Abstract
Making an error elicits activity from brain regions that monitor performance, especially the medial frontal cortex (MFC). However, uncertainty exists about whether the posterior or anterior/rostral MFC processes errors and to what degree affective responses to errors are mediated in the MFC, specifically the rostral anterior cingulate cortex (rACC). To test the hypothesis that rACC mediates a type of affective response, we conceptualized affect in response to an error as a reaction to loss and amplified this response with a monetary penalty. While subjects performed a cognitive interference task during functional magnetic resonance imaging, hemodynamic activity in the rACC was significantly greater when subjects lost money as a result of an error compared with errors that did not lead to monetary loss. A significant interaction between the incentive conditions and error events demonstrated that the effect was not merely attributable to working harder to win (or not lose) money, although an effect of motivation was noted in the mid-MFC. Activation foci also occurred in similar regions of the posterior MFC for error and interference processing, which were not modulated by the incentive conditions. However, at the level of the individual subject, substantial functional variability occurred along the MFC during error processing, including foci in the rostral/anterior extent of the MFC not appearing in the group analysis. The findings support the hypothesis that the rostral extent of the MFC (rACC) processes loss-related responses to errors, and individual differences may account for some of the reported variation of error-related foci in the MFC.
Keywords: motivation, anterior cingulate cortex, affect, response conflict, functional magnetic resonance imaging, error-related negativity
Introduction
When a person performs a task, a system in the brain monitors performance and responds vigorously when it detects the commission of an error. An event-related potential (ERP), known as the error-related negativity (ERN), begins at approximately the same time as the onset of an error response (Falkenstein et al., 1991; Gehring et al., 1993). Source localization of the ERN maps broadly to the medial frontal cortex (MFC), and neuroimaging studies have confirmed the presence of error-related activation along the MFC. Considerable variation exists between different studies in the exact location for error processing, from the supplementary motor area (SMA) [Brodman area (BA) 6] to the rostral anterior cingulate cortex (rACC), a range of >6 cm (Ridderinkhof et al., 2004). Theoretical debate exists about the process triggered by an error and whether it corresponds to conflict monitoring (Carter et al., 1998; Botvinick et al., 2001), an evaluative function signifying “worse-than-expected events” (Holroyd and Coles, 2002), an affective reaction (Luu et al., 2003), or some combination of these processes (Yeung, 2004).
Affective processes clearly play a role in error processing and may account for some anatomic variation. Larger ERNs (Gehring et al., 2000; Johannes et al., 2001) and greater error-related blood oxygenation level-dependent (BOLD) signals (Ursu et al., 2003; Fitzgerald et al., 2005; Maltby et al., 2005) have been observed in patients with obsessive-compulsive disorder, and larger ERNs have been identified in persons with anxious personality traits (Hajcak et al., 2003) and depression (Tucker et al., 2003). Based on ERP studies, it has been suggested that the rostral ACC (rACC) processes the emotional components of error processing (van Veen and Carter, 2002a; Luu et al., 2003). Relative to the caudal section of the ACC/MFC, the rACC has been designated the “affective division” (Devinsky et al., 1995; Bush et al., 2000). It receives more projections from limbic brain regions (Vogt and Pandya, 1987; Kunishio and Haber, 1994) and is more likely to be activated in studies using emotion probes (Phan et al., 2002). However, no neuroimaging studies have isolated affective processing during performance monitoring in the rACC.
To test the hypothesis that rACC activity reflects a type of emotional response to making an error, we measured hemodynamic activity while participants performed a response interference task in which errors were differentially penalized. Conceptualizing the affective response to an error as a reaction to loss, we reasoned that an added monetary penalty would heighten the sense of loss in emotion-related brain regions, i.e., rACC. In the experimental paradigm, errors could result in losing money, not winning money, or neither. For purposes of comparison, we also analyzed activation during cognitive interference for correct trials, which should occur in the dorsal, posterior aspect of the MFC (pMFC) (Barch et al., 2001; van Veen et al., 2001; Garavan et al., 2003). Last, subject-level activation maps were obtained to analyze individual differences in functional anatomy, which could contribute to the variance of error processing networks in the MFC.
Materials and Methods
Subjects.
Twelve subjects (five males; mean ± SD age, 27.9 ± 8.1 years) participated. All were healthy, not taking medications, and had no contraindications to participating in an functional magnetic resonance imaging (fMRI) experiment. They received verbal and written explanation of the purpose and risks of the study and gave informed consent to participate, as approved by the institutional review board of the University of Michigan Medical School.
Behavioral task.
We used an incentivized response interference task, with similarities to the Eriksen flanker task (Eriksen and Eriksen, 1974) but with features designed to increase error rates while manipulating incentive and motivating subjects. Subjects had to identify the odd letter (“target”) in a string of letters and make a response: right button press for “H” or “C” and left button press for “S” or “K.” Interference occurred because the nontarget letters consisted of letters from this same set of four letters. “High” interference occurred when the target response differed from the response indicated by the nontarget letters, e.g., HHHSHHH. “Low” interference occurred when the nontarget letters differed from the target letters but still indicated the same response as the target, e.g., HCHHHHH. The position of the target varied randomly in this string of letters, with the constraint that it never appeared at the beginning or the end of the string. Subjects had to respond within a deadline, which prevented them from responding slowly but more accurately, i.e., trading speed for accuracy (Ullsperger and von Cramon, 2004). They received immediate feedback for errors, which included both commission errors (wrong key press) and deadline errors (not responding quickly enough). To titrate error rates to ∼30%, a response deadline was set as 1.2–1.3 times the mean reaction time, determined from practice trials. Difficulty was also manipulated by having the subjects view either seven (subjects 1–6) or five (subjects 7–12) letters (target plus distractors). The performance of the two subgroups (five vs seven letters) did not differ.
