Abstract
Cell survival is an essential function in the development and maintenance of the nervous system. We demonstrate here a previously unappreciated role for extracellular nucleotide signaling through the P2Y2 receptor in the survival of neurons: PC12 (pheochromocytoma 12) cells and dorsal root ganglion neurons are protected from serum starvation-induced apoptosis by ATP, UTP, and ATPγS, an effect mediated via P2Y2 receptors, as demonstrated by small interfering RNA and genetic knock-out models. This protection occurs independently of neurophin signaling but requires Src activation of ERK (extracellular signal-regulated kinase) and Akt. Moreover, ATPγS and NGF act synergistically to enhance neuronal survival through enhanced TrkA signaling. The results, which define a novel mechanism for inhibition of apoptosis, implicate parallel, interacting systems—extracellular nucleotides/P2Y2 receptors and neurotrophin/TrkA—to sustain neuronal survival.
Keywords: dorsal root ganglion (DRG), nucleotide, ATP, purinergic, P2Y, Akt, Src, PC12, NGF
Introduction
The regulation of cell development and organismal growth is a highly regulated process that involves maintaining a balance between proliferation and apoptosis (Duque-Parra, 2005). A large body of data document that both intrinsic and extrinsic apoptotic pathways result in distinct morphological and biochemical cellular changes (Saunders, 1966; Twomey and McCarthy, 2005). Injury, oxidative stress, and reduced extracellular levels of trophic factors are examples of inciting stimuli that can result in cells initiating apoptotic pathways (Twomey and McCarthy, 2005).
The maintenance of cell survival is a crucial component of neuronal function. Survival of neurons, in particular the inhibition of apoptosis, is dependent on the presence of trophic and nontrophic factors to maintain function (Akassoglou, 2005; Shaw, 2005). Nerve growth factor (NGF) is one of the best studied examples of an extracellular stimulant that regulates neuronal survival by antiapoptotic effects. NGF acts via its cognate receptor, TrkA, with subsequent activation of extracellular signal-regulated kinase 1/2 (ERK1/2) and Akt kinases that inhibit apoptotic signaling in neurons (Greene, 1978; Chao, 2003).
Extracellular nucleotide signaling via nucleotide (P2) receptors is a mechanism that may regulate apoptosis (Burnstock and Williams, 2000). P2 receptors, which are comprised of P2X (ionotropic) and P2Y (metabotropic, G-protein-coupled) subtypes, respond to a variety of nucleotide agonists. The role of P2 receptors, particularly P2X receptors, in apoptosis has been demonstrated in both non-neuronal and neuronal cells, including most prominently the P2X7 receptor in initiating spinal neuron apoptosis (Franke et al., 2004; Wang et al., 2004; Coutinho-Silva et al., 2005). In contrast, the role of P2Y receptors in neuronal apoptosis remains mostly unexplored.
In this study, we tested the hypothesis that extracellular nucleotides, signaling through P2Y2 receptors, modulate neuronal apoptosis. Using a series of complementary approaches, we demonstrate a role for P2Y2-mediated inhibition of neuronal apoptosis through signaling pathways that are both neurotrophin-dependent and -independent, resulting in enhanced survival in response to trophic factor withdrawal.
Materials and Methods
Reagents.
The following reagents were used: ATPγS, ATP, UTP, histamine (Sigma, St. Louis, MO), NGF (Invitrogen, Carlsbad, CA), methyl-9-(S)-12(R)-epoxy-1H-diindolo[1,2,3-fg: 3′2′1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-2,3,9,10,11,12-hexahydro-10-(R)-hydroxy-9-methyl-1-oxo-10-carboxilate (K252a), 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2), 3-[1–3-(amidinothio)-propyl-1H-indol-3-yl]-3-(1-methyl-1H-indol-3-yl)maleimide (Ro-31-8220), 2-(2-diamino-3-methoxyphenyl-4H-1-benzopyran-4-one (PD98059), 2,3-dihydro-N,N-dimethyl-2-oxo-3-[(4,5,6,7-tetrahydro-1H-indol-2-yl)methylene]-1H-indole-5-sulfonamide (SU6656), 1-[6-((17β-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl]-1H-pyrrole-2,5-dione (U73122), BAPTA-AM, Raf kinase inhibitor (Calbiochem, San Diego, CA), 1,4-diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto)butadiene (U0126), and 2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one (LY294002) (Cell Signaling, Beverly, MA).
Cell isolation and culture.
DRG neurons were dissected and trypsin dissociated from adult wild-type (wt) or P2Y2−/− mice (Cressman et al., 1999) (gift from Dr. Beverly Koller, University of North Carolina, Chapel Hill, NC), as previously described (Arthur et al., 2005). Dissociated cultures were grown on laminin/poly-d-lysine/collagen-coated plates for 96 h in Neurobasal A (Invitrogen) with B27 supplement (Invitrogen) and FUDR (fluoro-2′-deoxyuridine) (Sigma). Pheochromocytoma 12 (PC12) cells were grown as described previously (Taupenot et al., 1999).
