Abstract
Adaptations in dopamine (DA) transmission in the prefrontal cortex (PFC) are thought to be critical to the development and persistence of drug addiction. Our previous findings showed that medial PFC (mPFC) neurons in rats treated repeatedly with amphetamine exhibit a decreased inhibitory response to iontophoretically applied DA, demonstrating altered DA receptor transmission. To determine the role postsynaptic DA D1 receptors play in this effect, we used whole-cell patch-clamp recordings of acutely dissociated pyramidal mPFC neurons and inhibition of transient voltage-sensitive sodium current (INaT) as a measure of D1 receptor function. After 3 d of withdrawal, neurons recorded from amphetamine-treated rats (5 mg/kg for 5 d) demonstrated a significant decrease in whole-cell INaT density and in the ability of D1 receptor stimulation to inhibit INaT. Application of a protein kinase A (PKA) inhibitor blocked the ability of D1 receptor activation to inhibit INaT and increased the current density of both groups to similar values. These results suggest that repeated amphetamine exposure results in subsensitivity of the INaT to D1 receptor-mediated inhibition because of a possible increase in basal PKA activity. This adaptation may contribute to perseverative behaviors in animals that self-administer psychostimulants as well as compromised PFC-dependent behaviors in human addicts.
Keywords: dopamine, sensitization, addiction, impulsivity, inhibitory control, drug abuse
Introduction
Considerable addiction research has focused on the ability of repeated drug exposure to enhance the incentive motivational qualities of drugs and their conditioned stimuli (Robinson and Berridge, 2003; Di Chiara et al., 2004). More recently, it has been recognized that a failure of inhibitory control, leading to impulsivity and compulsive stereotyped behaviors, is another major factor contributing to the development of addiction (Tiffany and Carter, 1998; Jentsch and Taylor, 1999; de Wit and Richards, 2004; Volkow and Li, 2004). A disruption in medial prefrontal cortex (mPFC) function is thought to be involved in the impaired behavioral inhibition seen in some psychiatric disorders, such as attention deficit hyperactive disorder and schizophrenia (Fuster, 1999), and may also contribute to the development of the perseverative behavioral characteristics seen in human drug addicts and animal models of addiction (Jentsch and Taylor, 1999; Spinella, 2003; Volkow and Li, 2004).
The goal of this study was to further investigate the role of mPFC dysfunction in addiction using behavioral sensitization, an animal model for the intensification of drug craving in human addicts (Robinson and Berridge, 2003). Behavioral sensitization is defined as the augmentation of behavioral responses to psychostimulants during and after their repeated administration. The mPFC plays a critical role in behavioral sensitization (Wolf, 1998; Vanderschuren and Kalivas, 2000) as well as in reinstatement of drug-seeking behavior after extinction of self-administration (Kalivas and McFarland, 2003). We reported previously that responsiveness of mPFC neurons (prelimbic/infralimbic layers V–VI) to the inhibitory effects of iontophoretic dopamine (DA) is reduced in rats treated repeatedly with amphetamine and withdrawn for 3 d (Peterson et al., 2000). To determine whether this effect was, in part, mediated via postsynaptic DA D1 receptors, the present study used whole-cell patch-clamp recordings of acutely dissociated mPFC neurons and examined inhibition of transient voltage-sensitive sodium currents (INaT) as a measure of D1 receptor activation efficacy. INaT plays a critical role in neuronal excitability and can also be used to study changes in intrinsic membrane properties (Li et al., 1992). Because recent studies suggest that there exists an optimal level of D1 receptor stimulation for proper frontal cortex function in normal animals (Williams and Goldman-Rakic, 1995; Zahrt et al., 1997; Dreher and Burnod, 2002), we hypothesized that disruption of D1 receptor-mediated signaling after repeated drug exposure could contribute to altered PFC-dependent behaviors associated with loss of control over drug-seeking and drug-taking behavior.
Materials and Methods
Drug treatment.
