Abstract
Peer-reared (PR) rhesus monkeys with early maternal separation later exhibit aggressiveness, impaired impulse control, alcohol abuse, and low CSF 5-hydroxyindoleacetic acid. This study compared regional brain serotonin transporter (SERT) binding between nine PR and seven mother-reared rhesus monkeys with [11C]DASB positron emission tomography (PET) imaging. Parametric images of binding potential (BP) (which is proportional to Bmax/KD, in which Bmax is transporter density and KD is dissociation constant) and relative blood flow (R1) were generated by the two-parameter multilinear reference tissue model. R1 images were used for coregistration and normalization of PET parametric data to the magnetic resonance imaging template space. Group BP differences were analyzed voxelwise by Student's t test in SPM2. Region of interest-based parameter values were also calculated to obtain the magnitude of regional BP differences between the two groups. For the PR group, SERT BP was decreased by 10–23% across a range of brain areas consisting of the raphe, thalamus, hypothalamus, caudate and putamen, globus pallidum, anterior cingulate gyrus, and medial temporal regions, including amygdala and hippocampus (cluster-level corrected p = 0.002). For the latter three regions, BP was decreased in the right hemisphere. These results agree with the hypothesis that early maternal deprivation affects the development of the serotonergic system and suggest that decreased serotonergic innervations in the critical brain regions may explain some of the behavioral and biochemical abnormalities in PR monkeys.
Keywords: positron emission tomography, serotonin transporter, early life stress, aggression, peer-reared rhesus monkey, impulse control
Introduction
Rhesus monkeys exposed to early life stress in the form of peer rearing are known to show behavior aberrations during adolescence characterized by impaired impulse control, aggressive behavior, anxiety-like behavior, social withdrawal, and excessive alcohol consumption (Higley et al., 1991a,b; Suomi et al., 1992). The pathophysiological mechanism underlying such behavior aberrations may be linked to the impaired central serotonin (5-HT) transmission and neuroendocrine stress axis. Low CSF concentrations of the major metabolite of 5-HT, 5-hydroxyindoleacetic acid (5-HIAA), and low basal plasma cortisol/ACTH levels with impaired response to acute stress have been consistently found in these animals (Higley et al., 1996a,b). Low CSF concentrations of 5-HIAA are also found in humans in association with impaired impulse control and aggressive behavior (Brown et al., 1979; Linnoila et al., 1983, 1989; Stanley et al., 1985; Kruesi et al., 1990), as well as type II alcoholism and antisocial behavior traits (Virkkunen and Linnoila, 1993). CSF concentrations of 5-HIAA are presumed to reflect central production of 5-HIAA, and differences in 5-HIAA productions may be attributable to changes in serotonergic innervations or altered turnover and metabolism of 5-HT.
During the early postnatal period, 5-HT plays a pivotal role in the development of the CNS (Buznikov et al., 2001), and 5-HT neurotransmission is involved in both activation and feedback control of the neuroendocrine stress axis (Weidenfeld et al., 2002). In turn, stress hormones are also involved in regulating the expression of certain serotonin system genes, including that for the serotonin transporter (SERT) (Tafet et al., 2001). SERT is located on the cell body and presynaptic terminals of the 5-HT neuron and terminates the action of intrasynaptic 5-HT by reuptake of 5-HT back into the presynaptic nerve terminal. SERT is also the site of action of commonly used antidepressant drugs (Shaskan and Snyder, 1970; Lesch, 1997). SERT density generally correlates with 5-HT nerve terminal density, and it can thus be used as a marker of serotonergic innervations (Soucy et al., 1994).
To delineate regional abnormalities of the serotonergic system in the brain of peer-reared (PR) rhesus monkeys, the authors used positron emission tomography (PET) with a recently developed radioligand, [11C]-3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)-benzonitrile ([11C]DASB) (Wilson et al., 2000, 2002). [11C]DASB binds reversibly to SERT with high affinity and selectivity. [11C]DASB PET allows accurate quantitative imaging of SERT binding noninvasively without requiring blood data (Ginovart et al., 2001; Ichise et al., 2003). The authors hypothesized that, in this exploratory study, [11C]DASB PET imaging would detect regionally decreased SERT binding in the brains of PR compared with mother-reared (MR) monkeys, the latter representing monkeys in natural conditions.
Materials and Methods
Animals.
