Abstract
To test the gliomagenic potential of adult glial progenitors, we infected adult rat white matter with a retrovirus that expresses high levels of PDGF and green fluorescent protein (GFP). Tumors that closely resembled human glioblastomas formed in 100% of the animals by 14 d postinfection. Surprisingly, the tumors were composed of a heterogeneous population of cells, <20% of which expressed the retroviral reporter gene (GFP). The vast majority of both GFP+ and GFP– tumor cells expressed markers of glial progenitors. Thus, the tumors arose from the massive expansion of both infected and uninfected glial progenitors, suggesting that PDGF was driving tumor formation via autocrine and paracrine stimulation of glial progenitor cells. To explore this possibility further, we coinjected a retrovirus expressing PDGF-IRES-DsRed with a control retrovirus expressing only GFP. The resulting tumors contained a mixture of red cells (PDGF-expressing/tumor-initiating cells) and green cells (recruited progenitors). Both populations were highly proliferative and infiltrative. In contrast, when the control GFP retrovirus was injected alone, the animals never formed tumors and the majority of infected cells differentiated along the oligodendrocyte lineage. Together, these results reveal that adult white matter progenitors not only have the capacity to give rise to gliomas, but resident progenitors are recruited to proliferate within the mitogenic environment of the tumor and in this way contribute significantly to the heterogeneous mass of cells that compose a malignant glioma.
Keywords: platelet-derived growth factor, tumor environment, oligodendrocyte, glioblastoma multiforme, animal model, tumor clonality
Introduction
Identifying the cells that give rise to human gliomas has long been a major goal of neuro-oncology research. Recent studies showing that many human gliomas contain a subpopulation of cells with stem cell-like properties have focused attention on the role of neural stem cells or progenitors as possible “cells of origin” (Reya et al., 2001; Ignatova et al., 2002; Singh et al., 2003). This idea is supported by studies that have shown that infecting progenitor cells in the neonatal rodent brain with PDGF-expressing retroviruses induces the formation of tumors that closely resemble human gliomas (Uhrbom et al., 1998; Dai et al., 2001; Shih et al., 2004). However, no previous studies have tested the effects of delivering a PDGF-expressing retrovirus to the adult brain, and it is not yet known whether adult progenitors possess the proliferative and self-renewing capacity to form tumors. This question is of great clinical relevance, because most malignant gliomas occur in adults.
The subcortical white matter contains one of the largest populations of cycling cells in the adult brain. It is estimated that glial progenitors account for ∼2% of the adult rat white matter and as many as 4% of the adult human white matter (Hommes and Leblond, 1967; Roy et al., 1999; Gensert and Goldman, 2001; Dawson et al., 2003). Stereotactic injections of replication incompetent retrovirus into adult rat white matter selectively infects the resident population of cycling glial progenitors, most of which belong to the oligodendrocyte lineage (Gensert and Goldman, 1996). We have chosen to test the effects of PDGF overexpression on glial progenitors in the adult white matter for several reasons. (1) Subcortical white matter is the most common location in which malignant gliomas arise. (2) Many human gliomas express markers also expressed by glial progenitors, including olig2, NG2, and PDGF receptor α (PDGFRα), suggesting a close relationship (Shoshan et al., 1999; Chekenya and Pilkington, 2002; Bouvier et al., 2003; Ligon et al., 2004). (3) PDGF is a powerful mitogen for both glioma cells and adult glial progenitors (Wolswijk et al., 1991; Wolswijk and Noble, 1992; Vassbotn et al., 1994). (4) Human gliomas often express both PDGF and its receptor, suggesting that autocrine and paracrine PDGF signaling plays a role in glioma growth and progression (Hermanson et al., 1992; Westermark et al., 1995; Di Rocco et al., 1998). This also raises the possibility that nontransformed glial progenitors entrapped within an infiltrating glioma will be driven to proliferate by paracrine PDGF signaling.
In this study, we show that infecting adult white matter progenitors with a PDGF-B-expressing retrovirus induces rapid and consistent formation of tumors that closely resemble glioblastomas. The retroviruses also express high levels of fluorescent reporter genes, allowing us to identify infected cells and their progeny. This analysis has led to the finding that the majority of cells comprising the tumors are uninfected progenitor cells, suggesting that adult glial progenitors not only have the proliferative and self-renewing capacity to form tumors when infected with the PDGF retrovirus but also that uninfected progenitor cells can be recruited and induced to proliferate via paracrine signaling.
Materials and Methods
Retrovirus production and injections.
A 0.8 kb fragment encoding PDGF-B-hemagglutinin (HA) (Shih et al., 2004) was ligated into the MCS1 region of the retroviral vectors pQ-MCS1-IRES-eGFP (pQ-GFP) and pQ-MCS1-IRES-DsRed. Replication-deficient viruses with vsv-G coats were generated from these constructs as well as from pNIT-GFP as described previously (Kakita and Goldman, 1999). Viral titers were determined in colony-forming units (CFU) by incubating C6 glioma cells with serial dilutions of retrovirus in 10-fold steps. At 48 h postinfection the number of GFP+ (or DsRed+) cell clusters were counted. The CFU were calculated by multiplying the number of GFP+ (or DsRed+) cell clusters by the dilution factor. Viruses were injected into the forceps minor of the corpus callosum (stereotactic coordinates relative to bregma: 2 mm lateral, 2.5 mm rostral, 3.5 mm deep) of adult Sprague Dawley rats using a Hamilton syringe with a 33 gauge needle (5 μl at 0.2 μl/min) (see Fig. 1). The injection of retrovirally infected progenitor cells (see below) was performed using the same procedure including the same volume, flow rate, and stereotactic coordinates. All animal work was performed according to Institutional Animal Care and Use Committee (IACUC) guidelines of Columbia University.
Figure 1.
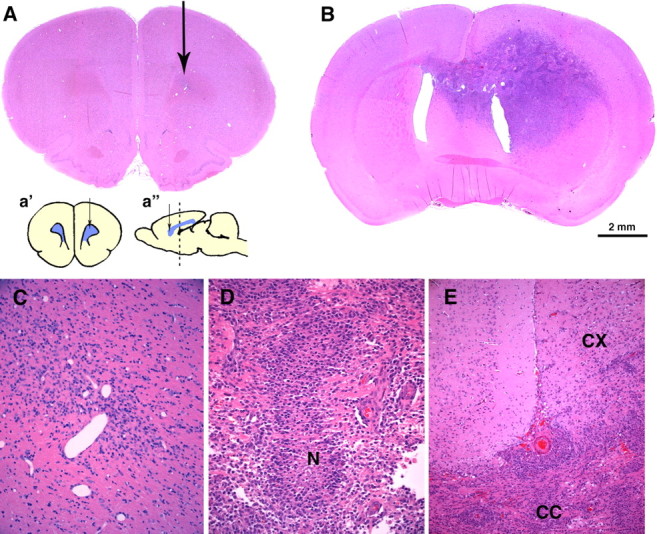
PDGF overexpression induces the formation of malignant gliomas. Hematoxylin and eosin stains were performed on coronal sections of adult rat brains 14 dpi with pQ-GFP (A, C) or PDGF-IRES-GFP (B, D, E). A, No tumors formed in brains injected with the control retrovirus, but a small area of reactive gliosis is seen around the needle track (arrow). C, Higher-magnification micrograph showing the injection site. B, Large infiltrative tumors with the histological features of human glioblastoma formed by 14 dpi with PDGF-IRES-GFP retrovirus. This section, 3 mm caudal from injection site, shows the tumor extending across the corpus callosum into the contralateral hemisphere. D, Higher-magnification micrograph showing an area of pseudopalisading necrosis (N), a hallmark of glioma malignancy seen in human glioblastomas. E, Tumor cells crossing the corpus callosum (CC) and infiltrating the cortex (CX). Scale bar: (in B) A, B, 2 mm. Insets in A are coronal (a′) and sagittal (a″) schematic diagrams of the injection site (arrow) at the level of the forceps minor corpus callosum. The dotted line in a″ shows the level of the coronal section shown in B.
Survival study.
Adult Sprague Dawley rats were injected with either pQ-GFP (six animals) or PDGF-IRES-GFP (six animals) as described above. Animals were monitored daily for signs of morbidity, such as weight loss, seizures, posturing, and nasal and/or periorbital hemorrhage. In addition to this survival study, 26 animals were injected with the PDGF-IRES-GFP retrovirus and followed until they showed signs of tumor morbidity (between 14 and 20 dpi). In accordance with IACUC guidelines, animals were killed at the first sign of morbidity. All animals were perfused with 4% paraformaldehyde, and the brains were examined histologically for the presence of tumor.
