Abstract
Functioning of the cerebral cortex requires the coordinated assembly of circuits involving glutamatergic projection neurons and GABAergic interneurons. Despite their segregated origin in different regions of the telencephalon, projection neurons and interneurons born synchronically end up adopting the same cortical layer, suggesting that layer acquisition is highly coordinated for both neuronal types. The radial migration and laminar arrangement of projection neurons depends on Reelin, a secreted glycoprotein expressed near the pial surface during embryogenesis. In contrast, the mechanisms controlling layer acquisition by cortical interneurons remain essentially unknown. Here, we have used an ultrasound-guided transplantation approach to analyze the mechanisms underlying the acquisition of laminar locations by cortical interneurons. We found that layer acquisition by cortical GABAergic interneurons does not directly depend on Reelin signaling. Moreover, interneurons invade their target layers well after synchronically generated projection neurons reach their final destination. These results suggest a model in which cues provided by projection neurons guide cortical interneurons to their appropriate layer, and reveal that, at least for some neuronal types, long-range radial migration does not directly require Reelin.
Keywords: ultrasound-guided transplantation, radial migration, layering, neocortex, inhibitory, Dab1
Introduction
The function of the cerebral cortex requires the coordinated activity of the two main classes of cortical neurons, glutamatergic projection neurons and GABA containing (GABAergic) interneurons. Recent studies have demonstrated that cortical projection neurons and interneurons follow mostly different developmental programs. In short, projection neurons originate throughout the ventricular zone of the pallium and migrate radially to form the developing cortex. Interneurons, in contrast, originate in the ventricular zone of the subpallium and migrate tangentially to the pallium (Corbin et al., 2001; Marín and Rubenstein, 2001). After reaching the pallium, migrating interneurons invade the cortical plate by changing their mode of migration from tangential to radial (Tanaka et al., 2003; López-Bendito et al., 2004).
Cell layers within the cortical plate (future cortical layers II–VI) are established according to an inside-out pattern (Angevine and Sidman, 1961; Rakic, 1974; Takahashi et al., 1999). Accordingly, projection neurons born simultaneously migrate and stop migrating roughly at the same time; thus, they all occupy the same cortical layer. Remarkably, GABAergic interneurons tend to adopt the same cortical layer as synchronically generated projection neurons (Miller, 1985; Fairén et al., 1986; Nery et al., 2002; Valcanis and Tan, 2003), although interneurons must migrate through much greater distances than projection neurons and thus require additional time to reach the pallium. In contrast to the expanding literature on the tangential migration of cortical interneurons (for review, see Flames and Marín, 2005; Métin et al., 2006), our knowledge of the mechanisms controlling the acquisition of their laminar position is still very limited.
The analysis of cortical lamination has been facilitated by animal models such as the mutant mouse reeler, in which disruption of the Reelin gene leads to severe layer disorganization in all cortical structures (Caviness, 1982). The function of Reelin, an extracellular protein expressed by Cajal-Retzius cells (D'Arcangelo et al., 1995; Ogawa et al., 1995; Soriano and Del Rio, 2005), is mediated by a signaling cascade that includes two receptors, the very low-density lipoprotein receptor (VLDLR) and the apolipoprotein receptor 2 (ApoER2 or Lrp8), as well as the intracellular adaptor protein Dab1 (D'Arcangelo et al., 1999; Hiesberger et al., 1999; Howell et al., 1999, 2000). Mice lacking Dab1 or both VLDLR and Lrp8 have lamination defects that are indistinguishable from those found in reeler, suggesting that they all participate in the same genetic pathway controlling laminar positioning (Howell et al., 1997; Rice and Curran, 1999; Trommsdorff et al., 1999), with Dab1 acting as a cell-autonomous requirement for Reelin signaling (Hammond et al., 2001; Sanada et al., 2004; Olson et al., 2006).
The differential origin of cortical projection neurons and interneurons has raised important questions regarding the mechanism used for their assembly in the cerebral cortex. It has been proposed that projection neurons may wait for the arrival of their interneuron counterparts to simultaneously invade the cortical plate, a coherent mechanism to explain how synchronically born projection neurons and interneurons end up in the same layer (Kriegstein and Noctor, 2004). Implicit to this model is the idea that both types of neurons rely on Reelin signaling to adopt their laminar position, although the role of this pathway in the migration of cortical interneurons remains to be fully elucidated.
Here, we investigated the function of Reelin signaling in controlling the migration of cortical GABAergic interneurons. Our results indicate that Reelin is neither required for tangential migration of cortical interneurons from the subpallium to the pallium, nor is it directly implicated in their laminar position, suggesting instead that projection neurons may contribute to the final arrangement of interneurons in the cerebral cortex.
Materials and Methods
Animals.
Wild-type, green fluorescent protein (GFP)-expressing transgenic mice (Hadjantonakis et al., 1998), Dlx5/6Cre-IRES-EGFP transgenic mice (Stenman et al., 2003), and Dab1 mutant mice (Howell et al., 1997), maintained in a CD1 background, were used in this study. The day of vaginal plug was considered embryonic day 0.5 (E0.5). Animals were kept at the Instituto de Neurociencias de Alicante under Spanish and European Union regulation.
Histology.
Mice were anesthetized with an overdose of sodium pentobarbital and transcardially perfused with 4% paraformaldehyde (PFA). Postnatal brains were removed, fixed for 3 h at 4°C, and cryoprotected in 30% sucrose in PBS. Brains were then cut frozen transversally on a sliding microtome at 40 μm and stored at −20°C in ethylene glycol until used. Embryonic brains were fixed overnight in 4% PFA, embedded in Tissue-Tek OCT compound (Sakura Finetek Europe, Zoeterwoude, The Netherlands), and sectioned at 20 μm in the cryostat. The following antibodies were used: chicken anti-GFP (1:1000; Aves Labs, Tigard, OR), rabbit anti-GFP (1:2000; Invitrogen, Eugene, OR), rabbit anti-GABA (1:2000; Sigma, St. Louis, MO), rat anti-bromodeoxyuridine (BrdU; 1:100; Accurate Chemical, Westbury, New York), rabbit anti-calbindin (1:5000; Swant, Bellinzona, Switzerland), rat anti-calretinin (1:3000; Swant), rabbit anti-nitric oxide synthase (NOS; 1:1000; ImmunoStar, Hudson, WI), rabbit anti-parvalbumin (1:5000; Swant), rabbit anti-Lhx6 (1:100; kindly provided by V. Pachnis, National Institute for Medical Research, London, UK), rabbit anti-Dab1 (1:750; Chemicon, Temecula, CA). The following secondary antibodies were used: goat anti-rabbit 488, goat anti-rat 546, goat anti-rabbit biotin, donkey anti-rabbit 594, rabbit anti-chicken 488 (all from Invitrogen), and cyanine 3-conjugated donkey anti-rat (Jackson ImmunoResearch, West Grove, PA). For BrdU double staining, sections were first processed for the GABA, Lhx6, or GFP immunohistochemistry, fixed in 4% PFA for 50 min, and then processed for BrdU staining.
For in situ hybridization, E13.5 brains were fixed overnight in 4% PFA, cryoprotected in 30% sucrose, embedded in OCT compound and stored frozen at −80°C. Twenty-micrometer coronal cryostat sections were hybridized as described previously (Flames et al., 2004). Images were obtained using a cooled-CCD camera (DC500; Leica, Nussloch, Germany).
Primary cultures.
The cortices of six E17.5 Dlx5/6Cre-IRES-EGFP embryos were dissected and incubated with Tripsine-EDTA for 20 min at 37°C. Cells were then mechanically dissociated in L-15 medium containing DNase I and centrifuged (5 min, 1000 rpm). Cells were resuspended with Neurobasal medium and counted in a Neubauer chamber. A total of 105 cells were plated on poly-lysine-coated glass coverslips. After 24 h, cells were fixed in 4% PFA for 30 min and processed for GFP and Dab1 immunohistochemistry.
Donor cells.
The medial ganglionic eminence (MGE) from 8 to 13 E12.5 or E15.5 wild-type, Dab1−/−, or GFP-expressing embryos were dissected under a stereomicroscope. Explants were washed in 0.5 ml of L-15 medium (Invitrogen) containing DNase I (100 μg/ml), and cells were mechanically dissociated by repeated pipetting (20–30 times) through a 200 μl plastic pipette tip. Dissociated cells were then pelleted by centrifugation (5 min, 1000 rpm), resuspended in 6 μl of L-15 medium with DNase I, and kept on ice until injection. All donor pregnant females were injected 12 h before dissection with BrdU (40 mg/kg). Dab1−/− embryos were genotyped as described previously (Howell et al., 1997).
In utero transplantation.
