Abstract
Previous research has shown that cAMP response element (CRE)-mediated transcription is activated in the nucleus accumbens, a major brain reward region, by a variety of environmental stimuli and contributes to neuroadaptations to these stimuli. CRE-binding protein (CREB) is the most studied activator of CRE transcription and has been implicated in this brain region as a gating mechanism for behavioral responses to emotional stimuli. Little attention, however, has been given to naturally occurring inhibitors of CRE-mediated transcription, such as the inducible cAMP early repressor (ICER), an inhibitory product of the CRE modulator gene. In the present study, we investigated the extent to which ICER is induced in the nucleus accumbens by two types of environmental stimuli, stress and amphetamine, and characterized how induction of ICER in this region affects complex behavior. We show that stress and amphetamine each induces ICER expression and that overexpression of ICER in the nucleus accumbens, using viral-mediated gene transfer, increases behavioral responses to both rewarding and aversive emotional stimuli. For example, ICER overexpression increases sensitivity to amphetamine-stimulated locomotor activity as well as to natural rewards such as sucrose and social grooming. However, ICER overexpression also increases measures of anxiety in the elevated plus maze and neophobia to novel tastes. Finally, ICER produces an antidepressant-like effect in the forced swim test, further indication of an enhanced active response to stress. These results suggest that ICER is an important mechanism for modulating CRE-mediated transcription in the nucleus accumbens.
Keywords: depression, addiction, anxiety, drugs of abuse, stimulants, CREB, CREM, striatum, cocaine, sucrose preference
Introduction
Gene transcription is one mechanism by which an organism can adapt to changing environmental conditions. The cAMP response element (CRE) is a promoter/enhancer element present in many genes (Impey et al., 2004; Zhang et al., 2005) that has proven sensitive in many brain areas to diverse environmental stimuli ranging from psychotropic drugs to psychological stress to natural reward (for review, see Konradi et al., 1994; Conti and Blendy, 2004; Carlezon et al., 2005). CRE-mediated transcription is increased when CRE-binding protein (CREB) or several related family members dimerize, bind to DNA, and are activated by phosphorylation, although not necessarily in that order (for review, see Mayr and Montminy, 2001). Previous work has shown that increases in CREB-mediated transcription in the shell region of the nucleus accumbens (ventral striatum), induced by drugs of abuse or stress, decrease behavioral responses to both positive and negative emotional stimuli, whereas blocking CRE transcription, via overexpression of a dominant-negative mutant CREB or via genetic knockdown of CREB, generally does the opposite (Carlezon et al., 1998; Pliakas et al., 2001; Walters and Blendy, 2001; Barrot et al., 2002; Newton et al., 2002; Walters et al., 2003). Although much attention has been directed at activation of CRE-mediated transcription by CREB itself, relatively little attention has been directed at naturally occurring inhibitors of CRE-mediated transcription.
The inducible cAMP early repressor (ICER) protein is a highly inducible and potent endogenous repressor of CRE-mediated transcription (Mioduszewska et al., 2003). ICER is an alternative product of the CRE modulator (CREM) gene driven by a second promoter (P2) located in an intron region of the gene. The full-length CREM protein is very similar to CREB in structure and function; the N terminal consists of glutamine-rich activation domains and a kinase-inducible domain also important for activation of transcription. The C terminal consists of a basic region of charged residues for binding DNA at CRE sites and a nearby leucine zipper allowing for dimerization. ICER, because it is missing the N terminus of the CREM gene, dimerizes and binds DNA but cannot be activated because of a lack of activation and kinase-inducible domains. Thus, ICER is an endogenous dominant-negative inhibitor of CRE-mediated transcription. The intronic promoter driving ICER has two pairs of CRE sequences in close proximity, which renders this protein highly inducible by stimuli that activate CRE transcription (Molina et al., 1993; Conti et al., 2004). Accordingly, ICER has been implicated in several biological systems as an important negative-feedback mechanism for shutting off CREB-induced transcription (Servillo et al., 2002; Shepard et al., 2005).
In the present study, we characterized the induction of ICER in the nucleus accumbens by stress or amphetamine and studied the implications of its induction on complex behavior. Our findings demonstrate an important role for ICER in opposing the actions of CREB on regulation of emotional behavior and thereby helping an individual adapt to a range of aversive and rewarding stimuli.
Materials and Methods
Animals.
Male Sprague Dawley rats (Harlan, Houston, TX), 250–350 g, were used for all experiments. Rats were pair-housed in an American Association for the Accreditation of Laboratory Animal Care-approved colony, and all experiments conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1995). Rats were housed on a 12 h light/dark cycle with lights on at 7:00 A.M. All procedures were conducted during the light phase of the cycle.
Quantification of mRNA.
Rats were killed by rapid decapitation, the entire striatum was dissected, and mRNA was isolated using the RNA Stat-60 reagent (Teltest, Houston, TX) according to the manufacturer’s directions. Contaminating DNA was removed with by DNase treatment (DNA-Free, catalog #1906; Ambion, Austin, TX). Purified RNA was reverse transcribed into cDNA (Superscript First Strand Synthesis, catalog #12371-019; Invitrogen, Carlsbad, CA). ICER transcript was quantified using quantitative real-time PCR (SYBR Green; Applied Biosystems, Foster City, CA) on a Stratagene (La Jolla, CA) Mx5000p 96-well thermocycler. Primers for rat ICER were designed and validated (forward primer, CTTTATTTTGGACTGTGGTACGG; reverse primer, AGTAGGAGCTCGGATCTGGTAA). The forward primer bound to the 5′ untranslated region of ICER (P2 of the CREM gene). Because full-length CREM lacks this region, these primers are specific to ICER mRNA. In addition, real-time PCR primers were designed to amplify the major excitatory (τCREM) and inhibitory (αCREM, βCREM, and γCREM) CREM splice variants driven by the P1 promoter. All PCR data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels, which themselves were not altered by stress or amphetamine treatments.
Amphetamine injection for real-time PCR.
Amphetamine hemi-sulfate (Sigma, Dallas, TX) was administered intraperitoneally at a dose of 0.5, 2, or 4 mg/kg. Rats received seven daily injections of saline or amphetamine. In other words, “acute” amphetamine animals received six daily injections of saline, followed by an amphetamine injection on day 7, whereas “7 d” amphetamine animals received seven daily amphetamine injections.
Stress procedures.