After a 3 d acclimation period, 4- to 5-week-old male Sprague Dawley rats (100–150 g body weight) were assigned randomly to two groups that received saline (1.0 ml/kg, i.p.) or amphetamine sulfate (5.0 mg/kg, i.p.) once daily for 5 consecutive days. Behavioral tests and electrophysiology recordings were performed 3 d after the final injection. This amphetamine regimen was used in our previous study of DA and glutamate transmission in the mPFC (Peterson et al., 2000). A different challenge dose (2.5 mg/kg free base, equivalent to 3.4 mg/kg amphetamine) and route of administration (subcutaneous) was used because this drug challenge enables excellent behavioral discrimination between sensitized and naive rats (Wolf et al., 1995).
Behavioral assessments.
Activity was measured using photobeam frames (San Diego Instruments, San Diego, CA). Each frame was 50 × 30 cm, with three photocells (separated by 10.5 cm) located lengthwise, 3.5 cm above the floor. A standard polyethylene rat cage (identical to home cages) was set inside each frame, and one rat was placed in each cage. Ambulation counts, which register after breaking of consecutive photobeams, were used as a measure of horizontal locomotor activity. To assess stereotypy, each rat was observed for 1 min of each 20 min interval during the first hour after amphetamine injection. Intervals were scored, using criteria described previously (Wolf et al., 1995), for the presence of two behaviors characteristic of amphetamine sensitization: (1) continuous in-place rearing and (2) nose-down, in-place stereotyped sniffing.
Electrophysiology.
Dissociated cell preparation and patch-clamp recordings were conducted as reported previously (Dong et al., 2005). Briefly, rats were anesthetized and decapitated rapidly. Brains were removed quickly, blocked, and sliced into coronal slices (400 μm) containing the mPFC. Slices were incubated for 1–6 h at room temperature in a NaHCO3-buffered Earle's balanced salt solution bubbled with 95% O2/5% CO2. The deep layers (V–VI) of the mPFC were isolated and digested in HEPES-buffered HBSS (Sigma, St. Louis, MO) containing protease type XIV (1–2 mg/ml; Sigma) at 35°C for 25–30 min. The tissue was then mechanically dissociated with a graded series of fire-polished Pasteur pipettes. The cell suspension was transferred to the recording chamber. Cells were allowed to settle for 5–10 min before starting a continuous background flow of PO4-free HEPES-buffered solution containing the following (in mm): 140 NaCl, 23 glucose, 13 HEPES, 2 KCl, 2 MgCl2, and 1 CaCl2 (pH 7.2, 300–310 mOsm/L). Sodium current was isolated using the following solutions (in mm): (1) internal: 120 N-methyl-glucamine, 35 HEPES, 20 CsCl, 1 NaCl, 4 MgCl2, 12 phosphocreatine, 2 Na2ATP, 0.2 NaGTP, and 0.1 leupeptin (pH 7.2 with H2SO4, 265–270 mOsm/L); (2) external: 30 NaCl, 110 choline chloride, 1 CaCl2, 1 MgCl2, 0.4 CdCl2, 5 CsCl, 10 glucose, and 10 HEPES (pH 7.4, 300–305 mOsm/L). Na+ current isolating solutions and pharmacological solutions were applied by a gravity-fed sewer pipe system. The array of application capillaries (0.78 mm inner diameter) was positioned a few hundred micrometers away from the cell under study, and solution changes were made by altering the position of the array with a rotating head rapid solution changer (Biologic, Grenoble, France). Complete solution changes were achieved within <1 s. Recordings were obtained with a Molecular Devices (Union City, CA) Axopatch 200A patch-clamp amplifier and controlled and monitored with a Dell pentium personal computer running pClamp8 (version 8.1) with a digiData 1200 interface (Molecular Devices). Electrode resistances were 1–2.5 MΩ in bath. After seal rupture, series resistance was compensated and monitored periodically. Recordings were discarded when series resistance exceeded 15 MΩ. Capacitative and leak currents were subtracted using a P/N technique. Data were low-pass filtered and digitized. All experiments were performed at room temperature (22–24°C).
Statistics.
Data are presented as mean ± SEM. See graphs for statistical measures. All data were collected and analyzed without knowledge of the treatment that the animals had received.
Drugs.
(+)-Amphetamine sulfate was obtained from the National Institute on Drug Abuse (Rockville, MD). All drug doses refer to salt weights unless noted otherwise. All other drugs and the protein kinase A (PKA) inhibitor fragment 14-24 trifluoroacetate salt were obtained from Sigma.
