Abstract
Purpose of review:
To provide an update on medication development efforts for alcohol use disorder (AUD) by reviewing recently published (past two years) human studies that evaluated medications’ effects on alcohol-related outcomes.
Recent findings:
Forty-five publications were found suitable for this review. A variety of compounds have been tested in the past two years as potential pharmacological options for AUD, including medications that act on multiple targets (topiramate, aripiprazole, quetiapine), calcium channels (gabapentin), GABA receptors (baclofen, diazepam), glutamate receptors (ifenprodil, memantine, glycine), nicotinic acetylcholine receptors (varenicline, mecamylamine), α1 adrenergic receptors (prazosin, doxazosin), neuroendocrine pathways (oxytocin, a vasopressin receptor 1b antagonist, a ghrelin receptor inverse agonist), and others (samidorphan, ibudilast, N-acetylcysteine, citoline). Important findings and limitations regarding the effects of these medications on alcohol-related outcomes are discussed.
Summary:
There is a critical need to increase the armamentarium of medications for AUD. Human laboratory studies may help screen and prioritize promising targets and compounds before running large clinical trials. Given the complexity of AUD and the heterogeneity of afflicted patients, future studies should also investigate potential moderators and predictors of response to each pharmacological intervention.
Keywords: Alcohol use disorder, addiction, medication, pharmacotherapy
1. Introduction
Alcohol represents a leading risk factor for premature death and disability, and alcohol use disorder (AUD) affects ~283 million people worldwide [1, 2]. In addition to psychosocial interventions that play an important role in AUD treatment, four medications have been approved by the United States Food and Drug Administration and/or the European Medicines Agency: disulfiram, acamprosate, naltrexone, and nalmefene (the latter is only approved in Europe). While meta-analyses show that currently approved medications are effective [3], there is a critical need to increase the armamentarium of pharmacotherapies for AUD. Neuroscientific advances have led to identification of novel therapeutic targets and development of new medications in this regard [4]. The goal of this paper is to provide an update on medication development efforts for AUD by reviewing human studies published in the past two years.
2. Methods
2.1. Literature search
We searched MEDLINE, Embase, PsycINFO, and Cochrane Library for human studies published in English from January 2017 to January 2019. Keywords and controlled vocabulary terms relevant to the concept of this review were used. The search strategy was developed, and literature search was performed by, an Informationist at the National Institutes of Health (NIH) Library, in consultation with the authors (see Appendix 1). EndNote X9 was used to collect, de-duplicate, and manage the records.
2.2. Study selection
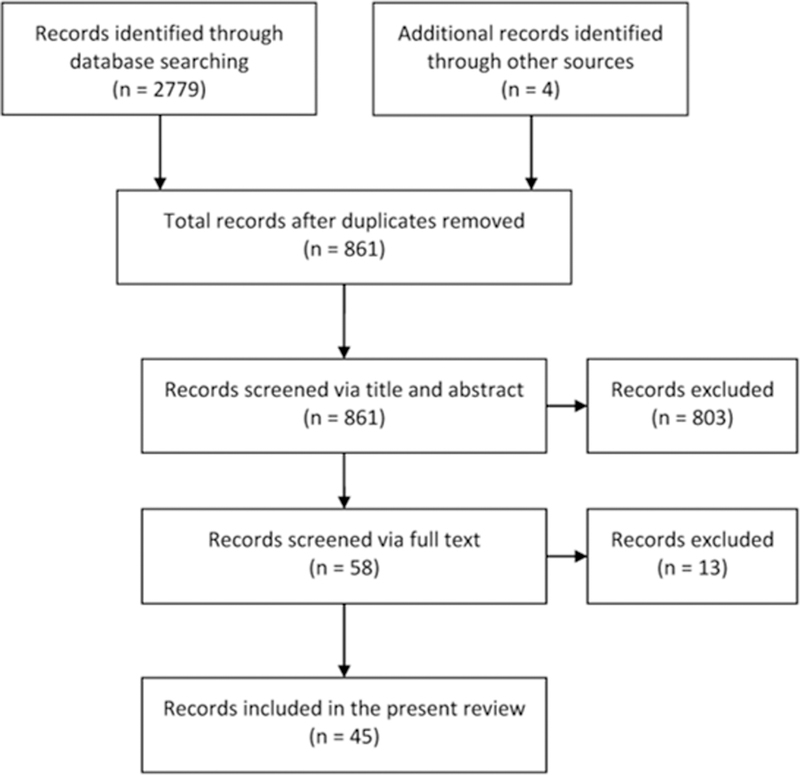
Original research studies that evaluated the effects of a medication or compound on alcohol-related outcomes (e.g., drinking, craving, subjective response, brain activity) in humans were included. Preclinical studies, review articles, single case reports, and conference abstracts were excluded. We also excluded studies that were exclusively focused on the four approved medications for AUD (disulfiram, acamprosate, naltrexone, and nalmefene), alcohol-related diseases (gastritis, liver problems, etc.), and/or non-pharmacological modalities (brain stimulation, cognitive behavior therapy, etc.). After removal of duplicates, the first authors (MF and BDB) independently screened the titles/abstracts and filtered the records according to the aforementioned eligibility criteria. The two resulting lists were compared, and discrepancies were discussed. In case of disagreement, the senior author (LL) was consulted to reach consensus. Following this initial screen, the full texts of potentially eligible records were retrieved. These papers were evaluated, using an identical process as the title/abstract stage, for final inclusion in this review (Figure 1).
Figure 1.
Flow diagram of the records identified, screened, and included in this review
3. Results
Forty-five records were found suitable for this review and are outlined in Table 1; selected studies are discussed below.
Table 1.
