Abstract
Cocaine primarily exerts its behavioral effects by enhancing dopaminergic neurotransmission, amplifying dopamine-encoded sensorimotor integration. The presumed mechanism for this effect is inhibition of the dopamine transporter, which blocks dopamine uptake and prolongs the duration of dopamine in the extracellular space. However, there is growing evidence that cocaine can also augment dopamine release. Here, we directly monitored the actions of cocaine on dopamine release by using electrochemical detection to measure extracellular dopamine in the striatum of anesthetized mice. Cocaine enhanced the levels of striatal dopamine produced by electrical stimulation of dopaminergic neurons. Even after pretreatment with α-methyl-p-tyrosine, which depletes the readily releasable pool of dopamine, cocaine was still capable of elevating dopamine levels. This suggests that cocaine enhances dopamine release by mobilizing a reserve pool of dopamine-containing synaptic vesicles. To test this hypothesis, we examined electrically evoked dopamine release in synapsin I/II/III triple knock-out mice, which have impaired synaptic vesicle reserve pools. Knock-out of synapsins greatly reduced the ability of cocaine to enhance dopamine release with long stimulus trains or after depletion of the newly synthesized pool. We therefore conclude that cocaine enhances dopamine release and does so by mobilizing a synapsin-dependent reserve pool of dopamine-containing synaptic vesicles. This capacity to enhance exocytotic release of dopamine may be important for the psychostimulant actions of cocaine.
Keywords: cocaine, synapsin, dopamine, in vivo voltammetry, storage pools, psychostimulant
Introduction
Dopamine neurons in the midbrain are synchronously and transiently activated by presentation of salient stimuli, including those that predict reinforcers (Schultz, 1998). These neurons project to areas of the forebrain that ultimately modulate motor performance. Indeed, striatal dopaminergic terminals are strategically located to play a central role in sensorimotor integration (Taghzouti et al., 1985). Recent findings demonstrate that transient dopamine surges occur in the striatum in response to important natural (Robinson et al., 2002; Roitman et al., 2004) or drug-related (Phillips et al., 2003) stimuli, and that these transients are involved in directing the animal's behavior. Cocaine, an important drug of abuse, enhances sensorimotor reactivity through its action on dopamine terminals (Davis, 1985). Indeed, physiological and emotional responses to drug-related sensory stimuli are enhanced after cocaine use in humans (Childress et al., 1999).
The mechanisms through which cocaine enhances dopaminergic neurotransmission have long been debated (Bauman and Maitre, 1976). It is well established that cocaine competitively inhibits the dopamine transporter (DAT), thereby elevating extracellular levels of dopamine (Jones et al., 1995). However, several reports point toward the possibility that cocaine and other psychostimulants can also affect release of dopamine (Stamford et al., 1989; Lee et al., 2001). The first indication of an effect of cocaine on dopamine release came from the observation that cocaine and other nonamphetamine psychostimulants can still stimulate the CNS even after dopamine availability is limited by inhibiting its synthesis (Shore, 1976). These results were interpreted to mean that the psychostimulants act by mobilizing a storage pool of dopamine. Support for this interpretation was provided by the demonstration that one of these psychostimulants, amfonelic acid, can restore dopamine release after depletion of newly synthesized dopamine with α-methyl-p-tyrosine (αMPT) (Ewing et al., 1983). Although these findings were intriguing, they were unable to elucidate cellular or molecular substrates for this postulated cocaine-sensitive storage pool.
More recent studies of other neurotransmitter systems have shown that several different pools of secretory vesicles exist (for review, see Neher, 1998). These include a releasable pool of vesicles that are available for immediate exocytosis and a reserve pool of vesicles that are spatially segregated and mobilized after prolonged synaptic activity (Pieribone et al., 1995; Rosenmund and Stevens, 1996; Kuromi and Kidokoro, 1998; Duncan et al., 2003; Richards et al., 2003). Therefore, it is possible that cocaine could also mobilize dopaminergic vesicles from such a reserve pool. Here, we consider this hypothesis by using mice in which all three known synapsin genes have been disrupted, synapsin I/II/III triple-knock-out (TKO) mice. Synapsins are phosphoproteins that interact with the surface of synaptic vesicles and segregate synaptic vesicles into the reserve pool (Greengard et al., 1993; Hilfiker et al., 1999). These mice are viable but have severe deficits in their synaptic vesicle reserve pools (Gitler et al., 2004b). We found that cocaine enhances dopamine released by stimuli that mobilize vesicles from the reserve pool in wild-type mice but has little effect on dopamine release in the TKO mice. These results indicate that cocaine enhances dopamine release by mobilizing a synapsin-dependent reserve pool of dopamine-containing synaptic vesicles.