For acute restraint stress, rats were placed in a plastic conical sleeve (DecapiCone, model DC200; Braintree Scientific, Braintree, MA) for up to 60 min. Rats were removed from the cone and placed back into their home cage for time points exceeding 60 min. For repeated unpredictable stress, procedures were adapted from Ortiz et al. (1996) and included the following: day 1: cage rotation 50 min at 12:00 P.M., swim stress 4 min at 1:00 P.M.; day 2: wet cage 60 min at 11:00 A.M., lights on overnight; day 3: lights off 180 min at 12:00 P.M., wet cage 15 min at 3:00 P.M.; day 4: cage rotation 50 min at 7:00 P.M., food/water deprivation overnight; day 5: swim stress 3 min at 1:00 P.M., isolation housing overnight; day 6: restraint stress 60 min at 11:00 A.M., lights off 120 min at 3:00 P.M.; day 7: swim stress 4 min at 10:00 A.M., restraint stress 60 min at 12:00 P.M.
ICER mRNA levels in the nucleus accumbens were also measured subsequent to other acute stressors. Separate groups of rats were subjected either to a 15 min forced swim (identical to the first session of the forced swim test described below) or to a 5 min confinement to the open arm of an elevated plus maze. Rats were killed 4 h after these stresses.
Construction of plasmids.
ICER IIγ and τCREM cDNAs were amplified from reverse-transcribed rat brain mRNA. Primers were designed encompassing the coding region of the genes with XbaI and/or BamHI restriction sites (ICER forward primer, TAGTCTAGATCTGTATGCAAAAGCCCAAC; CREM forward primer, TAGTCTAGAGGATCCGTATGACCATGGAA; reverse primers for both, AGATGGATCCCTACTAATCTGTTTTGGGAG) to insert into the herpes simplex virus (HSV) PrPUC amplicon (Neve and Gellar, 1995). HSV-mediated CREM and ICER transcription was driven by a viral IE4/5 promoter. The mutant CREB (mCREB) HSV vector has been described previously (Carlezon et al., 1998; Barrot et al., 2002). All amplicons were packaged using previously described methods (Neve and Gellar, 1995). A β-galactosidase or enhanced green fluorescent protein (GFP) viral vector was used as a control.
Stereotaxic surgery.
HSV vectors were injected bilaterally (1 μl/side in 10 min) into the nucleus accumbens shell using coordinates from Paxinos and Watson (1997) (anteroposterior, 1.7 mm; lateral, 2.4 mm; dorsal, −6.7 mm from bregma; 10° lateral angle). Behavioral measures were taken 48–96 h after surgery when HSV-mediated expression is highest (Barrot et al., 2002).
Validation of virus.
In initial experiments, the HSV–ICER vector was injected into rat nucleus accumbens shell, and expression was verified using fluorescent immunohistochemistry. Polyclonal rabbit antibody to the full-length human CREM protein (sc-440; Santa Cruz Biotechnology, Santa Cruz, CA) was used at 1:2000 to visualize viral-mediated transgene expression. These conditions do not label endogenous CREM or ICER proteins. The secondary antibody (donkey anti-rabbit; Jackson ImmunoResearch, West Grove, PA) was conjugated to CY2 fluorophore and used at a concentration of 1:200. To further verify expression, PC12 cells were infected with HSV–LacZ or HSV–ICER, and protein was harvested 12 h after infection. ICER protein was identified via Western blot using the CREM antibody at a concentration of 1:1000. To verify the ability of HSV–ICER to decrease expression of CRE− target genes, HSV–ICER and HSV–LacZ were each mixed with a small amount (10%) of HSV–GFP, and the two mixtures were injected into the nucleus accumbens of separate groups of rats. At 48 h after injection, rats were given injections of 4 mg/kg amphetamine. Brains were harvested 3 h later and frozen on dry ice. Brains were later sliced on a cryostat (8 μm), and slices were mounted on polyethylene naphthalate-membrane slides for laser microdissection. Using GFP as a marker for the site of infection, infected tissue was microdissected by laser capture, and RNA was purified using the PicoPure RNA isolation kit (Arcturus, Mountain View, CA). RNA was then amplified using the RiboAmp OA kit (Arcturus) according to the manufacturer’s instructions. RNA was subsequently reverse transcribed using the Superscript III first-strand kit (Invitrogen). Levels of brain-derived neurotrophic factor (BDNF) mRNA, a well characterized CREB target, were measured using real-time PCR. All PCR data are normalized to GAPDH, which was not itself affected by ICER.
To further verify the ability of HSV–ICER to decrease CRE-driven transcription, viral vectors were also injected into the nucleus accumbens of CRE–LacZ reporter mice. These transgenic mice express β-galactosidase driven by a synthetic promoter consisting of seven tandem repeats of the consensus CRE sequence upstream of a minimal promoter (Barrot et al., 2002; Shaw-Lutchman et al., 2003). For double-labeling experiments, a goat anti-β-galactosidase primary antibody (catalog #4600-1409; Biogenesis, Kingston, NH) was used at a concentration of 1:5000, with a CY3-conjugated secondary antibody. Brains were fixed with 4% paraformaldehyde before sectioning and staining. A laser-scanning confocal microscope was used for imaging. The Z-stack images were analyzed for pixel intensity using the Volocity (Improvision, Lexington, MA) computer program. Mean pixel intensity of uninfected LacZ-positive cells (n = 51) was compared with that of infected LacZ-positive cells (n = 21 in three mice).
Chromatin immuunoprecipitation (ChIP) assays were performed exactly as described by Kumar et al. (2005) to study the binding of endogenous ICER to target gene promoters. Briefly, chromatin was isolated from formalin-fixed rat nucleus accumbens, sheared, and immunoprecipitated with the anti-CREM antibody or with non-immune IgG. Levels of the BDNF gene present in the immunoprecipitates were then analyzed by real-time PCR. In contrast to the CREM antibody, non-immune IgG did not precipitate the BDNF gene.
Locomotor activity.
Activity was measured in a circular corridor (10 cm wide × 60 cm in diameter × 30 cm high; Med Associates, St. Albans, VT) via four photoelectric cells located every 90° along the circle as described previously (Rahman et al., 2003). For spontaneous locomotor activity, rats were placed in circular corridor chambers and total activity was measured for 120 min. For amphetamine-stimulated locomotor activity, rats were given two habituation sessions (60 min each day, beginning 48 h after surgery) before testing. On the test day (day 4 after surgery), rats were placed in corridors for 60 min before they were given intraperitoneal injections of 2 or 4 mg/kg amphetamine hemi-sulfate. Locomotor activity was assessed for an additional 120 min.
Sucrose preference and neophobia.
For sucrose preference, water was removed and rats were pre-exposed to a 1% (w/v) sucrose solution for 3 d ending at least 48 h before surgery. For testing, rats were first isolated at 5:00 P.M. with food but no water. When the lights went out at 7:00 P.M., preweighed water and 1% sucrose bottles were placed on the home cage and rats were allowed to drink for 10 min. The procedure was similar for sucrose neophobia, except that rats were not pre-exposed to sucrose before surgery and the duration of the test session was 30 min.