PC12 cell transfection.
PC12 cells were transfected with predesigned small interfering RNA (siRNA) (ID numbers 50110, 143692; Ambion, Austin, TX) for P2Y2 with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Apoptosis.
PC12 cells and DRG neurons were incubated in serum-free medium for 12 h with ATPγS (10 μm), ATP (100 μm), UTP (100 μm), NGF (10 ng/ml), K252a (10 nm), PP2 (10 μm), Raf kinase inhibitor (50 nm), SU6656 (5 μm), U0126 (10 μm), LY294002 (10 μm) (unless otherwise noted) where indicated. Cells were lysed and were ELISA assayed for DNA fragmentation (Roche, Indianapolis, IN), caspase 3 (Roche), and membrane inversion (APOPercentage; Biocolor, Newtonabbey, UK). All conditions were assessed in triplicate.
Immunoblot analysis.
Protein samples, loaded at equal concentrations, were separated on 10 or 12% precast SDS polyacrylamide gels (Invitrogen) and then transferred to polyvinylidene difluoride membranes. Membranes were blocked in 20 mm PBS, 1% Tween with 1.5% nonfat dry milk, and then incubated with primary antibody at 4°C overnight. Antibodies used were as follows: P-TrkA, P-ERK1/2, ERK1/2, P-Src, Src, P-B-Raf, P-Akt, Akt (Cell Signaling), P2Y2 (Alomone Labs, Jerusalem, Israel), actin, TrkA (Santa Cruz Biotechnology, Santa Cruz, CA), glyceraldehyde-3-phosphate dehydrogenase (Novus Biologicals, Littleton, CO), B-Raf (Abcam, Cambridge, MA). Secondary antibodies conjugated to horseradish peroxidase (Cell Signaling) were visualized with ECL reagent (Amersham Biosciences, Piscataway, NJ). All immunoblots were done in triplicate.
NGF ELISA.
DRG cultures were serum-deprived and treated with either 10 μm histamine (a stimulant of NGF secretion) or 10 μm ATPγS for 24 h. NGF concentrations in conditioned medium prepared from DRG cultures were measured in triplicate by ELISA (R&D Systems, Minneapolis, MN) as previously described (Lipnik-Stangelj and Carman-Krzan, 2004).
Statistical analysis.
All experiments were conducted in triplicate. Data were analyzed by a one-way ANOVA followed by Tukey's multiple comparison test or linear regression. Significance was assigned to p < 0.05.
Results
P2Y2 activation inhibits apoptosis of PC12 cells
Serum starvation for 12 h significantly (p < 0.001) increases DNA fragmentation in PC12 cells, a result indicative of apoptosis (Fig. 1A) (Batistatou and Greene, 1993). NGF treatment prevents apoptosis produced in this manner (Fig. 1A). Because of our recent findings demonstrating interaction between expression of nucleotide/P2Y2 and NGF/TrkA signaling in enhancing neuronal differentiation and growth (Arthur et al., 2005), we tested whether nucleotide/P2Y2 receptor activation might also promote neuronal survival. In initial studies, we treated cells with ATP (100 μm), UTP (100 μm), or ATPγS (10 μm), all agonists of P2Y2 receptors (Burnstock and Williams, 2000) and found that all three agonists reduced serum starvation-induced DNA fragmentation, ATPγS more significantly (p < 0.05) than ATP or UTP (Figs. 1A, 2A), an effect likely attributable to the resistance of ATPγS to hydrolysis. We used ATPγS for subsequent experiments.
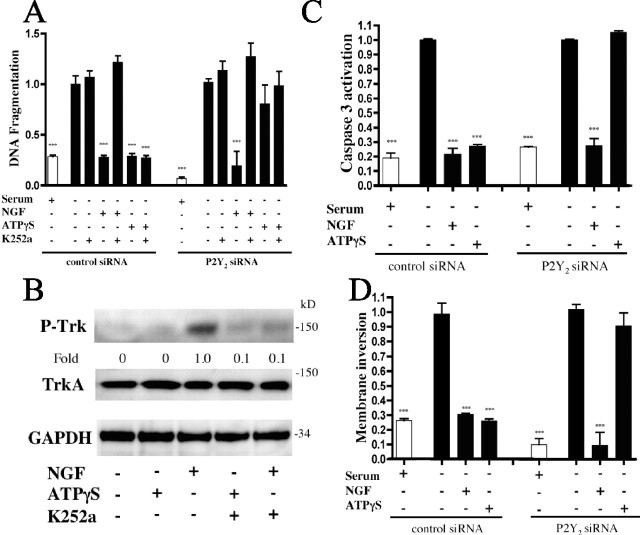
Figure 1.
