Abstract
Kisspeptins are neuropeptides encoded by the Kiss1 gene, which have been implicated in the neuroendocrine regulation of gonadotropin-releasing hormone (GnRH) secretion. The goal of this study was to test the hypothesis that activation of Kiss1 neurons in the anteroventral periventricular nucleus (AVPV) is linked to the induction of the preovulatory luteinizing hormone (LH) surge in the rat. First, we determined that levels of Kiss1 mRNA in the AVPV peaked during the evening of proestrus, whereas Kiss1 mRNA in the arcuate nucleus (Arc) was at its nadir. Second, we corroborated this observation by demonstrating that Kiss1 mRNA is increased in the AVPV at the time of an estrogen (E)- and progesterone-induced LH surge in ovariectomized animals, whereas in the Arc, the expression of Kiss1 mRNA was decreased. Third, we found that most Kiss1 neurons in the AVPV coexpress the immediate early gene Fos coincidently with the LH surge, but virtually none coexpressed Fos on diestrus. In contrast, Kiss1 neurons in the Arc were Fos negative at the time of the LH surge as well as on diestrus. Finally, we found that most Kiss1 neurons in the AVPV and Arc express estrogen receptor α mRNA, suggesting that E acts directly on these neurons. These results suggest that Kiss1 neurons in the AVPV play an active role in mediating the effects of E on the generation of the preovulatory GnRH/LH surge on proestrus.
Keywords: Kiss1 mRNA, kisspeptin, metastin, AVPV, arcuate, estrogen, progesterone
Introduction
Ovulation in mammals is triggered by a preovulatory surge of luteinizing hormone (LH). The LH surge is generated by the release of gonadotropin-releasing hormone (GnRH) from nerve terminals in the median eminence, whose cell bodies reside in the basal forebrain. Throughout most of the estrous cycle, GnRH secretion is under tonic negative feedback control by gonadal steroids. However, beginning on diestrus II and continuing into proestrus, estradiol (E) acts on the brain to initiate a shift from negative to positive feedback and generates the preovulatory GnRH/LH surge. Although it is evident that E acts in the forebrain to produce the GnRH surge, neither the cellular targets nor the molecular pathway that mediates this phenomenon have been elucidated. GnRH neurons do not express either estrogen receptor α (ERα) or the progesterone (P) receptor, and it is widely thought that other steroid-sensitive neurons in the forebrain mediate the effects of sex steroids on GnRH secretion (Herbison, 1998; Petersen et al., 2003; Freeman, 2006).
The Kiss1 gene codes for a family of RFamide-type neuropeptides called kisspeptins, which bind to a G-protein-coupled receptor, GPR54 (Kotani et al., 2001; Ohtaki et al., 2001). This receptor and its ligand(s) have been implicated in the neuroendocrine regulation of GnRH secretion (de Roux et al., 2003; Funes et al., 2003; Seminara et al., 2003; Smith et al., 2006). Kisspeptins stimulate GnRH and LH secretion in the rat, mouse, monkey, and human (Gottsch et al., 2004; Irwig et al., 2004; Matsui et al., 2004; Dhillo et al., 2005; Shahab et al., 2005). Kiss1 neurons are found in the anteroventral periventricular (AVPV) and arcuate (Arc) nuclei (Gottsch et al., 2004; Smith et al., 2005a,b), areas known to send projections to the preoptic area, in which GnRH cell bodies reside (Canteras et al., 1994; Simonian et al., 1999; Simerly, 2002).
The expression of Kiss1 in the brain is regulated by gonadal steroids (Irwig et al., 2004; Navarro et al., 2004; Smith et al., 2005a,b; Dungan et al., 2006; Roa et al., 2006), but the nature of this regulation differs dramatically among nuclei (Smith et al., 2005a,b). In the Arc, E inhibits the expression of Kiss1 mRNA, whereas in the AVPV, E stimulates the expression of Kiss1. We have suggested that the differential regulation of Kiss1 mRNA between the Arc and AVPV mediates the “negative” and “positive” feedback effects of E on GnRH secretion, respectively (Smith et al., 2005a,b, 2006). Circumstantial evidence supports the inference that an unidentified group of neurons in the AVPV, possibly Kiss1 neurons, triggers the preovulatory GnRH surge. We postulated that, if activation of Kiss1 neurons in the AVPV were involved in the generation of the GnRH/LH surge, then (1) the expression of Kiss1 in the AVPV would be elevated during the LH surge, (2) markers of transcriptional activation (e.g., Fos) would be induced in Kiss1 neurons concomitantly with the surge, and (3) Kiss1 neurons would express E receptors.
Materials and Methods
Animals
Adult female Sprague Dawley rats were used for all experiments. For experiments 1, 2, and 4, animals (weighing 300–320 g) were obtained from Charles River Laboratories (Wilmington, MA). For experiment 3, rats were obtained from Zivic Miller (Zeonople, PA). For experiments 1, 2, and 4, animals were maintained on a 12 h light/dark cycle, with lights on at 6:00 A.M.; for experiment 3, 12 h light/dark cycles were also used, but lights were turned on at 3:00 A.M. All animals had access to standard rodent chow and water ad libitum. All procedures used for experiments 1, 2, and 4 were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Washington, and those used in experiment 3 were approved by the IACUC at the University of Maryland, in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Experimental design
Experiment 1: regulation of Kiss1 mRNA during the estrous cycle
Objective. The purpose of this experiment was to examine the regulation of Kiss1 mRNA throughout the estrous cycle in the rat.
Animal and tissue preparation.
Daily vaginal cytology was examined to determine the stage of estrous cycle of each animal. Rats were observed for two complete cycles and then killed at 8:00 A.M. (2 h after lights on) at estrus, diestrus II, or proestrus (n = 8 per group). An additional group was killed at 5:00 P.M. (1 h before lights off) on the afternoon of proestrus (n = 8), the approximate time of the LH surge. At the time the animals were killed, they were weighed, anesthetized with isoflurane, and then killed by decapitation. Trunk blood was collected for measurement of serum LH and E by radioimmunoassay. In preparation for single-labeled in situ hybridization (ISH) for Kiss1 mRNA, brains were quickly removed from the skull, frozen on dry ice, and then stored at −80°C until sectioned. Five sets of sections were cut at 20 μm in the coronal plane from the diagonal band of Broca to the mammillary bodies, thaw mounted onto slides (SuperFrost Plus; VWR Scientific, West Chester, PA), and stored at −80°C. One set was used for ISH (at 100 μm intervals).
Radiolabeled Kiss1 cRNA riboprobe.