Magnetic resonance imaging study.
Adult Sprague Dawley rats were injected with PDGF-IRES-GFP (three animals) as described above. At 5, 10, and 17 d postinfection (dpi), the animals were anesthetized and 0.1 ml of gadolinium was given intraperitoneally. Animals were immobilized in a Plexiglas frame and T1 and T2 images were collected from a magnetic resonance imaging (MRI) unit with a 1.5 T magnet.
Immunohistochemistry, microscopy, and cell counting.
Brains were fixed at 3, 14, or 17 dpi by cardiac perfusion with 4% paraformaldehyde. Hematoxylin and eosin stains were performed on 5 μm sections from paraffin-embedded blocks. Immunofluorescence analysis was performed on 10 μm cryosections as described previously (Marshall et al., 2005) using the following antibodies: anti-Olig2 (kind gift from Dr. Thomas Jessel, Columbia University, New York, NY), mouse anti-Nestin (1:200; Chemicon, Temecula, CA), rabbit anti-GFAP (1:500; DakoCytomation, Carpinteria, CA), mouse anti-NeuN (1:500; Chemicon), rabbit anti-GFP (1:500; Invitrogen, Eugene, OR), rabbit anti-DsRed (1:100; BD Biosciences, Palo Alto, CA), mouse anti-HA (1:100; Covance, Berkeley, CA), rabbit anti-Ki67 (1:1000; NovoCastra, New Castle, UK), goat anti-PDGFRα (1:80; NeoMarkers, Fremont, CA), anti-NG2 (kind gift from Dr. William Stallcup, Burnham Institute, La Jolla, CA), mouse anti-smooth muscle actin (SMA) (1:200; DakoCytomation), and mouse anti-CC1 (1:50; Calbiochem, La Jolla, CA). Stained sections were examined and photographed using a Zeiss (Oberkochen, Germany) Axiophot 200 fluorescent microscope equipped with an Axiocam (Zeiss) and OpenLab imaging software (Improvision, Lexington, MA). Multiple sections from three to six brains from each condition were photographed, and the number of cells staining positive for each marker was manually counted. Statistical analysis was performed using InStat, version 3.0, program.
Cell culture.
White matter progenitors were isolated from adult Sprague Dawley rats as described previously (Gensert and Goldman, 2001; Mason and Goldman, 2002). The isolated cells were cultured in B104 conditioned media, as described previously (Canoll et al., 1996) for 5 d, and then infected with either pNIT-GFP or PDGF-IRES-DsRed retrovirus. Cells were injected into animals (10,000 cells in 5 μl) at 2 dpi, as described above. In some experiments, infected cells were kept in culture with basal media (DMEM, N2, T3, 0.5% FBS, and penicillin/streptomycin/amphotericin) for 10 dpi, and then fixed in 4% paraformaldehyde, and analyzed by fluorescence microscopy.
Flow cytometry and fluorescence activated cell sorting.
Tumors were dissected between 17 and 19 dpi and cells were isolated using the same method of isolation of adult white matter progenitors (Gensert and Goldman, 2001; Mason and Goldman, 2002) and then stained at 4°C with A2B5 antibody (American Type Culture Collection, Manassas, VA) for 1 h followed by anti-IgM Cy5 secondary for 30 min (Jackson ImmunoResearch, West Grove, PA). Cells were resuspended in PBS/10% FBS and FACS-sorted in a BD FACS ARIA system (BD Bioscience). The FlowJo program was used for flow cytometry data analysis. Cells isolated from the tumors that formed from the coinjection of pNIT-GFP and PDGF-IRES-DsRed were sorted on the basis of endogenous GFP and DsRed.
ELISA.
Confluent cultures of human glioma cell lines (U87, U251, U343, and U373) or primary cultures of PDGF-induced tumor cells (isolated from tumors at 17–19 dpi as described above) were rinsed with PBS and incubated for 24 h with basal medium. Quantikine kits from R&D Systems (Minneapolis, MN) were used for quantification of PDGF-AA or PDGF-BB of cell culture media according to the manufacturer’s instructions. Optical densities were obtained with a Bio-Rad (Hercules, CA) 680 plate reader, and concentrations were determined with the Microplate Manager, version II, program.
Results
PDGF-expressing retrovirus introduced into adult white matter progenitors induces tumors that closely resemble human glioblastomas
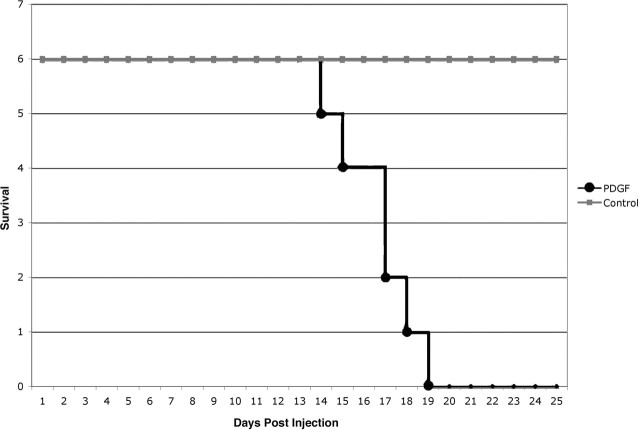
To determine whether PDGF overexpression could induce adult white matter progenitors to give rise to gliomas, we generated a Moloney-based retrovirus that coexpresses PDGF-B-HA and GFP, separated by an internal ribosomal entry site (PDGF-IRES-GFP). The infected cells expressed GFP at high levels, making it easy to identify the infected cells and their progeny in tissue sections by fluorescence microscopy and to isolate and analyze the cells by FACS. We stereotactically injected 5 μl of the retrovirus (at titers of ∼105 CFU/ml) into the rostral subcortical white matter (the forceps minor of the corpus callosum) of adult rats (Fig. 1). Brain tumors developed in 100% of the animals injected (86 of 86). Survival studies showed that all animals developed signs of tumor-induced morbidity between 14 and 20 dpi (Fig. 2). We were also able to follow tumor progression through MRI scanning at 5, 10, and 17 dpi. PDGF retrovirus-induced tumors were visible by 10 dpi with some edema around the small tumor. A large tumor was visible at 17 dpi with marked edema throughout the ipsilateral hemisphere and extending into the contralateral hemisphere (Fig. 3).
Figure 2.
PDGF retrovirus causes rapid tumor formation and morbidity. Kaplan–Meier survival curve showing the rapid onset of tumor-induced morbidity. Equal volumes and titers of pQ-GFP or PDGF-IRES-GFP were injected into the subcortical white matter of adult rats (6 rats in each group). All rats injected with the PDGF-IRES-GFP retrovirus showed signs of tumor-induced morbidity between 14 and 19 dpi. None of the rats injected with pQ-GFP showed any signs of tumor-induced morbidity at 35 dpi. The graph shows the results of one representative experiment. In total, we have performed survival studies on 32 rats injected with the PDGF-IRES-GFP retrovirus. All have developed tumor-induced morbidity between 14 and 20 dpi. In all cases, the presence of a malignant glioma was confirmed by histologic analysis.
Figure 3.
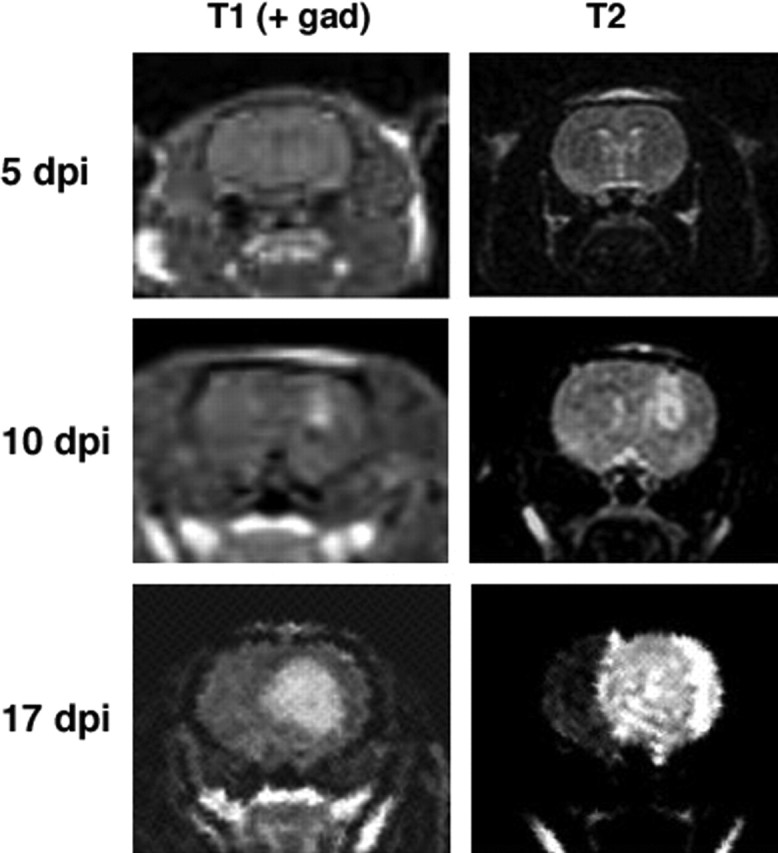
Serial MRI studies show tumor progression. MRI scans were performed on adult rats at 5, 10, and, 17 dpi with the PDGF-IRES-GFP retrovirus. Here, we show a representative series of coronal images from one animal. No tumor is visible at 5 dpi. By 10 dpi, a small tumor is seen at the injection site on postcontrast T1 images. Tumor-induced edema is visible on T2 images. At 17 dpi, a large tumor is visible on postcontrast T1 image, and edema involves the entire hemisphere and part of the contralateral hemisphere as seen in the T2 image. gad, Gadolinium.