List and summary of records found suitable for this review
| Medications | References |
|---|---|
| Topiramate | |
| Treatment with topiramate (up to 200 mg/day) was not superior to placebo in preventing alcohol relapse or helping smoking cessation in alcohol-dependent, nicotine-smoking male individuals. | [5] |
| Treatment with topiramate (up to 200 mg/day), compared to placebo, reduced alcohol drinking in a naturalistic setting but not in a laboratory experiment, suppressed alcohol craving, and increased alcohol-induced stimulation in non-treatment-seeking, alcohol-dependent individuals. | [6] |
| Aripiprazole | |
| Treatment with aripiprazole (up to 15 mg/day) was not superior to placebo in reducing alcohol drinking or craving in non-treatment-seeking, alcohol-dependent individuals. | [6] |
| Treatment with aripiprazole (15 mg/day), compared to placebo, reduced alcohol drinking in alcohol-dependent individuals with low self-control and prolonged the latency to drink alcohol in non-treatment-seeking, alcohol-dependent individuals with high impulsivity. | [7] |
| Variation of dopamine-related genes moderated the effect of aripiprazole treatment (15 mg/day), compared to placebo, on alcohol drinking and on cue-elicited brain activation (fMRI) in ventral striatum in non-treatment-seeking, alcohol-dependent individuals. | [8] |
| Quetiapine | |
| Treatment with quetiapine extended-release (up to 300 mg/day), compared to placebo, resulted in trend-level, but not significant, reduction of alcohol drinking in highly depressed and anxious patients with bipolar I or II disorder. | [9] |
| Gabapentin | |
| Treatment with gabapentin immediate-release (300–1,200 mg/day), compared to placebo, reduced the percentage of heavy drinking days, but did not affect overall amount of alcohol consumption in alcohol-dependent individuals. | [10] |
| Treatment with gabapentin enacarbil extended-release (1,200 mg/day) was not superior to placebo in reducing alcohol drinking or craving in patients with AUD. Higher exposure to gabapentin (based on population pharmacokinetic analysis) was associated with less alcohol consumption. | [11] |
| Receipt of gabapentin (≥ 1,500 mg/day) for any indication, compared to a propensity score matched group not receiving gabapentin, was associated with decreased AUDIT-C scores in patients with AUD. | [12] |
| Baclofen | |
| Treatment with baclofen (30 mg/day) was not superior to placebo in reducing alcohol drinking or craving in alcohol-dependent veterans with chronic HCV. | [14] |
| Treatment with baclofen (30 mg/day), compared to benfothiamine, increased abstinent days and reduced heavy drinking days and craving, but did not affect time to first relapse in alcohol-dependent individuals. | [44] |
| Treatment with baclofen (30 mg/day, open-label) reduced alcohol consumption and severity of alcohol dependence in alcohol-dependent individuals with alcohol-related liver problems. | [45] |
| Treatment with baclofen (50 mg/day) was not superior to placebo in reducing alcohol drinking or craving in alcohol-dependent individuals. | [15] |
| Treatment with baclofen (180 mg/day), compared to placebo, reduced alcohol craving, but did not improve abstinence in alcohol-dependent individuals. Baclofen, compared to placebo, resulted in a trend-level, but not statistically significant, reduction of alcohol consumption in a subgroup of participants with very high drinking levels at baseline. | [16] |
| Treatment with high-dose baclofen (> 150 mg/day), compared with medium-dose baclofen (90–150 mg/day), improved alcohol drinking outcomes. Treatment with medium-dose baclofen (90–150 mg/day), compared with low-dose baclofen (< 90 mg/day), resulted in less favorable drinking outcomes. No differences between high-dose and low-dose baclofen were found. | [19] |
| Treatment with baclofen (30 or 75 mg/day), compared to placebo, prolonged the time to first lapse and relapse in alcohol-dependent individuals with alcoholic liver disease. No differences were found between the two baclofen doses. | [21] |
| Treatment with baclofen (30 or 75 mg/day), compared to placebo, increased parietal concentrations of glutathione and N-acetylaspartate in recently drinking alcohol-dependent individuals. Higher glutathione predicted lower heavy drinking days at follow-up. | [46] |
| A single nucleotide polymorphism on GABBR1 (rs29220) moderated the clinical response to baclofen treatment (30 or 75 mg/day) in alcohol-dependent individuals. | [47] |
| Treatment with baclofen (75 mg/day), compared to placebo, attenuated alcohol cue-elicited brain activation (fMRI) in prefrontal regions in alcohol-dependent individuals. | [48] |
| Treatment with baclofen (up to 60 mg/day), compared to placebo, enhanced alcohol cue-elicited brain activation (fMRI) in DLPFC and ACC and reduced the IC activation in alcohol-dependent individuals. ACC activation and IC deactivation were associated with longer time to first lapse in baclofen-treated individuals. | [49] |
| Treatment with baclofen (30–270 mg/day), compared to placebo, attenuated alcohol cue-elicited brain activation (fMRI) in OFC, amygdala, and VTA, as well as alcohol cue-modulated functional connectivity (fMRI) between VTA and both ACC and MPFC. | [50] |
| Treatment with baclofen (30 mg/day), compared to placebo, increased subjective response to alcohol and decoupled the link between post-priming breath alcohol concentration and the amount of alcohol self-administration in non-treatment-seeking anxious alcohol-dependent individuals. | [22] |
| Treatment with baclofen (30 mg/day), compared to placebo, decoupled the link between post-priming variables (craving, sedation) and the amount of alcohol self-administration in non-treatment-seeking, anxious, alcohol-dependent individuals. Maximum concentration of baclofen in the blood negatively correlated with cue-induced alcohol craving and alcohol-induced rating of ‘like more’. | [24] |
| Single-dose administration of baclofen in healthy controls (0, 10, 60 mg), compared to abstinent alcohol-dependent individuals (0, 60, 90 mg), increased subjective response to alcohol, blood GH concentrations, and EEG theta power. No pharmacokinetic differences were found between alcohol-dependent individuals and healthy controls. | [23] |
| Pharmacokinetic parameters were characterized in 143 alcohol-dependent individuals treated with baclofen (up to 250 mg/day, open-label). A linear pharmacokinetic profile and high inter-individual variability was observed. | [26] |
| Pharmacokinetic parameters were characterized in 57 alcohol-dependent individuals treated with baclofen (up to 300 mg/day, open-label). A linear pharmacokinetic profile and high inter-individual variability was observed. | [27] |
| Diazepam | |
| Treatment with diazepam (up to 40 mg/day, open label) for 30 days, compared to 10 days, reduced alcohol drinking, craving, and anxiety in alcohol-dependent individuals at 3 months follow-up. NOTE: It is critical to keep in mind the abuse liability of diazepam and benzodiazepines in general. While benzodiazepines represent the gold standard treatment of alcohol withdrawal syndrome, there is no evidence that benzodiazepines may be effective for treating AUD. The lack of efficacy combined with the significant risk of abuse does not support any efforts to use or test this class of medications for AUD. There is also a significant risk of serious benzodiazepine-alcohol interactions in patients who take benzodiazepines and may relapse - another concern that should discourage any effort to consider benzodiazepines as a treatment for AUD. |
[51] |
| Ifenprodil | |
| Treatment with ifenprodil (60 mg/day), compared to placebo, reduced alcohol drinking in alcohol-dependent individuals. | [28] |
| Memantine | |
| Add-on treatment with memantine (5 mg/day, open label) reduced alcohol drinking in patients with bipolar II disorder and comorbid alcohol dependence who were being treated with valproic acid. | [52] |
| Glycine | |
| Add-on treatment with glycine (0.8 g/kg/daya) was not superior to placebo in reducing alcohol drinking or craving in patients with schizophrenia and comorbid alcohol dependence who were being treated with antipsychotics. | [29] |