Antisense and sense mouse Kiss1 probes were generated as described previously (Gottsch et al., 2004). The Kiss1-specific sequence spanned bases 76–486 of the mouse cDNA sequence (GenBank accession number AF472576). The Kiss1 riboprobe was generated against the mouse Kiss1 mRNA. There is 90% homology between mouse and rat in the cloned region, and we demonstrated previously that this probe can be used successfully in the rat (Irwig et al., 2004).
Single-labeled ISH.
ISH with a radiolabeled probe (33P) was performed as described previously (Cunningham et al., 2002). The Kiss1 cRNA probe (0.03 pmol/ml) was denatured, dissolved in hybridization solution with tRNA (2 mg/ml), and applied to brain sections. After hybridization (55°C for 16 h), slides were treated with RNase, washed, and dehydrated. Slides were dipped in NTB liquid emulsion (Eastman Kodak, Rochester, NY), protected from light, and stored at 4°C. Slides were developed 2–3 d later.
Quantification and analysis of Kiss1 mRNA.
All slides were assigned a random three-letter code and read (unilaterally) under dark-field illumination. Custom-designed software was used to count the number of cells and the number of grains over each cell (a semiquantitative index of Kiss1 mRNA content per cell) (Cunningham et al., 2002). Cells were counted as Kiss1 mRNA positive when the number of silver grains in a cluster exceeded that of background by threefold.
Experiment 2: regulation of Kiss1 mRNA during the E/P-induced LH surge
Objective. Having observed in experiment 1 that the expression of Kiss1 mRNA in the AVPV peaked on the afternoon of proestrus, the purpose of this experiment was to adduce additional evidence that the induction of Kiss1 expression in the AVPV is temporally linked to the occurrence of the LH surge under a different experimental paradigm.
Animal and tissue preparation.
Female rats were initially divided into three experimental groups (n = 4): ovary intact (at diestrus II), ovariectomized (OVX), and OVX plus E replacement. Ovaries were removed under ketamine (100 mg/ml)/xylazine (20 mg/ml)/acepromazine (10 mg/ml) anesthesia (5.0:2.5:1.0 ratio). Immediately after surgery, animals in the E-replacement group received an E-filled SILASTIC implant (inner diameter, 1.57 mm; outer diameter, 3.18 mm; Dow Corning, Midland, MI) whereas the other half received an empty (sham) implant. Tubing (4 mm lengths) was filled with crystalline E (or was empty), sealed at each end with silicone cement, and implanted (subcutaneously) through a small incision at the base of the neck.
On either diestrus II (intact animals) or 7 d after ovariectomy, the intact and the sham-treated OVX animals were anesthetized with isoflurane and quickly decapitated (2 h after lights on). Tissue collection and preparation for measuring Kiss1 mRNA by ISH was performed as described in experiment 1. OVX animals that had previously received E were given injections of P (5 mg in safflower oil, subcutaneously; n = 4) 6 h after lights on this seventh day after the initial surgery. Five hours later (1 h before lights off), the OVX/E/P-treated animals were anesthetized with isoflurane and rapidly decapitated, and tissues were collected and prepared for Kiss1 ISH as described above.
Experiment 3: double-labeled in situ hybridization for Kiss1 mRNA and immunohistochemistry for Fos
Objective. The purpose of this study was to determine whether Fos expression is induced in Kiss1 neurons coincidently with the LH surge and confirm that Fos is induced in GnRH neurons at the time of the surge.
Animals and tissue preparation.
Adult female rats displaying four consecutive estrous cycles, as determined by vaginal lavage and cytology, were selected on either the afternoon of proestrus or diestrus II (n = 4 for both groups killed 30 min after lights off). The animals were anesthetized with 100 mg/kg pentobarbital, and each rat was perfused transcardially with saline containing 2.5% sodium nitrite, followed by ∼100 ml of a solution of 4% buffered paraformaldehyde, pH 6.8 (Sigma, St. Louis, MO) and 2.5% acrolein (EM grade; Polysciences, Warrington, PA). After perfusion, the brain was removed from the skull, submersed in 30% aqueous sucrose solution, and serially sectioned at 30 μm on a freezing microtome. Sections were transferred to cryoprotectant antifreeze solution and were stored at −20°C, until they were processed for ISH and immunohistochemistry (IHC).
Double-labeled ISH for Kiss1 mRNA and IHC for nuclear Fos.
The general strategy involved ISH with a biotinylated riboprobe, followed by immunohistochemical detection of fluorescence-tagged secondary antibodies (Berghorn et al., 1994, 2001; Koban et al., 2006). Briefly, the same 442 bp mouse Kiss1 probe described above was subcloned, linearized, and biotinylated. For the in vitro transcription reaction, a 20 μl mixture contained 0.65 mm biotin-16-UTP (Roche, Indianapolis, IN), 1 μg of linearized Kiss1, 20 U of the RNAase inhibitor Protector (Roche), 40 U of T7 or SP6 RNA polymerase (Roche), 0.35 mm UTP, and 1.0 mm each of ATP, GTP, and CTP. The transcription reaction was developed at 37°C for 120 min and stopped by the addition of 1 μl of EDTA.
Hybridization and detection.
On the first day, under RNase-free conditions, free-floating 25 μm tissue sections from a 1-in-6 series comprising all of the AVPV and Arc were removed from the antifreeze solution. These sections were rinsed in KPBS (potassium phosphate buffered saline, 0.05 m, pH 7.4) made with 0.1% DEPC H20, incubated in 1% sodium borohydride/KPBS plus DEPC H2O for 15 min, and rinsed. Tissue was rinsed twice in 0.1 m triethanolamine (TEA) buffer, pH 8.0, placed in 0.25% acetic anhydride in 0.1 m TEA buffer for 10 min, and rinsed in 2× SSC for 10 min at room temperature (RT). The sections were hybridized with the biotinylated Kiss1 probe (denatured with torula yeast RNA) adjusted to a concentration of 800 ng · kb−1 · ml−1 and processed overnight at 50°C.
On the second day, the tissue was rinsed with 4× SSC, rinsed once with RNase buffer (10 mm Tris, pH 8.0, 500 mm NaCl, and 0.75 mm EDTA, pH 8.0) heated to 37°C, followed by a 30 min incubation of RNase (20 μg/ml) in RNase buffer at 37°C. Sections were rinsed with RNase buffer at 37°C and rinsed multiple times in 2× SSC and in 0.1× SSC (heated to 55°C) for 60 min. Tissue was rinsed three times with 0.1× SSC at RT, followed by 1 h of rinsing with KPBS (at RT). Sections were then incubated in goat anti-biotin (Vector Laboratories, Burlingame, CA) at a concentration of 1:70,000 in KPBS plus 0.4% Triton X-100 at 4°C for 48 h.