High-density cell suspensions (∼25,000 cells/μl) were front loaded into beveled glass micropipettes (∼50 μm diameter) prefilled with mineral oil and mounted in a pressure microinjector (VisualSonics, Toronto, Ontario, Canada). Recipient pregnant females (E12.5 or E15.5) were anesthetized with sodium pentobarbital (0.625 mg/10 g, i.p.), their uterine horns exposed, and mounted under an ultrasound microscope (VisualSonics). The tip of the micropipette was inserted into the MGE under real-time ultrasound guidance and 36–54 nl of cell suspension was injected. The position of the embryo and the path of the micropipette insertion were recorded for each embryo at the time of the injection. Embryos in which we detected leakage to the lateral ventricle were excluded from the analysis. Nearly 80% of the injected embryos were born and survived, of which only approximately half contained grafted cells. This is likely attributable to the uneven distribution of cells within the micropipette. Three to four successfully transplanted animals were analyzed for each experimental condition.
Quantification.
For the analysis of GABA/BrdU+ cells, images obtained in a confocal microscope (Leica DM-R/TCS-SL) were coupled using Canvas X (ACD Systems, Miami, FL) software. The number of double-positive cells was counted from a 375-μm-wide profile of the lateral ventricular wall in the somatosensory cortex at two different bregma levels (0.02, −0.46) from four different brains. The cortex (layers 1–6) was analyzed with a grid of 10 equal horizontal bins. In controls, bin 1 roughly corresponds to the marginal zone, and bin 10 corresponds to the lower end of layer 6. For the quantification of GFP/BrdU+ and BrdU+ neurons in transplanted animals, all cells located in motor, somatosensory, and visual cortices from bregma levels +1.42 to −3.40 were plotted and assigned to different layers of the cortex using photomontages with fluorescence nuclear staining as a cytoarchitectonic reference. For the quantification of Lhx6/BrdU+ and BrdU+ cells in E16.5 and neonate mice, confocal images coupled using Canvas software were analyzed. Cells were counted from a 375-μm-wide profile of the dorsal cortex at intermediate rostrocaudal levels from four different animals.
To examine differences across populations, data were statistically analyzed using χ2 tests. For those distributions of cells in which statistical differences were found for the whole population, each category (layers or bins, depending on the experiment) was then independently analyzed using two by two contingency tables to assign statistical differences to specific categories.
Results
Layer acquisition by cortical interneurons is altered in the absence of Reelin signaling
The function of Reelin on cortical neuronal migration has been classically studied with a unitary perspective, i.e., mutations affecting Reelin signaling would affect equally both projection neurons and interneurons (Rice and Curran, 2001). The discovery that cortical projection neurons and interneurons of the cerebral cortex follow different developmental programs (Anderson et al., 1997), however, has provided a novel framework to understand the role of Reelin on cortical neuronal migration. In this new context, we examined the laminar distribution of GABAergic neurons in the cortex of control and Dab1 mutant mice at postnatal day 14 (P14) (Fig. 1). To identify different cohorts of GABAergic neurons, we performed BrdU birth dating at two embryonic days, E12 and E15. In control mice, GABAergic neurons born at E12 were found in deep layers of the P14 cortex, whereas those born at E15 preferentially occupied superficial layers (n = 3) (Fig. 1A,C,E,F). In contrast, the laminar distribution of GABAergic neurons was abnormal in Dab1 mutant mice. Thus, GABAergic neurons born at E12 were found preferentially in superficial layers of the P14 cortex, whereas interneurons born at E15 preferentially occupied deep layers (n = 3) (Fig. 1B,D,E,F). These results essentially recapitulate those reported for reeler mice (Hevner et al., 2004; Yabut et al., 2006) and are not attributable to differences in the total number of GABAergic interneurons found in the cortex of control and Dab1 mutant mice [E12 interneurons: control, 24.30 ± 3.77 cells/mm2 (average ± SEM); mutant, 19.62 ± 2.94, p = 0.543, n = 3; for E15 interneurons: control, 42.09 ± 5.56; mutant, 44.87 ± 8.35, p = 0.629, n = 3].
Figure 1.
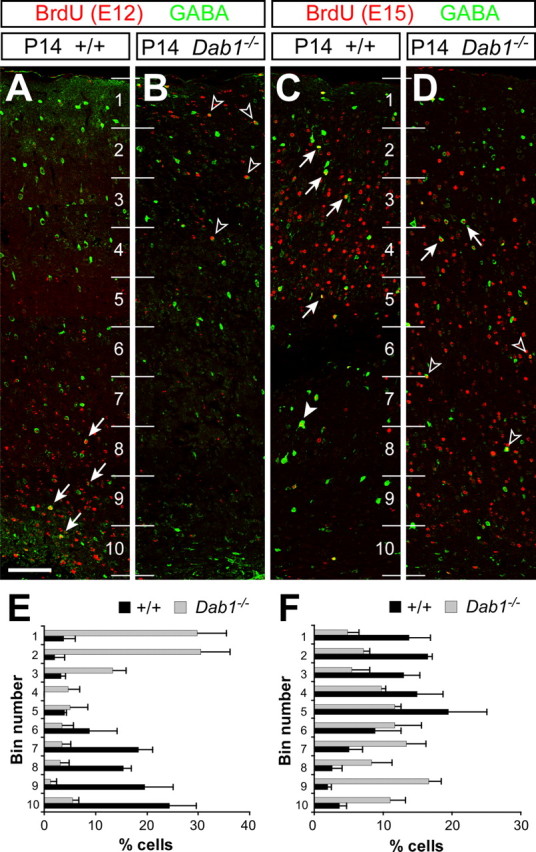
The laminar position of cortical interneurons is inverted in Dab1 mutant mice. A–D, Confocal microscopic reconstructions of the cerebral cortex of wild-type (A and C) and Dab1 mutant (B and D) mice stained for BrdU (red) and GABA (green) after injection of BrdU at E12 (A and B) or E15 (C and D). A, B, Early-born cortical interneurons (BrdU+/GABA+) acquire deep cortical positions in control mice (arrows), whereas they occupy superficial positions in Dab1 mutants (open arrowheads). C, D, Late-born interneurons (BrdU+/GABA+) populate mostly superficial layers (arrows) in wild-type mice, although a few also occupy deep layers (solid arrowhead). In Dab1 mutants, the majority of late-born interneurons occupy deep positions (open arrowheads), although some populate superficial layers (arrows). Numbers identify bins for quantification. E, F, Binned quantifications of the distribution of E12 (E) and E15 (F) interneurons in the cerebral cortex of wild-type (black bars) and Dab1 mutant (gray bars) mice (average + SEM). The width of the cortex proper (layers 1–6) was divided into 10 equal bins for laminar distribution analysis, with bin 1 at the top (marginal zone) and bin 10 at the bottom (white matter). Scale bar, 100 μm.
Analysis of specific neurochemical markers also revealed a disorganization of the laminar distribution of distinct interneuron populations. For example, GABAergic interneurons that express the enzyme NOS were preferentially found in deep layers of the somatosensory cortex at P14 in control mice but were frequently present in superficial layers in Dab1 mutant mice (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). Conversely, calretinin-expressing interneurons, which were most commonly found in superficial layers of the cortex in control mice, were abundantly distributed through deep layers of the cortex in Dab1 mutant mice (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). Moreover, reeler mice exhibited a phenotype identical to that of Dab1 mutants (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). Together, these results demonstrate that the laminar arrangement of GABAergic interneurons is severely impaired in the postnatal cortex of mouse mutants in which Reelin signaling is disrupted.
Loss of Reelin signaling does not impair tangential migration of cortical interneurons
The proper allocation of GABAergic interneurons in the cortex requires two different phases of migration. First, interneurons migrate tangentially from the subpallium to the pallium. Subsequently, interneurons migrate radially from the marginal zone or the subventricular zone into the cortical plate, in which they finally adopt a defined laminar position. We therefore reasoned that the atypical location of interneurons in the cortex of reeler and Dab1 mutants could be a direct consequence of abnormal tangential migration of these cells from the subpallium to the pallium. To test this hypothesis, we analyzed the pattern of interneuron tangential migration during embryonic stages in reeler and Dab1 mutants. Analysis of the expression of multiple markers on tangentially migrating interneurons in the embryonic telencephalon, such as Lhx6, Dlx2, and Gad67, did not reveal any prominent differences, both in number and routes of migration, between controls and Dab1 mutants (n = 3) (Fig. S2, available at www.jneurosci.org as supplemental material, and data not shown). Identical results were obtained when comparing control and reeler mutant embryos (supplemental Fig. S3, available at www.jneurosci.org as supplemental material, and data not shown). In conclusion, Reelin signaling does not seem to influence the tangential migration of interneurons from the subpallium to the cortex.
