) of myelinated axons were computed in the 3 groups of pups using Image J (NIH).
Statistics and Analysis:
Data are presented as means ± standard error of the mean (s.e.m.). To compare the levels of myelin basic protein (MBP), myelin associated glycoprotein (MAG) and cytokines as well as cell counts between 3 groups (no IVH, vehicle, and AR-A014418 or Wnt3A treated pups), we used one way ANOVA. To assess the difference in the protein and mRNA expression of Wnt signaling molecules between pups with and without IVH at D3 and D7 age, we employed two-way ANOVA. All post hoc comparisons between means were done by Tukey multiple comparison test at 0.05 significance.
RESULTS
IVH downregulates Wnt/ β–catenin-TCF4 pathway in rabbits:
Whereas canonical Wnt/β-catenin pathway is inhibited in hypoxia-ischemia and Alzheimer’s disease (Diaz et al., 2016), it is activated in amyotrophic lateral sclerosis, Parkinson’s disease, Huntington’s disease, multiple sclerosis, and Friedreich’s ataxia (Vallee and Lecarpentier, 2016). The effect of IVH on Wnt signaling remains unknown. Therefore, we asked whether IVH would affect Wnt-signaling and its downstream molecules. To this end, we assessed expressions of total β-catenin, activated β-catenin, TCF4 and Axin-2 transcription factors in rabbits with and without IVH at postnatal day (D) 3 and 7 (Fig. 1). Western blot analyses showed that total β-catenin levels were reduced in pups with IVH compared to glycerol-treated pups without IVH at postnatal day (D) 7 (P= 0.04, Fig. 1B), but not at D3. More importantly, activated β-catenin levels were also reduced in pups with IVH compared to both glycerol treated and untreated pups without IVH at D3 and 7 (P=0.007, 0.008 for D3 and both P<0.001 at D7, Fig. 1B). TCF4 is a transcription factor and a member of downstream Wnt signaling pathway that plays key role in oligodendrogenesis (Ye et al., 2009). Western blot analyses showed that TCF4 expression was also reduced in rabbits with IVH compared to glycerol-treated pups without IVH at postnatal day 7 (P=0.022), but not at D3. We next quantified Axin2 mRNA by RT-qPCR using TaqMan probes. Consistent with β-catenin and TCF4 protein quantification, Axin-2 mRNA expression was reduced in pups with IVH at D7 (P=0.03, Fig. 1C), but not at D3.
We next evaluated immunolabeled coronal sections to assess the expression of total β-catenin and TCF4 in pups with and without IVH. For each immunolabeling, we employed both negative (no primary antibody) and positive controls (image not shown). We found that β-catenin was expressed in the ventricular and subventricular zone (VZ and SVZ) and relatively weakly in the white matter at D3 and 7 (Fig. 1D). The total β-catenin was expressed primarily in the membrane and cytoplasm. More importantly, the immunoreactivity was weaker in the VZ and SVZ and relatively sparse in the white matter of rabbits with IVH compared to controls without IVH.
We next assessed TCF4 immunoreactivity in the preterm rabbits with and without IVH. We found that TCF4 was expressed in the VZ and SVZ of the dorsal telencephalon, white matter, and medial ganglionic eminence of preterm (E29) rabbit pups at D3 and D7 (Fig. 1D, 1E). Importantly, TCF4 immunoreactivity was reduced in the white matter and the adjacent SVZ of dorsal telencephalon in rabbits with IVH compared to controls without IVH (Fig. 1D). The PDGFRα+ OPCs co-expressed TCF4 abundantly in the white matter and dorsal SVZ, but weakly in the MGE (MGE data not shown). We next assessed the expression of TCF4 in Nkx2.2+ cells, which are immature oligodendrocytes. We found that TCF4 was widely expressed on Nkx2.2 in the white matter. Together, IVH results in downregulation of Wnt/β -catenin signaling; and TCF4 is expressed on both early OPCs and immature oligodendrocytes in preterm-born rabbits.
IVH downregulates Wnt-signaling in human preterm infants:
To define the translational potential of the study, we evaluated the expression of β-catenin and TCF4 in the forebrain of human premature infants of 23–30 gestational weeks (autopsy samples). Coronal sections at the head of caudate nucleus level were immunolabeled with β-catenin and TCF4 antibodies and Western blot analyses were performed to compare their protein levels between premature infants with and without IVH. We found that β-catenin was extensively expressed in the periventricular ganglionic eminence (Fig. 2A), just as in rabbits. β-catenin was expressed primarily in the cytoplasm and plasma membrane, and was weak-to-absent in the nucleus. Double labeling of β-catenin with PDGFRα (early OPC) and O4 (late OPC) specific antibodies showed β-catenin expression on few PDGFRα+ and O4+ OPC. β–catenin was extensively expressed on GFAP+ astrocytes and radial glia in the ganglionic eminences and SVZ of dorsal telencephalon, but not in the white matter (intermediate zone). Moreover, β–catenin expression was similar between infants with and without IVH in the three brain regions--cortex, white matter and ganglionic eminence. Accordingly, Western blot analyses showed that total β-catenin levels were comparable between infants with and without IVH in these three brain regions. However, activated β-catenin levels were reduced in the white matter of preterm infants with IVH compared to controls without IVH (P<0.009), but not in the cortex or ganglionic eminence.
Fig. 2: IVH reduced Active β-catenin (ABC) in human preterm infants:
A) Representative immunofluorescence of cryosections from dorsal SVZ, MGE and white matter (WM) of a 24 week premature human infant (without IVH) labeled with antibody to β-catenin. β-catenin immunoreactivity co-localized with GFAP+ radial glia (arrow) extensively in dorsal SVZ and moderately in MGE. Note β-catenin weakly expressed on some PDGFRα+ and O4+ OPC (arrowhead). B) A typical Western blot analyses for total β-catenin (TBC) and activated β-catenin (ABC) on brain homogenates of preterm infants with and without IVH. Rat brain was used as positive control. The bar charts are mean ± s.e.m. (n = 5 each). Values were normalized to β actin levels. ABC was decreased in the WM of infants with IVH and showed a trend towards reduction in other brain regions. **P<0.01 infant with vs. without IVH
We next assessed the expression of TCF4 on OPCs at different maturational stages in coronal sections double labeled with TCF4 and PDGFRα, O4, or Nkx2.2 specific antibodies (Fig. 3). We found that TCF4 was abundantly expressed in the periventricular white matter, VZ and SVZ of dorsal telencephalon, and medial as well as lateral ganglionic eminences. More importantly, TCF4 was expressed on PDGFRα+ and O4+ OPCs. Nkx2.2+ immature oligodendrocytes (differentiation committed OPC), which were abundant in the white matter and scarce in the ganglionic eminences and dorsal SVZ, also expressed TCF4. Western blot analyses revealed that there was significant reduction in TCF4 in the white matter of infants with IVH relative to controls without IVH (P<0.04), but not in the germinal matrix and cortex. Together, IVH downregulates Wnt signaling in humans as in rabbits. In addition, all stages of OPCs are enriched with TCF4, but poorly expressed β-catenin. This indicates that TCF4 might play an important role in maturation of OPCs.
