Abstract
We identified areas of the brain that are critical for naming pictures of objects, using a new methodology for testing which components of a network of brain regions are essential for that task. We identified areas of hypoperfusion and structural damage with magnetic resonance perfusion- and diffusion-weighted imaging immediately after stroke in 87 individuals with impaired picture naming. These individuals were reimaged after 3–5 d, after a subset of patients underwent intervention to restore normal blood flow, to determine areas of the brain that had reperfused. We identified brain regions in which reperfusion was associated with improvement in picture naming. Restored blood flow to left posterior middle temporal/fusiform gyrus, Broca's area, and/or Wernicke's area accounted for most acute improvement after stroke. Results show that identifying areas of reperfusion that are associated with acute improvement of a function can reveal the brain regions essential for that function.
Keywords: aphasia, stroke, perfusion, ischemia, cognitive, cortex, language, mapping, magnetic resonance imaging
Introduction
For hundreds of years, investigators have sought to identify areas of the brain that are responsible for a particular cognitive task, such as calculation or picture naming, with limited success. Early studies attempted to identify a single brain region in which damage caused disruption of a task. However, lesions in different brain regions were often found in individuals with the same impairment of higher cognitive function. One explanation of this finding is that any cognitive task demands a number of mental processes that might be performed in different brain regions. For example, naming a pictured object requires at least visual mechanisms that enable recognition of the object, semantic processes or representations that enable distinguishing the object from similar objects with different names, phonological processes that enable access to the learned pronunciation of the name, and motor processes that enable articulation of the name. These component processes plausibly take place in separate brain regions. Functional neuroimaging studies [positron emission tomography (PET) and functional magnetic resonance imaging (fMRI)] have recently confirmed that any cognitive task such as picture naming results in activation of a complex network of interacting brain areas (Price et al., 2005). However, some areas of activation may not be essential to the task. Thus, it is commonly held that lesion studies and functional imaging studies should be complementary (Chatterjee, 2005; Fellows et al., 2005; Muller and Knight, 2006). That is, functional imaging can reveal all brain regions engaged in a task, and lesions to each area can determine whether or not each area is essential to the task. However, this approach requires identifying the entire area of neural dysfunction that might be contributing to the functional deficit.
Magnetic resonance perfusion-weighted imaging (PWI) and diffusion-weighted imaging (DWI), which together reveal areas of dysfunctional tissue (areas of low blood flow as well as structural damage) in acute stroke, combined with cognitive assessment the same day, can reveal areas of brain that are essential for a complex task such as picture naming (Croquelois et al., 2003; Hillis et al., 2005a; Karnath et al., 2005). However, even stronger evidence that an area of the brain is essential for a particular task is obtained when dysfunction of that area is associated with impaired performance of the task, and restored tissue function in that area is associated with recovery of the performance. This sort of evidence is obtainable in the setting of acute ischemic stroke, when blood flow can be restored to an area that was receiving insufficient blood flow to function, as demonstrated previously only in single case studies (Hillis et al., 2001a, 2003a). Here we identify the areas of brain in which reperfusion (restored blood flow) contributed to improvement in picture naming in a large group of patients, revealing areas that are essential for the task.
Materials and Methods
Subjects.
The population consisted of a consecutive series of patients with acute left-hemisphere ischemic stroke who were admitted to Johns Hopkins Hospital and met the following inclusion criteria: (1) right handed; (2) premorbidly proficient in English; (3) able to provide informed consent for the study or indicate a family member who provided informed consent; (4) no contraindication to MRI (e.g., implanted ferrous metal); and (5) no previous diagnosis of dementia or other neurological disease, significant hearing loss, or blindness. All patients with language comprehension deficits assented to the study to the best of their understanding, and a family member provided informed consent using procedures and forms approved by the Johns Hopkins Institutional Review Board. A total of 217 patients met these criteria and consented to research participation. The current study was restricted to a subset of 87 patients who had impaired naming (as defined by accuracy in naming a set of pictures >2 SDs that of non-brain-damaged age-matched control subjects) and who completed language tests and imaging with both DWI and PWI at day 1 and days 3–5. The majority of patients who consented to research were excluded from this analysis because they did not complete the follow-up language testing or the imaging at days 3–5; others were excluded because they made no errors in picture naming at day 1 (and therefore could not show improvement) or because of technically inadequate scans.
Language tests.