ATPγS inhibits serum starvation-induced PC12 apoptosis via P2Y2 receptors independent of NGF/TrkA signaling. PC12 cells were transfected with P2Y2 siRNA and then grown for 12 h in the presence or absence of serum, ATPγS (10 μm), NGF (10 ng/ml), and/or K252a (10 nm) and analyzed for apoptosis by quantitation of DNA fragmentation (A). P2Y2 receptor expression in cells transfected with P2Y2 siRNA decreased by >70% versus cells transfected with a scrambled siRNA sequence. Treatment with the P2Y2-targeted siRNAs failed to alter serum starvation-induced apoptosis. An immunoblot of PC12 cells treated as in A and probed for TrkA activation is shown (B). Densitometry was measured as P-TrkA/TrkA and normalized to NGF treatment alone. PC12 cells transfected with P2Y2 siRNA and serum-starved for 12 h with the indicated treatments were analyzed for apoptosis by caspase 3 activation/expression (C) and an assay for plasma membrane inversion (D). ***p < 0.001 versus serum starvation alone, with values normalized to serum starvation alone. Error bars indicate SE.
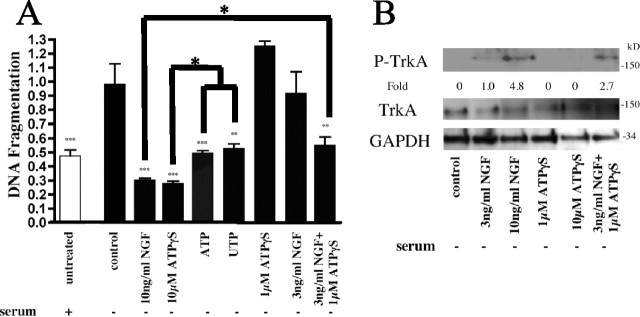
Figure 2.
ATPγS/NGF interact to enhance inhibition of apoptosis. Serum-starved PC12 cells were treated with ATP (100 μm), UTP (100 μm), NGF, and/or ATPγS at the indicated concentrations. Apoptosis was quantitated using DNA fragmentation (A). An immunoblot analysis of cells treated with NGF (10 or 3 ng/ml), ATPγS (10 or 1 μm), or 3 ng/ml NGF together with 1 μm ATPγS and probed for TrkA activation is shown (B). Densitometry was measured as P-TrkA/TrkA and normalized to 3 ng/ml NGF treatment alone. *p < 0.05, **p < 0.01, ***p < 0.001 versus serum starvation alone, with values normalized to serum starvation alone. Error bars indicate SE.
As a more direct test of the role of P2Y2 receptors, we transfected PC12 cells with each of two nonoverlapping siRNA sequences directed against P2Y2 (Arthur et al., 2005). Incubation with NGF reduced the level of DNA fragmentation in both scrambled and P2Y2 siRNA (sequence 1)-treated cells; ATPγS only significantly (p < 0.001) reduced serum starvation-induced DNA fragmentation in control (scrambled), but not P2Y2 siRNA-treated, cells (Fig. 1A). These results implicate the P2Y2 receptor as crucial for the ATPγS-promoted protection from apoptosis. Similar results were obtained using P2Y2 siRNA sequence 2 (data not shown). In addition to the effect on DNA fragmentation, ATPγS inhibited caspase 3 activation and membrane inversion, both measures of apoptosis (Fernandes-Alnemri et al., 1994; Martin et al., 1995; Zhou et al., 1997); these responses were also independent of NGF and reversed by reduction of P2Y2 receptor protein by the P2Y2 siRNA (Fig. 1C,D).
Inhibition of apoptosis of PC12 cells by P2Y2 activation is independent of NGF/TrkA
K252a, an inhibitor of Trk phosphorylation (P-TrkA) (MacInnis et al., 2003), reversed the NGF-mediated, but not the ATPγS-mediated, inhibition of DNA fragmentation (Fig. 1A). Immunoblotting of serum-starved PC12 cells showed activation of TrkA (P-TrkA) with NGF treatment (blocked by 10 nm K252a), but not with ATPγS (Fig. 1B). P2Y2 siRNA-treated PC12 cells showed similar levels of P-TrkA with NGF and lack of activation with ATPγS (data not shown).
