Abstract
The presence of endogenous stem cell populations in the adult mammalian CNS suggests an innate potential for regeneration and represents a potential resource for neuroregenerative therapy aimed at the treatment of neurodegenerative disorders, such as Parkinson's disease. However, it is first necessary to examine the microenvironmental signals required to activate these innate reparative mechanisms. The small molecule neurotransmitter dopamine has been shown to regulate cell cycle in developing and adult brain, and the D3 receptor is known to play an important role in dopaminergic development. Pharmacological activation of the dopamine D3 receptor has been shown to trigger neurogenesis in the substantia nigra of the adult rat brain. Here, we examined the cell proliferative, neurogenic, and behavioral effects of the dopamine D3 receptor agonist 7-OH-DPAT (7-hydroxy-N,N-di-n-propyl-2-aminotetralin) in a 6-hydroxydopamine model of Parkinson's disease. Consistent with previous findings, we observed a significant induction of cell proliferation in the substantia nigra pars compacta (SNC) with a time-dependent adoption of a neuronal dopaminergic phenotype in many of these cells. Indices of nigrostriatal integrity were also affected. Dopaminergic cell counts in the lesioned SNC recovered substantially in a time-dependent manner. Similarly, retrograde tracing revealed a restoration of striatal innervation from these cells, with evidence for projections arising from newly generated cells. Finally, we observed a substantial and persistent recovery of locomotor function in these animals. The results of these studies will further our understanding of the environmental signals regulating neurogenesis in the adult brain and could have significant implications for the design of novel treatment strategies for Parkinson's disease.
Keywords: Parkinson's disease, dopamine D3 receptor, substantia nigra, neurogenesis, proliferation, rat
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disorder characterized by a substantial loss of dopamine in the caudate–putamen (striatum) resulting from the gradual degeneration of dopaminergic neurons in the substantia nigra (SN) pars compacta (SNC). Although dopamine replacement therapy in the form of levodopa remains the primary mode of treatment for PD, long-term exposure is complicated by the emergence of fluctuations in motor function and a variety of involuntary movements (Marsden, 1984). These complications may result, at least in part, from ongoing cell loss. Thus, therapies aimed at neuronal integrity may achieve a more physiological restoration of the dopaminergic motor system, providing a more effective alternative.
Although the adult CNS has only limited potential to generate new neurons, discrete regions do retain the capacity for neurogenesis, including the dentate gyrus of the hippocampus (Palmer et al., 1997; Rietze et al., 2000) and the subventricular zone (SVZ), lining the lateral ventricles (Luskin, 1993). More recently, restricted progenitor cells have been identified in various “quiescent” regions of the adult brain including the striatum (Palmer et al., 1995; Pencea et al., 2001) and the SN (Lie et al., 2002; Zhao et al., 2003; Shan et al., 2006). Additional understanding of the microenvironmental signals regulating the proliferation of these cells would allow us the opportunity to enhance neuronal production and provide us with valuable tools for the development of new treatment strategies.
Dopamine receptor activation modulates neurogenesis in both the developing (Spencer et al., 1998; Ohtani et al., 2003) and adult (Coronas et al., 2004; Van Kampen et al., 2004; Van Kampen and Robertson, 2005) brain. Specifically, the dopamine D3 receptor appears to play an important role in neural development and shows a persistent expression through adulthood in the proliferative SVZ (Diaz et al., 1997). Furthermore, pharmacological stimulation of D3 receptors promotes proliferation and neuronal differentiation of adult SVZ cells, both in vitro (Coronas et al., 2004) and in vivo (Van Kampen et al., 2004), whereas activation of other dopamine receptor subtypes does not (Coronas et al., 2004; Van Kampen et al., 2004; Kippin et al., 2005). Removal of dopaminergic afferents reduces SVZ proliferation (Baker et al., 2004; Höglinger et al., 2004), an effect reversed by a dopamine D3 receptor agonist (Höglinger et al., 2004). The neurogenic capacity of D3 receptor activation may not be restricted to the SVZ, however. Indeed, low levels of dopamine D3 receptor expression have also been described in the SN (Diaz et al., 2000), a region of the adult brain shown to exhibit ongoing cytogenesis and neurogenic potential (Lie et al., 2002; Zhao et al., 2003; Shan et al., 2006). Furthermore, we have previously demonstrated a significant induction of neurogenesis in the SNC of the adult rat brain in response to D3 agonist treatment (Van Kampen and Robertson, 2005). Additional exploration of the neurogenic capacity of dopamine D3 receptor stimulation in the adult SNC could have serious implications for the development of novel therapeutic strategies targeting PD. Here, we examine the potential for dopamine neurogenesis and functional recovery after dopamine D3 receptor agonist treatment in hemiparkinsonian rats.
Materials and Methods
Animals.
All studies used 250 g female Sprague Dawley rats (Harlan, Indianapolis, IN). Animals were housed in a temperature-controlled environment with a 12 h light/dark cycle and ad libitum access to standard rat chow and water. All procedures used in this study were approved by the Mayo Foundation Institutional Animal Care and Use Committee.
Hemiparkinsonian model.
The 6-hydroxydopamine model is frequently used for studies involving cell replacement strategies (Bjorklund et al., 2002; Bartlett and Mendez, 2005; Richardson et al., 2005). Although other toxin-based models (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, rotenone, etc.) and transgenic mouse models of PD may be better suited for the study of disease process, 6-hydroxydopamine provides an ideal model for the study of cell replacement/neuroregenerative strategies. This is attributable, in large part, to a greater degree of control over the extent and kinetics of dopaminergic cell loss. For the studies outlined here, it was vital to restrict degeneration to a time, preceding therapeutic intervention, to properly differentiate between potential neuroprotective and neurorestorative events. The more progressive nature of other rodent PD models would have precluded this distinction and complicated data interpretation. For all surgeries, animals were anesthetized using isoflurane (1%) and placed in a Kopf stereotaxic frame for all surgical procedures. For nigrostriatal lesions, the dopaminergic neurotoxin, 6-hydroxydopamine hydrobromide, was injected unilaterally into three striatal sites [anteroposterior (AP), +1.0, mediolateral (ML), −3.0, dorsoventral (DV), −5.0; AP, −0.1, ML, −3.7, DV, −5.0; AP, −1.2, ML −4.5, DV, −5.0] (3.5 μg/μl/site) at a rate of 0.5 μl/min via an infusion cannula connected by polyethylene tubing (50 PE) to a 50 μl Hamilton syringe driven by a Harvard pump. After infusion, the toxin was permitted to diffuse away from the cannula for 2 min before withdrawal. Intrastriatal lesions were chosen in an effort to avoid nonspecific disruption of the nigrostriatal pathway, which could, potentially, impede the formation of novel nigrostriatal projections.
Drug delivery.
The preferential dopamine D3 receptor agonist 7-hydroxy-N,N-di-n-propyl-2-aminotetralin (7-OH-DPAT) was selected for this proposal based on previous studies demonstrating its mitogenic role both in vitro (Pilon et al., 1994; Coronas et al., 2004) and in vivo (Van Kampen et al., 2004; Van Kampen and Robertson, 2005). Thus, 4 weeks after intoxication (see Fig. 1), stainless steel indwelling cannulas were placed into the ventral third ventricle (AP, −2.00; ML, 0.00; DV, 8.00). The cannula (30 ga; Plastics One, Roanoke, VA) was fixed to the skull using dental acrylic and jeweler's screws. Each cannula was attached, by 50 PE polyethylene tubing, to an osmotic minipump [0.5 μl/h, 2 weeks (model 2002; Alza, Palo Alto, CA); 0.25 μl/h, 4 weeks (model 2004; Alza)], which was placed under the skin at the base of the neck. Each pump was filled with either 7-OH-DPAT (Sigma, St. Louis, MO) (2 μg/μl) or its vehicle, 0.9% saline. For those animals receiving 8 weeks of treatment, the pump was replaced at 4 weeks.