On the fourth day, tyramide amplification of the biotin signal was performed according to Berghorn et al. (1994) using a modified ABC protocol. After development of deposited biotin with a streptavidin cyanine 2 (Cy2) solution (5 μl/ml; Invitrogen, Carlsbad, CA) for 3 h at 37°C, tissue was rinsed and placed into rabbit anti-Fos [Oncogene Sciences (Uniondale, NY) antibody 4191 at 1:20,000 in KPBS plus 0.4% Triton X-100] for 48 h at 4°C. The sections were rinsed and processed with a Cy3-tagged secondary antibody [Cy3-labeled donkey anti-rabbit IgG heavy and light chains (Jackson ImmunoResearch, West Grove, PA), 1:300, 3 h at 37°C], rinsed in KPBS, transferred into normal saline, and mounted onto gelatin-subbed slides. After drying overnight, the sections were dehydrated rapidly in an ascending alcohol gradient, cleared in xylene, and coverslipped with Krystalon (Harleco, Voigt Global Distribution, Kansas City, MO).
Sections were visualized with a Nikon (Tokyo, Japan) Eclipse 800 microscope and appropriate cubes for each fluorophore (Chroma Technology, Rockingham, VT). Sections from the AVPV and Arc were captured with a Retiga EX cooled CCD camera and digitized with IP Spectrum Software (Biovision Technologies, Exton, PA). Adjustments for color and brightness/contrast were made in Photoshop (Adobe Systems, San Jose, CA). Counts were made with the use of a dual red/green filter cassette. For AVPV and Arc, all cells bearing Kiss1 mRNA were counted within a series of sections (for explanation of tissue sectioning, see above) and scored for double labeling with Fos. All counts were made by an individual blind to the condition of the animal. In addition, the total number of Fos-positive cells that did not show Kiss1 mRNA coexpression within the 100 μm distance from the ventricular wall in the posterior two-thirds of the AVPV and within the borders of the Arc nucleus was determined. Data are expressed as the percentage of Kiss1-expressing cells that were Fos positive and the percentage of Fos-positive cells that expressed Kiss1 mRNA.
Fos/GnRH double labeling.
A series of sections adjacent to those used for the Kiss1 ISH/Fos IHC were labeled sequentially for GnRH and Fos, by using NiDAB detection for Fos and DAB detection for GnRH as described previously (Hoffman et al., 2005). All identifiable GnRH neurons with a visible nucleus were scored for the presence or absence of Fos. All counts were made by an individual blind to the condition of the animal. Data are expressed as the percentage of GnRH neurons that coexpressed Fos.
Experiment 4: expression of ERα and ERβ mRNA in Kiss1 neurons
Objective. The purpose of this study was to determine whether Kiss1 neurons express either or both ERα and ERβ mRNA in the rat.
Animal and tissue preparation.
We performed double-labeled ISH on a set of coronal sections from brains of OVX and OVX plus E/P rats (used in experiment 2; n = 4). Sham-treated OVX rats were used to examine coexpression of Kiss1 and ERα in the Arc, in which the expression of Kiss1 is amplified in the absence of sex steroids (Smith et al., 2005b). OVX animals treated with E/P were used to evaluate coexpression of Kiss1 and ERα in the AVPV, in which Kiss1 expression is induced in the presence of E (Smith et al., 2005b). These treatments induced maximum expression and visualization of Kiss1 mRNA in the Arc and AVPV, respectively.
Double-labeled ISH.
The cDNA templates for ERα and ERβ riboprobes were generated by PCR as described previously (Smith et al., 2005b). The ERα mRNA-specific sequence spanned bases 1163–1990 of the mouse cDNA sequence (GenBank accession number NM_007956). We predicted the mouse riboprobe would hybridize to rat ERα mRNA because there is 94% homology between mouse and rat ERα cDNAs in the cloned region. The ERβ mRNA-specific sequence, spanning bases 662–1424 of the mouse cDNA (GenBank accession number NM_207707), was similarly 93% homologous to rat ERβ cDNAs in the cloned region.
The cDNA template for the Kiss1 riboprobe was prepared as above for single-labeled ISH. Digoxigenin (DIG)-labeled antisense cRNA was synthesized with T7 RNA polymerase and DIG labeling mix (Roche) according to the protocol of the manufacturer.
Slides were processed for ISH as described above with modifications. Radiolabeled antisense ERα or ERβ and DIG-labeled Kiss1 riboprobes (concentration determined empirically) were denatured, dissolved in the same hybridization buffer along with tRNA (2 mg/ml), and applied to the slides. Slides were hybridized as above. Kiss1 mRNA-positive cells were visualized by using anti-DIG fragments conjugated to alkaline phosphatase (diluted 1:300; Roche) and Vector Red substrate (SK-5100; Vector Laboratories), following the directions of the manufacturer. Slides were dipped in 70% ethanol, air dried, and then dipped in NTB liquid emulsion (Eastman Kodak). Slides were developed 14 d later.
Kiss1 mRNA-containing cells were identified under fluorescent illumination, and software described above was used to quantify silver grains, corresponding to radiolabeled ERα or ERβ mRNA over each cell (Smith et al., 2005b). Signal-to-background ratios (SBRs) for individual cells were calculated, and a cell was considered to be double labeled if it had an SBR of 3 or greater. For each animal, the amount of double labeling was calculated as a percentage of the total number of Kiss1 mRNA-expressing cells and then averaged across animals to produce a mean ± SEM.
Radioimmunoassays
Serum levels of E and LH were measured at Northwestern University (Evanston, IL). E was measured with a double-antibody kit (Diagnostics Production, Los Angeles, CA), which had a sensitivity of 2 pg/ml and intraassay coefficient of variation of 6%. The LH antiserum was anti-rLH-S11 and the standard was rLH-RP3. The sensitivity of the LH assay was 0.2 ng/ml, and the intraassay coefficient of variation was 4%.
Statistical analysis
All data are expressed as the mean ± SEM for each group. One-way ANOVAs were used to assess variation in Kiss1 mRNA expression across the estrous cycle in experiment 1 and among experimental groups in experiment 2. When the F test for the ANOVA reached statistical significance (p < 0.05), differences among means were assessed by least significant difference tests. All analyses were performed with Statview 5.0.1 or JMP 5.1 for Macintosh (SAS Institute, Cary, NC).