Fig. 3: IVH reduced TCF4 in human preterm infants:
A) Representative immunofluorescence of cryosections from dorsal SVZ, MGE and white matter (WM) of a 24 week premature human infant (without IVH) with antibodies to TCF4. TCF4 was extensively expressed in the dorsal SVZ, MGE, and WM. Immunosignals of TCF4 widely co-localized with reactivity of PDGFRα, O4, and Nkx2.2+ OPCs (arrowhead). Nkx2.2+ OPC were scarce in the MGE. Scale bar, 20μm. B) Western blot analyses for TCF4 was performed on brain homogenates of cortex (cortical plate), WM and MGE. Rat brain was used as positive control. The bar charts are mean ± s.e.m. (n = 5 each). Values were normalized to β actin levels. TCF4 was decreased in the WM of infants with IVH and showed a trend towards reduction in other brain regions. **P<0.01 infant with vs. without IVH. WM, white matter, GM, germinal matrix (ganglionic eminence).
IVH increases GSK-3β gene expression, and GSK-3β inhibition activates Wnt signaling:
GSK-3β controls both oligodendrogenesis and neurogenesis through modulation of Wnt signaling. It is dysregulated in a number of neurological disorders, including Parkinson’s disease, multiple sclerosis and Alzheimer’s disease (Kockeritz et al., 2006). Therefore, we quantified GSK-3β by RT-qPCR in rabbits with and without IVH. We found that GSK-3β mRNA expression was elevated in rabbits with IVH compared to controls without IVH at D7 (P=0.03), not at D3 (Fig. 4A). We could not find a satisfactory commercially available antibody reactive to GSK-3β protein for Western blot analyses.
Fig. 4: AR-A014418 inhibits GSK-3β and increases levels of ABC and TCF4.
A) Real time qPCR showed that p-GSK-3β mRNA expression was elevated in rabbits with IVH compared to glycerol controls without IVH at D7 and AR-A014418 treatment does not affect GSK-3β expression. In addition, AR-A014418 treatment elevated Axin-2 expression at D7. B) Representative Western blot analyses for p-GSK-3β (Ser9), p-GSK-3β (Tyr216), TCF4, and ABC on brain homogenates of preterm rabbits without IVH, AR-A014418- and vehicle-treated pups with IVH at D3. Rat brain was used as positive control. C) The bar charts are mean ± s.e.m. (n = 5 each). Values were normalized to β actin levels. p-GSK-3β (serine 9) levels were higher and p-GSK-3β (Tyr216) levels were reduced upon AR-A014418 treatment in rabbits with IVH. Active β-catenin (ABC) and TCF4 are reduced in rabbits with IVH compared to controls without IVH and AR-A014418 treatment increases the levels of ABC and TCF4. # P<0.05, ###P<0.001 for AR-A014418 vs. vehicle treatment. ***P<0.001 for IVH vs. no IVH controls. No IVH, glycerol no IVH
To downregulate GSK-3β enzyme levels, we selected AR-A014418, a specific GSK-3β inhibitor (Cohen and Goedert, 2004). Since Axin2 is a known Wnt-activated target and is transcriptionally induced with reception of Wnt/ βcatenin signal, we quantified Axin2 mRNA in AR-A014418 and vehicle treated pups. We found that AR-A014418 treatment increased Axin2 mRNA expression in rabbits with IVH relative to controls at D7 (P=0.04, Fig 4A), but not at D3. GSK-3 β activity is inhibited through phosphorylation of serine-9 and activated through phosphorylation of tyrosine-216 amino acid in GSK-3 β (Fang et al., 2000). Therefore, we evaluated GSK-3β phosphorylation to determine whether AR-A014418 treatment inhibited the GSK-3β enzyme. Western blot analyses revealed that p-GSK-3β (serine 9) levels were elevated (P=0.036) and p-GSK-3β (Tyr216) levels were reduced (P=0.03) upon AR-A014418 treatment in rabbits with IVH (Fig. 4B, 4C). This indicates that AR-A014418 treatment effectively inhibits GSK-3β enzyme in rabbits with IVH.
To further assess the effect of GSK-3β inhibition on Wnt signaling, we next quantified β-catenin and TCF4 expression in AR-A014418 treated animals with IVH compared to vehicle controls with IVH. We found that both activated β-catenin and TCF4 levels were elevated in AR-A014418 treated animals with IVH compared to vehicle controls with IVH at D7 (P=0.001 and 0.01, respectively; Fig. 4B, 4C). Together, GSK-3β is upregulated with the occurrence of IVH, and AR-A014418 treatment effectively inhibits GSK-3β and activates Wnt signaling in pups with IVH.
GSK-3β inhibition restores myelination in rabbit pups with IVH.
Since GSK-3β hinders oligodendrocyte maturation and myelination both in vivo and in vitro experiments (Azim and Butt, 2011), we postulated that GSK-3β inhibition by IM AR-A014418 treatment would enhance myelination in rabbits with IVH. To this end, we compared myelination among three groups of pups at D14: a) glycerol-treated pups without IVH, b) vehicle-treated pups with IVH, and c) AR-A014418-treated pups with IVH. IP glycerol was used to induce IVH, and subsequently the pups with IVH were treated with either IM AR-A014418 or vehicle to evaluate the effect of AR-A014418 on myelination. Severity of IVH, measured by head ultrasound, was comparable between groups. Stereological quantification of myelin basic protein (MBP) in immunostained sections demonstrated that the volume fractions of MBP in the corpus callosum and corona radiata were significantly reduced in pups with IVH relative to controls without IVH (P<0.001, Fig. 5A). More importantly, the expression of MBP was higher in AR-A014418 treated rabbits compared to vehicle controls (P=0.001 Fig. 5A). Consistent with stereological analyses, Western blot analyses revealed that MBP and MAG levels were reduced in pups with IVH compared to controls without IVH (P<0.01 both) and that AR-A014418 treatment significantly elevated MBP and MAG expression in pups with IVH (P=0.01 and 0.001, respectively, Fig. 5B,5C).
Fig. 5: GSK-3β inhibition by AR-A014418 improves myelination in rabbits with IVH:
A) Representative immunofluorescence of myelin basic protein (MBP) in the corona radiata of D 14 pups. Data are mean ± s.e.m. (n = 8 each group). Volume fractions of MBP were elevated in the corpus callosum and corona radiata of AR-A014418 treated pups compared with vehicle controls with IVH. Scale bar, 200 μm. V, ventricular side. B) Typical Western blot analysis for MBP in the forebrain of premature rabbit pups, as indicated, at D14. Adult rat brain was employed as positive control. Each lane represents lysate from a whole coronal slice taken at the level of mid septal nucleus of one brain. Bar chart shows mean ± s.e.m. (n=5 each group). MBP expression was higher in AR-A014418 treated pups compared with vehicle treated pups. C) Western blot analysis for MAG in the forebrain of pups as indicated at D14. Adult rat brain was used as positive control. Bar graph shows mean ± s.e.m. (n=5 each group). MAG expression was elevated in AR-A014418 treated pups compared with vehicle controls. D) Representative electron micrograph from rabbit pups without and with IVH, and pups with IVH treated with AR-A014418 at d 14. Note that myelinated axons were significantly decreased in pups with IVH compared to controls without IVH and that AR-A014418 treatment shows a trend towards increase in myelinated axons in pups with IVH. ***P<0.001 pups with vs. without IVH. #P<0.05, ###P<0.001 vehicle vs. AR-A014418 treated pups with IVH. Scale bar, 1 μm. no IVH, glycerol treated no IVH; IVH, IVH after glycerol treatment. No IVH implies glycerol no IVH.