Each trial began with a cue indicating the incentive condition: pictures of human hands in the thumbs-up orientation (Fig. 1) indicated the “gain” condition, thumbs down indicated the “loss” condition, and thumbs pointing inward indicated the “null” condition. Two thumbs were presented above and two thumbs below the target. Between the thumbs, subjects saw a monetary amount for the gain and loss conditions. To increase attention to the incentive manipulation, the amount varied between trials, from $2, $1, to 25¢ (10, 40, and 70% of trials, respectively). The variation in the incentive value served to raise subject anticipation and engage motivation to the incentive. Subjects were informed that they stood to gain, or lose, real money in the amount depicted for each trial. They began the experiment with a $10 “credit” and saw a cumulative tally of their earnings after each run. For the null condition, subjects saw “$0” between the thumbs. These cues, which appeared above and below the letter stimuli, preceded these stimuli by a 1.9–2.0 s stimulus onset asynchrony (depending on the deadline). After the subject’s response, letter stimuli were replaced by a set of asterisks, red in color if they responded within the deadline and white if they responded outside the deadline or if they made the incorrect response. Because the ERN is not sensitive to the physical characteristics of eliciting stimuli (Holroyd and Coles, 2002), the feedback cue color was not counterbalanced across subjects. Total duration of cue and letter string was 3.0 s, with a 2 s intertrial interval. Incentive conditions were also presented in equal proportions, pseudorandomized.
Figure 1.
Task design. a, The three panels indicate the three different types of cue that began each trial (gain, loss, null). The amounts for the gain and loss conditions varied between $2, $1, and 25¢. b, The cue panel appeared for ∼1.9 s, followed by the imperative stimulus. Subjects responded to the odd letter in the string.
Subjects performed 360 trials over six runs. Stimuli were presented and responses recorded using a computer running E-prime with IFIS (MRI Devices, Milwaukee, WI), interfaced to project stimuli onto MR-compatible liquid crystal display goggles (Resonance Technology, Northridge, CA).
Functional MRI acquisition.
MRI scanning occurred on a General Electric (Waukesha, WI) 3T Signa scanner [LX (8.3) release, neuro-optimized gradients]. Scanning began with structural acquisition of a standard T1 image (T1-overlay) for anatomic normalization and alignment. A T2*-weighted, reverse spiral acquisition sequence [GRE; repetition time, 2000 ms; echo time, 30 ms; flip angle, 90°; field of view (FOV), 20 cm; 40 slice; thickness/skip, 3.0/0 mm matrix size equivalent to 64 × 64] occurred in the same prescription as the T1-overlay, and 60 volumes were acquired for a run, after discarding four initial volumes to permit thermal equilibration of the MRI signal. This T2*-sensitive acquisition sequence was specifically designed to enable good signal recovery in ventral medial frontal regions, in which susceptibility artifact often impairs the T2* signal (Yang et al., 2002). After acquisition of functional volumes, a high-resolution T1 scan was obtained for anatomic normalization [three-dimensional spoiled gradient-recalled acquisition in a steady state (SPGR); 24 FOV; thickness/skip, 1.0/0 mm].
Data analysis.
Scans were reconstructed, slice-time corrected (interpolated with an eight-point sinc kernel multiplied by a Hanning window), realigned to the first scan in the experiment (Woods et al., 1998), and coregistered with the high-resolution SPGR T1. This high-resolution image was then anatomically normalized to the Montreal Neurological Institute (MNI) 152 template brain, as implemented in the SPM99 package (Wellcome Institute of Cognitive Neurology, London, UK). The resulting transformation parameters were applied to the time series of coregistered, normalized functional volumes, which were resliced and smoothed with a 6 mm full-width half-maximal (FWHM) isotropic Gaussian smoothing kernel. Each normalized image set was then high-pass filtered (100 s) and analyzed in a two-step process. The first step involved the construction of models for each subject, in which regressors of interest were convolved with a hemodynamic response function. Regressors at the onset of the letter stimuli modeled high-interference correct trials, low-interference correct trials, and error trials. Orthogonal to the high, low, and error trials were regressors for each incentive condition (gain, loss, and null) for a total of nine regressors of interest (plus five session regressors). This model permitted analysis of interference (high − low), error processing [error − correct(high + low)], and the effect of incentives (gain − null; loss − null) between errors committed in each incentive condition.
Because subjects could make two types of errors, either commission errors (pressing the wrong key) or deadline errors (not responding quickly enough), we ran a separate model comparing these errors. Although the pMFC signal was nominally larger for deadline errors than commission errors, there were no significant differences between the two error types in the pMFC; therefore, we did not separate these two error types in main analysis model (which would have reduced power across the various conditions).
Analysis was conducted at the subject and group level. An a priori region of interest was identified in the midline frontal cortex based on a published meta-analysis of MFC function (Ridderinkhof et al., 2004). This region comprised a volume of 202 cm3 (x = −18 to +18; y = 0–70; z = −18–72), and it included the rACC below the bicommissural line, in which we found error-related activation for patients with obsessive-compulsive disorder (Fitzgerald et al., 2005). Only results appearing within this region are reported. Analysis of individual subjects occurred only for error processing and interference processing, collapsed across the incentive conditions to maximize statistical power. This analysis of individual differences aimed to demonstrate the anatomic distribution of strongly significant activation foci in the MFC at the individual level. Therefore, a relatively stringent threshold was set, which minimized type 1 error rates. Voxels with p < 0.001 (Z > 3.09) were entered into the analysis, and the locus of maximum activation for each cluster was accepted if the voxel extent exceeded a corrected, familywise error rate of p < 0.05. The coordinates of these foci were mapped onto the MNI reference atlas for comparison between subjects.