| Varenicline | |
| Treatment with varenicline (2 mg/day), compared to placebo, reduced alcohol drinking in alcohol-dependent, nicotine-smoking individuals. | [30] |
| Treatment with varenicline (2 mg/day), compared to placebo, reduced alcohol drinking in alcohol-dependent, nicotine-smoking male individuals. | [31] |
| Treatment with varenicline (2 mg/day), compared to placebo, reduced cue-elicited alcohol craving in heavy-drinking individuals. | [32] |
| Treatment with varenicline (1 mg/day, 2 mg/day), compared to placebo, dose-dependently reduced alcohol drinking and tonic craving in depressed, heavy-drinking, alcohol-dependent individuals. | [33] |
| Treatment with varenicline (1 mg/day, 2 mg/day), compared to placebo, dose-dependently improved cognitive performance in heavy-drinking, alcohol-dependent individuals. This improvement was associated with less alcohol drinking among those treated with 2 mg/day. | [34] |
| Mecamylamine | |
| Treatment with mecamylamine (10 mg/day) was not superior to placebo in reducing alcohol drinking or craving in heavy-drinking, alcohol-dependent individuals. | [35] |
| Prazosin | |
| Treatment with prazosin (16 mg/day), compared to placebo, reduced alcohol drinking in heavy-drinking, alcohol-dependent individuals. | [36] |
| Treatment with prazosin (16 mg/day), compared to placebo, reduced alcohol drinking in heavy-drinking, alcohol-dependent individuals with optimal treatment exposure. Diastolic blood pressure was a significant moderator of prazosin’s effect. | [37] |
| Doxazosin | |
| Diastolic blood pressure was a significant moderator of response to treatment with doxazosin (up to 16 mg/day), compared to placebo, in reducing alcohol drinking in heavy-drinking, alcohol-dependent individuals. | [38] |
| Oxytocin | |
| Treatment with intranasal oxytocin (24 IU, single dosea), compared to placebo, reduced alcohol cue-elicited brain activity in insular cortex, hippocampal/parahippocampal formation, cingulate cortex, inferior/medial frontal gyrus, and visual/motor regions in social drinking males. | [39] |
| ABT-436 (Vasopressin Receptor 1b Antagonist) | |
| Treatment with ABT-436 (up to 800 mg/day), compared to placebo, increased abstinent days in heavy-drinking, alcohol-dependent individuals. | [40] |
| PF-5190457 (Ghrelin Receptor Inverse Agonist) | |
| Compared to placebo, PF-5190457 combined with alcohol was safe and well-tolerated, with no drug-alcohol interactions. Treatment with PF-5190457 (200 mg/day), compared to placebo, reduced alcohol craving and attention to alcohol in heavy-drinking individuals. | [43] |
| Samidorphan | |
| Treatment with samidorphan (1, 2.5, or 10 mg/day), compared to placebo, dose-dependently reduced alcohol drinking in heavy-drinking, alcohol-dependent individuals. Furthermore, the highest dose of samidorphan (10 mg/day), compared to placebo, reduced alcohol craving. | [53] |
| Ibudilast | |
| Treatment with ibudilast (100 mg/day), compared to placebo, reduced tonic craving for alcohol and improved mood (post cue- and stress-exposure) in nontreatment-seeking individuals with AUD. Baseline ratings of depression moderated the effect of ibudilast on subjective response to alcohol. | [54] |
| N-acetylcysteine | |
| Treatment with N-acetylcysteine (2,400 mg/day), compared to placebo, decreased alcohol drinking in cannabis-dependent individuals. | [55] |
| Citicoline | |
| Treatment with citicoline (up to 2,000 mg/day) was not superior to placebo in reducing alcohol drinking or craving in heavy-drinking, alcohol-dependent individuals | [56] |
Dosage was confirmed through communication with the authors.
Abbreviations: ACC, anterior cingulate cortex; AUD, alcohol use disorder; AUDIT-C, alcohol use disorders identification test-consumption; DLPFC, dorsolateral prefrontal cortex; EEG, electroencephalogram; fMRI, functional magnetic resonance imaging; GABBR1, GABA-B receptor subunit 1 gene; GH, growth hormone; HCV, hepatitis C virus; MPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; IC, insular cortex; VTA, ventral tegmental area.
3.1. Medications acting on multiple targets: Topiramate, Aripiprazole, Quetiapine
Topiramate is a drug that reduces neuronal excitability by inhibiting glutamatergic neurotransmission and enhancing GABAergic neurotransmission, among other mechanisms of action. Based on previous data suggesting that topiramate may reduce both alcohol drinking and nicotine smoking, Anthenelli and colleagues (2017) conducted a 12-week randomized controlled trial (RCT) with topiramate (up to 200 mg/day) in 129 alcohol-dependent (but abstinent) nicotine-smoking males who were motivated to remain abstinent from alcohol and quit smoking. Smoking cessation was the primary efficacy endpoint. Participants were exclusively males, the majority of whom were veterans with high percentage of other comorbid substance use disorders. At baseline, the Fagerstrom test for nicotine dependence (FTND) score was significantly higher in the placebo than topiramate group and, therefore, was entered as a covariate in the analyses. In this study, topiramate was not superior to placebo in terms of alcohol relapse prevention or nicotine smoking cessation [5]. A placebo-controlled human laboratory study investigated the effects of topiramate (up to 200 mg/day) and aripiprazole (up to 15 mg/day), alone or combined, on alcohol-related outcomes in 90 non-abstinent, non-treatment-seeking, heavy-drinking, alcohol-dependent individuals. An experimental session was conducted following a 5-week outpatient dosing (naturalistic phase). The two primary outcomes of the study were drinking during the week before the laboratory session and drinks self-administered during the laboratory session. While no significant effects were found on alcohol self-administration in the laboratory, topiramate, both alone and combined with aripiprazole, reduced alcohol drinking during the naturalistic phase. Topiramate also reduced craving for alcohol and increased alcohol-induced stimulation. Of note, participants randomized to topiramate had higher alcohol dependence scale (ADS) scores at baseline, which was entered as a covariate in the analyses. No significant effects were found for aripiprazole in this study [6]. Anton and colleagues (2017) found that 1-week treatment with aripiprazole (15 mg/day), compared to placebo, suppressed the amount of alcohol self-administration in a bar laboratory experiment. Non-treatment-seeking alcohol-dependent individuals were stratified by impulsivity scores based on Barratt impulsiveness scale (BIS). Within each BIS category (high: ≥ 68, low: ≤ 67), participants were further stratified by gender and smoking status. By chance, aripiprazole-treated individuals had higher baseline drinking levels than the placebo group, which was accounted for in the analyses as a covariate. Interestingly, individuals with low self-control and high impulsivity respectively showed a more favorable response to aripiprazole in terms of the amount of alcohol consumption and the latency to drink alcohol [7]. Another report from this study found that variation of dopamine-related genes moderated the effect of aripiprazole on alcohol self-administration in the bar laboratory, as well as the effect of aripiprazole on cue-elicited brain activation in ventral striatum. Of note, these pharmacogenetic effects were not moderated by participants’ impulsivity level [8]. Aripiprazole acts via a combination of partial agonist activity at dopamine D2 and serotonin 5HT1A receptors and antagonist activity at serotonin 5HT2A receptors. Quetiapine is another medication that modulates both dopaminergic and serotoninergic neurotransmission. An 8-week RCT tested quetiapine extended-release (up to 300 mg/day) in 90 highly depressed and anxious patients with bipolar I or II disorder, with and without recent alcohol and/or cannabis use disorder. Given the heterogeneity of the enrolled sample (e.g., multiple comorbidities), it is hard to interpret the results. Participants who received quetiapine reported less alcohol and cannabis use, compared to those who received placebo, although the differences were not statistically significant [9].
