Abstract
Guidelines recommend vaccination starting 12 months after autologous hematopoietic stem cell transplant (aHCT), but there is varying practice for patients on maintenance therapy, with some centers not immunizing at all. Due to decreased vaccine rates among the general population causing loss of herd immunity, we aimed to establish the safety and efficacy of revaccinating multiple myeloma patients on lenalidomide maintenance (LM). Of the 122 patients who were vaccinated after aHCT between 2010–2014 at MSKCC, 91 (75%) were on LM. Vaccine responses were defined by increases between pre- and post-vaccination titers. Reponses varied by vaccine type with 76% responding to pertussis, 70% diphtheria, 60% tetanus, 71% haemophilus influenzae, and 58% pneumococcal. All patients retained minimal levels of polio immunity, but 27% responded with increased titers. Fewer patients received Hepatitis A and B, but of those who did, 30% responded to hepatitis A and 40% to hepatitis B. No differences were seen in rates of response for those on LM at time of vaccination compared to those who were not. There were no vaccine related adverse effects. Re-immunization with inactivated vaccines in patients on LM is therefore both safe and effective, offering this population immunity to vaccine-preventable diseases.
Introduction:
Multiple myeloma (MM) is a clonal plasma disorder with an overproduction of monoclonal proteins in the serum or urine causing organ dysfunction, with an annual incidence of approximately 24,000 people in the United States1. Standard treatment includes combination therapy with proteasome inhibitors (ex. bortezomib, ixazomib, carfilzomib), immunomodulatory drugs (ex. lenalidomide, thalidomide, pomalidomide), and dexamethasone followed by autologous hematopoietic stem cell transplantation (aHCT) for eligible patients2,3. Continuous low dose lenalidomide maintenance (LM) is standard of care and prolongs progression free and overall survival4,5.
After aHCT, patients have prolonged susceptibility to infections until immune reconstitution. Particularly for pneumococcus, influenza, measles, mumps, and rubella, aHCT recipients rely on herd immunity for protection against vaccine preventable illnesses. Herd immunity is often accomplished through mandatory vaccination of school aged children, but has been compromised recently due to the increase in non-medical exemptions, leading to outbreaks in communities across the United States and elsewhere6–10. Consequently, re-immunization after aHCT is vital to the wellbeing of this vulnerable population.
Current guidelines of the American Society of Blood and Marrow Transplantation (ASBMT), European Society for Blood and Marrow Transplantation (EBMT), and the Infectious Disease Society of America (IDSA) recommend re-vaccination starting between 6–12 months after transplant following a set schedule11,12. However, recommendations suggest that patients on active therapy should not be vaccinated. LM is currently given until disease progression and re-immunization of these patients is not uniform between institutions. We therefore evaluated the safety and efficacy of vaccination in MM patients on LM after aHCT.
Methods:
Patients
MM patients who underwent their first aHCT between 2010–2014 were identified retrospectively from the Memorial Sloan Kettering Cancer Center (MSKCC) institutional registry and were included if the aHCT occurred less than one year after diagnosis, and they did not receive a tandem transplant. Inclusion in the analysis required at least one vaccine with pre-and post-vaccination titers. Demographic and treatment characteristics were collected through chart abstraction with approval from the MSKCC Institutional Review Board. Safety data was collected by retrospective chart review.
Vaccination
Immunizations started at approximately the one-year anniversary of the aHCT. The vaccination schedule is summarized here and presented in Supplementary Table 1 with combination vaccines used when possible. Haemophilus influenzae type B conjugate vaccine (HiB, ActHib, Sanofi Pasteur, Lyon, France), poliovirus inactivated vaccine (IPOL, Sanofi Pasteur, Lyon, France), pneumococcal conjugate vaccine (Prevnar 13-Valent, Wyeth-Ayerst, Collegeville, Pennsylvania), and tetanus/diphtheria/pertussis (Tdap) given as Boostrix (Glaxo Smith Kline, London, England) or Adacel for latex allergic patients (Sanofi Pasteur, Lyon, France) are typically administered at the same visit and given as a three dose series at 1–3 month intervals. Hepatitis A-hepatitis B vaccine (Twinrix, Glaxo Smith Kline, London, England) is also given as a 3 dose series at month 0, month 1 and month 6. If given separately, hepatitis B recombinant (Glaxo Smith Kline, London, England) is also at month 0, month 1 and month 6, while the hepatitis A vaccine (Havrix, Glaxo Smith Kline, London, England) is given as a 2 dose series 6–12 months apart. Measles, mumps, and rubella (MMR, Merck & Co. Inc, Kenilworth, New Jersey) is given as a 2 dose series at a 2–3 month interval.