For group analyses, subjects were treated as a random effect, and contrast images were derived for each subject and smoothed with a 6 mm FWHM Gaussian kernel to stabilize variance properties. The smoothed contrasts were then entered into a second-level analysis to examine effects of error processing. The initial threshold to enter a voxel into analysis was p < 0.005 (Z > 2.58), with a minimum cluster size k > 8 voxels (216 mm3), and foci were reported with a false discovery rate of p < 0.005 (Genovese et al., 2002). In contrast to the individual analysis, the group analysis was designed to maximize sensitivity to test specific hypothesis about the common behavior of the BOLD signal.
Behavioral results were analyzed with Statistical Package for the Social Sciences 11.0 (SPSS, Chicago, IL), using two-factor, repeated-measures ANOVAs, with incentive and interference as the two orthogonal factors, and Greenhouse–Geisser corrections for nonsphericity. For reaction time measurement, only correct trials, within the response deadline, were analyzed.
Results
Behavioral results
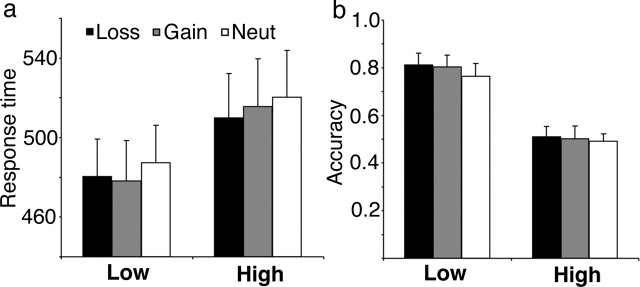
As expected, interference from the high conflict stimuli affected both accuracy and speed. Subjects were more accurate for the low-interference compared with the high-interference conditions (F(1,11) = 39.9; p < 0.000), and they responded faster during the low-interference trials (F(1,11) = 21.9; p < 0.001) (Fig. 2). The overall accuracy rate was 64.3 ± 13.6% (mean ± SD); errors of commission occurred at a rate of 26.9 ± 15.4%, and deadline errors (response too late) occurred at 8.8 ± 3.6%. The incentive manipulation affected latency, inducing faster responses (F(1.84,20.2) = 3.95; p = 0.04). There was no significant effect of incentive on accuracy (F(1.54,16.9) = 1.68; p = 0.22) and no significant interaction effect between incentive and interference for either latency or accuracy (p > 0.4).
Figure 2.
Behavioral results. Behavioral results depict response times (in milliseconds, ±SEM) for correct trials (a) and accuracy rates (±SEM) (b) for the low- and high-interference conditions and the three incentive conditions.
Subjects were debriefed after the experiment (data available for 11 subjects). All but one subject reported that they felt “flustered” after making a mistake at least “‘somewhat.” All subjects reported that they were at least somewhat frustrated by their performance. Six subjects said that they tried harder during loss and gain trials compared with neutral, and four of these subjects said that they tried harder during loss compared with potential gain trials. No subjects reported that they tried harder for the gain compared with the loss trials.
Neuroimaging results: error and incentive
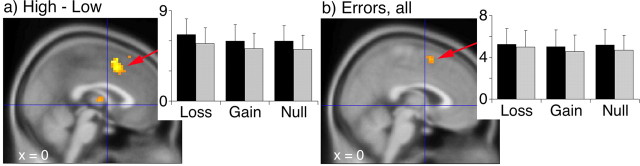
To test our primary hypothesis that errors made with a financial penalty would activate anterior/rostral portions of the medial frontal wall, we contrasted errors made during the incentive conditions with errors made in the null condition. As predicated, activation occurred in the rACC for the contrast of loss − null (k = 13; Z = 3.00 at −12, 36, −6; p < 0.004) (Fig. 3a). There was no difference between gain and null in this region (Fig. 3b) and no difference between gain and loss in the rACC, even at a threshold of p < 0.01 for both contrasts with gain. The only difference between gain and loss above threshold occurred in the dorsal pMFC, for gain > loss (k = 16; Z = 3.29 at 18, 33, 51; p < 0.005). There was a focus in the mid-MFC (BA 8/32) that appeared during both gain and loss conditions compared with the null condition (loss − null, k = 33, Z = 3.17 at 9, 30, 39, p < 0.004; gain − null, k = 40, Z = 3.03 at 9, 30, 42, p < 0.004) (Fig. 3a,b). For the loss − null contrast, we also observed a focus in the left and right caudate nuclei, which were included in our a priori region of interest (k = 60, Z = 4.19 at 9, 18, 3, p < 0.002; k = 46, Z = 3.51 at −12, 3, 15, p < 0.002).
Figure 3.