Social behavior.
To investigate social interaction, viral vectors were injected into the nucleus accumbens and social behavior was measured on day 4 after surgery. Rats were isolated for 24 h before testing. For testing, cage mates were reunited in novel square chambers (50 × 50 cm) and behavior was videotaped and later scored for 30 min. Pairs of rats were scored together using an observer-based scoring system (Noldus Observer; Noldus Information Technology, Wageningen, The Netherlands). Durations of social grooming (one rat grooming the other), exploring, playing, and resting were coded as state variables. This paradigm of short-term isolation before measuring social behavior has been shown to be sensitive to dopamine and opioid manipulations (Niesink and Van Ree, 1989).
Forced swim test.
The forced swim test was performed according to published procedures (Eisch et al., 2003). Rats were placed in 25° water in tubs (60 cm tall filled to 45 cm) for 15 min for the initial session and 5 min for the test session. The latency to immobility is defined as no struggling for a full 1 s and has been validated as an antidepressant-sensitive measure (Pliakas et al., 2001; Eisch et al., 2003).
Elevated plus maze.
Anxiety-like behavior in the elevated plus maze was measured according to published procedures (Barrot et al., 2002). The arms of the elevated plus maze measured 12 × 50 cm, and the maze was 1 m from the floor. Time on open arms was measured for 5 min (expressed as a percentage of total time). Behavior was scored using an automated video-tracking system (Noldus Ethovision; Noldus Information Technology).
Peanut butter chip neophobia.
Rats were isolated with access to food and water for 16 h before testing. A preweighed amount (∼15 g) of Reese’s peanut butter chips was placed in a cup in the home cage for 2 h during the light phase of the light/dark cycle, and the amount of chips consumed was recorded.
Results
Effect of amphetamine on ICER mRNA expression
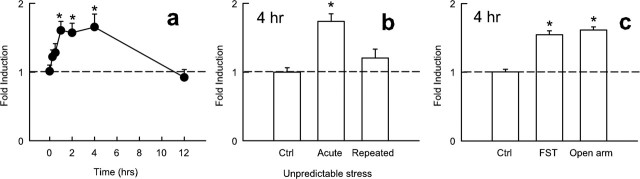
We first examined whether amphetamine induces ICER expression in the striatum including the nucleus accumbens. We chose amphetamine because it has been shown previously to dramatically induce CRE-mediated transcription within this brain region (Shaw-Lutchman et al., 2003). ICER mRNA levels were measured by real-time PCR. Acute amphetamine administration increased ICER mRNA levels in the striatum, an effect that peaked at 3 h and returned to control within 12 h (F(5,22) = 6.28; p < 0.005) (Fig. 1a). Amphetamine regulation of ICER expression was dose dependent (F(3,14) = 34.3; p < 0.001) (Fig. 1b). Interestingly, however, the induction of ICER mRNA diminishes progressively with repeated amphetamine administration (F(4,15) = 20.77; p < 0.001) (Fig. 1c–e). Thus, whereas the first amphetamine injection causes a maximal approximate threefold increase in ICER expression, only an ∼50% increase is seen after seven daily doses of the drug (Fig. 1d).
Figure 1.
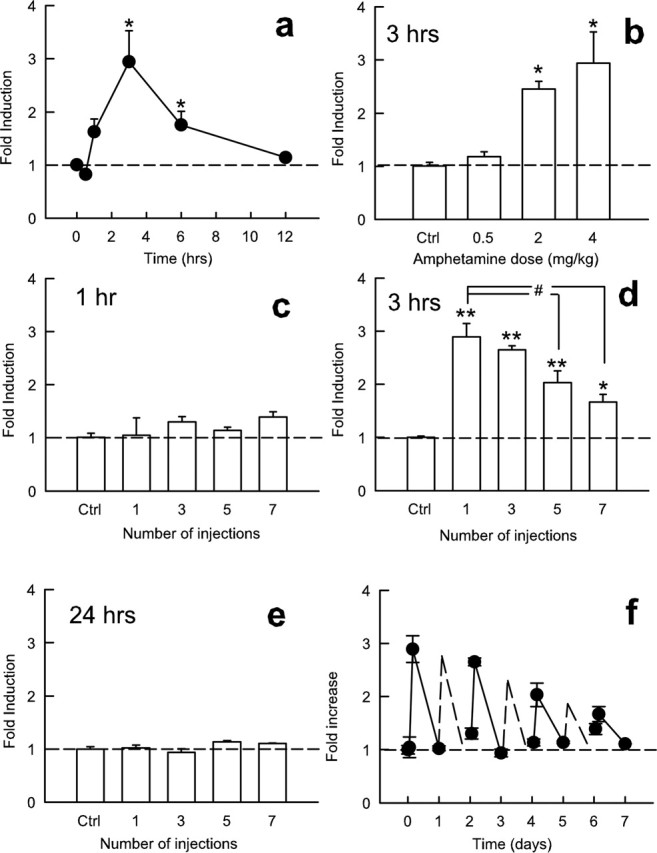
Effect of amphetamine on ICER mRNA expression in the striatum. a, Time course of ICER induction by amphetamine (4 mg/kg, i.p.). Data points represent mean ± SEM relative induction of ICER mRNA compared with saline-injected controls (n = 4). b, Dose–response function for amphetamine induction of ICER mRNA 3 h after injection (n = 4). c–e, Effect of acute and repeated amphetamine injections at 1 h (c), 3 h (d), or 24 h (e) after injection compared with saline-injected controls. *p < 0.05, statistical difference from saline; **p < 0.001, difference from saline; #statistical significance from acute amphetamine administration. f, Timeline of ICER mRNA induction with repeated injection of amphetamine using 1, 3, and 24 h time points. Dashed lines represent interpolated points of even-numbered injections; interpolated points were calculated as the average of the previous and subsequent sessions. Ctrl, Control.
The CREM gene encodes inhibitory products, in addition to ICER, via a distinct promoter, P1. These products have been proposed, like ICER, to mediate negative-feedback control over CRE transcription (Fitzgerald et al., 1996; Borsook et al., 1999; Servillo et al., 2002; Mioduszewska et al., 2003); however, virtually nothing is known about their function in brain. We found that the inhibitory P1-driven CREM isoforms (αCREM, βCREM, and γCREM) were not regulated in the nucleus accumbens by amphetamine (data not shown). mRNA levels of full-length τCREM isoforms were also not affected by amphetamine administration.