The tumors had all of the histologic features of glioblastoma multiforme, including marked vascular proliferation as seen with immunostain for SMA (Fig. 4C) and pseudopalisading necrosis (Figs. 1D, 4B). Fluorescence microscopy revealed that numerous GFP+ cells had infiltrated the surrounding brain tissue and migrated across the corpus callosum into the contralateral hemisphere (Fig. 4A). In contrast, none of the animals injected with pQ-GFP or pNIT-GFP control retroviruses developed tumors (0 of 72) (Figs. 1A,C, 7A; supplemental Fig. 2, available at www.jneurosci.org as supplemental material).
Figure 4.

GFP expression reveals the distribution of retrovirally infected cells. Immunofluorescence analysis for GFP and SMA was performed on sections of tumor at 17 dpi with the PDGF-IRES-GFP retrovirus. A, Micrograph shows GFP+ cells (green) crossing the corpus callosum (CC) and invading the contralateral hemisphere. Hoechst stain (blue) shows increased cellular density in and around the main tumor mass. Note that only a subset of the cells is GFP+. B, GFP+ and GFP− cells are seen intermingled throughout the tumor, including in areas of pseudopalisading necrosis (N). C, SMA immunofluorescence (red) shows marked vascular proliferation with recruitment of perivascular smooth muscle cells. Scale bar, 1 mm.
Figure 7.

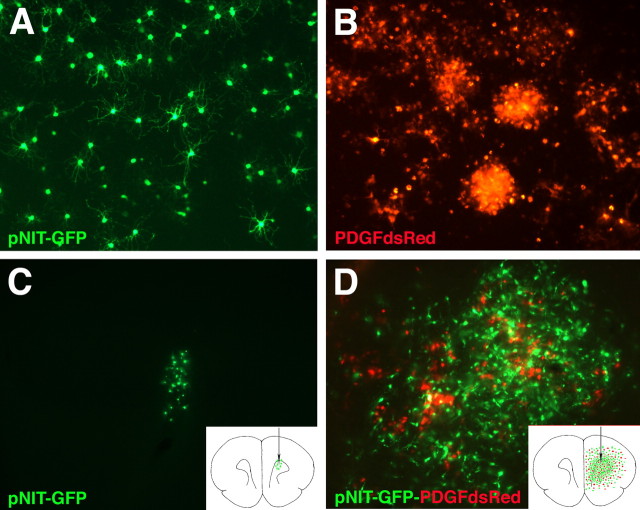
Coinjection studies show that PDGF-expressing cells recruit other progenitors to proliferate within the tumor. A, Low-power micrograph showing a small collection of GFP+ cells (green) along the injection track (white arrow) at 17 dpi with pNIT-GFP. B, In contrast, a large tumor containing many GFP+ cells (green) and DsRed+ cells (red) are seen at 17 dpi with pNIT-GFP and PDGF-IRES-DsRed. Note that this section, 3 mm caudal of the injection site, shows that many of the GFP+ cells are seen crossing the corpus callosum (CC) into the contralateral hemisphere. Scale bars: A, B, 2 mm: A, B, C, Higher-magnification micrograph showing red and green cells intermingled throughout the tumor, including areas of pseudopalisading necrosis (N). D, Cells isolated from tumors (17–19 dpi with pNIT-GFP and PDGF-IRES-DsRed) were FACS-sorted into four populations: GFP−/DsRed−, GFP+/DsRed−, DsRed+/GFP−, and DsRed+/GFP+. Totals of 1.5 × 106 GFP+/DsRed− cells and 2.8 × 105 DsRed+/GFP− cells were sorted from two pooled tumors in this representative experiment (the experiment was repeated five times). E–E″, In brains injected with pNIT-GFP (17 dpi), the GFP+ cells have a highly branched morphology and are negative for the proliferation marker Ki67 (labeling index of <5%). F–F″, In tumors generated by coinfection of pNIT-GFP and PDGF-IRES-DsRed, the GFP+ cells have an immature morphology and many are Ki67+ (labeling index of 26%). Insets in A and B are schematic diagrams illustrating the distribution and number of cells (green and red dots) in coronal sections of adult brain at their corresponding levels.
Retroviral delivery to the adult white matter predominantly infects NG2+/GFAP− glial progenitors
To characterize the identity of the cells that were infected with the retroviruses and gave rise to the PDGF-driven tumors, we performed immunohistochemistry of retrovirus-infected cells at 3 dpi with PDGF-IRES-GFP or a control retrovirus that expresses only GFP (pNIT-GFP). At 3 dpi, the infected (GFP+) populations were very similar in number, distribution, morphology, and immunophenotype for each retrovirus. Our results showed that the large majority of GFP+ cells were NG2+ (87.8 ± 1.7% for pNIT-GFP and 91.0 ± 1.1% for PDGF-IRES-GFP) (supplemental Fig. 1A,C, available at www.jneurosci.org as supplemental material). Less than 1% of the GFP+ cells expressed detectable levels of GFAP (0.5 ± 0.2% for pNIT-GFP and 0.4 ± 0.4% for PDGF-IRES-GFP) (supplemental Fig. 1B,D, available at www.jneurosci.org as supplemental material). The majority of cells was located in the subcortical white matter near the injection site. A few GFP+ cells were also seen in the overlying cortex adjacent to the injection tract and these cells were also NG2+/GFAP−. These results are consistent with previous BrdU-labeling studies showing that NG2+ glial progenitors account for the majority of cycling cells in the adult white matter and cortex (Dawson et al., 2003). By 14 dpi with the control retroviruses, the majority of the GFP+ cells had begun to differentiate along the oligodendrocyte lineage: 83.9 ± 2.5% expressed the oligodendrocyte marker CC1, and 1.3 ± 0.7% expressed GFAP (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). It is important to note that, although a gliotic response formed around the injection site, this did not lead to the retroviral labeling of reactive astrocytes. Previous studies have shown that there is a 3–4 d delay before GFAP+ astrocytes proliferate in response to a cortical stab wound (Latov et al., 1979; Takamiya et al., 1988). The retroviral titers decay rapidly and in vitro analysis has shown that these retroviruses have a half-life of ∼4 h at 37°C (Gensert and Goldman, 1997). Therefore, the effective viral titer has dropped to near zero by the time astrocytes begin to proliferate. These results are consistent with previous results showing that retroviral injection into the adult white matter predominantly labels glial progenitors, most of which belong to the oligodendrocyte lineage and does not label GFAP+ astrocytes (Gensert and Goldman, 1996).
The tumors are composed of infected and uninfected glial progenitors
To characterize the cellular composition of the tumors, we performed immunohistochemical analysis for retrovirally encoded genes, GFP and PDGF-HA, and a variety of neuronal and glial markers. Remarkably, only a subset of the cells in the tumor expressed detectable levels of GFP or PDGF-HA, suggesting that tumors were composed of both infected and uninfected cells (Figs. 5, 6). Furthermore, double immunofluorescence showed that GFP and HA colocalized to the same subset of cells (Fig. 5A), confirming that GFP is a reliable marker for cells that are expressing both retroviral genes. The vast majority of tumor cells expressed markers seen in immature glia, including olig2, NG2, nestin, and PDGFRα, and double-immunofluorescence analysis showed that the majority of both GFP+ and GFP− cells were positive for these markers (Figs. 5, 6). Numerous GFAP+/GFP− reactive astrocytes were scattered throughout the tumor (Fig. 5E–E″), but <3% of the GFP+ cells were GFAP+ (data not shown). None of the GFP+ cells expressed the neuronal marker, NeuN (data not shown).
