Abstract
Although neural stem cells hold considerable promise for treatment of the injured or degenerating nervous system, their current human sources are embryonic stem cells and fetally derived neural tissue. Here, we asked whether rodent and human skin-derived precursors (SKPs), neural crest-related precursors found in neonatal dermis, represent a source of functional, myelinating Schwann cells. Specifically, cultured SKPs responded to neural crest cues such as neuregulins to generate Schwann cells, and these Schwann cells proliferated and induced myelin proteins when in contact with sensory neuron axons in culture. Similar results were obtained in vivo; 6 weeks after transplantation of naive SKPs or SKP-derived Schwann cells into the injured peripheral nerve of wild-type or shiverer mutant mice (which are genetically deficient in myelin basic protein), the majority of SKP-derived cells had associated with and myelinated axons. Naive rodent or human SKPs also generated Schwann cells that myelinated CNS axons when transplanted into the dysmyelinated brain of neonatal shiverer mice. Thus, neonatal SKPs generate functional neural progeny in response to appropriate neural crest cues and, in so doing, provide a highly accessible source of myelinating cells for treatment of nervous system injury, congenital leukodystrophies, and dysmyelinating disorders.
Keywords: neural crest, stem cells, dermis, nerve injury, shiverer mouse, cell transplantation
Introduction
There is considerable interest in the use of stem cells for treatment of the injured or diseased nervous system. Although embryonic stem cells and fetally derived neural stem cells are candidate sources of transplantable neural precursors, their use is associated with both ethical and clinical issues, such as the requirement for immune suppression. The discovery of precursor cells with neural potential in peripheral adult tissues such as skin (Toma et al., 2001), gut (Kruger et al., 2002), and pancreas (Seaberg et al., 2004) provides a potential adult stem cell source for neural transplantation. The skin, the most accessible of these tissues, contains a neural crest-related precursor cell [skin-derived precursors (SKPs)] that is capable of differentiating in culture into neural crest-derived cell types, including cells with characteristics of peripheral neurons and Schwann cells (Toma et al., 2001; Fernandes et al., 2004). These dermal precursors can first be detected in embryonic murine skin at embryonic day 15 (E15) and persist in neonatal and adult skin in lower numbers, in which they reside within a niche in the dermal papillae of hair and whisker follicles. SKPs derive from the neural crest, at least within facial skin, and exhibit properties consistent with the idea that they are a neural crest precursor maintained within an adult tissue. Importantly, because SKPs can also be isolated and expanded from both neonatal and adult human skin (Joannides et al., 2004; Toma et al., 2005), they may well provide an accessible, autologous source of neural crest-derived cell types for transplantation.
Schwann cells are one neural crest-derived cell type that has been proposed for a number of clinical applications based on their ability to remyelinate demyelinated lesions (Blakemore and Crang, 1985; Kohama et al., 2001) and to promote regeneration and remyelination in the injured spinal cord (Takami et al., 2002; Pearse et al., 2004). Artificial grafts seeded with Schwann cells have also been shown to promote regeneration after peripheral nerve injury (Guenard et al., 1992). However, human Schwann cells are derived from invasive nerve biopsies, and their expansion is limited, making it desirable to identify a renewable, accessible Schwann cell source. In this regard, bone marrow stromal cells have been shown to generate Schwann cells by some groups (Dezawa et al., 2001; Akiyama et al., 2002), but others have reported that these same cells do not generate bona fide neural cell types (Vitry et al., 2003; Neuhuber et al., 2004). Here, we asked whether neonatal SKPs generate functional neural progeny and, if so, whether they represent an accessible, alternative Schwann cell precursor source. Our findings indicate that SKPs do indeed differentiate into Schwann cells in response to appropriate neural crest cues and that these Schwann cells are capable of myelinating the injured peripheral nerve and dysmyelinated CNS.
Materials and Methods
Cell culture.
Rodent and human SKPs were cultured as described previously (Toma et al., 2001, 2005; Fernandes et al., 2004). Briefly, for rodent SKPs, skin from mouse embryos (E15–E19), mouse, or rat neonates [postnatal day 2 (P2) to P6] was cut into 2–3 mm2 pieces. Tissue was digested with 0.1% trypsin or 1 mg/ml collagenase for 10–45 min at 37°C, mechanically dissociated, and filtered through a 40 μm cell strainer (Falcon, Franklin Lakes, NJ). Cells were plated at a density of 1–2.5 × 104 cells/ml in DMEM/F-12 at 3:1 (Invitrogen, Carlsbad, CA), with 20 ng/ml epidermal growth factor (EGF) and 40 ng/ml FGF2 (both from Collaborative Research, Bedford, MA), hereafter referred to as proliferation medium. SKPs were passaged by mechanically dissociating spheres and splitting one to three with 75% new medium and 25% conditioned medium. Human SKPs were generated from infant circumcisions as described previously (Toma et al., 2005). For Schwann cell differentiation, primary or secondary spheres were dissociated and plated on poly-d-lysine and laminin (BD Biosciences, Franklin Lakes, NJ), cultured in DMEM/F-12 at 3:1 with 10% FBS for 7 d, and then switched to the same medium plus 4 μm forskolin (Sigma, St. Louis, MO). For some experiments, SKPs were differentiated in DMEM/F-12 at 3:1 with 1% N2 supplement, 10 ng/ml neuregulin-1β (heregulin-β1; R & D Systems, Minneapolis, MN), and 4 μm forskolin, referred to as Schwann cell differentiation medium. Medium was changed every 3–4 d. Proliferating colonies of morphologically identifiable Schwann cells were isolated with cloning cylinders (Corning, Corning, NY), trypsinized from the dish, and replated in the same medium. Cultures of increasing purity were obtained by sequential passaging. Pure cultures (>95% purity) were passaged once a week, up to five times, with cells being frozen at early passages for later use. Clonal spheres were prepared as described previously (Fernandes et al., 2004) and were differentiated similarly with the addition of 1% serum for the first 3 d. Human Schwann cells were differentiated as for rodent SKPs with the addition of 1–5% serum.
Dorsal root ganglion cocultures.
Cocultures were prepared basically as described previously (Eldridge et al., 1987), with some modifications. Briefly, in rat dorsal root ganglion (DRG) experiments, DRGs were dissected from E15 Sprague Dawley rat embryos, suspended in drops of DMEM/F-12 medium (3:1) with N2 and 50 ng/ml NGF (Cedarlane, Hornby, Ontario, Canada), and plated on chamber slides coated in poly-d-lysine and laminin. Twenty-four hours later, yellow fluorescent protein (YFP)-tagged SKPs differentiated for 7 d in Schwann cell differentiation medium, or purified cultures of SKP-derived Schwann cells were injected into the ganglia with a nanoinjector (Drummond Scientific, Broomall, PA). For the shiverer DRG experiments, ganglia from E17–E18 homozygous shiverer mice (obtained from The Jackson Laboratory, Bar Harbor, ME) were dissected, and a single ganglion was placed into each chamber of an eight-chamber tissue-culture slide (Nunc, Naperville, IL). Ganglia were grown in DMEM/F-12 (3:1) supplemented with 1% N2, nerve growth factor (50 ng/ml), and, for the first 3 d of plating, cytosine arabinoside (7 μm). Ganglia were then washed three times with fresh medium and maintained in the same medium without cytosine arabinoside. Twenty-four hours later, ∼1000–2000 YFP-SKP-derived Schwann cells were plated within each chamber. In both sets of experiments, ascorbic acid (10 μm) and 10 ng/ml IGF-1 (R & D Systems) were added after 7 d for an additional 2 weeks before samples were processed for immunohistochemistry.
To quantify the percentage of SKP-derived Schwann cells that associated with axons and/or expressed a myelinating phenotype, cultures were triple labeled for YFP, βIII-tubulin, and either Ki67, myelin basic protein (MBP), or P0. These were then analyzed for the total number of cells within the circumference of the explant, the number of cells apposed to a βIII-tubulin-positive axon, and the number of cells coexpressing YFP and the other relevant protein. A total of 30 such cultures were performed and analyzed.
Cerebellar slice cultures.