Results
Four- to 5-week-old rats pretreated with amphetamine demonstrate behavioral sensitization
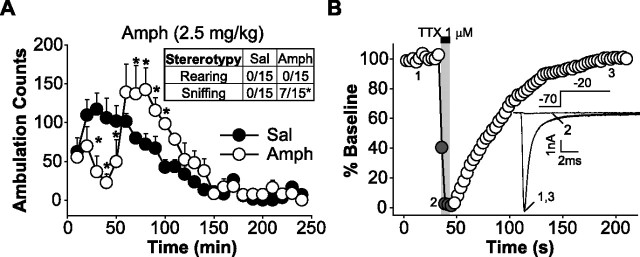
Rats 4–5 weeks old are an ideal age for the dissociated cell preparation, but it is unclear whether they develop behavioral sensitization to the amphetamine regimen used in these experiments (Tirelli et al., 2003). Therefore, we first determined whether behavioral sensitization to amphetamine could be demonstrated in 4- to 5-week-old rats using the same injection protocol and housing arrangements that we planned to use for electrophysiology experiments. Animals were administered daily injections of saline (1 ml/kg) or amphetamine (5 mg/kg) intraperitoneally for 5 d, followed by a 3 d withdrawal period. When both groups of rats were challenged with amphetamine (3.4 mg/kg, s.c.; see Materials and Methods), the amphetamine group exhibited a significant increase in stereotyped behavior and post-stereotypy locomotor hyperactivity compared with saline control rats [repeated-measures ANOVA, group × time effect: saline (n = 5), amphetamine (n = 5), F(23,184) = 3.96, p < 0.00001] (Fig. 1A). These results are similar to those obtained in adult rats (Wolf et al., 1995), verifying that 4- to 5-week-old rats manifest behavioral sensitization in response to repeated amphetamine treatment.
Figure 1.
Repeated administration of amphetamine produces behavioral sensitization in 4- to 5-week-old rats. A, On the third day of withdrawal, amphetamine (Amph) rats demonstrated sensitized stereotyped behaviors (during the first hour of the test) and post-stereotypy locomotor hyperactivity (during the second hour of the test) compared with saline (Sal) control rats (*p < 0.05; Duncan's post hoc) in response to an amphetamine challenge injection. Inset, Stereotyped behavior during the first hour of behavioral testing. See Materials and Methods for scoring criteria (Fisher's exact probability test; *p < 0.05). B, Application of TTX rapidly and reversibly blocks peak inward INaT of pyramidal mPFC neurons. A plot of the percentage of baseline of peak INaT evoked by a step in HP from −70 to −20 mV as function of time is shown. Inset, Representative traces of peak Na+ current before, during, and after TTX application.
INaT density is reduced in amphetamine-pretreated mPFC neurons
For electrophysiology experiments, rats received the same injection protocol as above but did not receive an amphetamine challenge injection. On the third day of withdrawal, deep-layer (layers V–VI) mPFC neurons were acutely dissociated and prepared for recording. Only mPFC neurons with a characteristic pyramidal shape with one apical dendrite and truncated or no basilar processes were studied. Inward currents elicited by stepping to −20 mV from a holding potential (HP) of −70 mV [approximate resting membrane potential in vitro (Nasif et al., 2005)] in a sodium current isolating extracellular recording solution were rapidly blocked by perfusion of the Na+ channel blocker tetrodotoxin (TTX; 1 μm) (Fig. 1B).