For patients without a response to Hib, IPOL, Prevnar 13, or Tdap, an additional (4th) dose of the needed vaccine is given prior to the booster round, which is given 6–12 months later. The same formulations are given for the booster round, except for the pneumococcal vaccine, which is given as Pneumovax 23 (Merk & Co. Inc, Kenilworth, New Jersey), for patients who did not respond to Prevnar 13. For patients without response to hepatitis A or B, the full series is repeated with the individual vaccine.
Our analysis is limited to the response after the completion of the initial series for each vaccine subtype.
Vaccine Response
Patients had pre-vaccine titers were measured on the first day of vaccination and titers were rechecked 1–3 months after completion of the full series of each vaccine. Protective levels and thresholds for response are defined in Table 1, and based on a combination of published thresholds, manufacturer recommendations, and expert opinion based on the most commonly used values. Diphtheria titers were measured by enzyme linked immunosorbent assay (ELISA), while for tetanus an immunoassay and for Haemophilus influenza an enzyme immunoassay were used. Pertussis and pneumococcal titers were measured by multi-analyte immunodetection (MAID), while for polio a culture/neutralization assay was done by Quest Diagnostics. The ABBOTT Architect i2000R instrument was used for hepatitis titers. Finally, titers for measles, mumps, rubella, and varicella were measured by enzyme linked fluorescent assays on the bioMeriuex VIDAS instrument.
Table 1:
Inactivated Vaccine Response Definitions
| Disease/Vaccine | MSKCC Lab Values | Definition of Response |
|---|---|---|
|
Haemophilus Influenzae -Haemophilus B conjugate Vaccine |
<0.15 Non-protective 0.15–0.99 micro g/mL indeterminate >= 1.00 mcg/ml - protective | 1. Non-protective to Protective level (<0.15 to >=1) 2. Four fold increase if in indeterminate range 3. If >1 in pre-titer, immune, but not evaluable for response. |
|
Polio -Poliovirus Vaccine Inactivated (IPOL) |
1:8 or greater = protective | 1. Not protective to protective (4 to >8) in all 3 serotypes* 2. If pre-titer >8 for any subtype, response = any increase in number 3. If all 3 post titers >8 but do not meet above, pt is immune, but not evaluable for response |
|
Pneumococcus -Pneumococcal Conjugate Vaccine (Prevnar 13-Valent) -Pneumococcal Vaccine (Pneumovax 23) (23-Valent) |
Range <0.3 to >35.4 > 2.0 mcg/mL protective** |
1. Two fold increase in 70% of pneumococcal serotypes*** |
|
Tetanus -Boostrix -Adacel |
Range <0.1 to >7 >0.15 International Units/mL healthy Immunized |
1. >0.5 |
|
Diphtheria -Boostrix -Adacel |
<0.01 IU/mL Non-protective > or = 0.01 IU/mL Protective | 1. Four fold increase |
|
Pertussis -Boostrix -Adacel |
Pertussis Toxin IU/mL | 1. Increase to >5 units/mL |
|
Hepatitis A -Hepatitis A-Hepatitis B Vaccine (Twinrix) -Hepatitis A Virus Vaccine |
Hep A IgG Ab = Negative, Positive Hep A Antibody Panel = Non-Reactive, Reactive |
1. Negative to Positive 2. If pre-titer positive, immune but not evaluable for response |
|
Hepatitis B -Hepatitis A-Hepatitis B Vaccine (Twinrix) -Hepatitis B Vaccine Recombinant |
Hep B Surface AB = Negative, Non-Reactive, Positive, Reactive | 1. Negative to Positive 2. If pre-titer positive, immune but not evaluable for response |
Subtypes = Poliovirus Type I, II, III
Protective lab value based on manufacturer.