P2Y2 and TrkA synergistically inhibit apoptosis
Based on findings showing that ATPγS enhances P-TrkA formation in the presence of NGF (Arthur et al., 2005), we tested the potential interaction of nucleotide and neurotrophin signaling in the inhibition of apoptosis. Lower concentrations of NGF (3 ng/ml) or ATPγS (1 μm) individually did not prevent DNA fragmentation in serum-starved PC12 cells (Fig. 2A), but the combination of these suboptimal concentrations resulted in a significant (p < 0.01) inhibition of DNA fragmentation (Fig. 2A). Immunoblot analysis of serum-starved PC12 cells treated with the combination of lower concentrations of NGF and ATPγS revealed that these cells expressed more P-TrkA than cells treated with a low concentration of NGF alone (Fig. 2B), implying that enhancement in TrkA signaling by ATPγS contributes to the promotion of survival by the synergistic combination of ATPγS and NGF.
P2Y2 activation inhibits apoptosis via both ERK and Akt
Agonists of P2Y2 receptors in PC12 cells are able to activate ERK1/2 (P-ERK1/2) (D'Ambrosi et al., 2001; Arthur et al., 2005), a kinase that inhibits apoptosis (Xia et al., 1995). We thus tested the role of ERK1/2 in inhibition of apoptosis by activation of P2Y2 receptors and found that PC12 cells serum-starved in the presence of ATPγS and the ERK1/2 inhibitor, U0126 (10 μm) (Xie et al., 2000), had similar levels of DNA fragmentation as did cells incubated with ATPγS alone (Fig. 3A). Serum-starved PC12 cells incubated with LY294002 (10 μm), an inhibitor of Akt activation (P-Akt formation), another kinase that can inhibit apoptosis (Crowder and Freeman, 1998), also showed no difference in the ability of ATPγS to reduce DNA fragmentation (Fig. 3A). However, combined inhibition of ERK1/2 and Akt by U0126 and LY294002 blocked ATPγS-mediated inhibition of serum starvation-induced DNA fragmentation (Fig. 3A).
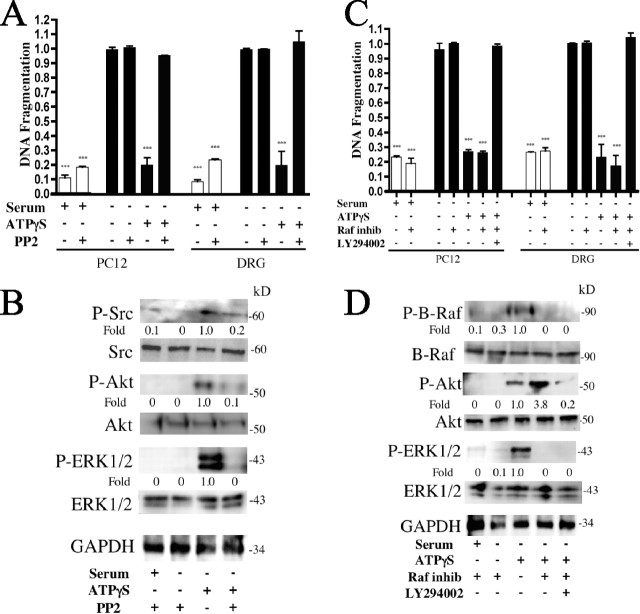
Figure 3.
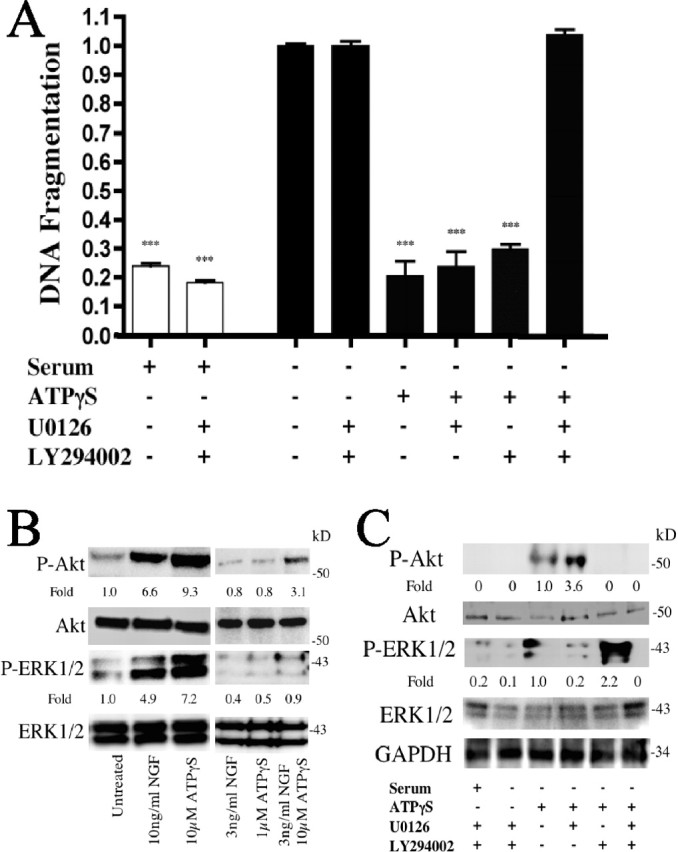
ATPγS inhibits apoptosis via activation of ERK1/2 and Akt. Serum-starved PC12 cells were treated with U0126 (10 μm) or LY294002 (10 μm), and the indicated treatments were analyzed for apoptosis by quantitation of DNA fragmentation (A). An immunoblot of serum-starved PC12 cells left untreated, treated with 10 ng/ml NGF, 10 μm ATPγS, or as indicated and probed for activated Akt and ERK1/2 is shown (B). Densitometry was measured as P-Akt/Akt and P-ERK1/2/ERK1/2 and normalized to untreated. Immunoblot of serum-starved PC12 cells treated as in A and probed for Akt and ERK1/2 activation is shown (C). Densitometry was measured as P-Akt/Akt and P-ERK1/2/ERK1/2 and normalized to ATPγS-treated alone. ***p < 0.001 versus serum starvation alone, with values normalized to serum starvation alone. Error bars indicate SE.