Results
Experiment 1: regulation of Kiss1 mRNA expression across the estrous cycle
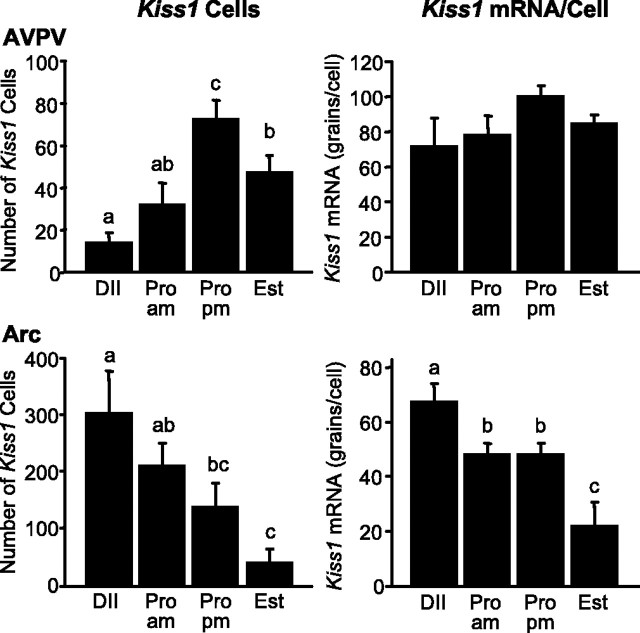
In the AVPV, on the afternoon of proestrus, the number of identifiable Kiss1-expressing cells was significantly higher than that observed on the morning of the same day (2.3-fold greater; p < 0.005) (Fig. 1). The number of Kiss1-expressing cells was significantly lower on estrus compared with the afternoon of proestrus (p < 0.05), and the number of Kiss1 cells was at its nadir in the cycle on diestrus II. No difference in the content of Kiss1 mRNA per cell (as reflected by grains per cell) was detectable in the AVPV during different stages of the estrous cycle.
Figure 1.
The number of identifiable Kiss1 mRNA-positive cells and grains per cell in the AVPV and Arc of the female rat during the estrous cycle. Values without common notations (a, b, c) differ significantly (p < 0.05). Values are the mean ± SEM. DII, Diestrus; Pro am, proestrus 2 h after lights on; Pro pm, proestrus 1 h before lights off; Est, estrus.
In the Arc, both the number of identifiable Kiss1 neurons and the content of Kiss1 mRNA per cell were highest on diestrus II and lowest on estrus (Fig. 1). No difference was observed in Kiss1 expression in the Arc between the morning and afternoon of proestrus.
Serum levels of E were within the normal physiological range, with concentrations elevated at proestrus compared with diestrus II and estrus (p < 0.05) (Table 1). Levels of LH were also as expected. During estrus, diestrus II, and the morning of proestrus, levels of LH were all below 1 ng/ml; however, on the afternoon of proestrus, the concentration of LH was increased ∼16-fold compared with all other groups (p < 0.0001) (Table 1). A small population of Kiss1-expressing neurons was also observed in the periventricular nucleus. The expression of Kiss1 mRNA in this region did not appear to change as a function of the estrous cycle (data not shown).
Table 1.
Serum concentrations of E and LH over the estrous cycle
| Diestrus | Proestrus |
Estrus | ||
|---|---|---|---|---|
| AM | PM | |||
| Estradiol (pg/ml) | 16 ± 4a | 55 ± 9b | 57 ± 9b | 23 ± 8a |
| LH (ng/ml) | 0.9 ± 0.3a | 0.9 ± 0.2a | 16.2 ± 1.1b | 0.8 ± 0.1a |
Data are presented as the mean ± SEM. Serum levels of E were elevated during the morning [2 hr after lights on (AM)] and evening [1 hr before lights off (PM)] of proestrus compared with diestrus II and estrus (all p < 0.05). Levels of serum LH were elevated only during the evening of proestrus (p < 0.0001 compared with all other groups).
Experiment 2: regulation of Kiss1 mRNA during the E/P-induced LH surge
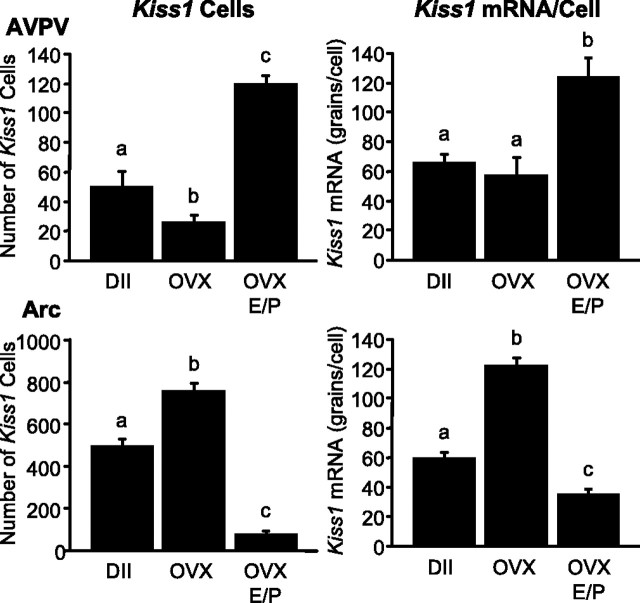
In the AVPV, the number of identifiable Kiss1-expressing cells and the cellular content of Kiss1 mRNA (reflected by grains per cell) were both approximately twofold higher in OVX/E/P-treated animals compared with intact controls at diestrus II (number of cells, diestrus II, 51 ± 10; OVX/E/P, 120 ± 5; grains per cell, diestrus II, 66 ± 6; OVX/E/P, 125 ± 6; p < 0.001 for both). The number of Kiss1-expressing cells in the AVPV of OVX animals (26 ± 4) was reduced by ∼50% compared with animals at diestrus II (p < 0.05), whereas the per cell content of Kiss1 mRNA (58 ± 4) was indistinguishable between these two groups (Fig. 2).
Figure 2.
Kiss1 mRNA expression in the AVPV and Arc of the female rat during the E/P-induced LH surge. Values without common notations (a, b, c) differ significantly (p < 0.05). Values are the mean ± SEM. DII, Diestrus.
In the Arc, sham-treated OVX animals had significantly more identifiable Kiss1 neurons (1.5-fold) and a higher cellular content of Kiss1 mRNA (twofold) compared with animals on diestrus II (number of cells, diestrus II, 502 ± 27; OVX, 763 ± 34; grains per cell, diestrus II, 60 ± 4; OVX, 123 ± 5; both p < 0.0001). OVX animals treated with E/P had fewer Kiss1 cells (78 ± 14) and reduced cellular content of Kiss1 mRNA (36 ± 2) in the Arc compared with intact, OVX controls at diestrus II (p < 0.0001 for both) (Fig. 2). Photomicrographs from these data areshown in Figure 3.
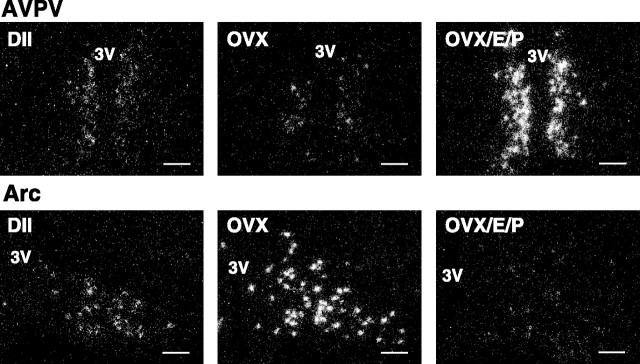
Figure 3.