In summary, recent evidence seems to support previous literature on the role of topiramate to treat AUD patients, but not in those who are already abstinent and have smoking comorbidity. Furthermore, compared to previous studies, those conducted in the past two years suggest that aripiprazole could play a role in AUD treatment, but only in specific subgroups of patients.
3.2. Medications acting on calcium channels: Gabapentin
Gabapentin is a structural analogue to GABA with no activity at GABA receptors. Blockade of voltage-gated calcium channels containing the α2δ-1 subunit appears to be gabapentin’s primary mechanism of action. In a 12-week RCT, 122 alcohol-dependent individuals received gabapentin immediate-release (300–1,200 mg/day, mean: 391 mg/day) or placebo, following discharge from an inpatient alcohol treatment program in Thailand. Despite high drop-out rates in both groups, mainly due to loss to follow-up, results showed that gabapentin-treated individuals had lower percentage of heavy drinking days per week, compared to the placebo group, while the amount of alcohol consumption per week was not significantly different between the two groups [10]. In a large multisite study, gabapentin enacarbil extended-release (GE-XR) was also tested. Compared to the immediate-release formulation, GE-XR has higher bioavailability and may potentially enhance adherence, as it only needs to be taken twice a day. In this 26-week RCT, 346 AUD patients received GE-XR (1,200 mg/day) or placebo, in addition to a computerized behavioral intervention. Percentage of subjects with no heavy drinking days was the primary outcome. There were no significant differences between the two groups in terms of alcohol drinking or craving. Population pharmacokinetic analyses showed that exposure to gabapentin was less than other studies using a similar dose of the immediate-release formulation, and that higher exposure to gabapentin was associated with less alcohol consumption [11]. Using propensity score matching, a recent study found that AUD patients who had received gabapentin, for any indication, experienced greater decrease in alcohol use disorders identification test-consumption (AUDIT-C) scores, compared to those with no exposure to gabapentin. No significant effects were found among patients treated with < 1,500 mg/day of gabapentin or those with baseline AUDIT-C score of ≥ 4 or no diagnosis of AUD [12].
In summary, the work conducted in the past two years supports previous evidence on the role of gabapentin in treating AUD, while bringing an open question on its pharmacokinetics profile and how lower exposure to the drug may result in lack of efficacy. It seems that gabapentin’s beneficial effects in relation to alcohol use appear only at high doses; treating patients with these doses should be balanced against the potential risk of side effects.
3.3. Medications acting on GABA receptors: Baclofen
Previous studies testing the GABA-B receptor agonist baclofen in AUD have yielded conflicting results in terms of efficacy [13]. Optimal dosage, predictors and moderators of response, and pharmacokinetic characteristics are among the main topics that recent studies with baclofen have attempted to address. Three RCTs found no superiority for baclofen, compared with placebo, in promoting abstinence or decreasing the amount of alcohol drinking in AUD individuals [14–16]. Of note, various dosages and durations were tested across these three studies: 30 mg/day of baclofen (or placebo) for 12 weeks [14], 50 mg/day of baclofen (or placebo) for 3 months [15], and 180 mg/day of baclofen (or placebo) for 20 weeks [16]. The latter study found that baclofen-treated individuals reported greater reduction in alcohol craving than the placebo group, and that baclofen tended to be effective in reducing alcohol consumption in a subgroup of patients with very high baseline drinking levels (post-hoc analysis). The study, however, was not powered to detect statistically significant differences between low and high drinking individuals [16].
Following some initial reports on the effectiveness of high-dose baclofen for AUD, the optimal dosing strategy, from both safety and efficacy standpoints, has been under debate [17, 18]. The relationship between baclofen dosage and treatment response in AUD individuals does not appear to be linear. Relevant to this notion, a 1-year cohort study found that high-dose (> 150 mg/day) baclofen resulted in better drinking outcomes, compared with medium-dose (90–150 mg/day), but not low-dose (< 90 mg/day) baclofen. Paradoxically, drinking outcomes were less favorable in individuals treated with medium-dose than low-dose baclofen, suggesting a possible U-shaped dose-response relationship [19]. In a multisite RCT, 104 individuals with alcohol dependence were treated with baclofen (30 or 75 mg/day) or placebo for 12 weeks. Based on previous work [20], participants were stratified according to presence or absence of alcoholic liver disease (ALD). Baclofen (composite of 30 and 75 mg/day), compared to placebo, prolonged the time to first lapse and relapse (i.e., primary outcomes); however, there were no differences between the two baclofen doses (i.e., 30 vs. 75 mg/day). Interestingly, comparison of ALD and non-ALD subgroups revealed that baclofen (composite of 30 and 75 mg/day) was more effective than placebo only in patients with ALD, while no significant effects were found within the non-ALD subgroup [21].
To better understand the biobehavioral effects of baclofen in relation to alcohol use, we conducted a human laboratory study with baclofen in non-treatment-seeking, alcohol-dependent outpatients with high anxiety levels. The experimental paradigm followed one week of baclofen or placebo intake and included a cue-reactivity procedure, an alcohol priming, and an alcohol self-administration session. Baclofen (30 mg/day), compared to placebo, had no significant effect on cue- or alcohol-induced craving, or the amount of alcohol self-administered. However, secondary analyses showed that baclofen significantly increased participants’ subjective response to alcohol consumption (i.e., feeling high and intoxicated) [22] – a finding consistent with the results of another recent study [23]. In addition, baclofen appeared to decouple the association between alcohol priming and self-administration. Specifically, higher breath alcohol concentration, higher craving, and lower sedation after consuming the priming drink were associated with more alcohol self-administration, only in the placebo and not the baclofen group [22, 24]. In addition, pharmacokinetic-behavior analyses showed that maximum plasma concentration (Cmax) of baclofen negatively correlated with cue-induced alcohol craving and alcohol-induced rating of ‘like more’ [24]. Pharmacokinetic analyses in AUD individuals consistently show significant interindividual variability in baclofen pharmacokinetic parameters [23–27], which may be partially responsible for the heterogeneity of response to baclofen.