Two groups of mean age- and weight-matched young adolescent male rhesus monkeys consisting of nine PR (3.3 ± 0.4 years and 5.0 ± 0.6 kg) and seven MR (3.4 ± 0.4 years and 5.4 ± 0.8 kg) animals were selected from two birth-year cohorts. We used only male animals to avoid potential effects of the female estrous cycle on the imaging results. The rearing conditions of these animals have been described in detail previously (Higley et al., 1991a,b; Suomi et al., 1992), and all rearing procedures were approved by the National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee. In brief, PR animals were separated from their mothers at birth and hand-reared in a neonatal nursery for the first 30 d of life. They were then placed in a cage with three other age mates (peers), with whom they had continuous contact, but they had no adult parental contact. The rearing conditions for MR animals approximated the natural rearing conditions. When both the PR and MR animals reached an average age of 6 months, they were placed together to form a larger permanent social group.
Positron emission tomography procedure.
Before PET scanning, anesthesia was induced with ketamine (10 mg/kg, i.m.) and endotracheal intubation was performed. After transportation to the PET suite, the animals were placed under isoflurane anesthesia (1.6 ± 0.1%). The head of the animal was immobilized in a stereotactic frame. To minimize the effects of ketamine, scans were started at least 2 h after ketamine administration. Vital signs were monitored throughout the experiment. All imaging and associated procedures were approved by the National Institute of Mental Health Animal Care and Use Committee.
PET scans were performed with a GE Advance scanner (GE Medical Systems, Waukesha, WI), with reconstructed resolution of 6 mm full-width half-maximum in all directions in three-dimensional mode. Coronal slices covering the whole brain were obtained. A transmission scan was initially performed for attenuation correction. PET scans were acquired for 120 min (33 frames with scan duration ranging from 30 s to 5 min) after bolus administration of 155 ± 37 Bq (specific activity, 29.6 ± 96.2 GBq/μmol) of [11C]DASB.
For image coregistration and normalization, each monkey had a T1-weighted magnetic resonance imaging (MRI) scan on a GE Signa 1.5 T scanner (spoiled gradient-recalled acquisition in a steady state; repetition time, 13.1 ms; echo time, 5.8 ms; flip angle, 45°; 0.4 × 0.4 × 1.5 mm; coronal acquisition on a 256 × 256 × 60 matrix).
CSF sampling.
Approximately 1 week before the scheduled PET scan study, a cisternal CSF tap was performed using a 5 ml syringe and a 23 or 22 gauge needle to obtain a 2–3 ml sample of CSF, and concentrations of CSF 5-HIAA were quantified with HPLC as described previously (Anderson et al., 1987). Additionally, CSF 5-HIAA data obtained previously when animals were at ∼6 months of age were included for the current data analysis.
Data analysis.
Analysis of the PET data were performed in two stages: generation of parametric images and statistical group comparisons. To generate parametric images of binding potential (BP) (Mintun et al., 1984) and relative tracer delivery (R1) from dynamic [11C]DASB PET data, we used a computationally fast and noise-resistant parameter estimation method, called two-parameter linearized reference tissue model (MRTM2) developed by Ichise et al. (2003). MRTM2 requires a priori estimation of k2′, which was estimated by the three-parameter MRTM using the regions of interest (ROIs) time activity curves of the cerebellum (reference tissue), raphe, and thalamus as described in detail previously (Ichise et al., 2003). The cerebellum ROI excluded the midline vermis and regions containing dentate nuclei. MRTM2 parametric imaging was performed by voxelwise weighted linear least-squares fitting with weights equal to the inverse of the data variance. The data variance was obtained by the method based on the noise equivalent counts (Pajevic et al., 1998) implemented in pixelwise modeling software, PMOD [version 2.5, PMOD group, Zurich, Switzerland (Mikolajczyk et al., 1998)].
To investigate regional differences in BP between the two animal groups, an exploratory statistical parametric analysis was implemented through SPM2 (www.fil.ion.ucl.ac.uk/spm). ROI-based parameter values were also calculated to obtain the magnitude of regional BP differences between the two groups.
For the SPM analysis, an MRI brain template was first created for rhesus monkey using methods described by Black et al. (1997). In brief, one monkey's MRI scan of 16 was randomly selected to which each of the remaining 15 MRI scans was normalized using a nonrigid-body 12-parameter algorithm in SPM2. All 16 scans were then averaged to obtain an initial template, which became the reference image for another iteration of registrations, and they were then averaged to get a second template. This process was repeated three times. Then, BP and R1 images were transformed into this group MRI template space using the product of two transformation matrices: (R1 to individual MRI) × (individual MRI to group template MRI). The first registration was accomplished with a rigid-body six-parameter algorithm in SPM2, whereas the second was accomplished with the same nonrigid-body algorithm used for the group template creation. The images were then smoothed with an 8 × 8 × 8 mm filter, and voxel-by-voxel paired t statistics were performed. Given the exploratory nature of this analysis, a threshold level of α = 0.01 uncorrected for multiple comparisons was chosen.