Effect of stress on ICER expression
To study the effect of aversive environmental stimuli on ICER expression in the striatum, we subjected rats to acute restraint stress. An ANOVA showed that this stress treatment produced a moderate but significant (∼75%) increase in ICER mRNA levels, which peaked 1–4 h after the stress and reverted to control levels within 12 h (F(6,20) = 4.97; p < 0.005) (Fig. 2a). Although this effect was smaller in magnitude compared with that seen with amphetamine, the duration of peak ICER induction was longer in response to restraint stress (compare Figs. 1a, 2a). In contrast to acute restraint stress, chronic (7 d) restraint stress failed to induce ICER mRNA levels in the striatum (data not shown). One limitation of this latter experiment is that habituation is known to occur to many effects of restraint stress. Therefore, it was important to study whether desensitization of ICER induction also occurs after a different type of chronic stress, chronic unpredictable stress, which shows much less habituation (Ortiz et al., 1996). Chronic unpredictable stress, like chronic restraint stress, failed to induce ICER mRNA levels in the striatum (F(2,11) = 11.7; p < 0.005) (Fig. 2b). In contrast, both the major inhibitory P1-driven CREM isoforms (αCREM, βCREM, and γCREM) as well as the excitatory isoform (τCREM) were not regulated in the nucleus accumbens by stress (data not shown).
Figure 2.
Effect of stress on ICER mRNA expression in the striatum. a, Time course of ICER induction by acute restraint stress. Points represent mean ± SEM relative induction from non-stressed controls (n = 4). b, Effect of acute restraint stress and repeated unpredictable stress on ICER mRNA levels compared with non-stressed controls. c, Effect of forced swimming or being confined to the open arm of an elevated plus maze on ICER mRNA levels at 4 h. *p < 0.05, statistical difference from non-stressed controls. Ctrl, Control; FST, forced swim test.
Other forms of acute stress, including anxiogenic stimuli, induce ICER expression in the striatum. Ten minutes of forced swimming, or being confined to the open arm of the elevated plus maze, significantly increased ICER mRNA levels (F(2,11) = 42.5; p < 0.001) 4 h after the stressor (Fig. 2c).
Validation of HSV–ICER vector
To study the functional role played by ICER induction in the striatum in response to amphetamine or stress, we constructed an HSV vector that expresses ICER under the control of a viral promoter. In cell culture, the vector induced the expression of a protein of the correct Mr recognized by an anti-ICER/CREM antibody as determined by Western blotting (Fig. 3a). HSV–ICER was next injected into the nucleus accumbens shell of rat brain. Viral-mediated expression of ICER protein was easily visualized using immunohistochemistry (Fig. 3b). To study the functional activity of HSV–ICER in rat brain, mRNA levels of BDNF, a well established CRE target gene (Finkbeiner et al., 1997; McClung and Nestler, 2003), was compared in nucleus accumbens tissue infected with HSV–ICER or HSV–LacZ. In animals examined 3 h after a single dose of amphetamine (4 mg/kg) to boost BDNF mRNA expression, we found that ICER robustly decreased BDNF mRNA levels by 73 ± 21% (p < 0.05). To further demonstrate an in vivo connection between ICER and BDNF, a ChIP experiment was performed on striatal tissue. We found significant binding of CREM/ICER to the P3 rat BDNF promoter, which contains a well characterized CRE sequence, but no significant binding to the P2 promoter (F(2,20) = 22.6; p < 0.001; Dunnet post hoc: P2, p = 0.98; P3, p < 0.001). This binding is likely attributable to ICER, because it was induced by acute amphetamine, conditions under which ICER is the only CREM gene product induced (2.3 ± 0.4-fold amphetamine vs saline; n = 3–4; p < 0.05).
Figure 3.
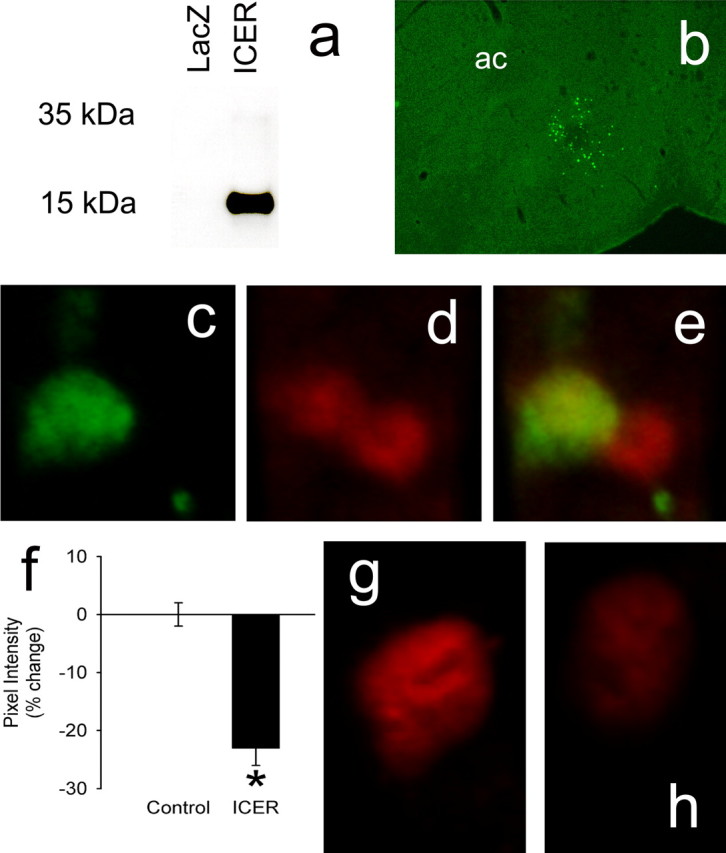
HSV-mediated ICER overexpression in the nucleus accumbens. a, Western blot depicting HSV-mediated ICER overexpression in PC12 cells, an effect not seen with HSV–LacZ. b, ICER overexpression in the nucleus accumbens shell visualized by immunohistochemistry. ac, Anterior commissure. c–e, HSV–ICER was injected into the nucleus accumbens of CRE–LacZ transgenic reporter mice. ICER (c) was visualized using a CY2-conjugated secondary antibody, whereas β-galactosidase (d) immunofluorescence was visualized using a CY3 secondary antibody. The merged image (e) shows colocalization of β-galactosidase (red) and ICER (green) in one representative cell and lack of ICER expression in a second nearby β-galactosidase-positive cell. f, Comparison of mean pixel intensity (±SEM) of β-galactosidase immunofluorescence in ICER+ and ICER− cells as a percentage of control intensity (n = 21 cells in 3 animals). The results show that ICER significantly (*p < 0.05) decreases CRE-mediated transcription. g, LacZ immunofluorescence in a representative cell infected with control (HSV–GFP) virus. h, LacZ immunofluorescence in a representative cell infected with HSV–ICER virus. Note the clear reduction in LacZ expression caused by ICER.