Materials and Methods
Animals.
Synapsin (I/II/III) TKO and wild-type (WT) mice were generated at The Rockefeller University (New York, NY) and bred at Duke University (Durham, NC), as described previously (Gitler et al., 2004a,b).
Surgery.
Animals were anesthetized with urethane (1.5 g/kg, i.p.) and placed in a stereotaxic frame. The coordinates for placement of the working electrode in the caudate–putamen are (in mm from bregma): anteroposterior (AP) +1.1, mediolateral (ML) +1.2, and dorsoventral (DV) −2.2. The stimulating electrode was placed in the medial forebrain bundle at AP −2.4, ML +1.1, DV −4.5. The dorsoventral placement of both the working and stimulating electrodes was adjusted in small increments to find maximal dopamine release. An Ag/AgCl reference electrode was inserted into the contralateral side of the brain.
Electrochemistry.
Dopamine was detected with 50-μm-long cylindrical carbon-fiber microelectrodes (Venton et al., 2002). Dopamine signals were identified with fast-scan cyclic voltammetry with a voltage scan from −0.4 to 1.0 V and back at 300 V/s, repeated every 100 ms. For pharmacology experiments, once a dopamine release site was identified with cyclic voltammetry, constant-potential amperometry (+0.3 V) was used, because it has a more rapid time response (Venton et al., 2002). Electrodes were calibrated in vitro after the experiment using known concentrations of dopamine.
Data analysis.
Amperometric data recorded during 24 pulse stimulations were modeled (Venton et al., 2003) by assuming that each stimulus pulse evokes an increase in the extracellular concentration of dopamine ([DA]p). In the time between stimulus pulses and after the stimulus train, uptake of dopamine by the DAT was assumed to follow Michaelis–Menten kinetics with an apparent affinity for dopamine (Km) of 0.2 μm in WT mice (Joseph et al., 2002; Venton et al., 2003) and a maximum rate of uptake of the DAT (Vmax) that is a function of the density of proximal uptake sites. The simulation also included an apparent distance (dapp) that dopamine can diffuse. After block of uptake by competitive inhibitors, Vmax was kept constant, and the remaining parameters were allowed to vary until an optimal fit to the data was obtained. Statistical comparisons were performed in Microsoft (Seattle, WA) Excel using t tests. Data are reported as mean ± SEM and were considered significant at p < 0.05.
Drugs.
Cocaine and αMPT were purchased from Sigma-Aldrich (St. Louis, MO). All drugs were dissolved in saline for intraperitoneal administration.
Results
Effects of cocaine on electrically evoked dopamine release
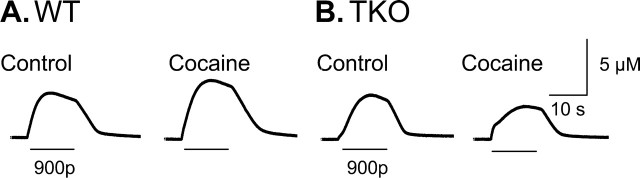
Dopamine release was measured in the caudate–putamen of anesthetized mice while the medial forebrain bundle was stimulated with long stimuli (900 pulses, 60 Hz) to probe the multiple compartments of releasable dopamine. At the onset of electrical stimulation in WT mice, dopamine rapidly appeared in the caudate–putamen (Fig. 1A). As the stimulation proceeded, dopamine concentration reached a maximum and then slowly diminished. This biphasic response during stimulation is similar to that characterized previously in rats and is attributed to a long-term depression of dopamine release (Montague et al., 2004). After the stimulation, dopamine concentration declined rapidly because of neuronal uptake of dopamine (Garris et al., 1994). Ten minutes after administration of cocaine (10 mg/kg), the amplitude of the evoked dopamine response ([DA]max) increased and reached levels that were 146 ± 26% of predrug values (n = 6 mice). The clearance of dopamine after stimulation appears in Figure 1 to be little affected by cocaine. This is because the dopamine concentrations evoked by the long stimuli greatly exceed Km, the uptake parameter that is affected by cocaine (Venton et al., 2003). However, when the rate of uptake is examined at low (submicromolar) dopamine concentrations, near the natural Km value, changes in Km after cocaine can be observed. The apparent value of Km determined under these conditions was 179 ± 49% of the predrug value in WT mice (n = 4).