Dark-field photomicrographs showing Kiss1 mRNA-expressing cells (as reflected by the presence of white clusters of silver grains) in representative sections of the AVPV and Arc from diestrus (DII), OVX, and OVX/E/P-treated female rats. 3V, Third ventricle. Scale bars, 100 μm.
Serum LH was increased approximately sixfold after OVX (7.3 ± 0.2 ng/ml) compared with diestrus II (1.3 ± 0.5 ng/ml; p < 0.01). LH was further increased with OVX plus E/P treatment (11.5 ± 1.9 ng/ml; 8.8-fold, p < 0.001 compared with diestrus; 1.6-fold, p < 0.05 compared with OVX).
Experiment 3: coexpression of Fos in Kiss1 and GnRH neurons
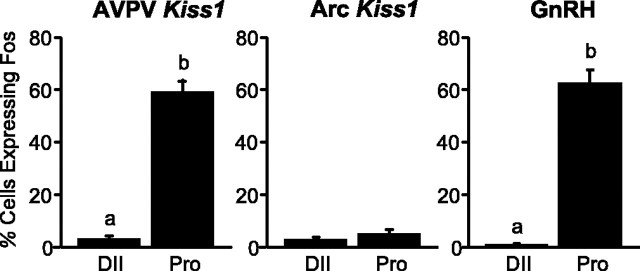
On the afternoon of diestrus II, only an occasional Kiss1 neuron in the AVPV showed Fos expression (Figs. 4, 5); however, on the afternoon of proestrus, Fos appeared in ∼60% of Kiss1 neurons (coincident with the LH surge) (Figs. 4, 5). In the AVPV on proestrus, Kiss1 neurons that expressed Fos represented >80% of the total number of Fos-activated neurons in this nucleus (data not shown). In the Arc, there were very few detectable Fos-positive nuclei on either diestrus II or the afternoon of proestrus, and <5% of the Kiss1 cells showed Fos labeling on either day (Fig. 6).
Figure 4.
Quantitative analysis showing the percentage of Kiss1 neurons that coexpress Fos 30 min after lights off on diestrus II (DII) and proestrus (Pro) in the AVPV (left) and Arc (middle) and the percentage of GnRH neurons that coexpress Fos on diestrus II and proestrus (right panel). Values without common notations (a, b) differ significantly (p < 0.05). Values are the mean ± SEM.
Figure 5.
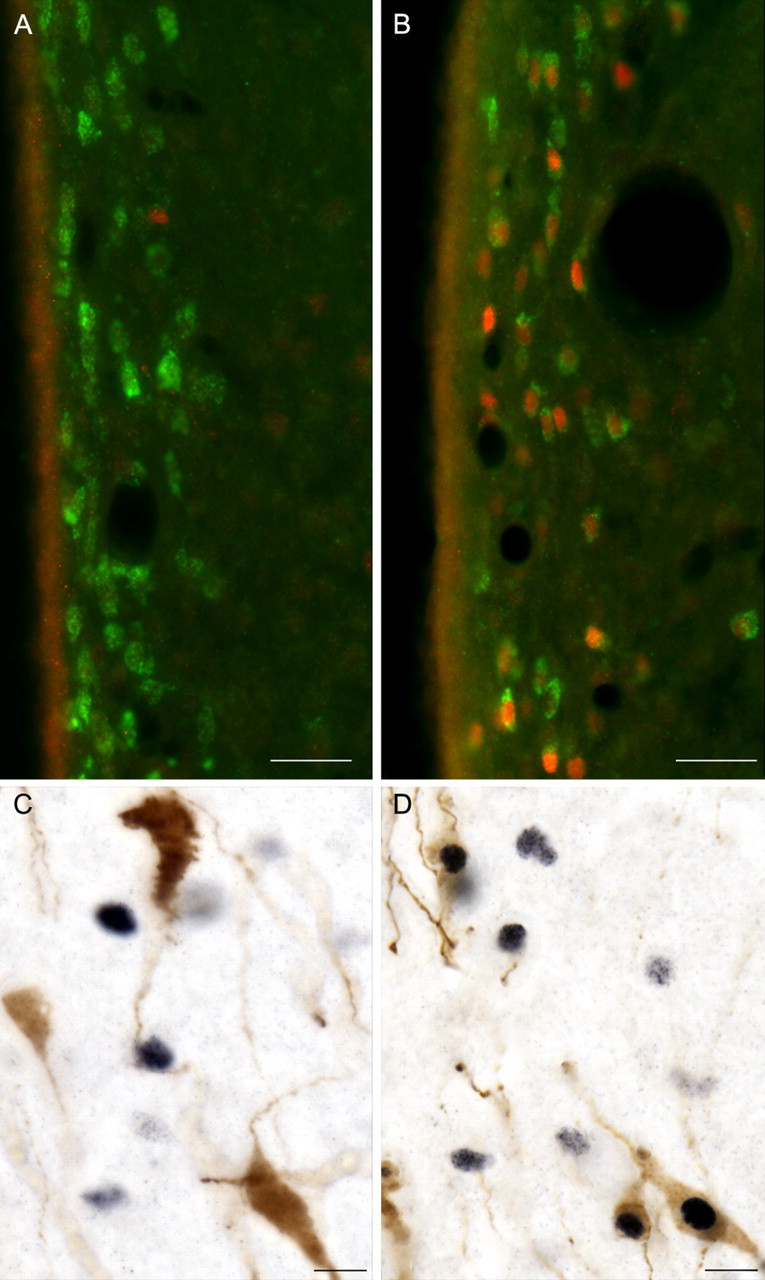
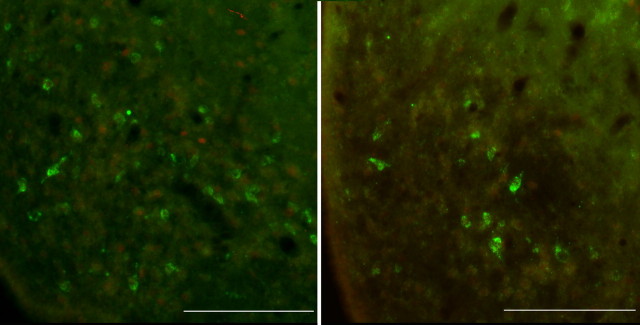
A, B, Photomicrographs showing Kiss1 neurons (green) in the AVPV on diestrus II (A) and proestrus (B), with the induction of Fos (red) in Kiss1 neurons on the afternoon of proestrus (compare A, B). Scale bars, 25 μm. C, D, Photomicrographs showing GnRH neurons (brown) and Fos (black) on diestrus II (C) and their coexpression on the afternoon of proestrus (D). Scale bars, 10 μm.