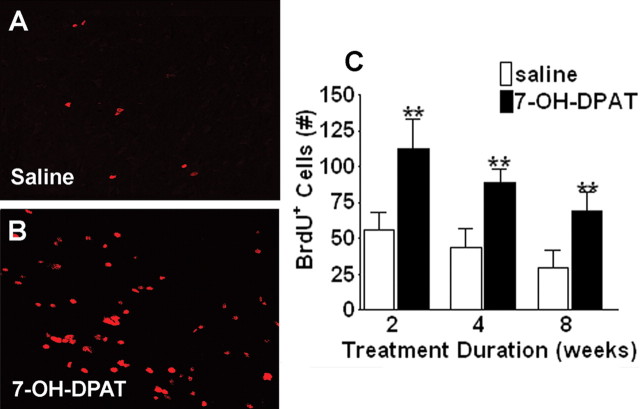
Figure 1.
7-OH-DPAT stimulates cell proliferation in the ipsilateral SNC. A, B, Representative fluorescent photomicrographs depicting BrdU immunolabeling in a coronal section through the ipsilateral SN in animals treated with saline (A) or 7-OH-DPAT (B) for 2 weeks. C, Quantitative analysis of BrdU-positive cells in the ipsilateral SNC. Each bar represents the mean (±SEM, n = 6) number of BrdU-positive cells counted in the ipsilateral SNC after 2, 4, or 8 weeks of treatment. Significant difference from saline-treated controls, ∗∗p < 0.001.
Bromodeoxyuridine administration.
During the first 2 weeks of 7-OH-DPAT treatment, all animals received daily injections of bromodeoxyuridine (BrdU; 100 mg/kg, i.p.; Sigma) to label proliferating cells.
Retrograde tracing.
Five days before being killed, animals received bilateral intrastriatal infusion of the retrograde fluorescent tracer FluoroGold (FG; Fluorochrome, Denver, CO) into six sites in the striatum (2% in 0.9% saline; 0.2 μl/site) at a rate of 0.05 μl/min via an infusion cannula connected by polyethylene tubing (50 PE) to a 10 μl Hamilton syringe driven by a Harvard pump.
Drug-induced rotations.
Before 7-OH-DPAT treatment began, animals were tested for lesion severity, as determined using standard amphetamine-induced rotation analysis. Animals were habituated to cylindrical Plexiglas test chambers for 30 min before testing. Animals were then administered the dopamine-releasing agent methamphetamine (2 mg/kg, i.p.), and rotations were recorded 30 min later for 1 h. Animals were tested again at the conclusion of the study after a minimum 3 d drug washout period. A subset of animals were tested once more, 2 or 4 months after termination of drug treatment.
Skilled reaching.
Skilled paw reaching was assessed in the “staircase test.” On each trial, the apparatus was baited with two 45 mg sucrose food pellets (Bioserv, Frenchtown, NJ). Rats were placed on a schedule of restricted feeding, in which animals received ad libitum access to food for 4 h/d immediately after training/testing. Training in the paw reaching apparatus was conducted for 1 trial/d, 5 d/week, over 3 weeks. On each trial, the maximum distance reached and the total number of pellets successfully retrieved in 15 min was recorded. The rats were returned to ad libitum feeding for 1 week before 6-hydroxydopamine lesioning. After 7-OH-DPAT treatment, animals were returned to a restricted feeding schedule and reaching performance was reassessed. Data were analyzed by separate multifactorial ANOVAs for each primary measure: (1) number of pellets consumed and (2) number of steps reached. Where significant F values were obtained, planned pairwise comparisons were made using Newman–Keuls.
Immunohistochemistry.
Animals were killed by transcardial perfusion with 4% paraformaldehyde. Brains were removed and postfixed for 24 h in 4% paraformaldehyde followed by cryoprotection in 30% sucrose. Symmetrical 40-μm-thick sections were cut on a freezing microtome and stored in a Millonigs solution. Every 12th section was processed for immunohistochemistry. Free-floating sections were pretreated with 50% formamide/280 mm, incubated in 2 m HCl at 37°C for 30 min, and rinsed in 0.1 m boric acid, pH 8.5, at room temperature for 10 min. Sections were incubated in 1% H2O2 in PBS for 15 min in blocking solution [3% goat or donkey serum/0.3% Triton X-100/Tris-buffered saline (TBS)] for 1 h at room temperature, followed by the appropriate antibody at 4°C overnight. Anti-BrdU antibodies were mouse monoclonal anti-BrdU (1:250; Chemicon, Temecula, CA) or sheep polyclonal anti-BrdU (1:10,000; Research Diagnostics Inc., Flanders, NJ). The other primary antibodies were monoclonal mouse anti-glial fibrillary acidic protein (GFAP) (1:1000; Chemicon), monoclonal mouse anti-Tuj1 (1:1000; Chemicon), polyclonal guinea pig anti-doublecortin (DCX; 1:5000; Chemicon), monoclonal mouse anti-neuronal-specific nuclear protein (NeuN; 1:1000; Chemicon), and proliferating cell nuclear antigen (PCNA; 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA). For proper identification of the SNC, all sections were incubated with either polyclonal mouse anti-tyrosine hydroxylase (TH; 1:10,000; Chemicon) or monoclonal mouse anti-TH (1:1000; Chemicon). For fluorescent visualization, sections were incubated with the respective secondary antibody conjugated to either Alexa 488, Alexa 594, Alexa 633, or Alexa 350 (Invitrogen, Eugene, OR). Between steps, sections were washed three times for 10 min in TBS. Sections were mounted on unsubbed glass slides and coverslipped in Fluoromount. Fluorescence signals were detected with a Zeiss (Oberkochen, Germany) axiophot laser-scanning confocal microscope at excitation/emission wavelengths of 323/340, 535/565, 470/505, and 585/615 nm.
Cell counting.
The number of immunopositive cell bodies was counted by an observer blinded to treatment history. Unbiased stereological cell counts were obtained using a computer-assisted image analysis system and Zeiss Axiovision 4.3 image analysis software, including Axiovision Interactive Measurement Module. For counting cells of the substantia nigra, the compacta regions were defined by the distribution of TH-positive cells within a set of clear anatomical landmarks/boundaries, used to delineate the SNC. Immunopositive cells were counted using a 20× objective (sampling frame area, 90,000 μm2) containing an optical grid. The counting frame was placed over the counting area and then systematically moved in the x–y direction until the entire delineated area was sampled. The number of immunopositive cells counted across four sections per animal (third ventricle: −2.12, −2.80, −3.60, −4.16 mm; SNc: −4.80, −5.30, −5.60, −6.04 mm) was totaled for each animal. Fluorescent double labeling was observed in microfine slices (1.5 μm) using a laser-scanning microscope (Zeiss LSM 510) under a 40× objective. A double-labeled BrdU/phenotypic marker-positive cell was defined as having the strongest intensity of both immunolabels within the same or neighboring 1.5-μm-thick optical sections through the cell in a consecutive Z-series of at least 10 sections, at 200× magnification and with a resolution of 1024 × 1024 pixels. Cells not present in their entirety were excluded. All double labeling was confirmed by rotating the image along each axis to ensure that signals were localized within the same cell rather than separate cells in close apposition. Slides were blind coded to eliminate experimenter bias.