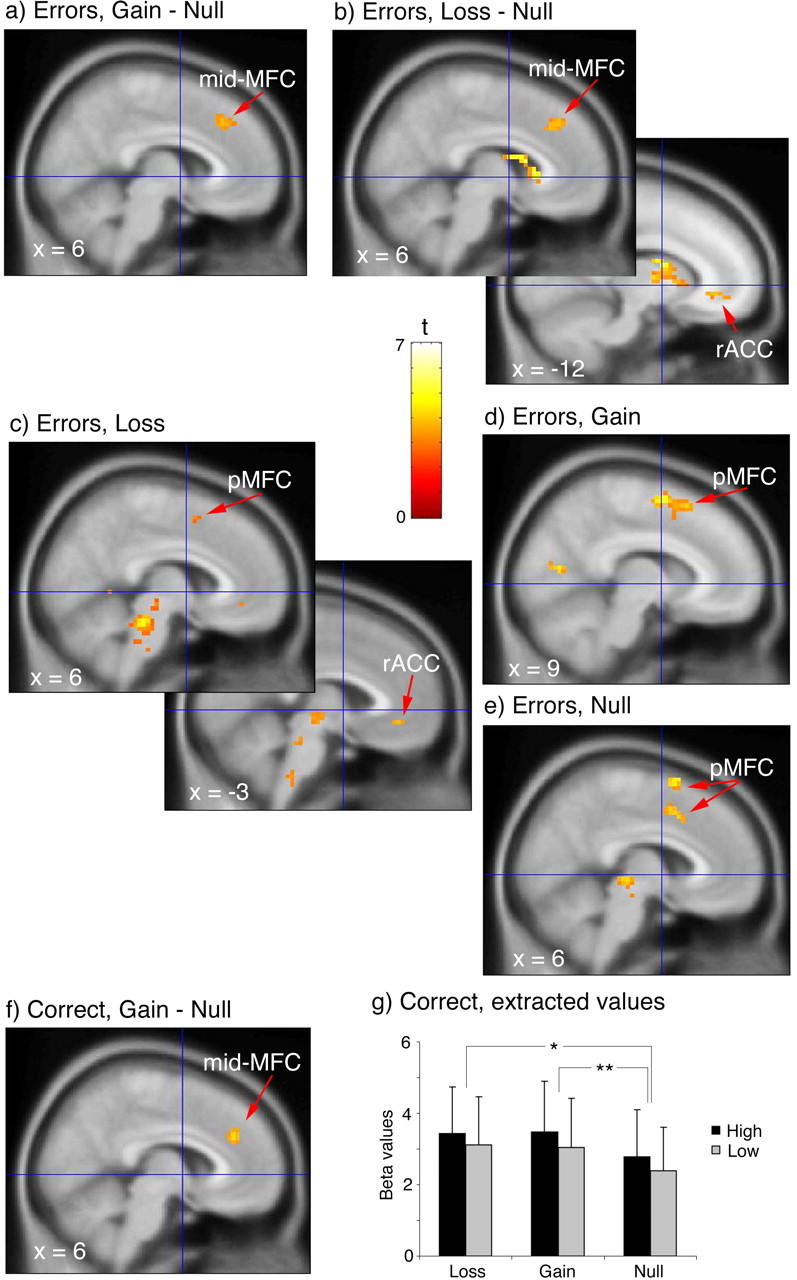
Error processing in the MFC. Contrasts for error trials between gain and null (a) and between loss and null (b) show a mid-MFC focus in both incentive conditions relative to null and rACC activation in the loss − null contrast. BOLD signal change for error trials minus correct trials for loss (c), gain (d), and null (e) reveals pMFC activation for each condition, plus rACC activation for loss. Activation occurs for correct trials in the mid-MFC for gain (f) relative to null. g, Extracted BOLD signal (regression coefficient values ± SEM) from an unbiased volume of interest derived from the mid-MFC activation during error trials demonstrates activity increases for gain and loss incentive conditions (*p < 0.05, loss > null; **p < 0.05, gain > null). All voxels are overlaid on the MNI 152 reference image and displayed at a threshold of p < 0.005, except for the pMFC focus in c (p < 0.01).
One possible interpretation of these results is that, during the incentive conditions, our subjects tried harder, and activity in the rACC and mid-MFC reflected greater effort, not greater response to an error. To examine this possibility, we first looked at activation maps for the effect of errors during each incentive condition (error − correct). When errors were made during each incentive condition, activation occurred in the pMFC (Table 1, Fig. 3c–e), although for the loss condition, this occurred below our chosen significance threshold. We also found an activation focus in the rACC for errors made during the loss condition, suggesting that activity in this region does not simply reflect greater motivation. To verify that the effect of loss during errors was significantly greater than any effect of loss during correct performance, we examined the interaction between incentive and error/correct in the rACC [error (loss − null) − correct (loss − null)] and found the focus in the rACC (k = 73, Z = 3.57 at −12, 45, −6; Z = 3.12 at −3, 39, −18, p < 0.004). There were no activation foci outside the a priori region occurring in both the contrast or error − correct and the contrast of incentive trials with the null condition during errors.
Table 1.
Activation foci in MFC during error and interference processing
| Region | Cluster sizea | x, y, zb | Zscorec |
|---|---|---|---|
| Error − correct, loss conditions | |||
| pMFC (BA 6) | 5 | 6, 6, 54 | 2.61d |
| rACC (BA 32) | 9 | −3, 39, −9 | 3.01 |
| Error − correct, gain conditions | |||
| pMFC (BA 6) | 100 | 9, 3, 63 | 3.48 |
| 0, 18, 54 | 3.35 | ||
| 12, 18, 57 | 3.13 | ||
| Error − correct, null conditions | |||
| pMFC (BA 6, 32) | 17 | 6, 9, 66 | 3.32 |
| 29 | 6, 9, 45 | 3.11 | |
| 3, 6, 57 | 2.86 | ||
| Error − correct, all conditions | |||
| pMFC (BA 6) | 23 | 3, 6, 57 | 2.86 |
| 6, 6, 51 | 2.68 | ||
| High–low interference | |||
| pMFC (BA 6, 32) | 139 | 0, 9, 54 | 3.72 |
| 3, 9, 45 | 3.68 | ||
| 9, 15, 42 | 3.72 | ||
| 6, 21, 60 | 3.04 |
aNumber of voxels (p < 0.005) exceeding contiguity threshold of 216 mm3.
bStereotactic coordinates according to the MNI atlas, right/left, anterior/posterior, and superior/inferior, respectively.
cZ score for peak magnitude(s) within a cluster, with false discovery rate of p < 0.005, corrected for search region.
dFalse discovery rate, p < 0.01.