Figure 6.
Photomicrographs showing Kiss1 neurons (green) and nuclear Fos (red) in the arcuate nucleus on diestrus II (left) and the afternoon of proestrus (right), revealing no evidence for colocalization of Kiss1 mRNA and Fos on either day. Scale bars, 100 μm.
In sections adjacent to those examined for Fos activation in Kiss1 neurons, we monitored the patterns of Fos in GnRH neurons. On the afternoon of diestrus II, virtually no GnRH neurons coexpressed Fos, whereas on the afternoon of proestrus, >60% of GnRH neurons were Fos positive (Figs. 4, 5).
Experiment 4: Kiss1 mRNA coexpression with ERα and ERβ mRNAs
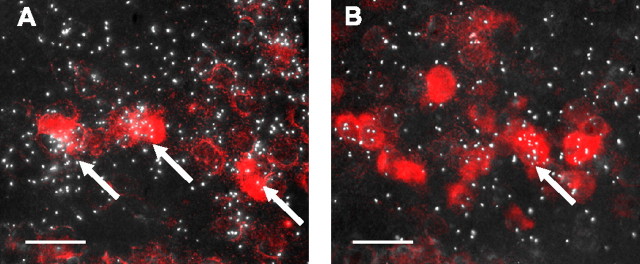
The majority of Kiss1 neurons in the Arc and AVPV expressed ERα mRNA (Fig. 7A). Quantitative analysis (with a criterion for double labeling of signal three times greater than background) indicated that 62 ± 3% of Kiss1 neurons in the AVPV and 70 ± 4% of Kiss1 neurons in the Arc expressed ERα mRNA. A much smaller fraction of Kiss1 neurons in the Arc (11 ± 2%) and AVPV (21 ± 3%) expressed ERβ mRNA (Fig. 7B).
Figure 7.
Representative photomicrographs showing coexpression of Kiss1 mRNA with ERα (A) and ERβ (B) in the anteroventral periventricular nucleus. Kiss1 mRNA-expressing cells were visualized with Vector Red substrate, and ERα (A) or ERβ (B) were marked by the presence of clusters of silver grains. Arrows indicate Kiss1 neurons that coexpress either ERα or ERβ. Scale bars, 20 μm.
Discussion
The brain triggers the preovulatory release of GnRH and LH during the estrous cycle of the rat (Freeman, 2006); however; the neuronal circuitry and molecular mechanisms that mediate this event have yet to be fully elucidated. On diestrus II, the rising tide of E in the plasma, produced by the maturing ovarian follicles, acts on E-sensitive neurons in the forebrain to stimulate GnRH secretion on the late afternoon of proestrus. GnRH neurons themselves are not the direct targets for the action of E. Instead, another population of neurons in the forebrain acts indirectly to relay the E signal through ERα-dependent mechanisms to GnRH neurons (Petersen et al., 2003). Although the phenotypic identity of these E-sensitive neurons remains uncertain, they appear to be located in the AVPV (Simerly, 1998; Le et al., 1999).
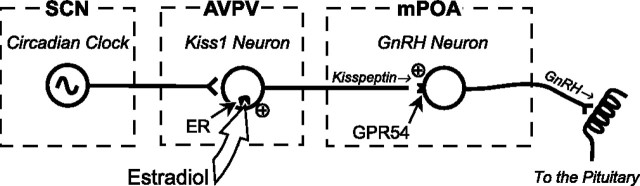
Here, we report that Kiss1 neurons in the AVPV are direct targets for E and that these cells become transcriptionally activated as a function of the LH surge in the female rat. Based on these and other observations, a strong circumstantial case can be made that Kiss1 neurons in the AVPV drive the E-induced GnRH/LH surge. First, kisspeptin is a potent secretagogue for GnRH. Kisspeptin administered into the brain stimulates GnRH and LH secretion through a direct action on GnRH neurons, most of which express the kisspeptin receptor GPR54 (Gottsch et al., 2004; Irwig et al., 2004; Matsui et al., 2004; Navarro et al., 2004). Moreover, antiserum to kisspeptin administered into the brain blocks the preovulatory LH surge in the rat (Kinoshita et al., 2005). Second, there is compelling evidence that the GnRH/LH surge is generated by E-sensitive neurons whose cell bodies reside in the AVPV and are activated by a circadian signal from the suprachiasmatic nucleus (SCN) (Gu and Simerly, 1997; de la Iglesia and Schwartz, 2006). Lesions of the AVPV block spontaneous and steroid-induced LH surges (Wiegand et al., 1978, 1980; Terasawa et al., 1980; Wiegand and Terasawa, 1982; Ronnekleiv and Kelly, 1986; Ronnekleiv and Kelly, 1988; Le et al., 1999). Third, the AVPV is sexually differentiated, with females having greater numbers of cells than males (Simerly, 1998), and the surge mechanism is only present in the female rodents (Pfeiffer, 1936; Barraclough, 1961; Gogan et al., 1980; Corbier, 1985; Hoffman et al., 2005). Fourth, the expression of Kiss1 is also sexually differentiated in the AVPV, again with females showing greater numbers of Kiss1 neurons and higher expression of Kiss1 mRNA than males (Smith et al., 2005a,b; Tena-Sempere et al., 2006). Fifth, ERα mediates the effects of E on the GnRH/LH surge mechanism, as well as the effects of E on Kiss1 expression (Petersen et al., 2003; Smith et al., 2005a,b), and we have demonstrated here that most Kiss1 neurons express ERα. Finally, we have shown that the expression of Kiss1 mRNA increases as a function of the LH surge, with highest levels of Kiss1 expression occurring in temporal association with the LH surge and coincidently with the coexpression of the transcription factor Fos. Together, these observations suggest that Kiss1 neurons in the AVPV are targets for transcriptional activation by E and that increased activity of these cells, gated by circadian afferents from the SCN, is the proximate cue for the preovulatory GnRH/LH surge (Fig. 8).
Figure 8.
Simplified model of the role of Kiss1 neurons in the generation of the GnRH/LH surge. According to this model, Kiss1 neurons in the AVPV receive circadian signals from the SCN and sense circulating E levels via estrogen receptors (ER), which are expressed within these neurons. When E levels and the circadian signal are both high, Kiss1 neurons become activated, causing kisspeptin to be released at synapses on GnRH neurons. Kisspeptin activates those GnRH neurons, driving the release of GnRH into the portal circulation. mPOA, Medial preoptic area.