Organotypic slice cultures (300 μm) were generated from the cerebellum of P12–P14 shiverer mutant mice with a McIlwain tissue chopper (Mickle Laboratory Engineering, Surrey, UK) and maintained as described previously (Stoppini et al., 1991). Briefly, slices were grown on Millicell-CM membranes (Millipore, Bedford, MA) over MEM (Invitrogen), HBSS (Invitrogen), and heat-inactivated horse serum (Invitrogen) in a ratio of 2:1:1, supplemented with 1% penicillin–streptomycin, glucose, and sodium bicarbonate. SKPs at ∼105 differentiated for 1 week in Schwann cell differentiation medium were transplanted into white matter tracts using a nanoinjector (Drummond Scientific). Medium was changed every 3 d, and immunocytochemistry was performed at 2 weeks.
Transplantation experiments.
For the peripheral nerve experiments, 6- to 8-week old-Sv129 (Charles River Laboratories, Wilmington, MA) or homozygous shiverer mice were used. Animals were anesthetized with isofluorane (3% induction and 1% maintenance) in 40% O2 and 60% N2O, and the sciatic nerve was exposed and crushed with #5 watchmaker forceps for 1 min ∼1 cm proximal to the nerve bifurcation. Immediately after, naive or differentiated SKPs, or purified SKP Schwann cells (1–4 × 105 cells in 1–2 μl of media) were transplanted into the distal nerve by injection with a 10 μl Hamilton syringe fitted with a 33 gauge needle. All animals received daily subcutaneous injections of cyclosporine A (20 mg/kg; Sandimmune IV; Novartis, Basel, Switzerland). After 6–8 weeks, animals were anesthetized with a lethal dose of Somnotol and perfused with saline, followed by 4% paraformaldehyde (PFA) with or without 1% glutaraldehyde for electron microscopy (EM) and immunocytochemistry, respectively.
To quantify the number of transplanted cells that survived within the regenerating shiverer nerve and expressed a myelinating phenotype, sciatic nerves (n = 5 mice) were longitudinally cryosectioned at a thickness of 16 μm, and every section was collected. Sections were double labeled for YFP and MBP (both present only in the transplanted cells) and counterstained with Hoechst. For each nerve, every eighth section throughout the thickness of the nerve was analyzed, and all YFP-positive cells containing a Hoechst-positive nucleus and/or coexpressing MBP were counted to determine the percentage of transplanted cells that expressed MBP. To determine the total number of surviving donor cells, the number of YFP-positive cells in all of the counted sections were multiplied by 8 to compensate for the sampling frequency. To ascertain the myelinating efficiency of naive SKPs versus predifferentiated SKP-derived Schwann cells, transplanted shiverer sciatic nerves (n = 3 mice each for naive and differentiated SKPs) were cut in semithin coronal sections (1 μm thick) and immunostained for MBP to detect MBP-positive myelin figures. Nontransplanted contralateral nerves (n = 3) were analyzed to verify the absence of MBP expression within shiverer nerves. Sections were sampled every 0.4 mm through the length of the nerve segment surrounding the transplantation site (∼4 mm). The total number of MBP-positive myelin profiles counted in each sample were summed but were not normalized for sampling frequency.
For the CNS transplant experiments, P0–P1 homozygous shiverer mice were cryoanesthetized, and dissociated murine or human SKPs (1 × 105 in 1 μl of F-12 medium) were injected through the skull into the cerebellum and midbrain via a 28 gauge Hamilton syringe fitted with a glass micropipette. To identify the transplant regions, murine SKPs were generated from YFP transgenic mice (Hadjantonakis et al., 1998), and human SKPs were labeled by adding Cell Tracker CM DiI (1:1000; Invitrogen) to the donor cell suspension 30 min before transplantation. Mice were killed at 4 weeks, transplanted regions were identified by fluorescence illumination, isolated, and trimmed, and then these tissue pieces were processed for EM. As controls, the homotopic regions that did not contain fluorescence label were isolated. All procedures were approved by the Hospital for Sick Children Research Institute, in accordance with guidelines of the Canadian Council on Animal Care.
Immunocytochemistry.
Immunocytochemical analysis was performed on plated cells in chamber slides (Nunc) as described previously (Toma et al., 2001, 2005; Fernandes et al., 2004). The following primary antibodies were used: anti-βIII-tubulin monoclonal (1:500; Covance Research Products, Berkeley, CA), rabbit anti-neurofilament-M (NFM) (1:500; Chemicon, Temecula, CA), polyclonal chicken anti-green fluorescent protein (1:1000; Chemicon), anti-GFAP polyclonal (1:500; Accurate Chemical, Westbury, NY), anti-GFAP polyclonal (1:500; DakoCytomation, Carpinteria, CA), anti-p75 neurotrophin receptor (p75NTR) polyclonal (1:500; Promega, Madison, WI), anti-S100β monoclonal (1:1000; Sigma), anti-peripheral myelin protein 22 (PMP22) monoclonal (1:200; NeoMarkers, Fremont, CA), anti-MBP monoclonal (1:200; Chemicon), anti-MBP monoclonal (1:100; Serotec, Indianapolis, IN), polyclonal chicken anti-P0 (1:500; Aves Labs, Tigard, OR), or anti-Ki67 monoclonal (1:200; BD Biosciences PharMingen, San Diego, CA). Secondary antibodies used were as follows: Alexa488-conjugated goat anti-mouse, Alexa488-conjugated goat anti-chicken, Alexa555 goat anti-mouse IgM, Alexa555 goat anti-rabbit, and Alexa350 goat anti-mouse (1:1000; all from Invitrogen). Alternatively, semithin nerve sections were incubated with rat anti-MBP (1:20; Serotec), followed by a peroxidase conjugated anti-rat secondary (Jackson ImmunoResearch, West Grove, PA) and visualized with nickel-enhanced diaminobenzidine (Vector Laboratories, Burlingame, CA). Positive controls for myelinating Schwann cells used wild-type sciatic nerve sections. Negative controls either (1) excluded primary antibodies and showed an absence of staining or (2) were performed on non-neural tissues and also showed no immunostaining above background.
Confocal and electron microscopy.
Confocal imaging was done using a Zeiss (Oberkochen, Germany) LSM 5 Pascal confocal microscope and image processing software (Zeiss). Colocalization was verified by imaging adjacent 1 or 2 μm optical slices. Samples for EM were postfixed in 4% PFA/1% glutaraldehyde for 1 h, washed in PBS, fixed for 1.5 h in 1% osmium tetroxide, washed in phosphate buffer, and dehydrated through a graded ethanol series. Samples were then washed three times for 10 min each in propylene oxide, incubated 1.5 h in 1:1 Spurr resin/ethanol, two times for 2 h in 100% Spurr resin, and polymerized overnight at 70°C. Thin sections were collected on copper grids and stained with uranyl acetate and lead citrate. Samples for immuno-EM were postfixed in 4%PFA/1% glutaraldehyde for 1 h, washed in PBS, cryoprotected in 30% glycerol for 4 h, and immersed in liquid nitrogen. Freeze substitution was performed over 48 h at −90°C in 1% uranyl acetate in methanol using an automatic freeze-substitution system (Leica, Nussloch, Germany). The temperature was raised to −35°C over 18 h, and samples were washed in methanol and embedded in Lowicryl HM20 as follows: 1:1 HM20 methanol for 2 h, 3:1 HM20/methanol for 2 h, followed by 100% HM20 for 18 h, all at −35°C. The plastic was polymerized with UV for 48 h at −35°C. Sections on nickel grids were incubated with rat-anti MBP (1:20; Serotec) for 4 h, washed in PBS, and stained with 10 nm colloidal gold-conjugated anti-rat secondary (1:20; Sigma) for 2 h, all at room temperature. Sections were then fixed for 5 min in 2% glutaraldehyde in PBS and stained with uranyl acetate and lead citrate. Sections were examined using an FEI Company (Hillsboro, OR) Tecnai 20 transmission electron microscope.