To calculate ROI-based parameter values, several anatomical ROIs were manually drawn on the sagittal template MRI images, in which the SERT density is high enough to provide a reliable signal, with reference to a stereotaxic rhesus brain MRI atlas (Paxinos et al., 2000). These ROIs included the frontal, temporal, parieto-occipital, striatum, and thalamus regions in each hemisphere, as well as the midline dorsal raphe, with the ROI size ranging from 11 to 40 voxels (voxel size of 2 × 2 × 2 mm). Regional BP values were obtained by applying this ROI set on the individual parametric images that were normalized to the group MRI template. For bilateral regions, right and left values were averaged. Regional SERT BP values were compared between the PR and MR groups with Student's t test for independent samples. No corrections were made for multiple comparisons.
Finally, CSF 5-HIAA concentrations were compared between the PR and MR groups with Student's t test for independent samples. The relationship between CSF 5-HIAA concentrations and regional SERT BP values was evaluated by the Pearson's correlation analysis. Statistical significance was defined as p < 0.05.
Results
PET data
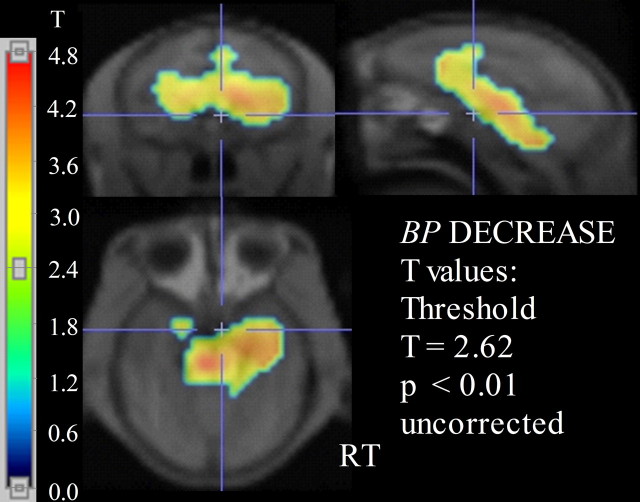
Parametric images of BP (Fig. 1) and R1 were successfully generated in all animals. Exploratory statistical parametric analysis of BP differences between the two animal groups by SPM2 (threshold, T = 2.62; p < 0.01) showed a single large cluster of decreased BP for the PR group (cluster extent, kE = 1418, multiple-comparisons cluster-level corrected p = 0.002; T = 4.84, p < 0.001 for the peak voxel within the cluster). This interconnected cluster extended across a range of brain areas consisting of the thalamus, hypothalamus, caudate, putamen, globus pallidum, anterior cingulate gyrus (areas 24a and 24b), and medial temporal regions, including amygdala and hippocampus (Fig. 2). For the latter three regions consisting of the globus pallidum, anterior cingulate gyrus (particularly area 24b), and medial temporal regions, BP was decreased asymmetrically involving predominantly the right hemisphere. The ROI analysis showed that BP was lower by 10–23% in the raphe, thalamus, striatum, and temporal regions of the PR group (Table 1). Finally, SPM2 analysis showed that there were no increases of BP in any region of PR compared with MR monkeys.
Figure 1.
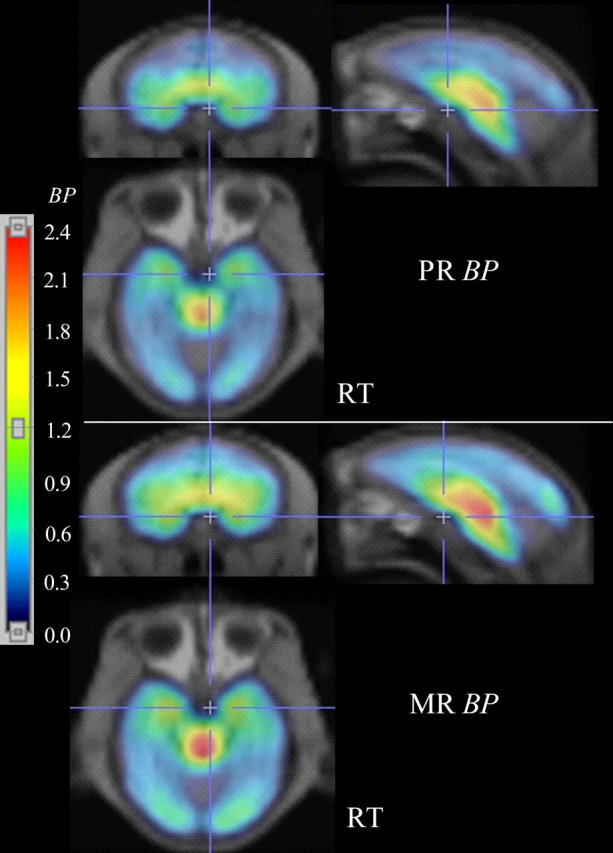
Spatially normalized parametric images of SERT BP fused onto the rhesus MRI template. BP images are shown in color, whereas the template MRIs are shown in grayscale. One PR monkey and one MR monkey are shown, respectively, in the top two rows and bottom two lower separated by a horizontal white line. For each monkey, coronal (top left), sagittal (top right), and transaxial (bottom left) slices are shown. The color scale on the left represents the range of BP values from 0 to 2.4. RT, Right.