Modern serotypes tested = 1, 3, 4, 8, 9 (9N), 12 (12F), 14, 19 (19F), 23 (23F), 26 (6B), 51 (7F), & 56 (18C)
For analysis, we defined four groups based on the pre- and post-vaccine titers for each vaccine. “Not evaluable” defined patients who were missing titer results or titers were drawn >6 months after the completion a vaccines series. Patients were noted to have “retained immunity” if pre-titers met the defined cutoffs for protection and the post-titers continued to demonstrate immunity. “Non-responders” either did not achieve the titer threshold for immunity or did not have the appropriate fold increase between the pre- and post-titer. In cases where the vaccine response was defined as a fold increase, patients could meet criteria for “retained immunity,” but were classified as “non-responders” if they did not achieve the fold increase required in their titers (Diphtheria and Pneumococcal vaccines). “Responders” attained the threshold or fold increase between the pre- and post-vaccine titers.
Statistics
Descriptive statistics were used to summarize the results for each vaccine. Univariate analysis using the Fisher’s exact test was conducted to identify associations between response and factors at the time of vaccination including being on LM, steroids, or chemotherapy, receiving IVIg after transplant, or having relapsed. SAS 9.4 statistical software was used for all analyses.
Results:
Patients
Of the 122 patients who met our inclusion criteria, 91 (75%) were on LM at the time of vaccination with a median age of 58 years (range, 42–75) (Table 2). The median age of patients not on LM was similar (57 years, range 38–71). The majority of patients in each group were Caucasian with 54% of those on LM and 48% of those not on LM being male. Lenalidomide was included in the induction therapy for 79 vs 65% of LM and non-LM patients, respectively. The portion of patients receiving steroids or multiagent therapy at the initiation of vaccination was low in both groups, coinciding with the small percent of patients with relapsed disease by the 1 year time point. Similarly, intravenous immunoglobulin use in these patients was minimal. The median time to first vaccination was 12.6 months (range 8.1–26.4), and was not different between LM and non-LM patients (12.63 months (range 11.05–26.45) vs 12.63 months (8.06–24.70), p=0.48). Complete vaccination of with HiB, pneumococcus, polio, and Tdap occurred in 79%, while only 37% had a complete series of those vaccines and HepA and HepB. Titers were drawn at a median of 5 months (range 1–20 months) after the last vaccine in the primary series. No patients had vaccine related adverse events on retrospective chart evaluation.
Table 2:
Patient Characteristics
| LM (n=91, %) | No LM (n=31, %) | |
|---|---|---|
| Age, median (range) | 58 (42–75) | 57 (38–71) |
| Male | 49 (54) | 15 (48) |
| Caucasian | 74 (81) | 22 (71) |
| Lenalidomide in Induction | 72 (79) | 20 (65) |
| Steroid use at 1 year after aHCT | 7 (8) | 5 (16) |
| On multi agent therapy at 1 year after aHCT | 5 (5) | 6 (19) |
| IVIg within the 1st year after aHCT | 0 | 1 (3) |
| Relapse by 1 year after aHCT | 2 (2) | 3 (10) |
LM, lenalidomide maintenance; IVIG, intravenous immunoglobulin; aHCT, autologous hematopoietic stem cell transplant
Vaccine Responses
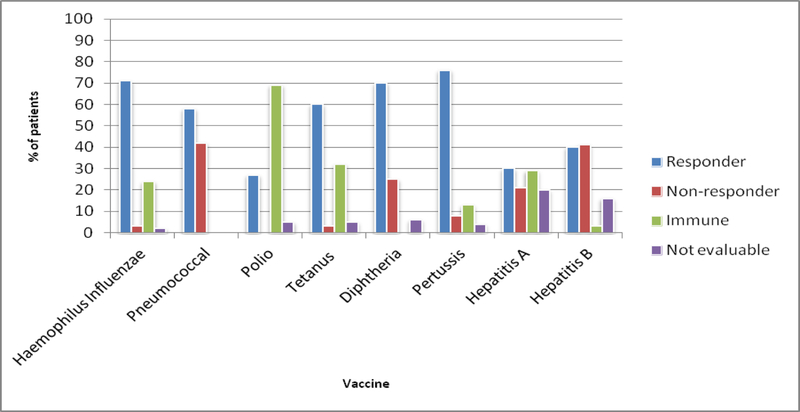
Of the 120 patients who received any HiB vaccine, 118 (98.3 %) completed the HiB vaccination series. With 28 patients (24%) retaining immunity and 2 patients (1.6 %) not evaluable, response was demonstrated in 84 patients (71%) (Figure 1, Supplement 2).