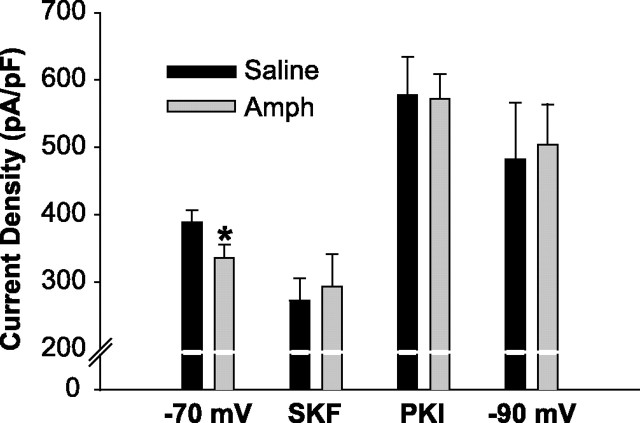
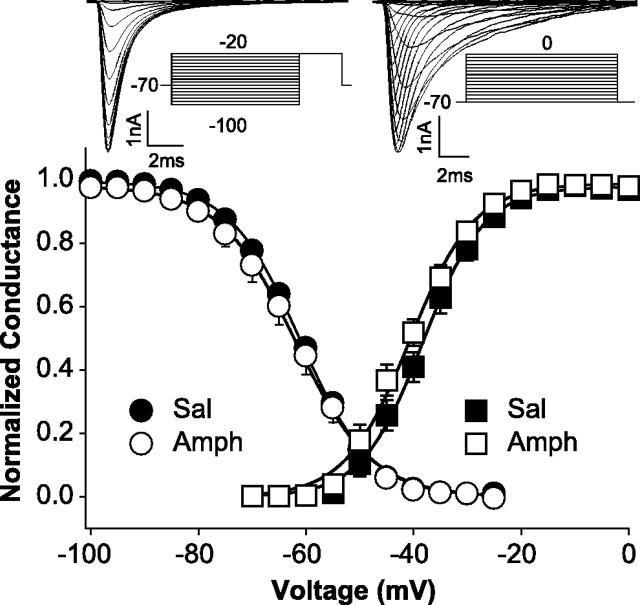
Whole-cell INaT density (peak INaT/capacitance) and voltage dependence of steady-state activation and inactivation were studied to determine whether intrinsic membrane properties had been affected by repeated amphetamine administration. Whole-cell INaT density was significantly reduced [13.6%; ANOVA: F(1,78) = 4.074, p < 0.05] in mPFC neurons obtained from amphetamine rats (336.14 ± 19.3 pA/pF; n = 36) compared with neurons taken from saline controls (389.01 ± 17.9 pA/pF; n = 44) (see Fig. 4). Voltage-dependent, steady-state half-maximal activation or inactivation (V1/2) and slope factor (k) values were obtained individually for each neuron tested for steady-state activation or inactivation and compared using ANOVA. There was no significant difference between groups in the kinetics or voltage dependence of steady-state activation [saline (n = 11): V1/2 = −38.6 ± 3.9 mV, k = 5.65 ± 2.0 mV; amphetamine (n = 10): V1/2 = −41.4 ± 3.3 mV, k = 6.3 ± 2.6 mV; V1/2: F(1,19) = 3.31, p = 0.085; k: F(1,19) = 0.37, p = 0.552] or inactivation [saline (n = 16): V1/2 = −60.9 ± 3.2 mV, k = 6.60 ± 0.5 mV; amphetamine (n = 10): V1/2 = −61.9 ± 5.3 mV, k = 6.98 ± 1.4 mV; V1/2: F(1,24) = 0.366, p = 0.551; k: F(1,24) = 0.936, p = 0.343] (Fig. 2). Activation of the protein kinase A (PKA) pathway produces effects on peak INaT and the voltage dependence of steady-state activation and inactivation of INaT similar to those observed in the present study of amphetamine-treated rats (Li et al., 1992), suggesting that the PKA pathway may be involved in the reduced INaT density produced by repeated amphetamine administration.
Figure 4.
Repeated amphetamine (Amph) treatment reduces the INaT density in mPFC pyramidal neurons; however, PKI or a −90 mV HP increases INaT density to similar values. INaT density is significantly reduced (*p < 0.05) in mPFC pyramidal neurons taken from rats pretreated with amphetamine compared with neurons taken from saline-pretreated controls when holding neurons at −70 mV. INaT density in the presence of SKF 81297 (SKF) is shown for representation of raw data values (as opposed to the percentage of inhibition shown in Fig. 3C) and is not significantly different (saline, 272.73 ± 33.1 pA/pF; amphetamine, 293.58 ± 48 pA/pF; ANOVA: F(1,25) = 0.136, p = 0.716). INaT density of mPFC neurons is increased to a similar level in rats treated with amphetamine or saline in the presence of PKI or when neurons were held at −90 mV. Error bars indicate SE.
Figure 2.