Figure 2.
Voxelwise comparison of SERT BP between PR and MR rhesus monkeys by SPM2. The panels show coronal (left top), sagittal (right top), and axial (left bottom) views of the rhesus MRI template represented in grayscale, with superimposed p values for a t test (thresholded at p < 0.01; T = 2.62) representing an interconnected cluster of voxels with decreases of BP in the PR group. The color scale on the left represents the range of t values from 0 to 4.8. RT, Right.
Table 1.
Comparison of BP between PR and MR groups based on the ROI analysis (Student's t test for independent samples)
| ROI | BP | |||
|---|---|---|---|---|
| PR | MR | % decrease | p | |
| Raphe | 1.84 ± 0.21 | 2.11 ± 0.15 | 13 | <0.01 |
| Thalamus | 1.55 ± 0.19 | 1.72 ± 0.12 | 10 | <0.05 |
| Striatum | 0.77 ± 0.18 | 1.01 ± 0.19 | 23 | <0.05 |
| Temporal | 0.78 ± 0.14 | 0.96 ± 0.10 | 19 | <0.01 |
| Frontal | 0.34 ± 0.06 | 0.42 ± 0.10 | 18 | 0.11 |
| Parieto-occipital | 0.41 ± 0.06 | 0.47 ± 0.13 | 12 | 0.27 |
CSF data
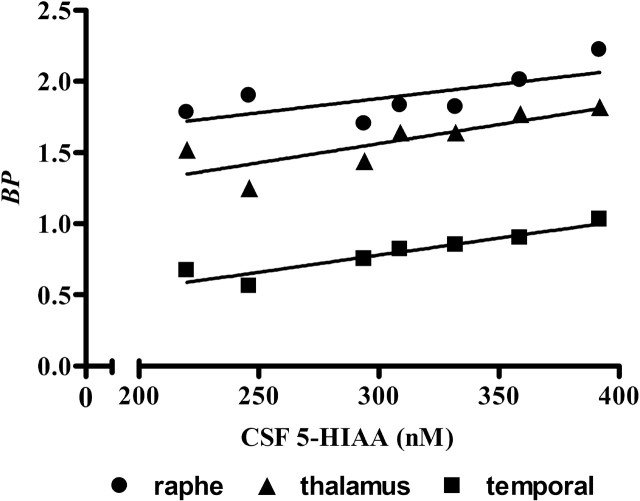
The CSF samples obtained approximately 1 week before the scheduled PET scan study were available for eight of nine PR and only four of seven MR monkeys. For these data, 5-HIAA concentrations tended to be lower by 17% (p = 0.19) in the PR (339 ± 91 nm) than in the MR (407 ± 170 nm) animals. There were no significant correlations between the CSF 5-HIAA concentrations and the regional SERT BP values. The CSF data obtained when these animals were at ∼6 months were available for seven of nine PR and seven of seven MR monkeys. For these data, 5-HIAA concentrations also tended to be lower (307 ± 61 nm) by 13% in the PR than in the MR animals (352 ± 61 nm; p = 0.19). In addition, for the PR group, there was a positive linear correlation between the 5-HIAA concentrations and SERT BP in the raphe (r2 = 0.49; p = 0.08), thalamus (r2 = 0.68; p = 0.02), and temporal region (r2 = 0.88; p = 0.002) (Fig. 3), although the correlation did not reach statistical significance for the raphe.
Figure 3.
Correlation between CSF 5-HIAA concentrations and SERT BP. For the PR monkey group, there was a positive linear correlation between CSF 5-HIAA concentrations and SERT BP in the raphe (r2 = 0.49; p = 0.08), thalamus (r2 = 0.68; p = 0.02), and temporal region (r2 = 0.88; p = 0.002). Oblique straight lines indicate linear regression lines.