Figure 5.
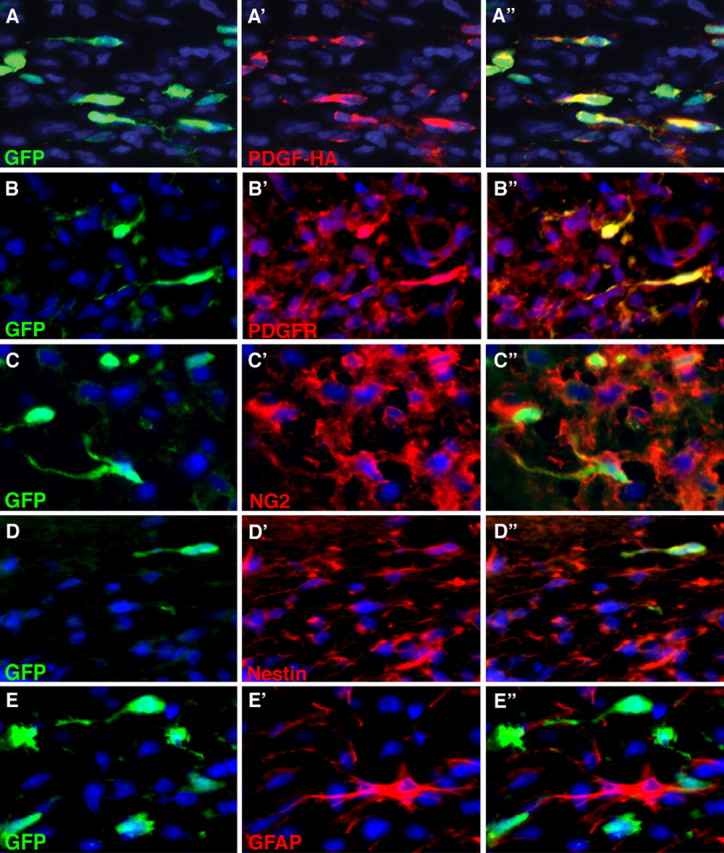
GFP+ and GFP− tumor cells express markers of glial progenitor cells. Double-immunofluorescence analysis of tumors, 17 dpi with the PDGF-IRES-GFP retrovirus, shows that GFP (green) is expressed in only a subset of tumor cells. A–A″, PDGF-HA expression (red) is seen in the same subset of cells that express GFP. However, PDGFRα (B′, B″), NG2 (C′, C″), and nestin (D′, D″), each stained red, are expressed in the vast majority of GFP+ and GFP− tumor cells. E′, E″, GFAP+/GFP− reactive astrocytes (red) are seen scattered throughout the tumor. Rare GFAP+/GFP+ cells were seen (<3%).
Figure 6.
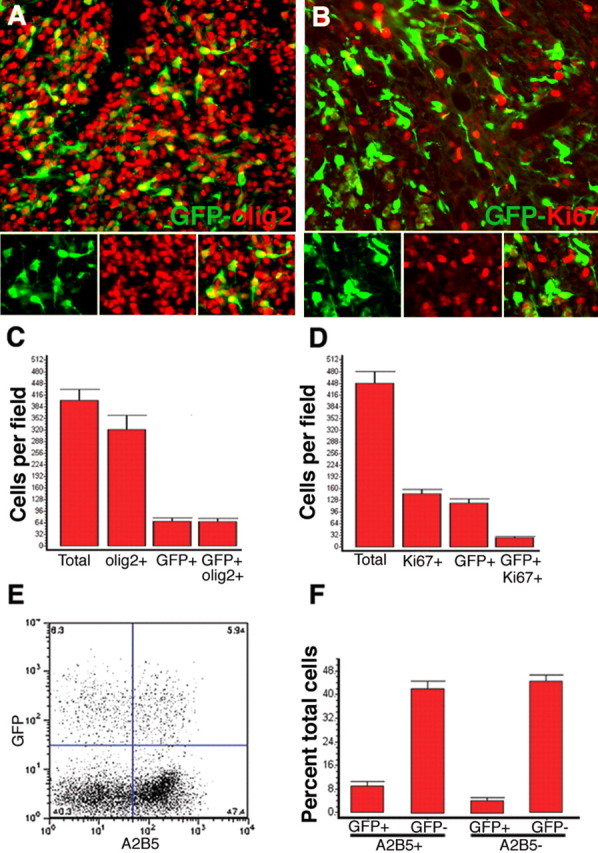
Quantitative analysis shows that the majority of cells in the PDGF-driven tumors are GFP− progenitor cells. A, Double immunofluorescence of tumors, 17 dpi with PDGF-IRES-GFP, shows that the vast majority of tumor cells are olig2+ (red), but only a subset of olig2+ cells are GFP+ (green). B, Double immunofluorescence for GFP (green) and Ki67 (red), a marker for cycling cells, shows that tumor cells are highly proliferative, but only a subset of Ki67+ cells are GFP+. The triptych at the bottom of A and B show green, red, and green/red overlay. C, Bar graph showing the number of total cells (identified by Hoechst nuclear staining), olig2+, GFP+, and GFP+/olig2+, cells per high-powered field. D, Bar graph showing the number of total, Ki67+, GFP+, and GFP+/Ki67+ cells per high-powered field. Data represent the mean ± SEM of multiple high-powered fields from three separate brain tumors. E, F, Cells isolated from freshly dissected tumors (17 dpi with PDGF-IRES-GFP retrovirus) were immunostained with the progenitor cell marker A2B5 and analyzed by flow cytometry. E, Scatter plot from a single representative experiment showing the relative abundance of four populations of tumor cells: GFP+/A2B5+, GFP−/A2B5+, GFP+/A2B5−, and GFP/A2B5−. F, Bar graph showing the percentage of cells in each population. Data represent the mean ± SEM from three separate experiments.
To determine the relative abundance and proliferation rates of GFP+ and GFP− cells in the tumors, we performed quantitative analysis after double immunofluorescence for GFP and olig2, or GFP and the proliferation marker Ki67 on sections of tumors 17 dpi with the PDGF-IRES-GFP retrovirus, and counted the number of cells positive for each marker. The majority of cells in the tumor (77.6 ± 4.6%) expressed olig2. However, only 16.8 ± 2.0% of the total cells expressed GFP, almost all of which (96.5 ± 0.5%) were olig2+ (Fig. 6A,C). Similarly, a large fraction of tumor cells (34.0 ± 4.1%) were Ki67+, but only 18.2 ± 1.8% of the Ki67+ cells were GFP+ (Fig. 6B,D). Thus, the majority of olig2+ and Ki67+ cells in the tumor were GFP negative, suggesting that most of the proliferating cells in the tumor were uninfected glial progenitors that had been recruited via paracrine signaling.
As a second approach to quantitate the relative abundance of the different cell types in the tumor, we performed flow cytometry on cells isolated from tumors (17–19 dpi) using the A2B5 monoclonal antibody, which is a well established marker for a subset of progenitor cells found in the adult white matter (Mason and Goldman, 2002; Nunes et al., 2003). Flow cytometry showed that 13.2 ± 1.1% of the isolated cells expressed GFP (consistent with our immunohistochemical analysis noted above). Of the total cells, 51.2 ± 1.9% were A2B5+, but only 9.0 ± 2.6% were A2B5+/GFP+ (Fig. 6E,F). From a typical experiment, we isolated ∼5 × 106 A2B5+ cells per tumor (of which the majority was GFP−). In contrast, using the same procedure, we typically isolate on the order of 104 A2B5+ cells from a normal adult brain (data not shown). Thus, the PDGF retrovirus caused a massive expansion of the A2B5+ population, most of which are GFP− (uninfected) cells.