Repeated amphetamine treatment does not affect steady-state activation or inactivation of mPFC pyramidal neurons. A plot of peak conductance normalized to maximum conductance as a function of test pulse voltage (▪, activation) and peak INaT normalized to maximum peak INaT as function of prepulse voltage (•, inactivation) with representative current traces of INaT and voltage protocols (activation: HP, −70 mV; test pulse, 20 ms from −65 to 0 mV; increments, 5 mV; intertrial interval, 5 s; inactivation: HP, −70 mV; prepulse, 120 ms from −100 to −20 mV; increments, 5 mV; test pulse, 20 mV for 20 ms; intertrial interval, 5 s) is shown. There was no significant difference between groups. Sal, Saline; Amph, amphetamine.
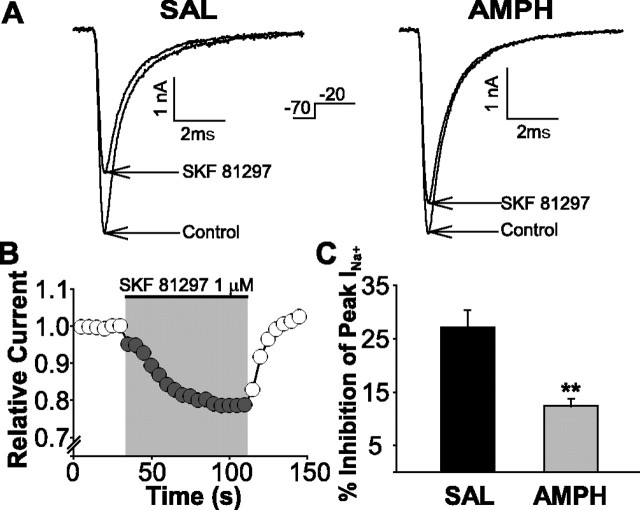
D1 receptor-mediated suppression of INaT in mPFC neurons is reduced in animals pretreated with amphetamine
Maurice et al. (2001) showed that activation of PKA, either via G-protein-coupled receptors positively coupled to adenylyl cyclase (e.g., D1 receptors) or directly using cAMP analogs, inhibits INaT in mPFC neurons. To test the functional efficacy of D1 receptors in mPFC neurons, application of the D1 receptor full agonist 6-chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine (SKF; SKF 81297) (1 μm) was used to reversibly decrease peak INaT (Fig. 3B). An average peak suppression of 27.1 ± 3.2% was observed in saline-treated rats (Fig. 3C). Coapplication of the D1 receptor antagonist SCH 23390 (7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine; 1 μm) blocked the SKF-induced inhibition of INaT (average peak suppression, 6.25 ± 1.3%; n = 4; data not shown). In amphetamine-treated rats, SKF was only about half as effective (∼46%; ANOVA: F(1,25) = 14.82; p < 0.001) at suppressing the INaT (average peak suppression, 12.4% ± 1.3%) (Fig. 3C), indicating that D1 receptor efficacy is reduced by repeated amphetamine administration. The number of neurons responding to D1 receptor stimulation did not differ between groups [n = 12 of 15 neurons responded (73.3%) in the amphetamine group, and n = 15 of 19 neurons responded (77.8%) in the saline group].
Figure 3.
Repeated amphetamine treatment reduces the magnitude of the inhibitory effect of D1 receptor stimulation on the INaT. A, Representative traces of INaT, elicited by a change in voltage command (−70 to −20 mV), before and during application of the D1 receptor full agonist SKF 81297 onto neurons from saline- and amphetamine-pretreated animals. B, Plot of relative peak INaT current as a function of time. Application of SKF 81297 reversibly suppressed peak INaT (21.4%; example taken from a saline control rat demonstrating a drug effect time course). C, Bar graph summary of the percentage of inhibition of peak INaT by SKF 81297 application onto neurons from saline- and amphetamine-pretreated animals (**p < 0.001). Error bars indicate SE. SAL, Saline; AMPH, amphetamine.