These observations on the effects of sex steroids on Kiss1 expression in the rat are consistent with our previous studies in the mouse, which demonstrated that the expression of Kiss1 in the AVPV is increased by chronic exposure to gonadal steroids such as E (Smith et al., 2005a,b). However, during the estrous cycle, there are unique features that add complexity to understanding Kiss1 regulation. The transition between the morning and evening of proestrus is associated with relatively steady-state levels of circulating E, yet the expression of Kiss1 in the AVPV increases during this interval. It is plausible that the increase in Kiss1 mRNA that occurs during the afternoon of proestrus reflects a phase lag in the responsiveness of the Kiss1 gene to the action of E. Alternatively, this induction of Kiss1 may reflect additional afferent input to Kiss1 neurons from other circuits that become activated as a prelude to the preovulatory GnRH/LH surge. For example, circadian projections from the SCN to the AVPV constrain the timing of the GnRH/LH surge to the evening of proestrus (Gu and Simerly, 1997; Barbacka-Surowiak et al., 2003; de la Iglesia and Schwartz, 2006), and Kiss1 neurons may be the targets for these afferents. It is conceivable that projections from the SCN to the AVPV stimulate (or amplify beyond E alone) the expression of Kiss1 (and Fos) at the time of the GnRH/LH surge. This is consonant with our previous observation in the mouse that increased expression of Kiss1 mRNA in the AVPV alone is insufficient to generate an LH surge (Smith et al., 2005a,b). Another possibility is that progesterone, which peaks on the afternoon of proestrus (Smith et al., 1975), augments the expression of Kiss1 and the expression of Fos in Kiss1 neurons, as is the case in GnRH neurons (Lee et al., 1990). These possibilities remain to be explored.
Here, we have shown that the expression of Kiss1 in the Arc is regulated in precisely the opposite manner as that in the AVPV, with Kiss1 mRNA in the Arc reaching its peak on diestrus II and its nadir on proestrus and estrus. These results corroborate our previous findings in mice that sex steroids inhibit the expression of Kiss1 in the Arc, in contrast to their stimulatory effects on Kiss1 in the AVPV (Smith et al., 2005a,b). We argued that Kiss1 neurons in the Arc, in both the male and female, provide a tonic and direct stimulatory drive to GnRH neurons. In this model, when circulating levels of sex steroids gradually decline, the activity of Kiss1 neurons in the Arc becomes amplified. This increases the facilitatory drive to GnRH neurons (and gonadotropes), which then reverses the decline in sex steroids. Likewise, when circulating levels of sex steroids rise in the face of high circulating levels of LH, the activity of Kiss1 neurons in the Arc declines. This reduces the drive to GnRH neurons (and gonadotropes), which in turn diminishes the secretion of sex steroids. The Arc plays a critical role in the negative feedback regulation of gonadotropin secretion (Greeley et al., 1978; Dyer et al., 1981; Inkster and Whitehead, 1987), and we suggest that Kiss1 neurons in the Arc provide the cellular conduit for mediating this process. This is in contrast to Kiss1 neurons in the AVPV, which we postulate play a role in E-mediated positive feedback, but with physiological relevance here only in the female.
It is notable that two previous reports concerning the effects of gonadal steroids on Kiss1 expression might appear to be at variance with the results reported here (Navarro et al., 2004; Kinoshita et al., 2005). However, neither of these previous reports had anatomical resolution beyond the total hypothalamus, because measurements of Kiss1 mRNA were performed by reverse transcription-PCR on grossly dissected segments of the medial forebrain. The report by Kinoshita et al. (2005) testifying to the appearance of Fos labeling of kisspeptin neurons in the Arc is more difficult to reconcile with our own findings (i.e., no Fos labeling of Kiss1 neurons in the Arc on either diestrus or the evening of proestrus). There are several possible explanations for this discrepancy. First, there was a difference in the time of day the animals were killed. In the study by Kinoshita et al., animals were killed in the early afternoon, whereas in our study, they were killed in the evening. Second, the methods used to identify Kiss1/kisspeptin neurons were different between the two studies. We used ISH to visualize Kiss1 mRNA-positive neurons, whereas the Kinoshita group used IHC to detect kisspeptin-positive cells. We note that the antibody that was used by Kinoshita et al. to detect kisspeptin-containing cells by IHC has not been rigorously validated for possible cross-reactivity with other RFamide peptides (such as gonadotropin inhibitory hormone). There are also unresolved discrepancies between the distribution of Kiss1 mRNA-positive neurons (visualized by ISH) and “kisspeptin (metastin)-positive” neurons by IHC (Irwig et al., 2004; Brailoiu et al., 2005; Kinoshita et al., 2005), which may contribute to the discrepancy in Kiss1/Fos and kisspeptin/Fos coexpression between the two reports.
In summary, we have shown that Kiss1 neurons in the AVPV and Arc are direct targets for the action of E (through ERα) and that Kiss1 mRNA and Fos are induced in Kiss1 neurons in the AVPV in temporal association with the LH surge in the female rat. We suggest that Kiss1 neurons in the AVPV, whose activity is induced by E, play a role in generating the preovulatory GnRH/LH surge, whereas Kiss1 neurons in the Arc, whose activity is inhibited by E, provide tonic stimulatory drive to GnRH neurons and mediate the negative feedback effects of sex steroids on GnRH/LH secretion.
Footnotes
This work was supported by National Institutes of Health Grants SCCPRR U54 HD 12629, R01 HD27142, R01 DK61517, R01 NS28730, R01 MH57759, and K02 MH01349. We thank Kathy Lee, Yeqin Ma, and Blake Acohido for their technical assistance, and we thank Michelle Gottsch, Heather Dungan, and Alexander Kauffman for their editorial commentary on this manuscript. We are grateful to Brigitte Mann at Northwestern University for performing the radioimmunoassays.