Dab1 is expressed by GABAergic interneurons in the cortex
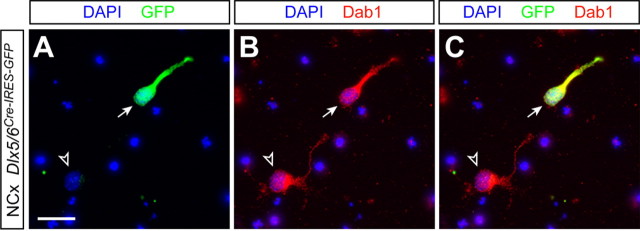
Because GABAergic interneurons reach the cortex in the absence of Reelin signaling, we reasoned that the abnormal lamination of cortical interneurons observed in reeler and Dab1 mutants was likely caused by a defect in their radial translocation into the cortical plate. High levels of Dab1, VLDLR, and ApoER2 expression have been reported in the embryonic cortex as migrating neurons invade the cortical plate (Rice et al., 1998; D'Arcangelo et al., 1999; Hiesberger et al., 1999), consistent with the proposed role of Reelin/Dab1 signaling in radial migration. However, it is not known whether GABAergic interneurons also express Dab1 at the stages at which they begin to enter the cortical plate. To answer this question, we took advantage of a strain of transgenic mice in which a bicistronic cassette containing CRE recombinase and GFP is expressed under the control of a Dlx5/6 enhancer element (Stenman et al., 2003), which marks virtually every GABAergic interneuron in the mouse cerebral cortex (Stühmer et al., 2002). Immunohistochemical analysis of dissociated cells obtained from the cortex of Dlx5/6Cre-IRES-EGFP embryos at E17.5, an age at which large numbers of GABAergic interneurons invade the cortical plate (Tanaka et al., 2003; López-Bendito et al., 2004), revealed that every GFP-positive (i.e., GABAergic) cell in the cortex at this stage expressed Dab1 (100% of 59 GFP+ counted cells) (Fig. 2). As expected, the vast majority of cells obtained from the E17.5 cortex that expressed Dab1 did not stain for GFP (91.29% of 678 Dab1+ counted cells), reflecting the fact that projection neurons essentially outnumber GABAergic interneurons in the cortex. Thus, cortical interneurons express Dab1 as laminar formation proceeds, thereby reinforcing the view that they may respond to Reelin during this process.
Figure 2.
Expression of Dab1 in cortical interneurons during corticogenesis. Images of dissociated cultures obtained from E17.5 cortices of Dlx5/6Cre-IRES-GFP embryos showing nuclear staining [4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI)] and immunohistochemistry for GFP (A and C) and Dab1 (B and C) are shown. Scale bar, 20 μm.
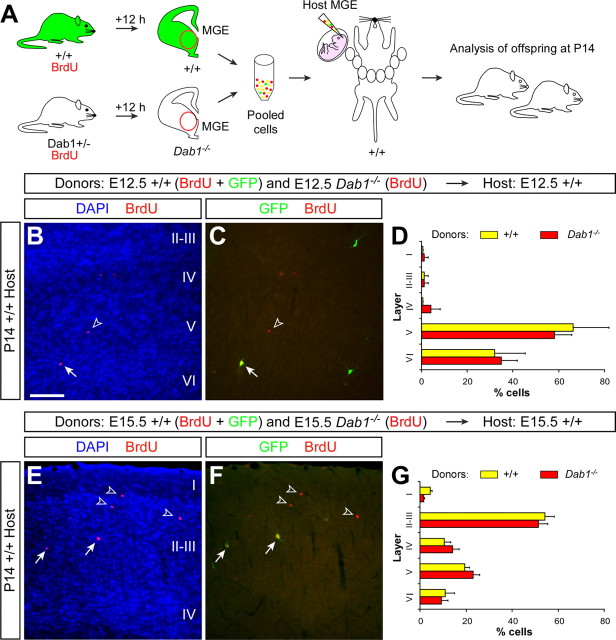
Transplantation of MGE-derived cells in utero recapitulates the acquisition of laminar positions in vivo
Over the past few years, the analysis of interneuron tangential migration has greatly benefited from the development of an organotypic slice preparation that roughly recapitulates, both in time and space, the movement of cortical interneurons from the basal telencephalon to the cortex (Anderson et al., 1997). The analysis of layer acquisition by cortical interneurons is, however, unfeasible using in vitro techniques, primarily because it occurs through a much longer period of time. To overcome this problem, we used a recently developed ultrasound-guided microtransplantation technique (Olsson et al., 1997), which allows long-term in vivo fate-mapping analysis of transplanted cells.
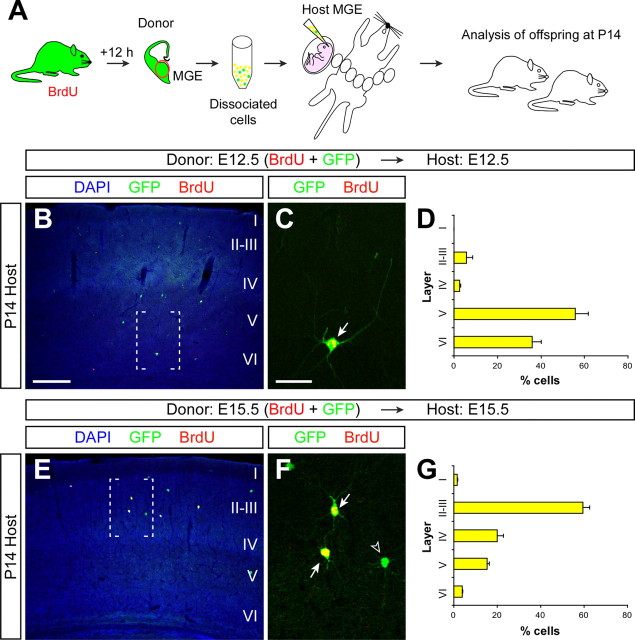
To validate this method for the analysis of layer acquisition by cortical interneurons, we performed a series of experiments intended to replicate previous birth-dating studies on GABAergic interneurons. GABAergic interneurons tend to adopt the same cortical layer as projection neurons born roughly at the same time, i.e., early-born interneurons (e.g., E12) tend to occupy deep, infragranular layers of the cortex, whereas late-born interneurons (e.g., E15) have a preference for superficial, supragranular layers (Miller, 1985; Fairén et al., 1986; Peduzzi, 1988). In this context, we performed homotopic and isochronic transplants of cells derived from the MGE of E12.5 or E15.5 GFP-expressing embryos and analyzed the final distribution of transplanted cells in the neocortex of P14 host animals (Fig. 3A). We chose the MGE for our transplantation assays because it is the primary source of cortical GABAergic interneurons in the basal telencephalon (Lavdas et al., 1999; Sussel et al., 1999; Wichterle et al., 1999, 2001; Butt et al., 2005). Donor pregnant females were BrdU injected 12 h before dissection of the MGE to ensure that the analysis was performed only on transplanted cells that divided last in the donor environment (Fig. 3A). Remarkably, GFP-expressing MGE-derived cells labeled with BrdU at E12 and transplanted at E12.5 into the MGE of E12.5 host embryos were found to occupy deep layers of the cortex in P14 host animals (n = 3, a total of 159 counted cells) (Fig. 3B–D and supplemental Fig. S4, available at www.jneurosci.org as supplemental material), whereas GFP-expressing MGE-derived cells labeled with BrdU at E15 and transplanted at E15.5 into the MGE of E15.5 host embryos consistently occupied upper layers of the cortex (n = 4, a total of 949 counted cells) (Fig. 3E–G and supplemental Fig. S4, available at www.jneurosci.org as supplemental material). Thus, our experiments showed that in utero homotopic transplantation of MGE-derived cells using ultrasound-guided transplantation faithfully recapitulates the acquisition of laminar fates by cortical GABAergic interneurons in vivo, validating this approach for the analysis of the mechanisms controlling this process.
Figure 3.
Cortical interneurons transplanted homotypically and isochronically in utero retain their program for laminar specificity. A, Schematic diagram of the experimental design. GFP+ donor pregnant mice received a single injection of BrdU, and 12 h later the MGE was dissected from their embryos and dissociated. GFP+ donor MGE cells [also BrdU+ (yellow), or BrdU− (green)] were then injected into the MGE of age-matched host embryos. Host embryos were allowed to be born and analyzed at P14. B, E, Coronal sections through the somatosensory cortex of P14 mice showing the distribution of E12.5 (B) and E15.5 (E) GFP+ MGE-derived cells after nuclear staining [4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI)] and immunohistochemistry for BrdU (red) and GFP (green). C, F, Magnifications of the areas boxed in B and E, respectively. Arrows show BrdU+/GFP+ cells; the open arrowhead indicates an example of BrdU−/GFP+ cell not included in the analysis. D, G, Quantification of the distribution of isochronically transplanted E12.5 (D) and E15.5 (G) interneurons in the P14 cortex (average + SEM). Scale bars: (in B) B, E, 300 μm; (in C) C, F, 50 μm.