Results
Neural crest cues promote the differentiation of rodent and human SKPs into Schwann cells
We have shown previously that both rodent and human SKPs can generate, under basal differentiation conditions, a low number of cells with characteristics of Schwann cells, including a bipolar morphology and coexpression of p75NTR, GFAP, CNPase, and S100β (Fernandes et al., 2004; Toma et al., 2005). Because SKPs have characteristics of neural crest precursors, we predicted that cues that enhance the generation and expansion of Schwann cells from neural crest stem cells would do the same for SKPs. To test this prediction, we used two extrinsic cues, forskolin (which increases intracellular cAMP) and neuregulin-1β, which are known to enhance the genesis, proliferation, and differentiation of Schwann cells from embryonic neural crest precursors (Morgan et al., 1991; Shah et al., 1994). When neonatal mouse or rat SKPs were differentiated in serum alone, immunocytochemistry revealed the presence of a minor subpopulation of cells with bipolar morphology that expressed the Schwann cell markers S100β, CNPase, and/or GFAP (data not shown), as we reported previously (Toma et al., 2001; Fernandes et al., 2004). However, when sister cultures were differentiated in the presence of either serum and forskolin, or neuregulin-1β and forskolin, this led to the appearance of parallel arrays of bipolar, S100β-positive cells (Fig. 1a), many of which coexpressed the myelin proteins MBP or PMP22 (Fig. 1b,c), as well as GFAP and p75NTR (data not shown).
Figure 1.
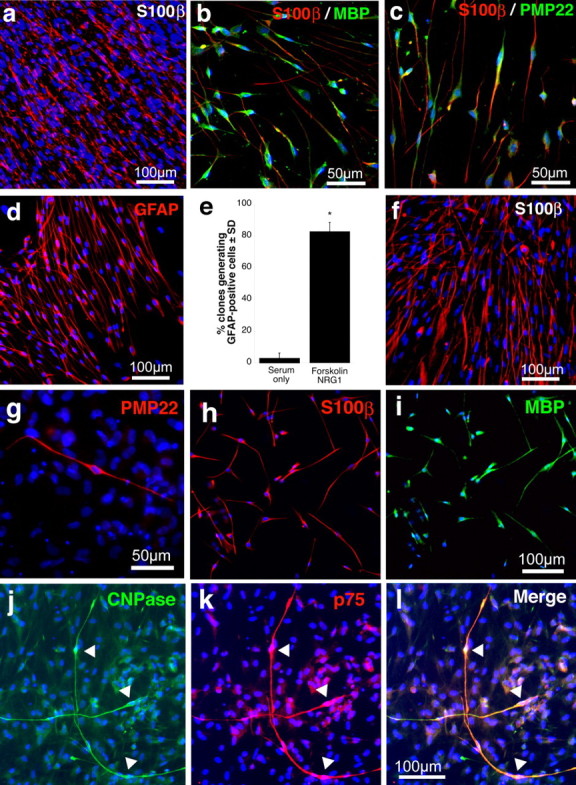
Rodent and human SKPs differentiate into Schwann cells in response to neural crest cues. a, Neonatal rat SKPs differentiated in serum and forskolin and immunostained for the Schwann cell marker S100β (red). b, c, Neonatal rat SKPs differentiated in serum and forskolin for 10 d and then immunostained for S100β (red in b, c) and MBP (b) or PMP22 (c) (both in green). Panels show the merged images. Most cells coexpress both proteins and exhibit a bipolar morphology. d, A representative clonal rat SKPs sphere differentiated for 10 d in forskolin and neuregulin-1β (NRG1) and then immunostained for GFAP (red). e, Quantitation of the percentage of neonatal rat SKP clones containing at least one bipolar, GFAP-positive cell (similar to those shown in d) after differentiation in serum alone versus forskolin and neuregulin-1β. Error bars indicate the mean ± SD. ∗p < 0.001, Student’s t test; n = 3. f–l, Human neonatal foreskin SKPs passaged three times, differentiated in serum, forskolin, and neuregulin-1β, and immunostained for S100β (red, f) and PMP22 (red, g). Many cells expressing S100β (green, h) also expressed MBP (green, i). Similarly, many cells expressed CNPase (green, j) and coexpressed p75NTR (red, k), as shown in the merged image (l). Arrows indicate individual cells expressing both markers. f–l each show differentiation of a different line of human SKPs. In all panels, nuclei were visualized by Hoechst 33258 staining (blue).
To more quantitatively assess the effects of neuregulin-1β and forskolin on the ability of SKPs to differentiate into Schwann cells, we performed clonal analysis. Single, clonal rat SKP spheres were generated as we described previously (Fernandes et al., 2004), were proliferated adherently on poly-d-lysine/laminin in the presence of FGF2 and EGF for 3 d, and then were differentiated for 10–12 d in forskolin and neuregulin-1β in the absence of serum. For comparison, we differentiated sister cultures in the presence of 5% serum with or without N2 (similar results were obtained in both conditions). Immunocytochemistry revealed that 83% of these clones contained GFAP-positive cells with a bipolar morphology (Fig. 1d), presumably Schwann cells, compared with only 3% when cultured in the absence of these cues (n = 3) (Fig. 1e). Thus, neuregulin-1β and forskolin instruct SKPs to generate Schwann cells, as they do for embryonic neural crest precursors (Shah et al., 1994).
We then asked whether similar conditions could be used to differentiate human SKPs into Schwann cells. When human SKPs were cultured from neonatal foreskin tissue (Toma et al., 2005), passaged two or three times, and differentiated under basal differentiation conditions, which included 5% serum plus or minus 1% N2, bipolar, S100β, or GFAP-positive cells could only rarely be identified, and cells were never seen that expressed myelin proteins such as MBP, as reported previously (Toma et al., 2005). In contrast, when human SKPs were differentiated in 1% serum with forskolin and neuregulin-1β, ∼5% of cells expressed the glial marker CNPase (two experiments; 8.97 and 1.24% of total cells were CNPase positive) (Fig. 1j) and parallel arrays of many bipolar S100β-positive cells were observed (Fig. 1f). Immunocytochemistry also revealed that many of these cells coexpressed GFAP or p75NTR (Fig. 1j–l), and a subpopulation also coexpressed the myelin proteins PMP22 or MBP (Fig. 1g–i). Thus, human SKPs also respond to these neural crest cues to generate Schwann cells.
Having derived conditions in which a robust percentage of SKPs differentiated into cells with characteristics of Schwann cells, we then asked whether we could purify these cells. When mass cultures of rodent (both rat and mouse) SKPs were differentiated with forskolin and neuregulin-1β for 7 d, small colonies of putative SKP-derived Schwann cells could be identified on the basis of their morphology (Fig. 2a). These colonies could be isolated with cloning cylinders and replated in the same conditions for 1–2 weeks to ultimately yield cultures of >95% purity, as judged by their bipolar cellular morphology (Fig. 2b,c). Immunocytochemistry confirmed this estimate; >95% of the cells in these cultures expressed GFAP (Fig. 2b,c), the peripheral myelin protein P0, and the p75 neurotrophin receptor (Fig. 2d–f), a phenotypic profile characteristic of Schwann cells.
Figure 2.
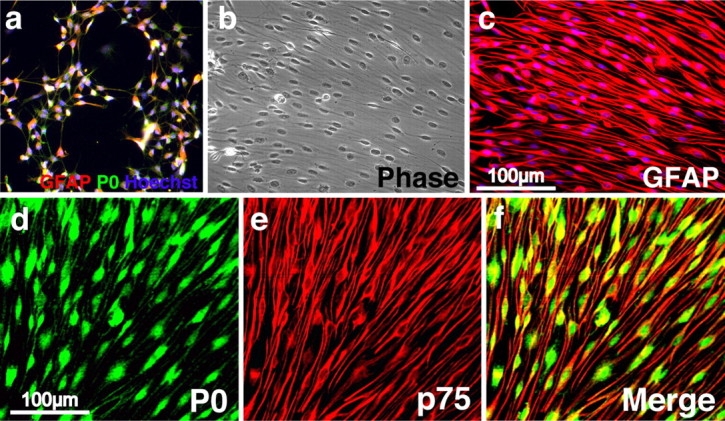
Characterization of purified, SKP-derived Schwann cell cultures. a, Fluorescence photomicrograph of SKPs differentiated in forskolin and neuregulin-1β for 1 week and then immunostained for P0 (green) and GFAP (red). Note the cluster of cells coexpressing both proteins (yellow). Similar putative Schwann cell clones were identified by morphology and were isolated using cloning cylinders and expanded. b, c, Almost all of the cells in these purified, expanded cultures were bipolar, as shown by phase microscopy (b) and expressing the Schwann cell marker GFAP (c, red), as demonstrated by immunocytochemistry. Nuclei in a–c were counterstained with Hoechst 33258 (blue), and b and c are photographs of the same field. d–f, Purified cultures were double labeled for the Schwann cell markers P0 (d, green) and the p75 neurotrophin receptor (e, red; f shows the merged image). Similar results were obtained in six independent purifications.