To further study the functional activity of virally encoded ICER on CRE-mediated transcription, we injected HSV–ICER into the nucleus accumbens of CRE–LacZ reporter mice and 3 d later, when HSV expression is maximal, administered a moderate dose of amphetamine (1 mg/kg) to boost the normally low levels of LacZ expression in the nucleus accumbens of these mice (Shaw-Lutchman et al., 2003). Animals were analyzed 4 h later, a time point that provides a stable and reliable measure of LacZ induction (Barrot et al., 2002; Shaw-Lutchman et al., 2002, 2003). ICER expression significantly decreased the mean intensity of β-galactosidase immunofluorescence (F(1,70) = 52.75; p < 0.001) (Fig. 3c–f), an effect not seen after viral-mediated expression of a control protein (e.g., GFP) (Fig. 3g,h). These results show that viral infection per se does not affect CRE activity and confirm the biological activity of viral-encoded ICER as an inhibitor of CRE transcription in the nucleus accumbens in vivo.
As observed with many other HSV vectors (Carlezon et al., 1998; Barrot et al., 2002), viral expression was seen in neurons only and was not associated with any toxicity beyond that observed with vehicle injections alone (data not shown).
Effect of ICER overexpression on locomotor activity
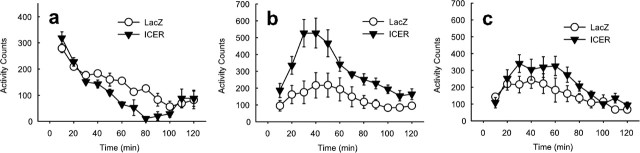
Using this HSV–ICER vector, we wanted to study the influence of ICER overexpression in the nucleus accumbens on an animal’s responses to a wide range of emotional stimuli, both aversive and rewarding. The first behavior examined was locomotor activity. We found that ICER overexpression in the nucleus accumbens decreased spontaneous activity compared with an HSV–LacZ vector that overexpresses β-galactosidase as a control protein. A two-factor ANOVA (virus × time) revealed a main effect of virus (F(1,77) = 18.3; p < 0.005) (Fig. 4a) and a virus × time interaction (F(11,77) = 2.69; p < 0.05). Contrasts revealed a significant quadratic component of the interaction, meaning that ICER decreased spontaneous locomotor activity only during the middle part of the test session (F(1,7) = 27.0; p < 0.001). When challenged with a 2 mg/kg dose of amphetamine, however, ICER overexpression in the nucleus accumbens strongly potentiated amphetamine-induced locomotor activity throughout the session compared with LacZ-overexpressing animals (F(1,110) = 11.6; p < 0.01) (Fig. 4b). The time × virus interaction also yielded significant results (F(11,110) = 2.69, p < 0.005), meaning that most of the potentiation occurred from 20 to 60 min after amphetamine injection. There was a nonsignificant trend for ICER to potentiate locomotor activity at a higher amphetamine dose (4 mg/kg; F(1,110) = 2.15; p = 0.17) (Fig. 4c). The lack of significant influence of ICER was likely attributable to a ceiling effect and the appearance of stereotypy, which is known to limit locomotor activity at higher amphetamine doses.
Figure 4.
Effect of ICER overexpression in the nucleus accumbens on spontaneous and amphetamine-stimulated locomotor activity. HSV–ICER or HSV–LacZ was injected bilaterally in the nucleus accumbens. a, Time course of spontaneous locomotor activity. Points represent mean ± SEM beam breaks in 10 min bins. b, Time course of amphetamine-stimulated locomotor activity at 2 mg/kg. Points represent mean ± SEM beam breaks in 10 min bins. Rats were allowed 60 min to habituate to the test chamber before amphetamine (2 mg/kg) was injected. c, Effect of ICER on amphetamine-stimulated locomotor activity at 4 mg/kg.
Effect of ICER overexpression on responses to natural rewards
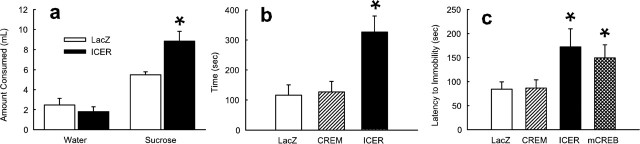
To determine whether levels of ICER in the nucleus accumbens also affect an animal’s responses to natural rewards, we studied the animals in a sucrose preference paradigm. ICER overexpression in this brain region produced an increase in sucrose preference in animals that were already familiar with sucrose compared with LacZ overexpression (F(1,14) = 7.88; p < 0.05) (Fig. 5a).
Figure 5.
Effect of ICER overexpression in the nucleus accumbens on natural reward and depression-related behavior. HSV–ICER or HSV–LacZ was injected bilaterally in the nucleus accumbens. a, Effect of ICER overexpression on sucrose preference. Data represent mean ± SEM milliliter of water or 1% sucrose solution consumed over 10 min using a two-bottle choice procedure. b, Effect of ICER overexpression on social grooming. Bars represent mean ± SEM seconds one rat groomed another rat during a 30 min session. Rats were tested after 24 h of isolation. Lack of effect of HSV–τCREM is shown for comparison. c, Effect of ICER on latency to immobility in the forced swim test. Bars represent mean ± SEM seconds latency to immobility (1 s immobility) during a 5 min test session. The lack of effect of τCREM and known antidepressant-like effect of mCREB are shown for comparison. *Significant difference from HSV–LacZ control.
As with humans, rats find nonaggressive social contact to be rewarding and prefer social contact (e.g., grooming) to isolation (Calcagnetti and Schechter 1992). ICER overexpression increased social reward as indicated by an increased amount of time rats groomed each other in a test of social interaction (F(1,10) = 21.85; p < 0.005) (Fig. 5b). In contrast, the full-length CREM protein (τCREM), in comparison, had no effect on social grooming and was indistinguishable from HSV–LacZ-injected animals.
Effect of ICER overexpression on depression-related behavior
One hallmark of depression is subsensitivity to hedonic stimuli. As described above, ICER overexpression in the nucleus accumbens increases social reward (grooming) and sucrose reward. It was therefore of interest to directly study the influence of ICER in a test of depression-related behavior. We found that ICER overexpression in the nucleus accumbens lengthened the latency to immobility in the forced swim test compared with LacZ overexpression (F(1,12) = 4.64; p < 0.05) (Fig. 5c). This is an antidepressant-like effect that is seen in normal animals after administration of any of several chemical antidepressants (Lucki, 2001; Pliakas et al., 2001). An HSV–mCREB virus, which overexpresses a synthetic dominant-negative mutant of CREB, was used as a positive control. This vector caused a similar increase in latency to immobility as seen for HSV–ICER and thereby replicates the findings of Pliakas et al. (2001) with mCREB (F(1,10) = 4.34; p < 0.05; one-tailed test). In contrast, the full-length CREM protein (τCREM) had no antidepressant effect (Fig. 5c).