Layer acquisition of most cortical interneurons is determined at birth
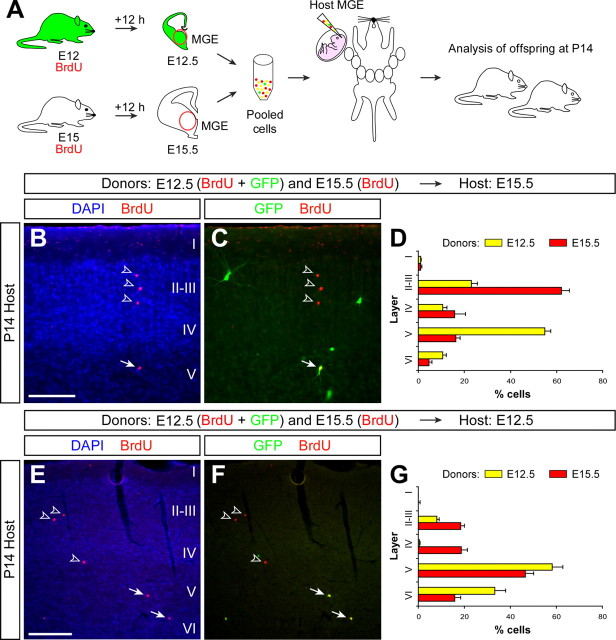
According to the current view on Reelin function during cortical lamination, cohorts of newly generated neurons migrate radially through the developing intermediate zone and cortical plate until they reach the maximum concentration of Reelin, just below the marginal zone, at which they stop and differentiate. To test this hypothesis in relation to the development of cortical interneurons, we designed experiments in which two different populations of MGE-derived cells were simultaneously transplanted into a common host environment (Fig. 4A). In a first series of experiments, we simultaneously transplanted GFP-expressing MGE-derived cells labeled with BrdU at E12 and MGE-derived cells labeled with BrdU at E15 into the MGE of E15.5 host embryos (Fig. 4A). As expected from the initial isochronic transplants, MGE-derived cells labeled with BrdU at E15 were found to primarily occupy upper layers of the cortex in P14 host animals (n = 4, a total of 1289 counted cells) (Fig. 4B–D). Remarkably, GFP-expressing MGE-derived cells labeled with BrdU at E12 were found to occupy deep layers of the cortex despite having been transplanted into an environment older than that in which they were born (Fig. 4B–D). This suggests that most early-born interneurons are able to retain their laminar fate when transplanted into an older environment. Nevertheless, >20% of the early-born interneurons were found in upper cortical layers (layers II–III) (Fig. 4D), suggesting that at least some interneurons changed their laminar fate and accommodated to the new environment in which they were transplanted despite becoming postmitotic in a younger environment.
Figure 4.
Cortical interneurons adopt multiple laminar fates after heterochronic transplantation. A, Schematic diagram of the experimental design. GFP+ donor pregnant mice received a single injection of BrdU at E12, and wild-type donor pregnant mice received a single injection of BrdU at E15. Twelve hours after BrdU injection, the MGE was dissected from embryos and dissociated. Pooled donor MGE cells were then injected into the MGE of either E12.5 or E15.5 host embryos. Host embryos were allowed to be born and analyzed at P14. B, C, Coronal section through the somatosensory cortex of a transplanted P14 mouse showing the distribution of E12.5 (BrdU+/GFP+) and E15.5 (BrdU+/GFP−) MGE-derived cells after nuclear staining [4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI)] and immunohistochemistry for BrdU (red) and GFP (green). Arrows point to a BrdU+/GFP+ cell; open arrowheads indicate BrdU+/GFP− cells. D, Quantification of the distribution of heterochronically transplanted E12.5 (yellow) and isochronically transplanted E15.5 (red) interneurons in the P14 cortex (average + SEM). E, F, Coronal section through the somatosensory cortex of a transplanted P14 mouse showing the distribution of E12.5 (BrdU+/GFP+) and E15.5 (BrdU+/GFP−) MGE-derived cells after nuclear staining (DAPI) and immunohistochemistry for BrdU (red) and GFP (green). Arrows point to BrdU+/GFP+ cells; open arrowheads indicate BrdU+/GFP− cells. G, Quantification of the distribution of isochronically transplanted E12.5 (yellow) and heterochronically transplanted E15.5 (red) interneurons in the P14 cortex (average + SEM). Scale bars: B, C, 100 μm; E, F, 200 μm.
In a second series of experiments, GFP-expressing MGE-derived cells labeled with BrdU at E12 and MGE-derived cells labeled with BrdU at E15 were now simultaneously transplanted into the MGE of E12.5 host embryos (Fig. 4A). As expected from the isochronic transplants, GFP-expressing MGE-derived cells labeled with BrdU at E12 were found to occupy deep layers of the cortex in P14 host animals (n = 4, a total of 421 counted cells) (Fig. 4E–G). In the case of late-born interneurons (E15), ∼40% of the cells maintained their laminar fate and occupied upper layers of the cortex (Fig. 4E–G). However, a large percentage of MGE-derived cells labeled with BrdU at E15 were no longer committed to upper layers of the cortex and instead were located in deep layers (layers V–VI) (Fig. 4G). This shift in the distribution of cells born at E15 indicates that, after transplantation in a younger environment, many late-born interneurons have changed their laminar fates and behaved like early-born neurons. In summary, most interneurons tend to maintain the laminar fate they acquire at the time of birth, especially early-born interneurons, but a proportion is also susceptible to signals found in the host environment.
Cortical interneurons born at different times can simultaneously invade the cortical plate
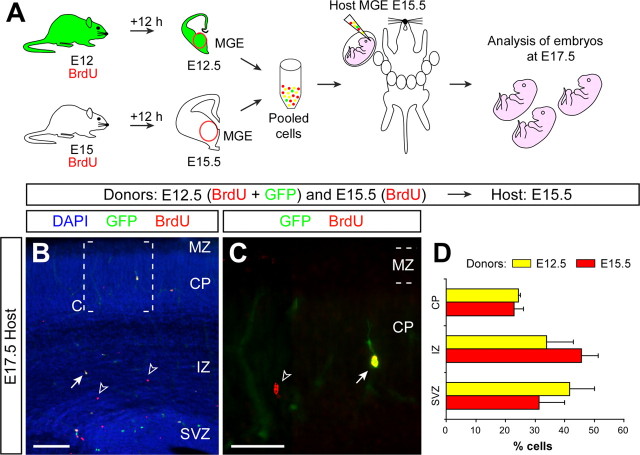
The previous experiments demonstrate that simultaneously transplanted populations of interneurons born at different stages can, at least to a large extent, segregate into different layers of the postnatal cortex (Fig. 4D). One important conclusion that can be inferred from this observation is that these results are mostly incompatible with the classical model in which Reelin signaling dictates the laminar position of cortical neurons. Thus, either the different cohorts of interneurons respond differently to the same concentration of Reelin, or this signaling system does not directly contribute to the lamination of cortical interneurons. One alternative possibility would be that, despite being simultaneously transplanted, both populations may reach the cortical plate at different times and therefore may still respond to Reelin according to the common rule, i.e., they migrate until they reach the maximum concentration of Reelin. To evaluate this possibility, we performed another series of mixed transplants. As before, we transplanted GFP-expressing MGE-derived cells labeled with BrdU at E12 and MGE-derived cells labeled with BrdU at E15 into the MGE of E15.5 host embryos. In this case, however, the distribution of early- and late-born interneurons was evaluated 2 d after transplantation (Fig. 5A). At this early stage of migration, a large number of neurons were still confined to the cortical subventricular zone, through which most interneurons migrate tangentially toward the cortex (Fig. 5B,D). Nevertheless, many transplanted neurons were also found in the intermediate zone of the cortex or even in the developing cortical plate (Fig. 5B–D). Remarkably, both early- and late-born cells were present in the different layers at equivalent proportions (n = 3, a total of 233 counted cells, p = 0.2, χ2 test) (Fig. 5D), demonstrating that interneurons born at different dates and transplanted simultaneously can initiate radial migration and invade the cortical plate at the same time.
Figure 5.
Interneurons born at different times of development invade the cortex synchronically after pooled transplantation. A, Schematic diagram of the experimental design. GFP+ donor pregnant mice received a single injection of BrdU at E12, and wild-type donor pregnant mice received a single injection of BrdU at E15. Twelve hours after BrdU injection, the MGE was dissected from embryos and dissociated. Pooled donor MGE cells were then injected into the MGE of E15.5 host embryos. Host embryos were analyzed at E17.5. B, Coronal sections through the somatosensory cortex of a transplanted E17.5 embryo showing the distribution of E12.5 (BrdU+/GFP+) and E15.5 (BrdU+/GFP−) MGE-derived cells after nuclear staining [4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI)] and immunohistochemistry for BrdU (red) and GFP (green). C, Magnification of the area boxed in B. The arrow points to a BrdU+/GFP+ cell; open arrowheads indicate BrdU+/GFP− cells. D, Quantification of the distribution of heterochronically transplanted E12.5 (yellow) and isochronically transplanted E15.5 (red) interneurons in the E17.5 cortex (average + SEM). CP, Cortical plate; IZ, intermediate zone; MZ, marginal zone; SVZ, subventricular zone. Scale bars: B, 100 μm; C, 50 μm.