Rodent SKPs associate with peripheral sensory neuron axons in culture and express a myelinating phenotype
To determine whether the Schwann cells that differentiate from SKPs are bona fide, functional Schwann cells, we asked whether they could myelinate the axons of peripheral neurons. Initially, we asked this question in vitro by coculturing SKPs with DRG explants under conditions in which primary Schwann cells will myelinate sensory neurons (Eldridge et al., 1987). Neonatal murine SKPs were cultured from a transgenic mouse that expresses YFP in all tissues to provide a genetic tag for SKP-derived cells. These YFP-tagged SKPs were differentiated with forskolin and neuregulin-1β for 7 d, and then the proliferating Schwann cells were purified and expanded as described above (Fig. 2). As transplant recipients for these experiments, we used DRG explants derived from homozygous shiverer mutant mice, which are genetically deficient in MBP; explants were dissected from E17–E18 shiverer mice and were then cultured 3 d in the presence of cytosine arabinoside to reduce the number of endogenous Schwann cells in the cultures. After removal of the cytosine arabinoside, 1000–2000 SKP-derived Schwann cells were cocultured with these DRG explants for 1 week in NGF to support survival of the DRG neurons and then for an additional 2 weeks (3 weeks total) in the presence of NGF, IGF-1, and ascorbic acid to induce myelination. Immunocytochemical analysis of these cultures revealed that, at all time points examined, commencing at 2 d, the majority of YFP-expressing cells were associated with βIII-tubulin-positive DRG axons (Fig. 3a,b). Quantification of seven experiments revealed that 75.6 ± 3.2% (SD) of the YFP-positive cells were axon associated at 3 weeks in culture. At this same time point, a significant number of associated SKP-derived Schwann cells were dividing (10.8 and 13.4% in two separate experiments) as indicated by their expression of the proliferation marker Ki67. Interestingly, Ki67-positive SKP-derived cells that were not axon associated were not observed, suggesting that axons themselves, and not a secreted cue from the conditioned medium, provided the proliferative cue. Immunocytochemistry at this 3 week time point also revealed that these axon-associated, YFP-expressing cells were virtually all positive for the peripheral myelin protein P0 (n = 7) (Fig. 3d) and that many were positive for PMP22 (data not shown). Moreover, approximately half (52%, one experiment quantified of seven performed) were YFP positive and MBP positive (Fig. 3e), consistent with a myelinating phenotype. Thus, in these cocultures, the majority of SKP-derived Schwann cells associate with axons, proliferate to some degree in response to axon-derived cues, and then induce a myelinating phenotype, behavior analogous to that displayed by primary cultured Schwann cells (Eldridge et al., 1987; Cheng et al., 1999).
Figure 3.
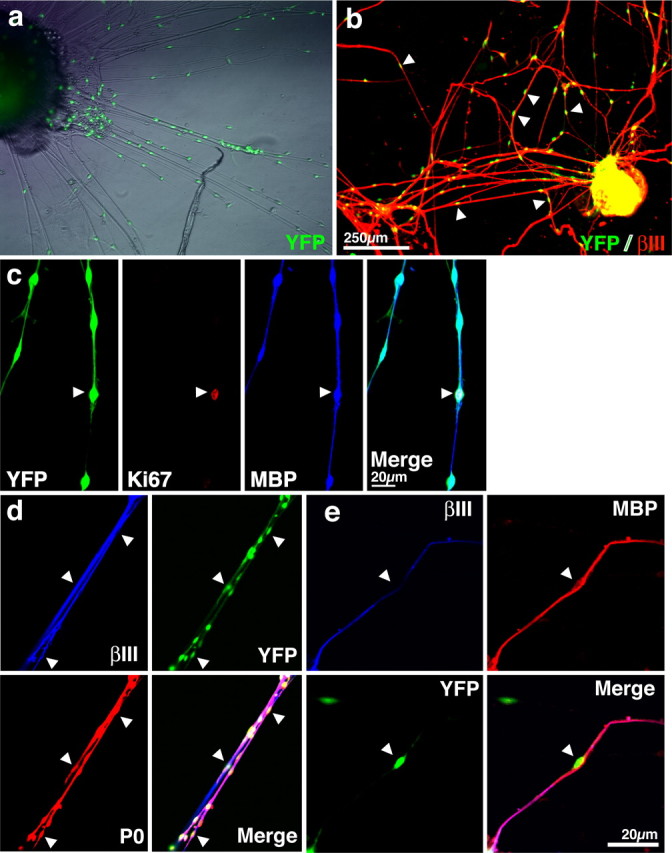
Purified SKP-derived Schwann cells cocultured with shiverer DRG explants associate with sensory axons, proliferate, and express a myelinating phenotype. a, b, Low-magnification photomicrographs of purified, YFP-tagged SKP-derived Schwann cells cocultured with embryonic DRG explants from shiverer mutant mice, which are genetically deficient in MBP. Phase combined with fluorescence microscopy (a) shows that the majority of the YFP-positive cells were associated with axons. This conclusion was confirmed by double labeling similar cocultures for YFP (green) and for the axonal protein βIII-tubulin (red). Arrows indicate YFP-positive cells associated with axons. c, Confocal microscopy of cocultures triple labeled after 3 weeks for YFP (green, left), the proliferation marker Ki67 (red, left middle panel), and MBP, which is expressed only by the transplanted cells (blue, right middle panel; right panel is the merge) reveals that SKP-derived Schwann cells populate axons, divide, and express a myelinating phenotype. d, e, Confocal microscopy of cocultures triple labeled for βIII-tubulin (blue), YFP (green), and either P0 (d, red) or MBP (e, red; in the bottom right panel is the merge) demonstrate that axon-associated SKP-derived Schwann cells express a myelinating phenotype. Similar results were obtained in 10 independent cocultures, three of which were quantitatively analyzed (see Results).
SKP-derived Schwann cells associate with and myelinate regenerating axons in the injured sciatic nerve
To definitively establish whether SKP-derived Schwann cells were capable of myelinating axons, we transplanted them into the sciatic nerve. Because in the uninjured nerve, all axons are already associated with Schwann cells, we performed our transplants into the distal segment of the crushed sciatic nerve, in which the newly regenerated axons will associate with Schwann cells de novo. To perform these experiments, we cultured neonatal YFP-tagged murine SKPs, differentiated them in neuregulin-1β and forskolin for 7 d, and then transplanted this mixed population of SKP-derived cells 5 mm distal to a sciatic nerve crush immediately after injury in immunosuppressed adult mice. Alternatively, we transplanted naive SKPs that had not been predifferentiated. Immunocytochemical analysis of nerve cross sections by confocal microscopy 2 weeks after transplant revealed that many of the transplanted, YFP-tagged cells associated with neurofilament-positive axons over a series of adjacent optical slices. Remarkably, this occurred regardless of whether the original starting population was SKP-derived Schwann cells or naive SKPs (Fig. 4a). Some YFP-positive cells coexpressed MBP or PMP22 and exhibited a morphology consistent with that of myelinating Schwann cells (Fig. 4b,c), although the percentage of transplanted cells expressing these myelin proteins appeared to be lower with naive SKPs than with SKP-derived Schwann cells. Similar profiles were observed up to 4 weeks after injury, the longest time point examined.
Figure 4.