The PKA inhibitor PKI and a more hyperpolarized HP increased INaT density in both groups to similar values
Because D1 receptor stimulation inhibits INaT in naive rats via PKA, we hypothesized that increased basal activation of the PKA pathway in mPFC neurons from amphetamine-treated rats could account for our observations of reduced INaT density and reduced D1 receptor efficacy. First, we confirmed that D1 receptor activation was inhibiting INaT via PKA by applying SKF 81297 in the presence of a PKA inhibitor (PKI). The addition of PKI (10 U/ml) in the recording pipette completely blocked the SFK-induced suppression of INaT (average peak suppression, 2.37 ± 2.1%; n = 5; data not shown). We then looked at the effect of PKI on INaT density. PKI increased INaT density to similar values (ANOVA: F(1,39) = 0.0012; p = 0.972) in pyramidal neurons taken from both amphetamine-treated (560 ± 36.7 pA/pF; n = 22) and saline-treated (558 ± 48.4 pA/pF; n = 19) animals (Fig. 4), indicating that the INaT capacity of mPFC neurons taken from both treatment groups is similar in the absence of PKA activity.
A key aspect of PKA modulation of INaT is its voltage dependence (Cantrell et al., 1999; Carr et al., 2003). Cantrell et al. (1999) demonstrated in hippocampal neurons and Carr et al. (2003) demonstrated in mPFC neurons that PKA-induced inhibition of INaT is reduced when holding neurons at more hyperpolarized potentials (e.g., −90 mV). We therefore elicited INaT by stepping to −20 mV from a more hyperpolarized HP of −90 mV. INaT density was increased to similar values (ANOVA: F(1,23) = 0.0459; p = 0.832) in pyramidal neurons taken from both amphetamine-treated (504 ± 59.5 pA/pF; n = 12) and saline-treated (482 ± 83.7 pA/pF; n = 13) animals when using this protocol (Fig. 4). Taken together, these data suggest that the basal activity of the PKA pathway in amphetamine-treated animals is increased and may, in part, be responsible for the reduced ability of D1 receptor activation to inhibit INaT.
Discussion
Repeated exposure to drugs of abuse causes many neuroadaptations within the mesocorticolimbic system (Wolf, 1998; Vanderschuren and Kalivas, 2000). The current experiments were designed to characterize changes in D1 receptor function in the mPFC after repeated amphetamine administration. An optimal level of D1 receptor function in the mPFC is critical to the successful execution of different PFC-dependent behaviors, such as behavioral inhibition and working memory, and disruptions in D1 receptor signaling have profound effects on these behaviors (Williams and Goldman-Rakic, 1995; Zahrt et al., 1997; Goldman-Rakic et al., 2000; Dreher and Burnod, 2002). In addition, it is hypothesized that altered D1 receptor signaling is a major contributing factor to aberrant behavioral inhibition in human drug addicts (Jentsch and Taylor, 1999). Our in vivo recordings demonstrated that the iontophoretic application of DA was less efficacious at inhibiting the firing rate of mPFC neurons in rats tested after discontinuing repeated amphetamine injections compared with controls (Peterson et al., 2000), possibly contributing to the impaired behavioral inhibition seen in amphetamine-treated rats (Peterson et al., 2003).
The current experiments demonstrate a reduction in whole-cell INaT density in mPFC neurons from amphetamine-sensitized rats, most likely because of increased activation of the PKA pathway. When comparing the INaT densities at baseline (HP, −70 mV), during SKF 81297 application, in the presence of PKI, and at a −90 mV HP, it is only the baseline INaT density of mPFC neurons taken from amphetamine rats that is significantly different (reduced) compared with controls (Fig. 4). This suggests an increase in tonic PKA activity in the mPFC of amphetamine-sensitized rats, resulting in a tonic decrease in the open probability of voltage-sensitive sodium channels at activating potentials (Li et al., 1992), possibly because of increased entry of the channels into a slow inactivated-like state (Carr et al., 2003). Similarly, Dong et al. (2005) demonstrated that, after repeated cocaine administration, basal PKA activity is increased in mPFC neurons, which results in a decrease in the current density of voltage-gated potassium (K+) currents. It is significant that we found the same results in rats treated repeatedly with amphetamine because, despite similar initial effects of cocaine and amphetamine on extracellular DA levels, the identification of common downstream effects of repeated cocaine or amphetamine on the function of DA-receptive neurons has been a surprisingly elusive goal (Tzschentke, 2001). Activation of the PKA pathway has also been reported in other brain regions, such as the nucleus accumbens, after discontinuing repeated stimulant exposure (Nestler, 2001; Hope et al., 2005).
