Abstract
G-protein-coupled inwardly rectifying K+ channels (Kir3 channels) coupled to metabotropic GABAB receptors are essential for the control of neuronal excitation. To determine the distribution of Kir3 channels and their spatial relationship to GABAB receptors on hippocampal pyramidal cells, we used a high-resolution immunocytochemical approach. Immunoreactivity for the Kir3.2 subunit was most abundant postsynaptically and localized to the extrasynaptic plasma membrane of dendritic shafts and spines of principal cells. Quantitative analysis of immunogold particles for Kir3.2 revealed an enrichment of the protein around putative glutamatergic synapses on dendritic spines, similar to that of GABAB1. Consistent with this observation, a high degree of coclustering of Kir3.2 and GABAB1 was revealed around excitatory synapses by the highly sensitive SDS-digested freeze–fracture replica immunolabeling. In contrast, in dendritic shafts receptors and channels were found to be mainly segregated. These results suggest that Kir3.2-containing K+ channels on dendritic spines preferentially mediate the effect of GABA, whereas channels on dendritic shafts are likely to be activated by other neurotransmitters as well. Thus, Kir3 channels, localized to different subcellular compartments of hippocampal principal cells, appear to be differentially involved in synaptic integration in pyramidal cell dendrites.
Keywords: GABAB1, Kir3, G-protein-coupled receptors, electron microscopy, immunocytochemistry, spillover
Introduction
Inwardly rectifying potassium channels play a crucial role in the control of neuronal excitation by mediating slow inhibitory synaptic responses and contributing to the resting membrane potential (Hille, 1992; Chen and Johnston, 2005). A subfamily of inwardly rectifying channels (Kir3) is directly coupled to G-proteins and mediates the effect of metabotropic receptors in a membrane-delimited manner (Wickman et al., 1994; Huang et al., 1995; Wickman and Clapham, 1995; Schreibmayer et al., 1996; Dascal, 1997; Yamada et al., 1998; Stanfield et al., 2002; Bichet et al., 2003). Kir3 channels serve as a common effector for various neurotransmitters including the major inhibitory transmitter GABA acting on type B receptors (GABABRs) (Andrade et al., 1986; Mihara et al., 1987; North et al., 1987; Trussell and Jackson, 1987; Lüscher et al., 1997; Sharon et al., 1997; Kaupmann et al., 1998; Torrecilla et al., 2002; Chen and Johnston, 2005; Koyrakh et al., 2005; Marker et al., 2005).
The mammalian Kir3 channel subfamily comprises four subunits designated Kir3.1, Kir3.2, Kir3.3, and Kir3.4 (Dascal, 1997). The functional channels exist as homotetrameric or heterotetrameric complexes (Inanobe et al., 1995; Kofuji et al., 1995; Krapivinsky et al., 1995; Slesinger et al., 1996; Spauschus et al., 1996; Wischmeyer et al., 1997). In the CNS, channels are thought to be mainly composed of the Kir3.1 and Kir3.2 subunits (Duprat et al., 1995; Lesage et al., 1995; Leaney, 2003). Recent evidence further suggests that the Kir3.2 subunit is an essential part of the functional channel, determining its assembly and surface localization (Inanobe et al., 1999; Ma et al., 2002). Indeed, lack of Kir3.2 leads to reduced Kir3.1 expression (Liao et al., 1996; Signorini et al., 1997) and to loss of slow inhibitory postsynaptic responses in hippocampal pyramidal cells (Lüscher et al., 1997).
Neurons in the hippocampal formation express high levels of Kir3 subunit transcripts (Dixon et al., 1995; Kobayashi et al., 1995; Karschin et al., 1996; Liao et al., 1996). Although a large body of electrophysiological and pharmacological data are available on the function of these channels (Gähwiler and Brown, 1985; Andrade et al., 1986; Andrade and Nicoll, 1987; Nicoll, 1988; Sodickson and Bean, 1996; Lüscher et al., 1997; Takigawa and Alzheimer, 2002, 2003; Leaney, 2003; Chen and Johnston, 2005), the localization of Kir3 in various subcellular compartments of principal cell remains mostly unknown. Previous immunohistochemical studies showed high levels of Kir3 channels in the dendrites of pyramidal cells (Ponce et al., 1996; Drake et al., 1997), suggesting a subcellular distribution similar to that of GABAB receptors (Kulik et al., 2003). To investigate the spatial relationship of the channel and receptor, we studied the subcellular localization of the Kir3.2 subunit and determined the compartment-dependent colocalization of Kir3.2 and GABAB1 in pyramidal cells by using high-resolution immunocytochemical techniques. Interestingly, we found that Kir3.2 and GABAB1 are mostly segregated on dendritic shafts, contacted by inhibitory GABAergic boutons, whereas the two proteins are highly colocalized on dendritic spines adjacent to the excitatory synapses.
Materials and Methods
Antibodies and controls
Antibodies.
An affinity-purified polyclonal antibody specific for the C-terminal domain of the Kir3.2 subunit (Lesage et al., 1994; Isomoto et al., 1996) was purchased from Alomone Labs (Jerusalem, Israel). This antibody recognizes the Kir3.2a and Kir3.2c splicing isoforms. It additionally recognizes the Kir3.2d isoform, which is predominantly expressed in testis (Inanobe et al., 1999). To localize GABAB receptors composed of GABAB1 and GABAB2 subunits (Kaupmann et al., 1998), two affinity-purified polyclonal antibodies recognizing both a and b splice variants of the GABAB1 subunit were used. The first antibody (B17) was raised in rabbits, and its characteristics and specificity have been described previously (Kulik et al., 2002, 2003). The second antibody (B62) was raised in guinea pigs against a GST fusion protein containing amino acid residues 857–960 of the GABAB1 protein (Kaupmann et al., 1997). Its specificity was confirmed by immunoblot analysis: it gave rise to two immunoreactive bands with molecular masses of 130 and 100 kDa corresponding to GABAB1a and GABAB1b proteins (supplemental Fig. 1A, available at www.jneurosci.org as supplemental material). The B62 antibody yielded an immunoreactive pattern in the hippocampus (supplemental Fig. 1B, available at www.jneurosci.org as supplemental material) similar to that obtained with the well characterized B17 antibody. To identify the postsynaptic density of excitatory synapses, a monoclonal anti-postsynaptic density-95 (PSD-95) antibody was also used (Upstate Biotechnology, Lake Placid, NY). To determine the spatial relationship of GABAB receptors and the K+–Cl− cotransporter 2 (KCC2) an antibody against the cotransporter (Upstate Biotechnology) was used.
Figure 1.