Adult white matter progenitors infected with the PDGF-expressing retrovirus in vivo and in vitro are able to recruit and activate normal progenitors
To test the hypothesis that PDGF-expressing cells were recruiting other glial progenitors to proliferate, we coinjected a GFP-expressing retrovirus (pNIT-GFP) and the PDGF-IRES-DsRed retrovirus into adult white matter. All of the rats coinjected with pNIT-GFP and PDGF-IRES-DsRed retrovirus formed malignant gliomas by 17 dpi (35 of 35) (Fig. 7B). Immunofluorescence analysis and FACS for GFP and DsRed, showed that tumors were composed of four populations of cells: GFP−/DsRed−, GFP+/DsRed−, GFP−/DsRed+, and a small subpopulation was GFP+/DsRed+, demonstrating that coinfection was a rare event (Fig. 7D). The red cells (PDGF-expressing/tumor-initiating cells) and green cells (recruited progenitors) were intermingled within the tumor, including regions of pseudopalisading necrosis (Fig. 7C). The large majority of both populations had an immature morphology and were nestin+, NG2+, and olig2+ (data not shown). Both red and green cells diffusely infiltrated the brain, crossing the corpus callosum and invading the contralateral hemisphere (Fig. 7B).
To further characterize the normal fate and behavior of the retrovirally infected cells, we injected the pNIT-GFP virus alone, in the same volume and at the same titer (106 CFU/ml). As anticipated, none of these animals developed tumors (0 of 19). By 17 dpi, the pNIT-GFP cells remained few (∼100 cells per brain) and all of the pNIT-GFP cells were distributed within 500 μm of the injection track (Fig. 7A). The majority of pNIT-GFP cells had acquired a complex morphology with numerous thin processes characteristic of differentiating oligodendrocytes (Fig. 7E–E″). Immunofluorescence analysis showed that 77% of the pNIT-GFP+ cells stained positive for the oligodendrocyte marker CC1 (61 of 79 cells counted), and <1% of the pNIT-GFP cells expressed GFAP (consistent with the result of our analysis at 14 dpi described above). Less than 1% of the pNIT-GFP cells were Ki67+ in brains injected with the control virus (Fig. 7E–E″). In contrast, 26% of the pNIT-GFP/DsRed− cells were Ki67+ in tumors formed by the coinjection of the two viruses (Fig. 7F–F″). Together, these results show that, in normal adult white matter, the majority of retrovirally infected cells differentiated along the oligodendrocyte lineage. However, within the mitogenic rich environment of the tumor, the pNIT-GFP-infected cells were induced to proliferate and migrate, and thus mimic the behavior of malignant glioma cells.
As another test of the recruitment hypothesis, and to eliminate the possibility of any cells being coinfected with both viruses, we isolated progenitors from adult white matter, expanded them in vitro for 5 d in B104 conditioned media, and infected them in vitro. Equal numbers of progenitors were separately infected with either pNIT-GFP or PDGF-IRES-DsRed. In some experiments, the infected progenitor cells were grown in culture with basal media for 10 dpi (Fig. 8A,B). Cells infected with pNIT-GFP stopped proliferating and acquired a highly branched morphology characteristic of maturing oligodendrocytes (Fig. 8A). In contrast, cells infected with PDGF-IRES-DsRed remained immature and highly proliferative, forming large clusters of cells by 10 dpi (Fig. 8B). In a separate set of experiments, the in vitro-infected progenitor cells were transplanted into adult rat subcortical white matter (same coordinates as shown in Fig. 1) 2 d after infection, by either coinjecting pNIT-GFP and PDGF-IRES-DsRed cells (2000 of each cell type), or pNIT-GFP+ cells alone (2000). Coinjected cells formed infiltrative tumors by 20 dpi (Fig. 8D). The tumors were composed of three populations: DsRed+/GFP−, DsRed−/GFP+, and DsRed−/GFP−. No double-positive cells were observed. Both the PDGF-IRES-DsRed and pNIT-GFP cells in the coinjections remained immature, infiltrative, and highly proliferative (Fig. 8D). In contrast, pNIT-GFP cells injected alone remained few, had branched morphologies characteristic of differentiating glia, and did not develop into tumors (Fig. 8C). All of the pNIT-GFP cells were located within a few hundred micrometers of the injection site, demonstrating that, when injected alone, the transplanted adult progenitors do not accumulate or migrate far from the injection site (Fig. 8C).
Figure 8.
Adult white matter progenitors infected with PDGF-IRES-DsRed in vitro form malignant gliomas through autocrine and paracrine signaling. Normal adult white matter progenitors were isolated and expanded for 5 d in vitro with B104-containing media. Proliferating progenitors were then infected with pNIT-GFP or PDGF-IRES-DsRed. A, B, Adult white matter progenitors grown in culture for 10 dpi in basal media. A, pNIT-GFP-infected cells stopped proliferating and acquired a highly branched morphology. B, PDGF-IRES-DsRed-infected cells retained a simple morphology and remained highly proliferative, forming large clusters of cells. C, D, At 2 d postinfection, the cells were implanted into adult brains. Brains were analyzed at 20 dpi. C, pNIT-GFP-infected cells injected alone remained close to the injection site and no tumors formed. D, When pNIT-GFP-infected cells were coinjected with PDGF-IRES-DsRed-infected cells, a tumor formed by 20 dpi, and this tumor is composed of a mixture of red and green cells. Equal numbers of pNIT-GFP cells were injected in C and D. Note the marked proliferation and dispersion of pNIT-GFP cells in the presence of PDGF-IRES-DsRed cells (D). Insets in C and D are schematic diagrams illustrating the distribution and number of cells (green and red dots) in coronal sections of adult brain at the level of the injection site.
Secretion of PDGF-A and PDGF-B into glioma cell conditioned medium
The above results strongly suggest that retrovirus-infected cells secrete PDGF at high enough levels to drive proliferation of progenitors cells in their local environment. How do the levels of PDGF produced and secreted by the retrovirus-induced tumor cells compare with those produced by human glioma cells? Do human glioma cells secrete high enough levels of PDGF to drive glial progenitor proliferation? To address these questions, we collected conditioned medium from confluent cultures of the retrovirus-induced tumors (17 dpi) and several human glioma cell lines (U87, U251, U343, and U373). The levels of PDGF-A and PDGF-B in conditioned medium were measured by ELISA (Table 1). These results show that the primary cultures from the retrovirus-induced tumors secreted detectable levels of both PDGF-A (1.4 ± 0.1 ng/ml) and high levels of PDGF-B (29.2 ± 7.4 ng/ml). The human glioma cell lines we tested did not secrete detectable levels of PDGF-B but did secrete significant levels of PDGF-A. In fact, one of the cell lines (U343) expressed very high levels of PDGF-A (52.1 ± 1.6 ng/ml). Previous studies have shown that many glioma cells express PDGF-A, PDGF-B, or both, but most glioma cell lines secrete predominantly PDGF-A (Betsholtz et al., 1986; Nister et al., 1986, 1991). The levels of PDGF measured in these previous studies (20–30 ng/ml) are very similar to our results. It is important to note that these levels are well within the range of PDGF concentrations needed to drive glial progenitor proliferation. In vitro studies have shown that both PDGF-A and PDGF-B are potent mitogens for glial progenitors with PDGF-A having half-maximal effects at 2 ng/ml and PDGF-B having half-maximal effects at 10 ng/ml (Pringle et al., 1989). Together, these results suggest that some human glioma cells produce high enough levels of PDGF to drive the proliferation of glial progenitors that are in and around the tumor.
Table 1.
Secretion of PDGF-A and PDGF-B into culture medium
| Conditioned medium | PDGF-A concentration | PDGF-B concentration |
|---|---|---|
| PDGF-IRES-GFP tumor cells | 1.46 ± 0.12 ng/ml | 29.2 ± 7.4 ng/ml |
| U87 | 31.6 ± 16.2 pg/ml | ND |
| U251 | 1.41 ± 0.38 ng/ml | ND |
| U343 | 52.1 ± 1.64 ng/ml | ND |
| U373 | 1.40 ± 0.38 ng/ml | ND |
Confluent cultures of human glioma cell lines (U87, U251, U343, and U373) or PDGF-induced tumor cells were grown in basal medium for 24 h. The level of PDGF-A and PDGF-B in each conditioned medium was measured by ELISA. The values represent the mean ± SEM from three separate experiments. ND, Not detected.
Discussion
We have shown that infecting glial progenitors in adult rat white matter with a retrovirus that expresses high levels of PDGF-B induces the rapid and consistent formation of tumors that closely resemble human glioblastomas. The tumors are composed of infected and uninfected progenitors, suggesting that tumor formation was being driven by both autocrine and paracrine growth factor stimulation. This conclusion was further supported by our coinjection experiments that allowed us to compare the behavior (and fate) of GFP-tagged glial progenitors (and their progeny) in the presence or absence of PDGF-IRES-DsRed-expressing cells. When the GFP-expressing virus was injected alone, tumors never formed and the majority of GFP-tagged cells differentiated along the oligodendrocyte lineage by 14 dpi. In contrast, when both viruses were injected, the animals formed tumors that were composed of a mixture of red and green cells. Both populations were highly proliferative and migratory. We also infected adult glial progenitors in vitro with one or the other of these retroviruses and then injected the pNIT-GFP cells alone or with PDGF-IRES-DsRed cells. This approach again showed that the GFP-tagged progenitors were recruited to proliferate within the tumor and effectively ruled out the possibility that any of the green cells had been infected with the PDGF-expressing retrovirus.