Discussion
In the present [11C]DASB PET study of rhesus monkeys, the SPM analysis showed that SERT BP was lower in PR than in MR monkeys across a range of brain areas consisting of the raphe, thalamus, hypothalamus, caudate and putamen, globus pallidum, anterior cingulate gyrus, and medial temporal regions, including amygdala and hippocampus (cluster-level corrected p = 0.002). The ROI analysis showed that BP was lower by 10–23% in the raphe, thalamus, striatum, and temporal regions of the PR group (Table 1). As a term of reference, our recent test/retest [11C]DASB PET study showed that the test/retest variability of parametric image-based BP measurements in human was <5% in the thalamus, striatum, and raphe, and 9–13% in the cortical regions (Kim et al., 2006). In addition, in the same study, the method of parametric image-based BP measurements in an ROI was virtually identical to that of the conventional method of estimating a BP from the time activity curve of the same ROI. Thus, the 10–23% reductions of SERT BP in substantial areas of the brain of the PR group detected with [11C]DASB PET in the present study appear to be a robust finding.
The reductions of SERT BP for the PR group may be attributable to reduced serotonergic innervations across a range of brain areas. Alternatively, altered regulation of SERT protein expression may explain the BP decreases found in the present study. In the case of dopamine transporter (DAT), extracellular dopamine levels are known to regulate the transporter density (Gordon et al., 1996; Han et al., 1999). However, evidence suggests that extracellular 5-HT levels typically show no influence on regional SERT density (Graham et al., 1987; Dewar et al., 1992; Benmansour et al., 1999). In addition, [11C]DASB binding appears insensitive to competition with extracellular 5-HT levels (Praschak-Rieder et al., 2005; Talbot et al., 2005). A reduced production of serotonin secondary to the widespread reductions of central serotonergic innervations, as opposed to altered turnover and metabolism of 5-HT, may then account for the reduced CSF levels of a metabolite of 5-HT (5-HIAA) typically found in these animals.
In the present study, 5-HIAA levels tended to be lower in the PR than in the MR animals in which the statistical comparison may have been underpowered because of the small limited CSF sample size. In addition, the CSF data obtained when these PR animals were at ∼6 months showed a significant positive correlation between CSF 5-HIAA concentrations and SERT BP in multiple brain regions. Typically, low CSF 5-HIAA concentrations are found in PR monkeys early, and these reductions of 5-HIAA concentrations are known to persist into adolescence and adulthood (Higley et al., 1996b; Shannon et al., 2005). From a psychiatric perspective, PR monkeys with decreased CSF 5-HIAA concentrations are known to be at risk for a variety of serotonin-mediated psychopathological problems. For example, PR animals tend to show anxiety-like behavior, hyperarousal and high anxiety during minor stressors, high rates of violent aggression, and social withdrawal (Mineka and Suomi, 1978; Higley and Suomi, 1989; Higley et al., 1991a,b; Suomi et al., 1992). PR animals are also more likely to show despair in studies using nonhuman primates to model depression, and these depressive symptoms can be reversed using standard selective serotonin reuptake inhibitor (SSRI) treatments (Mineka and Suomi, 1978; Higley and Suomi, 1989; Higley et al., 1991a,b). PR animals are furthermore more likely to consume alcohol in excessive quantities in free-access paradigms (Higley et al., 1996a,b). This alcohol-consuming behavior is also reversible using SSRIs such as sertraline (Higley et al., 1998). These behavioral, biochemical, and current imaging data taken together suggest that parental input may be of critical importance for the development of the central serotonergic system and that, in the absence of such input, substantial brain areas rich in serotonin may be impaired. However, not all animals were affected equally, and there was some overlap between the PR and MR groups in the present study.
Low CSF concentrations of 5-HIAA are also found in human children and adults in association with impaired impulse control and aggressive behavior (Linnoila et al., 1983, 1989; Kruesi et al., 1990). Using the SERT tracer, [11C]McN 5652 ([″C]-(+)-6β-(4-methylthiophenyl)-1,2,3,5,6α, 10β-hexahydropyrrolo[2, 1-a]isoquinoline), Frankle et al. (2005) found decreased SERT densities in the anterior cingulate cortex of 10 human subjects with impulsive aggression compared with 10 healthy subjects. Frankle et al. (2005) found that decreased SERT binding was more marked on the left side, whereas we found greater losses on the right side in PR monkeys. As discussed by Frankle et al. (2005), traumatic brain lesions to the left frontal cortex have been shown by others to give rise to aggression and hostility, whereas right-sided lesions lead to anxiety/depression (Grafman et al., 1996). As noted above, PR animals tend to show anxiety-like behavior and social withdrawal among other behavioral aberrations. This is to our knowledge the first report of laterality differences in differentially reared primates. Heinz et al. (1998, 2003) also studied PR and MR monkeys with [123I]β-carbomethoxy-3β-(4-iodophenyl)-tropane ([123I]β-CIT), a single photon emission computed tomography (SPECT) radioligand that labels both DAT and SERT. Contrary to the current findings, these investigators found a negative correlation between the raphe SERT binding and CSF 5-HIAA concentrations. The discrepant findings may well be attributable to the specific radioligand used. Although the vast majority of striatal uptake of [123I]β-CIT reflects DAT, perhaps only 50% of midbrain reflects SERT. Because of the lower spatial resolution of SPECT and the poor selectivity of [123I]β-CIT for SERT, the current findings with [11C]DASB are more likely to be correct.