Treatment of serum-starved PC12 cells with either ATPγS or NGF activated both Akt and ERK1/2 (Fig. 3B) and cells treated with low concentrations of NGF and ATPγS had a synergistic enhancement in P-Akt and P-ERK1/2 formation, consistent with the impact of the two classes of agonists on DNA fragmentation and P-TrkA expression (compare Figs. 2, 3B). ATPγS treatment of serum-starved PC12 cells increased expression of both P-Akt and P-ERK1/2, responses that could be blocked by inhibition of the kinases by LY294002 or U0126, respectively (Fig. 3C). Inhibition of the two kinases blocked formation of both phosphorylated species, implying that both of these signaling molecules contribute to the prevention of apoptosis (Fig. 3A) in response to activation of P2Y2 receptors.
ERK and Akt activation by P2Y2 requires Src
To further elucidate components involved in the inhibition of apoptosis via P2Y2 activation, we assayed several additional signaling molecules that might contribute to the downstream responses. Inhibitors of phospholipase C (U73122) (Nussenzveig et al., 1993), protein kinase C (PKC) (Ro-31-8220; 1 μm) (Bacon and Camp, 1990), and intracellular calcium (BAPTA-AM; 50 μm) had no effect on the ATPγS-mediated inhibition of serum starvation-induced apoptosis (supplemental Fig. 1, available at www.jneurosci.org as supplemental material) (data not shown). In contrast, we found that Src appears to play an important regulatory role in the signal transduction pathway that mediates this inhibition. PP2, an inhibitor of Src phosphorylation (Nagao et al., 1998), reversed the ATPγS/P2Y2-mediated inhibition of DNA fragmentation in both PC12 cells and dorsal root ganglion (DRG) neurons, a peripheral nerve cell known to express P2Y2 receptors (Sanada et al., 2002; Arthur et al., 2005) (Fig. 4A). Another Src inhibitor, SU6656 (Blake et al., 2000), produced similar results (data not shown). Immunoblot analysis of serum-starved PC12 cells treated with ATPγS showed activated Src (P-Src) expression, whereas inhibition of ATPγS-mediated P-Src formation by PP2 (10 μm) abolished P-Akt and P-ERK1/2 formation (Fig. 4B). Together, these results indicate that Src activation is a necessary step in the ATPγS/P2Y2-receptor-mediated inhibition of neuronal apoptosis.
Figure 4.
ATPγS inhibits apoptosis via Src activation of Akt and B-Raf-mediated ERK1/2 activation. Serum-starved PC12 cells and DRG neurons were treated with 10 μm PP2 and 10 μm ATPγS. Cells were analyzed for apoptosis by quantitation of DNA fragmentation (A). An immunoblot of PC12 cells treated as in A and probed for Src, Akt, and ERK activation is shown (B). Densitometry was measured as P-Src/Src, P-Akt/Akt, and P-ERK1/2/ERK1/2 and normalized to ATPγS-treated alone. Serum-starved PC12 cells and DRG neurons were treated with a Raf kinase inhibitor (50 nm) and/or an Akt inhibitor (LY294002; 10 μm). Cells were analyzed for apoptosis by quantitation of DNA fragmentation (C). An immunoblot of PC12 cells treated as in C and probed for B-Raf, Akt, and ERK activation is shown (D). Densitometry was measured as P-B-Raf/Raf, P-Akt/Akt, and P-ERK1/2/ERK1/2 and normalized to ATPγS-treated alone. ***p < 0.001 versus serum starvation alone, with values normalized to serum starvation alone. Error bars indicate SE.