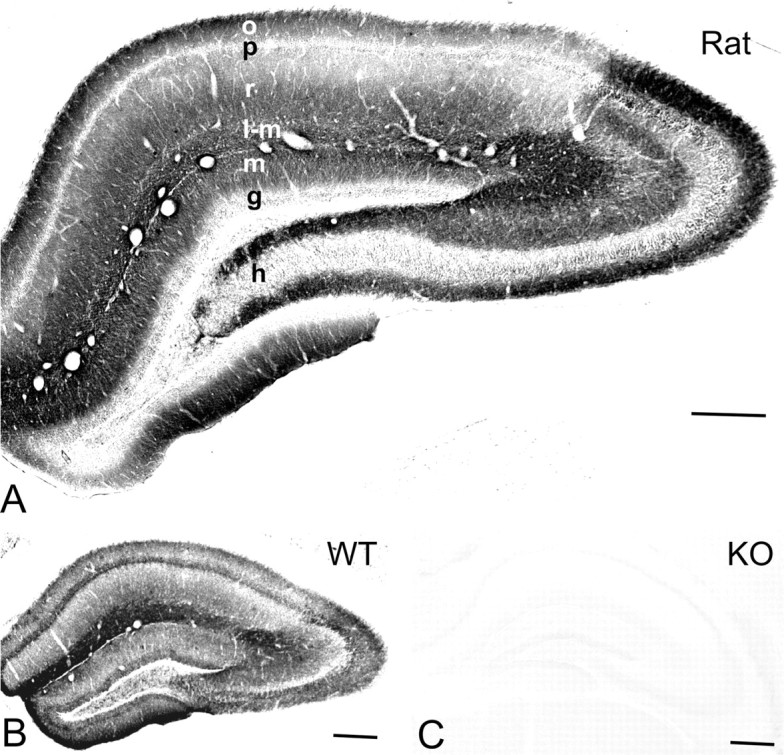
Distribution of immunoreactivity for the Kir3.2 subunit in the hippocampus. A, B, The immunostaining was moderate to strong in dendritic layers of the CA area and dentate gyrus in the rat (A) and wild-type (WT) mouse (B). In the CA1, the immunolabeling for the protein was strong and homogeneous in the stratum lacunosum-moleculare, whereas the strata oriens and radiatum showed uneven immunostaining with moderate intensity of immunoreactivity in the proximal half and high intensity in the distal half of these layers. In CA3, the immunoreactivity for Kir3.2 was strong in the strata oriens, radiatum, and lacunosum-moleculare. In the dentate gyrus, strong immunostaining was detected in the hilus and moderate in the molecular layer. C, No immunoreactivity for Kir3.2 was found in the hippocampus of the Kir3.2-deficient (KO) mice. Scale bars, 200 μm. o, Stratum oriens; p, stratum pyramidale; r, stratum radiatum; l-m, stratum lacunosum-moleculare; m, stratum moleculare; g, stratum granulosum; h, hilus.
Controls.
The specificity of the immunolabeling for Kir3.2 and GABAB1 in these experiments was controlled by (1) staining of sections obtained from either Kir3.2- (Torrecilla et al., 2002) or GABAB1-deficient mice (Schuler et al., 2001), and (2) in case of double- and triple-labeling experiments, omitting one of the primary antibodies. No immunolabeling was detected on sections and replicas derived from Kir3.2- or GABAB1-deficient mice (supplemental Fig. 1C, available at www.jneurosci.org as supplemental material) stained with the respective primary antibodies in preembedding and replica experiments, further confirming specificity of the antibodies. When one of the primary antibodies was omitted, but secondary antibodies were included, no immunolabeling was detected for the respective protein excluding the possibility of cross-reactivity of the primary and secondary antibodies.
Immunoblot analysis
Immunoblot analysis was performed as described previously (Shigemoto et al., 1997). The crude membrane preparations from adult rat forebrain were separated by 7.5% SDS-PAGE and transferred onto polyvinylidene difluoride (Bio-Rad, Hercules, CA) membrane. The membrane was blocked with Block-Ace (Dainippon Pharmaceutical, Suita, Japan) and then reacted with the affinity-purified GABAB1 (B62) antibody (0.5 μg/ml). An alkaline phosphatase-labeled secondary antibody (1:5000; Chemicon, Temecula, CA) was used to visualize protein bands.
Immunocytochemistry
A total of 19 adult male Wistar rats (Charles River, Freiburg, Germany), 7 adult wild-type mice, 5 Kir3.2-deficient mice, and 6 GABAB1-deficient mice were used in the present study. Care and handling of the animals before and during the experiments followed European Union regulations and was approved by the animal care and use committees of the authors’ institutions.
Preembedding immunocytochemistry
Immunohistochemical labeling for light and electron microscopy was performed as described previously (Kulik et al., 2002). For light microscopy, animals (n = 5 rats; n = 3 wild-type mice; n = 2 Kir3.2-deficient mice; n = 3 GABAB1-deficient mice) were deeply anesthetized with Narkodorm-n (pentobarbital; 180 mg/kg, i.p.) (Alvetra, Neumünster, Germany) and perfused transcardially with 4% paraformaldehyde (Merck, Darmstadt, Germany), 15% saturated picric acid, and 0.05% glutaraldehyde (Polyscience, Warrington, PA) made up in 0.1 m phosphate buffer (PB). Tissue blocks were cryoprotected and freeze-thawed, and sections were cut (40 μm). Sections were incubated with 0.6 μg/ml primary antibody for Kir3.2 in 25 mm PBS containing 3% normal goat serum (NGS) (Vector Laboratories, Burlingame, CA) and 0.1% Triton X-100. After washes in PBS, the sections were incubated with biotinylated goat anti-rabbit IgG antibody (1:100; Vector Laboratories), then reacted with avidin–biotin peroxidase complex (ABC kit; 1:100; Vector Laboratories), and finally incubated with 0.025% 3,3′-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO) and 0.003% hydrogen peroxide. For electron microscopy, animals (n = 8 rats; n = 2 wild-type mice; n = 1 Kir3.2-deficient mouse; n = 1 GABAB1-deficient mouse) were perfused with the same fixative as described for light microscopy. Sections (50 μm) were incubated in a blocking solution followed by the primary antibodies (2.0–3.0 μg/ml) diluted in Tris-buffered saline (TBS) containing 3% NGS. After washing, the sections were incubated with 1.4 nm gold-coupled goat anti-rabbit or goat anti-guinea pig secondary antibodies (Fab fragment; 1:100; Nanogold; Nanoprobes, Stony Brook, NY), and then reacted with HQ Silver kit (Nanoprobes). After treatment with OsO4, the sections were stained with uranyl acetate, dehydrated, and flat-embedded in epoxy resin (Durcupan; ACM; Fluka; Sigma).
Three-dimensional reconstruction and quantification of Kir3.2 and GABAB1 immunoreactivity
The three-dimensional (3D) reconstruction of CA1 pyramidal cell dendritic spines and shafts immunoreactive for either the Kir3.2 or GABAB1 subunits was performed from serial ultrathin sections obtained from preembedding material as described previously (Kulik et al., 2002). Samples were taken from the very surface (<3 μm) of blocks containing strata oriens, radiatum, or lacunosum-moleculare of CA1. For each immunoparticle (located within 25 nm from the membrane), the distances to the closest edge of asymmetrical and symmetrical synapses were measured along the surface of the 3D reconstructed profiles.
SDS-digested freeze–fracture replica immunolabeling
Animals (n = 6 rats; n = 2 wild-type mice; n = 2 Kir3.2-deficient mice; n = 2 GABAB1-deficient mice) were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p), and the hearts were surgically exposed for perfusion fixation. First, the vascular system was flushed by circulating 25 mm PBS for 1 min. This was followed by transcardial perfusion with a fixative containing 2% paraformaldehyde and 15% saturated picric acid made up in 0.1 m PB. Sagittal sections from the CA1 or the CA3 area were cut on a microslicer at a thickness of 90 μm. The slices were cryoprotected in a solution containing 30% glycerol made up in 0.1 m PB overnight at 4°C, and then were frozen by a high-pressure freezing machine (HPM 101; BAL-TEC, Balzers, Lichtenstein). Frozen samples were inserted into a double replica table and then fractured into two pieces at −115°C. Fractured faces were replicated by deposition of carbon (2–3 nm thickness), platinum (2 nm), and carbon (20 nm) in a freeze–fracture replica machine (BAF 060; BAL-TEC). They were digested in a solution containing 2.5% SDS and 20% sucrose made up in 15 mm Tris buffer, pH 8.3, at 105°C for 15 min followed by their incubation in the same solution overnight at room temperature. The replicas were washed in 25 mm TBS containing 0.05% bovine serum albumin (BSA) (Nacalai Tesque, Kyoto, Japan) and incubated in a blocking solution containing 5% BSA in 25 mm TBS for 1 h. Subsequently, the replicas were incubated in the primary antibody for Kir3.2 or, in double- and triple-immunolabeling experiments, with mixtures of primary antibodies (20–25 μg/ml) for Kir3.2 and GABAB1 or Kir3.2, GABAB1, and PSD-95 diluted in TBS containing 5% BSA overnight at room temperature. In control experiments, replicas were incubated in a mixture of primary antibodies for KCC2, GABAB1, and PSD-95 diluted in the same solution as described above. After several washes, the replicas were reacted with a mixture of gold-coupled goat anti-rabbit (for Kir3.2 or KCC2), goat anti-guinea pig (for GABAB1), and goat anti-mouse (for PSD-95) secondary antibodies (1:30; BioCell Research Laboratories, Cardiff, UK) made up in 25 mm TBS containing 5% BSA overnight at room temperature. They were then washed and picked up on 100-mesh grids. For quantitative analysis, samples were taken from layers of CA1 and CA3 double-immunolabeled for the Kir3.2 and GABAB1 subunits. Clusters of immunoparticles for Kir3.2 and GABAB1 were determined by outlining the areas covered by immunogold particles (three particles or more within a distance of 50 nm) on dendritic shafts and on dendritic spines of pyramidal cells. Distances between the center of clusters of Kir3.2 and the closest clusters of GABAB1 were measured along the surface of the profiles. The relative frequency for the cluster pairs was determined by binning the data set at 50 nm. Values are expressed as mean ± SEM, and for statistical comparison the nonparametric double-sided nonpaired Wilcoxon–Mann–Whitney test was used.