Ultrastructural appraisal of the corpus callosum and corona radiata showed that numbers of myelinated axons were fewer in pups with IVH compared to controls without IVH (P<0.05, Fig. 5D). AR-A014418 treatment increased the mean density of myelinated axons to ~150% in rabbits with IVH, however the difference was not statistically significant. Moreover, the g-ratio was comparable in the three groups of pups (0.71±0.008 vs.0.73 ±0.016 vs. 0.71±0.01, in pups without IVH, IVH with vehicle and AR-A014418 treatment, respectively). G-ratios were not affected in rabbit pups with IVH (reduced myelination) and upon successful treatment (improved myelination) in our developmental and other models of brain injury in previous studies (Vinukonda et al., 2016; Vose et al., 2013).
We next assessed the effect of AR-A014418 treatment on preterm rabbits without IVH. These animals were treated with IM AR-A014418 for 7 days in a dose similar to rabbits with IVH. Western blot analyses using MBP antibody and stereological quantification of MBP reactivity in immunolabeled sections did not show difference in the myelination between AR-A014418- and vehicle-treated pups without IVH (data not shown).
Together, the data suggests that GSK-3β inhibition restores myelination of the white matter in preterm rabbit pups with IVH.
rh-Wnt3A treatment does not enhance myelination.
Since GSK-3β inhibition and Wnt3A administration differently affect OPC maturation in culture experiments (Azim and Butt, 2011), we evaluated the effect of rh-Wnt3A treatment on myelination in rabbit pups with IVH. We first examined whether rh-Wnt3A treatment activated Wnt-β-catenin signaling cascade. To this end, we compared activated β-catenin and TCF4 expression between ICV rh-Wnt3A and ICV vehicle treated pups by Western blot analyses. We found that activated β-catenin were elevated in rh-Wnt3A treated pups compared to vehicle controls at D3 (P= 0.029, Fig. 6A). Tcf4 levels were also elevated in rh-Wnt3A treated pups, however comparison was not significant (P=0.06). We next compared mRNA expression of Axin2 between ICV rh-Wnt3A and ICV vehicle treated pups. Axin 2 mRNA expression was higher in rh-Wnt3A treated pups relative to controls at D7 (P=0.035), but not at D3. This suggested that rh-Wnt3A treatment effectively upregulated Wnt-catenin-β cascade.
Fig. 6: rh-Wnt3A treatment does not improve myelination in rabbits with IVH:
A) Representative Western blot analyses for active β-catenin and TCF4 on brain homogenates of preterm pups without IVH, rh-Wnt3A- and vehicle-treated pups with IVH at D3. rh-Wnt3A treatment enhanced the levels of active β-catenin, but not of TCF4 levels in rabbits with IVH. B) Representative immunofluorescence of myelin basic protein (MBP) in the corona radiata of D 14 pups. Data are mean ± s.e.m. (n = 8 each group). Volume fractions of MBP were similar in the corpus callosum and corona radiata of Wnt3A treated pups compared with vehicle controls with IVH. Scale bar, 200 μm. C) Typical Western blot analysis for MBP and MAG in the forebrain of premature rabbit pups, as indicated, at D14. Adult rat brain was employed as positive control. Each lane represents lysate from a whole coronal slice taken at the level of mid septal nucleus of one brain. Bar chart shows mean ± s.e.m. (n=5 each group). MBP and MAG expression showed an insignificant trend toward increase in Wnt3A treated pups compared with vehicle treated controls. *P<0.05, ***P<0.001 pups with vs. without IVH. #P<0.05, vehicle vs. rh-Wnt3A treated pups with IVH. No IVH implies “glycerol no IVH”
We next determined the effect of ICV rh-Wnt3A on myelination and thus, compared glycerol treated pups without IVH, ICV rh-Wnt3A and ICV vehicle treated pups with IVH for the expression of MBP in the white matter at D14. The severity of IVH was balanced between the vehicle and rh-Wnt3A treated pups. Stereological quantification showed no significant difference in the expression of MBP in the immunostained sections between vehicle and rh-Wnt3A treated pups (Fig. 6B). Accordingly, MBP and MAG protein levels measured by Western blot analyses were similar between rh-Wnt3A and vehicle treated rabbits (Fig. 6C).
Together, the data suggests that rh-Wnt3A treatment does not restore myelination of the white matter in preterm rabbit pups with IVH, unlike GSK-3β inhibition.
GSK-3β inhibition reduces apoptosis and promotes maturation of OPC:
Since GSK3 β inhibition protects both hippocampal and cerebellar neurons from apoptosis in culture experiments (Thotala et al., 2012; Yeste-Velasco et al., 2008), we asked whether GSK-3β inhibition by AR-A014418 treatment would reduce apoptosis of OPCs in rabbits with IVH. To this end, we labeled brain sections with Olig2 antibody and then performed TUNEL staining. We found that the total number of apoptotic cells were significantly more abundant in the corpus callosum and corona radiata of pups with IVH compared with glycerol-treated controls without IVH at D 3 (P<0.01) and that AR-A014418 treatment substantially reduced the density of total apoptotic cells (P<0.02, Fig. 7A). OIig2+ apoptotic cells also showed a trend toward reduction with AR-A014418 treatment, however the comparison was not significant.
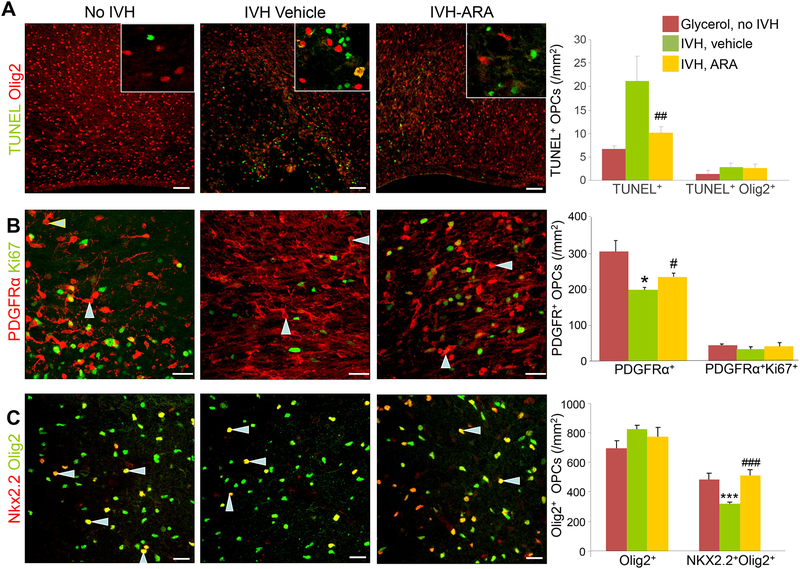
Fig. 7: AR-A014418 treatment reduces apoptosis and promotes maturation of OPC in rabbits with IVH.