Within 24 h of onset of stroke symptoms (day 1) and at days 3–5, patients were administered tests of oral naming of 17 pictured objects and 17 objects presented for tactile exploration; written naming of 17 pictured objects; repetition and spelling of 34 words; oral reading of 34 words; visual and auditory lexical decision; and spoken and written word comprehension. Each word comprehension task required word/picture verification, each with 17 items presented three times (once with a semantically related foil, once with a phonologically related foil, and once with the target). A correct response required rejection of both foils and acceptance of the target. Items across tests were matched for word frequency and length. On days 3–5, patients had repeat testing with separate forms of each test, with items matched for frequency, word length (in letters and syllables), and grammatical word class.
Treatment to restore blood flow.
A subset of 24 patients had some medical or surgical intervention to restore or improve cerebral blood flow in ischemic areas. Interventions included the following: (1) temporary blood pressure elevation with fluids or pressor medications, which results in a linear increase in regional cerebral blood flow to poorly perfused but noninfarcted areas (Skyhøj Olsen et al., 1983; Rordorf et al., 2001; Hillis et al., 2003b) in 18 patients; (2) urgent carotid endarterectomy in one patient; (3) intra-arterial thrombolysis in one patient; (4) carotid stents placed in two patients; and (5) immunosuppressive treatment for vasculitis in two patients. Many of the untreated patients showed some spontaneous reperfusion on the follow-up scans.
Imaging and image analysis.
DWI trace images [bmax = 1000 s/mm2; repetition time (TR)/echo time (TE), 10,000/120 ms] and PWI (TR/TE, 2000/60 ms) scans were obtained on a GE Signa 1.5 tesla echo planar imaging-capable scanner (GE Healthcare, Chalfont St. Giles, UK), with whole-brain coverage and 5 mm slice thickness; flip angle was 90°, and field of view was 240 mm. Apparent diffusion coefficient (ADC) maps were generated from the b = 1000 and b = 0 images to confirm the acuity of the DWI lesion. PWI images were obtained with injections of 20 cc gadolinium diethylene triamine pentaacetic acid at a rate of 5 cc/s and postprocessed to yield maps of time-to-peak (TTP) bolus concentration. Regions of hypoperfusion were defined as >4 s delay in time-to-peak delay in arrival of contrast relative to the delay in the homologous region of in the intact hemisphere. Although this threshold of hypoperfusion does not necessarily correspond to tissue that is at risk for progressing to infarction in the absence of restored blood flow (Thijs et al., 2001), it is a threshold that corresponds to dysfunction as indicated by a strong association with impaired performance (Neumann-Haefelin et al., 1999; Hillis et al., 2001b) and positron emission tomography (Sobesky et al., 2004). Regions of hypoperfusion on TTP maps were transferred to a Brodmann's atlas and to the Montreal Neurological Institute (MNI) atlas using MRICro (http://www.sph.sc.edu/comd/rorden/mricro.html) by technicians without knowledge of the patients' performance on language tasks. DWI abnormalities were also manually transferred to the Brodmann's atlas and to the MNI atlas in the same way. Abnormal areas on DWI were defined as areas that were bright on DWI and dark on ADC maps. The entire region of hypoperfusion plus the area of infarct (usually overlapping areas) was considered the area of tissue dysfunction on each scan. Reperfusion of a given Brodmann's area (BA) (an area of the brain with distinct cytoarchitecture) was defined as hypoperfusion of that region at day 1 and normal perfusion (<4 s delay in TTP and at least 2 s improvement in TTP compared with day 1) and the absence of DWI abnormality in that area at follow-up (days 3–5). The following BAs were examined for reperfusion: 19, 20, 21, 22, 37, 38, 39, 40, and 44/45, because these are areas that show activation in functional imaging studies of object naming (Howard et al., 1992; Price et al., 2006)
Statistical analysis.
Areas of reperfusion that contributed to improvement in oral naming of pictures from day 1 to days 3–5 were identified using stepwise linear regression. To confirm the findings, we also evaluated the association between reperfusion in each of the regions and improvement in naming by at least 10 percentage points, using χ2 tests for each individual area. An α level of <0.05 after Bonferroni correction for multiple comparisons was used to identify significant associations. To determine whether the identified regions of reperfusion contributed specifically to improvement of oral naming, versus improvement of all language tasks, we identified areas of reperfusion that contributed to improvement in spoken word comprehension (tested with word/picture verification) as well.
