An entrenched organizational principle regarding the human hand region of primary somatosensory (SI) cortex holds that it is exclusively devoted to processing afferent input from the contralateral side of the body. However, the hypothesis that SI cortex also receives input from the ipsilateral hand dates back several decades (Tamura, 1972). The responsiveness of ipsilateral SI to distal upper-limb stimulation is supported by converging evidence from multiple lines of research, including the following: (1) cortical ablation studies in rats; (2) single-neuron electrophysiology and functional magnetic resonance imaging (fMRI) with nonhuman primates; and (3) intracranial recording, magnetoencephalographic (MEG), transcranial magnetic stimulation, and fMRI studies with humans, as well as behavioral results from bimanual tasks (for most recent discussion, see Tommerdahl et al., 2006). Despite this evidence, general acceptance has not been achieved, potentially because of the incomplete characterization of ipsilateral input within SI cortex. In a recent article in The Journal of Neuroscience, Hlushchuk and Hari (2006) provide additional support for and further elucidate the nature of the ipsilateral SI response.
Using a 3 T scanner, the authors dissociate ipsilateral sensorimotor responses by assessing positive and negative blood oxygenation level-dependent (BOLD) responses, activations and deactivations, respectively. In two experiments, each with 10 subjects, Hlushchuk and Hari (2006) demonstrate that unilateral stimulation of the fingers can be accompanied by activation of Brodmann area (BA) 2 and deactivation of area 3b in ipsilateral SI cortex, as well as by deactivation of primary motor cortex (MI) in both hemispheres.
Stimuli consisted of tactile pulses delivered at various frequencies to three fingers (index, middle, and ring) of the left or right hands by means of balloon diaphragms driven by compressed air. Compared with electrical median nerve stimulation, which has been commonly used to explore the somatosensory system, mechanical tactile pulses offer a more physiological means to assess cortical responses. Whereas electrical stimulation of a peripheral nerve may be regarded as un-physiological because it bypasses mechanoreceptors and produces a large afferent volley, mechanical pulses approximate natural tactile stimuli.
In an initial assessment, used to describe the time course of the ipsilateral responses, the authors delivered stimuli to right-hand fingers during 25 s blocks. Right-hand stimulation produced, in addition to the previously well characterized contralateral SI and bilateral secondary somatosensory (SII) responses, activation of SI and deactivation of sensorimotor rolandic cortex in the ipsilateral (right) hemisphere [Hlushchuk and Hari (2006), their Fig. 1 (http://www.jneurosci.org/cgi/content/full/26/21/5819/F1)]. The ipsilateral SI activation presumably corresponded to BA 2, but precise delineation of the deactivations required additional examination. The time course of the averaged BOLD signals indicated that tonic contralateral activation lasted ∼45 s, whereas phasic ipsilateral deactivations lasted ∼18 s. The time course of the ipsilateral SI activation (BA 2) was not reported but would be of interest because it could provide additional insight into ipsilateral SI function.
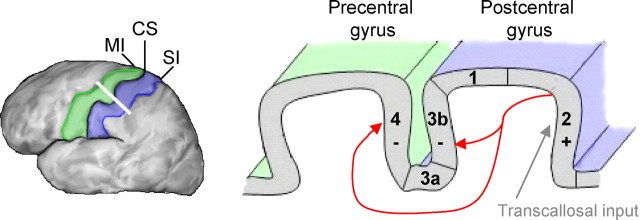
Figure 1.
Schematic illustration of potential routes responsible for the observed activation and deactivations in ipsilateral SI (blue) and MI (green) cortex. Ipsilateral SI receives transcallosal input via area 2 (gray arrow), resulting in activation (+). Corticocortical influences from BA 2 to areas 3b and 4 (red arrows) reduce neuronal activity, resulting in deactivation (−). CS, Central sulcus.
In a subsequent experiment, intended to confirm that the observed deactivations were not confined to the right hemisphere, stimuli were unilaterally delivered to the left and right hands in separate blocks (20 s per block). Using the mean time course of the previously described deactivations, the authors constructed custom-built hemodynamic response models to optimally detect phasic ipsilateral deactivations. Additionally, the data preprocessing step of spatial smoothing was avoided. Analysis was performed in each hemisphere within a region of interest encompassing MI and SI cortex. Sets of contrasts were used to reveal areas exhibiting either tonic activation in response to contralateral stimulation and phasic deactivation to ipsilateral stimulation or areas showing phasic deactivation to ipsilateral as well as contralateral stimulation. Whereas the former set of contrasts revealed an area within SI cortex, presumably corresponding to BA 3b, the latter set implicated a region of MI cortex [Hlushchuk and Hari (2006), their Fig. 3 (http://www.jneurosci.org/cgi/content/full/26/21/5819/F3)]. In other words, the posterior bank of the central sulcus (BA 3b) displayed tonic activation to contralateral stimulation and phasic deactivation to ipsilateral stimulation, and the anterior bank of the central sulcus (BA 4) displayed transient deactivation to both contralateral and ipsilateral stimulation. Thus, the authors were able to dissociate contiguous areas of deactivation in ipsilateral sensorimotor cortex and demonstrate that such responses are detectable in both hemispheres.