Because Bonhomme et al. (1995) found no difference in D1 or D2 receptor binding in the mPFC at 1 or 15 d withdrawal from a repeated amphetamine regimen similar to ours, a change in D1 receptor expression is unlikely to be responsible for the reduced ability of SKF 81297 to inhibit the INaT in mPFC neurons from amphetamine rats. Rather, our results indicate that SKF 81297 is less efficacious, because the PKA pathway is already activated in the mPFC of amphetamine-sensitized rats.
Although an increase in basal PKA activity might suggest a decrease in membrane excitability attributable to reduced INaT, the effects of DA receptor activation on mPFC pyramidal neurons are very complex (Seamans and Yang, 2004). Under some conditions, D1 receptor-mediated PKA activation can actually augment responses to glutamate agonists (Wang and O'Donnell, 2001; Tseng and O'Donnell, 2004; Sun et al., 2005). Moreover, as mentioned above, mPFC neurons recorded after repeated cocaine administration show an increase in PKA activity that decreases voltage-gated potassium channel current density, resulting in increased membrane excitability (Dong et al., 2005; Nasif et al., 2005). In this context, an increase in basal PKA activity would help explain results of our previous in vivo recording studies, in which we found that the iontophoretic application of glutamate onto mPFC neurons was more efficacious at increasing firing rates in amphetamine rats compared with control rats (Peterson et al., 2000). However, this may be an oversimplification of this complex subject.
In summary, these results demonstrate that INaT and its modulation by D1 receptors in mPFC neurons are reduced after repeated amphetamine administration. These reductions are most likely attributable to an increase in basal activity of the PKA pathway, which could possibly result in an increase in mPFC excitability. These findings clarify mechanisms underlying our previous in vivo observations (Peterson et al., 2000) and support the hypothesis that increased excitatory output of mPFC projection neurons after psychostimulant treatment contributes to an increased firing rate of ventral tegmental area DA neurons, probably through polysynaptic pathways (Wolf, 2002), which in turn is critical for initiation of behavioral sensitization (Henry et al., 1998; You et al., 1998). The observed changes in mPFC neuronal function may contribute to the disruption in some mPFC-dependent behaviors seen in human drug addictions, such as impaired behavioral inhibition (Jentsch and Taylor, 1999).
Footnotes
This work was supported by National Institutes of Health Grants DA12618 (F.J.W.), DA00456 (F.J.W.), DA05930 (J.D.P.), DA00453 (M.E.W.), and DA09621 (M.E.W.). We thank Kerstin Ford and Lorinda Baker for excellent technical assistance and Drs. Xiu-Ti Hu and Yan Dong for their critical advice and guidance.
References
- Bonhomme N, Cador M, Stinus L, Le Moal M, Spampinato U (1995). Short and long-term changes in dopamine and serotonin receptor binding sites in amphetamine-sensitized rats: a quantitative autoradiographic study. Brain Res 675:215–223. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Scheuer T, Catterall WA (1999). Voltage-dependent neuromodulation of Na+ channels by D1-like dopamine receptors in rat hippocampal neurons. J Neurosci 19:5301–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Day M, Cantrell AR, Held J, Scheuer T, Catterall WA, Surmeier DJ (2003). Transmitter modulation of slow, activity-dependent alterations in sodium channel availability endows neurons with a novel form of cellular plasticity. Neurons 39:793–806. [DOI] [PubMed] [Google Scholar]
- de Wit H, Richards JB (2004). Dual determinants of drug use in humans: reward and impulsivity. Nebr Symp Motiv 50:19–55. [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D (2004). Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology 47:227–241. [DOI] [PubMed] [Google Scholar]
- Dong Y, Nasif FJ, Tsui JJ, Ju WY, Cooper DC, Hu XT, Malenka RC, White FJ (2005). Cocaine-induced plasticity of intrinsic membrane properties in prefrontal cortex pyramidal neurons: adaptations in potassium currents. J Neurosci 25:936–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Burnod Y (2002). An integrative theory of the phasic and tonic modes of dopamine modulation in the prefrontal cortex. Neural Netw 15:583–602. [DOI] [PubMed] [Google Scholar]
- Fuster JM (1999). Synopsis of function and dysfunction of the frontal lobe. Acta Psychiatr Scand Suppl 395:51–57. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC III, Williams GV (2000). D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev 31:295–301. [DOI] [PubMed] [Google Scholar]