Results
Kir3.2 immunoreactivity in dendritic layers of the hippocampus
The use of the Kir3.2 affinity-purified antibody revealed a specific pattern of immunostaining in the rat (Fig. 1A) and wild-type mouse hippocampus (Fig. 1B). The immunoreactivity for the protein was widely distributed in the hippocampus with moderate to strong staining in dendritic layers. In CA1, the immunolabeling for Kir3.2 was homogeneously strong in the stratum lacunosum-moleculare, whereas the strata radiatum and oriens showed an uneven immunostaining with moderate intensity in the proximal half and high intensity in the distal half of these layers. In CA3, the immunoreactivity was strongest in strata oriens, radiatum, and lacunosum-moleculare, whereas in the stratum lucidum it was moderate. In the dentate gyrus, strong immunostaining for the subunit was detected in the hilus and moderate in the molecular layer. In the pyramidal and granule cell layers, weak labeling was observed. No immunoreactivity for Kir3.2 was detected in the white matter. In sections obtained from Kir3.2-deficient mice, the specific immunolabeling pattern was completely abolished (Fig. 1C).
Kir3.2 is preferentially localized to extrasynaptic membrane of dendrites
To determine the subcellular localization of Kir3.2 responsible for the immunostaining in dendritic layers, we used preembedding immunogold labeling. For electron-microscopic investigation, tissue blocks were taken from the CA1 area. Immunoreactivity for the Kir3.2 subunit was primarily found in postsynaptic elements, namely, on dendritic shafts and spines of putative pyramidal cells (Fig. 2). Clusters of immunogold particles were localized to the extrasynaptic plasma membrane of dendritic shafts (Fig. 2A,B,F,G,I) establishing symmetrical (putative GABAergic) synapses with presynaptic boutons (Fig. 2B,G). Strong immunolabeling was also found on the extrasynaptic membrane of dendritic spines (Fig. 2A,C–F,H,I). Immunoparticles also appeared occasionally at the edge (Fig. 2D,F) and over the postsynaptic membrane specialization of asymmetrical, putative glutamatergic synapses on dendritic spines (Fig. 2C). This predominantly extrasynaptic localization of the channel is in good agreement with the finding of Nehring et al. (2000), who showed that the Kir3.2 subunit is unable to form a complex with PSD-95 at postsynaptic sites. In addition to pyramidal cells, immunoreactivity for Kir3.2 was also seen on the extrasynaptic plasma membrane of putative interneuron dendrites (Fig. 2I), identified by the lack of dendritic spines and the presence of asymmetrical synapses. In contrast to the strong dendritic labeling, very little immunolabeling was seen in somata of pyramidal cells under our experimental conditions. Presynaptically, weak immunoreactivity for Kir3.2 was occasionally detected in axon terminals making asymmetrical synapses with dendritic spines in strata oriens and radiatum (Fig. 2C,E). Immunoparticles were localized either to the extrasynaptic plasma membrane (Fig. 2C) or to the presynaptic membrane specialization of boutons (Fig. 2E). The specificity of the labeling in preembedding material was confirmed by the absence of labeling in Kir3.2-deficient animals.
Figure 2.
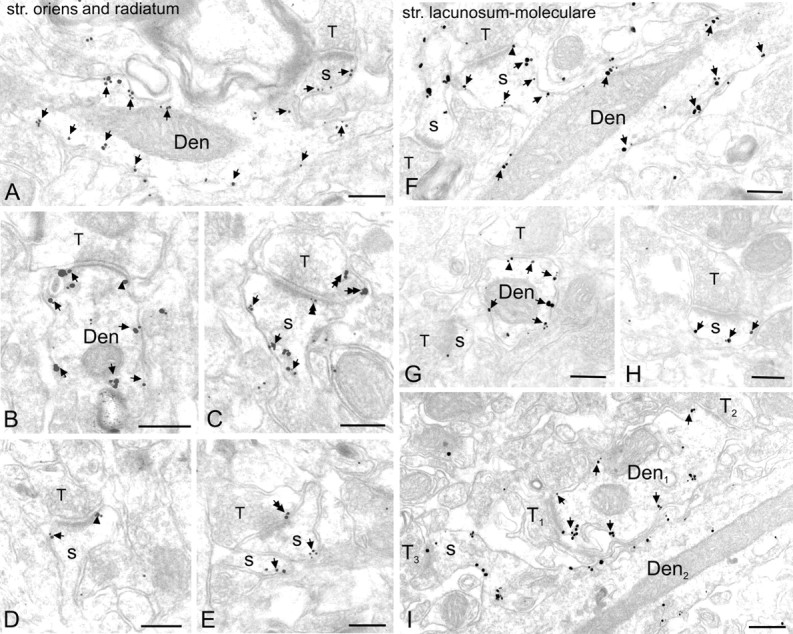
Preferential postsynaptic localization of Kir3.2 in dendritic layers of the CA1 area. Electron micrographs show immunoreactivity for the Kir3.2 subunit in the strata oriens (A, C), radiatum (B, D, E), and lacunosum-moleculare (>F–I) as detected by the preembedding immunogold method. A, B, F, G, I, Clusters of immunogold particles were seen along the extrasynaptic plasma membrane (arrows) of dendritic shafts (Den) of pyramidal cells contacted by terminals (T) of presumed GABAergic cells. Labeling was occasionally found at the edge of symmetrical synaptic specializations (arrowheads in B, G). A, C–F, H, I, Immunoparticles were abundant on the extrasynaptic plasma membrane (arrows) of dendritic spines of pyramidal cells (s). They also appeared occasionally over the postsynaptic specialization (double arrowhead in C) at synapses between axon terminals (T) of putative pyramidal cells and dendritic spines and at the edge of asymmetrical synapses (arrowheads in D, F). C, E, Presynaptically, immunogold particles (double arrows) were localized to the extrasynaptic plasma membrane and to the presynaptic membrane specialization of axon terminals (T) establishing asymmetrical synapses. I, Immunolabeling was also visible in dendritic shafts of presumed interneurons (Den1) establishing asymmetrical synapses with presynaptic boutons (T1, T2). Note that the dendritic spine (s), contacted by an axon terminal (T3), and the dendritic shaft (Den2) of a pyramidal cell are also labeled. Scale bars, 0.2 μm.