Figure 1.
Effects of cocaine (10 mg/kg, i.p.) on dopamine release evoked by 900 pulse, 60 Hz electrical stimulations. Predrug traces are displayed on the left, and postinjection traces (10–13 min after cocaine administration and 40 min after the previous stimulation) are shown on the right. A, Representative traces from WT mice. B, Representative traces from TKO mice. The data are traces from cyclic voltammetric recordings.
This experiment was repeated in synapsin TKO mice to examine the potential role of a reserve pool of dopamine in the response to cocaine. Dopamine responses evoked by the same prolonged stimuli described above had a similar waveform in the TKO mice as in WT mice (Fig. 1B). However, 10 min after cocaine, the amplitude of the stimulated dopamine release was diminished, reaching levels that were only 78 ± 2% of predrug values (n = 6 mice). Thus, the pool mobilized by cocaine appears to be absent in synapsin TKO mice. The apparent value of Km was 206 ± 77% of the predrug value in TKO mice (n = 6), a value that is not statistically different from that found in WT mice (p > 0.05).
Effects of cocaine on dopamine release after synthesis inhibition
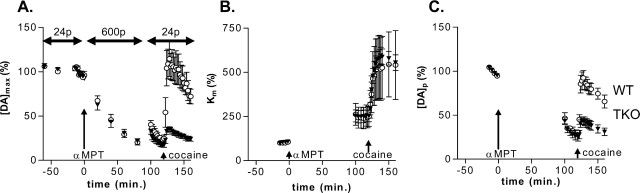
We next examined more directly the ability of cocaine to mobilize the reserve pool of dopamine. This was done by depleting the releasable pool to ∼25% of its predrug value (Ewing et al., 1983) by repeatedly applying long-duration stimulus trains (600 pulses at 60 Hz) after administration of αMPT (200 mg/kg, i.p.), a dopamine synthesis inhibitor. Shorter-duration stimuli (24 pulses at 60 Hz) were then applied to probe the effects of cocaine. In the absence of αMPT, maximal responses to these stimuli were relatively constant over time (Fig. 2A). However, injection of αMPT caused the amount of dopamine released by either type of stimulus to be reduced by ∼75% (Fig. 2A). Consistent with previous work with other psychostimulants done in rats (Ewing et al., 1983), cocaine (10 mg/kg, i.p.) restored the amplitude of responses to electrical stimuli (Fig. 2A, open symbols). Thus, cocaine can enhance dopamine responses, even after the readily releasable pool of dopamine is mostly depleted by αMPT. This suggests that cocaine releases dopamine from a pool that is distinct from the readily releasable pool.
Figure 2.
Effects of αMPT (200 mg/kg, i.p.) followed by cocaine (10 mg/kg, i.p.) administration on stimulated dopamine release in WT (open circles) and synapsin TKO (filled triangles) mice. Dopamine release was electrically evoked by short (24 pulse, 60 Hz, every 2 min) stimulation trains except for the 100 min interval after αMPT when long (600 pulse, 60 Hz, every 20 min) stimulations were used to deplete dopamine stores, and dopamine was measured by constant potential amperometry. The stimulation pulses (p) in these time domains are indicated at the top of the figure. A, The peak neurochemical response ([DA]max) to 24- and 600-pulse stimulations as a percentage of the predrug values. B, Km values obtained from modeling of the experimental data expressed as a percentage of the predrug values. C, [DA]p values obtained by neurochemical modeling of the experimental data expressed as a percentage of the predrug value. The data are presented as the mean ± SEM from measurements in eight animals for each genotype.
To evaluate the role of synapsins in the cocaine-sensitive pool, similar experiments were done in the synapsin TKO mice. As in the WT mice, 600 pulse, 60 Hz stimuli delivered to TKO mice diminished releasable dopamine to ∼25% after αMPT (200 mg/kg, i.p.) (Fig. 2A). However, subsequent administration of cocaine (10 mg/kg, i.p.) to synapsin TKO mice caused a much smaller increase in the maximal evoked release of dopamine (Fig. 2A). Fitting the responses to 24 pulses with the mathematical model indicated that αMPT caused Km values to increase slightly in both types of mice; we attribute this to the metabolism of αMPT to p-hydroxyamphetamine (Joseph et al., 2002). Cocaine administration also caused the same relative change in Km for dopamine uptake in both WT and TKO mice (Fig. 2B). In contrast, [DA]p values, the amount released per stimulus pulse, remained low in TKO mice, whereas they returned to near pre-αMPT values in WT mice (Fig. 2C). The [DA]p values were 42 ± 4% of initial levels after cocaine in TKO mice and 87 ± 8% of initial levels in WT mice, values that are statistically different (p < 0.05). Thus, the absence of synapsins significantly reduced mobilization of the reserve pool of dopamine in response to cocaine. These findings indicate that synapsins play a major role in maintaining the dopamine reserve pool and that these proteins serve as a target for cocaine.