The tumor formation seen with this model was remarkably rapid and consistent. Although this makes the model very convenient and attractive for the use of controlled studies, such as preclinical trials for new therapies, one may question the degree to which this resembles the growth of human tumors. It is not known how long human gliomas have been growing before they manifest clinically; however, by the time of diagnosis, human glioblastomas can grow remarkably fast. Serial imagining studies have shown that humans gliomas can grow with volume doubling times as short as 1 week (Blankenberg et al., 1995; Swanson et al., 2003). Furthermore, human glioblastomas often have Ki67 labeling indices as high or higher than that seen in the PDGF glioma model. The major difference in survival times (<3 weeks for the rats vs <1 year for treated human glioblastomas) has more to do with the size of the skull and the room available to accommodate the tumor than it has to do with growth rate of the tumor per se.
Adult glial progenitors as a cell of origin for malignant gliomas
Several different types of brain cells have been implicated in the formation of human gliomas including mature astrocytes, progenitors, and neural stem cells (Bachoo et al., 2002; Singh et al., 2004; Fomchenko and Holland, 2005; Sanai et al., 2005). Using the RCAS system, Holland and coworkers have shown that, in the neonatal brain, GFAP+ astrocytes and nestin+ progenitors have the capacity to form tumors when infected with a PDGFB-HA-expressing retrovirus (Dai et al., 2001). In these studies, the frequency of tumor formation and the histologic features of the tumor (grade and tumor type) depended on the genetic background of the mice used and the population of cells targeted by the retrovirus (Uhrbom et al., 1998; Dai et al., 2001; Hesselager et al., 2003). Shih et al. (2004) compared the tumorigenic effects of retroviruses expressing different levels of PDGFB-HA. The PDGFB-HA retrovirus expressing higher levels of growth factor caused more malignant tumors with a shorter latency and in a higher percentage of animals.
In this study, we used the same high expressing PDGFB-HA construct (cloned into an amphoteric retrovirus) to selectively infect progenitors in the adult brain. Our results show that adult white matter progenitors have a remarkable capacity to form tumors. This is perhaps surprising considering that these cells are normally nonmigratory and slowly proliferating (Noble et al., 1992; Wren et al., 1992; Gensert and Goldman, 1996; Roy et al., 1999; Dawson et al., 2003). In contrast, neonatal progenitors are highly migratory and proliferative and resemble glioma cells in their capacity to infiltrate long distances through brain tissue (Goldman et al., 1997; Kakita and Goldman, 1999; Suzuki and Goldman, 2003). However, in vitro studies have shown that adult glial progenitors can be induced to become more migratory and proliferative when treated with growth factors including PDGF, basic fibroblast growth factor, and glial growth factor (Wolswijk et al., 1991; Wolswijk and Noble, 1992; Shi et al., 1998). Our results provide the first in vivo evidence that PDGF can drive adult glial progenitors to acquire a more migratory and proliferative behavior. When this behavior proceeds unchecked, it leads to the formation of malignant tumors with all of the histologic features of glioblastoma. In particular, the patterns of infiltration seen in this model, with a predilection for fiber tracks, closely resemble those seen in human gliomas. This also resembles the migration of glial progenitors that occurs during brain development (Kakita and Goldman, 1999; Kakita et al., 2003; Suzuki and Goldman, 2003), suggesting that the infiltrative capacity of glioma cells may be a re-expression of a neonatal glial progenitor phenotype.
Constitutive overexpression of PDGF is sufficient to drive malignant behavior
Molecular and cytogenetic analysis has shown that human glioblastomas often contain multiple genetic lesions (Ohgaki and Kleihues, 2005). These findings have led to the assumption that malignant behavior requires the accumulation of multiple genetic lesions. However, studies using molecular and cytogenetic analysis of human tumors are correlative in nature, and it is not known how many genetic lesions are actually required for malignant transformation. One very important implication to our study is that the histologic hallmarks of malignancy can be driven by overexpression of a single growth factor. By 14 dpi, 100% of the PDGF-driven tumors have shown features of glioblastoma, including marked vascular proliferation and pseudopalisading necrosis. Furthermore, the areas of pseudopalisading necrosis contained a mixture of infected and uninfected cells, suggesting that even genetically normal cells can be driven to display a malignant phenotype. These findings point to an important concept; the progression to malignancy does not merely reflect the autonomous misbehavior of genetically deranged cells, but rather results from complex interactions between tumor cells and their environment. We propose that PDGF-expressing retroviruses drive the formation of malignant tumors so quickly because they not only transform the infected cells but they also transform the environment.
Although PDGF overexpression clearly initiates tumor formation in this model, it is possible that other genetic or epigenetic alterations are at play. Other growth factors may be upregulated (either by the progenitor cells or by other cells within the tumor) and cooperate with PDGF in the paracrine signaling, as has been shown to occur with neonatal and adult glial progenitors in vitro (Bogler et al., 1990; Wolswijk and Noble, 1992; Shi et al., 1998). It is also possible that the retrovirally infected cells acquire additional genetic lesions as a result of insertional mutagenesis. Previous studies using a Moloney-based retrovirus expressing PDGF have shown evidence that insertional mutagenesis may cooperate with PDGF in the development of gliomas (Johansson et al., 2004, 2005). However, the speed and consistency with which tumors formed in our model (100% of animals formed tumors by 14 dpi), and the fact that tumors never formed when animals were injected with control virus alone, make it unlikely that insertional mutagenesis and subsequent clonal selection played an essential role in tumor formation. Rather, the tumors are primarily driven by autocrine and paracrine growth factor stimulation. Still, the acquisition of additional genetic lesions (either by insertion mutagenesis or some other mechanism) may confer a selective advantage to a subpopulation of tumor cells (infected or recruited).
Recruitment as a mechanism of glioma growth
Human gliomas are composed of a heterogeneous mass of tumor cells intermingled with entrapped and reactive brain cells. Several studies have provided evidence that paracrine growth factor signaling and/or recruitment may play an important role in the rapid growth and progression of gliomas (Hermanson et al., 1992; Westermark et al., 1995; van der Valk et al., 1997; Shih and Holland, 2005). Vascular proliferation, which is one of the most reliable markers of malignant progression of gliomas, is well known to involve recruitment of endothelial cells, pericytes, and smooth muscle cells. This recruitment is mediated, in part, by paracrine signaling of multiple growth factors, including PDGF (Dunn et al., 2000; Guo et al., 2003; Lamszus et al., 2004). Recent studies have shown that gliomas can also recruit GFAP+ astrocytes and nestin+ neural stem cells/progenitors from the subventricular zone (Duntsch et al., 2005; Glass et al., 2005). In this study, we show, for the first time, that local recruitment of glial progenitors that reside in the adult white matter can contribute significantly to the proliferative mass. Immunohistochemical analysis showed that the majority of the recruited cells were of the oligodendroglial lineage (PDGFRα+, NG2+, olig2+, GFAP−) (Figs. 5, 6). PDGFRα+/NG2+ glial progenitors are abundant and widely distributed in the subcortical white matter of adult humans and rodents (Gogate et al., 1994; Nishiyama et al., 1996; Scolding et al., 1998; Dawson et al., 2000). As with our model, human gliomas grow by diffusely infiltrating the brain and the tumor cells intermingle with normal brain cells. As a result, glial progenitors entrapped within the infiltrative margins of the tumor are subject to paracrine growth factor stimulation. Therefore, in the case of human tumors that express a high level of PDGF, one would expect recruited glial progenitors to contribute to tumor growth regardless of the cell of origin. However, because of the remarkable phenotypic similarities between glial progenitors and glioma cells, it may be difficult to distinguish a recruited glial progenitor from a “true glioma cell.”
How can we define the difference between a true glioma cell and a recruited glial progenitor? In human gliomas, the “true tumor cells” most likely arise from the clonal expansion of a genetically transformed cell of origin. In principle, these cells would have the capacity to initiate the formation of new tumors if they were isolated and reinjected into the brain of a nude rodent. The recruited glial progenitors would be proliferating cells that are not clonally derived from the genetically transformed cell of origin. Some of the recruited cells may acquire genetic lesions as a result of their abnormally driven proliferation, but many may be genetically normal and their proliferation would be dependent on the mitogenic environment of the tumor. Such cells would not have the capacity to form new tumors if isolated and transplanted into a nude rodent.