Enrichment of Kir3.2 around excitatory synapses on dendritic spines
The pattern of the subcellular distribution of Kir3.2, particularly the strong labeling on dendritic spines of hippocampal pyramidal cells, is very similar to that of GABAB receptors (Kulik et al., 2003). To compare the distribution of the Kir3.2 and GABAB1 on dendrites of pyramidal cells in relation to putative GABAergic and glutamatergic synapses, three-dimensional (3D) reconstructions were made from serial ultrathin sections and the distances of the immunoparticles from the edge of symmetrical and asymmetrical synaptic specializations were measured. This approach revealed that, on dendritic shafts, the channel and the receptor showed no association to symmetrical, putative GABAergic synapses (Fig. 3A) (209 particles on eight dendrites for Kir3.2 and 241 particles on seven dendrites for GABAB1). In contrast, on dendritic spines, both Kir3.2 and GABAB1 were found to be preferentially localized around asymmetrical synapses. The distribution for Kir3.2 showed a peak between 0 and 240 nm from the synapses (Fig. 3B) (313 particles on 66 spines). Similarly, for GABAB1 the peak of the distribution was located between 60 and 240 nm (Fig. 3B) (325 particles on 49 spines), consistent with previous data obtained from CA1 and CA3 areas (Kulik et al., 2003). For both proteins ∼60% of the immunoparticles were located within a distance of 240 nm from the edge of asymmetrical synapses indicating an enrichment of the molecules in the vicinity of putative glutamatergic synapses on dendritic spines.
Figure 3.
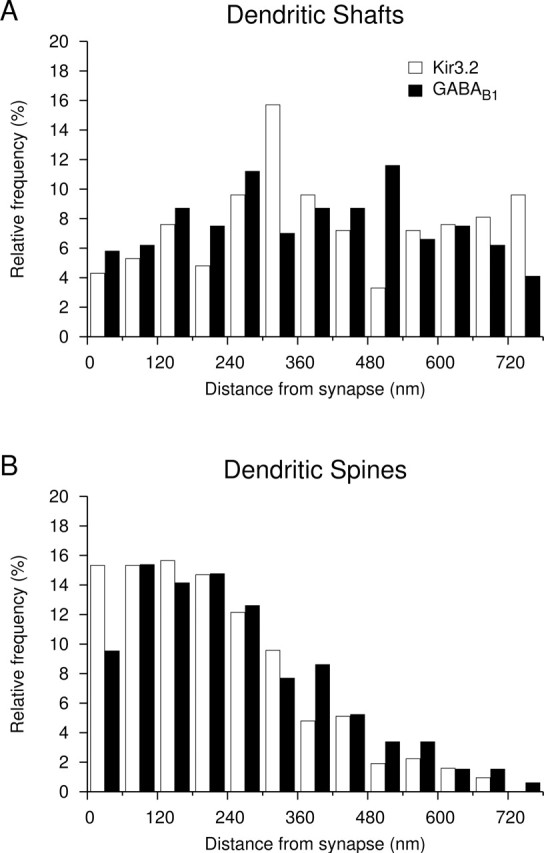
Distribution of immunoparticles for the Kir3.2 and GABAB1 subunits relative to symmetrical and asymmetrical synapses on dendrites of CA1 pyramidal cells as assessed by preembedding immunogold labeling. A, Histogram showing the spatial distribution of immunoparticles for Kir3.2 (open bars; n = 209) and GABAB1 (filled bars; n = 241) around symmetrical synapses on dendritic shafts. Distances of immunogold particles were measured from the closest edge of the synapses along the surface of dendritic shafts reconstructed from serial ultrathin sections. Values were allocated to 60-nm-wide bins and expressed as relative frequencies. B, Histogram showing the spatial distribution of immunoparticles for Kir3.2 (n = 313) and GABAB1 (n = 325) around asymmetrical synapses on dendritic spines. These data show that there is no association of Kir3.2 and GABAB1 to symmetrical, putative GABAergic synapses on dendritic shafts, but there is an enrichment of both proteins in the vicinity of asymmetrical, putative glutamatergic synapses on dendritic spines.
Preferential colocalization of Kir3.2 and GABAB1 on dendritic spines of CA1 pyramidal cells
To directly investigate the colocalization of Kir3.2 and GABAB1 in subcellular compartments of CA1 pyramidal cells, we performed double- and triple-labeling immunocytochemistry by using the highly sensitive SDS-digested freeze–fracture replica immunolabeling technique (Hagiwara et al., 2005; Tanaka et al., 2005). Consistent with the results of preembedding experiments, strong immunolabeling for Kir3.2 was found postsynaptically. Clusters of immunoparticles were observed on the protoplasmic face of the membrane of dendritic shafts and spines of putative pyramidal cells (Fig. 4A). Double immunolabeling for Kir3.2 and GABAB1 further revealed that, on dendritic shafts, the channels and receptors were mainly segregated (Fig. 4B), whereas on dendritic spines, a high degree of coclustering of the immunogold particles for the two proteins was observed (Fig. 4C). To quantify the spatial relationship of the channel and receptor subunits, the distances between clusters of Kir3.2 and the closest clusters of GABAB1 were measured on dendritic shafts and spines (Fig. 4D). This analysis revealed that, on dendritic shafts, only 84 of 302 clusters (28%) were within 100 nm, whereas on dendritic spines 86 of 90 clusters (96%) were within this distance (Fig. 4D). The location of the Kir3.2–GABAB1 complexes relative to excitatory synaptic sites, demarcated by the presence of PSD-95, an essential component of the excitatory postsynaptic specialization, was investigated in triple-immunolabeling experiments. The Kir3.2–GABAB1 coclusters were found on the extrasynaptic membrane close to the location of PSD-95 immunoreactivity on dendritic spines (Fig. 4E,F). Weak Kir3.2 and GABAB1 immunolabeling was found in putative excitatory terminals, but no coclustering of the proteins was observed. Thus, our results demonstrate that, on dendritic shafts, where mostly GABAergic synapses are located, the Kir3.2-containing inwardly rectifying K+ channels and GABAB receptors are mainly segregated, whereas on dendritic spines, adjacent to glutamatergic synapses, the two proteins show a close association.
Figure 4.
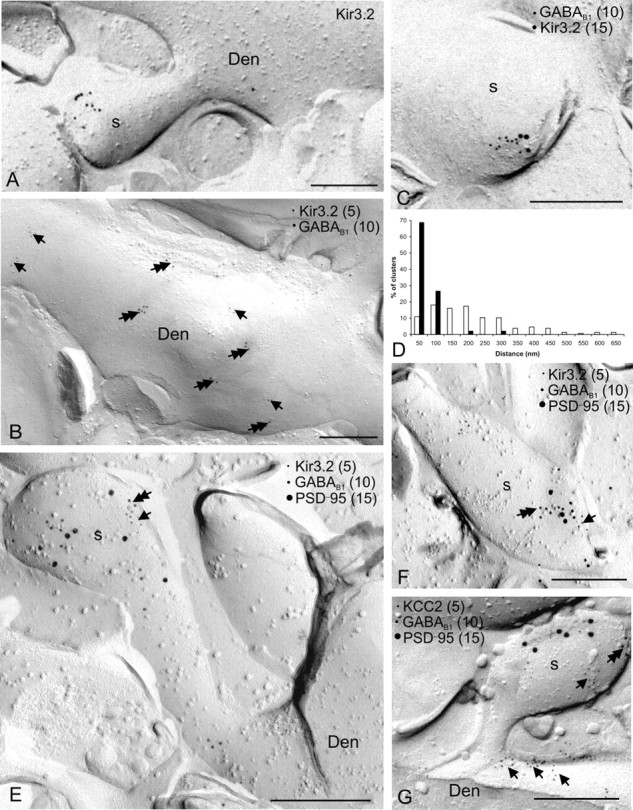
Colocalization of Kir3.2 and GABAB1 on dendritic spines of CA1 pyramidal cells. Localization of the Kir3.2 subunit and its colocalization with the GABAB1 subunit is demonstrated by the SDS-digested freeze–fracture replica labeling technique. A, Immunoparticles for Kir3.2 were found on dendritic spines (s) of principal cells. B, Double immunogold labeling for Kir3.2 (5 nm particles; arrows) and GABAB1 (10 nm; double arrows) revealed that the two proteins were mainly segregated on dendritic shafts of pyramidal cells (Den). C, Double labeling for Kir3.2 (15 nm) and GABAB1 (10 nm) showed that the two proteins coclustered on dendritic spines of pyramidal cells (s). D, Histogram showing the spatial relationship between clusters of Kir3.2 and GABAB1 on dendritic shafts (n = 302 clusters; open bars) and on dendritic spines (n = 90 clusters; filled bars). Distances were measured between the center of Kir3.2 clusters and the closest GABAB1 cluster. Values were allocated to 50-nm-wide bins and expressed as relative frequencies. E, F, Triple immunolabeling for Kir3.2 (5 nm), GABAB1 (10 nm), and PSD-95 (15 nm) demonstrated the coclustering of the Kir3.2 (arrows) and GABAB1 (double arrows) subunits around the site of the location of the PSD-95, indicating a close localization of Kir3.2-GABAB1 to glutamatergic synapses on dendritic spines of pyramidal cell. G, The spatial relationship of GABAB (double arrows) receptors and the functionally unrelated KCC2 (arrows) was also investigated on dendritic spines. Two proteins were found to be preferentially segregated in this subcellular compartment. Scale bars, 0.2 μm.