In addition, using MRICro, we identified areas of reperfusion in all patients who showed improvement in naming by at least 10 percentage points by subtracting the combined areas of dysfunctional tissue on the initial scans of these patients (transferred to the MNI atlas) from the combined areas of dysfunctional tissue on the follow-up scans done at days 3–5. This subtraction reveals the overlap of the brain regions that were initially hypoperfused but were no longer hypoperfused at days 3–5.
Results
Among the 87 patients who met our selection criteria, the mean ± SD accuracy in picture naming on day 1 was 45.9 ± 39% (compared with 99.2 ± 2.1% for 50 healthy controls; age, 64.7 ± 10.7 years) and on days 3–5 was 59.4 ± 40.1%. On day 1, patients scored from 0 to 94.1% correct; 22% made only one error. On days 3–5, patients scored from 0 to 100% correct; 39.1% of patients made only one or no errors (17.2% made no errors). A total of 42.5% improved by at least 10 percentage points.
The degree of improvement in oral naming of pictures was best predicted by the following model derived from the stepwise linear regression, where IN stands for improvement in naming accuracy and RP stands for “reperfusion of” as defined above: IN = RP BA 37 × 0.71 + RP BA 44/45 × 0.25 + RP BA 22 × 0.20 + 0.13 (r = 0.81; p < 0.0001).
The coefficients indicate the “weight” of the contribution to predicting improvement in naming. Areas in which reperfusion was not associated with the degree of improvement independently of the identified areas are not included in the model. This model indicates that reperfusion of left BA 37 (posterior middle and inferior temporal/fusiform gyrus) was the strongest predictor of acute improvement in picture naming after stroke (p < 0.0001). Reperfusion of left BA 44/45 (Broca's area; p = 0.027) and reperfusion of left BA 22 (Wernicke's area; p = 0.047) also independently contributed to the prediction of early improvement in naming after adjustment for each other and for left BA 37 reperfusion. Although improvement in some patients may have occurred with reperfusion of two or more of these areas, stepwise regression demonstrated that there was at least some independent contribution from each of these three areas in a multivariate model.
Furthermore, all 27 (100%) patients who showed reperfusion of left BA 37 showed improved oral picture naming by at least 10 percentage points (see Fig. 1 for an illustrative case). Only 10 of 60 (17%) patients who failed to reperfuse BA 37 showed that degree of improvement in picture naming (χ21 = 53; p < 0.0001). These patients showed reperfusion of BA 22 or BA 44/45 (or both). The fact that 50 patients failed to show improvement by at least 10 percentage points indicates that improvement by this degree (which was strongly associated with reperfusion of BA 37) is unlikely to be caused by a practice effect or rapid reorganization of structure–function relationships.
Figure 1.
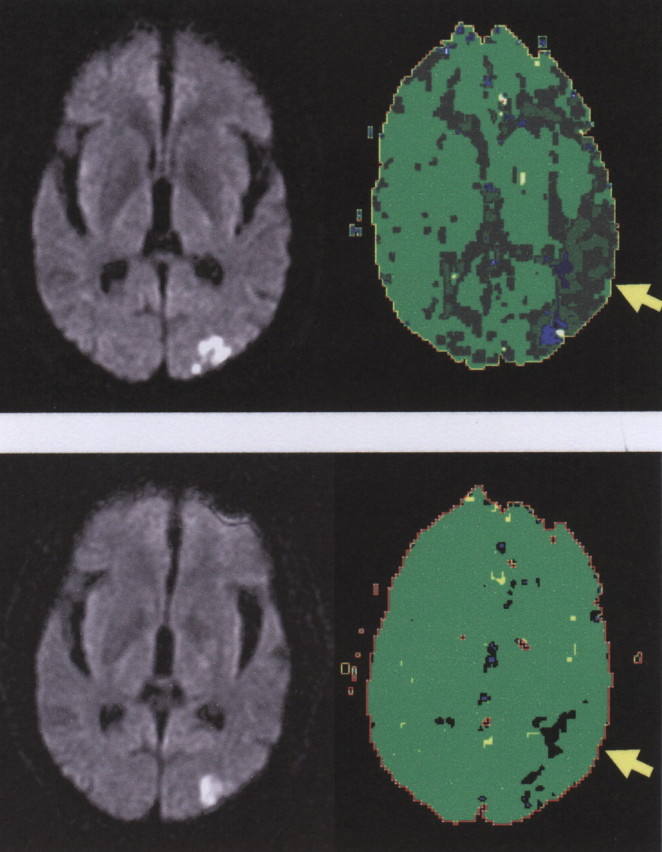
An illustrative case in which reperfusion of BA 37 was associated with an improvement of naming. Top, Day 1 DWI (left) and PWI (right) scans of a patient with impaired naming at day 1 of stroke. Bottom, DWI (left) and PWI (right) scans of the same patient on day 3, when naming had recovered. Dark green and blue areas on PWI are hypoperfused. Light green areas are normally perfused. The yellow arrow points to BA 37.