BP is commonly used a receptor parameter. BP (= f2Bmax/KD) reflects the receptor (transporter) density (Bmax), assuming that the tissue-free fraction, f2, and the dissociation constant, KD, are the same between the two groups, PR and MR groups in the present study. Although the foregoing assumptions are widely accepted, there are PET methods that can potentially allow separate estimation of Bmax and KD (Carson et al., 2002). The authors evaluated the feasibility of performing the Scatchard analysis described by Carson et al. (2002) with bolus/infusion administration of [11C]DASB at high and low specific radioligand activities (data not shown). In this attempt, it was feasible to establish equilibrium within the practical timespan of the PET experiment (2 h) in selected regions of the brain (striatum). The lower striatal BP in one PR monkey than that of another MR monkey was in fact attributable to a lower Bmax value of the former than that of the latter, although this finding needs to be replicated with a larger sample size. The PET data analysis model MRTM2 used in the present study generates parametric images of relative tracer delivery, R1, in addition to BP. The tracer kinetic model-based approach ensures that R1 estimation is independent of BP changes. Conversely, BP values are independent of regional blood flow (tracer delivery) or plasma tracer clearance (Ichise et al., 2001, 2003). The statistical quality of R1 images obtained noninvasively without blood data by MRTM2 is virtually identical to those obtained by nonlinear kinetic analysis with blood data (Ichise et al., 2003). The advantage of R1 images is that these images can be easily coregistered to the corresponding MRI, which allows more accurate three-dimensional alignment of PET and MRI space.
In conclusion, the current study showed widespread decreases of SERT binding in the brains of rhesus monkeys exposed to early life stress of maternal deprivation. These results agree with the hypothesis that early maternal deprivation affects the development of the serotonergic system and suggest that decreased serotonergic innervations in the critical brain regions may explain some of the behavioral and biochemical abnormalities in PR monkeys.
Footnotes
*S.J.S., J.D.H., and R.B.I. contributed equally to this work. The laboratories of affiliations 1, 2, and 4 also contributed equally.
This work was supported by the Intramural Research Program of the National Institutes of Health (NIH)/National Institute of Mental Health (NIMH)/National Institute on Alcohol Abuse and Alcoholism/ National Institute of Child Health and Human Development. We gratefully acknowledge Richard E. Carson for valuable input and suggestions, Dr. Cyrill Burger for providing the PET data analysis software PMOD, the staff of the NIH Clinical Center PET Department, the veterinary staff of the NIMH, including Drs. John Bacher and Tom Thomas, and Jae Seung Kim, Vanessa Cropley, Jeih-Sang Liow, Amira Brown, Michelle L. Becker, Melanie Schwandt, Stephen Lindell, and Christina S. Barr, for assistance in the PET and MRI studies.
References
- Anderson GM, Teff KL, Young SN (1987). Serotonin in cisternal cerebrospinal fluid of the rat: measurement and use as an index of functionally active serotonin. Life Sci 40:2253–2260. [DOI] [PubMed] [Google Scholar]
- Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, Frazer A (1999). Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci 19:10494–10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black KJ, Gado MH, Videen TO, Perlmutter JS (1997). Baboon basal ganglia stereotaxy using internal MRI landmarks: validation and application to PET imaging. J Comput Assist Tomogr 21:881–886. [DOI] [PubMed] [Google Scholar]
- Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF (1979). Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res 1:131–139. [DOI] [PubMed] [Google Scholar]
- Buznikov GA, Lambert HW, Lauder JM (2001). Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res 305:177–186. [DOI] [PubMed] [Google Scholar]
- Carson RE, Channing MA, Der MG, Herscovitch P, Eckelman WC (2002). Scatchard analysis with bolus/infusion administration of [11C]raclopride; amphetamine effects in anesthetized monkeys. In: Brain imaging using PET (Senda M, Kimura Y, Herscovitch P, eds) pp. 63–96. San Diego: Academic.