An exceptional advantage of the PDGF tumor model is that the retroviral reporters enable us to identify the infected cells and their progeny, and thus to distinguish the cells that arise from clonal expansion of the “tumor-initiating cells” from cells that have been recruited. We must consider the possibility that transcriptional silencing of retroviral genes could cause us to underestimate the number of infected cells. The established mechanisms of retroviral silencing (including methylation of promotor regions and histone acetylation) inhibit gene expression at the level of transcription. The retrovirus encodes PDGF and GFP on a single bicistronic message; therefore, transcriptional silencing, if it were to occur, should block expression of both viral genes. The immunohistochemical data showing a colocalization of PDGF-HA and GFP (Fig. 5A–A″) provides direct evidence that GFP is a reliable reporter of retroviral gene expression. Our coinjection studies also address this issue, allowing us to tag a separate population of progenitors with a control retrovirus and track the fate of their progeny. The massive expansion of progenitors infected with the pNIT-GFP control retrovirus (which were never infected with PDGF-expressing virus) cannot be explained by retroviral silencing and provides direct evidence of the recruitment phenomenon.
Progenitor recruitment is not inconsistent with the clonal character of human gliomas
Clonal analysis has shown that most (but not all) human gliomas contain a prominent (or at least detectable) clonal population (Berkman et al., 1992; Morse et al., 1994; Kattar et al., 1997). These studies have used X chromosome inactivation analyses, such as the HUMARA assay, which can detect a clonal population even if it accounts for only 15% of the total cells in a polyclonal background (Willman, 1994). Thus, a clonal character does not indicate that human gliomas are solid tumors composed exclusively of a monoclonal population of cells. They are not. Because of their highly infiltrative nature, glioma cells intermingle with the surrounding brain tissue. As a result, human gliomas are composed of a mixture of neoplastic and non-neoplastic cells, including entrapped neurons, astrocytes, microglia, blood vessels, and progenitor cells. The relative abundance of these different cell types varies from one region to the next. In some regions, the tumor may be composed predominantly of a clonal population of glioma cells, but in the more infiltrative regions (which, for many gliomas accounts for the vast majority of the tumor) the true tumor cells are a minority. Not surprisingly, clonal analysis of these more infiltrative regions of gliomas has shown that the clonal population accounts for a minority of the total cells, and in some cases the clonal population cannot be detected (Kattar et al., 1997). It is within these regions in which glioma cells are intermingling with and interacting with non-neoplastic cells, including many PDGF-responsive glial progenitors, that recruitment is likely to be most robust. Thus, the finding that most human gliomas contain a clonal population is not inconsistent with the possibility that extensive recruitment of glial progenitors is occurring (supplemental Fig. 3, available at www.jneurosci.org as supplemental material).
These findings shed new light on the relationship between gliomas and adult glial progenitors and suggest a novel mechanism of tumor growth by progenitor recruitment, which may play a role in the rapid progression and heterogeneity that characterize malignant gliomas. In addition to the novel implications regarding the relationship between progenitors and glioma cells, the utility of this model as a tool for additional studies of gliomas should not be understated. The ability to generate tumors that closely resemble human glioblastomas in a rapid and highly reproducible manner makes this an ideal model for preclinical animal studies involving new treatment strategies.
Footnotes
This work was supported by National Institutes of Health Grants NS17125 and NS045070. We thank Drs. Eric Holland, Alan Shih, and Chankai Dai for their PDGF-HA construct; Drs. Phyllis Faust and Carol Mason for sharing their microscope systems with us; Dr. Truman Brown and Jaime Cruz for their expert help with the magnetic resonance imaging; Kristie Gordon for her expert help with the FACS; and Dr. Fred Gage for the pNIT retrovirus construct.
References
- Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, Rowitch DH, Louis DN, DePinho RA (2002). Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell 1:269–277. [DOI] [PubMed] [Google Scholar]
- Berkman RA, Clark WC, Saxena A, Robertson JT, Oldfield EH, Ali IU (1992). Clonal composition of glioblastoma multiforme. J Neurosurg 77:432–437. [DOI] [PubMed] [Google Scholar]
- Betsholtz C, Johnsson A, Heldin CH, Westermark B (1986). Efficient reversion of simian sarcoma virus-transformation and inhibition of growth factor-induced mitogenesis by suramin. Proc Natl Acad Sci USA 83:6440–6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenberg FG, Teplitz RL, Ellis W, Salamat MS, Min BH, Hall L, Boothroyd DB, Johnstone IM, Enzmann DR (1995). The influence of volumetric tumor doubling time, DNA ploidy, and histologic grade on the survival of patients with intracranial astrocytomas. AJNR Am J Neuroradiol 16:1001–1012. [PMC free article] [PubMed] [Google Scholar]
- Bogler O, Wren D, Barnett SC, Land H, Noble M (1990). Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proc Natl Acad Sci USA 87:6368–6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier C, Bartoli C, Aguirre-Cruz L, Virard I, Colin C, Fernandez C, Gouvernet J, Figarella-Branger D (2003). Shared oligodendrocyte lineage gene expression in gliomas and oligodendrocyte progenitor cells. J Neurosurg 99:344–350. [DOI] [PubMed] [Google Scholar]
- Canoll PD, Musacchio JM, Hardy R, Reynolds R, Marchionni MA, Salzer JL (1996). GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron 17:229–243. [DOI] [PubMed] [Google Scholar]
- Chekenya M, Pilkington GJ (2002). NG2 precursor cells in neoplasia: functional, histogenesis and therapeutic implications for malignant brain tumours. J Neurocytol 31:507–521. [DOI] [PubMed] [Google Scholar]
- Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC (2001). PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev 15:1913–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MR, Levine JM, Reynolds R (2000). NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res 61:471–479. [DOI] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R (2003). NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci 24:476–488. [DOI] [PubMed] [Google Scholar]
- Di Rocco F, Carroll RS, Zhang J, Black PM (1998). Platelet-derived growth factor and its receptor expression in human oligodendrogliomas. Neurosurgery 42:341–346. [DOI] [PubMed] [Google Scholar]
- Dunn IF, Heese O, Black PM (2000). Growth factors in glioma angiogenesis: FGFs, PDGF, EGF, and TGFs. J Neurooncol 50:121–137. [DOI] [PubMed] [Google Scholar]
- Duntsch C, Zhou Q, Weimar JD, Frankel B, Robertson JH, Pourmotabbed T (2005). Up-regulation of neuropoiesis generating glial progenitors that infiltrate rat intracranial glioma. J Neurooncol 71:245–255. [DOI] [PubMed] [Google Scholar]
- Fomchenko EI, Holland EC (2005). Stem cells and brain cancer. Exp Cell Res 306:323–329. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE (1996). In vivo characterization of endogenous proliferating cells in adult rat subcortical white matter. Glia 17:39–51. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE (1997). Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron 19:197–203. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE (2001). Heterogeneity of cycling glial progenitors in the adult mammalian cortex and white matter. J Neurobiol 48:75–86. [PubMed] [Google Scholar]
- Glass R, Synowitz M, Kronenberg G, Walzlein JH, Markovic DS, Wang LP, Gast D, Kiwit J, Kempermann G, Kettenmann H (2005). Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival. J Neurosci 25:2637–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogate N, Verma L, Zhou JM, Milward E, Rusten R, O’Connor M, Kufta C, Kim J, Hudson L, Dubois-Dalcq M (1994). Plasticity in the adult human oligodendrocyte lineage. J Neurosci 14:4571–4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JE, Zerlin M, Newman S, Zhang L, Gensert J (1997). Fate determination and migration of progenitors in the postnatal mammalian CNS. Dev Neurosci 19:42–48. [DOI] [PubMed] [Google Scholar]