In summary, the baclofen studies conducted in the past two years suggest that its efficacy or lack of efficacy may, at least partially, be related to the variability in its pharmacokinetics profile and to the heterogeneity of AUD patients. On the latter point, studies confirm that baclofen may be effective in AUD patients with ALD. While dosage has been a primary point of discussion in the baclofen field, many other factors (e.g., severity of alcohol drinking, comorbidities, baclofen pharmacokinetics) may also play integral roles in shaping individuals’ response to baclofen in the context of AUD.
3.4. Medications acting on glutamate receptors: Ifenprodil, Glycine
Antagonists of the N-Methyl-D-aspartate (NMDA) receptor have shown promise in reducing alcohol drinking. In a 3-month RCT in 68 detoxified alcohol-dependent outpatients, treatment with a novel NMDA receptor antagonist, ifenprodil (60 mg/day), compared to placebo, significantly reduced the alcohol use score (primary endpoint), calculated as the weighted mean of the frequency of alcohol drinking and presence of heavy drinking [28]. On the other hand, a 12-week pilot trial showed no benefit for glycine, an agonist of the glycine B co-agonist site of the NMDA receptor, over placebo in reducing alcohol drinking or craving in patients with schizophrenia and comorbid alcohol dependence, suggesting that augmentation of the glutamatergic system may not be an effective approach to suppress alcohol consumption [29].
In summary, clinical studies conducted in the past two years provide preliminary support of NMDA receptor antagonism (ifenprodil), but not agonism (glycine), in AUD treatment. These results are very preliminary, and more studies are required in this regard.
3.5. Medications acting on nicotinic acetylcholine receptors: Varenicline, Mecamylamine
Previous data suggest that varenicline, a partial agonist of the α4β2 nicotinic acetylcholine receptor (nAChR), may suppress alcohol seeking and consumption. In one RCT, 33 alcohol-dependent nicotine-smoking individuals who were interested in quitting smoking received varenicline (2 mg/day) or placebo for 12 weeks. Varenicline, compared to placebo, significantly improved smoking outcomes and reduced drinks per drinking days. Smoking abstinence was the primary endpoint in this study, while alcohol use outcomes were secondary endpoints [30]. In a 2-site 17-week RCT, 131 alcohol-dependent nicotine-smoking individuals received varenicline (2 mg/day) or placebo, in addition to medical management. Randomization was stratified by site and gender. Varenicline improved smoking abstinence rates and reduced alcohol drinking, but the latter effect was more favorable in males than females. Percentage of heavy drinking days was the primary endpoint in this study [31]. Roberts and colleagues published 3 secondary reports from previously published human laboratory studies in heavy alcohol drinkers with alcohol-related outcomes as their primary endpoint: varenicline (2 mg/day) attenuated cue-elicited craving for alcohol in both smokers and non-smokers, with no effect on craving for nicotine [32]; varenicline (1 mg/day, 2 mg/day) dose-dependently reduced alcohol drinking and tonic (pre-alcohol) craving for alcohol, only among depressed individuals, with no effect on alcohol-elicited craving for alcohol [33]; varenicline (1 mg/day, 2 mg/day) dose-dependently improved cognitive performance, which was associated with less alcohol drinking, only among those treated with 2 mg/day [34]. In the latter study, blood varenicline concentrations negatively correlated with alcohol drinking levels, but only if varenicline concentrations were > 3 ng/ml, suggesting that favorable behavioral outcomes may be observed only after varenicline concentrations reach a certain therapeutic threshold [34]. Another nAChR antagonist, mecamylamine, was tested in a 12-week RCT, where no significant differences in alcohol drinking outcomes were found between mecamylamine-treated (10 mg/day) participants and those who received placebo (primary endpoint: percentage of heavy drinking days) [35].
In summary, human work conducted in the past two years further supports the role of varenicline, but not mecamylamine, in AUD treatment. Factors influencing varenicline’s efficacy in AUD may include gender, smoking status, and variability in pharmacokinetics profile.
3.6. Medications acting on α1 adrenergic receptors: Prazosin, Doxazosin
Consistent with the role of the noradrenergic system in alcohol reinforcement and relapse, the α1 adrenergic receptor antagonists, prazosin and doxazosin, have been studied as potential treatments for AUD. In a 12-week RCT, 92 treatment-seeking, heavy-drinking, alcohol-dependent individuals received prazosin (16 mg/day) or placebo, in addition to medical management. Randomization was stratified by gender, veteran status, and drinking frequency. Prazosin, compared to placebo, reduced alcohol drinking (number of drinks and number heavy drinking days, but not number of drinking days per week) and systolic blood pressure, with no significant effects on alcohol craving or diastolic blood pressure (DBP). Blood pressure did not moderate prazosin’s effects on alcohol consumption in this study [36]. Another study tested prazosin (16 mg/day) for a shorter period of time (6 weeks) and in a smaller sample (n = 27 completers). Randomization was stratified based on presence of an anxiety disorder diagnosis, given the beneficial effects of prazosin for post-traumatic stress disorder (PTSD). While prazosin did not have a significant effect on alcohol-related outcomes in intention-to-treat analyses (n = 36), when the assessment was limited to participants with optimal treatment exposure, it became apparent that prazosin, compared to placebo, significantly reduced the amount of alcohol consumption. Also, prazosin-treated patients, compared to the placebo group, demonstrated more negative slope of drinking over time, indicating faster reduction in alcohol consumption. Interestingly, DBP was found to be a significant moderator, i.e., prazosin reduced the rate of alcohol consumption in participants with high, but not low, DBP [37]. Consistent with this finding, secondary analysis of a 10-week RCT with doxazosin (up to 16 mg/day) found significant moderation effect for DBP in the same direction, i.e., individuals with high, but not low, DBP reduced their alcohol drinking under doxazosin, compared to placebo [38].
In summary, human studies conducted in the past two years tentatively suggest that α1 blockade may represent an effective pharmacological approach to treat AUD, although the benefits may be limited to specific subgroups of patients (e.g., those with higher adrenergic tone, as suggested by a potential moderation role for blood pressure).