Effect of ICER overexpression on anxiety-related behavior
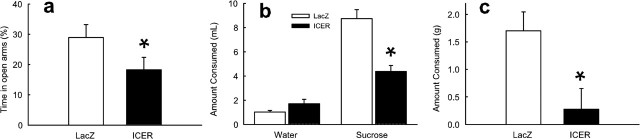
To further understand the functional relevance of ICER induction in the nucleus accumbens by emotional stimuli, we examined the influence of HSV–ICER in several tests of anxiety-like behavior. Viral-mediated overexpression of ICER in the nucleus accumbens produced a robust anxiogenic-like effect. Specifically, it decreased the time an animal spent in the open arms of an elevated plus maze (F(1,27) = 3.14; p < 0.05) (Fig. 6a). ICER overexpression also produced an anxiogenic-like effect in increasing an animal’s avoidance of novel tastes (a phenomenon known as “neophobia”), including sucrose (F(1,6) = 23.5; p < 0.005) (Fig. 6b) and peanut butter chips (F(1,32) = 6.21; p < 0.05) (Fig. 6c). This increased aversion to sucrose as a novel taste is interesting in light of the enhanced preference for sucrose when it is a familiar taste (compare Figs. 5a, 6b). Thus, although rats find sucrose and other sweets (like peanut butter chips) rewarding once they are familiar with the taste, they, like many other species, exhibit a strong aversion to novel tastes. This neophobia has been shown to have a strong anxiety component (Hall et al., 1997) and is regulated by the nucleus accumbens (Burns et al., 1996).
Figure 6.
Effect of ICER overexpression in the nucleus accumbens on anxiety-related behavior. HSV–ICER or HSV–LacZ was injected bilaterally in the nucleus accumbens. a, Effect of ICER in the elevated plus maze. Bars represent mean ± SEM percentage of time spent on the open arms of the elevated plus maze during a 5 min session. b, Effect of ICER on sucrose neophobia using a two-bottle choice procedure. Bars represent milliliters of water or a 1% sucrose solution consumed by naive rats across a 30 min session. c, Effect of ICER on peanut butter chip neophobia. Data represent mean ± SEM grams consumed during a 2 h test session. *Significant difference from HSV–LacZ control.
Discussion
The major findings of the present study are that emotionally salient environmental stimuli, both rewarding and aversive, increase ICER expression in the striatum and that mimicking this effect within the nucleus accumbens, the ventral subregion of the striatum, by use of viral-mediated gene transfer affects a wide range of behavior domains, from anxiety to depression to reward.
The results show that aversive or rewarding environmental factors, including stress on the one hand or amphetamine on the other, induce expression of ICER mRNA in the striatum. ICER mRNA peaks within 2–4 h after the inducing stimulus and returns to baseline within 12 h. In agreement with other stimuli in other brain regions, ICER mRNA induction is slower than that of other inducible transcription factors such as c-Fos (Konopka et al., 1998; T. A. Green and E. J. Nestler, unpublished data). The somewhat delayed time course of ICER induction is consistent with the hypothesized role of ICER as a negative-feedback mechanism for CREB-mediated transcription (Servillo et al., 2002; Mioduszewska et al., 2003; Shepard et al., 2005). According to this view, the delay in ICER induction allows CREB to induce transcription of its myriad of target genes; as ICER (one of those target genes) is translated, it subsequently impedes additional CREB-induced transcription. Consequently, ICER is hypothesized to function as an endogenous dominant-negative regulator of CREB function. A previous study showed CREM induction in the striatum by dopaminergic agonists, but it is unclear which CREM products (excitatory and/or inhibitory) were identified in the analysis (Berke et al., 1998).
The loss of induction of ICER mRNA after repeated amphetamine or stress exposure parallels effects seen with other inducible transcription factors after these and other repeated treatments, such as c-Fos, c-Jun, and JunB (Hope et al., 1992; Daunais et al., 1993; Persico et al., 1993; Hiroi and Graybiel, 1996; O’Donovan et al., 1999; Perrotti et al., 2004). Interestingly, all of these genes (including ICER) have now been identified as part of the CREB regulon (Impey et al., 2004) (i.e., they are all targets of CREB). To the extent that ICER binds the same CRE or CRE-like sites as CREB, all of these genes should be targets of ICER regulation as well, a possibility that now requires direct investigation. It is unlikely, however, that ICER itself is responsible for the desensitization of induction of these genes seen after repeated administration of amphetamine or stress, because ICER, like other immediate-early genes, shows this desensitization. Hence, it would seem that another factor, yet to be identified, is responsible for this form of tolerance.
To understand the functional relevance of ICER induction in the nucleus accumbens by amphetamine or stress, we used HSV-mediated gene transfer to overexpress ICER selectively within the nucleus accumbens and then examined the effect on an animal’s responses to a wide range of emotional stimuli, both aversive and rewarding. One observation is that ICER overexpression in the nucleus accumbens increases behavioral responses to amphetamine. This finding raises the interesting possibility that ICER induction contributes to the mechanisms by which a previous exposure to amphetamine or to stress increases an animal’s sensitivity to subsequent amphetamine exposure, a phenomenon that has been well established (Covington and Miczek, 2001; Robinson and Berridge, 2001). Our findings with amphetamine-induced locomotor activity are also in agreement with previous research, which showed that inhibition of CREB function in the nucleus accumbens, achieved via overexpression of the dominant-negative mutant mCREB, made animals more sensitive to the behavioral effects of cocaine and morphine (Carlezon et al., 1998; Barrot et al., 2002). In contrast, overexpression of CREB blunted responses to these drugs of abuse.
Furthermore, the current behavioral findings are consistent with the results of previous work, which demonstrated that CREB, acting in the nucleus accumbens, blunts behavioral responses to a wide range of emotional stimuli in addition to drugs of abuse (Barrot et al., 2002). In those previous studies, overexpression of CREB in the nucleus accumbens decreased responses to a range of aversive and rewarding stimuli and induced certain features of depression-like behavior, including symptoms of anhedonia and increased immobility in the forced swim test. However, these animals also show reduced anxiety-like behavior, consistent with a general numbing in behavioral responsivity. In these previous experiments, overexpression of mCREB, which is a synthetic dominant-negative mutant that lacks the serine 133 required for CREB phosphorylation and activation, in the nucleus accumbens caused the opposite behavioral effects: animals showed increased responsiveness to aversive and rewarding stimuli. In the present study, overexpression of ICER in the nucleus accumbens has similar effects as mCREB: increased ICER levels enhance an animal’s responses to anxiogenic and rewarding stimuli and also decrease depression-like behavior in the forced swim test. However, unlike mCREB, which is an artificial mutant, ICER is a naturally occurring protein, which we show is induced in the nucleus accumbens in response to aversive and rewarding stimuli. Thus, our data demonstrate that emotional stimuli induce both CREB and ICER, which produce opposing effects on behavioral responses to subsequent emotional stimuli.