Dab1-deficient cortical interneurons adopt a normal laminar fate in a wild-type environment
The previous experiments are in agreement with the hypothesis that interneurons may find their laminar position in response to cues other than Reelin. To unequivocally test this hypothesis, we simultaneously transplanted GFP-expressing MGE-derived cells labeled with BrdU at E12 and Dab1−/− MGE-derived cells labeled with BrdU at E12 into the MGE of E12.5 wild-type embryos (Fig. 6A). We reasoned that because Dab1 mutant MGE-derived cells could not respond to Reelin, their distribution in a wild-type cortex would reveal whether interneurons directly required this signaling system to acquire their laminar position. As expected from our previous experiments, wild-type transplanted cells (GFP-expressing MGE-derived cells labeled with BrdU at E12, the internal control) were mostly confined to deep layers of the P14 cortex (n = 2, a total of 177 counted cells) (Fig. 6B–D). Remarkably, Dab1−/− MGE-derived cells displayed an equivalent distribution in the postnatal cortex (n = 2, a total of 59 counted cells, p = 0.3, χ2 test) (Fig. 6B–D).
Figure 6.
The laminar distribution of cortical interneurons is independent of Reelin signaling. A, Schematic diagram of the experimental design. GFP+ and Dab1+/− donor pregnant mice received a single injection of BrdU at E12 or E15. Twelve hours after BrdU injection, the MGE of Dab1−/− embryos was collected and dissociated and pooled together with cells obtained from the MGE of GFP+ embryos. Pooled donor MGE cells were then injected into the MGE of either E12.5 or E15.5 wild-type host embryos. Host embryos were allowed to be born and analyzed at P14. B, C, Coronal section through the somatosensory cortex of a transplanted P14 mouse showing the distribution of E12.5 wild-type (BrdU+/GFP+) and E12.5 Dab1−/− (BrdU+/GFP−) MGE-derived interneurons after nuclear staining [4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI)] and immunohistochemistry for BrdU (red) and GFP (green). Arrows point to a wild-type (BrdU+/GFP+) cell; open arrowheads indicate a Dab1−/− (BrdU+/GFP−) cell. D, Quantification of the distribution of wild-type E12.5 (yellow) and Dab1−/− E12.5 (red) interneurons in the P14 cortex (average + SEM). E, F, Coronal section through the somatosensory cortex of a transplanted P14 mouse showing the distribution of E15.5 wild-type (BrdU+/GFP+) and E15.5 Dab1−/− (BrdU+/GFP−) MGE-derived interneurons after nuclear staining (DAPI) and immunohistochemistry for BrdU (red) and GFP (green). Arrows point to wild-type (BrdU+/GFP+) cells; open arrowheads indicate Dab1−/− (BrdU+/GFP−) cells. G, Quantification of the distribution of wild-type E15.5 (yellow) and Dab1−/− E15.5 (red) interneurons in the P14 cortex (average + SEM). Scale bar, 200 μm.
The ability of Dab1 mutant interneurons to adopt a normal laminar fate in a wild-type environment was not exclusive of early-born cells, because similar results were obtained in equivalent experiments in which we simultaneously transplanted GFP-expressing MGE-derived cells labeled with BrdU at E15 and Dab1−/− MGE-derived cells labeled with BrdU at E15. As expected, wild-type cells were found primarily in upper layers of the P14 cortex, with a smaller fraction spread through deeper layers (n = 3, a total of 251 counted cells) (Fig. 6E–G), and Dab1−/− MGE-derived cells displayed an equivalent distribution in the postnatal cortex (n = 3, a total of 428 counted cells, p = 0.1, χ2 test) (Fig. 6E–G). These experiments demonstrate that interneurons do not cell-autonomously require Reelin signaling to adopt their proper laminar position in the developing cortex.
Projection neurons invade the cortical plate before synchronically generated interneurons
The results of the previous experiments are incompatible with a model in which Reelin plays a direct role in the laminar positioning of cortical interneurons, and yet cortical GABAergic interneurons are abnormally located in the postnatal cortex of mice lacking Reelin signaling (Fig. 1 and supplemental Fig. S1, available at www.jneurosci.org as supplemental material) (Hevner et al., 2004). An alternative possibility is that interneurons may respond to information provided by cortical projection neurons as these settle in the developing cortical plate. We reasoned that if interneurons were to follow information presented in the cortical plate by their glutamatergic counterparts, it would be necessary that they invade the corresponding layer of the cortex after their companion projection neurons were already in place. To test this hypothesis, we performed experiments aimed to reveal the relative distribution of isochronically generated projection neurons and interneurons a few days after their birth. In a first series of experiments, we injected pregnant females with BrdU at E12 and killed their progeny at E16.5. Double-labeling immunohistochemistry for BrdU and Lhx6, a marker of cortical interneurons derived from the MGE (Lavdas et al., 1999), revealed that the distribution of projection neurons (BrdU+/Lhx6−) and MGE-derived interneurons (BrdU+/Lhx6+) in the cortex at birth was significantly different (n = 8, a total of 3233 counted cells, p < 0.001, χ2 test) (Fig. 7A–H). At this stage, most BrdU+/Lhx6− cells were located in the cortical plate (Fig. 7A,D,F). Remarkably, very few BrdU+/Lhx6+ cells were found within the cortical plate at the same stage (Fig. 7D,F). Instead, most BrdU+/Lhx6+ cells resided in the marginal zone or, to a minor extent, in the cortical subventricular zone (Fig. 7B,C,E,F). In a second series of experiments, we injected pregnant females with BrdU at E15 and killed their progeny at P0. Double-labeling immunohistochemistry for BrdU and Lhx6 revealed that the distribution of projection neurons (BrdU+/Lhx6−) and MGE-derived interneurons (BrdU+/Lhx6+) in the cortex at birth was also significantly different (n = 4, a total of 953 counted cells, p < 0.001, χ2 test) (Fig. 7G–L). At this stage, most BrdU+/Lhx6− cells were located in the upper cortical plate (Fig. 7G,J,L). In contrast, very few BrdU+/Lhx6+ cells born at E15 were found within the cortical plate at P0 (Fig. 7J,L). Instead, most BrdU+/Lhx6+ cells resided in the marginal zone or the cortical subventricular zone (Fig. 7H,I,K,L). Because synchronically generated projection neurons and interneurons end up occupying the same layer in the cortex (Miller, 1985; Fairén et al., 1986), these experiments demonstrate that MGE-derived GABAergic interneurons invade their corresponding layer in the cortex well after their glutamatergic counterparts are already in their final position.
Figure 7.