Statistical analysis.
Data were analyzed using a multivariant ANOVA. Where significant F-values were obtained, planned pairwise comparisons were made using Newman–Keuls. Differences were considered statistically significant at p < 0.05.
Results
Cell proliferation in the SN
Intraventricular infusion of the dopamine D3 receptor agonist 7-OH-DPAT resulted in a significant, more than twofold, increase in BrdU-positive cell counts in the SNC, ispilateral to the lesion, at all time points examined, when compared with the saline-treated controls (Fig. 1) (p < 0.001; n = 6). For comparison with a known neurogenic region, we also examined BrdU labeling in the ipsilateral SVZ, in which BrdU incorporation was almost doubled after 2 weeks of 7-OH-DPAT treatment (1231.63 ± 125.18) compared with saline treatment (627.83 ± 113.76) (Fig. 2) (p < 0.001; n = 6), consistent with previous findings in intact animals (Van Kampen et al., 2004). Although the contralateral, uninjured, hemisphere is not the primary focus of the current report and has been discussed previously (Van Kampen and Robertson, 2005), we did note a significant elevation in BrdU labeling in the contralateral SNC (data not shown), consistent with previous findings in intact animals. Compared with the contralateral SNC, overall levels of BrdU labeling were slightly but significantly (p < 0.05; n = 6) reduced in the ipsilateral SNC 4 weeks after intoxication (data not shown). This is consistent with previous reports of impaired cell proliferation in other regions, such as the SVZ, after dopaminergic denervation (Baker et al., 2004; Höglinger et al., 2004).
Figure 2.
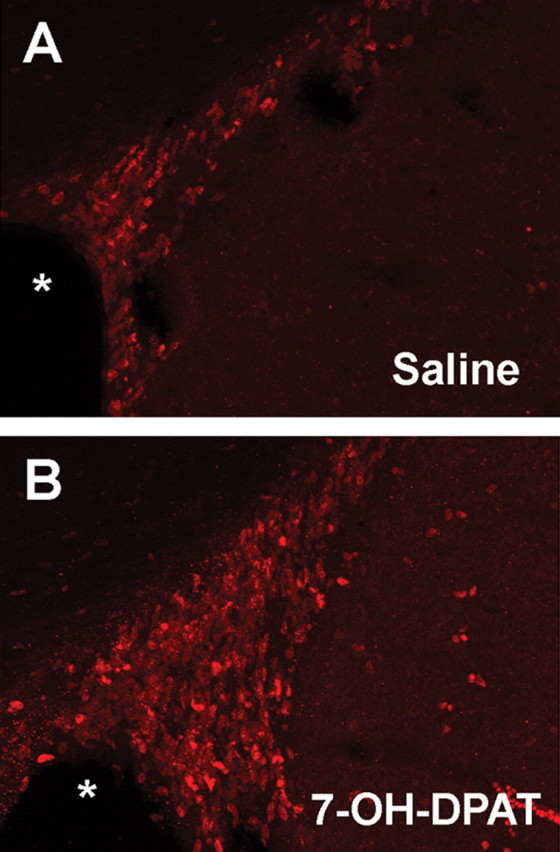
7-OH-DPAT stimulates cell proliferation in the ipsilateral SVZ. A, B, Representative fluorescent photomicrographs depicting BrdU immunolabeling in a coronal section through the ipsilateral SVZ in animals treated with saline (A) or 7-OH-DPAT (B) for 2 weeks. The asterisk indicates the lateral ventricle.
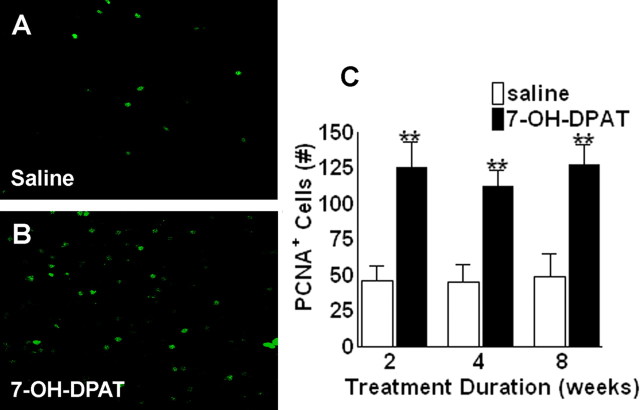
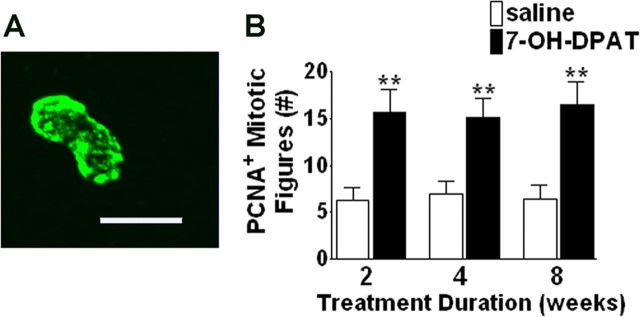
As a complement to the BrdU labeling, we also examined immunolabeling for PCNA, an endogenous marker of cell proliferation. Treatment with 7-OH-DPAT resulted in significant increases in PCNA labeling again at all time points examined (Fig. 3) (p < 0.001; n = 6). Because PCNA is an endogenous marker, antibodies only label cells proliferating at the time of death, in contrast to BrdU, which is taken within the cell and maintained through migration and differentiation. Thus, the increase in PCNA labeling within the SNC suggests that 7-OH-DPAT-induced increases in BrdU-positive cell counts are likely to reflect an effect on cell proliferation rather than an indirect effect on cell survival (Carvey et al., 2001; Du et al., 2005). It has been reported that cell cycle markers, including PCNA, may be expressed by injured cells undergoing DNA repair (Maga and Hubscher, 2003). Thus, we also examined the number of PCNA-positive cells displaying a mitotic morphology, as depicted in Figure 4A. After 7-OH-DPAT treatment, the number of PCNA+ mitotic figures was elevated by ∼146% (Fig. 4B) (p < 0.001; n = 6), comparable with changes observed in total PCNA+ cell counts (Fig. 3C).
Figure 3.
7-OH-DPAT stimulates expression of endogenous markers of cell proliferation in the ipsilateral SNC. A, B, Representative fluorescent photomicrographs depicting PCNA immunolabeling in a coronal section through the ipsilateral SN in animals treated with saline (A) or 7-OH-DPAT (B) for 2 weeks. C, Quantitative analysis of PCNA-positive cells in the ipsilateral SNC. Each bar represents the mean (±SEM, n = 6) number of PCNA-positive cells counted in the ipsilateral SNC after 2, 4, or 8 weeks of treatment. Significant difference from saline-treated controls, ∗∗p < 0.001.
Figure 4.
7-OH-DPAT increases the number of PCNA-positive mitotic figures in the ipsilateral SNC. A, Representative fluorescent photomicrograph depicting PCNA immunolabeling in a pair of proliferating cells appearing to be undergoing mitosis in the ipsilateral SNC. B, Quantitative analysis of PCNA-positive mitotic figures in the ipsilateral SNC. Each bar represents the mean (±SEM, n = 6) number of PCNA-positive mitotic pairs counted in the ipsilateral SNC after 2, 4, or 8 weeks of treatment. Significant difference from saline-treated controls, ∗∗p < 0.001. Scale bar, 20 μm.