Reperfusion of left BA 37 was not associated with improvement in all language tasks. The degree of improvement in spoken word comprehension (assessed with spoken word/picture verification) was best predicted by the following model derived from the stepwise linear regression analysis: Improvement in Spoken Comprehension = RP BA 22 × 0.81 + 0 (r = 0.60; p < 0.0001). That is, only reperfusion of BA 22 (Wernicke's area) was significantly associated with improvement in spoken word comprehension.
To illustrate the most common regions or voxels of reperfusion in patients who improved in naming by at least 10 percentage points, we subtracted the sum of the regions of dysfunctional tissue at day 1 from the sum of the regions of dysfunctional tissue at days 3–5 in these patients (Fig. 2). This illustration is consistent with the results of the statistical tests above, showing that the majority of patients who improved in naming showed reperfusion of a portion of left BA 37 (and that small areas of BA 22 and BA 44/45 were also commonly reperfused in this group). The subtraction cannot show which voxels were reperfused independently versus together.
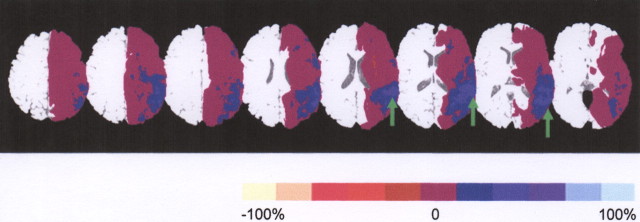
Figure 2.
Summary of MR perfusion changes in patients who improved in picture naming. Blue represents areas in which 20% (dark blue) to 100% (light blue) of patients who improved in naming showed hypoperfusion on the first but not the second scan. Purple areas represent areas in which no patients who improved showed a change in perfusion between the first and second scan. Red represents areas in which up to 20% of patients showed hypoperfusion on the second but not the first scan. Green arrows point to part of BA 37, in which 70–90% of patients who improved in naming showed reperfusion.
Discussion
These data show that reperfusion of each of three cortical regions (left BA 37, BA 44/45, and BA 22) was at least somewhat independently associated with improvement in oral naming in the first few days after stroke. These results provide novel evidence for the hypothesis that a network of perisylvian and extrasylvian areas of the left hemisphere together support naming. The results also explain why patients with quite different loci of damage often have deficits in naming (Goodglass and Wingfield, 1997). Damage to any of these critical areas should interfere with oral naming.
Converging evidence from previous lesion studies and functional imaging studies indicate that these three areas have different functions in naming. Chronic lesion studies have long pointed to an essential role of left BA 22 (Wernicke's area) in linking spoken words to their meanings, which is an important component of naming (Hart and Gordon, 1990). Likewise, direct cortical stimulation of Wernicke's area interferes with word meaning (Lesser et al., 1986) and naming (Ojemann, 1994). Furthermore, previous studies of acute stroke patients have demonstrated that the severity of hypoperfusion of Wernicke's area was linearly related to the severity of word comprehension deficits (Hillis et al., 2001b). Functional imaging studies (PET and fMRI) also provide evidence for the involvement of Wernicke's area in accessing the meanings of words in both naming and word comprehension (Wise et al., 2001; Leff et al., 2002; Saur et al., 2006).
Left BA 44/45 (Broca's area) has long been thought to be critical for motor programming/planning of speech articulation, which is another important component of oral naming. Studies of patients with chronic lesions (Broca, 1865; Mohr, 1978; Schiff et al., 1983; Alexander et al., 1990) and acute dysfunction (Hillis et al., 2004) show that tissue dysfunction in Broca's area is associated with impaired orchestration of speech articulation. PET and fMRI studies have also demonstrated activation of left BA 44/45 during speech articulation in both naming and oral reading (Price et al., 2005) or in propositional and nonpropositional speech (Blank et al., 2002). In light of these results, it is not surprising that reperfusion (restored function) of left BA 22 and/or BA 44/45 was associated with improved spoken naming.