A) Representative images of TUNEL labeling (green) and O4 immunostaining for OPCs (red) in the corona radiate of E29 pups at D3. Insets show high magnification views of the boxed area in the image. TUNEL+ cells were reduced in AR-A014418 treated pups with IVH compared to controls. Scale bar, 50μm. B) A representative image from cryosections of E29 pup double-labeled with PDGFRα and Ki67 specific antibodies at D3. Scale bar, 20 μm. Graphs show mean ± SEM (n = 5 each). Note the cycling PDGFRα+ cells are comparable in the three groups, as indicated. The total PDGFRα+ cells (arrowhead) are reduced in pups with IVH, and AR-A014418 treatment shows a significant increase in the density of these cells compared to vehicle controls. C) Representative immunofluorescence of cryosections from D 7 pups double-labeled with Olig2 and Nkx2.2 specific antibodies. Graphs show mean ± SEM (n = 5 each). Note an increase in Nkx2.2+Olig2+ cells (arrowhead) in AR-A014418 treated pups relative to vehicle controls. Scale bar, 20 μm. *P<0.05, ***P<0.001 pups with vs. without IVH. # P<0.05, ###P<0.001 vehicle vs. AR-A014418 treated pups with IVH.
As AR-A014418 treatment enhanced myelination in our preterm pups with IVH, we postulated that AR-A014418 treatment might promote proliferation and maturation of OPCs. To assess the proliferation of OPC in the corona radiata and corpus callosum, we compared cycling and total PDGFRα+ progenitor cells in glycerol-treated pups without IVH, vehicle-treated and AR-A014418-treated pups with IVH at D3. The densities of total PDGFRα+ cells were reduced in rabbits with IVH, and AR-A014418 treatment increased their densities (P<0.05 both). However, the densities of proliferating PDGFRα+ cells were also not significantly affected by AR-A014418 treatment (Fig. 7B).
Olig2, and Nkx2.2 play specific roles during OPC differentiation and remyelination, and has been used to assess OPC maturation (Fancy et al., 2004; Labombarda et al., 2009; Ligon et al., 2006). We immuno-labeled the sections with Olig2 and Nkx2.2 specific antibodies. Our analyses revealed that Olig2+ OPCs were similar between the three groups--glycerol-treated pups without IVH, vehicle-treated and AR-A014418-treated pups with IVH. However, the population of Olig2+Nkx2.2+ cells was significantly reduced in pups with IVH relative to controls without IVH (P=0.005), and AR-A014418 treatment substantially increased their density (P = 0.004) in the corona radiata and corpus callosum (Fig. 7C). Together, AR-A014418 treatment
enhances specification and maturation of OPCs in pups with IVH.
GSK-3β inhibition enhances neurological recovery, not rh-Wnt3A treatment.
To determine whether AR-A014418 treatment enhances neurological recovery of preterm rabbits with IVH, we performed neurobehavioral assessments of three sets of preterm pups at D 14: a) glycerol-treated pups without IVH (n=10), b) vehicle-treated pups with IVH (n=10), and c) AR-A014418 treated pups with IVH (n=9) (Table 2), as previously described (Vinukonda et al., 2010; Vose et al., 2013). Vehicle and AR-A014418 treated groups with IVH were balanced with respect to the severity of IVH. All glycerol treated pups without IVH were neurologically normal. In contrast, we found symmetric quadriparesis in 2 pups (20%), and left leg weakness in one pup (10%) in vehicle-treated group. The pups with quadriparesis were not able to walk and pups with monoparesis showed clumsiness in the gait. Among pups treated with AR-A014418, one pup (11%) showed quadriparesis and the remaining pups were neurologically normal. The scores for gait were significantly higher in AR-A014418 treated pups than in vehicle controls (P < 0.05). The average distance walked in 60s was farther in AR-A014418 treated pups compared with vehicle controls (P=0.045). Scores for the righting reflex, and hind arm movement were significantly better in AR-A014418 treated pups compared to vehicle controls (P <0.05, for all). There was no significant difference in sensory and cranial nerve assessment of the three sets of rabbit pups. Importantly, we did not observe any apparent side effect attributable to AR-A014418 treatment among pups with IVH receiving this medication.
Table 2:
Neurobehavioral evaluation of ARA- and vehicle-treated pups with IVH and controls without IVH at the postnatal day 14
| System | Test | No IVH (n=10) | IVH vehicle (n=10) | IVH, ARA (n=9) |
|---|---|---|---|---|
| Cranial Nerve | Aversive response to alcohol | 3 (3,3) | 3 (3,3) | 3 (3,3) |
| Sucking and Swallowing | 3 (3,3) | 3 (3,3) | 3 (3,3) | |
| Motor | Motor activity | |||
| Head | 3 (3,3) | 3 (2,3) | 3 (3,3) | |
| Fore Legs | 3 (3,3) | 3 (1.25,3) | 3 (3,3)* | |
| Hind Legs | 3 (3,3) | 3 (1,3)# | 3 (3,3)* | |
| Righting reflexa | 5 (5,5) | 4 (0.5,5)## | 5 (5,5)* | |
| Toneb: Forelimb | 0 (0,0) | 0 (0,0) | 0 (0,0) | |
| Toneb Hind Limb | 0 (0,0) | 0 (0,0) | 0 (0,0) | |
| Inability to hold their position at 60° Inclination for 20 s or less (latency to slip down the slope, if <20s) | 0% | 30% | 0% | |
| Gaitc | 4 (4,4) | 3 (1,4)# | 4 (3.5,4)* | |
| Distance travelled in one min (inches) | 155.5±13.6 | 89.0±15.8## | 145.5±12.7* | |
| Sensory | Facial touch | 3 (3,3) | 3 (3,3) | 3 (3,3) |
| Pain | 3 (3,3) | 3 (3,3) | 3 (3,3) |
Values are median and interquartile range. Zero is the worst response and 3 is the best response, unless otherwise noted.
P<0.05 for vehicle treated vs. ARA treated pups with IVH
P<0.05,
P<0.01for glycerol treated pups without IVH and vehicle treated pups with IVH
Score (range, 1–5): no. of times turns prone within 2 seconds when placed in supine out of 5 tries.
Score (range, 1–3): 0, no increase in tone; 1, slight increase in tone; 2, considerable increase in tone; 3, limb rigid in flexion or extension.
Gait was graded as 0 (no locomotion), 1 (crawls with trunk touching the ground for few steps and then rolls over), 2 (walks taking alternate steps, trunk low and cannot walk on inclined surface), 3 (walks taking alternate steps, cannot propel its body using synchronously the hind legs, but walks on 30° inclined surface), 4 (walks, runs, and jumps without restriction, propels the body using synchronously the back legs, but limitation in speed, balance), or 5 (normal walking).
We next compared ICV vehicle (saline) and rh-Wnt3A treated pups with IVH (n=8 each). We found 3 pups in vehicle group manifesting with abnormal motor signs—one with right lower limb weakness and others with bilateral lower leg weakness and quadriparesis. Similarly, two of the rh-Wnt3A treated pups exhibited motor signs—one with left arm and left leg weakness (hemiparesis) and the other with left leg weakness (monoparesis). There was no significant difference between ICV vehicle and rh-Wnt3A treated pups with IVH for metrics, including average distance walked in 60s, righting reflex, scores of gait and arm movement. Together, the neurological evaluation suggests that AR-A014418 treated groups showed significant neurological recovery compared to the matched controls, but not Wnt3A treated pups.
GSK-3β inhibition suppresses, while Wnt3A treatment promotes inflammation.
As GSK-3β promotes LPS induced inflammation (Huang et al., 2009), we asked whether IVH-induced cerebral inflammation and production of inflammatory mediators can be alleviated by AR-A014418 treatment. To this end, we quantified Iba1+ microglia in immunolabeled sections and performed RT-qPCR to quantify mRNA expression of proinflammatory cytokines, including IL1β, IL6, TNFα, and LIF, using TaqMan probes (Fig. 8).