Perhaps even more importantly, these newly generated cells appear to go on to develop a mature neuronal phenotype. We performed multiple immunolabeling experiments examining the number of BrdU+ cells colabeled with antibodies against the mature neuronal marker NeuN or the glial marker GFAP (Fig. 5F). After 2 weeks of treatment, ∼47% of the BrdU+ cells colabeled for either GFAP or NeuN. The distribution between these two cell-type markers was ∼50%. This is consistent with the TH immunolabeling results at the same time point, described below. At 4 and 8 weeks of age, however, we observed an increase in the number of BrdU+/NeuN+ cells and a decrease in the number of BrdU+/GFAP+ cells, with ∼56% of all BrdU+ cells then staining for one of these two phenotypic markers (p < 0.02; n = 6). These data indicate that, as treatment progresses, the total number of cells taking on distinct cell-type-specific phenotypes has increased, with many of these labeling for NeuN at later time points. Colabeling for BrdU and the immature neuronal marker Tuj1 was restricted primarily to the 2 week time point. No colabeling with DCX was evident at any time point, despite robust labeling in the SVZ and dentate gyrus. Although Tuj1 and DCX are both markers for immature neurons, DCX is primarily expressed by newly generated, migrating neurons (Gleeson et al., 1999). Thus, the absence of this marker in BrdU+ cells may suggest that these cells arise within the SN, itself, as opposed to migrating from another site of origin.
Figure 5.
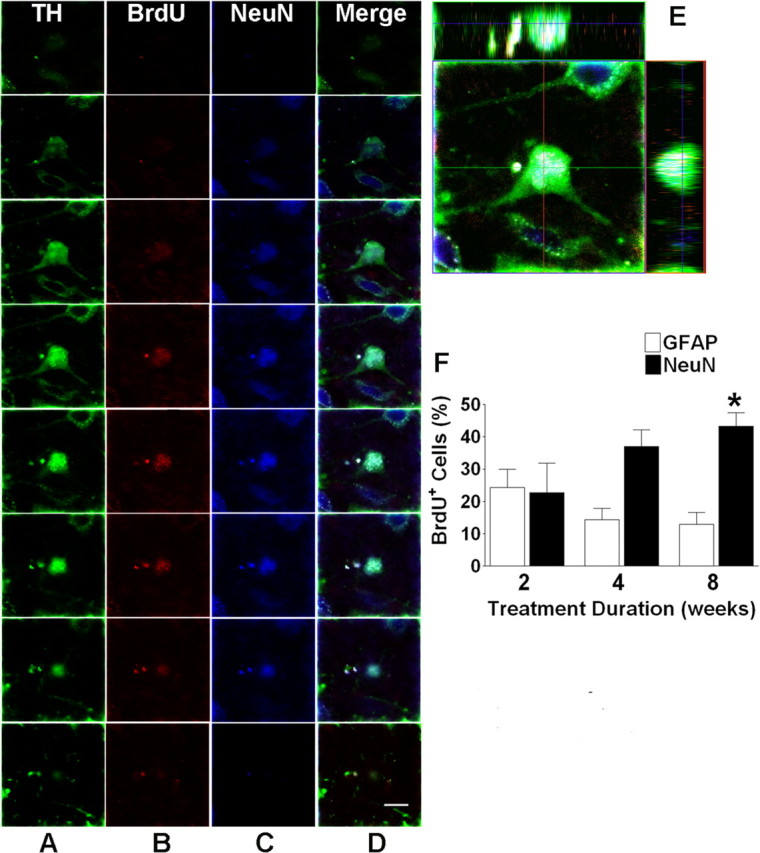
7-OH-DPAT promotes the adoption of a neuronal phenotype in newly generated cells of the SNC. A–C, Representative fluorescent photomicrographs depicting serial sections through a single cell immunolabeled for TH (A), BrdU (B), and NeuN (C). D, Merged image depicting immunolabeling for all three markers. E, Images were rotated in orthogonal planes to confirm the presence of all three markers within a single cell rather than individual cells in close proximity. F, Counts of BrdU-positive cells also labeled for either NeuN or GFAP in the ipsilateral SNc. Each bar represents the mean (±SEM, n = 6) number of BrdU-positive cells immunolabeled for GFAP or NeuN after 2, 4, or 8 weeks of treatment. Cells were counted in the ipsilateral SNC. The asterisk indicates significant difference from saline-treated controls. Scale bar, 10 μm.
Some of these newly generated neurons express a dopaminergic marker. To further determine the final neuronal type of these BrdU+/NeuN+ cells, we performed careful confocal analysis, including orthogonal rotations. Approximately 28% of all of the BrdU+ cells in the ipsilateral SNc of 7-OH-DPAT-treated animals were positive for both NeuN and TH, indicative of dopaminergic neurons (Fig. 5). These data indicate that the majority of all BrdU+/NeuN+ cells have adopted a dopaminergic phenotype after 8 weeks of treatment.
Nigrostriatal integrity
Nigral TH+ cell counts stabilize before initiation of 7-OH-DPAT treatment. After 6-hydroxydopamine lesioning, TH+ cell counts in the ipsilateral SNC were reduced to ∼23% of that in the contralateral hemisphere, reflecting a 77% depletion of dopaminergic neurons in the ipsilateral SNC, approximating levels of cell loss presented in PD. This effect remained unchanged across the various time points in saline-treated controls (Fig. 6D). This is consistent with previous reports (Yuan et al., 2005) and our own experience, indicating that this model is not progressive in nature, with the majority of cell loss observed within the first 2 weeks. Although TH+ cell counts are only a marker of dopaminergic phenotype and do not necessarily imply cell death, the number of cells estimated by TH and NeuN immunostaining correlated well in our model. The remaining 23% of cells may be important here, because it has been shown that when some intact cells remain, as opposed to the complete destruction of the nigrostriatal pathway, the potential for plasticity may be enhanced (Song and Haber, 2000; Stanic et al., 2003). Furthermore, 6-hydroxydopamine lesions have been shown to reduce dopamine D3 receptor expression in the SN (Bordet et al., 2000; Stanwood et al., 2000), possibly compromising the neurogenic potential of 7-OH-DPAT treatment. This would be consistent with the findings, reported above, of an overall reduction in the capacity of 7-OH-DPAT to induce proliferation after intoxication.
Figure 6.