Figure 1.
Response by Vaccine Subtype
No patients maintained immunity to pneumococcus by their pre-titer. Pneumococcal vaccination was completed in 119 of 120 patients (99.2 %), and all patients were evaluable. Response was demonstrated in 69 patients (58%).
Polio vaccination was completed in 109 of 120 patients (90.8 %). All evaluable patients demonstrated a baseline minimum level of immunity to polio considered protective. Polio response by the pre-specified titer increase was demonstrated in 29 patients (26.6%) with 5 patients (4.6%) non-evaluable.
The three dose series of Tdap vaccination was completed in 106 patients (88.3 %). Tetanus response was demonstrated in 64 patients (60%), with 5 patients (5%) not evaluable and 34 patients (32%) retaining immunity. With all of the evaluable patients starting with at least the minimum level of immunity to be considered protected, diphtheria response was demonstrated in 74 patients (70%) with 6 patients (5.6%) not evaluable. Pertussis response was demonstrated in 80 patients (76%) with 4 patients (3.5 %) not evaluable and 14 patients (13%) retaining immunity.
Hepatis A and B vaccination response was evaluated as long as at least 2 doses of these vaccinations were received (56/120 patients for Hep A (46.7%), 68/120 patients for Hep B (56.7 %). Hepatitis A vaccine response was demonstrated in 17 patients (30%) with 11 patients (20%) non-evaluable and 16 patients (29%) retaining immunity. Finally, with 2 patients (3%) maintaining immunity, hepatitis B response was demonstrated in 27 patients (40%) with 11 patients (16%) non-evaluable.
Rates of live vaccinations were much lower in this cohort, possibly due to being on LM and possibly due to our follow-up time, as these vaccines are not given until 2 years after transplant. Thirty two patients received at least one dose of MMR, with 15 (48%) completing the series. For the majority of these patients, post-MMR titers were not available, and therefore, we are not able to evaluate response. Nine (60% of those completing the series), 3 (20%), and 7 (47%) patients retained immunity to measles, mumps, and rubella, respectively. No patients developed documented side effects or acute infections after vaccination.
Impact of Lenalidomide Maintenance
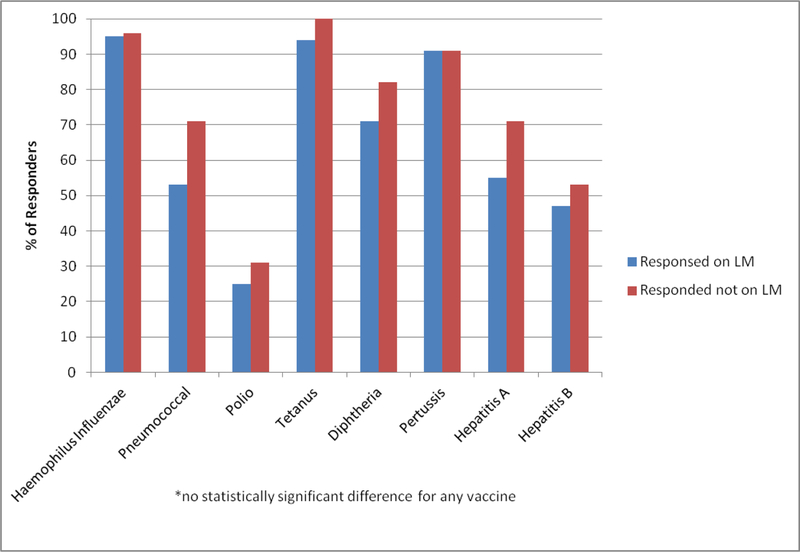
Univariate analysis was conducted to evaluate factors associated with response for all vaccines except polio, for which no patients were categorized as non-responders (Figure 2). There was no statistical difference in the rate of response for those patients who were receiving lenalidomide maintenance versus those who were not for HiB (p>0.95), pneumococcus (p=0.1), tetanus (p=0.56), diphtheria (p=0.31), pertussis (p>0.95), Hep A (p=0.66), or Hep B (p=0.78). Furthermore, no associations with response were seen with the use of steroids or chemotherapy at initiation of vaccination, IVIg after transplant, or relapsed disease for any vaccine; however, the numbers of patients in each of these groups was very small.