To assess the functional relevance of the observed association, we investigated the spatial relationship of molecules on dendritic spines that are functionally unrelated to GABAB receptors. To this end, we have performed immunogold labeling for KCC2, GABAB1, and PSD-95. This experiment revealed that, although immunoreactivity for KCC2 is abundantly localized to the dendritic spines (Gulyás et al., 2001), the cotransporter and the receptor were found to be mainly segregated (Fig. 4G) in this compartment.
Colocalization of Kir3.2 and GABAB1 on CA3 pyramidal cells
Finally, we investigated the distribution and colocalization of Kir3.2 and GABAB1 in the CA3 region. Samples were taken from the stratum oriens and processed for Kir3.2, GABAB1, and PSD-95 immunolabeling using the replica technique. Similarly to the CA1 area, clusters of immunogold particles for Kir3.2 were found on dendritic shafts (Fig. 5A) and spines (Fig. 5B). The mean number of particles per cluster on the dendritic shafts of the CA3 area was, however, higher (6.4 ± 0.2 particles/cluster; 162 clusters) compared with the CA1 area (4.2 ± 0.2 particles/cluster; 93 clusters; p < 0.01) in a good agreement with the difference in the staining intensity observed at the light-microscopic level (Fig. 1A). Despite this difference in cluster size, the colocalization pattern of Kir3.2 and GABAB1 was similar to that of the CA1 area. On dendritic shafts, the channel and receptor were mainly segregated (Fig. 5A), whereas on dendritic spines, the proteins showed a high level of colocalization (Fig. 5B).
Figure 5.
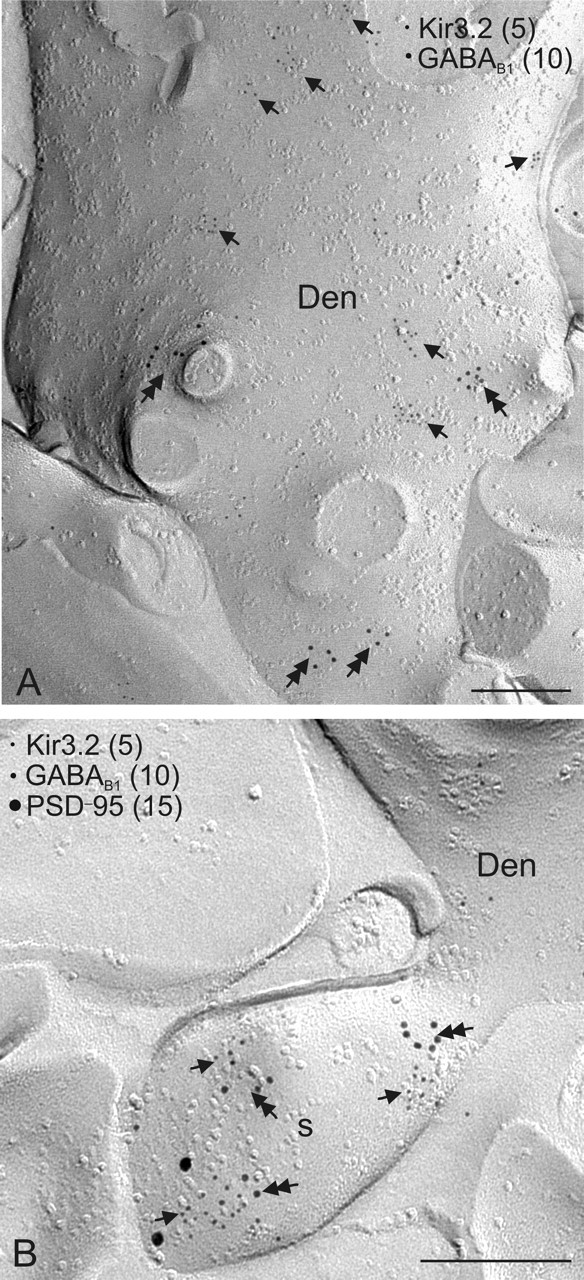
Colocalization of Kir3.2 and GABAB1 on CA3 pyramidal cells as assessed by the SDS-digested freeze–fracture replica labeling technique. The spatial relationship of Kir3.2 and GABAB was investigated as described in Figure 4.A, B, The proteins were found to be mainly segregated on dendritic shafts of pyramidal cells (Den), whereas the channel and the receptor were coclustered on dendritic spines (s) around glutamatergic synapses. Scale bars, 0.2 μm.
Discussion
The present study describes the subcellular localization of the Kir3.2 subunit of the G-protein-coupled inwardly rectifying K+ channel and its spatial relationship to GABAB receptor in the adult rat hippocampus. Kir3 channel proteins were primarily found postsynaptically and localized to dendritic shafts and dendritic spines of pyramidal cells. Double immunolabeling for Kir3.2 and GABAB1 using the replica technique revealed that, on dendritic shafts, the two proteins were mostly segregated, whereas on dendritic spines, around putative glutamatergic synapses, a high degree of coclustering of the ion channel and receptor subunits was observed. Immunolabeling for KCC2, a protein with no known functional association with GABAB receptors, showed that the transporter and the receptor were mainly segregated on dendritic spines. Thus, the observed close spatial relationship of Kir3.2 and GABAB1 likely reflects their functional interaction in this subcellular compartment.
Kir3.2 is preferentially localized to dendritic shafts and spines in hippocampal pyramidal cells
The Kir3.2 is the most abundant subunit of the Kir3 channel in the hippocampus as shown by previous in situ hybridization (Kobayashi et al., 1995; Karschin et al., 1996; Liao et al., 1996) and immunocytochemical studies (Liao et al., 1996; Signorini et al., 1997; Inanobe et al., 1999; Koyrakh et al., 2005). It has been further suggested that this subunit plays an essential role in the assembly and surface localization of functional channels (Inanobe et al., 1999; Ma et al., 2002). Accordingly, our results, obtained by using an antibody recognizing the Kir3.2a and Kir3.2c isoforms, but not the ubiquitously expressed Kir3.2b splice variant (Isomoto et al., 1996), show that the Kir3.2 protein was widely distributed in the hippocampus and the immunolabeling was particularly strong in dendritic layers.