3.7. Medications acting on neuroendocrine pathways: Oxytocin, ABT-436, PF-5190457
Building upon previous work with oxytocin, a pilot study tested the effects of intranasal oxytocin (24 IU, single dose) on brain activity in response to alcohol cues in social drinking males, and found that, compared to placebo, oxytocin decreased cue-reactivity in insular cortex, hippocampal/parahippocampal formation, cingulate cortex, inferior/medial frontal gyrus, and visual/motor regions [39]. Vasopressin is also emerging as a potential treatment target for AUD; a novel vasopressin 1b receptor antagonist, ABT-436, was studied in a 12-week multisite RCT. Randomization to the ABT-436 or placebo group was stratified by site and trait anxiety score. Heavy-drinking, alcohol-dependent individuals had higher percentage of abstinent days under ABT-436 (up to 800 mg/day) than placebo, while no differences were found on heavy drinking days (i.e., primary outcome) or craving. Furthermore, subgroup analysis showed that participants with high anxiety levels at baseline had a better response to ABT-436 [40]. Finally, growing evidence suggests a potential role for appetitive hormones, such as ghrelin, in AUD [41, 42]. Our group examined the safety and preliminary efficacy of a novel ghrelin receptor inverse agonist, PF-5190457, in 12 heavy-drinking individuals. PF-5190457 combined with alcohol was safe and well tolerated, with no drug-alcohol interactions. Preliminary data also showed that PF-5190457 (200 mg/day), compared to placebo, reduced craving for alcohol and attention to alcohol during a cue-reactivity experiment in a bar laboratory [43].
In summary, work conducted in the past two years provides support for future studies on stress- and feeding-related neuroendocrine pathways as treatment targets for AUD.
4. Conclusion
The studies summarized in this review reflect medication development efforts conducted in the past two years in the alcohol field. While some studies suffer from significant limitations (e.g., small samples, open-label design), other studies are promising and point to the importance and need for increased efforts toward developing new medications for AUD. In addition to the compounds discussed above, our search identified other medications that were tested in relation to alcohol use, namely diazepam, memantine, samidorphan, ibudilast, N-acetylcysteine, and citoline (Table 1). Human laboratory paradigms provide an efficient tool to screen and prioritize promising targets and compounds before running large clinical trials. Given the complexity and heterogeneity of AUD, it is important to increase the number and diversity of pharmacological options, and to study potential moderators/predictors of response to each medication. Finally, treatment regimens that include more than one medication may have synergistic beneficial effects by targeting multiple pathways; therefore, the safety and efficacy of combined therapies for AUD should also be investigated.
Supplementary Material
Key Points:
While currently approved medications are effective, there is a critical need to increase the armamentarium of pharmacotherapies for alcohol use disorder.
Neuroscientific advances have led to identification of novel therapeutic targets, and a variety of compounds have been studied in the past two years as potential pharmacological treatments for alcohol use disorder.
Human laboratory paradigms provide an efficient tool to screen and prioritize promising medications for alcohol use disorder before running large clinical trials.
Given the complexity and heterogeneity of alcohol use disorder, it is important to study potential moderators and predictors of response to different pharmacological options.
Acknowledgments:
The authors would like thank Ms. Alicia A. Livinski from the National Institutes of Health (NIH) Library for bibliographic assistance.
Financial support and sponsorship: The authors of this work are supported by and grateful to: A) NIH intramural funding ZIA-AA000218 (Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology; PI: Dr. Lorenzo Leggio), jointly supported by NIAAA Division of Intramural Clinical and Biological Research and NIDA Intramural Research Program, and B) NIH Center on Compulsive Behaviors, funded by the NIH Deputy Director for Intramural Research Innovation Award. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the funders, which had no role in the development of this article.
Footnotes
Conflicts of interest: None
References
- 1.World Health Organization (WHO), Global status report on alcohol and health 2018.
- 2.Griswold MG, et al. , Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet, 2018. 392(10152): p. 1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonas DE, et al. , Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. Jama, 2014. 311(18): p. 1889–900. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, Koob GF, and McLellan AT, Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med, 2016. 374(4): p. 363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anthenelli RM, et al. , A Randomized Trial Evaluating Whether Topiramate Aids Smoking Cessation and Prevents Alcohol Relapse in Recovering Alcohol-Dependent Men. Alcohol Clin Exp Res, 2017. 41(1): p. 197–206.* While some data suggest that topiramate may be an effective medication for alcohol use disorder, the results of this study indicate that topiramate may not work in abstinent alcohol-dependent individuals and in those with cigarette smoking comorbidity. These findings are consistent with the hypothesis generated by previous studies indicating the efficacy of topiramate in abstinence initiation.
- 6.Haass-Koffler CL, et al. , Comparing and Combining Topiramate and Aripiprazole on Alcohol-Related Outcomes in a Human Laboratory Study. Alcohol Alcohol, 2018. 53(3): p. 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anton RF, et al. , Aripiprazole Suppression of Drinking in a Clinical Laboratory Paradigm: Influence of Impulsivity and Self-Control. Alcohol Clin Exp Res, 2017. 41(7): p. 1370–1380.** This study applied a human laboratory approach to study the effects of aripiprazole on alcohol drinking versus an alternative reinforcer (i.e., delayed monetary reward). The finding that individuals with high impulsivity and low self-control respond better to aripiprazole is of particular importance.
- 8.Schacht JP, et al. , Dopaminergic Genetic Variation Influences Aripiprazole Effects on Alcohol Self-Administration and the Neural Response to Alcohol Cues in a Randomized Trial. Neuropsychopharmacology, 2018. 43(6): p. 1247–1256.* This study applied a human laboratory and brain imaging approach to study whether variations in dopamine-related genes may influence response to aripiprazole treatment. Results suggest that aripiprazole may work better in individuals with genetic predisposition to higher dopaminergic tone.
- 9.Gao K, et al. , A placebo controlled study of quetiapine-XR in bipolar depression accompanied by generalized anxiety with and without a recent history of alcohol and cannabis use. Psychopharmacology, 2017. 234(15): p. 2233–2244. [DOI] [PubMed] [Google Scholar]
- 10.Chompookham P, et al. , A randomized trial of low-dose gabapentin for post hospitalization relapse prevention in a Thai clinical sample of alcohol dependence. Psychiatry Research, 2018. 270: p. 34–40. [DOI] [PubMed] [Google Scholar]
- 11.Falk DE, et al. , Gabapentin Enacarbil Extended-Release for Alcohol Use Disorder: A Randomized, Double-Blind, Placebo-Controlled, Multisite Trial Assessing Efficacy and Safety. Alcohol Clin Exp Res, 2019. 43(1): p. 158–169.** This was the first phase 2 clinical trial testing gabapentin enacarbil extended-release (GE-XR) for alcohol use disorder. The large sample size, being a multisite trial, and testing a novel formulation are major strengths. While there was no difference between GE-XR and placebo on the study outcomes, the finding of a pharmacokinetic analysis indicating that higher exposure to gabapentin was associated with less alcohol consumption is of particular importance.