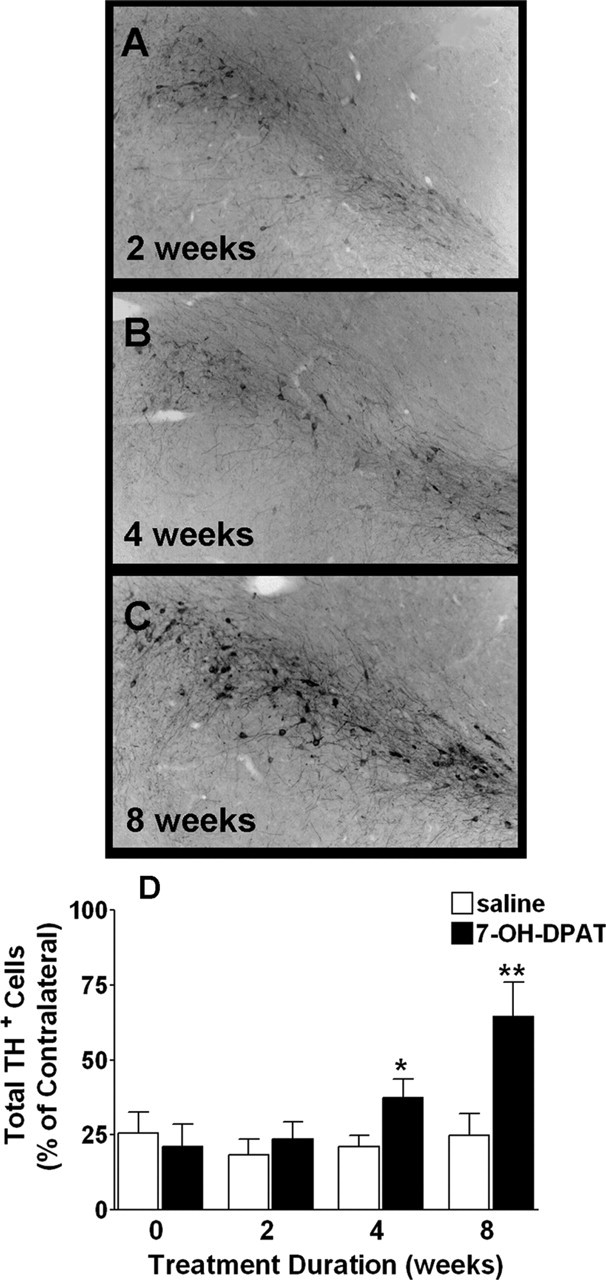
7-OH-DPAT treatment partially restores TH immunolabeling in the ipsilateral SNC. A–C, Photomicrographs depicting TH immunolabeling in the ipsilateral SNC after 2, 4, and 8 weeks of 7-OH-DPAT treatment. D, TH+ cell counts in the ipsilateral SNC. Each bar represents the mean percentage (±SEM, n = 6) of TH+ cells remaining in the ipsilateral SNC relative to the contralateral, intact hemisphere based on total cell counts. Significant difference from saline-treated controls, ∗∗p < 0.001; ∗p < 0.02.
Although the degree of nigral cell loss remained stable across time points in saline-treated controls, treatment with the preferential dopamine D3 receptor agonist 7-OH-DPAT reduced lesion severity in a time-dependent manner. After 4 weeks of 7-OH-DPAT treatment, we observed a significant, robust increase in the total (p < 0.01; n = 6) and relative (p < 0.02; n = 6) number of TH+ cells counted in the ipsilateral SNC (Fig. 6). This increase was even more pronounced in animals treated for 8 weeks, with the number of TH+ cells in the ipsilateral SNc approaching 65% of contralateral counts. Again, NeuN+ cell counts closely approximated these numbers. Interestingly, significant recovery of TH+ cells was only observed after extended treatment, with no changes seen after only 2 weeks of treatment, suggestive of progressive cell maturation.
Dopaminergic neurons in the SNc normally send projections to the striatum in the forebrain, and it is this loss of dopaminergic innervation that underlies key features of PD symptomatology. To assess the integrity of the nigrostriatal pathway, we used the retrograde tracer FG. Counting the number of FG-labeled cells in the SNC after injection of the tracer into the striatum permits a direct assessment of the number of SNC neurons with intact connections to the striatum and provides a better measure of pathway integrity than simpler measures of dopaminergic cell body numbers in the SNc (Sauer and Oertel, 1994). We examined changes in FG labeling and TH immunostaining in the ipsilateral and contralateral SNc after 6-hydroxydopamine lesioning. We found that FG labeling closely approximated TH+ cell counts in the SNc, including a significant decline in both TH and FG labeling ipsilateral to the lesion. After chronic intraventricular 7-OH-DPAT treatment, we observed a time-dependent, significant elevation in the number of FG-labeled cells in the ipsilateral SNC (p < 0.001; n = 6) (Fig. 7). In animals treated with 7-OH-DPAT for a full 8 weeks, FG-labeled cell counts in the SNC reached ∼75% of that observed in the contralateral, intact, hemisphere. Thus, recovery of nigrostriatal projections is dependent on treatment duration in a manner similar to that observed for TH+ cell counts. No significant changes in FG labeling were observed in saline-treated controls, indicating that the agonist treatment was directly responsible for this increase in apparent connectivity. Unlike other markers of dopaminergic innervation such as striatal TH or dopamine transporter, cell counts of nigral FG labeling are likely to reflect true changes in the number of nigral cells projecting to the striatum rather than axonal branching of existing neurons, which would alter labeling intensity rather than actual cell numbers. Some of these FG-labeled cells were also found to be BrdU+ (Fig. 8), suggesting that the increase in nigrostriatal projections, as evidenced by changes in nigral FG+ cell counts, may be attributable, at least in part, to the generation of new cells, described above.
Figure 7.
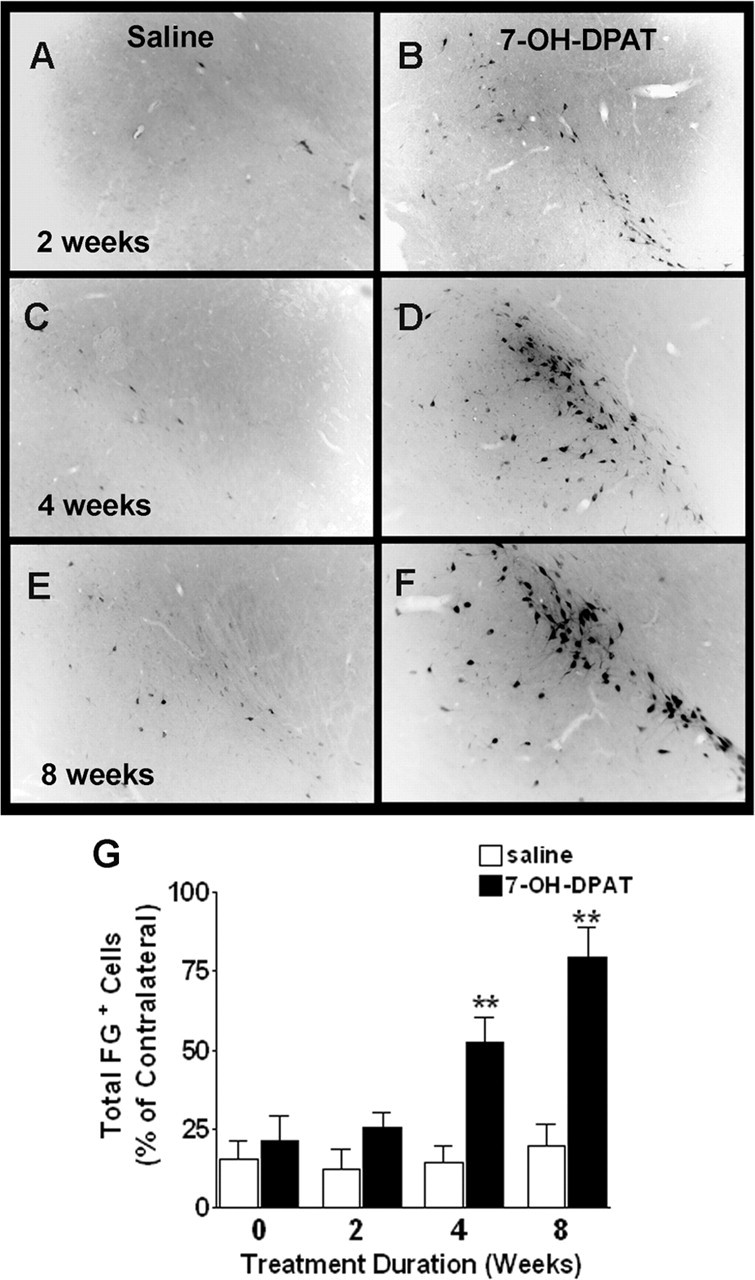
7-OH-DPAT partially restores retrograde FG labeling in the SNC. A–F, Representative photomicrographs depicting FG labeling in the ipsilateral SN after 2 (A, B), 4 (C, D), and 8 (E, F) weeks of saline (A, C, E) or 7-OH-DPAT (B, D, F) treatment. G, Quantitative analysis of FG-positive cells counted across four sections through the ipsilateral SN. Each bar represents the mean percentage (±SEM, n = 6) of FG-labeled cells remaining in the ipsilateral SNC relative to the contralateral, intact hemisphere based on total cell counts. Significant difference from saline-treated controls, ∗∗p < 0.001.