Discussion
Cocaine, like many other psychostimulants, is a competitive inhibitor of the DAT (Wu et al., 2001). Here, we demonstrate that cocaine can also promote dopamine release from the reserve pool. The results are consistent with previous hypotheses that striatal dopamine is segregated into releasable and storage compartments, and that the latter pool can be mobilized by nonamphetamine psychostimulants. The molecular bases of the segregation of these pools of dopamine were revealed through the use of mice lacking synapsin genes. To minimize the possibility of compensation resulting from redundant functions of synapsin isoforms, we used mice with deletions of all known isoforms of synapsin. In these synapsin TKO mice, both the cocaine-mediated augmentation of release after long stimulus trains and the ability of cocaine to restore release after synthesis inhibition were both dramatically reduced, suggesting that cocaine mobilizes a synapsin-dependent compartment of dopamine vesicles in striatal neurons. These results demonstrate that cocaine increases extracellular dopamine not only by blocking its uptake but also from mobilizing a synapsin-dependent storage pool.
Cocaine increases dopamine release probability
In addition to inhibiting dopamine uptake, many psychostimulants enhance the amount of releasable dopamine both in vivo (Ewing et al., 1983; Stamford et al., 1986, 1989) and in vitro (Hafizi et al., 1992; Lee et al., 2001). Our work confirms that cocaine also can promote enhanced release probed with long stimuli. DAT apparently is required for the psychostimulant-induced increase in dopamine release, because this increase in dopamine release is not observed in DAT-deficient mice (Jones et al., 1998).
Several lines of evidence point toward the conclusion that cocaine increases dopamine release by mobilizing dopamine from a reserve pool. Early work indicated that dopamine is stored in multiple compartments, with 80% unavailable for immediate release (Javoy and Glowinski, 1971; Korf et al., 1976). This normally unavailable dopamine, presumably in a reserve pool, becomes available for release after cocaine as well as other psychostimulants (Ewing et al., 1983). Our data extend this conclusion by demonstrating that enhanced release after cocaine treatment is most evident when the contribution of the readily releasable pool is minimized: cocaine increased dopamine release by ∼50% in control conditions (Fig. 1A), but increased release approximately fourfold after dopamine synthesis was inhibited by αMPT (Fig. 2A). Thus, cocaine preferentially acts on a pool of dopamine that is not readily releasable and does not depend on continuous synthesis of dopamine.
Synapsins regulate releasable stores in dopamine neurons
Although early investigators of catecholamine metabolism described a reserve compartment for the intracellular storage of dopamine (Javoy and Glowinski, 1971; Doteuchi et al., 1974), its nature and subcellular location have remained essentially unclear. In nerve terminals using other transmitters such as glutamate and GABA, a subset of vesicles is sequestered to the cytoskeleton by synapsins (Greengard et al., 1993; Gitler et al., 2004b). In synapsin TKO mice, we found that dopamine release could still be evoked electrically, but cocaine was much less able to enhance electrically evoked dopamine release than in WT animals. This demonstrates that synapsins also are important for the cocaine-sensitive storage pool in dopaminergic terminals. In fact, dopamine release evoked by long stimuli was actually diminished by cocaine in synapsin TKO mice. This presumably is a result of the absence of a reserve pool in combination with the action of cocaine on dopamine uptake, which would be expected to impair recycling of dopamine into the releasable pool and cause a net decrease in dopamine release.
These findings indicate that cocaine can increase dopamine release by mobilizing synapsin-bound vesicles that comprise the dopamine storage pool. How cocaine interacts with synapsin is unclear. Cocaine is known to increase dopamine release from striatal terminals even when isolated from their cell bodies (Lee et al., 2001), meaning that cocaine must act locally within the presynaptic terminal. In some cells, cocaine can enhance presynaptic Ca2+ influx (Premkumar, 1999; Yermolaieva et al., 2001), and chronic administration of cocaine increases the sensitivity of released dopamine to Ca2+ channel blockers (Pierce and Kalivas, 1997). It is thought that Ca2+-dependent phosphorylation of synapsins is required for mobilization of vesicles from the reserve pool during electrical stimulation (Greengard et al., 1993). Thus, it is possible that cocaine and other psychostimulants enhance dopamine release by increasing presynaptic Ca2+ influx and thereby mobilizing synaptic vesicles as a result of Ca2+-dependent phosphorylation of synapsins.