ERK activation by P2Y2 requires B-Raf
Based on previous data showing that activation of ERK1/2 by TrkA occurs via B-Raf (Chao, 2003), we tested the potential role of B-Raf in the regulation of ERK1/2 activation in the ATPγS/P2Y2 receptor-mediated inhibition of neuronal apoptosis. Inhibition of Raf kinase (Calbiochem; 50 nm; catalog #553008) in ATPγS-treated serum-starved PC12 cells and DRG neurons did not alter the reduction in apoptotic DNA fragmentation (Fig. 4C) but decreased P-ERK1/2 formation by ATPγS in serum-starved PC12 cells (Fig. 4D). However, simultaneous inhibition of Raf and Akt activation (by LY294002) blocked formation of P-ERK1/2 and P-Akt (Fig. 4D) and inhibited DNA fragmentation ascribed to ATPγS activation of P2Y2 receptors (Fig. 4C). These results place Raf activation upstream of ERK1/2 activation, but not Src or Akt activation.
P2Y2 activation in DRG neurons inhibits apoptosis independent of TrkA
Because no specific antagonists exist for the P2Y2 receptor (Burnstock and Williams, 2000), we used two alternative approaches, siRNA (Fig. 1) and genetic knock-outs (Fig. 5) to evaluate the role of these receptors in the inhibition of apoptosis by extracellular nucleotides. Using P2Y2−/− mice, we assessed adult DRG neurons, which do not require NGF for survival (Lindsay, 1988). Serum starvation of DRG neurons for 12 h in the absence of presence of NGF or ATPγS revealed that ATPγS inhibited DNA fragmentation and caspase 3 activation to levels similar to those produced by treatment with NGF (Fig. 5A,B). In contrast, DRG neurons derived from P2Y2−/− mice responded to NGF but did not demonstrate inhibition by ATPγS of serum starvation-induced apoptotic DNA fragmentation or caspase 3 activation (Fig. 5A,B).
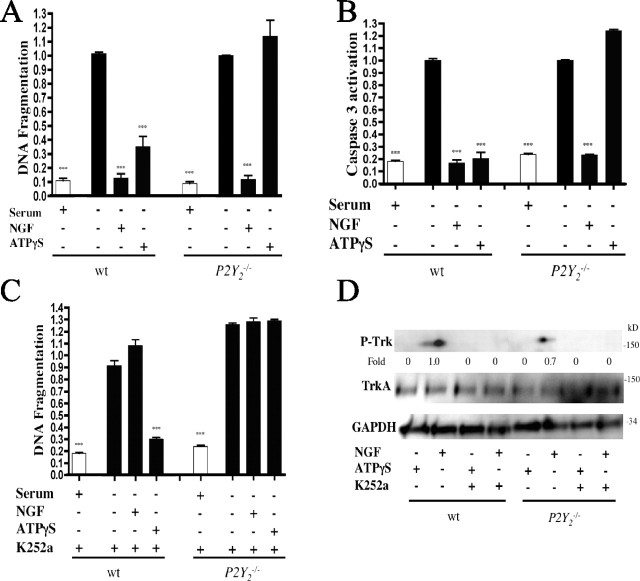
Figure 5.
ATPγS inhibits serum starvation-induced DRG apoptosis via P2Y2 independent of NGF/TrkA signaling. DRG neurons from wt and P2Y2−/− mice were serum-deprived for 12 h alone, with 10 ng/ml NGF, or with 10 μm ATPγS. Apoptosis was quantitated by DNA fragmentation (A) or caspase 3 expression (B). DRG neurons from wt and P2Y2−/− mice were serum-starved for 12 h in the presence of the TrkA inhibitor K252a (10 nm) and the indicated treatments (compare with A). Cells were lysed and analyzed for apoptosis by DNA fragmentation (C). An immunoblot analysis of DRG neurons treated as in C and probed for TrkA activation is shown (D). Densitometry was measured as P-TrkA/TrkA and normalized to NGF treatment alone. ***p < 0.001 versus serum starvation alone, values normalized to serum starvation alone. Error bars indicate SE.
Because a synergistic inhibition of apoptosis occurs with ATPγS and NGF (Fig. 2A), we assessed the concentration of NGF in DRG cultures (supplemental Fig. 2, available at www.jneurosci.org as supplemental material) and found that the concentration of NGF was <80 pg/ml after 72 h growth in serum-containing medium. Serum starvation, alone or together with ATPγS, did not stimulate NGF secretion, whereas histamine, a known stimulator of NGF secretion (Lipnik-Stangelj and Carman-Krzan, 2004), significantly (p < 0.01) increased NGF concentrations in DRG cultures (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). These NGF concentrations are lower than those needed for synergistic inhibition of apoptosis by ATPγS and NGF in PC12 cell cultures [compare Fig. 2A, supplemental Fig. 2 (available at www.jneurosci.org as supplemental material)].