The role of left BA 37 in naming has been more controversial. A few studies of acute or subacute stroke have indicated that lesions or hypoperfusion of left BA 37 is associated with impaired naming without comprehension deficits (Raymer et al., 1997; Foundas et al., 1998), but these studies have not evaluated the possibility that other regions are also essential for naming. Functional imaging studies often show activation of left BA 37 in modality-independent lexical processing (Price et al., 2003; Tranel et al., 2005), indicating that this area may be recruited in a variety of lexical tasks, including naming. Cases in which oral naming of objects presented for tactile exploration and oral reading, as well as oral naming, were impaired when this region was poorly perfused provide additional support for this hypothesis (Hillis et al., 2005a). The present results provide new evidence that left BA 37 is critical for naming, by showing that reperfusion of left BA 37, resulting in recovery of tissue function, was associated with improvement in oral naming in a large group of acute stroke patients.
Moreover, our results illustrate a novel methodology for testing hypotheses generated from functional imaging studies about the networks of brain regions essential for a complex cognitive task. Although many authors have argued that lesion studies complement functional imaging studies by demonstrating areas of the brain that are necessary for (and not simply engaged in) a task, a number of limitations of the lesion–deficit approach to studying brain–behavior relationships have been identified. Typically, investigators have identified individuals who developed impairment of some task after focal brain damage (usually stroke), and then identified the areas of brain most commonly damaged in those individuals. However, because the cerebral vasculature is similar across individuals, the area most commonly damaged after stroke may simply be the area most vulnerable to ischemia. Particularly when patients are studied months or years after stroke, most infarcts are quite large (because patients with small infarcts generally recover within days or weeks after stroke). Therefore, when only patients with residual deficits are studied, most patients with small lesions are excluded. To identify areas most commonly associated with a deficit that failed to recover, investigators have relied on finding the area of “overlap” in the large lesions. However, this area of overlap tends to be an area, such as the insula, that is commonly infarcted in all large strokes, which are generally caused by occlusion of the internal carotid or middle cerebral artery (Finley et al., 2003; Hillis et al., 2004). To avoid this limitation, it is necessary to determine the probability that damage to an area will cause the deficit, as well as the probability that the deficit will be associated with the lesion site. However, to determine the frequency with which damage to a given area results in the impairment requires studying patients immediately after stroke, because there is often extensive reorganization of structure–function relationships after stroke (Jenkins and Merzenich, 1987; Jenkins et al., 1990). That is, intact areas of brain often assume the functions of the damaged area over time.
Studying lesion–deficit relationships acutely after stroke is complicated by the fact that areas of low blood flow surrounding the structural damage may contribute to the observed deficits (Hillis et al., 2002a; Croquelois et al., 2003). Most studies have identified only the structural damage after stroke and assumed that the permanently damaged area caused the deficit. In this study and previous studies, we have identified areas of dysfunctional tissue with DWI and PWI at the very onset of stroke, and we have identified deficits with cognitive assessment at nearly the same time, to identify areas of brain in which acute tissue dysfunction causes impairment of a particular cognitive function (Hillis et al., 2002b, 2005b; Karnath et al., 2005). However, even stronger evidence that specific areas of the brain are essential for naming was obtained in this study when dysfunction of those areas was associated with impaired performance of the task and restored tissue function in those areas was associated with recovery of the performance. Recovery of normal performance was important to demonstrate that the naming deficit was not present before the stroke (e.g., because of a developmental abnormality or aging). Furthermore, recovery of naming did not occur without reperfusion of one or more of these critical areas, demonstrating that improvement in naming was likely caused by improved tissue function and not by reorganization of structure–function relationships in the brain.
In summary, there are several advantages of this methodology over traditional lesion studies. Identifying areas of hypoperfusion as well as dense ischemia or infarct before and after attempts to restore blood flow can reveal the entire region of dysfunctional brain (including areas that might be distant but functionally connected to the lesioned area) associated with the deficit at each time point. This method also reveals the probability that tissue dysfunction in a particular area is associated with the deficit, as well as the probability that the deficit is associated with tissue dysfunction in that area. Finally, deficits that recover with reperfusion cannot be attributed to premorbid deficits, and deficits that recover only with reperfusion cannot be attributed to reorganization of structure–function relationships. We have demonstrated the usefulness of this methodology in this study by identifying areas of brain in which reperfusion was associated with improvement in picture naming in a large group of patients, to reveal areas of the brain that are essential for naming.