Fig. 8: GSK-3β inhibition by AR-A014418 suppresses microglia infiltration and pro-inflammatory cytokines.
A) Representative immunofluorescence form corona radiata of cryosections from D3 pups stained with Iba-1 specific antibody. Insets (on right side of image) show high magnification views of the boxed area in the image. Iba1+ microglia (arrow) are more abundant in pups with IVH relative to controls without IVH and AR-A014418 treatment reduces their density. Scale bar, 50 μm. Bar charts are mean ± s.e.m. (n=5 each group). IVH elevates the density of Iba1+ microglia in both ganglionic eminences (lateral ventricular wall) and corona radiata; AR-A014418 treatment reduces their densities in both regions. B) mRNA expressions of TNFα, IL1β, IL 6, and LIF were elevated in IVH compared to controls with IVH at D3, and AR-A014418 treatment significantly reduced IL1β levels at D3. Data are mean ± s.e.m. (n=5 each group). *P<0.05, **P<0.01, ***P<0.001 pups with vs. without IVH. #P<0.05, ##P<0.01, ###P<0.001 vehicle vs. AR-A014418 treated pups with IVH.
Our analyses demonstrated that the onset of IVH significantly elevated the density of Iba1+ microglia in both periventricular ganglionic eminence and corona radiata (P=0.001 both, respectively) and more importantly, AR-A014418 treatment reduced their density at D3 (P=0.001 both, Fig. 8A). Consistent with these findings, the occurrence of IVH led to an elevation in the expression of IL1β, IL6, TNFα, and LIF mRNA at both D3 (P=0.003, 0.002, 0.001 and 0.001, respectively) and D7 (P=0.02, 0.04, 0.001, 0.001). More importantly, AR-A014418 treatment reduced the IL1 β levels at D3 (P=0.005, Fig. 8B), but not at D7 (P=0.08). AR-A014418 treatment also reduced the levels of IL6, TNFα, and LIF mRNA, however the difference was not statistically significant. The data suggest that IVH-induced inflammation was minimized by AR-A014418 treatment.
Wnt3A induces pro-inflammatory cytokine production in macrophages and microglia (Halleskog et al., 2011; Yu et al., 2014). Therefore we postulated that rh-Wnt3A treatment might worsen microglial density and cytokine production in rabbits with IVH. To this end, we compared Iba1+ microglia in immunolabeled sections between Wnt3A and vehicle treated pups with IVH at D3. We demonstrated that Iba1+ microglia was comparable between the two groups (Fig. 9A). However, TNFα, IL-1β, and IL-6 were elevated in Wnt3A treated rabbits with IVH compared to controls (P=0.45, 0.048, 0.003, Fig. 9B) but not LIF. Interestingly, IL-1β levels were reduced in Wnt3A treated rabbits with IVH relative to controls at D7 (P=0.031), but not TNFα, IL-6 or LIF. Together the data suggests that Wnt3A treatment elevates pro-inflammatory cytokines. Failure of microglia density to increase in Wnt3A treated pups with IVH could be because of an inability of Wnt3A treatment to make a perceivable difference in existing preponderance of inflammation in the pups with IVH.
Fig. 9: Wnt3A treatment does not affect microglia infiltration, but elevates pro-inflammatory cytokines.
A) Representative immunofluorescence form corona radiata of cryosections from D3 pups stained with Iba-1 specific antibody. Insets (on right side of image) show high magnification views of the boxed area in the image. Scale bar, 50 μm. Bar charts are mean ± s.e.m. (n=5 each group). rh-Wnt3A treatment does not affect the density of Iba1+ microglia in ganglionic eminences (lateral ventricular wall) and corona radiate of rabbits with IVH. B) mRNA expressions of TNFα, IL1β, IL 6, and LIF was assayed in saline and rh-Wnt3A treated pups with IVH at D3 and D7. TNFα, IL-1β, and IL-6 were higher in Wnt3A treated rabbits with IVH compared to controls. IL-1β levels were decreased in Wnt3A treated rabbits with IVH relative to controls at D7. *P<0.05, **P<0.01 pups with vs. without IVH. #P<0.05, ## P<0.01 vehicle vs. rh-Wnt3A treated pups with IVH
Together, GSK-3β inhibition by AR-A014418 treatment and Wnt3A administration has opposite effects; AR-A014418 treatment suppresses and Wnt3A administration potentiates inflammation.
GSK-3β inhibition suppresses, while Wnt3A potentiates Notch signaling.
Since GSK-3β has been implicated in regulation of Notch, sonic hedgehog and other signaling pathways (Hur and Zhou, 2010; Kim and Snider, 2011), we evaluated the effect of AR-A014418 treatment on the level of key molecules of Notch (NICD, Hes1 and Hes5) and Sonic Hedgehog (Shh and Gli1) signaling pathways.
Western blot analyses showed that IVH reduced the levels of NICD (P=0.001) at D3 and that AR-A014418 treatment further reduced the levels of NICD (P=0.045, Fig. 10A). RT-qPCR also showed reduction in mRNA expression for Hes5 at D7, but not at D3 (Fig. 10A). mRNA accumulation of Hes1 was comparable between vehicle and AR-A014418 treated pups.
Fig. 10. GSK-3β inhibition by AR-A014418 suppresses and Wnt3A treatment enhances Notch signaling.
A) Representative Western blot analyses for NICD from pups without IVH, AR-A014418-treated and vehicle treated pups with IVH. Adult rat brain was employed as positive control. Each lane represents lysate from a whole coronal slice taken at the level of mid septal nucleus of one brain. Bar chart shows mean ± s.e.m. (n=5 each group). NICD levels were reduced in AR-A014418 treated pups relative to vehicle controls. mRNA expression of Hes5 levels were reduced in AR-A014418 treated pups compared to vehicle controls at D7, but not at D3. Hes1 mRNA expression was similar between groups. B) Representative Western blot analyses for NICD from pups without IVH, Wnt3A-treated and vehicle-treated pups with IVH. Adult rat brain was employed as positive control. Each lane represents lysate from a whole coronal slice taken at the level of mid septal nucleus of one brain. Bar chart shows mean ± s.e.m. (n=5 each group). NICD levels were elevated in Wnt3A treated pups relative to vehicle controls. mRNA expression of Hes1 levels were elevated in Wnt3A treated pups compared to vehicle controls at D3, but not at D7. Hes5 mRNA expression was similar between groups. *P<0.05, **P<0.01 pups with vs. without IVH. #P<0.05, ## P<0.01 vehicle vs. rh-Wnt3A treated pups with IVH.
We next quantified NICD level between glycerol treated pups without IVH, vehicle-treated and Wnt3A treated pups with IVH using Western blot analyses. We found that NICD levels were elevated in Wnt3A treated pups compared to vehicle treated controls (P<0.001, Fig. 10B). Consistent with this finding, Hes1 levels were elevated in Wnt3A treated rabbits with IVH compared to vehicle controls at D3 (P=0.002), but not at D7. However, Hes5 mRNA levels were comparable between two groups.