Figure 2.
Percent of patients on lenalidomide maintenance versus not on lenalidomide maintenance who responded (excluding patients who retained immunity or were not evaluable).
Discussion:
This is the first study to evaluate the safety and efficacy of the standard inactivated vaccine series when given to MM patients on LM after aHCT. With the majority of patients completing the complete primary series, we show that response rates to the majority of vaccines are high and are not affected by the use of LM. The retention of immunity prior to vaccination differs based on the infectious agent, with many patients retaining immunity to polio and diphtheria, but no patients retaining immunity to pneumococcus. Revaccination with the complete schedule is therefore important for prevention of disease in this population with prolonged survivorship, known decrease in titers over time after aHCT11, and recent decreases in herd immunity.
Definitions of immunity after vaccination vary between studies and are partially dependent on the response assays used. In addition, some centers do not obtain pre-vaccination titers and define responses solely on the achievement of post-vaccination titers11. Nevertheless, response rates in our series are similar to previously published studies of vaccine responses after aHCT13–18. In a study of 20 patients vaccinated after aHCT, van del Velden et al report response rates of 94% for HiB, 78% for heptavalent pneumococcal conjugate vaccine (Prevnar-7), and 61% for the non-conjugated 23-valent pneumococcal vaccine (Pneumovax) 16. In our study, 71% responded to HiB, with the difference likely due to the exclusion of patients who retained immunity based on the pre-titer. Antin et al found a slightly lower rate of 60% responded to Prevnar-7 when given at 3, 6, and 12 months after aHCT, which was similar to our response rate of 58% with the Prevnar-1314. In a prior study at our center evaluating the Adacel vaccine, only 2/28 patients had a response to pertussis, which was thought to be secondary to the lower dose of pertussis toxin (PT) in the vaccine17. In our current study, patients received Boostrix which contains a higher dose of PT and 76% of patients responded. Therefore, in the adult population, it does appear that the higher dose of PT is needed for response. Overall, the response rates for the majority of vaccines were high. Notable exceptions included hepatitis A, hepatitis B, and polio in the few patients who were evaluable for response. The exact etiology of this is unclear, and further study into the immunogenicity of the vaccine or the schedule on which it is given after transplant is needed. These results also show the need for demonstration of response by titer and the rates of retained immunity which may wane over time necessitating intermittent monitoring of titers or the need for booster vaccinations.
Lenalidomide is an immunomodulatory agent that enhances immune response19,20. In a study of 17 MM patients, Noonan et al evaluated the ability of lenalidomide to augment vaccine responses20. They found that patients vaccinated with 2 doses of pneumococcal 7-valent conjugate vaccine while on lenalidomide had higher humoral and cellular responses compared to those who received the first dose prior to the initiation of lenalidomide. In our study, there was no difference in the response rates of patients on LM compared to those who were not on LM. This may be related to the larger number of patients in our study or the high rates of response in both groups.
Finally, analysis of live vaccines including measles, mumps, rubella (MMR)15 or varicella was limited due to the small number of patients who received these in this cohort (32/120 for MMR and 5/120 for varicella), and further research is needed to define the safety and efficacy of these vaccines for MM patients on LM after aHCT, especially given the recent outbreaks of measles around the country. Current recommendations by the CDC are that MMR and varicella vaccines should be administered 24 months after transplantation if the recipient is presumed to be immunocompetent21. Definitions for immunocompetence after aHCT are not well defined, and we plan to study this further in the patients who have been transplanted in more recent years when our center had an advanced practice provider who focused on vaccination for HCT patients. However, no patients contracted these illnesses after vaccination, and we believe these results are hypothesis generating for studies on safety and efficacy in this population. Issa et al. reported on the varicella vaccine in the post-transplant setting and also found minimal complications22.