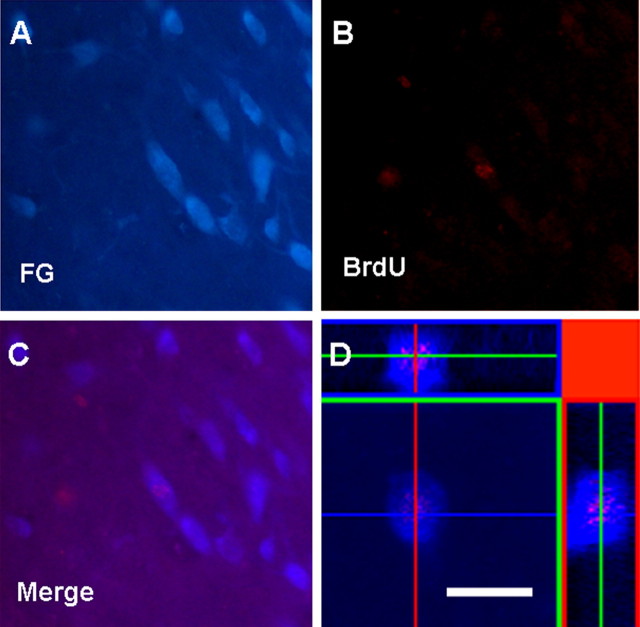
Figure 8.
Newly generated cells in the ipsilateral SNC have projections in the striatum. Representative fluorescent photomicrographs depicting double immunolabeling for FG (A) and BrdU (B) in the ipsilateral SNC after 8 weeks of 7-OH-DPAT treatment. C, Merged images indicate colocalization of both markers within cells of the ipsilateral SNC. D, Images were rotated in orthogonal planes to confirm the presence of both markers within a single cell rather than individual cells in close proximity. Scale bar, 20 μm.
Behavioral recovery
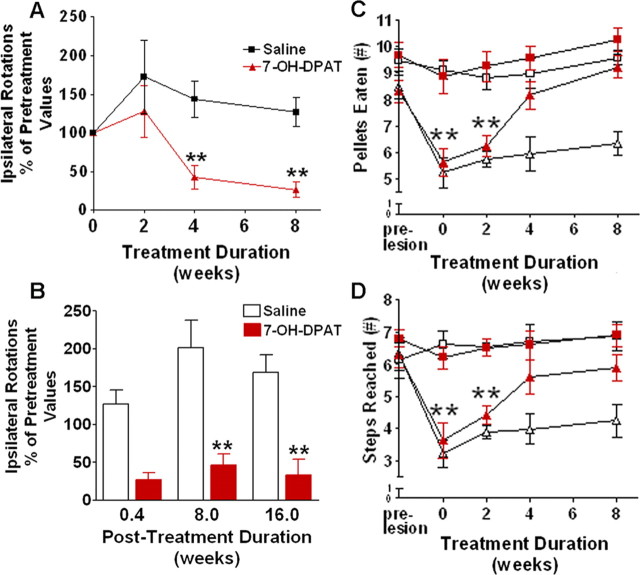
To assess the impact of these anatomical changes on the behavior of these animals, we incorporated two measures of nigrostriatal dopamine function: drug-induced rotational analysis and a skilled reaching task. To make certain that we were not assessing acute symptomatic effects of the compound, we incorporated a 3 d drug washout period before behavioral testing (supplemental Fig. 1S, available at www.jneurosci.org as supplemental material). Locomotor asymmetry, as determined using classical amphetamine-induced rotation analysis, showed significant improvements after 4 and 8 weeks of chronic intraventricular infusion of 7-OH-DPAT (p < 0.001; n = 6) (Fig. 9A) over pretreatment measures, coincident with the changes in TH+ and FG+ cell counts described above. No changes were observed in rotational response at any time point in the saline-treated controls or in animals receiving only 2 weeks of agonist treatment. Remarkably, animals receiving 7-OH-DPAT for a full 8 weeks displayed ≈80% fewer amphetamine-induced rotations than the controls, indicative of a substantial behavioral recovery. In light of the drug washout, these effects cannot be attributed to symptomatic benefits of acute receptor activation. However, to further demonstrate this, we retested a subset of animals 2 and 4 months after termination of 7-OH-DPAT treatment. As shown in Figure 9B, the reductions in rotational responses were evident even after this extended period of drug withdrawal, clearly indicating an improvement in the underlying pathology rather than simply a symptomatic response to the agonist.
Figure 9.
7-OH-DPAT promotes functional recovery after denervation in a rodent model of PD. Functional asymmetry was assessed before and after 7-OH-DPAT treatment using two standard behavioral measures. A, Counts of amphetamine-induced rotations 3 d after 0, 2, 4, or 8 weeks of treatment. Each point represents the mean (±SEM, n = 6) number of ipsilateral rotations expressed as a percentage of pretreatment values. Significant difference from saline-treated controls, ∗∗p < 0.001. B, Counts of amphetamine-induced rotations at various time points after the termination of 8 weeks of treatment. Each bar represents the mean (±SEM, n = 3–6) number of ipsilateral rotations expressed as a percentage of pretreatment values. Significant difference from saline-treated controls, ∗∗p < 0.001. C, Counts of pellets eaten in the staircase test. D, Counts of the number of steps reached in the staircase test. Each point represents the mean (±SEM, n = 6) number of pellets eaten or steps reached. Significant difference from ipsilateral paw, ∗∗p < 0.001. Squares, Ipsilateral paw; triangles, contralateral paw; open symbol, saline treatment; filled symbol, 7-OH-DPAT treatment.
Although drug-induced rotation remains the standard measure of functional asymmetry in hemiparkinsonian rats, striatal dopamine depletion has also been shown to impair skilled forelimb use (Montoya et al., 1991; Barnéoud et al., 1995). To assess skilled reaching, we used a “staircase test” (Montoya et al., 1991; Abrous and Dunnett, 1994). As expected, nigrostriatal 6-hydroxydopamine-induced lesions resulted in a significant impairment of skilled reaching with the contralateral paw but caused no disruption of reaching with the ipsilateral paw (Fig. 9C,D). Consistent with the behavioral improvement noted in the rotation analysis, we observed a significant improvement in skilled reaching after both 4 and 8 weeks of intraventricular 7-OH-DPAT infusion, with no changes observed in either the saline treatment animals or those animals treated for only 2 weeks.
Collectively, these data indicate that chronic intraventricular administration of 7-OH-DPAT results in significant functional recovery, as determined by both amphetamine-induced rotations and skilled reaching. Furthermore, behavioral improvements continue to persist long after drug washout, suggestive of changes in underlying pathology.