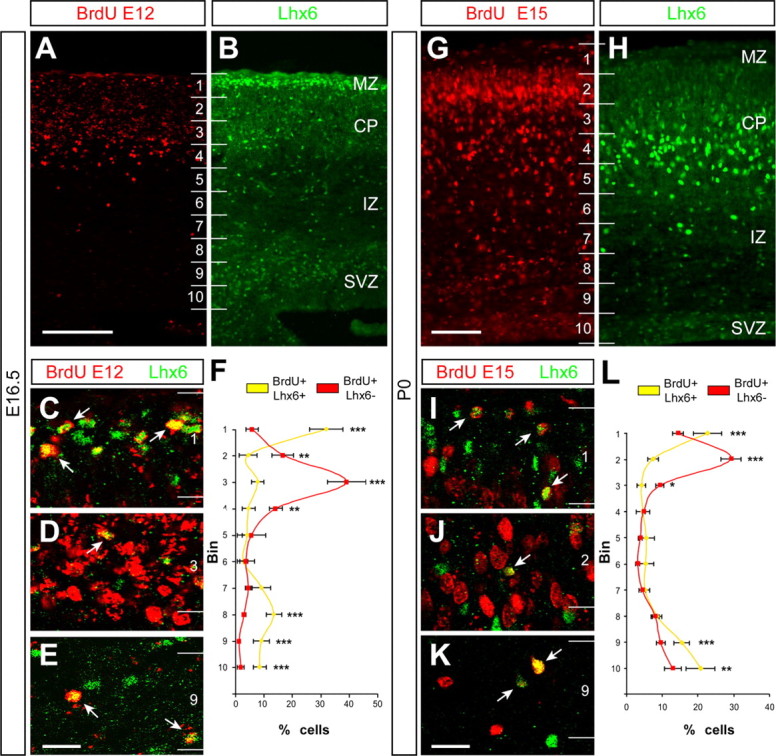
The timing of cortical plate invasion is different for interneurons than for projection neurons. A, B, Coronal sections through the somatosensory cortex of an E16.5 mouse embryo that received a single BrdU injection at E12, showing immunohistochemistry for BrdU (A) and Lhx6 (B). The majority of BrdU+ cells occupy the cortical plate (CP), although labeled cells are scattered through all layers. In turn, Lhx6 staining is more abundant in the marginal zone (MZ), cortical plate, and subventricular zone (SVZ). Numbers indicate bins for quantification. C–E, Confocal microscopic images of the marginal zone (MZ) (C), the CP (D), and the SVZ (E) showing BrdU (red) and Lhx6 (green) staining at E16.5. The large majority of BrdU+/Lhx6+ cells (arrows) were found in the MZ (C) and SVZ (E). Only a small fraction of BrdU+/Lhx6+ cells were located in the CP. F, Quantification of the relative distribution of BrdU+/Lhx6+ cells (yellow) and BrdU+/Lhx6− cells in the E16.5 cortex (average + SEM). ∗∗p < 0.01, ∗∗∗p < 0.001 (χ2 test). G, H, Coronal section through the somatosensory cortex of a P0 mouse that received a single BrdU injection at E15, showing immunohistochemistry for BrdU (G) and Lhx6 (H). Although the majority of BrdU+ cells occupy the top portion of the CP, labeled cells are scattered through all layers. Lhx6 staining is also present in all layers, but high levels of staining predominate in the bottom portion of the CP. I–K, Confocal microscopic images of the MZ (I), the CP (J), and the SVZ (K) showing BrdU (red) and Lhx6 (green) staining at P0. The large majority of BrdU+/Lhx6+ cells (arrows) were found in the MZ (I) and SVZ (K). Only a small fraction of BrdU+/Lhx6+ cells were located in the CP. L, Quantification of the relative distribution of BrdU+/Lhx6+ cells (yellow) and BrdU+/Lhx6− cells in the P0 cortex (average + SEM). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (χ2 test). Scale bars: A, B, 150 μm; G, H, 100 μm; C–E, I–K, 40 μm.
Wild-type cortical interneurons fail to adopt a normal laminar position in the Dab1 mutant cortex
Our previous results showed that interneurons invade the cortical plate well after synchronically generated projection neurons, suggesting that projection neurons may somehow contribute to the final positioning. If this were the case, wild-type interneurons should fail to adopt a normal laminar position when transplanted into a Dab1 mutant environment, because the laminar distribution of projection neurons is severely compromised in these mice. To test this hypothesis, we transplanted GFP-expressing MGE-derived cells labeled with BrdU at E15 into the MGE of E15.5 Dab1−/− embryos (Fig. 8A). In addition, to label the distribution of all neurons born at E15 in the Dab1−/− embryos, host mothers also received an injection of BrdU at E15, 12 h before transplantation (Fig. 8A). As expected from our previous experiments (Fig. 3), wild-type interneurons born on approximately E15.5 (GFP+/BrdU+) and transplanted into E15.5 wild-type host embryos were primarily located in upper layers of the P14 cortex (Fig. 8C,D). In contrast, the distribution of wild-type interneurons born at E15.5 (GFP+/BrdU+) and transplanted into E15.5 Dab1−/− embryos was significantly different from controls (n = 2, a total of 142 counted cells, p < 0.001, χ2 test) (Fig. 8F,G), with cells abnormally distributed throughout the cortex. Thus, wild-type cortical interneurons fail to adopt a normal laminar position in the Dab1 mutant cortex.
Figure 8.
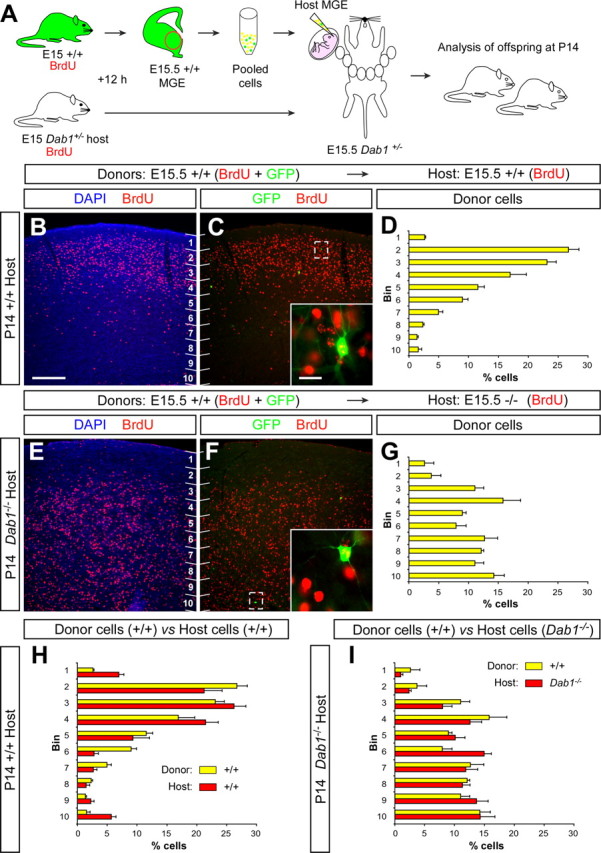
The laminar distribution of cortical interneurons correlates with the allocation of projection neurons. A, Schematic diagram of the experimental design. GFP+ donor and Dab1+/− host pregnant mice received a single injection of BrdU at E15. Twelve hours after BrdU injection, the MGE of GFP+ embryos was collected, dissociated, and then injected into the MGE of E15.5 Dab1+/? and Dab1−/− host embryos. Host embryos were allowed to be born and analyzed at P14. B, C, Coronal section through the somatosensory cortex of a transplanted P14 wild-type mouse showing the distribution of E15.5 wild-type (BrdU+/GFP+, see inset) MGE-derived interneurons and E15.5 host-derived neurons (BrdU+/GFP−) after nuclear staining [4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI)] and immunohistochemistry for BrdU (red) and GFP (green). Numbers indicate bins for quantification. D, Quantification of the distribution of transplanted wild-type E15.5 interneurons in the P14 wild-type cortex (average + SEM). E, F, Coronal section through the somatosensory cortex of a transplanted P14 Dab1−/− mouse showing the distribution of E15.5 wild-type (BrdU+/GFP+, see inset) MGE-derived interneurons and E15.5 host-derived neurons (BrdU+/GFP−) after nuclear staining (DAPI) and immunohistochemistry for BrdU (red) and GFP (green). G, Quantification of the distribution of transplanted wild-type E15.5 interneurons in the P14 Dab1−/− cortex (average + SEM). H, I, Quantification of the relative distribution of transplanted wild-type E15.5 interneurons (yellow) in relation to host (red) wild-type (H) or Dab1−/− (I) E15.5 neurons in the postnatal cortex (average + SEM). Scale bars: 200 μm (B, C, E, F); 20 μm (insets in C, F).
Analysis of the relative distribution of transplanted interneurons in relation to isochronically generated host neurons in both wild-type and Dab1 mutants shed further light on the possible mechanism underlying the laminar distribution of cortical interneurons. As expected from early birth-dating studies (Miller, 1985; Fairén et al., 1986; Peduzzi, 1988), the distribution of E15.5 wild-type interneurons (GFP+/BrdU+) transplanted into the wild-type cortex tends to follow the distribution of E15.5 host neurons (GFP−/BrdU+; mostly projection neurons but also includes host-derived interneurons) (Fig. 8H). Most notably, the distribution of E15.5 wild-type interneurons (GFP+/BrdU+) transplanted into the Dab1 mutant cortex also perfectly matches the distribution of E15.5 Dab1 mutant host neurons (GFP−/BrdU+; p = 0.3, χ2 test) (Fig. 8I), demonstrating that the final allocation of interneurons highly correlates with the distribution of their projection neuron counterparts.
Discussion
The requirement of the Reelin/Dab1 signaling pathway in radial migration and lamination of the cerebral cortex is a firmly established concept in the field. However, whether it directly influences both projection neurons and interneurons is still a matter of debate. Here we have adapted an ultrasound-guided microtransplantation technique (Olsson et al., 1997) to perform in vivo long-term fate-mapping analysis of cortical interneurons. Using this approach, we have studied cell-autonomous and non-cell-autonomous determinants governing the acquisition of laminar positions by cortical interneurons. Our results indicate that Reelin is neither required for tangential migration, nor is it directly implicated in the acquisition of particular laminar positions by cortical interneurons. Instead, our experiments suggest that this process may depend on the interaction between projection neurons and cortical interneurons, leading to a new model in which already settled projection neurons provide positional information for their inhibitory counterparts.