Inhibition of TrkA activation by K252a reversed NGF-promoted inhibition of DNA fragmentation in response to serum starvation in both wt and P2Y2−/− DRG neurons (compare Fig. 5A,C) and blocked P-TrkA formation in both wt and P2Y2−/− DRG neurons (Fig. 5D). Immunoblotting demonstrated that ATPγS did not activate TrkA in either wt or P2Y2−/− DRG neurons (Fig. 5D), confirming results obtained with PC12 cells (compare Figs. 5D; 1B,D) and providing additional evidence for the role of P2Y2 receptors in mediating extracellular nucleotide inhibition of apoptosis in a TrkA-independent manner.
Discussion
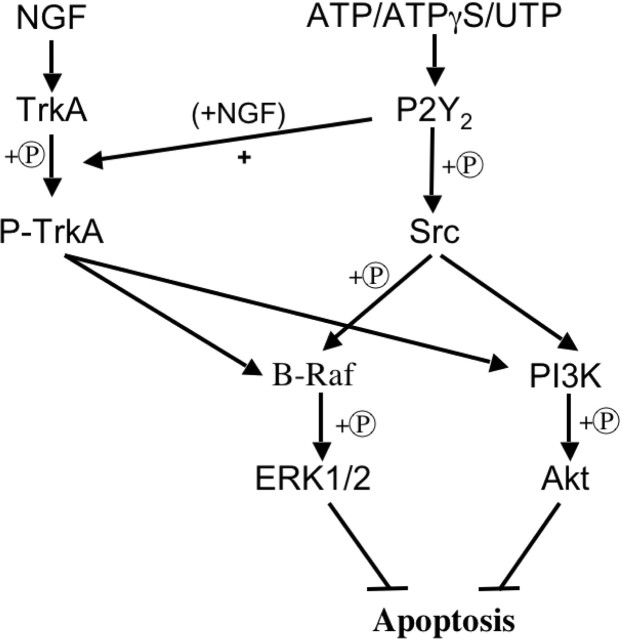
The ability of ATPγS, ATP, or UTP to inhibit neuronal apoptosis through the P2Y2 receptor, as demonstrated by both siRNA and genetic knock-out mice, represents a previously unidentified receptor target for the modulation of programmed cell death in the nervous system. Our data also reveal key elements in the signal transduction pathway that mediate this antiapoptotic effect (Fig. 6): P2Y2 receptor activation leads to Src activation/phosphorylation, which, in turn, activates B-Raf and PI3 kinase. These events lead to activation of ERK1/2 and Akt, respectively. Activation of ERK1/2 and Akt inhibits apoptosis by suppression of molecules such as c-Jun N-terminal kinase (JNK), p38, and various caspases (Berra et al., 1998; Shimoke et al., 1999; Horn et al., 2005). In addition to its direct activation of antiapoptotic events, agonist stimulation of P2Y2 receptors can indirectly inhibit apoptosis by potentiating NGF-promoted activation of TrkA, leading to enhanced ERK1/2 and Akt activation.
Figure 6.
Model of P2Y2-mediated inhibition of neuronal apoptosis. Agonists (e.g., ATP, ATPγS, or UTP) activate P2Y2 receptors leading to Src activation/phosphorylation (+P). Src activates/phosphorylates B-Raf and PI3K (phosphatidylinositol 3-kinase) leading to ERK1/2 and Akt activation/phosphorylation, respectively. Activation of ERK1/2 and Akt inhibits apoptosis. Activation of P2Y2 receptors in the presence of NGF increases TrkA activation/phosphorylation, thereby increasing the activation of ERK1/2 and Akt, resulting in inhibition of apoptosis via a NGF-dependent pathway.
P2Y, unlike P2X, receptors respond to UTP; P2Y2 are the only human P2Y receptors that respond to ATP and UTP with similar affinity (Burnstock and Williams, 2000). P2Y2 receptors couple to Gq/11-proteins, which activate phospholipase C, leading to formation of inositol-1,4,5-trisphosphate, which increases levels of intracellular Ca2+ and diacylglycerol, which activates protein kinase C (Gonzalez et al., 2005). Activation of ERK1/2 by P2Y2 receptors has been shown to occur through elevated intracellular Ca2+ and PKC activation (Soltoff et al., 1998), whereas activation of Akt by P2Y2 receptors has been demonstrated in renal mesangial cells (Huwiler et al., 2002), but not previously in neuronal cells.