Footnotes
This work was supported by National Institutes of Health Grants R01: DC05375 and P41 RR15241.
References
- Alexander MP, Naeser MA, Palumbo C (1990). Broca's area aphasias: aphasia after lesions including the frontal operculum. Neurology 40:353–362. [DOI] [PubMed] [Google Scholar]
- Blank SC, Scott SK, Murphy K, Warburton E, Wise RJ (2002). Speech production: Wernicke, Broca and beyond. Brain 125:1829–1838. [DOI] [PubMed] [Google Scholar]
- Broca P (1865). Sur la faculte du langage articule. Paris Bull Soc Anthr 6:337–393. [Google Scholar]
- Chatterjee A (2005). A madness to the methods in cognitive neuroscience? J Cogn Neurosci 17:847–849. [DOI] [PubMed] [Google Scholar]
- Croquelois A, Wintermark M, Reichhart M, Meuli R, Bogousslavsky J (2003). Aphasia in hyperacute stroke: language follows brain penumbra dynamics. Ann Neurol 54:321–329. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Heberlein AS, Morales DA, Shivde G, Waller S, Wu DH (2005). Method matters: an empirical study of impact in cognitive neuroscience. J Cogn Neurosci 17:850–858. [DOI] [PubMed] [Google Scholar]
- Finley A, Saver J, Alger J, Pregenzer M, Leary M, Ovbiagele B (2003). Diffusion weighted imaging assessment of insular vulnerability in acute middle cerebral artery infarctions. Stroke 34:259. [Google Scholar]
- Foundas A, Daniels SK, Vasterling JJ (1998). Anomia: case studies with lesion localization. Neurocase 4:35–43. [Google Scholar]
- Goodglass H, Wingfield A (1997). In: Word-finding deficits in aphasia: Brain–behavior relations and symptomatology. In: Anomia (Goodglass H, ed), pp 3–27. London: Academic.
- Hart J, Gordon B (1990). Delineation of single-word semantic comprehension deficits in aphasia, with anatomical correlation. Ann Neurol 27:226–231. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Barker P, Beauchamp N, Winters B, Mirski M, Wityk R (2001a). Restoring blood pressure reperfused Wernicke's area and improved language. Neurology 56:670–672. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Tuffiash E, Beauchamp NJ, Jacobs MA, Barker PB, Selnes OA (2001b). Hypoperfusion of Wernicke's area predicts severity of semantic deficit in acute stroke. Ann Neurol 50:561–566. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Barker PB, Beauchamp NJ, Gailloud P, Murphy K, Cooper O, Metter EJ (2002a). Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion. Brain 125:1094–1104. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Tuffiash E, Wityk RJ, Barker PB (2002b). Regions of neural dysfunction associated with impaired naming of actions and objects in acute stroke. Cogn Neuropsychol 19:523–534. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk R, Barker PB (2003a). Neural regions essential for writing verbs. Nat Neurosci 6:19–20. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Ulatowski JA, Barker PB, Torbey M, Ziai W, Beauchamp N, Oh S, Wityk R (2003b). A pilot randomized trial of induced blood pressure elevation: effects on function and focal perfusion in acute and subacute stroke. Cerebrovasc Dis 16:236–246. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Work M, Breese EL, Barker PB, Jacobs MA, Maurer K (2004). Re-examining the brain regions crucial for orchestrating speech articulation. Brain 127:1479–1487. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker PB, Herskovits E, Degaonkar M (2005a). The roles of the “visual word form area” in reading. Neuroimage 24:548–559. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker PB, Degaonkar M (2005b). Anatomy of spatial attention: insights from perfusion imaging and hemispatial neglect in acute stroke. J Neurosci 25:3161–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D, Patterson KE, Wise R, Brown WD, Friston K, Weiller C, Frackowiak R (1992). The cortical localization of the lexicons. Brain 115:1769–1782. [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM (1987). Reorganization of neocortical representations after brain injury: a neurophysiological model of the bases of recovery from stroke. Prog Brain Res 71:249–266. [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Recanzone G (1990). Neocortical representational dynamics in adult primates: implications for neuropsychology. Neuropsychologia 28:573–584. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Zopf R, Johannsen L, Fruhmann Berger M, Nagele T, Klose U (2005). Normalized perfusion MRI to identify common areas of dysfunction: patients with basal ganglia neglect. Brain 128:2462–2469. [DOI] [PubMed] [Google Scholar]