Discussion
These studies are the first to describe agonist-induced repair in a rodent model of PD. In these experiments, we have shown cellular proliferation in the SNC of hemiparkinsonian rats in response to intraventricular infusion of the dopamine D3 receptor agonist, 7-OH-DPAT. Not only was there evidence of increased cell proliferation in these regions, but a proportion of these newly generated cells appeared to adopt a mature neuronal phenotype, suggesting a potential neurogenic role for this compound. Indeed, this confirms previous findings involving intact animals, indicating that 7-OH-DPAT treatment promotes neurogenesis in the adult rat brain (Van Kampen et al., 2004; Van Kampen and Robertson, 2005). Dopamine neurons with incorporated BrdU label were discovered in the lesioned SNC after extended treatment, and a time-dependent recovery of nigral dopaminergic neurons and their projections was observed. Evidence for this anatomical restoration was further corroborated by the substantial functional recovery observed in two behavioral measures of locomotor asymmetry, an effect that persisted at least 4 months after treatment ended.
Evidence for cell proliferation, as measured by BrdU incorporation, was further reinforced in these studies by cell counts of mitotic figures and PCNA labeling. PCNA is an endogenous marker of cell proliferation, and antibodies for this protein label only those cells proliferating at the time the animals were killed, as opposed to BrdU, which is taken within the cell and maintained through migration and differentiation. Here, we demonstrate both the appearance of PCNA-positive cells within the SNC and their induction after chronic intraventricular administration of 7-OH-DPAT. This increase in PCNA labeling suggests that 7-OH-DPAT-induced increases in BrdU-positive cell counts reflect an effect on cell proliferation rather than an indirect effect on cell survival (Carvey et al., 2001; Du et al., 2005). These findings also suggest that cells are proliferating within the SNC itself. This would be consistent with the absence of DCX labeling in the SNC. Both Tuj1 and DCX are markers for immature neurons, but DCX is localized primarily in migrating neurons. Labeling for this neuronal marker was robust in regions known for neuronal migration, including the SVZ and dentate gyrus, with no labeling found in the SNC. Thus, the current data suggest that 7-OH-DPAT-induced proliferation in the SNC may occur in situ from resident neural progenitor cells (Lie et al., 2002) rather than progeny migrating from periventricular regions.
Although the incorporation of BrdU has long been considered the gold standard for the study of cytogenesis, BrdU can potentially incorporate into damaged or dying cells. For this reason, care should be taken when studying neurogenesis in disease models. In light of the neuroprotective effects attributed to D3 receptor agonists (Vu et al., 2000), 7-OH-DPAT could potentially have protected existing neurons from additional damage before degeneration was complete. However, this is unlikely to account for the findings reported in these studies. First, animals were treated with BrdU beginning a full 4 weeks after 6-hydroxydopamine intoxication, and there was no evidence for residual degeneration occurring beyond this point, because TH+ and NeuN+ cell counts did not differ across time points in the saline-treated controls. Furthermore, degeneration in this model occurs in a retrograde manner. Had cells begun to die back without actual cell loss, there would be a discrepancy between measures of striatal innervation and nigral cell counts before initiation of treatment. Instead, FG labeling closely approximated TH+ and NeuN+ cell counts at all time points. Also, had BrdU incorporation been attributable to unscheduled DNA synthesis/repair, 6-hydroxydopamine intoxication might be expected to actually enhance BrdU labeling. In contrast, BrdU labeling in the SNC was reduced overall in the lesioned hemisphere when compared with the contralateral, uninjured hemisphere. Thus, nonspecific uptake of BrdU by injured cells is unlikely to account for the findings reported here.
As mentioned, we observed a substantial, time-dependent improvement in behavioral measures after treatment. Although the dopamine D3 receptor is known to influence locomotor behavior in the rat (Ouagazzal and Creese, 2000), acute dopamine receptor activation is unlikely to explain the restoration of behavioral function observed here, because we used a 3 d washout before testing the animals. Furthermore, the greatest behavioral improvement was observed only after prolonged treatment, with no significant changes observed after only 2 weeks of treatment. Perhaps most convincing is the persistence of the observed behavioral improvement, which lasted as long as 4 months after drug treatment ended. Together, this would suggest a more permanent structural change underlying these behavioral improvements.
The most effective means of reinstating behavioral function in PD is to restore the nigrostriatal dopamine projection. The data provided here indicate a behaviorally relevant restoration of nigrostriatal projections. The strongest evidence for this is the retrograde incorporation of FG into cells of the SNC after injection into the striatum and an accompanying increase in the density of nigral TH+ cells. The direct correlation between the behavioral improvement and increased FG labeling and number of TH+ cells supports our hypothesis that restoration of the dopaminergic nigrostriatal circuit has occurred. The colabeling of these same SNC neurons with FG and the proliferation marker BrdU further supports the contention that 7-OH-DPAT treatment promotes regeneration of nigrostriatal cells. We are unaware of any other treatment modality capable of triggering such substantial neurorestorative effects.
The findings reported here in rats may have relevance for human PD. Although levodopa continues to be the most effective therapy for the cardinal features of PD, direct-acting dopamine receptor agonists have now become an important part of PD therapy. These drugs have been shown to modify the course of the disease, being associated with a reduced incidence of dyskinesias and motor fluctuations. Furthermore, recent evidence indicates that the advantages of ropinirole and pramipexole, two preferential dopamine D3 receptor agonists used to treat PD, extend beyond purely symptomatic benefits (Stern, 2004). Indeed, using imaging markers of dopamine function, treatment with these agonists has been found to be associated with a reduction in disease progression (Parkinson Study Group, 2002; Whone et al., 2003). Thus, observations in humans would be consistent with the data reported here and further highlight their relevance to PD. Indeed, dopaminergic modulation of forebrain precursor proliferation is not restricted to rodents but has also been identified in monkeys (Freundlieb et al., 2006) and humans (Höglinger et al., 2004), suggesting that pharmacological manipulation of precursor cell proliferation by small-molecule receptor agonists could represent an exciting means of using endogenous neural progenitor cells to repair the damaged PD brain.
Footnotes
This work was supported by grants from the National Institute of Neurological Disorders and Stroke and by the Mayo Foundation for Medical Education and Research (J.V.K., C.B.E.). J.V.K. was a fellow of the Robert and Clarice Smith Foundation. We thank Dr. H. A. Robertson and the Department of Anatomy and Neurobiology at Dalhousie University (Halifax, Nova Scotia, Canada) for counsel and use of their imaging facility for part of this work. We also wish to thank J. N. Joyce for his critical reading of this manuscript and helpful suggestions.