Examination of activity in the mid-MFC revealed a pattern more consistent with an effect of motivation/effort, because we did not find an activation focus in the mid-MFC for the contrast of error and correct in the loss and gain conditions. If activity of the mid-MFC does represent motivation, then we should see activation for the contrast of the incentive conditions with null during correct trials. Indeed, we did find, for the gain − null contrast, a focus in mid-MFC (BA 8/32; k = 27; Z = 3.13 at 6, 36, 30; p < 0.004) (Fig. 3f). For the corresponding comparison with the loss condition, activation appeared in this region only when we lowered the threshold to p < 0.05. There was no difference between gain and loss conditions in this area. The activation focus for gain − null during correct trials appeared very close to the focus of activation for gain − null and loss − null (Fig. 3a,b) during error trials. To determine whether the incentive manipulation did affect the same region of cortex, we used the incentive conditions from the error trials [(gain + loss) − null] to define a mask (coordinates at 6, 36, 39; cluster size, 107), which was used to extract adjusted BOLD signal (regression coefficient values) from the correct trials at the focus of activity in which the incentive manipulation affected error processing. The extractions revealed that activity (Fig. 3g) was significantly elevated for the incentive conditions compared with the null condition (gain vs null, t = 1.83, df = 11, p = 0.045; loss vs null, t = 1.86, df = 11, p = 0.047; one-tailed t test). A 3 × 2 repeated-measures ANOVA, with incentive (loss, gain, null) and interference (high, low) as repeated factors, showed a trend toward a main effect of incentive (F(1.38,15.2) = 3.03; p = 0.09), although this analysis lacked the benefit of the specific directional hypothesis of the paired t tests. A trend toward a main effect of interference (F(1,11) = 4.46; p = 0.06) was noted, but there was no evidence of any interaction (F(2,22) = 0.2; p = 0.98).
Neuroimaging results: comparing error and interference activations in the pMFC
We also conducted analyses comparing pMFC activation for errors with the predicted pMFC activation for interference. Figure 4and Table 1 demonstrate that both errors and interference processing (high − low) activated foci in the most dorsal, posterior extent of the MFC, corresponding to the presupplementary region (pre-SMA). Two questions were addressed: (1) was the pMFC focus sensitive to the incentive manipulation, and (2) did the pMFC focus for error processing also process interference, as suggested by conflict theory (Carter et al., 1998)? To test for any effects of incentive in the pMFC, we extracted activity (regression coefficient values) at each focus depicted in Figure 4. For interference (Fig. 4a, inset graph), there was no effect of incentive (F(1.74,19.1) = 1.6; p = 0.23) and no interaction with interference (F(1.5,16.3) = 0.1; p = 0.9). For the extracted values of the three incentive regressors in the pMFC error focus (graph not shown), there was no effect of incentive on the error signal (F(1.6,16.4) = 0.08; p = 0.45). To address the question of whether or not the this pMFC error focus also processed an interference signal, we used the mask derived from the error focus in Figure 4b to extract activity from the six regressors for the correct trials (Fig. 4b, inset graph), revealing a significant effect of interference (F(1,10) = 13.7; p = 0.004). In this region also, there was no effect of incentive (F(1.86,18.6) = 0.47; p = 0.63) and no interaction with interference (F(1.4,14.4) = 0.3; p = 0.75).
Figure 4.
Interference and error signal in the pMFC. a, BOLD activation for interference (high − low) collapsed across incentive conditions revealed a pMFC focus. The inset graph of extracted signal from the cluster defined by this contrast shows a similar pattern of interference across the three incentive conditions. b, Activation for error − correct occurs in the pMFC, and, when data are extracted from this cluster for the correct trials, the interference effect persists. Values on the ordinate axis of both inset graphs represent regression coefficient values ± SEM, whereas the categories on the abscissa represent the three incentive conditions. Voxels displayed for p < 0.005.
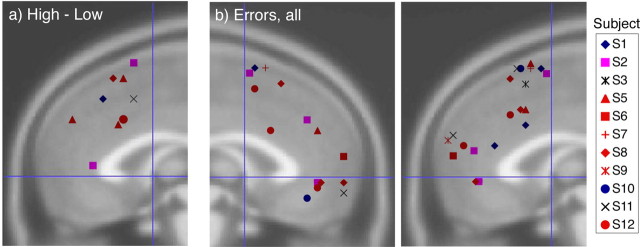
Neuroimaging results: individual results for error and interference trials
Group activation maps reveal little information about the variability among individuals in anatomic location and functional magnitude of activation. To explore these factors, we analyzed the effect of error and interference processing in each individual subject. Activation peaks for significant clusters for each individual are depicted in Figure 5. Significant functional anatomic variability was noted within the MFC for error and interference processing. For the high − low interference contrast, 6 of the 12 subjects had nine foci that exceeded significance thresholds, and all of these subjects had foci in the pMFC, corresponding to the pre-SMA focus identified in the group activation. Only two clusters occurred in the anterior/ventral region of the MFC. Z scores for each cluster ranged between 3.68 and 5.12 (Fig. 5a). For the error − correct contrast, 30 significant activation foci from 11 subjects exceeded threshold, with Z scores in excess of 4.2 and >5.2 in 23 of 30 foci. A cluster of foci from eight subjects occurred in the pre-SMA, corresponding to the focus found in the group activation (Fig. 4b). However, many foci occurred in the anterior and ventral areas of the MFC, including several foci near the rACC focus identified in Figure 3, a and e.
Figure 5.
Individual subject results for error processing. a, Peak activation foci for each subject (legend on the left of figure) for the high − low contrast, plotted on the right MFC for x = −3 to +18. No foci appeared for x < −3 in this contrast. b, Activation foci for error − correct contrasts are plotted on the left MFC (left) from x = +3 to −18 and on the right MFC (right) for x = −3 to +18.