At the ultrastructural level, the majority of the Kir3.2 subunits were observed on the extrasynaptic membrane of dendritic shafts and spines and was hardly detectable on somata of pyramidal cells. These immunocytochemical data thus underlie the dominant postsynaptic role of Kir3 channels observed in previous electrophysiological studies (Andrade et al., 1986; Lüscher et al., 1997; Kurachi and Ishii, 2003). Furthermore, the dendritic localization of the subunit corresponds well to the fact that Kir3-mediated currents are significantly larger in dendrites than in somata of hippocampal neurons (Newberry and Nicoll, 1985; Inanobe et al., 1999; Takigawa and Alzheimer, 1999; Chen and Johnston, 2005). This preferential dendritic localization offers an optimal position, on the one hand, for the modulation of the channels by various G-protein-coupled receptors residing in the dendritic compartments (Dournaud et al., 1996; Kia et al., 1996; Lujan et al., 1997, Shigemoto et al., 1997; Kulik et al., 2003). On the other hand, dendritic channels can be efficiently involved in the integration of synaptic inputs. Kir3 channels were shown to contribute to the resting membrane potential on dendrites (Chen and Johnston, 2005) and can thereby modulate the amplification of synaptic potentials by voltage-gated channels (Johnston et al., 1996). Kir3 channels associated with glutamatergic synapses can counteract excitatory postsynaptic responses by hyperpolarization and by shunting the excitatory synaptic currents (Takigawa and Alzheimer, 2003). Furthermore, after activation by GABAB receptors, these channels can also act as a brake on NMDA receptor responses by favoring their Mg2+ block and resulting in reduced synaptic plasticity (Otmakhova and Lisman, 2004). Conversely, activation of NMDA receptors results in the potentiation of the GABAB- and Kir3-mediated slow inhibitory synaptic response (Huang et al., 2005) that parallels with the long-term potentiation of excitatory transmission.
In addition to the strong postsynaptic labeling, a low but consistent presynaptic immunoreactivity for Kir3.2 was detected. Similar results were obtained for three subunits, Kir3.1, Kir3.2, and Kir3.3, in various brain regions in previous immunocytochemical studies (Liao et al., 1996; Morishige et al., 1996; Ponce et al., 1996; Drake et al., 1997; Grosse et al., 2003). Although the function of the presynaptic Kir3 channels remains unknown (Lüscher et al., 1997), their proximity to the axonal active zones strongly suggests an involvement in the regulation of neurotransmitter release.
Predominant colocalization of Kir3.2 with GABAB1 on dendritic spines of pyramidal cells
The coupling between Kir3 channels and receptors is mediated by G-proteins in a membrane-delimited manner (Pfaffinger et al., 1985; Dascal, 1997; Yamada et al., 1998). Theoretical considerations suggest that the distance between receptor and effector should be small (e.g., <500 nm) (Karschin, 1999) to enable their interaction. Moreover, it was hypothesized that preformed receptor–ion channel complexes could exist ensuring reliable and efficient coupling. The rapid activation of Kir3 channels by GABAB receptors in response to synaptically released GABA would support this latter hypothesis (Otis et al., 1993). To address the spatial relationship of Kir3.2 and the GABAB receptors, we used the highly sensitive SDS-digested freeze–fracture replica immunolabeling method, which provides a means for visualizing the distribution of molecules over the surface of the plasma membrane (Hagiwara et al., 2005). The results of this approach revealed that, on dendritic shafts of the pyramidal cells, contacted by GABAergic boutons, ion channels and receptors were mainly segregated, whereas on dendritic spines, contacted by excitatory terminals, a high degree of coclustering of the proteins was detected.
The observed distribution of the ion channels and GABAB receptors on dendritic shafts raises the question how segregated channels are activated. First, the Kir3.2-containing channels may cocluster with and couple to other G-protein-coupled receptors (e.g., adenosine A1, 5-HT1A, D2) (Andrade et al., 1986; Nicoll, 1988; Liao et al., 1996; Ehrengruber et al., 1997; Lüscher et al., 1997; Takigawa and Alzheimer, 1999). This possibility is supported by the results of electrophysiological experiments in which the GABAB receptor agonist baclofen evoked Kir3 currents only in a subset of isolated patches of pyramidal cell dendrites, whereas agonists of other metabotropic receptors could elicit currents in nonresponsive ones (Takigawa and Alzheimer, 1999; Chen and Johnston, 2005). Second, these channels may also be activated by GABAB receptors. Despite the segregation of Kir3.2 and GABAB1, the mean distance between molecules may be sufficient for functional interaction (Karschin, 1999), although, in this scenario, the coupling is expected to be less efficient and less reliable. Finally, constitutively active dendritic Kir3 channels have been observed in CA1 pyramidal cells (Chen and Johnston, 2005). Although the identity and the subunit composition of these ion channels are unclear, they may correspond to a segregated channel population.
It was proposed that GABAB receptor localization to dendritic spines is important for the modulation of metabotropic glutamate receptors (Hirono et al., 2001; Tabata et al., 2004). However, the intimate spatial relationship of GABAB receptors and Kir3 channels on dendritic spines around excitatory synapses, observed in this study, is suggestive of a functional coupling of the two proteins and may reflect the existence of preformed complexes. This scenario is further supported by the observation that the functionally unrelated KCC2 and GABAB1 were mainly found to be segregated in this subcellular compartment. In turn, the high level of colocalization of Kir3.2 and GABAB1 indicates that the ion channels strategically located to interact with individual glutamatergic synapses are primarily under the control of the inhibitory transmitter GABA spilling over from GABAergic synapses (Isaacson et al., 1993; Kulik et al., 2003; Cryan and Kaupmann, 2005).
In summary, the present study shows that the Kir3.2-containing K+ channels are preferentially located on the extrasynaptic membrane of hippocampal pyramidal cells and can be divided into two major populations with different roles in synaptic integration. Kir3 channels on dendritic shafts can mediate the effect of various transmitter systems including subcortical projections that show behavior state-dependent activity (Pace-Schott and Hobson, 2002). In contrast, Kir3 channels on dendritic spines appear to preferentially mediate the effect of GABA released from local feedback and feedforward inhibitory circuits via GABAB receptors. Thus, this channel population can provide a spatially and temporally well defined control to excitatory transmission by the GABAergic system.
Footnotes
This work was supported by Deutsche Forschungsgemeinschaft, Sonderforschungsbereiche 505 (A.K., I.V., M.F.), the Swiss Science Foundation (B.B.), Neurex (B.B., M.F.), and National Institutes of Health Grants MH61933 and DA011806 (K.W.). We are grateful to Prof. Peter Jonas for his helpful comments on this manuscript. We thank Dr. Andrea Lörincz, Sanae Hara, Emi Kamiya, Anikó Schneider, and Sigrun Nestel for their support with tissue preparation for the SDS-FRL and electron microscopy.