- Dewar KM, Grondin L, Carli M, Lima L, Reader TA (1992). [3H]paroxetine binding and serotonin content of rat cortical areas, hippocampus, neostriatum, ventral mesencephalic tegmentum, and midbrain raphe nuclei region following p-chlorophenylalanine and p-chloroamphetamine treatment. J Neurochem 58:250–257. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Ilise Lombardo I, New AS, Marianne Goodman M, Talbot PS, Huang Y, Dah Hwang D-R, Slifstein S, Curry S, Abi-Dargham A, Laruelle M, Siever LJ (2005). Brain serotonin transporter distribution in subjects with impulsive aggressivity: a positron emission study with [11C]McN 5652. Am J Psychiatry 162:915–923. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Wilson AA, Meyer JH, Hussey D, Houle S (2001). Positron emission tomography quantification of [11C]DASB binding to the human serotonin transporter: modeling strategies. J Cereb Blood Flow Metab 21:1342–1353. [DOI] [PubMed] [Google Scholar]
- Gordon I, Weizman R, Rehavi M (1996). Modulatory effect of agents active in the presynaptic dopaminergic system on the striatal dopamine transporter. Eur J Pharmacol 298:27–30. [DOI] [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM (1996). Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology 46:1231–1238. [DOI] [PubMed] [Google Scholar]
- Graham D, Tahraoui L, Langer SZ (1987). Effect of chronic treatment with selective monoamine oxidase inhibitors and specific 5-hydroxytryptamine uptake inhibitors on [3H]paroxetine binding to cerebral cortical membranes of the rat. Neuropharmacology 26:1087–1092. [DOI] [PubMed] [Google Scholar]
- Han S, Rowell PP, Carr LA (1999). D2 autoreceptors are not involved in the down-regulation of the striatal dopamine transporter caused by alpha-methyl-p-tyrosine. Res Commun Mol Pathol Pharmacol 104:331–338. [PubMed] [Google Scholar]
- Heinz A, Higley JD, Jones DW, Gorey JG, Saunders R, Zajicek K (1998). In vivo observation of an association between serotonin transporters and sensitivity to alcohol intoxication. Am J Psychiatry 155:1023–1028. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Gorey JG, Bennet A, Suomi SJ, Weinberger DR, Higley JD (2003). Serotonin transporter availability correlates with alcohol intake in non-human primates. Mol Psychiatry 8:231–234. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ (1989). Temperamental reactivity in nonhuman primates. In: Temperament in childhood (Kohnstamm D, Bates JE, Rothbart MK, eds) West Sussex, UK.: pp. 153–167.
- Higley JD, Hasert MF, Suomi SJ, Linnoila M (1991a). Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proc Natl Acad Sci USA 88:7261–7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M (1991b). CSF monoamine metabolite concentrations vary according to age, rearing, and sex, and are influenced by the stressor of social separation in rhesus monkeys. Psychopharmacology 103:551–556. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M (1996a). A nonhuman primate model of type II excessive alcohol consumption? Part 1. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations and diminished social competence correlate with excessive alcohol consumption. Alcohol Clin Exp Res 20:629–642. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M (1996b). A nonhuman primate model of type II alcoholism? Part 2. Diminished social competence and excessive aggression correlates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Alcohol Clin Exp Res 20:643–650. [DOI] [PubMed] [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M (1998). The serotonin reuptake inhibitor sertraline reduces excessive alcohol consumption in nonhuman primates. Neuropsychopharmacology 18:431–443. [DOI] [PubMed] [Google Scholar]
- Ichise M, Meyer JH, Yonekura Y (2001). An introduction to PET/SPECT neuroreceptor quantification models. J Nucl Med 42:755–763. [PubMed] [Google Scholar]
- Ichise M, Liow J-S, Lu J-Q, Takano A, Modell K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE (2003). Linearized reference tissue parametric imaging methods: application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J Cereb Blood Flow Metab 23:1096–1112. [DOI] [PubMed] [Google Scholar]
- Kim JS, Ichise M, Sangare J, Innis RB (2006). PET imaging of serotonin transporters with [11C]DASB: test-retest reproducibility using a multilinear reference tissue parametric imaging method. J Nucl Med 47:208–214. [PubMed] [Google Scholar]
- Kruesi MJ, Rapoport JL, Hamburger S, Hibbs E, Potter WZ, Lenane M, Brown GL (1990). Cerebrospinal fluid monoamine metabolites, aggression, and impulsivity in disruptive behavior disorders of children and adolescents. Arch Gen Psychiatry 47:419–426. [DOI] [PubMed] [Google Scholar]
- Lesch KP (1997). Molecular biology, pharmacology, and genetics of the serotonin transporter: psychobiological and clinical implications. In: Handbook of experimental pharmacology, Serotoninergic neurons and 5-HT receptors in the CNS (Baumgarten HG, Gothert M, eds) pp. 671–705. Berlin: Springer.