Implications of synapsin regulation of dopamine release
Dopamine neurons are phasically activated by incentive cues. Dopamine release then modulates neuronal signals passing through medium spiny neurons in striatum by gating glutamatergic afferents (Floresco et al., 2001) and promotes selection of an appropriate motor response. By interacting with synapsins, cocaine can switch dopamine neurons into a mode of sustained dopamine release that would be expected to elevate sensory cue reactivity. Indeed, amplification of sensorimotor integration by cocaine is consistent with the effects of this drug on mobilization of dopamine from the storage pool, because neither effect is prevented by inhibiting dopamine synthesis (Davis, 1985). Overall, the synapsin-mediated effect of cocaine on the dopamine reserve pool, in combination with other forms of plasticity in the mesolimbic system (Wolf, 1998; Robinson and Kolb, 1999; Ungless et al., 2001; Liu et al., 2005), may contribute considerably to the highly addictive nature of psychostimulants.
Footnotes
This work was supported by National Institutes of Health Grants NS-38879 and DA-10900 (R.M.W.) and by National Institute of Mental Health Grants MH-067044 (G.J.A.), MH39327, and DA10044 (P.G.). D.G. was a Pfizer fellow of the Life Sciences Research Foundation and a European Molecular Biology Organization fellow.
References
- Bauman PA, Maitre L (1976). Is drug inhibition of dopamine uptake a misinterpretation of in vitro experiments? Nature 264:789–790. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP (1999). Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M (1985). Cocaine: excitatory effects on sensorimotor reactivity measured with acoustic startle. Psychopharm (Berl) 86:31–36. [DOI] [PubMed] [Google Scholar]
- Doteuchi M, Wang C, Costa E (1974). Compartmentation of dopamine in rat striatum. Mol Pharmacol 10:225–234. [PubMed] [Google Scholar]
- Duncan RR, Greaves J, Wiegand UK, Matskevich I, Bodammer G, Apps DK, Shipston MJ, Chow RH (2003). Functional and spatial segregation of secretory vesicle pools according to vesicle age. Nature 422:176–180. [DOI] [PubMed] [Google Scholar]
- Ewing AG, Bigelow JC, Wightman RM (1983). Direct in vivo monitoring of dopamine released from two striatal compartments. Science 221:169–170. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG (2001). Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci 21:2851–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Ciolkowski EL, Pastore P, Wightman RM (1994). Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci 14:6084–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler D, Xu Y, Kao HT, Lin D, Lim S, Feng J, Greengard P, Augustine GJ (2004a). Molecular determinants of synapsin targeting to presynaptic terminals. J Neurosci 24:3711–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler D, Takagishi Y, Feng J, Ren Y, Rodriguiz R, Wetsel W, Greengard P, Augustine GJ (2004b). Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J Neurosci 24:11368–11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P, Valtorta F, Czernik AJ, Benfenati F (1993). Synaptic vesicle phosphoproteins and regulation of synaptic function. Science 259:780–785. [DOI] [PubMed] [Google Scholar]
- Hafizi S, Palij P, Stamford JA (1992). Activity of two primary human metabolites of nomifensine on stimulated efflux and uptake of dopamine in the striatum: in vitro voltammetric data in slices of rat brain. Neuropharmacol 31:817–824. [DOI] [PubMed] [Google Scholar]
- Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, Greengard P (1999). Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci 354:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javoy F, Glowinski J (1971). Dynamic characteristic of the “functional compartment” of dopamine in dopaminergic terminals of the rat striatum. J Neurochem 18:1305–1311. [DOI] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Wightman RM (1995). Different effects of cocaine and nomifensine on dopamine uptake in the caudate-putamen and nucleus accumbens. J Pharmacol Exp Ther 274:396–403. [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG (1998). Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA 95:4029–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JD, Wang YM, Miles PR, Budygin EA, Picetti R, Gainetdinov RR, Caron MG, Wightman RM (2002). Dopamine autoreceptor regulation of release and uptake in mouse brain slices in the absence of D(3) receptors. Neuroscience 112:39–49. [DOI] [PubMed] [Google Scholar]