References
- Andrade R, Nicoll RA (1987). Pharmacologically distinct actions of serotonin on single pyramidal neurons of the rat hippocampus recorded in vitro. J Physiol (Lond) 394:99–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade R, Malenka RC, Nicoll RA (1986). A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science 234:1261–1265. [DOI] [PubMed] [Google Scholar]
- Bichet D, Haass FA, Jan LY (2003). Merging functional studies with structures of inward rectifier K+ channels. Nat Rev Neurosci 4:957–967. [DOI] [PubMed] [Google Scholar]
- Chen X, Johnston D (2005). Constitutively active G-protein-gated inwardly rectifying K+ channels in dendrites of hippocampal CA1 pyramidal neurons. J Neurosci 25:3787–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Kaupmann K (2005). Don’t worry “B” happy!: a role for GABAB receptors in anxiety and depression. Trends Pharmacol Sci 26:36–43. [DOI] [PubMed] [Google Scholar]
- Dascal N (1997). Signalling via the G protein-activated K+ channels. Cell Signal 9:551–573. [DOI] [PubMed] [Google Scholar]
- Dixon AK, Gubitz AK, Ashford ML, Richardson PJ, Freeman TC (1995). Distribution of mRNA encoding the inwardly rectifying K+ channel, BIR1 in rat tissues. FEBS Lett 374:135–140. [DOI] [PubMed] [Google Scholar]
- Dournaud P, Gu YZ, Schonbrunn A, Mazella J, Tannenbaum GS, Beaudet A (1996). Localization of the somatostatin receptor SST2A in the rat brain using a specific anti-peptide antibody. J Neurosci 16:4468–4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Bausch SB, Milner TA, Chavkin C (1997). GIRK1 immunoreactivity is present predominantly in dendrites, dendritic spines, and somata in the CA1 region of the hippocampus. Proc Natl Acad Sci USA 94:1007–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Guillemare E, Fink M, Hugnot J-P, Bigay J, Lazdunski M, Romey G, Barhanin J (1995). Heterologous multimeric assembly is essential for K+ channel activity of neuronal and cardiac G-protein-activated inward rectifiers. Biochem Biophys Res Commun 212:657–663. [DOI] [PubMed] [Google Scholar]
- Ehrengruber MU, Doupnik CA, Xu Y, Garvey J, Jasek MC, Lester HA, Davidson N (1997). Activation of heteromeric G protein-gated inward rectifier K+ channels overexpressed by adenovirus gene transfer inhibits the excitability of hippocampal neurons. Proc Natl Acad Sci USA 94:7070–7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gähwiler BH, Brown DA (1985). GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci USA 82:1558–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse G, Eulitz D, Thiele T, Pahner I, Schröter S, Takamori S, Grosse J, Wickman K, Tapp R, Weh RW, Ottersen OP, Ahnert-Hilger G (2003). Axonal sorting of Kir3.3 defines a GABA-containing neuron in the CA3 region of rodent hippocampus. Mol Cell Neurosci 24:709–724. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Sik A, Payne JA, Kaila K, Freund TF (2001). The KCl cotransporter, KCC2, is highly expressed in the vicinity of excitatory synapses in the rat hippocampus. Eur J Neurosci 13:2205–2217. [DOI] [PubMed] [Google Scholar]
- Hagiwara A, Fukazawa Y, Deguchi-Tawarada M, Ohtsuka T, Shigemoto R (2005). Differential distribution of release-related proteins in the hippocampal CA3 area as revealed by freeze-fracture replica labeling. J Comp Neurol 489:195–216. [DOI] [PubMed] [Google Scholar]
- Hille B (1992). In: Ionic channels of excitable membranes Sunderland, MA: Sinauer.
- Hirono M, Yoshioka T, Konishi S (2001). GABAB receptor activation enhances mGluR-mediated responses at cerebellar excitatory synapses. Nat Neurosci 4:1207–1216. [DOI] [PubMed] [Google Scholar]
- Huang C-L, Slesinger P, Casey P, Jan YN, Jan LY (1995). Evidence that direct binding of Gβγ to the GIRK1 G protein-gated inwardly rectifying K+ channel is important for channel activation. Neuron 15:1133–1143. [DOI] [PubMed] [Google Scholar]
- Huang CS, Shi S-H, Ule J, Ruggiu M, Barker LA, Darnell RB, Jan YN, Jan LY (2005). Common molecular pathways mediate long-term potentiation of synaptic excitation and slow synaptic inhibition. Cell 123:105–118. [DOI] [PubMed] [Google Scholar]
- Inanobe A, Ito H, Ito M, Hosoya Y, Kurachi Y (1995). Immunological and physical characterization of the brain G protein-gated muscarinic potassium channel. Biochem Biophys Res Commun 217:1238–1244. [DOI] [PubMed] [Google Scholar]
- Inanobe A, Yoshimoto Y, Horio Y, Morishige K-I, Hibino H, Matsumoto S, Tokunaga Y, Maeda T, Hata Y, Takai Y, Kurachi Y (1999). Characterization of G-protein-gated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J Neurosci 19:1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Solis JM, Nicoll RA (1993). Local and diffuse synaptic actions of GABA in the hippocampus. Neuron 10:165–175. [DOI] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Takahashi N, Matsumoto S, Yamada M, Takumi T, Horio Y, Kurachi Y (1996). A novel ubiquitously distributed isoform of GIRK2 (GIRK2B) enhances GIRK1 expression of the G-protein-gated K+ current in Xenopus oocytes. Biochem Biophys Res Commun 218:286–291. [DOI] [PubMed] [Google Scholar]
- Johnston D, Magee JC, Colbert CM, Christie BR (1996). Active properties of neuronal dendrites. Annu Rev Neurosci 19:165–186. [DOI] [PubMed] [Google Scholar]
- Karschin A (1999). G protein regulation of inwardly rectifying K+ channels. News Physiol Sci 14:215–220. [DOI] [PubMed] [Google Scholar]
- Karschin C, Dissmann E, Stühmer W, Karschin A (1996). IRK1–3 and GIRK1–4 inwardly rectifying K+ channel mRNAs are differentially expressed in the adult rat brain. J Neurosci 16:3559–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, McMaster G, Angst C, Bittiger H, Froestl W, Bettler B (1997). Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature 386:239–246. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Schuler V, Mosbacher J, Bischoff S, Bittiger H, Heid J, Froestl W, Leonhard S, Pfaff T, Karschin A, Bettler B (1998). Human γ-aminobutyric acid type B receptors are differentially expressed and regulate inwardly rectifying K+ channels. Proc Natl Acad Sci USA 95:14991–14996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, Hamon M, Verge D (1996). Immunocytochemical localization of serotonin 1A receptors in the central nervous system. J Comp Neurol 365:289–305. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ikeda K, Ichikawa T, Abe S, Togashi S, Kumanishi T (1995). Molecular cloning of a mouse G-protein-activated K+ channel (mGIRK1) and distinct distribution of three GIRK (GIRK1, 2 and 3) mRNAs in mouse brain. Biochem Biophys Res Commun 208:1166–1173. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Davidson N, Lester HA (1995). Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by Gβγ subunits and function as heteromultimers. Proc Natl Acad Sci USA 92:6542–6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyrakh L, Lujan R, Colon J, Karschin C, Kurachi Y, Karschin A, Wickman K (2005). Molecular and cellular diversity of neuronal G-protein-gated potassium channels. J Neurosci 25:11468–11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, Clapham DE (1995). The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature 374:135–141. [DOI] [PubMed] [Google Scholar]
- Kulik A, Nakadate K, Nyiri G, Notomi T, Malitschek B, Bettler B, Shigemoto R (2002). Distinct localization of GABAB receptors relative to synaptic sites in the rat cerebellum and ventrobasal thalamus. Eur J Neurosci 15:291–307. [DOI] [PubMed] [Google Scholar]
- Kulik A, Vida I, Lujan R, Haas CA, López-Bendito G, Shigemoto R, Frotscher M (2003). Subcellular localization of metabotropic GABAB receptor subunits GABAB1a/b and GABAB2 in the rat hippocampus. J Neurosci 23:11026–11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurachi Y, Ishii M (2003). Cell signalling control of the G protein-gated potassium channel and its subcellular localization. J Physiol (Lond) 554:285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaney JL (2003). Contribution of Kir3.1, Kir3.2A and Kir3.2C subunits to native G protein-gated inwardly rectifying potassium currents in cultured hippocampal neurons. Eur J Neurosci 18:2110–2118. [DOI] [PubMed] [Google Scholar]