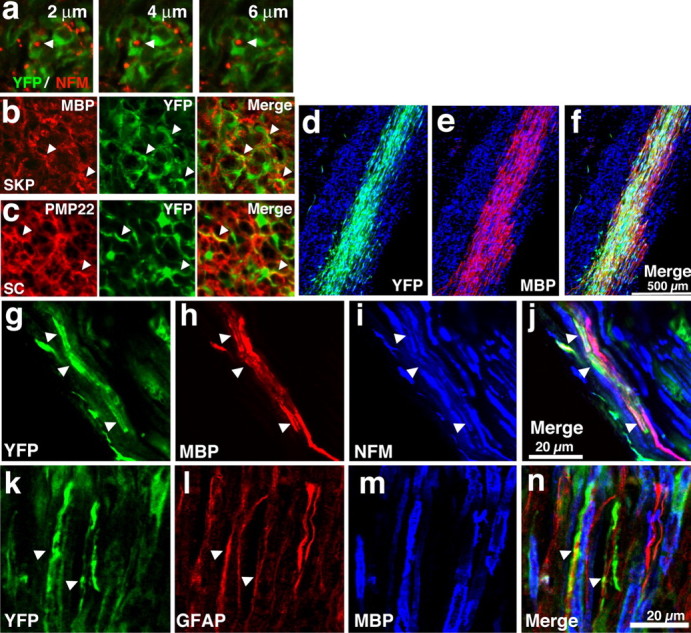
SKP-derived cells associate with regenerating axons in the injured peripheral nerve and express a myelinating phenotype. a–c, YFP-tagged naive SKPs or SKPs differentiated for 1 week in neuregulin-1β and forskolin, were transplanted into the sciatic nerve immediately distal to a nerve crush in immunosuppressed mice. Two weeks later, nerves were cut in cross section and analyzed by immunocytochemistry and confocal microscopy. a, Photomicrographs of nerves transplanted with differentiated SKPs and double labeled for YFP (green) and the axonal protein NFM, showing a series of 2 μm optical slices at high magnification, demonstrating that NFM-positive axons were associated with transplanted cells (arrows). b, c, Photomicrographs of nerves transplanted with naive (b, SKP) or differentiated [c, Schwann cell (SC)] SKPs and immunostained for YFP (green, middle panels) and MBP (b) or PMP22 (c) (red, left panels; right panels show the merged images), with arrowheads indicating colocalization. Similar results were obtained with seven transplanted animals in each group. d, Purified, YFP-tagged SKP-derived Schwann cells were transplanted into the distal crushed sciatic nerve of immunosuppressed shiverer mice and were analyzed 6 weeks after transplantation. d–f, Low-magnification photomicrographs of longitudinal sections of the nerve adjacent to the crush site showing the robust reconstitution of the nerve by YFP-positive (d, green), MBP-positive (e, red; f shows the merge of the two) SKP-derived Schwann cells. Sections were counterstained with Hoechst 33258 (blue) to show all of the cells. Note the absence of MBP expression in regions that do not contain the transplanted SKPs. g–j, Higher-magnification photomicrographs of a transplanted longitudinal nerve section triple labeled for YFP (g, green), MBP (h, red), and NFM (i, blue; the merge is shown in j). Note that YFP-positive, MBP-positive cells are associated with NFM-positive axons. k–n, Photomicrograph of a longitudinal section similar to that in d–f, triple labeled for YFP (k, green), GFAP (l, red), and MBP (m, blue; n shows the merged image). The arrows denote transplanted YFP-positive cells that are positive for GFAP but not MBP. Similar results were obtained in 10 transplanted nerves, some of which were quantitatively analyzed (see Results).
To more quantitatively and definitively demonstrate myelination, we transplanted purified, SKP-derived Schwann cells into the crushed sciatic nerve of shiverer mice, which have been used extensively to study myelination by transplanted cells (Yandava et al., 1999; Liu et al., 2000; Windrem et al., 2004). Whereas the shiverer PNS exhibits grossly normal compact myelin, myelination by wild-type transplanted cells can be visualized by MBP immunostaining, thereby providing two genetic tags for any myelinating SKP-derived cells, YFP and MBP. To perform these experiments, the sciatic nerve of adult shiverer mice was unilaterally crushed, and, distal to the crush, we transplanted purified, YFP-tagged, murine SKP-derived Schwann cells. Six to 8 weeks later, we isolated these transplanted nerves, sectioned them longitudinally, and performed immunocytochemistry for YFP and MBP (Fig. 4d–f). This analysis revealed that the transplanted SKP-derived Schwann cells had survived and robustly populated the regenerated distal nerve (Fig. 4d–f), with YFP-positive cells being present over distances of at least 1.5 cm. Quantitation revealed that, at 6 weeks after transplantation, ∼26,000 YFP-positive cells (n = 5) had integrated into this 1.5 cm segment of the nerve, of the 400,000 that had originally been injected. Moreover, triple labeling for YFP, MBP, and NFM revealed that most of these YFP-positive cells were aligned longitudinally along NFM-positive axon tracts, and many of them were MBP positive (Fig. 4g–j). Quantitative confocal analysis of these longitudinal sections revealed that 70.4 ± 4.4% of YFP-SKP-derived Schwann cells coexpressed MBP (n = 4). Importantly, MBP-positive, YFP-negative cells were never observed, confirming that MBP (in addition to the YFP tag) could be used as a reliable genetic marker for myelinating transplanted cells within the shiverer nervous system. We also asked whether any of the MBP-negative, YFP-positive cells had differentiated into nonmyelinating Schwann cells by performing immunocytochemistry for YFP and GFAP. This analysis revealed that a subset of transplanted, MBP-negative cells expressed GFAP but not MBP (see Fig. 6k–m) and were also aligned with axons, indicating that they were likely nonmyelinating Schwann cells.
Figure 6.
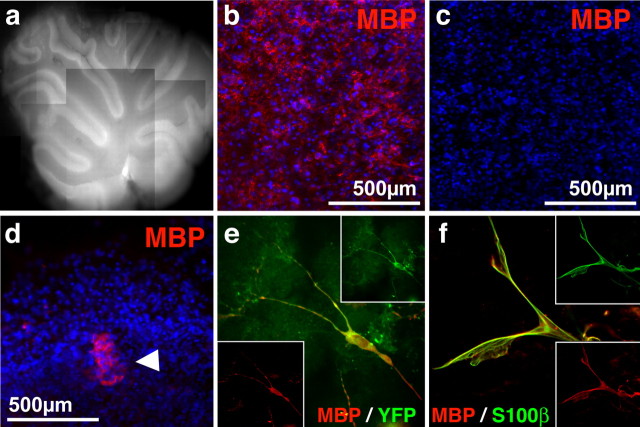
Transplanted SKP-derived Schwann cells integrate into white matter and express a myelinating phenotype in the dysmyelinated shiverer cerebellum ex vivo. a–c, Representative 2-week-old cerebellar slice culture as visualized by autofluorescence (a). Immunostaining confirmed that slice cultures from wild-type (b) but not shiverer (c) cultures expressed MBP (red). d, Fluorescence micrograph of differentiated SKPs transplanted into slice cultures and immunostained for MBP (red) after 3 d. The arrowhead indicates transplanted cells. In b–d, nuclei were visualized with Hoechst 33258 (blue). e, f, Fluorescence micrographs of SKP-derived cells on cerebellar slices 2 weeks after transplantation as visualized by immunostaining for MBP (red) and YFP (green) (e) or MBP (red) and S100β (green) (f). Similar results were obtained in three independent slice culture experiments.