- Linnoila M, Vikkunen M, Scheinin M, Muutila A, Rimon R, Goodwin FK (1983). Low cerebrospinal fluid 5-hydroxyindolacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci 33:2609–2614. [DOI] [PubMed] [Google Scholar]
- Linnoila M, DeJong J, Virkkunen M (1989). Monoamines, glucose metabolism, and impulse control. Psychopharmacol Bull 25:404–406. [PubMed] [Google Scholar]
- Mikolajczyk K, Szabatin M, Rudnicki P, Grodzki M, Burger C (1998). A JAVA environment for medical image data analysis: initial application for brain PET quantitation. Med Inform 23:207–214. [DOI] [PubMed] [Google Scholar]
- Mineka S, Suomi SJ (1978). Social separation in monkeys. Psychol Bull 85:1376–1400. [PubMed] [Google Scholar]
- Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ (1984). A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol 15:217–227. [DOI] [PubMed] [Google Scholar]
- Pajevic S, Daube-Witherspoon ME, Bacharach SL, Carson RE (1998). Noise characteristics of 3-D and 2-D PET images. IEEE Trans Med Imaging 17:9–23. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang X-F, Toga AW (2000). In: The rhesus monkey brain in stereotaxic coordinates San Diego: Academic.
- Praschak-Rieder N, Wilson AA, Hussey D, Carella A, Wei C, Ginovart N, Schwarz MJ, Zach J, Houle S, Meyer JH (2005). Effects of tryptophan depletion on the serotonin transporter in healthy humans. Biol Psychiatry 58:825–830. [DOI] [PubMed] [Google Scholar]
- Shannon C, Schwandt ML, Champoux M, Shoaf SE, Suomi SJ, Linnoila M, Higley JD (2005). Maternal absence and stability of individual differences in CSF 5-HIAA concentrations in rhesus monkey infants. Am J Psychiatry 162:1658–1664. [DOI] [PubMed] [Google Scholar]
- Shaskan EG, Snyder SH (1970). Kinetics of serotonin accumulation into slices from rat brain: relationship to catecholamine uptake. J Pharmacol Exp Ther 175:404–418. [PubMed] [Google Scholar]
- Soucy JP, Lafaille F, Lemoine P, Mrini A, Descarries L (1994). Validation of the transporter ligand cyanoimipramine as a marker of serotonin innervation density in brain. J Nucl Med 35:1822–1830. [PubMed] [Google Scholar]
- Stanley M, Traskman-Bendz L, Dorovini-Zis K (1985). Correlations between aminergic metabolites simultaneously obtained from human CSF and brain. Life Sci 37:1279–1286. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Rasmussen KLR, Higley JD (1992). Primate models of behavioral and physiological change in adolescence. In: Textbook of adolescent medicine (McAnarney K, Kreipe RE, Orr DP, Gormerci GD, eds) pp. 135–140. Philadelphia: Saunders.
- Tafet GE, Toister-Achituv M, Shinitzky M (2001). Enhancement of serotonin uptake by cortisol: a possible link between stress and depression. Cogn Affect Behav Neurosci 1:96–104. [DOI] [PubMed] [Google Scholar]
- Talbot PS, Frankle WG, Hwang DR, Huang Y, Suckow RF, Slifstein M, Abi-Dargham A, Laruelle M (2005). Effects of reduced endogenous 5-HT on the in vivo binding of the serotonin transporter radioligand 11C-DASB in healthy humans. Synapse 55:164–175. [DOI] [PubMed] [Google Scholar]
- Virkkunen M, Linnoila M (1993). Brain serotonin, type II alcoholism and impulsive violence. J Stud Alcol Suppl 11:163–169. [DOI] [PubMed] [Google Scholar]
- Weidenfeld J, Newman ME, Itzk A, Gur E, Feldman S (2002). The amygdala regulates the pituitary adrenocortical response and release of hypothalamic serotonin following electrical stimulation of the dorsal raphe nucleus in the rat. Neuroendocrinology 76:63–69. [DOI] [PubMed] [Google Scholar]
- Wilson AA, Ginovart N, Schmidt M, Meyer JH, Threlkeld PG, Houle S (2000). Novel radiotracers for imaging the serotonin transporter by positron emission tomography: synthesis, radiosynthesis, and in vitro and ex vivo evaluation of 11C-labeled 2-(phenylthio)araalkylamines. J Med Chem 43:3103–3110. [DOI] [PubMed] [Google Scholar]
- Wilson AA, Ginovart N, Hussey D, Meyer J, Houle S (2002). In vitro and in vivo characterization of [11C]DASB: a probe for in vivo measurements of the serotonin transporter by positron emission tomography. Nucl Med Biol 29:509–515. [DOI] [PubMed] [Google Scholar]