References
- Barbacka-Surowiak G, Surowiak J, Stoklosowa S (2003). The involvement of suprachiasmatic nuclei in the regulation of estrous cycles in rodents. Reprod Biol 3:99–129. [PubMed] [Google Scholar]
- Barraclough CA (1961). Production of anovulatory, sterile rats by single injections of testosterone propionate. Endocrinology 68:62–67. [DOI] [PubMed] [Google Scholar]
- Berghorn KA, Bonnett JH, Hoffman GE (1994). cFos immunoreactivity is enhanced with biotin amplification. J Histochem Cytochem 42:1635–1642. [DOI] [PubMed] [Google Scholar]
- Berghorn KA, Le WW, Sherman TG, Hoffman GE (2001). Suckling stimulus suppresses messenger RNA for tyrosine hydroxylase in arcuate neurons during lactation. J Comp Neurol 438:423–432. [DOI] [PubMed] [Google Scholar]
- Brailoiu GC, Dun SL, Ohsawa M, Yin D, Yang J, Chang JK, Brailoiu E, Dun NJ (2005). KiSS-1 expression and metastin-like immunoreactivity in the rat brain. J Comp Neurol 481:314–329. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW (1994). Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 348:41–79. [DOI] [PubMed] [Google Scholar]
- Corbier P (1985). Sexual differentiation of positive feedback: effect of hour of castration at birth on estradiol-induced luteinizing hormone secretion in immature male rats. Endocrinology 116:142–147. [DOI] [PubMed] [Google Scholar]
- Cunningham MJ, Scarlett JM, Steiner RA (2002). Cloning and distribution of galanin-like peptide mRNA in the hypothalamus and pituitary of the macaque. Endocrinology 143:755–763. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Schwartz WJ (2006). Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology 147:1148–1153. [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E (2003). Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR (2005). Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab 90:6609–6615. [DOI] [PubMed] [Google Scholar]
- Dungan HM, Clifton DK, Steiner RA (2006). Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology 147:1154–1158. [DOI] [PubMed] [Google Scholar]
- Dyer RG, Weick RF, Mansfield S, Corbet H (1981). Secretion of luteinizing hormone in ovariectomized adult rats treated neonatally with monosodium glutamate. J Endocrinol 91:341–346. [DOI] [PubMed] [Google Scholar]
- Freeman ME (2006). The neuroendocrine control of the ovarian cycle of the rat. In: Knobil and Neill's physiology of reproduction (Neill JD, ed.) Vol 2: pp. 2327–2388. Philadelphia: Elsevier. [Google Scholar]
- Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL (2003). The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun 312:1357–1363. [DOI] [PubMed] [Google Scholar]
- Gogan F, Beattie IA, Hery M, Laplante E, Kordon D (1980). Effect of neonatal administration of steroids or gonadectomy upon oestradiol-induced luteinizing hormone release in rats of both sexes. J Endocrinol 85:69–74. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA (2004). A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077. [DOI] [PubMed] [Google Scholar]
- Greeley GH Jr, Nicholson GF, Nemeroff CB, Youngblood WW, Kizer JS (1978). Direct evidence that the arcuate nucleus-median eminence tuberoinfundibular system is not of primary importance in the feedback regulation of luteinizing hormone and follicle-stimulating hormone secretion in the castrated rat. Endocrinology 103:170–175. [DOI] [PubMed] [Google Scholar]
- Gu GB, Simerly RB (1997). Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J Comp Neurol 384:142–164. [PubMed] [Google Scholar]
- Herbison AE (1998). Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 19:302–330. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Le WW, Schulterbrandt T, Legan SJ (2005). Estrogen and progesterone do not activate Fos in AVPV or LHRH neurons in male rats. Brain Res 1054:116–124. [DOI] [PubMed] [Google Scholar]
- Inkster SE, Whitehead SA (1987). Increased responsiveness of the hypothalamic-pituitary axis to steroid feedback effects in ovariectomized rats treated neonatally with monosodium l-glutamate. Experientia 43:606–608. [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA (2004). Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80:264–272. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K (2005). Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 146:4431–4436. [DOI] [PubMed] [Google Scholar]
- Koban M, Le WW, Hoffman GE (2006). Changes in hypothalamic corticotropin-releasing hormone, neuropeptide Y, and proopiomelanocortin gene expression during chronic rapid eye movement sleep deprivation of rats. Endocrinology 147:421–431. [DOI] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M (2001). The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 276:34631–34636. [DOI] [PubMed] [Google Scholar]
- Le WW, Berghorn KA, Rassnick S, Hoffman GE (1999). Periventricular preoptic area neurons coactivated with luteinizing hormone (LH)-releasing hormone (LHRH) neurons at the time of the LH surge are LHRH afferents. Endocrinology 140:510–519. [DOI] [PubMed] [Google Scholar]
- Lee WS, Smith MS, Hoffman GE (1990). Progesterone enhances the surge of luteinizing hormone by increasing the activation of luteinizing hormone-releasing hormone neurons. Endocrinology 127:2604–2606. [DOI] [PubMed] [Google Scholar]
- Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T (2004). Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun 320:383–388. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M (2004). Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145:4565–4574. [DOI] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M (2001). Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411:613–617. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Ottem EN, Carpenter CD (2003). Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod 69:1771–1778. [DOI] [PubMed] [Google Scholar]
- Pfeiffer CA (1936). Sexual differences of the hypophyses and their determination by the gonads. Am J Anat 58:195–226. [Google Scholar]
- Roa J, Vigo E, Castellano JM, Navarro VM, Fernandez-Fernandez R, Casanueva FF, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M (2006). Hypothalamic expression of KiSS-1 system and gonadotropin releasing effects of Kisspeptin in different reproductive states of the female rat. Endocrinology 147:2864–2878. [DOI] [PubMed] [Google Scholar]
- Ronnekleiv OK, Kelly MJ (1986). Luteinizing hormone-releasing hormone neuronal system during the estrous cycle of the female rat: effects of surgically induced persistent estrus. Neuroendocrinology 43:564–576. [DOI] [PubMed] [Google Scholar]
- Ronnekleiv OK, Kelly MJ (1988). Plasma prolactin and luteinizing hormone profiles during the estrous cycle of the female rat: effects of surgically induced persistent estrus. Neuroendocrinology 47:133–141. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF Jr, Aparicio SA, Colledge WH (2003). The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM (2005). Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 102:2129–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB (1998). Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav Brain Res 92:195–203. [DOI] [PubMed] [Google Scholar]
- Simerly RB (2002). Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci 25:507–536. [DOI] [PubMed] [Google Scholar]
- Simonian SX, Spratt DP, Herbison AE (1999). Identification and characterization of estrogen receptor alpha-containing neurons projecting to the vicinity of the gonadotropin-releasing hormone perikarya in the rostral preoptic area of the rat. J Comp Neurol 411:346–358. [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA (2005a). Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA (2005b). Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692. [DOI] [PubMed] [Google Scholar]
- Smith JT, Clifton DK, Steiner RA (2006). Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction 131:623–630. [DOI] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD (1975). The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96:219–226. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M, Kauffman AS, Roa J, Navarro VM, Gottsch ML, Clifton DK, Steiner RA (2006). Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. In: Sixth International Congress of Neuroendocrinology June Pittsburgh, PA:.
- Terasawa E, Wiegand SJ, Bridson WE (1980). A role for medial preoptic nucleus on afternoon of proestrus in female rats. Am J Physiol 238:E533–E539. [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E (1982). Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology 34:395–404. [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE (1978). Persistent estrus and blockade of progesterone-induced LH release follows lesions which do not damage the suprachiasmatic nucleus. Endocrinology 102:1645–1648. [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE, Goy RW (1980). Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology 31:147–157. [DOI] [PubMed] [Google Scholar]