To confirm that the association of MBP-positive SKP-derived cells with axons reflected myelination, we immunostained semithin cross sections of shiverer nerves for MBP 4 weeks after transplantation and analyzed them by light (Fig. 5a–e) and electron (Fig. 5f,g) microscopy. The light microscopic analysis revealed the presence of many MBP-positive myelin figures in nerves transplanted with either naive SKPs (Fig. 5b) or SKP-derived Schwann cells (Fig. 5c,d) but not in control nerves (Fig. 5a). Immunoelectron microscopy of similar thin nerve sections revealed that these MBP-positive myelin figures were bona fide compact myelin with major dense repeating lines (Fig. 5f,g). To quantitatively assess the engraftment of naive SKPs versus SKP-derived Schwann cells, we counted all of the MBP-positive myelin figures in these 1 μm thin sections, sampling every 0.4 mm throughout a 4 mm length of the nerve segment surrounding the transplantation site. We then summed the numbers obtained from these 10 sections per nerve and used this number as a relative index of engraftment. This analysis revealed that 4146 ± 832 and 6677 ± 1579 MBP-positive myelin sheaths were observed in nerves transplanted with SKPs or SKP-derived Schwann cells, respectively, although none were observed in the nontransplanted, contralateral nerves (n = 3 for each population) (Fig. 5e). Thus, even without normalizing for the sampling frequency (1 μm every 400 μm), these counts revealed a highly robust contribution of myelinating SKP-derived Schwann cells to the regenerating nerve.
Figure 5.
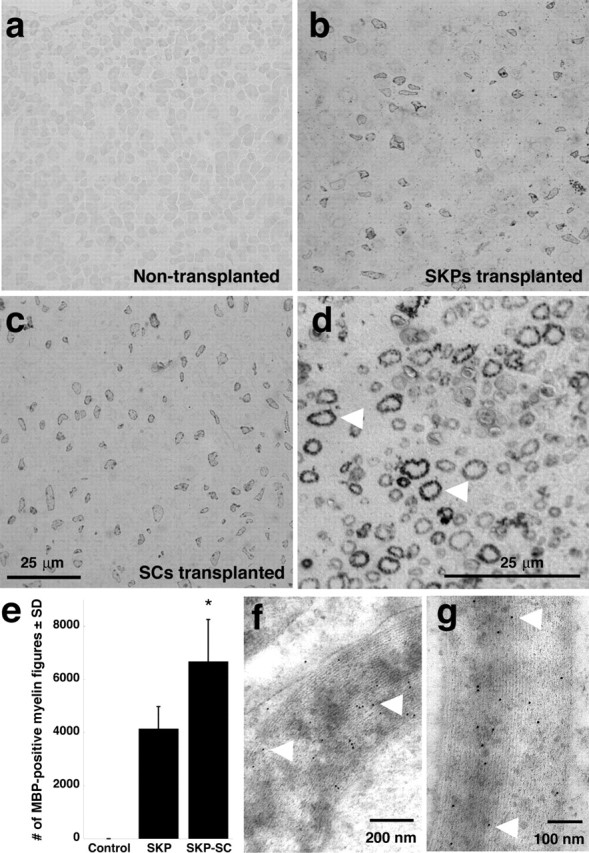
Structural evidence of myelination in the injured peripheral shiverer nerve by SKP-derived Schwann cells. Either naive SKPs or purified, SKP-derived Schwann cells were transplanted distal to a sciatic nerve crush in immunosuppressed shiverer mice, and nerves were analyzed as semithin cross sections 4 weeks after transplantation. a–c, Photomicrographs of semithin cross sections immunostained for MBP and visualized with DAB, showing the contralateral, uninjured, nontransplanted shiverer nerve (a), a nerve transplanted with naive SKPs (b), or a nerve transplanted with SKP-derived Schwann cells (c). Note the relatively robust number of MBP-positive myelin figures in the transplanted but not contralateral nerves. d, Higher-magnification micrograph illustrating the morphology of the MBP-positive myelin figures (arrowheads). Similar results were obtained in five transplanted nerves in each group. e, Quantitation of MBP-positive myelin figures in cross sections similar to those shown in a–d, comparing the relative number of myelin figures in nerves transplanted with naive SKPs versus SKP-derived Schwann cells. Details about the method of relative quantitation are provided in Results. Error bars indicate mean ± SD. ∗p < 0.05 for the comparison between the naive SKPs and SKP-derived Schwann cells, ANOVA; n = 3 in each group. f, g, Electron micrographs of ultrathin cross sections of injured shiverer sciatic nerve 4 weeks after transplantation of purified SKP-derived Schwann cells. Sections were immunostained for MBP and visualized with gold-labeled secondary antibody. Note the gold particles on the compact myelin (arrowheads).
Rodent and human SKPs myelinate CNS axons in shiverer mice
Nerve-derived Schwann cells can myelinate CNS axons, and their transplantation has therefore been suggested as a potential treatment for multiple sclerosis (Blakemore and Crang, 1985) and spinal cord injury (Takami et al., 2002; Pearse et al., 2004). To ask whether SKP-derived Schwann cells could also myelinate the dysmyelinated CNS, we initially transplanted neonatal YFP-tagged SKPs differentiated for 7 d in Schwann cell conditions into white matter tracts of organotypic cerebellar slice cultures derived from shiverer mice (Fig. 6a–d). As predicted, immunocytochemistry revealed that MBP expression was limited to the transplanted cells (Fig. 6b–d). Immunocytochemical analysis 2 weeks after transplantation revealed that many of these YFP-positive cells (at least 20%) coexpressed MBP within the cerebellar white matter (Fig. 6e). These MBP-positive cells also coexpressed S100β, confirming their identity as Schwann cells (Fig. 6f). Thus, SKP-derived Schwann cells integrate into CNS white matter, and this integration induces them to adopt a myelinating phenotype.
We next asked whether SKP-derived cells could myelinate CNS axons in vivo. Based on our studies with the regenerating nerve, we transplanted naive YFP-tagged SKPs into the brains of newborn shiverer mice, at a time point when myelination is commencing in vivo. Unlike the PNS, the shiverer CNS is characterized by extensive dysmyelination, such that any compact myelin can only be attributed to transplanted cells (Yandava et al., 1999; Liu et al., 2000; Vitry et al., 2003; Windrem et al., 2004). Four weeks after transplantation, we identified areas containing transplanted YFP-positive cells and processed these regions for electron microscopy. This analysis revealed that compact myelin was present in transplanted regions of shiverer brains (Fig. 7a) but not in adjacent regions that did not contain labeled cells or in control shiverer brains (Fig. 7b). At higher magnification, compact myelin with repeating major dense lines was evident in transplanted brains (Fig. 7c), whereas nontransplanted brains exhibited loosely wrapped axons characteristic of shiverer myelin (Fig. 7d). Thus, as seen in the regenerating nerve, even naive SKPs will differentiate into myelinating Schwann cells and myelinate axons, presumably in response to axonal cues within the developing postnatal CNS.
Figure 7.