- Korf J, Grasdijk L, Westerink BHC (1976). Effects of electrical stimulation on the nigrostriatal pathway of the rat on dopamine metabolism. J Neurochem 26:579–584. [DOI] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y (1998). Two distinct pools of synaptic vesicles in single presynaptic boutons in a temperature-sensitive Drosophila mutant, shibire. Neuron 20:917–925. [DOI] [PubMed] [Google Scholar]
- Lee TH, Balu R, Davidson C, Ellinwood EH (2001). Differential time-course profiles of dopamine release and uptake changes induced by three dopamine uptake inhibitors. Synapse 41:301–310. [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM (2005). Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature 437:1027–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, McClure SM, Baldwin PR, Phillips PEM, Budygin EA, Stuber GD, Kilpatrick MR, Wightman RM (2004). Dynamic gain control of dopamine delivery in freely moving animals. J Neurosci 24:1754–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E (1998). Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron 20:389–399. [DOI] [PubMed] [Google Scholar]
- Phillips PEM, Stuber GD, Heien MLAV, Wightman RM, Carelli RM (2003). Subsecond dopamine release promotes cocaine seeking. Nature 422:614–618. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW (1997). Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J Neurosci 17:3254–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone VA, Shupliakov O, Brodin L, Hilfiker-Rothenfluh S, Czernik AJ, Greengard P (1995). Distinct pools of synaptic vesicles in neurotransmitter release. Nature 375:493–497. [DOI] [PubMed] [Google Scholar]
- Premkumar LS (1999). Selective potentiation of L-type calcium channel currents by cocaine in cardiac myocytes. Mol Pharmacol 56:1138–1142. [DOI] [PubMed] [Google Scholar]
- Richards DA, Guatimosim C, Rizzoli SO, Betz WJ (2003). Synaptic vesicle pools at the frog neuromuscular junction. Neuron 39:529–541. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Heien MLAV, Wightman RM (2002). Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J Neurosci 22:10477–10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B (1999). Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci 11:1598–1604. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PEM, Wightman RM, Carelli RM (2004). Dopamine operates as a subsecond modulator of food seeking. J Neurosci 24:1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF (1996). Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron 16:1197–1207. [DOI] [PubMed] [Google Scholar]
- Schultz W (1998). Predictive reward signal of dopamine neurons. J Neurophysiol 80:1–27. [DOI] [PubMed] [Google Scholar]
- Shore PA (1976). Actions of amfonelic acid and other non-amphetamine stimulants on the dopamine neuron. J Pharm Pharmacol 28:855–857. [DOI] [PubMed] [Google Scholar]
- Stamford JA, Kruk ZL, Millar J (1986). Measurement of stimulated dopamine release in the rat by in vivo voltammetry: the influence of stimulus duration on drug responses. Neurosci Lett 69:70–73. [DOI] [PubMed] [Google Scholar]
- Stamford JA, Kruk ZL, Millar J (1989). Dissociation of the actions of uptake blockers upon dopamine overflow and uptake in the rat nucleus accumbens: in vivo voltammetric data. Neuropharmacology 28:1383–1388. [DOI] [PubMed] [Google Scholar]
- Taghzouti K, Simon H, Louilot A, Herman JP, Le Moal M (1985). Behavioral study after local injection of 6-hydroxydopamine into the nucleus accumbens in the rat. Brain Res 344:9–20. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A (2001). Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411:583–587. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Troyer KP, Wightman RM (2002). Response times of carbon fiber microelectrodes to dynamic changes in catecholamine concentration. Anal Chem 74:539–546. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Zhang H, Garris PA, Phillips PE, Sulzer D, Wightman RM (2003). Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing. J Neurochem 87:1284–1295. [DOI] [PubMed] [Google Scholar]
- Wolf ME (1998). The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol 54:679–720. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Kuhar MJ, Carroll FI, Garris PA (2001). Preferential increases in nucleus accumbens dopamine after systemic cocaine administration are caused by unique characteristics of dopamine neurotransmission. J Neurosci 21:6338–6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yermolaieva O, Chen J, Couceyro PR, Hoshi T (2001). Cocaine- and amphetamine-regulated transcript peptide modulation of voltage-gated Ca2+ signaling in hippocampal neurons. J Neurosci 21:7474–7480. [DOI] [PMC free article] [PubMed] [Google Scholar]