Neuroimaging results: correct trials
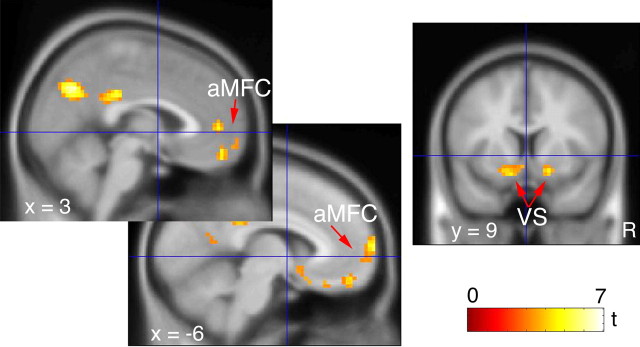
Last, we analyzed the effect of a correct, relative to an error, trial (correct − error). Results are tabulated in Table 2and depicted in Figure 6. Notably, the ventral, anterior aspect of the MFC (BA 10, BA 11, and BA 32) was activated. Because the striatum was included in the region of interest, bilateral activation of the ventral striatum (VS) is also identified. The effects of loss and gain incentives on correct trials were analyzed in contrast to the null condition. The ventral, anterior MFC activations found in the correct − error contrast did not appear as an effect of either gaining money or not losing money relative to the null condition, even when the threshold was lowered to p < 0.01.
Table 2.
Activation foci in MFC during correct trials
| Region | Cluster sizea | x, y, zb | Zscorec |
|---|---|---|---|
| Correct − error | |||
| Anterior MFC (BA 32) | 16 | 3, 48, 3 | 3.44 |
| Anterior MFC (BA 10) | 126 | −9, 69, 9 | 3.97 |
| Anterior MFC (BA 11) | 0, 51, −18 | 3.76 | |
| 9, 63, −9 | 2.91 | ||
| Ventral striatum (left) | 53 | −18, 6, −12 | 3.72 |
| −3, 12, −9 | 3.43 | ||
| −12, 9, −15 | 3.36 | ||
| Ventral striatum (right) | 26 | 18, 9, −12 | 3.43 |
aNumber of voxels (p < 0.005) exceeding contiguity threshold of 216 mm3.
bStereotactic coordinates according to the MNI atlas, right/left, anterior/posterior, and superior/inferior, respectively.
cZscore for peak magnitude(s) within a cluster, with false discovery rate of p < 0.005, corrected for search region.
Figure 6.
Incentive effect on correct trials. Activation for correct − error occurs in anterior MFC and the VS. Voxels displayed for p < 0.005.
Discussion
Monitoring task performance and detecting errors entails a variety of functions and anatomic structures. In this report, we focused on the MFC and the “affective” response to making an error, in which affect has been operationalized as a monetary loss. As predicted, activity in the rACC was significantly greater when subjects lost money as a result of an error compared with the null condition. Individual subjects showed substantial anatomic variability of the error-related BOLD signal along the MFC, although group activation maps revealed similar foci of activation in the pMFC, not modulated by the incentive conditions. On objective performance measures, group averages demonstrated significant improvements in response latency and nominal increases in accuracy for the incentive conditions. These behavioral changes suggest that the MFC signal increase for the incentive conditions did not reflect a shift toward slower and more accurate responding, which increases the size of the ERN (Gehring et al., 1993) and the fMRI BOLD signal in the pMFC (Ullsperger and von Cramon, 2004).
Error processing and the rACC
These findings are consistent with the hypothesis that the rostral MFC processes affective components of an error (van Veen and Carter, 2002a; Luu et al., 2003), although the relevant process may be more general than a simple affective reaction. The link between affect and bad outcomes is indirect here, and one could posit an alternative process, such as computations about expected value, which would not necessarily evoke an affect (Holroyd and Coles, 2002). Nevertheless, error commission can elicit affect, and almost all subjects reported frustration with making mistakes. The rACC focus was associated with error-related loss, although it was not activated by error-related failure to gain. Although we considered the failure to gain as a type of loss and predicted activation of the rACC, the experience of losing money may have had more power to amplify activity in this region. This result is consistent with the phenomenon of “loss aversion,” i.e., people tend to place greater value on avoiding a loss than on failing to gain something (Kahneman and Tversky, 1979). Our subjects endorsed this phenomenon: one-third tried harder during loss conditions (compared with gain), whereas none tried harder for gain. Other neuroimaging work implicates this ventral area of the MFC in negative affective states. The rACC focus lies close to a “medial orbitofrontal” cortex area (MNI coordinates −8, 32, −14) associated with the feeling of regret (Coricelli et al., 2005). Ventral MFC (rACC and subgenu ACC) has been activated by sad mood (George et al., 1995; Mayberg et al., 1999; Liotti et al., 2000) and implicated in depression (Drevets et al., 1997; Mayberg et al., 1999). Using a similar interference paradigm without incentives, we found excessive activity in the rACC in obsessive-compulsive disorder (Fitzgerald et al., 2005). More generally, the rACC and adjacent MFC has also been associated with the receipt of reward (Elliott et al., 2000; Rogers et al., 2004) and the recognition of self-relevant information (Kircher et al., 2000). Thus, beyond loss-related components of an error, this region may also process the motivational significance of events.