Induction of ICER protein in the nucleus accumbens produced a clear antidepressant phenotype in the forced swim test and in experiments measuring natural reward. However, overexpression of ICER also increases anxiety-like behavior in the elevated plus maze and in taste neophobia experiments. The ICER protein itself does not affect behavior directly; rather, it affects expression of other proteins that then affect neuronal functioning. As an example, we show that ICER repressed BDNF expression in the nucleus accumbens, and previous studies have shown that reduced levels of BDNF in this brain region cause antidepressant-like responses in animal models (Eisch et al., 2003; Berton et al., 2006). One caveat of the present studies is that they rely on overexpression of ICER, and exogenous ICER may interact with genes not affected by endogenous ICER. This is not the case for the BDNF gene, which we show by ChIP to bind ICER, but a thorough examination of this possibility will require development of tools not yet available to selectively remove endogenous ICER, but not other CREM gene products.
One potential path toward exploiting these findings for the development of new treatments for depression and other stress-related disorders is to identify the target genes that mediate the antidepressant- and anxiogenic-like effects of ICER. It would be important, for example, to determine whether the genes responsible for the antidepressant and anxiety phenotypes can be dissociated. Although we use viral-mediated gene transfer as an experimental tool with which to study the role of given genes in complex behavior, this line of research does raise the eventual possibility of using similar approaches in the treatment of depression in humans (Green and Nestler, 2005). The use of viral-mediated gene therapy to treat depression is clearly years in the future, but we should emphasize that recent research supports the role of invasive neurosurgical interventions to treat particularly severe, treatment-refractory cases (Mayberg et al., 2005).
Although the current behavioral experiments concern specific induction of ICER in the nucleus accumbens shell, another study found that deletion of all CREM gene products in mice causes hyperactivity and an anxiolytic-like phenotype (Maldonado et al., 1999). Our data raise the possibility that the anxiolytic phenotype seen in these mice may be attributable to the loss of ICER per se in the nucleus accumbens.
The CREM gene also produces inhibitory (αCREM, βCREM, and γCREM) and excitatory (τCREM) isoforms driven by the P1 promoter. These transcripts are increased in other brain regions by electroconvulsive seizure (Fitzgerald et al., 1996) and lipopolysaccharide administration (Borsook et al., 1999), but these studies did not distinguish between inhibitory versus excitatory isoforms. The lack of induction of P1 inhibitory isoforms in the nucleus accumbens by stress or amphetamine demonstrates that ICER variants (driven by the P2 promoter) are the major inhibitory CREM products in the nucleus accumbens responsive to environmental stimuli and the ones that mediate changes in behavior resulting from past experience.
The current experiments expand previous work by showing the “emotional gating” effect of CREB has an endogenous opposing mechanism that serves to increase responses to emotion-eliciting stimuli. This opposing mechanism, however, decreases with repeated exposure to stress or amphetamine, even as the CREB response persists or even increases with repeated exposure (Shaw-Lutchman et al., 2003). Hence, ICER induction is a homeostatic negative-feedback mechanism that appropriately shuts down CREB-driven transcription acutely. However, repeated stimulation causes this induction to wane (Fig. 1e), such that the subsequent exaggerated CREB response could contribute to the pathological behavior caused by chronic stress (e.g., certain subtypes of depression) or chronic administration of drugs of abuse (e.g., addiction). These results also provide an additional mechanism for the close relationship between drug abuse and depression at least in some individuals (Nunes and Levin, 2004; Nestler and Carlezon, 2005). In summary, these experiments provide evidence that behavioral responses to emotional stimuli do not depend on CREB alone, but rather are dependent on CREB–ICER competition at CRE sites. Therefore, a complete understanding of the role of CRE-mediated transcription in the nucleus accumbens, or any other brain region of interest, on complex behavior will require an integrated analysis of CREB and ICER and their functional interactions.
Footnotes
R. J. DiLeone’s present address: Department of Psychiatry, Yale University School of Medicine, New Haven, CT 06508.
This work was supported by grants from the National Institute of Mental Health and the National Institute on Drug Abuse.
References
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ (2002). CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci USA 99:11435–11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Paletzki RF, Aronson GJ, Hyman SE, Gerfen CR (1998). A complex program of striatal gene expression induced by dopaminergic stimulation. J Neurosci 18:5301–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, DiLeone RJ, Krishnan V, Russo S, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ (2006). Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311:864–868. [DOI] [PubMed] [Google Scholar]
- Borsook D, Smirnova O, Behar O, Lewis S, Kobierski LA (1999). Phospho CREB and CREM/ICER: positive and negative regulation of proenkephalin gene expression in the paraventricular nucleus of the hypothalamus. J Mol Neurosci 12:35–51. [DOI] [PubMed] [Google Scholar]
- Burns LH, Annett L, Kelley AE, Everitt BJ, Robbins TW (1996). Effects of lesions to amygdala, ventral subiculum, medial prefrontal cortex, and nucleus accumbens on the reaction to novelty: implication for limbic-striatal interactions. Behav Neurosci 110:60–73. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD (1992). Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav 51:667–672. [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ (1998). Regulation of cocaine reward by CREB. Science 282:2272–2275. [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr, Duman RS, Nestler EJ (2005). The many faces of CREB. Trends Neurosci 28:436–445. [DOI] [PubMed] [Google Scholar]
- Conti AC, Blendy JA (2004). Regulation of antidepressant activity by cAMP response element binding proteins. Mol Neurobiol 30:143–155. [DOI] [PubMed] [Google Scholar]
- Conti AC, Kuo YC, Valentino RJ, Blendy JA (2004). Inducible cAMP early repressor regulates corticosterone suppression after tricyclic antidepressant treatment. J Neurosci 24:1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, Miczek KA (2001). Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges.”. Psychopharmacology 158:388–398. [DOI] [PubMed] [Google Scholar]
- Daunais JB, Roberts DC, McGinty JF (1993). Cocaine self-administration increases preprodynorphin, but not c-fos, mRNA in rat striatum. NeuroReport 4:543–546. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ (2003). Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry 54:994–1005. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME (1997). CREB: a major mediator of neuronal neurotrophin responses. Neuron 19:1031–1047. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LR, Vaidya VA, Terwilliger RZ Duman RS (1996). Electroconvulsive seizure increases the expression of CREM (cyclic AMP response element modulator) and ICER (inducible cyclic AMP early repressor) in rat brain. J Neurochem 66:429–432. [DOI] [PubMed] [Google Scholar]
- Green TA, Nestler EJ (2005). Psychiatric applications of viral vectors. In: Gene therapy in the central nervous system (Kaplitt MG, During MJ, eds) pp. 181–193. New York: Elsevier.