We next evaluated the effect of AR-A014418 or Wnt3A treatment on Sonic Hedgehog and Gli1 level using Western blot analyses. We found that none of the two treatments affected Shh signaling (data not shown). Together, GSK-3β inhibition inhibits and Wnt3A treatment potentiates Notch signaling, consistent with the previous study (Hofmann et al., 2004); and they do not influence Shh signaling. It is plausible that inhibition of Notch by AR-A014418 may promote OPC maturation and Notch activation by rh-Wnt3A administration might suppress OPC differentiation.
Discussion
Despite major advances in the neonatal care of premature infants and striking increase in their survival, there has not been any major breakthrough in the treatment of white matter injury and associated neurological deficits in extremely preterm neonates. IVH and resultant white matter injury remains a major public health concern. In the present study, we evaluated the effect of IVH on Wnt/β-catenin signaling pathway and determined the impact of Wnt signaling activation by treatment with rh-Wnt3A or GSK-3β inhibition on myelination and neurological function. We demonstrated that the occurrence of IVH elevated transcription of GSK-3β and downregulated Wnt signaling. More importantly, GSK-3β inhibition by AR-A014418 treatment reduced apoptosis and enhanced maturation of OPCs and myelination in preterm rabbits with IVH. Conversely, intracerebroventricular rh-Wnt3A treatment failed to enhance myelination in rabbits with IVH. AR-A014418 treatment reduced inflammation, whereas rh-Wnt3A administration potentiated. IM AR-A014418 inhibited Notch signaling, but ICV rh-Wnt3A stimulated it. The study underscores the dissimilarity between the two approaches of activating Wnt signaling and highlights a viable option to enhance myelination in premature infants with IVH.
The most important and novel observation in this study was that GSK-3β inhibition enhanced proliferation and maturation of OPCs and myelination in preterm rabbits with IVH. To our knowledge, this is the first report that GSK-3β inhibition increased survival and differentiation of OPCs, and enhanced myelination of the white matter in an animal model of IVH. Consistent with our studies, GSK-3β inhibition by lithium or AR-A014418 enhances proliferation and maturation of OPCs as well as myelination in P8–11 rats (Azim and Butt, 2011). Moreover, GSK-3β inhibition restores myelination in models of demyelination, including experimental autoimmune encephalitis and lysolecithin-induced demyelination (Azim and Butt, 2011; De Sarno et al., 2008). GSK-3β suppression also has offered neuroprotection in neonatal and adult model hypoxia-ischemia, Alzheimer’s and Parkinson’s disease (Chen et al., 2004; Huang et al., 2017; Ren et al., 2016; Wang et al., 2007). Indeed, a number of GSK-3β inhibitors have reached clinical trial for neurological disorders, psychiatric conditions, diabetes and cancer. For example, Tideglusib has undergone randomized clinical trial for Alzheimer’s disease and supranuclear palsy, LY2090314 for pancreatic cancer, and lithium for bipolar disorders (del Ser et al., 2013; Hampel et al., 2009; Tolosa et al., 2014; Zamek-Gliszczynski et al., 2013). Together, all these studies reinforce the concept that GSK-3β inhibition confers neuroprotection and promotes myelination and neurologic recovery. Hence, we speculate that GSK-3β inhibition might improve the neurological outcome of premature infants.
In our studies, rh-Wnt3A treatment influenced neither myelination nor neurologic recovery. Wnt/β-catenin signaling exhibits context-dependent effect on maturation of OPCs and myelination. For example, inhibition of Wnt signaling (Langseth et al., 2010; Shimizu et al., 2005), inactivating Wnt signaling in knock –out mouse experiments, or stabilizing Axin-2 to inhibit Wnt pathway increases production of OPCs and enhance myelination (Fancy et al., 2011; Feigenson et al., 2009). In conflict to these studies, activation of Wnt signaling by GSK-3β inhibition promotes OPC differentiation (Azim and Butt, 2011; Azim et al., 2014), overexpression of the dominant-negative form of TCF4 reduces PDGFRα+ and Olig2+ cells (Ortega et al., 2013); and Wnt/catenin pathway drives myelin gene expression (Tawk et al., 2011). The present study showed a lack of effect of Wnt-3A treatment on myelination in our animal model of IVH-induced hypomyelination, despite reduced Wnt signaling in these animals. This suggests that downregulation of Wnt signaling might not be the principal cause of myelination failure in IVH. Moreover, the premature animals and humans with IVH exhibit a unique paradigm of white matter injury, and thus the effect of these interventions in our model cannot be compared with the effects in other disease model.
Alteration in Wnt/β-catenin signaling has been evaluated in a number of neurological disorders. Canonical Wnt/β-catenin pathway is downregulated in hypoxia-ischemia and Alzheimer’s disease (Diaz et al., 2016), while it is activated in amyotrophic lateral sclerosis, Parkinson’s disease, Huntington’s disease, multiple sclerosis, and Friedreich’s ataxia (Vallee and Lecarpentier, 2016). In our studies, we found that IVH reduces the expression of total and activated β-catenin, TCF4, and Axin-2, which reflects downregulation of Wnt signaling in pups with IVH. We also found that GSK-3β mRNA expression is elevated in pups with IVH, which might be contributing to Wnt signaling inhibition. Since a large number of molecules, including Wnt, frizzled, LRP, axin, APC and others, affect Wnt signaling and as we are uncertain about the changes in expression of these molecules with the occurrence of IVH, it is difficult to point out the mechanism underlying the downregulation in Wnt signaling in newborns with IVH. Together, IVH downregulates Wnt/β-catenin signaling, just as in hypoxia-ischemia and Alzheimer’s disease; and an elevation in GSK-3β might be contributing to Wnt inhibition in rabbits with IVH.
We demonstrated that GSK-3β inhibition by AR-A014418 treatment reduced inflammation in our model of IVH. Specifically, we showed that AR-A014418 treatment diminished microglial density and IL1β cytokine levels in rabbits with IVH compared to vehicle controls. As inflammation is an important mediator of brain injury in IVH and since inhibition of inflammation promotes myelination in our model (Vinukonda et al., 2010), it is plausible that AR-A014418 treatment enhanced OPC maturation and myelination in rabbits with IVH by suppressing inflammation. Consistent with our study, several lines of evidence have shown that GSK-3β is an important target of inflammation-mediated diseases and plays a key role in facilitating inflammatory responses (Beurel, 2011). Moreover, in culture experiments, inhibitors of GSK-3β attenuate microglia responses to inflammatory stimuli (Yuskaitis and Jope, 2009). GSK-3β inhibition alleviates inflammation by regulating NFκB and cyclic AMP response element binding protein (CREB) signaling pathways (Beurel, 2011; Martin et al., 2005). In contrast to the anti-inflammatory effects of GSK3β, we observed that Wnt3A treatment elevated pro-inflammatory cytokine levels in rabbits with IVH without increasing microglial density in rabbits with IVH. Since IVH induces microglial infiltration and elevates pro-inflammatory cytokines levels, Wnt3A administration might have potentiated cytokine levels without causing perceptible increase in microglial density (Fig. 11). Consistent with these findings, previous studies have reported that Wnt3A treatment stimulates microglia and macrophages to produce pro-inflammatory cytokines (Halleskog et al., 2011; Yu et al., 2014). Together, these findings reinforces the notion that anti-inflammatory accomplishment of GSK-3β inhibition might be promoting OPC maturation and myelination, and that pro-inflammatory action of Wnt3A administration would be blocking the process of myelination.