- Guo P, Hu B, Gu W, Xu L, Wang D, Huang HJ, Cavenee WK, Cheng SY (2003). Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol 162:1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanson M, Funa K, Hartman M, Claesson-Welsh L, Heldin CH, Westermark B, Nister M (1992). Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res 52:3213–3219. [PubMed] [Google Scholar]
- Hesselager G, Uhrbom L, Westermark B, Nister M (2003). Complementary effects of platelet-derived growth factor autocrine stimulation and p53 or Ink4a-Arf deletion in a mouse glioma model. Cancer Res 63:4305–4309. [PubMed] [Google Scholar]
- Hommes OR, Leblond CP (1967). Mitotic division of neuroglia in the normal adult rat. J Comp Neurol 129:269–278. [DOI] [PubMed] [Google Scholar]
- Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA (2002). Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia 39:193–206. [DOI] [PubMed] [Google Scholar]
- Johansson FK, Brodd J, Eklof C, Ferletta M, Hesselager G, Tiger CF, Uhrbom L, Westermark B (2004). Identification of candidate cancer-causing genes in mouse brain tumors by retroviral tagging. Proc Natl Acad Sci USA 101:11334–11337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson FK, Goransson H, Westermark B (2005). Expression analysis of genes involved in brain tumor progression driven by retroviral insertional mutagenesis in mice. Oncogene 24:3896–3905. [DOI] [PubMed] [Google Scholar]
- Kakita A, Goldman JE (1999). Patterns and dynamics of SVZ cell migration in the postnatal forebrain: monitoring living progenitors in slice preparations. Neuron 23:461–472. [DOI] [PubMed] [Google Scholar]
- Kakita A, Zerlin M, Takahashi H, Goldman JE (2003). Some glial progenitors in the neonatal subventricular zone migrate through the corpus callosum to the contralateral cerebral hemisphere. J Comp Neurol 458:381–388. [DOI] [PubMed] [Google Scholar]
- Kattar MM, Kupsky WJ, Shimoyama RK, Vo TD, Olson MW, Bargar GR, Sarkar FH (1997). Clonal analysis of gliomas. Hum Pathol 28:1166–1179. [DOI] [PubMed] [Google Scholar]
- Lamszus K, Heese O, Westphal M (2004). Angiogenesis-related growth factors in brain tumors. Cancer Treat Res 117:169–190. [DOI] [PubMed] [Google Scholar]
- Latov N, Nilaver G, Zimmerman EA, Johnson WG, Silverman AJ, Defendini R, Cote L (1979). Fibrillary astrocytes proliferate in response to brain injury: a study combining immunoperoxidase technique for glial fibrillary acidic protein and radioautography of tritiated thymidine. Dev Biol 72:381–384. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Alberta JA, Kho AT, Weiss J, Kwaan MR, Nutt CL, Louis DN, Stiles CD, Rowitch DH (2004). The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol 63:499–509. [DOI] [PubMed] [Google Scholar]
- Marshall CA, Novitch BG, Goldman JE (2005). Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells. J Neurosci 25:7289–7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Goldman JE (2002). A2B5+ and O4+ cycling progenitors in the adult forebrain white matter respond differentially to PDGF-AA, FGF-2, and IGF-1. Mol Cell Neurosci 20:30–42. [DOI] [PubMed] [Google Scholar]
- Morse RP, Darras BT, Ye Z, Wu JK (1994). Clonal analysis of human astrocytomas. J Neurooncol 21:151–157. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB (1996). Interaction between NG2 proteoglycan and PDGFα-receptor on O2A progenitor cells is required for optimal response to PDGF. J Neurosci Res 43:315–330. [DOI] [PubMed] [Google Scholar]
- Nister M, Heldin CH, Westermark B (1986). Clonal variation in the production of a platelet-derived growth factor-like protein and expression of corresponding receptors in a human malignant glioma. Cancer Res 46:332–340. [PubMed] [Google Scholar]
- Nister M, Claesson-Welsh L, Eriksson A, Heldin CH, Westermark B (1991). Differential expression of platelet-derived growth factor receptors in human malignant glioma cell lines. J Biol Chem 266:16755–16763. [PubMed] [Google Scholar]
- Noble M, Wren D, Wolswijk G (1992). The O-2A(adult) progenitor cell: a glial stem cell of the adult central nervous system. Semin Cell Biol 3:413–422. [DOI] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G II, Jiang L, Kang J, Nedergaard M, Goldman SA (2003). Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med 9:439–447. [DOI] [PubMed] [Google Scholar]
- Ohgaki H, Kleihues P (2005). Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol 64:479–489. [DOI] [PubMed] [Google Scholar]
- Pringle N, Collarini EJ, Mosley MJ, Heldin CH, Westermark B, Richardson WD (1989). PDGF A chain homodimers drive proliferation of bipotential (O-2A) glial progenitor cells in the developing rat optic nerve. EMBO J 8:1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL (2001). Stem cells, cancer, and cancer stem cells. Nature 414:105–111. [DOI] [PubMed] [Google Scholar]
- Roy NS, Wang S, Harrison-Restelli C, Benraiss A, Fraser RA, Gravel M, Braun PE, Goldman SA (1999). Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J Neurosci 19:9986–9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, Alvarez-Buylla A, Berger MS (2005). Neural stem cells and the origin of gliomas. N Engl J Med 353:811–822. [DOI] [PubMed] [Google Scholar]
- Scolding N, Franklin R, Stevens S, Heldin CH, Compston A, Newcombe J (1998). Oligodendrocyte progenitors are present in the normal adult human CNS and in the lesions of multiple sclerosis. Brain 121:2221–2228. [DOI] [PubMed] [Google Scholar]
- Shi J, Marinovich A, Barres BA (1998). Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J Neurosci 18:4627–4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AH, Holland EC (2005). Platelet-derived growth factor (PDGF) and glial tumorigenesis. Cancer Lett 232:139–147. [DOI] [PubMed] [Google Scholar]
- Shih AH, Dai C, Hu X, Rosenblum MK, Koutcher JA, Holland EC (2004). Dose-dependent effects of platelet-derived growth factor-B on glial tumorigenesis. Cancer Res 64:4783–4789. [DOI] [PubMed] [Google Scholar]
- Shoshan Y, Nishiyama A, Chang A, Mork S, Barnett GH, Cowell JK, Trapp BD, Staugaitis SM (1999). Expression of oligodendrocyte progenitor cell antigens by gliomas: implications for the histogenesis of brain tumors. Proc Natl Acad Sci USA 96:10361–10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB (2003). Identification of a cancer stem cell in human brain tumors. Cancer Res 63:5821–5828. [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004). Identification of human brain tumour initiating cells. Nature 432:396–401. [DOI] [PubMed] [Google Scholar]
- Suzuki SO, Goldman JE (2003). Multiple cell populations in the early postnatal subventricular zone take distinct migratory pathways: a dynamic study of glial and neuronal progenitor migration. J Neurosci 23:4240–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson KR, Bridge C, Murray JD, Alvord EC Jr (2003). Virtual and real brain tumors: using mathematical modeling to quantify glioma growth and invasion. J Neurol Sci 216:1–10. [DOI] [PubMed] [Google Scholar]
- Takamiya Y, Kohsaka S, Toya S, Otani M, Tsukada Y (1988). Immunohistochemical studies on the proliferation of reactive astrocytes and the expression of cytoskeletal proteins following brain injury in rats. Brain Res 466:201–210. [DOI] [PubMed] [Google Scholar]
- Uhrbom L, Hesselager G, Nister M, Westermark B (1998). Induction of brain tumors in mice using a recombinant platelet-derived growth factor B-chain retrovirus. Cancer Res 58:5275–5279. [PubMed] [Google Scholar]
- van der Valk P, Lindeman J, Kamphorst W (1997). Growth factor profiles of human gliomas. Do non-tumour cells contribute to tumour growth in glioma? Ann Oncol 8:1023–1029. [DOI] [PubMed] [Google Scholar]
- Vassbotn FS, Ostman A, Langeland N, Holmsen H, Westermark B, Heldin CH, Nister M (1994). Activated platelet-derived growth factor autocrine pathway drives the transformed phenotype of a human glioblastoma cell line. J Cell Physiol 158:381–389. [DOI] [PubMed] [Google Scholar]
- Westermark B, Heldin CH, Nister M (1995). Platelet-derived growth factor in human glioma. Glia 15:257–263. [DOI] [PubMed] [Google Scholar]
- Willman CL (1994). Detection of clonal histiocytes in Langerhans cell histiocytosis: biology and clinical significance. Br J Cancer Suppl 23:S29–S33. [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G, Noble M (1992). Cooperation between PDGF and FGF converts slowly dividing O-2Aadult progenitor cells to rapidly dividing cells with characteristics of O-2Aperinatal progenitor cells. J Cell Biol 118:889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G, Riddle PN, Noble M (1991). Platelet-derived growth factor is mitogenic for O-2Aadult progenitor cells. Glia 4:495–503. [DOI] [PubMed] [Google Scholar]
- Wren D, Wolswijk G, Noble M (1992). In vitro analysis of the origin and maintenance of O-2Aadult progenitor cells. J Cell Biol 116:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]