Limitations of our study include the smaller portion of patients not on LM at the time of vaccination. Maintenance therapy is standard of care at our institution for patients without contraindications and therefore the number of patients not receiving it in the more recent years has decreased. Patients undergoing aHCT in earlier years were not included as vaccination and titer monitoring was less systematic during that time. Minimal data for the meningococcal vaccine were available at our center, so we did not include it in this study, but Mahler et al. have reported minimal response with a single dose after allogeneic HCT23. The ASBMT and CDC recommend that post-HCT meningococcal re-immunization with the 2 dose series to follow the criteria for the general population24. At our center, we would vaccinate those who are going to college, who are functionally or anatomically asplenic, military recruits, and those travelling to endemic areas, which would be a small number of the adult patient population, and response in this population requires further study. Receiving the full series of vaccinations is often challenging, especially without a dedicated survivorship/vaccination team, partly due to the extra visits required which may not fit the planned follow-up schedule. In this study, this was especially true for the hepatitis vaccines, and additional study of barriers may be helpful. Furthermore, the cutoffs for titer response are not uniformly accepted. We have combined criteria from several prior studies, manufacturer recommendations, and expert opinion to create our cutoffs in Table 1, but acknowledge that the response rates may change with alternative definitions. Finally, as vaccination is often deferred in patients on active therapy and the number of patients in our study with relapsed disease or on combination therapy was minimal, further study is needed for this population. It is therefore possible that our population includes mostly patients doing well and able to comply with necessary follow-up, though patients receiving vaccinations locally were also not included in our study.
An additional area of future investigation is the timing of vaccination given the wide variation reported in both the guidelines and expert opinion11,12,25. Though we did not evaluate the influenza vaccine in our study as titers are not checked or readily available, Sokol et al recently reported that 1½4 patients responded after a median time to vaccination of 4.2 months26. Hence, the influenza vaccine may be worthwhile to administer regardless of the timing flu season in the post transplant course.
Overall, we show similar rates of vaccine responses between MM patients who were or were not receiving LM after aHCT. Re-immunization with inactivated vaccines in patients on LM is therefore both safe and effective, and as patients on LM after aHCT have a prolonged survival, efforts should be made to offer this population immunity to vaccine preventable diseases.
Supplementary Material
Highlights:
Inactivated vaccines are safe on lenalidomide maintenance after auto transplant.
Response rates are similar to previously reported after transplant.
Additional study is needed for live vaccines in this setting.
Acknowledgements
This research was supported in part by National Institutes of Health award number P01 CA23766 and NIH/NCI Cancer Center Support Grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest statement
No authors declare competing financial interests
References:
- 1.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013 NCI. http://seer.cancer.gov/csr/1975_2013#sthash.UgkMcBA3.dpuf. Published 2016. Accessed April 19, 2017.
- 2.Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. AJH 2016;91(7):719–734. doi: 10.1002/ajh.24402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah N, Callander N, Ganguly S, et al. Hematopoietic Stem Cell Transplantation for Multiple Myeloma: Guidelines from the American Society for Blood and Marrow Transplantation. BBMT 2015;21(7):1155–1166. doi: 10.1016/j.bbmt.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after Stem-Cell Transplantation for Multiple Myeloma. NEJM 2012;366(19):1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide Maintenance after Stem-Cell Transplantation for Multiple Myeloma. NEJM 2012;366(19):1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 6.Advisory Committee on Immunization Practices. Recommended Immunization Schedule for Persons Age 0 Through 18 Years CDC. http://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent.html. Published 2014.