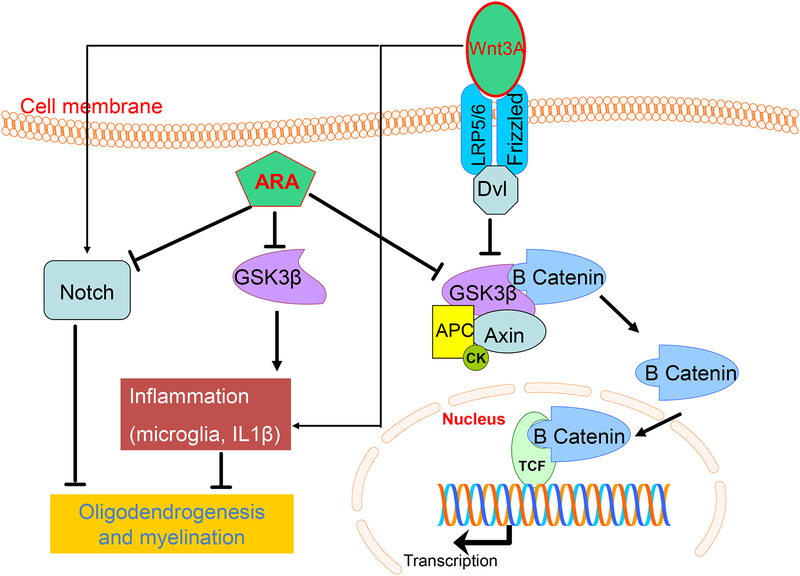
Fig.11: GSK3β inhibition promotes myelination by inhibiting inflammation and Notch signaling:
The schematic shows that both Wnt3A and GSK3β inhibition activates Wnt signaling leading to an elevation in the cytoplasmic β-catenin and its translocation in the nucleus. ARA treatment (GSK3β inhibition) suppresses inflammation by reducing microglia infiltration and IL1β levels, whereas Wnt3A treatment promotes inflammation by elevating TNFα and IL-1β levels. In addition, GSK3β inhibition downregulates notch signaling, while Wnt3a activates Notch signaling. Hence, ARA treatment promotes myelination by Notch inhibition and attenuating inflammation.
GSK-3β is an important regulator of numerous signaling pathways, including Wnt, Notch, Shh and others. Wnt signaling also interacts with Notch and Shh signaling pathways. Since Notch and Shh are key controller of oligodendrogenesis and myelination, we evaluated the effect of GSK-3β inhibition or Wnt3A administration on Notch and Shh signaling pathways. We demonstrated that AR-A014418 treatment inhibited Notch signaling, whereas Wnt3A treatment showed synergy with Notch pathway (Fig. 11). In agreement with our findings, AR-A014418 treatment downregulated NICD on optic nerves in tissue culture experiments (Azim and Butt, 2011). Moreover, GSK-3β positively regulates Notch in vascular smooth muscle cells in vitro (Guha et al., 2011), and Wnt signaling directly and synergistically regulate Dll1 transcription and Notch activity in presomatic mesoderm of mouse embryo (Hofmann et al., 2004). Importantly, Notch signaling inhibits oligodendrogenesis by inducing Hes5, which directly represses Sox10 (Kondo and Raff, 2000; Park and Appel, 2003). In addition, it promotes astrocytogenesis by diverting progenitors toward astrocyte lineage (Park and Appel, 2003). Hence, it is plausible that AR-A014418-induced Notch downregulation contributed to OPC maturation and myelination in rabbits with IVH, and that Wnt3A-activated Notch signaling inhibited myelination.
In conclusion, the present study discovered that GSK-3β expression is elevated and Wnt signaling is downregulated in rabbits with IVH. More importantly, GSK-3β inhibition enhanced OPC maturation, myelination and neurological recovery, but Wnt-3A treatment did not. In addition, GSK-3β inhibition reduced inflammation and Notch signaling, which might have contributed to OPC maturation and clinical recovery. Since, GSK-3β inhibitors have undergone clinical trials for wide range of diseases (Mangialasche et al., 2010; Palomo et al., 2017), GSK-3β inhibition could be a viable alternative to treat premature infants will IVH to promote myelination and clinical recovery.
Highlights:
IVH reduces activated β-catenin, TCF-4, and Axin-2 levels in preterm newborns
Both GSK3 β inhibition and Wnt-3A treatment activates Wnt signaling
GSK3β inhibition accelerates myelination, and clinical recovery in preterm rabbits with IVH, but rh-Wnt3A does not
GSK3β inhibition attenuates, but rh-Wnt3A accentuates inflammation and Notch signaling.
Acknowledgments:
Study was supported by NIH/NINDS grant # 1R01NS083947–01 (PB).
References
- Azim K, et al. , 2017. Pharmacogenomic identification of small molecules for lineage specific manipulation of subventricular zone germinal activity. PLoS Biol. 15, e2000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim K, Butt AM, 2011. GSK3beta negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia. 59, 540–53. [DOI] [PubMed] [Google Scholar]
- Azim K, et al. , 2014. GSK3beta regulates oligodendrogenesis in the dorsal microdomain of the subventricular zone via Wnt-beta-catenin signaling. Glia. 62, 778–9. [DOI] [PubMed] [Google Scholar]
- Ballabh P, 2010. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 67, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh P, et al. , 2007. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med. 13, 477–85. [DOI] [PubMed] [Google Scholar]
- Beurel E, 2011. Regulation by glycogen synthase kinase-3 of inflammation and T cells in CNS diseases. Front Mol Neurosci. 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystron I, et al. , 2008. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 9, 110–22. [DOI] [PubMed] [Google Scholar]
- Chen G, et al. , 2004. Glycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxydopamine-induced neuronal death. FASEB J. 18, 1162–4. [DOI] [PubMed] [Google Scholar]
- Chua CO, et al. , 2009. Consequences of intraventricular hemorrhage in a rabbit pup model. Stroke. 40, 3369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Goedert M, 2004. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 3, 479–87. [DOI] [PubMed] [Google Scholar]
- De Sarno P, et al. , 2008. Lithium prevents and ameliorates experimental autoimmune encephalomyelitis. J Immunol. 181, 338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Ser T, et al. , 2013. Treatment of Alzheimer’s disease with the GSK-3 inhibitor tideglusib: a pilot study. J Alzheimers Dis. 33, 205–15. [DOI] [PubMed] [Google Scholar]
- Diaz R, et al. , 2016. Environmental enrichment attenuates the blood brain barrier dysfunction induced by the neonatal hypoxia-ischemia. Int J Dev Neurosci. 53, 35–45. [DOI] [PubMed] [Google Scholar]
- Dummula K, et al. , 2011. Bone morphogenetic protein inhibition promotes neurological recovery after intraventricular hemorrhage. J Neurosci. 31, 12068–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, et al. , 2011. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci. 14, 1009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, et al. , 2004. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol Cell Neurosci. 27, 247–54. [DOI] [PubMed] [Google Scholar]
- Fang X, et al. , 2000. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci U S A. 97, 11960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenson K, et al. , 2009. Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol Cell Neurosci. 42, 255–65. [DOI] [PubMed] [Google Scholar]
- Georgiadis P, et al. , 2008. Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke. 39, 3378–88. [DOI] [PubMed] [Google Scholar]
- Guha S, et al. , 2011. Glycogen synthase kinase 3 beta positively regulates Notch signaling in vascular smooth muscle cells: role in cell proliferation and survival. Basic Res Cardiol. 106, 773–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, et al. , 2015. Canonical Wnt signaling in the oligodendroglial lineage--puzzles remain. Glia. 63, 1671–93. [DOI] [PubMed] [Google Scholar]
- Halleskog C, et al. , 2011. WNT signaling in activated microglia is proinflammatory. Glia. 59, 119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, et al. , 2009. Lithium trial in Alzheimer’s disease: a randomized, single-blind, placebo-controlled, multicenter 10-week study. J Clin Psychiatry. 70, 922–31. [PubMed] [Google Scholar]