The tangential migration of cortical interneurons does not require Reelin signaling
The abnormal laminar distribution of GABAergic interneurons in the neocortex of reeler and Dab1 mutants could be attributable to multiple causes. Among them, defects in the tangential migration of cortical interneurons could influence their final distribution in the neocortex. However, analysis of the routes and number of migrating neurons in reeler and Dab1 mutant embryos did not reveal major differences with control mice. These results are in agreement with the observation that the number of GABAergic interneurons is normal in reeler neonates (Hevner et al., 2004), reinforcing the view that Reelin/Dab1 signaling does not influence the tangential migration of cortical interneurons. This conclusion contradicts previous results in which a reduction in the number of cortical interneurons in neonatal reeler Orleans mutants has been reported (Morante-Oria et al., 2003). It should be noted, however, that the Orleans allele exhibits a deletion of the last 205 residues of Reelin, a region required for the secretion of the protein (D'Arcangelo et al., 1997; de Bergeyck et al., 1997). Because interneurons also express Reelin as they settle in the cortex (Alcántara et al., 1998), it is conceivable that the accumulation of a truncated Reelin protein in these cells may abnormally affect interneurons in reeler Orleans mutants.
Acquisition of laminar fates by cortical interneurons
The analysis of the mechanisms controlling the acquisition of laminar identities by cortical GABAergic interneurons has been constrained because of methodological limitations. In the case of projection neurons, the development of new transplantation paradigms by McConnell and colleagues during the late 1980s and 1990s led to a series of studies that constituted a major step forward in our understanding of cortical lamination (McConnell, 1988). Two major conclusions were obtained from those studies: (1) the laminar identity of cortical projection neurons is determined early in the cell cycle of their progenitors and depends on environmental cues encountered just before mitosis (McConnell and Kaznowski, 1991); and (2) there is a progressive temporal restriction in the potential of cortical progenitors to produce deep layer projection neurons (Frantz and McConnell, 1996).
The remote origin of cortical interneurons in the subpallium has greatly limited the application of in vitro transplantation paradigms to study their laminar distribution. To solve this question, Tan and colleagues injected dissociated cells from the interneuron progenitor pool directly into the lateral ventricle of host embryos (Valcanis and Tan, 2003). Using this protocol, transplanted interneuron precursors attach to the ventricular zone of the telencephalon in random locations and infiltrate the mantle, migrating eventually toward the cortical plate. Although this method is hampered by the fact that interneurons do not follow their normal route of migration and therefore may fail to encounter important factors required for their final laminar location, this study led to important observations. Of note, they demonstrated that cells undergoing a final division in the host environment change their laminar fate to adopt one in register with the cells being born at the same time in the host (Valcanis and Tan, 2003). Thus, similar to projection neurons, local cues influence the laminar fate of interneuron precursors at the time of their final division.
Our study has extended these findings in a number of ways. In particular, a more complex perspective of the mechanisms controlling laminar acquisition by cortical interneurons is obtained from the experiments in which we combined BrdU-labeled, heterochronic and isochronic homotypic transplants in the same host environment. On one hand, we found that a large number of transplanted cells that divided last in the donor environment did not modify their laminar fate when transplanted to a younger or older environment (Fig. 4D,G). These observations are in agreement with the idea that embryonic progenitors undergo cyclical changes in their ability to respond to local cues, and therefore those progenitors transplanted later in the cell cycle are already committed to their normal fate (McConnell and Kaznowski, 1991; Valcanis and Tan, 2003). This was particularly obvious for early-born interneurons transplanted into an older environment, which were still primarily committed to deep layers of the neocortex. In contrast, we also found that many transplanted interneurons labeled with the same BrdU pulse (and therefore also late in their cell cycle) dramatically changed their laminar fate when transplanted into a different environment. This was especially noticeable for late-born interneurons, which were primarily confined to deep layers when transplanted into a young environment (Fig. 4D). The fact that not every interneuron transplanted late in the cell cycle retains its laminar fate demonstrates that the host environment can indeed influence the laminar distribution of interneurons. This specific observation extends the conclusion that interneurons are specified with respect to their future layer address as they start migrating from the subpallium (Valcanis and Tan, 2003) and suggests that either the route of migration or the cortex itself can additionally affect the final arrangement of interneurons, independently of their birth date. In conclusion, the subcortical environment in which cortical interneurons are born imprints them to adopt specific laminar fates, but the cortical environment that migrating interneurons encounter during their migration also contributes to refine their final position.
Layer acquisition by cortical interneurons does not directly require Reelin
The distribution of both cortical projection neurons and interneurons is severely disrupted in the absence of Reelin signaling. Despite this alteration, the distribution of projection neurons and interneurons is never uncoupled, suggesting that either interneurons also depend on Reelin signaling or that they require a normal distribution of projection neurons to adopt their laminar position. Our experiments provide several lines of evidence supporting this second hypothesis. First, interneurons born at different times but transplanted simultaneously have the ability to segregate into different layers of the cortex. This observation is incompatible with a mechanism in which Reelin would directly determine the laminar location of interneurons, or at least it would be necessary that interneurons born at different times would respond differently to Reelin. Second, projection neurons settle in the cortical plate before their synchronically generated interneuron counterparts. This suggests that projection neurons do not wait for their interneuron counterparts to simultaneously invade the cortex (Kriegstein and Noctor, 2004). This conclusion is also compatible with the fact that migrating interneurons enter the cortical plate from both superficial (marginal zone) and deep (subplate, subventricular zone) positions (Polleux et al., 2002; Ang et al., 2003; Tanaka et al., 2003; Hevner et al., 2004), whereas cortical projection neurons invade the cortical plate in a unidirectional migration from the ventricular zone. Third, Dab1−/− interneurons transplanted in a wild-type environment can adopt a normal laminar distribution, demonstrating that Reelin/Dab1 signaling is not directly required for the acquisition of laminar fates by cortical interneurons. Finally, when wild-type interneurons are transplanted into a Dab1−/− environment, they fail to adopt their normal laminar position. These results suggest that the distribution of projection neurons and/or a normal radial glia organization is required for interneurons to distribute appropriately in the cortex. These findings challenge recent experiments in which wild-type interneurons were found to distribute normally when transplanted into the lateral ventricle of Dab1 mutant embryos (Hammond et al., 2006). Other than the obvious methodological differences that exist between both experiments (transplantation in the MGE versus transplantation in the lateral ventricle), we cannot explain the origin of this discrepancy. However, the conclusion reached by Hammond et al. (2006) with this experiment, i.e., that late-born interneurons respond to Reelin to adopt their normal laminar fate, seems arbitrary because, even in the event that wild-type interneurons could allocate normally in the cortex of Dab1 mutants (which our results strongly contradict), this would by no means demonstrate that interneurons require Reelin for doing so. In contrast, our experiments in which Dab1−/− interneurons were transplanted into a wild-type environment clearly demonstrate that both early- and late-born interneurons can autonomously adopt a normal laminar distribution in the absence of Reelin/Dab1 signaling. Moreover, in view of the evidence summarized above, we also suggest that the organization of projection neurons, which depends on Reelin/Dab1 signaling, influences the final allocation of cortical interneurons.
The molecular nature of the factors that regulate the laminar distribution of cortical interneurons remains unknown. Because both fate determinants and local cues are important to specify the laminar fate of interneurons, we favor a model in which interneurons born at specific times during development are committed to distinct layers of the cortex in response to cues provided by projection neurons (Hammond et al., 2001; Hevner et al., 2004). These cues, however, do not need to be homogeneously present throughout the thickness of the cortex but may emerge as cortical layers progressively differentiate, defining a temporal window in which a specific cortical layer becomes particularly permissive for a population of interneurons. Unraveling the molecular nature of such signal(s) will greatly contribute to our understanding of the mechanisms controlling the formation of laminar structures in the brain.
Footnotes
This work was supported by grants to O.M. from the Spanish Government (BFU2005-04773/BMC), fundació “la Caixa,” the European Commission through STREP contract number 005139 (INTERDEVO), and the European Young Investigator Award (EURYI) program. R.P. was supported by a Formación de Personal Investigator fellowship from the Spanish Ministerio de Educación y Ciencia (MEC). V.B. is a “Ramón y Cajal” Investigator from the Consejo Superior de Investigaciones Científicas. N.F. was supported by a Formación de Profesorado Universitario fellowship from the Spanish MEC. O.M. is a European Molecular Biology Organization Young Investigator, a National Alliance for Research on Schizophrenia and Depression Young Investigator, and an EURYI Awardee. We thank T. Gil, M. Pérez, and M. Bonete for excellent technical assistance; B. Condie, V. Pachnis, and J. L. R. Rubenstein for plasmids and reagents; and G. D'Arcangello and K. Campbell for providing Dab1 and Dlx5/6CRE-IRES-GFP mice, respectively. We are particularly grateful to G. Fishell for his extraordinary support in the implementation of the ultrasound-guided transplantation technique in our laboratory and to M. Fuccillo for tips and guidance. We are thankful to B. Rico and members from the Marín and Rico laboratories for useful discussions, comments, and critical reading of this manuscript.