- 12.Rentsch CT, et al. , Association between gabapentin receipt for any indication and AUDIT-C scores among clinical sub-populations with and without alcohol use disorder. Alcohol Clin Exp Res, 2019. 43(3): p. 522–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agabio R, et al. , Baclofen for the treatment of alcohol use disorder: the Cagliari Statement. Lancet Psychiatry, 2018. 5(12): p. 957–960. [DOI] [PubMed] [Google Scholar]
- 14.Hauser P, et al. , The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction, 2017. 112(7): p. 1173–1183.* Given some previous evidence on the efficacy of baclofen in alcohol-dependent individuals with liver problems, this large multisite clinical trial tested baclofen in veterans with chronic hepatitis C. The negative results on alcohol-related outcomes are in line with previous controversial results with baclofen and highlight the need for more research in this regard. Notably, baseline alcohol drinking levels of the enrolled patients were low, supporting the previous work that baclofen may only work in patients with high severity of alcohol use disorder.
- 15.Krupitskii EM, et al. , Efficacy and Safety of the Use of Baclofen in the Treatment of Alcohol Dependent (a Double-Blind, Randomized, Placebo-Controlled Pilot Study). Neuroscience and Behavioral Physiology, 2017. 47(2): p. 153–162. [Google Scholar]
- 16.Reynaud M, et al. , A Randomized, Placebo-Controlled Study of High-Dose Baclofen in Alcohol-Dependent Patients-The ALPADIR Study. Alcohol Alcohol, 2017. 52(4): p. 439–446.* This was a large clinical trial testing high-dose baclofen (180 mg/day) as a treatment for alcohol use disorder. While baclofen was not superior to placebo overall, the finding that individuals with very high drinking level responded better to baclofen treatment is of particular importance.
- 17.Thompson A, et al. , Systematic review: Baclofen dosing protocols for alcohol use disorders used in observational studies. Eur Neuropsychopharmacol, 2017. 27(11): p. 1077–1089. [DOI] [PubMed] [Google Scholar]
- 18.Costa M, Rolland B, and Carrieri P, The need for patient-tailored dosing of baclofen in future clinical trials. Eur Neuropsychopharmacol, 2018. 28(5): p. 656–657. [DOI] [PubMed] [Google Scholar]
- 19.Pignon B, et al. , The dose–effect relationship of baclofen in alcohol dependence: A 1-year cohort study. Human Psychopharmacology, 2017. 32(4). [DOI] [PubMed] [Google Scholar]
- 20.Addolorato G, et al. , Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet, 2007. 370(9603): p. 1915–22. [DOI] [PubMed] [Google Scholar]
- 21.Morley KC, et al. , Baclofen in the treatment of alcohol dependence with or without liver disease: multisite, randomised, double-blind, placebo-controlled trial. Br J Psychiatry, 2018. 212(6): p. 362–369.** This study tested two doses of baclofen, compared to placebo, in a multisite trial. Stratifying participants based on presence or absence of alcoholic liver disease was novel and informative. The finding that only patients with alcoholic liver disease responded to baclofen is of particular importance.
- 22.Farokhnia M, et al. , Biobehavioral effects of baclofen in anxious alcohol-dependent individuals: a randomized, double-blind, placebo-controlled, laboratory study. Transl Psychiatry, 2017. 7(4): p. e1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durant CF, et al. , Using Baclofen to Explore GABA-B Receptor Function in Alcohol Dependence: Insights from Pharmacokinetic and Pharmacodynamic Measures. Front Psychiatry, 2018. 9: p. 664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farokhnia M, et al. , A deeper insight into how GABA-B receptor agonism via baclofen may affect alcohol seeking and consumption: lessons learned from a human laboratory investigation. Molecular Psychiatry, 2018. [in press]** This study applied a human laboratory approach to investigate how baclofen may affect the relationship between different alcohol-related outcomes. The finding that baclofen decoupled the link between an initial drink and subsequent alcohol consumption is of particular importance. Furthermore, pharmacokinetic-behavior results highlight the importance of considering this aspect in future studies with baclofen.
- 25.Marsot A, et al. , High variability in the exposure of baclofen in alcohol-dependent patients. Alcohol Clin Exp Res, 2014. 38(2): p. 316–21. [DOI] [PubMed] [Google Scholar]
- 26.Chevillard L, et al. , Population pharmacokinetics of oral baclofen at steady-state in alcoholic-dependent adult patients. Fundam Clin Pharmacol, 2018. 32(2): p. 239–248. [DOI] [PubMed] [Google Scholar]
- 27.Simon N, et al. , Full-Profile Pharmacokinetic Study of High Dose Baclofen in Subjects with Alcohol Use Disorder. Front Psychiatry, 2018. 9: p. 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugaya N, et al. , A randomized controlled study of the effect of ifenprodil on alcohol use in patients with alcohol dependence. Neuropsychopharmacology reports, 2018. 38(1): p. 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serrita J, et al. , A Pilot Randomized, Placebo-Controlled Trial of Glycine for Treatment of Schizophrenia and Alcohol Dependence. Journal of Dual Diagnosis, 2018. 11: p. 1–10. [DOI] [PubMed] [Google Scholar]
- 30.Hurt RT, et al. , Varenicline for tobacco-dependence treatment in alcohol-dependent smokers: A randomized controlled trial. Drug Alcohol Depend, 2018. 184: p. 12–17.* This study provides evidence that varenicline may be effective in both promoting smoking abstinence and reducing alcohol drinking in cigarette smokers with alcohol use disorder.
- 31.O’Malley SS, et al. , Effect of Varenicline Combined with Medical Management on Alcohol Use Disorder with Comorbid Cigarette Smoking: A Randomized Clinical Trial. JAMA Psychiatry, 2018. 75(2): p. 129–138.** This was a large 2-site trial testing varenicline in alcohol-dependent nicotine-smoking individuals. The finding that varenicline’s effects on alcohol drinking was more favorable in males than females is of particular importance as it points to a possible gender difference in response to varenicline.
- 32.Roberts W, Harrison ELR, and McKee SA, Effects of varenicline on alcohol cue reactivity in heavy drinkers. Psychopharmacology (Berl), 2017. 234(18): p. 2737–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts W, et al. , Effects of varenicline on alcohol self-administration and craving in drinkers with depressive symptoms. Journal of Psychopharmacology, 2017. 31(7): p. 906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts W and McKee SA, Effects of varenicline on cognitive performance in heavy drinkers: Dose-response effects and associations with drinking outcomes. Exp Clin Psychopharmacol, 2018. 26(1): p. 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrakis IL, et al. , Mecamylamine treatment for alcohol dependence: a randomized controlled trial. Addiction, 2018. 113(1): p. 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simpson TL, et al. , Double-Blind Randomized Clinical Trial of Prazosin for Alcohol Use Disorder. Am J Psychiatry, 2018. 174(12): p. 1216–1224* This was the first fully powered phase 2 clinical trial testing prazosin for alcohol use disorder. Participants with posttraumatic stress disorder were excluded in order to isolate the effects of prazosin on alcohol-related outcomes alone. Some beneficial effects were shown on drinking outcomes.