Performance monitoring in the pMFC
Our data are also consistent with performance monitoring by the pMFC. In contrast to error-related processing, interference elicited no discernible affective response. As predicted (Barch et al., 2001; van Veen et al., 2001; Garavan et al., 2003), conflicting response choices recruited the pMFC. Likewise, error processing recruited a similar region in the pMFC sensitive to interference but not incentives. This pMFC region may correspond to the module posited by conflict theory, one theoretical account of the error signal (Carter et al., 1998; Botvinick et al., 2001). Cognitive conflict occurs when a strong response tendency competes with, and must be overcome by, the intended response, e. g. nontarget letters that indicated a different response than the target letter. According to conflict theory, the dorsal ACC (dACC), within the pMFC, monitors for the presence of cognitive conflict (Carter et al., 1998; Botvinick et al., 2001; Yeung et al., 2004). Errors are a special case of high conflict when the stronger, but undesired, response tendency reaches a sufficient threshold to command an actual response. Accordingly, errors and conflict recruit the same region of the MFC (Carter et al., 1998; van Veen and Carter, 2002b), as Figure 4 demonstrates, although the group foci lie posterior to the dACC.
The wide distribution of individual activation foci for error processing may reveal one reason for disagreement about error and interference processing in the MFC. For example, some investigators have noted a tendency for conflict-related foci to fall into BA 6/8 (pre-SMA) and error-related foci to fall into BA 24/32 (dACC), in addition to areas of overlap (Kiehl et al., 2000; Braver et al., 2001; Ullsperger and von Cramon, 2001, 2004; Garavan et al., 2002). If enough subjects in a random sample have more rostral/anterior activation to errors, that would shift the group focus away from the pMFC. We suggested that affective responses, mediated by the rACC, might be one source of anatomic variability between individuals, but other sources of individual difference probably also contribute, such as differences in individual performance and age, as others have reported (Hester et al., 2004). Although a preliminary analysis of age and subjective responses with the extent and magnitude of activation revealed no significant associations, the power of our dataset limits any negative conclusions.
Motivational effects in the mid-MFC
In the mid-MFC (BA 8/32), we found activation for both incentive conditions, relative to null, occurring for error and correct trials, which could reflect a motivational signal to enhance performance. Evidence suggests that systems in the MFC may play a primary role in motivation, serving to increase behavioral tendencies in the direction of perceived value. For example, lesions of the ACC in rodents impair the choice of a high-cost/high-reward option, without impairing the choice of a less-demanding and less-rewarding option (Walton et al., 2003). We reported previously an increased BOLD response in the mid-MFC to monetary incentives during a working memory task (Taylor et al., 2004). Alternatively, this region may interact with a general arousal signal from widely ramifying, neuromodulatory nuclei, such as brainstem monoaminergic neurons (Aston-Jones et al., 1999), in response to task demands to increase attention. In support of this possibility, correlations between sympathetic arousal and hemodynamic responses have been identified in the ACC during effortful tasks (Paus, 2000; Critchley et al., 2003).
Responses to correct trials in the anterior MFC and VS
When subjects made a correct response, they activated anterior/ventral MFC regions, including medial orbitofrontal cortex, and bilateral VS. The VS has been implicated in the receipt of a reward or avoiding a loss (Berns et al., 2001; Knutson et al., 2001; Elliott et al., 2003; Nieuwenhuis et al., 2005b). Activation of the anterior/ventral MFC, including the medial orbitofrontal cortex, occurs in tasks using monetary performance incentives, similar to the present design (O’Doherty et al., 2001; Elliott et al., 2003; Knutson et al., 2003; Rogers et al., 2004). These regions did not exhibit differential sensitivity to incentives, although the absence of a difference may reflect either the insensitivity of the neural system or insufficient experimental power. Although the BA 32 focus for correct responses appears very close to the rACC focus for errors, Wager et al. (2003) found a similar close anatomic association between approach-related behavior (MPC) and withdraw-related behavior (rACC) in a meta-analysis of neuroimaging studies of emotion.
Implications and conclusions
The study involved certain choices about the design relevant to conclusions drawn from the results. Subjects could have made two types of errors: commission errors and deadline errors. Although some performance monitoring processes must differ between these two types of errors, we focused on the processes common to both types. From the theoretical perspective of conflict theory (Carter et al., 1998; Botvinick et al., 2001), deadline errors would not entail the same type of conflict as commission errors, but our study was not designed to discern such differences. For both error types, subjects received immediate feedback but in neither case were they operating with any uncertainty about the required response. In this respect, the feedback of our task differs from the feedback of a time estimation task (van Veen et al., 2004; Nieuwenhuis et al., 2005a), which has been reported to not activate the pMFC. Future work will be required to investigate differences arising from the two types of errors used in our study.
In summary, the present study found that error processing does occur in the anterior aspect of the MFC, specifically the rACC, when a worse-than-desired outcome occurs in the form of a monetary penalty. Emotional responses to poor performance may also contribute to the anatomic variability of error-related processing in the MFC, although other explanations also need to be considered. However, the anatomic pattern of these BOLD changes may hold important keys for disorders of emotion, such as depression and obsessive-compulsive disorder.
Parts of this work were presented previously at the 43rd Annual Meeting of the American College of Neuropsychopharmacology (San Juan, Puerto Rico, December, 2004) and the 11th Annual Meeting of the Organization for Human Brain Mapping (Toronto, Canada, June, 2005).
Footnotes
This work was supported by the Rachel Upjohn Fund from the University of Michigan Depression Center and National Institute of Mental Health Grant R01 MH078281 (S.F.T.). We thank K. Newnham for assistance with data acquisition.