- Lesage F, Duprat F, Fink M, Guillemare E, Coppola T, Lazdunski M, Hugnot J-P (1994). Cloning provides evidence for a family of inward rectifier and G-protein coupled K+ channels in the brain. FEBS Lett 353:37–42. [DOI] [PubMed] [Google Scholar]
- Lesage F, Guillemare E, Fink M, Duprat F, Heurteaux C, Fosset M, Roemy G, Barhanin J, Lazdunski M (1995). Molecular properties of neuronal G-protein-activated inwardly rectifying K+ channels. J Biol Chem 270:28660–28667. [DOI] [PubMed] [Google Scholar]
- Liao YJ, Jan YN, Jan LY (1996). Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J Neurosci 16:7137–7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Roberts JDB, Shigemoto R, Somogyi P (1997). Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1a, mGluR2, and mGluR5, relative to neurotransmitter release sites. J Chem Neuroanat 13:219–241. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Jan LY, Stöffel M, Malenka RC, Nicoll RA (1997). G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 19:687–695. [DOI] [PubMed] [Google Scholar]
- Ma D, Zerangue N, Raab-Graham K, Fried SR, Jan YN, Jan LY (2002). Diverse trafficking patterns due to multiple traffic motifs in G protein-activated inwardly rectifying potassium channels from brain and heart. Neuron 33:715–729. [DOI] [PubMed] [Google Scholar]
- Marker CL, Lujan R, Loh HH, Wickman K (2005). Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of μ- and δ- but not κ-opioids. J Neurosci 25:3551–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara S, North RA, Surprenant A (1987). Somatostatin increases an inwardly rectifying potassium conductance in guinea pig submucous plexus neurons. J Physiol (Lond) 390:335–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishige KI, Inanobe A, Takahashi N, Yoshimoto Y, Kurachi H, Miyake A, Tokunaga Y, Maeda T, Kurachi Y (1996). G protein-gated K+ channel (GIRK1) protein is expressed presynaptically in the paraventricular nucleus of the hypothalamus. Biochem Biophys Res Commun 220:300–305. [DOI] [PubMed] [Google Scholar]
- Nehring RB, Wischmeyer E, Döring F, Veh RW, Sheng M, Karschin A (2000). Neuronal inwardly rectifying K+ channels differentially couple to PDZ proteins of the PSD-95/SAP90 family. J Neurosci 20:156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry NR, Nicoll RA (1985). Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol (Lond) 360:161–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA (1988). The coupling of neurotransmitter receptors to ion channels in the brain. Science 241:545–551. [DOI] [PubMed] [Google Scholar]
- North RA, Williams JT, Surprenant A, Christie MJ (1987). μ and δ receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci USA 84:5487–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, De Koninck Y, Mody I (1993). Characterization of synaptically elicited GABAB responses using patch-clamp recordings in rat hippocampal slices. J Physiol (Lond) 463:391–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE (2004). Contribution of Ih and GABAB to synaptically induced afterhyperpolarizations in CA1: a brake on the NMDA response. J Neurophysiol 92:2027–2039. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Hobson JA (2002). The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci 3:591–605. [DOI] [PubMed] [Google Scholar]
- Pfaffinger PJ, Martin JM, Hunter D, Nathanson NM, Hille B (1985). GTP-binding proteins couple cardiac muscarinic receptors to a K+ channel. Nature 317:536–538. [DOI] [PubMed] [Google Scholar]
- Ponce A, Bueno E, Kentros C, de Miera EV-S, Chow A, Hillman D, Chen S, Zhu L, Wu MB, Rudy B, Thornhill WB (1996). G-protein-gated inward rectifier K+ channel proteins (GIRK1) are present in the soma and dendrites as well as in nerve terminals of specific neurons in the brain. J Neurosci 16:1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibmayer W, Dessauer CW, Vorobiov D, Gilman AG, Lester HA, Davidson N, Dascal N (1996). Inhibition of an inwardly rectifying K+ channels by G-protein α-subunits. Nature 380:624–627. [DOI] [PubMed] [Google Scholar]
- Schuler V, Lüscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, Fox A, Spooren W, Jaton AL, Vigouret J, Pozza M, Kelly PH, Mosbacher J, Froestl W, Kaslin E, Korn R (2001). Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)). Neuron 31:47–58. [DOI] [PubMed] [Google Scholar]
- Sharon D, Vorobiov D, Dascal N (1997). Positive and negative coupling of the metabotropic glutamate receptors to a G protein-activated K+ channel, GIRK, in Xenopus oocytes. J Gen Physiol 109:477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Nakanishi S, Mizuno N (1997). Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci 17:7503–7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorini S, Liao YJ, Duncan SA, Jan LY, Stöffel M (1997). Normal cerebellar development but susceptibility to seizures in mice lacking G protein-coupled, inwardly rectifying K+ channel GIRK2. Proc Natl Acad Sci (USA) 94:923–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slesinger PA, Patil N, Liao J, Jan YN, Jan LY, Cox DR (1996). Functional effects of the mouse weaver mutation on G protein-gated inwardly rectifying K+ channels. Neuron 16:321–331. [DOI] [PubMed] [Google Scholar]
- Sodickson DL, Bean BP (1996). GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J Neurosci 16:6374–6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spauschus A, Lentes K-U, Wischmeyer E, Dissmann E, Karschin C, Karschin A (1996). A G-protein-activated inwardly rectifying K+ channel (GIRK4) from human hippocampus associates with other GIRK channels. J Neurosci 16:930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield PR, Nakajima S, Nakajima Y (2002). Constitutively active and G-protein coupled inward rectifier K+ channels: Kir2.0 and Kir3.0. Rev Physiol Biochem Pharmacol 145:47–179. [DOI] [PubMed] [Google Scholar]
- Tabata T, Araishi K, Hashimoto K, Hashimotodani Y, van der Putten H, Bettler B, Kano M (2004). Ca2+ activity at GABAB receptors constitutively promotes metabotropic glutamate signalling in the absence of GABA. Proc Natl Acad Sci USA 101:16952–16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigawa T, Alzheimer C (1999). G protein-activated inwardly rectifying K+ (GIRK) currents in dendrites of rat neocortical pyramidal cells. J Physiol (Lond) 517:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigawa T, Alzheimer C (2002). Phasic and tonic attenuation of EPSPs by inward rectifier K+ channels in rat hippocampal pyramidal cells. J Physiol (Lond) 539:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigawa T, Alzheimer C (2003). Interplay between activation of GIRK current and deactivation of Ih modifies temporal integration of excitatory input in CA1 pyramidal cells. J Neurophysiol 89:2238–2244. [DOI] [PubMed] [Google Scholar]
- Tanaka J-I, Matsuzaki M, Tarusawa E, Momiyama A, Molnar E, Kasai H, Shigemoto R (2005). Number and density of AMPA receptors in single synapses in immature cerebellum. J Neurosci 25:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrecilla M, Marker CL, Cintora SC, Stöffel M, Williams JT, Wickman K (2002). G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J Neurosci 22:4328–4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO, Jackson MB (1987). Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci 7:3306–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickman K, Clapham DE (1995). Ion channel regulation by G proteins. Physiol Rev 75:865–885. [DOI] [PubMed] [Google Scholar]
- Wickman KD, Iniguez-Lluhl JA, Davenport PA, Taussig R, Krapivinsky GB, Linder ME, Gilman AG, Clapham DE (1994). Recombinant G-protein βγ-subunits activate the muscarinic-gated atrial potassium channel. Nature 368:255–257. [DOI] [PubMed] [Google Scholar]
- Wischmeyer E, Döring F, Wischmeyer E, Spauschus A, Thomzig A, Veh R, Karschin A (1997). Subunit interactions in the assembly of neuronal Kir3.0 inwardly rectifying K+ channels. Mol Cell Neurosci 9:194–206. [DOI] [PubMed] [Google Scholar]
- Yamada M, Inanobe A, Kurachi Y (1998). G protein regulation of potassium ion channels. Pharmacol Rev 50:723–757. [PubMed] [Google Scholar]