- 37.Wilcox CE, et al. , A Randomized, Placebo-controlled, Clinical Trial of Prazosin for the Treatment of Alcohol Use Disorder. J Addict Med, 2018. 12(5): p. 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haass-Koffler CL, et al. , Higher pretreatment blood pressure is associated with greater alcohol drinking reduction in alcohol-dependent individuals treated with doxazosin. Drug Alcohol Depend, 2017. 177: p. 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansson AC, et al. , Oxytocin reduces alcohol cue-reactivity in alcohol-dependent rats and humans. Neuropsychopharmacology, 2018. 43(6): p. 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan ML, et al. , A Phase 2, Double-Blind, Placebo-Controlled Randomized Trial Assessing the Efficacy of ABT-436, a Novel V1b Receptor Antagonist, for Alcohol Dependence. Neuropsychopharmacology, 2017. 42(5): p. 1012–1023.** This was the first phase 2 clinical trial testing a vasopressin 1b receptor antagonist (ABT-436) for alcohol use disorder. The findings that ABT-436 improved both alcohol drinking and nicotine smoking outcomes is of particular importance. Furthermore, the results suggest that alcohol-dependent individuals with high anxiety levels may particularly benefit from treatments that target the vasopressin 1b receptor.
- 41.Zallar LJ, et al. , The Role of the Ghrelin System in Drug Addiction. Int Rev Neurobiol, 2017. 136: p. 89–119. [DOI] [PubMed] [Google Scholar]
- 42.Farokhnia M, et al. , Ghrelin: From a gut hormone to a potential therapeutic target for alcohol use disorder. Physiol Behav, 2019. 204: p. 49–57. [DOI] [PubMed] [Google Scholar]
- 43.Lee MR, et al. , The novel ghrelin receptor inverse agonist PF-5190457 administered with alcohol: preclinical safety experiments and a phase 1b human laboratory study. Mol Psychiatry, 2018. [In press].* This was the first study testing a ghrelin receptor blocker (PF-5190457) in a sample of heavy alcohol drinkers. The study showed the safety and tolerability of this compound, co-administered with alcohol. Furthermore, while preliminary, the finding that PF-5190457 reduced cue-induced craving and attention to alcohol cues is of potential importance.
- 44.Gupta M, et al. , Randomized open-label trial of baclofen for relapse prevention in alcohol dependence. Am J Drug Alcohol Abuse, 2017. 43(3): p. 324–331. [DOI] [PubMed] [Google Scholar]
- 45.Owens L, et al. , A prospective cohort study examining the effectiveness of baclofen in the maintenance of abstinence in alcohol use disorder patients attending a joint liver and alcohol treatment clinic. Alcohol, 2017. 62: p. 11–15. [DOI] [PubMed] [Google Scholar]
- 46.Morley KC, et al. , Neurometabolite levels in alcohol use disorder patients during baclofen treatment and prediction of relapse to heavy drinking. Frontiers in Psychiatry, 2018. 9: p. 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morley KC, et al. , Moderation of baclofen response by a GABAB receptor polymorphism: results from the BacALD randomized controlled trial. Addiction, 2018. 113(12): p. 2205–2213. [DOI] [PubMed] [Google Scholar]
- 48.Logge WB, et al. , Baclofen attenuates fMRI alcohol cue reactivity in treatment-seeking alcohol dependent individuals. Psychopharmacology (Berl), 2019. [In press]. [DOI] [PubMed]
- 49.Holla B, et al. , Brain functional magnetic resonance imaging cue-reactivity can predict baclofen response in alcohol use disorders. Clinical Psychopharmacology and Neuroscience, 2018. 16(3): p. 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beck A, et al. , Effects of high-dose baclofen on cue reactivity in alcohol dependence: A randomized, placebo-controlled pharmaco-fMRI study. European Neuropsychopharmacology, 2018. 28(11): p. 1206–1216. [DOI] [PubMed] [Google Scholar]
- 51.Simioni N, et al. , Thirty- Versus Ten-Day Diazepam Treatment for Alcohol Detoxification and a Comparison of Drinking Patterns, Craving, and Anxiety for up to 12 Weeks: A “Proof-of-Concept” Open-Label Randomized Controlled Trial. J Clin Psychopharmacol, 2017. 37(6): p. 722–728. [DOI] [PubMed] [Google Scholar]
- 52.Lee SY, et al. , Add-On Memantine Treatment for Bipolar II Disorder Comorbid with Alcohol Dependence: A 12-Week Follow-Up Study. Alcohol Clin Exp Res, 2018. 42(6): p. 1044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Malley SS, et al. , Effects of the Opioid System Modulator, Samidorphan, on Measures of Alcohol Consumption and Patient-Reported Outcomes in Adults with Alcohol Dependence. Alcoholism: Clinical and Experimental Research, 2018. 42(10): p. 2011–2021.* This was the first phase 2 trial testing samidorphan for alcohol use disorder. While the effect on the primary outcome (percentage of subjects with no heavy drinking days) was negative, participants reported better treatment response under samidorphan than placebo, highlighting the importance of prioritizing patient-centered outcomes in alcohol clinical trials.
- 54.Ray LA, et al. , Development of the Neuroimmune Modulator Ibudilast for the Treatment of Alcoholism: A Randomized, Placebo-Controlled, Human Laboratory Trial. Neuropsychopharmacology, 2017. 42(9): p. 1776–1788.** This was the first phase 2 clinical trial testing ibudilast for alcohol use disorder. The finding that ibudilast reduced stimulant and mood-altering effects of alcohol among highly depressed individuals is of particular importance, as it suggests that depression levels may moderate the effects of neuroimmune pharmacotherapies in relation to alcohol use.
- 55.Squeglia LM, et al. , The effect of N-acetylcysteine on alcohol use during a cannabis cessation trial. Drug Alcohol Depend, 2018. 185: p. 17–22.* This study utilized data from a multisite clinical trial with N-acetylcysteine for treatment of cannabis dependence. The finding that N-acetylcysteine reduced alcohol drinking is of particular importance, suggesting that this compound may be further studied as a potential treatment for alcohol use disorder.
- 56.Brown ES, et al. , A Randomized, Double-Blind, Placebo-Controlled Trial of Citicoline in Patients with Alcohol Use Disorder. Alcohol Clin Exp Res, 2018. 43(2): p. 317–323* This was the first pilot study testing citicoline for alcohol use disorder. The finding that citicoline did not improve alcohol-related outcomes creates questions about the need for further investigation on this over-the-counter supplement as a potential treatment for alcohol use disorder.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.