References
- Aston-Jones G, Rajkowski J, Cohen J (1999). Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry 46:1309–1320. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A (2001). Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex 11:837–848. [DOI] [PubMed] [Google Scholar]
- Berns GS, McClure SM, Pagnoni G, Montague PR (2001). Predictability modulates human brain response to reward. J Neurosci 21:2793–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD (2001). Conflict monitoring and cognitive control. Psychol Rev 108:624–652. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A (2001). Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex 11:825–836. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4:215–222. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD (1998). Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280:747–749. [DOI] [PubMed] [Google Scholar]
- Coricelli G, Critchley HD, Joffily M, O’Doherty JP, Sirigu A, Dolan RJ (2005). Regret and its avoidance: a neuroimaging study of choice behavior. Nat Neurosci 8:1255–1262. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ (2003). Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain 126:2139–2152. [DOI] [PubMed] [Google Scholar]
- Devinsky W, Morrell MJ, Vogt BA (1995). Contributions of anterior cingulate cortex to behavior. Brain 118:279–306. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, Raichle ME (1997). Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386:824–827. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ (2000). Dissociable neural responses in human reward systems. J Neurosci 20:6159–6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Newman JL, Longe OA, Deakin JF (2003). Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci 23:303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW (1974). Effects of noise letters upon the identifcation of a target letter in a nonsearch task. Percept Psychophys 16:143–149. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L (1991). Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol 78:447–455. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SF (2005). Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biol Psychiatry 57:287–294. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA (2002). Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. NeuroImage 17:1820–1829. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA (2003). A midline dissociation between error-processing and response-conflict monitoring. NeuroImage 20:1132–1139. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin EA (1993). Neural system for error-detection and compensation. Psychol Sci 4:385–390. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG (2000). Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol Sci 11:1–6. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T (2002). Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 15:870–878. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM (1995). Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry 152:341–351. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF (2003). Anxiety and error-related brain activity. Biol Psychol 64:77–90. [DOI] [PubMed] [Google Scholar]
- Hester R, Fassbender C, Garavan H (2004). Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cereb Cortex 14:986–994. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH (2002). The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev 109:679–709. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Nager W, Rada D, Dengler R, Emrich HM, Munte TF, Dietrich DE (2001). Discrepant target detection and action monitoring in obsessive-compulsive disorder. Psychiatry Res 108:101–110. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A (1979). Prospect theory: an analysis of decision making under risk. Econometrica 47:263–291. [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB (2000). Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology 37:216–223. [PubMed] [Google Scholar]
- Kircher TT, Senior C, Phillips ML, Benson PJ, Bullmore ET, Brammer M, Simmons A, Williams SC, Bartels M, David AS (2000). Towards a functional neuroanatomy of self processing: effects of faces and words. Brain Res Cogn Brain Res 10:133–144. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D (2001). Dissociation of reward anticipation and outcome with event-related fMRI. NeuroReport 12:3683–3687. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D (2003). A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. NeuroImage 18:263–272. [DOI] [PubMed] [Google Scholar]
- Kunishio K, Haber SN (1994). Primate cingulostriatal projection: limbic striatal versus sensorimotor striatal input. J Comp Neurol 350:337–356. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT (2000). Differential limbic–cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol Psychiatry 48:30–42. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C (2003). Electrophysiological responses to errors and feedback in the process of action regulation. Psychol Sci 14:47–53. [DOI] [PubMed] [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, O’Keefe TM, Kiehl KA (2005). Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. NeuroImage 24:495–503. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT (1999). Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 156:675–682. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Slagter HA, von Geusau NJ, Heslenfeld DJ, Holroyd CB (2005a). Knowing good from bad: differential activation of human cortical areas by positive and negative outcomes. Eur J Neurosci 21:3161–3168. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Heslenfeld DJ, von Geusau NJ, Rogier BM, Holroyd CB, Yeung N (2005b). Activity in human reward-sensitive brain areas is strongly context dependent. NeuroImage 25:1302–1309. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C (2001). Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci 4:95–102. [DOI] [PubMed] [Google Scholar]
- Paus T (2000). Functional anatomy of arousal and attention systems in the human brain. Prog Brain Res 126:65–77. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I (2002). Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage 16:331–348. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S (2004). The role of the medial frontal cortex in cognitive control. Science 306:443–447. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM (2004). Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry 55:594–602. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Welsh RC, Wager TD, Phan KL, Fitzgerald KD, Gehring WJ (2004). A functional neuroimaging study of motivation and executive function. NeuroImage 21:1045–1054. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Luu P, Frishkoff G, Quiring J, Poulsen C (2003). Frontolimbic response to negative feedback in clinical depression. J Abnorm Psychol 112:667–678. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY (2001). Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. NeuroImage 14:1387–1401. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY (2004). Neuroimaging of performance monitoring: error detection and beyond. Cortex 40:593–604. [DOI] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS (2003). Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychol Sci 14:347–353. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS (2002a). The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci 14:593–602. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS (2002b). The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav 77:477–482. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS (2001). Anterior cingulate cortex, conflict monitoring, and levels of processing. NeuroImage 14:1302–1308. [DOI] [PubMed] [Google Scholar]
- van Veen V, Holroyd CB, Cohen JD, Stenger VA, Carter CS (2004). Errors without conflict: implications for performance monitoring theories of anterior cingulate cortex. Brain Cogn 56:267–276. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN (1987). Cingulate cortex of the rhesus monkey. II. Cortical afferents. J Comp Neurol 262:256–270. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF (2003). Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage 19:513–531. [DOI] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Alterescu K, Rushworth MF (2003). Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. J Neurosci 23:6475–6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC (1998). Automated image registration. II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr 22:153–165. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gu H, Zhan W, Xu S, Silbersweig D, Stern E (2002). Simultaneous perfusion and BOLD imaging using reverse spiral scanning at 3T: characterization of functional contrast and susceptibility artifacts. Magn Reson Med 48:278–289. [DOI] [PubMed] [Google Scholar]
- Yeung N (2004). Relating cognitive and affective theories of the error-related negativity. In: Errors, conflicts, and the brain. Current opinions on performance monitoring (Ullsperger M, Falkenstein M, eds) pp. 63–70. Leipzig, Germany: Max Planck Institute of Cognitive Neuroscience.
- Yeung N, Cohen JD, Botvinick MM (2004). The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev 111:931–959. [DOI] [PubMed] [Google Scholar]