- 7.Elam-Evans L, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years--United States, 2013. MMWR 2014;63(29):625–633. [PMC free article] [PubMed] [Google Scholar]
- 8.Seither R, Masalovich S, Knighton C, Mellerson J, Singleton J, Greby S. Vaccination coverage among children in kindergarten - United States, 2013–14 school year. MMWR 2014;63(41):913–920. [PMC free article] [PubMed] [Google Scholar]
- 9.Omer S, Enger K, Moulton L, Halsey N, Stokley S, Salmon D. Geographic clustering of nonmedical exemptions to school immunization requirements and associations with geographic clustering of pertussis. Am J Epidemiol 2008;168(12):1389–1396. doi: 10.1093/aje/kwn263. [DOI] [PubMed] [Google Scholar]
- 10.Omer SB, Salmon DA, Orenstein WA, Halsey N. Vaccine Refusal, Mandatory Immunization, and the Risks of Vaccine-Preventable Diseases. NEJM 2009;360(19):1981–1988. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter PA, Englund JA. How I vaccinate blood and marrow transplant recipients. Blood 2016;127(23):2824–2832. doi: 10.1182/blood-2015-12-550475. [DOI] [PubMed] [Google Scholar]
- 12.Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. CID 2014;58(3):e44–100. doi: 10.1093/cid/cit684. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi MK, Egner W, Sizer L, et al. Antibody responses to vaccinations given within the first two years after transplant are similar between autologous peripheral blood stem cell and bone marrow transplant recipients. BMT 2001;28(8):775–781. doi: 10.1038/sj.bmt.1703239. [DOI] [PubMed] [Google Scholar]
- 14.Antin JH, Guinan EC, Avigan D, et al. Protective antibody responses to pneumococcal conjugate vaccine after autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2005;11(3):213–222. doi: 10.1016/j.bbmt.2004.12.330. [DOI] [PubMed] [Google Scholar]
- 15.Small TN, Rice RD, McCullagh E, et al. Vaccine Efficacy Following Autologous Peripheral Blood Stem Cell Transplant for Lymphoma. Blood 2007;110:603 http://www.bloodjournal.org/content/110/11/603?sso-checked=true. Accessed April 23, 2017. [Google Scholar]
- 16.van der Velden AMT, Claessen AME, van Velzen-Blad H, et al. Vaccination responses and lymphocyte subsets after autologous stem cell transplantation. Vaccine 2007;25(51):8512–8517. doi: 10.1016/j.vaccine.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Small TN, Zelenetz AD, Noy A, et al. Pertussis immunity and response to tetanus-reduced diphtheria-reduced pertussis vaccine (Tdap) after autologous peripheral blood stem cell transplantation. BBMT 2009;15(12):1538–1542. doi: 10.1016/j.bbmt.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Small T, Cowan M. Immunization of hematopoietic stem cell transplant recipients against vaccine-preventable diseases. Expert Rev Clin Immunol 2011;7(2):193–203. doi: 10.1586/eci.10.103. [DOI] [PubMed] [Google Scholar]
- 19.Wu L, Adams M, Carter T, et al. Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res 2008;14(14):4650–4657. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 20.Noonan K, Rudraraju L, Ferguson A, et al. Lenalidomide-induced immunomodulation in multiple myeloma: impact on vaccines and antitumor responses. Clin Cancer Res 2012;18(5):1426–1434. doi: 10.1158/1078-0432.CCR-11-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. The Pink Book: General Recommendations on Immunization https://www.cdc.gov/vaccines/pubs/pinkbook/genrec.html. Published 2016. Accessed August 1, 2017.
- 22.Issa NC, Marty FM, Leblebjian H, et al. Live attenuated varicella-zoster vaccine in hematopoietic stem cell transplantation recipients. BBMT 2014;20(2):285–287. doi: 10.1016/j.bbmt.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Mahler MB, Taur Y, Jean R, Kernan NA, Prockop SE, Small TN. Safety and Immunogenicity of the Tetravalent Protein-Conjugated Meningococcal Vaccine (MCV4) in Recipients of Related and Unrelated Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant 2012;18(1):145–149. doi: 10.1016/j.bbmt.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. BBMT 2009;15(10):1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alemu A, Richards JO, Oaks MK, Thompson MA. Vaccination in Multiple Myeloma: Review of Current Literature. Clin Lymphoma Myeloma Leuk 2016;16(9):495–502. doi: 10.1016/j.clml.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Sokol KA, Kim-Schulze S, Robins H, et al. Timing of Influenza Vaccine Response in Patients That Receive Autologous Hematopoietic Cell Transplantation. BBMT 2017;23(3):S143–S144. doi: 10.1016/j.bbmt.2016.12.266. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.