References
- Abrous DN, Dunnett SB (1994). Skilled paw reaching in rats: the staircase test. Neurosci Protoc 3:1–11. [Google Scholar]
- Baker SA, Baker KA, Hagg T (2004). Dopaminergic nigrostriatal projections regulate neural precursor cell proliferation in the adult mouse subventricular zone. Eur J Neurosci 20:575–579. [DOI] [PubMed] [Google Scholar]
- Barnéoud P, Parmentier S, Mazadier M, Miquet JM, Boireau A, Dubédat P, Blanchard JC (1995). Effects of complete and partial lesions of the dopaminergic mesotelencephalic system on skilled forelimb use in the rat. Neuroscience 67:837–848. [DOI] [PubMed] [Google Scholar]
- Bartlett LE, Mendez I (2005). Dopaminergic reinnervation of the globus pallidus by fetal nigral grafts in the rodent model of Parkinson's disease. Cell Transplant 14:119–127. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Sanchez-Pernaute R, Chung S, Andersson T, Chen IY, McNaught KS, Brownell AL, Jenkins BG, Wahlstedt C, Kim KS, Isacson O (2002). Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson's rat model. Proc Natl Acad Sci USA 99:2344–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Schwartz JC, Sokoloff P (2000). Involvement of the direct striatonigral pathway in levodopa-induced sensitization in 6-hydroxydopamine-lesioned rats. Eur J Neurosci 12:2117–2123. [DOI] [PubMed] [Google Scholar]
- Carvey PM, McGuire SO, Ling ZD (2001). Neuroprotective effects of D3 dopamine receptor agonists. Parkinsonism Relat Disord 7:213–223. [DOI] [PubMed] [Google Scholar]
- Coronas V, Bantubungi K, Fombonne J, Krantic S, Schiffmann SN, Roger M (2004). Dopamine D3 receptor stimulation promotes the proliferation of cells derived from the post-natal subventricular zone. J Neurochem 91:1292–1301. [DOI] [PubMed] [Google Scholar]
- Diaz J, Ridray S, Mignon V, Griffon N, Schwartz JC, Sokoloff P (1997). Selective expression of dopamine D3 receptor mRNA in proliferative zones during embryonic development of the rat brain. J Neurosci 17:4282–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J, Pilon C, Le Foll B, Gross C, Triller A, Schwartz JC, Sokoloff P (2000). Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci 20:8677–8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Li R, Huang Y, Li X, Le W (2005). Dopamine D3 receptor-preferring agonists induce neurotrophic effects on mesencephalic dopamine neurons. Eur J Neurosci 22:2422–2430. [DOI] [PubMed] [Google Scholar]
- Freundlieb N, François C, Tandé D, Oertel WH, Hirsch EC, Höglinger GU (2006). Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J Neurosci 26:2321–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA (1999). Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 23:257–271. [DOI] [PubMed] [Google Scholar]
- Höglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC (2004). Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci 7:726–735. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Kapur S, van der Kooy D (2005). Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. J Neurosci 25:5815–5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Dziewczapolski G, Willhoite AR, Kaspar BK, Shults CW, Gage FH (2002). The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci 22:6639–6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB (1993). Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 11:173–189. [DOI] [PubMed] [Google Scholar]
- Maga G, Hubscher U (2003). Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J Cell Sci 116:3051–3060. [DOI] [PubMed] [Google Scholar]
- Marsden CD (1984). The pathophysiology of movement disorders. Neurol Clin 2:435–459. [PubMed] [Google Scholar]
- Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB (1991). The staircase test: A measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods 36:219–228. [DOI] [PubMed] [Google Scholar]
- Ohtani N, Goto T, Waeber C, Bhide PG (2003). Dopamine modulates cell cycle in the lateral ganglionic eminence. J Neurosci 23:2840–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouagazzal AM, Creese I (2000). Intra-accumbens infusion of D3 receptor agonists reduces spontaneous and dopamine-induced locomotion. Pharmacol Biochem Behav 67:637–645. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Ray J, Gage FH (1995). FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci 6:474–486. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Takahashi J, Gage FH (1997). The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci 8:389–404. [DOI] [PubMed] [Google Scholar]
- Parkinson Study Group. (2002). Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA 287:1653–1661. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Weigand SJ, Luskin MB (2001). Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci 21:6706–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon C, Levesque D, Dimitriatdou V, Griffon N, Martres MP, Schwartz JC, Sokoloff P (1994). Functional coupling of the human dopamine D3 receptor in a transfected NG 108–15 neuroblastoma-glioma hybrid cell line. Eur J Pharmacol 268:129–139. [DOI] [PubMed] [Google Scholar]
- Richardson RM, Broaddus WC, Holloway KL, Fillmore HL (2005). Grafts of adult subependymal zone neural progenitor cells rescue hemiparkinsonian behavioral decline. Brain Res 1032:11–22. [DOI] [PubMed] [Google Scholar]
- Rietze R, Poulin P, Weiss S (2000). Mitotically active cells that generate neurons and astrocytes are present in multiple regions of the adult mouse hippocampus. J Comp Neurol 424:397–408. [PubMed] [Google Scholar]
- Sauer H, Oertel WH (1994). Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience 59:401–415. [DOI] [PubMed] [Google Scholar]
- Shan X, Chi L, Bishop M, Luo C, Lien L, Zhang Z, Liu R (2006). Enhanced de novo neurogenesis and dopaminergic neurogenesis in the substantia nigra of MPTP-induced Parkinson's disease-like mice. Stem Cells 24:1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DD, Haber SN (2000). Striatal responses to partial dopaminergic lesion: Evidence for compensatory sprouting. J Neurosci 20:5102–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer GE, Klumperman J, Syed NI (1998). Neurotransmitters and neurodevelopment: role of dopamine in neurite outgrowth, target selection and specific synapse formation. Perspect Dev Neurobiol 5:451–467. [PubMed] [Google Scholar]
- Stanic D, Finkelstein DI, Bourke DW, Drago J, Horne MK (2003). Timecourse of striatal re-innervation following lesions of dopaminergic SNpc neurons of the rat. Eur J Neurosci 18:1175–1188. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Artymyshyn RP, Kung MP, Kung HF, Licki I, McGonigle P (2000). Quantitative autoradiographic mapping of rat brain dopamine D3 binding with [(125)I]7-OH-DPAT: evidence for the presence of D3 receptors on dopaminergic and nondopaminergic cell bodies and terminals. J Pharmacol Exp Ther 295:1223–1231. [PubMed] [Google Scholar]
- Stern MB (2004). Dopamine agonists modify the course of Parkinson's disease. Arch Neurol 61:1969–1971. [DOI] [PubMed] [Google Scholar]
- Van Kampen JM, Robertson HA (2005). A possible role for dopamine D3 receptor stimulation in the induction of neurogenesis in the adult rat substantia nigra. Neuroscience 136:381–386. [DOI] [PubMed] [Google Scholar]
- Van Kampen JM, Hagg T, Robertson HA (2004). Induction of neurogenesis in the adult rat subventricular zone and neostriatum following dopamine D3 receptor stimulation. Eur J Neurosci 19:2377–2387. [DOI] [PubMed] [Google Scholar]
- Vu TQ, Ling ZD, Ma SY, Robie CW, Tong CW, Chen EY, Lipton JW, Carvey PM (2000). Pramipexole attenuates the dopaminergic cell loss induced by intraventricular 6-hydroxydopamine. J Neural Transm 107:159–176. [DOI] [PubMed] [Google Scholar]
- Whone AL, Watts RL, Stoessl AJ, Davis M, Reske S, Nahmias C, Lang AE, Rascol O, Ribeiro MJ, Remy P, Poewe WH, Hauser RA, Brooks DJ (2003). Slower progression of Parkinson's disease with ropinirole versus levodopa: the REAL-PET study. Ann Neurol 54:93–101. [DOI] [PubMed] [Google Scholar]
- Yuan H, Sarre S G. E., Michotte Y (2005). Histological, behavioural and neurochemical evaluation of medial forebrain bundle and striatal 6-OHDA lesions as rat models of Parkinson's disease. J Neurosci Methods 144:35–45. [DOI] [PubMed] [Google Scholar]
- Zhao M, Momma S, Delfani K, Carlen M, Cassidy RM, Johansson CB, Brismar H, Shupliakov O, Frisen J, Janson AM (2003). Evidence for neurogenesis in the adult mammalian substantia nigra. Proc Natl Acad Sci USA 100:7925–7930. [DOI] [PMC free article] [PubMed] [Google Scholar]