- Leff A, Crinion J, Scott S, Turkheimer F, Howard D, Wise R (2002). A physiological change in the homotopic cortex following left posterior temporal lobe infarction. Ann Neurol 51:553–558. [DOI] [PubMed] [Google Scholar]
- Lesser R, Luders H, Morris HH, Dinner DS, Klem G, Hahn J, Harrison M (1986). Electrical stimulation of Wernicke's area interferes with comprehension. Neurology 36:658–663. [DOI] [PubMed] [Google Scholar]
- Mohr JP (1978). Broca aphasia: pathological and clinical. Neurology 28:311–324. [DOI] [PubMed] [Google Scholar]
- Muller NG, Knight RT (2006). The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience 139:51–58. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Wittsack HJ, Wenserski F, Siebler M, Seitz RJ, Modder U, Freund HJ (1999). Diffusion and perfusion-weighted MRI: the DWI/PWI mismatch region in acute stroke. Stroke 30:1591–1597. [DOI] [PubMed] [Google Scholar]
- Ojemann GA (1994). In: Cortical stimulation and recording in language. London: Academic.
- Price CJ, Winterburn D, Giraud AL, Moore CJ, Noppeney U (2003). Cortical localisation of visual and auditory word form areas: a reconsideration of the evidence. Brain Lang 86:272–286. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT, Moore CJ, Morton C, Laird AR (2005). Meta-analyses of object naming: effect of baseline. Hum Brain Mapp 25:70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, McCrory E, Noppeney U, Mechelli A, Moore CJ, Biggio N, Devlin JT (2006). How reading differs from object naming at the neuronal level. Neuroimage 29:643–648. [DOI] [PubMed] [Google Scholar]
- Raymer A, Foundas AL, Maher LM, Greenwald ML, Morris M, Rothi LG, Heilman KM (1997). Cognitive neuropsychological analysis and neuroanatomical correlates in a case of acute anomia. Brain Lang 58:137–156. [DOI] [PubMed] [Google Scholar]
- Rordorf G, Koroshetz WJ, Ezzeddine MA, Segal AZ, Buonanno FS (2001). A pilot study of drug-induced hypertension for treatment of acute stroke. Neurology 56:1210–1213. [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, Weiller C (2006). Dynamics of language reorganization after stroke. Brain 129:1371–1384. [DOI] [PubMed] [Google Scholar]
- Schiff HB, Alexander MP, Naeser MA, Galaburda AM (1983). Aphemia. Clinical-anatomic correlations. Arch Neurol 40:720–727. [DOI] [PubMed] [Google Scholar]
- Skyhøj Olsen T, Larsen B, Herning M, Skriver EB, Lassen N (1983). Blood flow and vascular reactivity in collaterally perfused brain tissue. Stroke 14:332–341. [DOI] [PubMed] [Google Scholar]
- Sobesky J, Zaro Weber O, Lehnhardt FG, Hesselmann V, Thiel A, Dohmen C, Jacobs A, Neveling M, Heiss WD (2004). Which time-to-peak threshold best identifies penumbral flow? A comparison of perfusion-weighted magnetic resonance imaging and positron emission tomography in acute ischemic stroke. Stroke 35:2843–2847. [DOI] [PubMed] [Google Scholar]
- Thijs VN, Adami A, Neumann-Haefelin T, Moseley ME, Marks MP, Albers GW (2001). Relationship between severity of MR perfusion deficit and DWI lesion evolution. Neurology 57:1205–1211. [DOI] [PubMed] [Google Scholar]
- Tranel D, Grabowski TJ, Lyon J (2005). Naming the same entities from visual or from auditory stimulation engages similar regions of left inferotemporal cortices. J Cogn Neurosci 17:1293–1305. [DOI] [PubMed] [Google Scholar]
- Wise RJS, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA (2001). Separate neural subsystems within “Wernicke's area.”. Brain 124:83–95. [DOI] [PubMed] [Google Scholar]