References
- Alcántara S, Ruiz M, D'Arcangelo G, Ezan F, de Lecea L, Curran T, Sotelo C, Soriano E (1998). Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci 18:7779–7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JLR (1997). Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science 278:474–476. [DOI] [PubMed] [Google Scholar]
- Ang ES Jr, Haydar TF, Gluncic V, Rakic P (2003). Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex. J Neurosci 23:5805–5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angevine JB, Sidman RL (1961). Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 192:766–768. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G (2005). The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron 48:591–604. [DOI] [PubMed] [Google Scholar]
- Caviness VS Jr (1982). Neocortical histogenesis in normal and reeler mice: a developmental study based upon [3H]thymidine autoradiography. Brain Res 256:293–302. [DOI] [PubMed] [Google Scholar]
- Corbin JG, Nery S, Fishell G (2001). Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain. Nat Neurosci 4:1177–1182. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T (1995). A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374:719–723. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Curran T (1997). Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J Neurosci 17:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T (1999). Reelin is a ligand for lipoprotein receptors. Neuron 24:471–479. [DOI] [PubMed] [Google Scholar]
- de Bergeyck V, Nakajima K, Lambert de Rouvroit C, Naerhuyzen B, Goffinet AM, Miyata T, Ogawa M, Mikoshiba K (1997). A truncated Reelin protein is produced but not secreted in the “Orleans” reeler mutation (Reln[rl-Orl]). Brain Res Mol Brain Res 50:85–90. [DOI] [PubMed] [Google Scholar]
- Fairén A, Cobas A, Fonseca M (1986). Times of generation of glutamic acid decarboxylase immunoreactive neurons in mouse somatosensory cortex. J Comp Neurol 251:67–83. [DOI] [PubMed] [Google Scholar]
- Flames N, Marín O (2005). Developmental mechanisms underlying the generation of cortical interneuron diversity. Neuron 46:377–381. [DOI] [PubMed] [Google Scholar]
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, Lai C, Rubenstein JL, Marín O (2004). Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron 44:251–261. [DOI] [PubMed] [Google Scholar]
- Frantz GD, McConnell SK (1996). Restriction of late cerebral cortical progenitors to an upper-layer fate. Neuron 17:55–61. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Gertsenstein M, Ikawa M, Okabe M, Nagy A (1998). Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech Dev 76:79–90. [DOI] [PubMed] [Google Scholar]
- Hammond V, Howell B, Godinho L, Tan SS (2001). disabled-1 functions cell autonomously during radial migration and cortical layering of pyramidal neurons. J Neurosci 21:8798–8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond V, So E, Gunnersen J, Valcanis H, Kalloniatis M, Tan SS (2006). Layer positioning of late-born cortical interneurons is dependent on Reelin but not p35 signaling. J Neurosci 26:1646–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF, Daza RA, Englund C, Kohtz J, Fink A (2004). Postnatal shifts of interneuron position in the neocortex of normal and reeler mice: evidence for inward radial migration. Neuroscience 124:605–618. [DOI] [PubMed] [Google Scholar]
- Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J (1999). Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron 24:481–489. [DOI] [PubMed] [Google Scholar]
- Howell BW, Hawkes R, Soriano P, Cooper JA (1997). Neuronal position in the developing brain is regulated by mouse disabled-1. Nature 389:733–737. [DOI] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Cooper JA (1999). Reelin-induced tyrosine phosphorylation of disabled 1 during neuronal positioning. Genes Dev 13:643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Hildebrand JD, Zhang Y, Cooper JA (2000). Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr Biol 10:877–885. [DOI] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor SC (2004). Patterns of neuronal migration in the embryonic cortex. Trends Neurosci 27:392–399. [DOI] [PubMed] [Google Scholar]
- Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG (1999). The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci 19:7881–7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bendito G, Sturgess K, Erdelyi F, Szabo G, Molnar Z, Paulsen O (2004). Preferential origin and layer destination of GAD65-GFP cortical interneurons. Cereb Cortex 14:1122–1133. [DOI] [PubMed] [Google Scholar]
- Marín O, Rubenstein JLR (2001). A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci 2:780–790. [DOI] [PubMed] [Google Scholar]
- McConnell SK (1988). Fates of visual cortical neurons in the ferret after isochronic and heterochronic transplantation. J Neurosci 8:945–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SK, Kaznowski CE (1991). Cell cycle dependence of laminar determination in developing neocortex. Science 254:282–285. [DOI] [PubMed] [Google Scholar]
- Miller MW (1985). Cogeneration of retrogradely labeled corticocortical projection and GABA-immunoreactive local circuit neurons in cerebral cortex. Brain Res 355:187–192. [DOI] [PubMed] [Google Scholar]
- Morante-Oria J, Carleton A, Ortino B, Kremer EJ, Fairen A, Lledo PM (2003). Subpallial origin of a population of projecting pioneer neurons during corticogenesis. Proc Natl Acad Sci USA 100:12468–12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métin C, Baun JP, Rakic S, Parnavelas JG (2006). Cell and molecular mechanisms involved in the migration of cortical interneurons. Eur J Neurosci 23:894–900. [DOI] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG (2002). The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci 5:1279–1287. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K (1995). The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron 14:899–912. [DOI] [PubMed] [Google Scholar]
- Olson EC, Kim S, Walsh CA (2006). Impaired neuronal positioning and dendritogenesis in the neocortex after cell-autonomous Dab1 suppression. J Neurosci 26:1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Campbell K, Turnbull DH (1997). Specification of mouse telencephalic and mid-hindbrain progenitors following heterotopic ultrasound-guided embryonic transplantation. Neuron 19:761–772. [DOI] [PubMed] [Google Scholar]
- Peduzzi JD (1988). Genesis of GABA-immunoreactive neurons in the ferret visual cortex. J Neurosci 8:920–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Whitford KL, Dijkhuizen PA, Vitalis T, Ghosh A (2002). Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development 129:3147–3160. [DOI] [PubMed] [Google Scholar]
- Rakic P (1974). Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science 183:425–427. [DOI] [PubMed] [Google Scholar]
- Rice DS, Curran T (1999). Mutant mice with scrambled brains: understanding the signaling pathways that control cell positioning in the CNS. Genes Dev 13:2758–2773. [DOI] [PubMed] [Google Scholar]
- Rice DS, Curran T (2001). Role of the reelin signaling pathway in central nervous system development. Annu Rev Neurosci 24:1005–1039. [DOI] [PubMed] [Google Scholar]
- Rice DS, Sheldon M, D'Arcangelo G, Nakajima K, Goldowitz D, Curran T (1998). Disabled-1 acts downstream of Reelin in a signaling pathway that controls laminar organization in the mammalian brain. Development 125:3719–3729. [DOI] [PubMed] [Google Scholar]
- Sanada K, Gupta A, Tsai LH (2004). Disabled-1-regulated adhesion of migrating neurons to radial glial fiber contributes to neuronal positioning during early corticogenesis. Neuron 42:197–211. [DOI] [PubMed] [Google Scholar]
- Soriano E, Del Rio JA (2005). The cells of Cajal-Retzius: still a mystery one century after. Neuron 46:389–394. [DOI] [PubMed] [Google Scholar]
- Stenman J, Toresson H, Campbell K (2003). Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci 23:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer T, Puelles L, Ekker M, Rubenstein JL (2002). Expression from a Dlx gene enhancer marks adult mouse cortical GABAergic neurons. Cereb Cortex 12:75–85. [DOI] [PubMed] [Google Scholar]
- Sussel L, Marín O, Kimura S, Rubenstein JL (1999). Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development 126:3359–3370. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Goto T, Miyama S, Nowakowski RS, Caviness VS Jr (1999). Sequence of neuron origin and neocortical laminar fate: relation to cell cycle of origin in the developing murine cerebral wall. J Neurosci 19:10357–10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka D, Nakaya Y, Yanagawa Y, Obata K, Murakami F (2003). Multimodal tangential migration of neocortical GABAergic neurons independent of GPI-anchored proteins. Development 130:5803–5813. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J (1999). Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97:689–701. [DOI] [PubMed] [Google Scholar]
- Valcanis H, Tan SS (2003). Layer specification of transplanted interneurons in developing mouse neocortex. J Neurosci 23:5113–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A (1999). Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat Neurosci 2:461–466. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A (2001). In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development 128:3759–3771. [DOI] [PubMed] [Google Scholar]
- Yabut O, Renfro A, Niu S, Swann JW, Marín O, D'Arcangelo G (2006). Abnormal laminar position and dendrite development of interneurons in the reeler forebrain. Brain Res Mol Brain Res in press. [DOI] [PubMed]