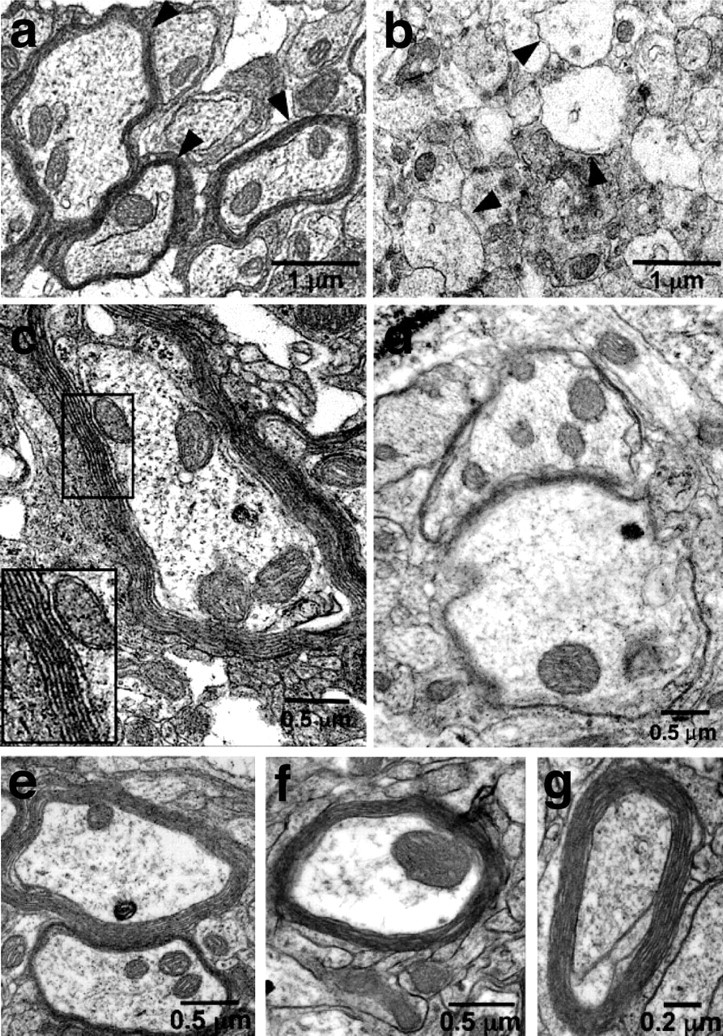
Transplanted, naive rodent and human SKPs generate Schwann cells to myelinate axons in the dysmyelinated shiverer brain. Electron micrographs of ultrathin sections of the shiverer midbrain 6 weeks after transplantation at birth with undifferentiated murine (a–d) or human (e–g) SKPs. a, c, Naive, YFP-tagged SKPs were transplanted, and those regions containing YFP-positive cells were microdissected, processed, and photographed at lower (a) or higher (c) magnification. In a, the arrowheads indicate myelin sheaths surrounding axons in regions of the shiverer midbrain that had been transplanted with SKPs; in c, the boxed area is shown at higher magnification to illustrate the compact myelin with major dense lines. b, d, Control shiverer brains were analyzed in the same way, and photomicrographs at lower (b) and higher (d) magnification illustrate the lack of compact myelin in these brains. Similar results were obtained in three transplanted shiverer animals that were analyzed by electron microscopy. e–g, Human SKPs were labeled with DiI before transplantation, and, at 6 weeks after transplantation, the regions containing DiI fluorescence were microdissected and processed. Each panel shows an electron micrograph of compact myelin generated by human SKPs transplanted into the brain of a newborn shiverer mouse. Similar results were obtained in three transplanted shiverer animals that were analyzed by electron microscopy.
Finally, we asked whether human SKPs could generate myelinating Schwann cells in vivo, as they apparently could in vitro (Fig. 1f–h). Undifferentiated neonatal human foreskin SKPs were labeled with DiI and transplanted into the neonatal shiverer mouse brain. After 6 weeks, transplant sites were visualized by DiI and processed for EM. Compact myelin similar to that seen in the rodent SKPs transplants was observed in transplanted regions (Fig. 7e–g) but not in adjacent DiI-negative regions. Similar results were obtained in three shiverer mice transplanted with human SKPs and analyzed by electron microscopy. Because compact myelin was never observed in the CNS of six control shiverer mice that were analyzed by electron microscopy and because it has never been reported previously (Yandava et al., 1999; Liu et al., 2000; Vitry et al., 2003; Windrem et al., 2004), we conclude that, like their rodent counterparts, human SKPs were able to differentiate into Schwann cells within the neonatal shiverer brain environment and myelinate these dysmyelinated CNS axons.
Discussion
The data reported here support four major conclusions. First, our culture studies indicate that neonatal human and rodent SKPs respond to environmental cues such as neuregulins by generating Schwann cells, a response very similar to that displayed by embryonic neural crest stem cells. Second, the coculture studies demonstrate that SKP-derived Schwann cells associate with axons, proliferate, and ultimately display a myelinating phenotype, presumably in response to axon-derived cues. Third, results of our peripheral nerve transplantation studies indicate that, as seen in culture, SKP-derived Schwann cells robustly associate with and myelinate regenerating axons, generating compact myelin that is indistinguishable from that made by resident Schwann cells in the nerve. The robustness of the responses demonstrated here and the coincidence between the culture and in vivo studies argue that SKP differentiation into Schwann cells is not a rare event, nor is it attributable to phenomena such as cell fusion. Fourth, and perhaps most surprisingly, the in vivo environments of the regenerating peripheral nerve and the neonatal brain are sufficient to direct naive SKPs to differentiate into Schwann cells that myelinate both PNS and CNS axons. Thus, our findings indicate that neonatal SKPs provide a highly accessible source of human Schwann cells for cell therapy and/or discovery research. Moreover, these data, together with our previous work (Toma et al., 2001, 2005; Fernandes et al., 2004), argue that SKPs represent a bona fide postnatal neural crest precursor cell that can generate functional neural progeny, a finding of importance from both basic and clinical perspectives.
Our previous work indicated that SKPs represent an embryonic neural crest-related precursor that migrated into the dermis during embryogenesis and then persisted into adulthood in lower numbers (Fernandes et al., 2004). Moreover, our work demonstrated that SKPs generated from facial skin were neural crest derived (Fernandes et al., 2004). However, we were unable to determine whether SKPs isolated from nonfacial skin were neural crest-derived because of the low penetrance of the fate-mapping strategy that was used (Fernandes et al., 2004). Here, we demonstrated that SKPs cultured from dorsal skin generate functional Schwann cells, a cell type that is only ever derived from the neural crest. Such a finding, together with our previous work, strongly supports the idea that SKPs are indeed neural crest-derived precursors. However, we cannot formally exclude the possibility that SKPs have a different embryonic origin but unexpectedly have the capacity to generate neural crest-derived cell types.
Is it possible that SKPs originate from previously uncharacterized Schwann cell precursors that are resident within postnatal dermis? It is clear that SKPs do not derive from dedifferentiation of Schwann cells themselves because (1) SKP-originating cells express Sca-1 (stem cell antigen-1) and Schwann cells do not (Fernandes et al., 2004), (2) cultured Schwann cells do not generate self-renewing spheres under SKP culture conditions (J. Biernaskie and F. D. Miller, unpublished data), (3) SKPs cannot be cultured from postnatal sciatic nerve, which primarily comprises Schwann cells and axons (Toma et al., 2001), and (4) SKPs can be generated from dissected vibrissal dermal papillae, a niche that does not contain Schwann cells (Fernandes et al., 2004). It is, however, possible that endogenous SKPs within the dermis function as precursors for resident neural crest cell types, including the Schwann cells found associated with nerve terminals. In this case, we predict that differentiation of these endogenous multipotent precursors is primarily determined by their environment because, once they are cultured, they generate neural crest-derived cell types that are never found within the dermis, such as neurons (Fernandes et al., 2004, 2006) and osteocytes (Biernaske, K.J. Fernandes, and Miller, unpublished data).
One of the surprising findings reported here is that the environments of the regenerating peripheral nerve and the neonatal shiverer CNS are sufficient to direct naive SKPs to generate myelinating Schwann cells. Moreover, this induction is highly efficient, at least within the regenerating nerve, because both naive and purified SKP-derived Schwann cells contributed robustly to the nerve distal to a crush injury, with the purified Schwann cells only being approximately twofold more efficient than the naive SKPs. The robust nature of this differentiation argues that regenerating axons provide a very strong instructive cue for naive SKPs. Based on culture work reported here and on previous work with developing Schwann cells (Shah et al., 1994; Morris et al., 1999; Woldeyesus et al., 1999; Garratt et al., 2000; Lemke, 2001; Leimeroth et al., 2002; Michailov et al., 2004; Taveggia et al., 2005), we propose that this axon-derived cue(s) is likely neuregulin-1, which has been shown to instruct neural crest precursors to generate Schwann cells, to promote Schwann cell proliferation, and to induce a myelinating phenotype. Because developing CNS neurons also secrete axonal neuregulins (Fernandez et al., 2000), it is possible that a similar axon-derived cue instructs SKPs to generate myelinating Schwann cells in the neonatal shiverer CNS, particularly because the endogenous shiverer oligodendrocytes might well be at a competitive disadvantage because of their genetic deficiency in MBP.