- Henry DJ, Hu XT, White FJ (1998). Adaptations in the mesoaccumbens dopamine system resulting from repeated administration of dopamine D1 and D2 receptor-selective agonists: relevance to cocaine sensitization. Psychopharmacology (Berl) 140:233–242. [DOI] [PubMed] [Google Scholar]
- Hope BT, Crombag HS, Jedynak JP, Wise RA (2005). Neuroadaptations of total levels of adenylate cyclase, protein kinase A, tyrosine hydroxylase, cdk5 and neurofilaments in the nucleus accumbens and ventral tegmental area do not correlate with expression of sensitized or tolerant locomotor responses to cocaine. J Neurochem 92:536–545. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR (1999). Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 146:373–390. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K (2003). Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology 168:44–56. [DOI] [PubMed] [Google Scholar]
- Li M, West JW, Lai Y, Scheuer T, Catterall WA (1992). Functional modulation of brain sodium channels by cAMP-dependent phosphorylation. Neuron 8:1151–1159. [DOI] [PubMed] [Google Scholar]
- Maurice N, Tkatch T, Meisler M, Sprunger LK, Surmeier DJ (2001). D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent sodium currents in prefrontal cortex pyramidal neurons. J Neurosci 21:2268–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasif FJ, Sidiropoulou K, Hu XT, White FJ (2005). Repeated cocaine administration increases membrane excitability of pyramidal neurons in the rat medial prefrontal cortex. J Pharmacol Exp Ther 312:1305–1313. [DOI] [PubMed] [Google Scholar]
- Nestler EJ (2001). Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2:119–128. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Wolf ME, White FJ (2000). Altered responsiveness of medial prefrontal cortex neurons to glutamate and dopamine after withdrawal from repeated amphetamine treatment. Synapse 36:342–344. [DOI] [PubMed] [Google Scholar]
- Peterson JD, Wolf ME, White FJ (2003). Impaired DRL 30 performance during amphetamine withdrawal. Behav Brain Res 143:101–108. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (2003). Addiction. Annu Rev Psychol 54:25–53. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR (2004). The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 74:1–57. [DOI] [PubMed] [Google Scholar]
- Spinella M (2003). Relationship between drug use and prefrontal-associated traits. Addict Biol 8:67–74. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME (2005). Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci 25:7342–7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Carter BL (1998). Is craving the source of compulsive drug use? J Psychopharmacol 12:23–30. [DOI] [PubMed] [Google Scholar]
- Tirelli E, Laviola G, Adriani W (2003). Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev 27:163–178. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P (2004). Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci 24:5131–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM (2001). Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog Neurobiol 63:241–320. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW (2000). Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 151:99–120. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Li T-K (2004). Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci 5:963–970. [DOI] [PubMed] [Google Scholar]
- Wang J, O'Donnell P (2001). D(1) dopamine receptors potentiate NMDA-mediated excitability increase in layer V prefrontal cortical pyramidal neurons. Cereb Cortex 11:452–462. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS (1995). Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 376:572–575. [DOI] [PubMed] [Google Scholar]
- Wolf ME (1998). The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol 54:679–720. [DOI] [PubMed] [Google Scholar]
- Wolf ME (2002). Addiction: Making the connection between behavioral changes and neuronal plasticity in specific circuits. Mol Interventions 2:146–157. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Dahlin SL, Hu XT, Xue CJ, White K (1995). Effects of lesions of prefrontal cortex, amygdala, or fornix on behavioral sensitization to amphetamine: comparison with N-methyl-d-aspartate antagonists. Neuroscience 69:417–439. [DOI] [PubMed] [Google Scholar]
- You ZB, Tzschentke TM, Brodin E, Wise RA (1998). Electrical stimulation of the prefrontal cortex increases cholecystokinin, glutamate, and dopamine release in the nucleus accumbens: an in vivo microdialysis study in freely moving rats. J Neurosci 18:6492–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF (1997). Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci 17:8528–8535. [DOI] [PMC free article] [PubMed] [Google Scholar]