- Hofmann M, et al. , 2004. WNT signaling, in synergy with T/TBX6, controls Notch signaling by regulating Dll1 expression in the presomitic mesoderm of mouse embryos. Genes Dev. 18, 2712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, et al. , 2017. GSK-3beta inhibitor TDZD-8 reduces neonatal hypoxic-ischemic brain injury in mice. CNS Neurosci Ther. 23, 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, et al. , 2009. Glycogen synthase kinase-3 negatively regulates anti-inflammatory interleukin-10 for lipopolysaccharide-induced iNOS/NO biosynthesis and RANTES production in microglial cells. Immunology. 128, e275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur EM, Zhou FQ, 2010. GSK3 signalling in neural development. Nat Rev Neurosci. 11, 539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS, et al. , 2007. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res. 32, 577–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichev M, Dawson LA, 2011. Evidence for antimanic efficacy of glycogen synthase kinase-3 (GSK3) inhibitors in a strain-specific model of acute mania. Int J Neuropsychopharmacol. 14, 1051–67. [DOI] [PubMed] [Google Scholar]
- Kim WY, Snider WD, 2011. Functions of GSK-3 Signaling in Development of the Nervous System. Front Mol Neurosci. 4, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kockeritz L, et al. , 2006. Glycogen synthase kinase-3--an overview of an over-achieving protein kinase. Curr Drug Targets. 7, 1377–88. [DOI] [PubMed] [Google Scholar]
- Kondo T, Raff M, 2000. Basic helix-loop-helix proteins and the timing of oligodendrocyte differentiation. Development. 127, 2989–98. [DOI] [PubMed] [Google Scholar]
- Labombarda F, et al. , 2009. Effects of progesterone on oligodendrocyte progenitors, oligodendrocyte transcription factors, and myelin proteins following spinal cord injury. Glia. 57, 884–97. [DOI] [PubMed] [Google Scholar]
- Lang J, et al. , 2013. Adenomatous polyposis coli regulates oligodendroglial development. J Neurosci. 33, 3113–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langseth AJ, et al. , 2010. Wnts influence the timing and efficiency of oligodendrocyte precursor cell generation in the telencephalon. J Neurosci. 30, 13367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, et al. , 2016. Wnt3a upregulates brain-derived insulin by increasing NeuroD1 via Wnt/beta-catenin signaling in the hypothalamus. Mol Brain. 9, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon KL, et al. , 2006. Olig gene function in CNS development and disease. Glia. 54, 1–10. [DOI] [PubMed] [Google Scholar]
- Mangialasche F, et al. , 2010. Alzheimer’s disease: clinical trials and drug development. Lancet Neurol. 9, 702–16. [DOI] [PubMed] [Google Scholar]
- Martin M, et al. , 2005. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 6, 777–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, et al. , 2004. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 5, 691–701. [DOI] [PubMed] [Google Scholar]
- Mouton PR, et al. , 2009. Caloric restriction attenuates amyloid deposition in middle-aged dtg APP/PS1 mice. Neurosci Lett. 464, 184–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega F, et al. , 2013. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat Cell Biol. 15, 602–13. [DOI] [PubMed] [Google Scholar]
- Palomo V, et al. , 2017. Subtly Modulating Glycogen Synthase Kinase 3 beta: Allosteric Inhibitor Development and Their Potential for the Treatment of Chronic Diseases. J Med Chem. 60, 4983–5001. [DOI] [PubMed] [Google Scholar]
- Park HC, Appel B, 2003. Delta-Notch signaling regulates oligodendrocyte specification. Development. 130, 3747–55. [DOI] [PubMed] [Google Scholar]
- Ren ZX, et al. , 2016. Dihydromyricetin protects neurons in an MPTP-induced model of Parkinson’s disease by suppressing glycogen synthase kinase-3 beta activity. Acta Pharmacol Sin. 37, 1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, et al. , 2007. Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer’s disease are associated with reduced amyloid precursor protein phosphorylation. J Neurosci. 27, 1981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno L, et al. , 2009. A novel GSK-3beta inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol Dis. 35, 359–67. [DOI] [PubMed] [Google Scholar]
- Shimizu T, et al. , 2005. Wnt signaling controls the timing of oligodendrocyte development in the spinal cord. Dev Biol. 282, 397–410. [DOI] [PubMed] [Google Scholar]
- Tawk M, et al. , 2011. Wnt/beta-catenin signaling is an essential and direct driver of myelin gene expression and myelinogenesis. J Neurosci. 31, 3729–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thotala DK, et al. , 2012. Glycogen synthase kinase 3beta inhibitors protect hippocampal neurons from radiation-induced apoptosis by regulating MDM2-p53 pathway. Cell Death Differ. 19, 387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolosa E, et al. , 2014. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov Disord. 29, 470–8. [DOI] [PubMed] [Google Scholar]
- Vallee A, Lecarpentier Y, 2016. Alzheimer Disease: Crosstalk between the Canonical Wnt/Beta-Catenin Pathway and PPARs Alpha and Gamma. Front Neurosci. 10, 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinukonda G, et al. , 2010. Neuroprotection in a rabbit model of intraventricular haemorrhage by cyclooxygenase-2, prostanoid receptor-1 or tumour necrosis factor-alpha inhibition. Brain. 133, 2264–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinukonda G, et al. , 2016. Epidermal growth factor preserves myelin and promotes astrogliosis after intraventricular hemorrhage. Glia. 64, 1987–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose LR, et al. , 2013. Treatment with thyroxine restores myelination and clinical recovery after intraventricular hemorrhage. J Neurosci. 33, 17232–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, et al. , 2007. Inhibition of glycogen synthase kinase-3beta protects dopaminergic neurons from MPTP toxicity. Neuropharmacology. 52, 1678–84. [DOI] [PubMed] [Google Scholar]
- Ye F, et al. , 2009. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 12, 829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeste-Velasco M, et al. , 2008. GSK-3 beta inhibition and prevention of mitochondrial apoptosis inducing factor release are not involved in the antioxidant properties of SB-415286. Eur J Pharmacol. 588, 239–43. [DOI] [PubMed] [Google Scholar]
- Yu CH, et al. , 2014. Recombinant Wnt3a and Wnt5a elicit macrophage cytokine production and tolerization to microbial stimulation via Toll-like receptor 4. Eur J Immunol. 44, 1480–90. [DOI] [PubMed] [Google Scholar]
- Yuskaitis CJ, Jope RS, 2009. Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity. Cell Signal. 21, 264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, et al. , 2013. Pharmacokinetics, metabolism, and excretion of the glycogen synthase kinase-3 inhibitor LY2090314 in rats, dogs, and humans: a case study in rapid clearance by extensive metabolism with low circulating metabolite exposure. Drug Metab Dispos. 41, 714–26. [DOI] [PubMed] [Google Scholar]