The demonstration that neonatal human skin contains a multipotent precursor cell that can generate functional Schwann cells provides a proof-of-concept with regard to a number of potential therapeutic uses. First, our findings indicate that SKPs represent a potential source of human Schwann cells for transplantation in disorders ranging from spinal cord injury to dysmyelinating disorders. However, we have not yet demonstrated that adult human skin-derived SKPs generate Schwann cells (neonatal foreskin was the human tissue source used here), nor have we determined the efficiency of human SKP differentiation into Schwann cells. Nonetheless, SKPs can be isolated and expanded from adult human skin biopsies (Toma et al., 2001; Joannides et al., 2004), and these adult human SKPs generate GFAP-positive glial cells that may be Schwann cells. If these adult biopsy-derived SKPs do retain the ability to differentiate into Schwann cells, then they would represent a possible autologous Schwann cell precursor source. Second, the finding of a precursor within skin that can generate Schwann cells suggests that these cells, SKPs, may represent a candidate initiating cell for tumors, such as those seen in neurofibromatosis-1, which partially manifest in skin and are thought to involve a transformed Schwann cell or Schwann cell precursor (Ward and Gutmann, 2005). Finally, because SKPs clonally generate both neural and mesodermal cells types in culture (Toma et al., 2001, 2005; Fernandes et al., 2004), the finding that at least some of these progeny are functional in vivo, as shown here, provides strong support for the concept that SKPs represent an expandable, clinically useful source of human neural crest-derived cell types for a variety of purposes.
Footnotes
*I.A.M. and J.B. contributed equally to this work.
This work was supported by grants from the Canadian Stroke Network (F.D.M.), Canadian Stem Cell Network (F.D.M., I.A.M.), Canadian Heart and Stroke Foundation (F.D.M.), NeuroSciences Canada (F.D.M.), Canadian Institutes of Health Research (F.D.M.), Hospital for Sick Children Foundation (I.A.M., J.B.), and Parkinson’s Foundation of Canada (J.B.) F.D.M. holds a Canada Research Chair. We thank Nao Kobayashi, Robert Temkin, and Anne Aumont for experimental help and members of the Miller/Kaplan laboratories for valuable input.
References
- Akiyama Y, Radtke C, Kocsis JD (2002). Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J Neurosci 23:6623–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore WF, Crang AJ (1985). The use of cultured autologous Schwann cells to remyelinate areas of persistent demyelination in the central nervous system. J Neurol Sci 70:207–223. [DOI] [PubMed] [Google Scholar]
- Cheng HL, Russell JW, Feldman EL (1999). IGF-I promotes peripheral nervous system myelination. Ann NY Acad Sci 883:124–130. [PubMed] [Google Scholar]
- Dezawa M, Takahashi I, Esaki M, Takano M, Sawada H (2001). Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur J Neurosci 14:1771–1776. [DOI] [PubMed] [Google Scholar]
- Eldridge CF, Bunge MB, Bunge RP, Wood PM (1987). Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J Cell Biol 105:1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabe-Heider F, Biernaskie J, Junek A, Kobayashi NR, Toma JG, Kaplan DR, Labosky PA, Rafuse V, Hui CC, Miller FD (2004). A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol 6:1082–1093. [DOI] [PubMed] [Google Scholar]
- Fernandes KJ, Kobayashi NR, Gallagher CJ, Barnabe-Heider F, Aumont A, Kaplan DR, Miller FD (2006). Analysis of the neurogenic potential of multipotent skin-derived precursors. Exp Neurol in press. [DOI] [PubMed]
- Fernandez PA, Tang DG, Cheng L, Prochiantz A, Mudge AW, Raff MC (2000). Evidence that axon-derived neuregulin promotes oligodendrocyte survival in the developing rat optic nerve. Neuron 28:81–90. [DOI] [PubMed] [Google Scholar]
- Garratt AN, Voiculescu O, Topilko P, Charnay P, Birchmeier C (2000). A dual role of erB2 in myelination and in expansion of the Schwann cell precursor pool. J Cell Biol 148:1035–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenard V, Kleitman N, Morrissey TK, Bunge RP, Aebischer P (1992). Syngeneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve regeneration. J Neurosci 12:3310–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjantonakis AK, Gertsenstein M, Ikawa M, Okabe M, Nagy A (1998). Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech Dev 76:79–90. [DOI] [PubMed] [Google Scholar]
- Joannides A, Gaughwin P, Schwiening C, Majed H, Sterling J, Compston A, Chandran S (2004). Efficient generation of neural precursors from adult human skin: astrocytes promote neurogenesis from skin-derived stem cells. Lancet 364:172–178. [DOI] [PubMed] [Google Scholar]
- Kohama I, Lankford KL, Preiningerova J, White FA, Vollmer TL, Kocsis JD (2001). Transplantation of cryopreserved adult human Schwann cells enhances axonal conduction in demyelinated spinal cord. J Neurosci 21:944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ (2002). Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron 35:657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimeroth R, Lobsiger C, Lussi A, Taylor V, Suter U, Sommer L (2002). Membrane-bound neuregulin1 type III actively promotes Schwann cell differentiation of multipotent progenitor cells. Dev Biol 246:245–258. [DOI] [PubMed] [Google Scholar]
- Lemke G (2001). Glial control of neuronal development. Annu Rev Neurosci 24:87–105. [DOI] [PubMed] [Google Scholar]
- Liu S, Qu Y, Stewart TJ, Howard MJ, Chakrabortty S, Holekamp TF, McDonald JW (2000). Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci USA 97:6126–6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA (2004). Axonal neuregulin-1 regulates myelin sheath thickness. Science 304:700–703. [DOI] [PubMed] [Google Scholar]
- Morgan L, Jessen KR, Mirsky R (1991). The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP−, N-CAM−, NGF-receptor−) depends on growth inhibition. J Cell Biol 112:457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Lin W, Hauser C, Marchuk Y, Getman D, Lee KF (1999). Rescue of the cardiac defect in ErB2 mutant mice reveals essential roles of Erb2 in peripheral nervous system development. Neuron 23:273–283. [DOI] [PubMed] [Google Scholar]
- Neuhuber G, Gallo G, Howard L, Kostura L, Mackay A, Fischer I (2004). Reevaluation of in vitro differentiation protocols for bone marrow stromal cells: disruption of actin cytoskeleton induces rapid morphological changes and mimics neuronal phenotype. J Neurosci Res 77:192–204. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MT (2004). cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med 10:610–616. [DOI] [PubMed] [Google Scholar]
- Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, van der Kooy D (2004). Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol 22:1115–1124. [DOI] [PubMed] [Google Scholar]
- Shah NM, Marchionni MA, Isaacs I, Stroobant P, Anderson DJ (1994). Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell 77:349–360. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D (1991). A simple method for organotypic cultures of nervous tissue. J Neurosci Methods 37:173–182. [DOI] [PubMed] [Google Scholar]
- Takami T, Oudega M, Bates ML, Wood PM, Kleitman N, Bunge MB (2002). Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J Neurosci 22:6670–6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL (2005). Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 47:681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma JG, Akhavan M, Fernandes KJL, Fortier MP, Barnabe-Heider F, Sadikot A, Miller FD (2001). Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol 3:778–784. [DOI] [PubMed] [Google Scholar]
- Toma JG, McKenzie IA, Bagli D, Miller FD (2005). Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells 23:727–737. [DOI] [PubMed] [Google Scholar]
- Vitry S, Bertrand JY, Cumano A, Dubois-Dalcq M (2003). Primordial hematopoietic stem cells generate microglia but not myelin-forming cells in a neural environment. J Neurosci 23:10724–10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BA, Gutmann DH (2005). Neurofibromatosis 1: from lab bench to clinic. Pediatr Neurol 32:221–228. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Nunes MC, Rashbaun WK, Schwartz TH, Goodman RA, McKhann G 2nd, Roy NS, Goldman SA (2004). Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat Med 10:93–97. [DOI] [PubMed] [Google Scholar]
- Woldeyesus MT, Britsch S, Riethmacher D, Xu L, Sonnenberg-Riethmacher E, Abou-Rebyeh F, Harvey R, Caroni P, Birchmeier C (1999). Peripheral nervous system defects in erB2 mutants following genetic rescue of heart development. Genes Dev 13:2538–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yandava BD, Billinghurst LL, Snyder EY (1999). “Global” cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain. Proc Natl Acad Sci USA 96:7029–7034. [DOI] [PMC free article] [PubMed] [Google Scholar]