The precise pathway mediating the ipsilateral response is unclear. Three possibilities have been considered: (1) transcallosal input from contralateral SI (Allison et al., 1989); (2) direct uncrossed afferent projections to ipsilateral SI (Kanno et al., 2003); and (3) top-down input from higher-level processing areas such as SII (Tommerdahl et al., 2006). Hlushchuk and Hari (2006) suggest that the observed ipsilateral responses result from transcallosal input. After contralateral activation, ipsilateral SI could receive input via area 2, which possesses the densest transcallosal connections among SI regions. Subsequently, corticocortical projections from BA 2 to areas 3b and 4 could be responsible for the observed deactivations (Fig. 1). Estimates from intracranial and MEG recordings suggest an ipsilateral somatosensory evoked response latency of ∼40–50 ms to median nerve stimulation (Allison et al., 1989; Kanno et al., 2003). The delay of the ipsilateral response relative to the onset of contralateral activity (∼25 ms for area 2) is consistent with the transcallosal hypothesis. Conversely, this response latency appears to be inconsistent with the notion that projections from SII (latency of 60–70 ms) give rise to ipsilateral responses. Although uncrossed ascending projections to ipsilateral SI remain a viable alternative explanation, the exact pathway has been left unspecified. Additional characterization of the ipsilateral pathway, as well as the correspondence between BOLD activations/deactivations and ipsilateral responses observed using intracranial and MEG recordings, is needed. A detailed mechanistic account relating negative BOLD responses and decreased neuronal firing (possibly involving presynaptic inhibition) would also improve understanding of ipsilateral SI functions.
The integration of somatosensory information from the two hands is an important prerequisite for coordinated bimanual tactile exploration and tasks. Primates’ high capacity for sophisticated bimanual tasks indicates that fusion of information from the hands occurs within the CNS and possibly underlies essential behaviors (Tommerdahl et al., 2006). Psychophysical studies have demonstrated that finger stimulation to one hand can alter perception at the opposite hand (Harris et al., 2001; Braun et al., 2005). These findings suggest a functional role for the fusion of somatosensory information. Although much of this integration likely takes place at higher-level processing areas, such as SII and parietal association cortex, the effects of ipsilateral SI input should not be overlooked. Hlushchuk and Hari (2006) offer a coherent description of ipsilateral influences within primary sensorimotor cortex, providing an important step toward a more complete understanding of how the hands work together.
Editor’s Note: These short reviews of a recent paper in the Journal, written exclusively by graduate students or postdoctoral fellows, are intended to mimic the journal clubs that exist in your own departments or institutions. For more information on the format and purpose of the Journal Club, please see http://www.jneurosci.org/misc/ifa_features.shtml.
Footnotes
References
- Allison T, McCarthy G, Wood CC, Williamson PD, Spencer DD (1989). Human cortical potentials evoked by stimulation of the median nerve. II. Cytoarchitectonic areas generating long-latency activity. J Neurophysiol 62:711–722. [DOI] [PubMed] [Google Scholar]
- Braun C, Hess H, Burkhardt M, Wuhle A, Preissl H (2005). The right hand knows what the left hand is feeling. Exp Brain Res 162:366–373. [DOI] [PubMed] [Google Scholar]
- Harris JA, Harris IM, Diamond ME (2001). The topography of tactile learning in humans. J Neurosci 21:1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlushchuk Y, Hari R (2006). Transient suppression of ipsilateral primary somatosensory cortex during tactile finger stimulation. J Neurosci 26:5819–5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno A, Nakasato N, Hatanaka K, Yoshimoto T (2003). Ipsilateral area 3b responses to median nerve somatosensory stimulation. NeuroImage 18:169–177. [DOI] [PubMed] [Google Scholar]
- Tamura K (1972). Ipsilateral somatosensory evoked responses in man. Folia Psychiatr Neurol Jpn 26:83–94. [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Simons SB, Chiu JS, Favorov O, Whitsel BL (2006). Ipsilateral input modifies the primary somatosensory cortex response to contralateral skin flutter. J Neurosci 26:5970–5977. [DOI] [PMC free article] [PubMed] [Google Scholar]