- Hall FS, Humby T, Wilkinson LS, Robbins TW (1997). The effects of isolation-rearing of rats on behavioural responses to food and environmental novelty. Physiol Behav 62:281–290. [DOI] [PubMed] [Google Scholar]
- Hiroi N, Graybiel AM (1996). Atypical and typical neuroleptic treatments induce distinct programs of transcription factor expression in the striatum. J Comp Neurol 374:70–83. [DOI] [PubMed] [Google Scholar]
- Hope B, Kosofsky B, Hyman SE, Nestler EJ (1992). Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci USA 89:5764–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, McCorkle SR, Cha-Molstad H, Dwyer JM, Yochum GS, Boss JM, McWeeney S, Dunn JJ, Mandel G, Goodman RH (2004). Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell 119:1041–1054. [DOI] [PubMed] [Google Scholar]
- Konopka D, Szklarczyk AW, Filipkowski RK, Trauzold A, Nowicka D, Hetman M, Kaczmarek L (1998). Plasticity- and neurodegeneration-linked cyclic-AMP responsive element modulator/inducible cyclic-AMP early repressor messenger RNA expression in the rat brain. Neuroscience 86:499–510. [DOI] [PubMed] [Google Scholar]
- Konradi C, Cole RL, Heckers S, Hyman SE (1994). Amphetamine regulates gene expression in rat striatum via transcription factor CREB. J Neurosci 14:5623–5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Choi K-H, Renthal W, Tsankova NM, Theobald DEH, Truong H-T, Russo SJ, LaPlant Q, Whistler K, Neve RL, Self DW, Nestler EJ (2005). Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron 48:303–314. [DOI] [PubMed] [Google Scholar]
- Lucki I (2001). A prescription to resist proscription for murine models of depression. Psychopharmacology 153:395–398. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Smadja C, Mazucchelli C, Sassone-Corsi P (1999). Altered emotional and locomotor responses in mice deficient in the transcription factor CREM. Proc Natl Acad Sci USA 96:14094–14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH (2005). Deep brain stimulation for treatment-resistant depression. Neuron 45:651–660. [DOI] [PubMed] [Google Scholar]
- Mayr B, Montminy M (2001). Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2:599–609. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ (2003). Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci 6:1208–1215. [DOI] [PubMed] [Google Scholar]
- Mioduszewska B, Jaworski J, Kaczmarek L (2003). Inducible cAMP early repressor (ICER) in the nervous system—a transcriptional regulator of neuronal plasticity and programmed cell death. J Neurochem 87:1313–1320. [DOI] [PubMed] [Google Scholar]
- Molina CA, Foulkes NS, Lalli E, Sassone-Corsi P (1993). Inducibility and negative autoregulation of CREM: an alternative promoter directs the expression of ICER, an early response repressor. Cell 75:875–886. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA Jr (2006). The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59:1151–1159. [DOI] [PubMed] [Google Scholar]
- Neve RL, Geller AI (1995). A defective herpes simplex virus vector system for gene delivery into the brain: comparison with alternative gene delivery systems and usefulness for gene therapy. Clin Neurosci 3:262–267. [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, Chen J, Neve R, Nestler EJ, Duman RS (2002). Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci 22:10883–10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesink RJ, Van Ree JM (1989). Involvement of opioid and dopaminergic systems in isolation-induced pinning and social grooming of young rats. Neuropharmacology 28:411–418. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Levin FR (2004). Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA 291:1887–1896. [DOI] [PubMed] [Google Scholar]
- O’Donovan KJ, Tourtellotte WG, Millbrandt J, Baraban JM (1999). The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci 22:167–173. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Lane S, Terwilliger R, Nestler EJ (1996). Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacology 14:443–452. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1997). In: The rat brain in stereotaxic coordinates San Diego: Academic.
- Perrotti LI, Hadeishi Y, Ulery P, Barrot M, Monteggia L, Duman RS, Nestler EJ (2004). Induction of ΔFosB in reward-related brain regions after chronic stress. J Neurosci 24:10594–10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persico AM, Schindler CW, O’Hara BF, Brannock MT, Uhl GR (1993). Brain transcription factor expression: effects of acute and chronic amphetamine and injection stress. Mol Brain Res 20:91–100. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA Jr (2001). Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci 21:7397–7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman Z, Schwarz J, Zachariou V, Gold SJ, Wein M, Choi KH, Kovoor A, Chen CK, DiLeone RJ, Schwarz SC, Selley DE, Sim-Selley LJ, Barrot M, Leudtke RR, Self DW, Neve RL, Lester HA, Simon MI, Nestler EJ (2003). RGS9 modulates dopamine signaling in striatum. Neuron 38:941–952. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (2001). Incentive-sensitization and addiction. Addiction 96:103–114. [DOI] [PubMed] [Google Scholar]
- Servillo G, Della FM, Sassone-Corsi P (2002). Coupling cAMP signaling to transcription in the liver: pivotal role of CREB and CREM. Exp Cell Res 275:143–154. [DOI] [PubMed] [Google Scholar]
- Shaw-Lutchman TZ, Barrot M, Wallace T, Gilden L, Zachariou V, Impey S, Duman RS, Storm D, Nestler EJ (2002). Regional and cellular mapping of CRE-mediated transcription during naltrexone-precipitated morphine withdrawal. J Neurosci 22:3663–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw-Lutchman TZ, Impey S, Storm D, Nestler EJ (2003). Regulation of CRE-mediated transcription in mouse brain by amphetamine. Synapse 48:10–17. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Liu Y, Sassone-Corsi P, Aguilera G (2005). Role of glucocorticoids and cAMP-mediated repression in limiting corticotropin-release hormone transcription during stress. J Neurosci 25:4073–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CL, Blendy JA (2001). Different requirements for cAMP response element binding protein in positive and negative reinforcing properties of drugs of abuse. J Neurosci 21:9438–9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters CL, Kuo YC, Blendy JA (2003). Differential distribution of CREB in the mesolimbic dopamine reward pathway. J Neurochem 87:1237–1244. [DOI] [PubMed] [Google Scholar]
- Zhang X, Odom DT, Koo SH, Conkright MD, Canettieri G, Best J, Chen H, Jenner R, Herbolsheimer E, Jacobsen E, Kadam S, Ecker JR, Emerson B, Hogenesch JB, Unterman T, Young RA, Montminy M (2005). Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci USA 102:4459–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]