The studies here thus present a novel pathway for P2Y2 signaling and regulation of neuronal apoptosis by both neurotrophin-dependent and neurotrophin-independent mechanisms. Inhibition of “classical” components of P2Y2 G-protein signal transduction (i.e., Ca2+ and PKC) did not affect the inhibition of apoptosis, as measured by DNA fragmentation, caspase 3 activation, and membrane inversion (supplemental Fig. 1, available at www.jneurosci.org as supplemental material) (data not shown). G-protein-coupled receptors, such as P2Y2 receptors, are capable of transducing signals independent of G-proteins, particularly with respect to modulation of signals involved in neurotransmission (Heuss and Gerber, 2000; Pierce et al., 2002). Signal transduction by GPCRs independent of G-proteins can occur through molecules such as β-arrestins, which scaffold and regulate kinases such as JNK, p38, Src, and ERK1/2 (Luttrell and Luttrell, 2004; Lefkowitz and Shenoy, 2005; Shenoy et al., 2005). Such nontraditional (i.e., G-protein-independent) signal transduction pathways may be involved in the ability of P2Y2 receptors to activate Src, ERK, and Akt, leading to the inhibition of apoptosis.
A possible mechanism for the linkage to Src may involve a unique property of the P2Y2 receptor itself: association with Src via a SH3 (Src homology 3) binding domain located in the C-terminal region of the P2Y2 receptor; mutations to this domain alter signaling and receptor association with tyrosine kinases (Zhang et al., 2001; Liu et al., 2004; Gonzalez et al., 2005; Weisman et al., 2005). Because Src is able to activate ERK1/2 [via Raf (Troppmair et al., 1994) and Akt in PC12 cells and DRG neurons (Figs. 3, 4) as well as in other cell types (Zachary, 2003; Mehdi et al., 2005)], activation of Src by P2Y2 receptors may provide a mechanism that contributes to the maintenance of neuronal survival.
Neurotrophins such as NGF are well established inhibitors of neuronal apoptosis, but the current data imply that P2Y2 receptors, stimulated by ATP and UTP, are another physiologically relevant means by which neuronal survival is regulated. In addition, adenosine, a metabolic product of ATP hydrolysis, is able to inhibit neuronal apoptosis in a TrkA-dependent manner by activation of the A2A P1 receptors (Lee and Chao, 2001; Wakade et al., 2001; Lee et al., 2002). Our results define a TrkA-independent mechanism for inhibition of apoptosis that does not require ATP hydrolysis. P2Y receptor transcripts are widely expressed in central and peripheral nervous tissue samples, and P2Y2 receptors have been shown to play an important role in neuronal differentiation (Moore et al., 2001; Arthur et al., 2005; Franke and Illes, 2005). We propose that release of nucleotides from glia, neurons, or perhaps other cell types (e.g., vascular elements) (Lazarowski and Boucher, 2001; Hansson and Ronnback, 2003; Newman, 2003; Brockhaus et al., 2004; Wang et al., 2005) may serve as autocrine–paracrine sources of extracellular nucleotides that promote survival, either acting alone or through potentiation of neurotrophin signaling. As such, ATP (and perhaps UTP) may serve as key extracellular regulators of neuronal development that protect developing neurons from proapoptotic stimuli. Moreover, because neurotrophin signaling and innervation of peripheral target tissues declines with age (Gavazzi and Cowen, 1996; Santer et al., 2002), based on the current findings, drugs that activate P2Y2 receptors would appear to have potential to prevent this age-related decline, as well as apoptosis triggered by disease or injury.
The current findings define a previously unappreciated aspect of function of nucleotides/P2Y2 receptors in the nervous system in addition to enhancement of neuronal differentiation by these receptors (Arthur et al., 2005). Together with the latter results and recent evidence obtained with P2Y2−/− mice indicating that P2Y2 receptors are critically involved in allodynia and processing of pain stimuli (Davis et al., 2005), the data described herein identify extracellular nucleotides and their activation of P2Y2 receptors as a physiologically important system involved in the regulation of development, survival, and function of neurons.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke Grants R01-NS051470 and NS052189 (K.A.); a University of California, San Diego, Academic Senate grant; National Institute on Drug Abuse Training Grant DA073103; National Institute of General Medical Sciences (NIGMS) Cellular, Molecular, and Genetics Training Grant GM007240; National Heart, Lung, and Blood Institute Grant P01-HL58120; and NIGMS Grant R01-GM66232 (P.A.I.).
References
- Akassoglou K (2005). Nerve growth factor-independent neuronal survival: a role for NO donors. Mol Pharmacol 68:952–955. [DOI] [PubMed] [Google Scholar]
- Arthur DB, Akassoglou K, Insel PA (2005). P2Y2 receptor activates nerve growth factor/TrkA receptor signaling to enhance neuronal differentiation. Proc Natl Acad Sci USA 102:19138–19143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon KB, Camp RD (1990). Interleukin (IL)-8-induced in vitro human lymphocyte migration is inhibited by cholera and pertussis toxins and inhibitors of protein kinase C. Biochem Biophys Res Commun 169:1099–1104. [DOI] [PubMed] [Google Scholar]
- Batistatou A, Greene LA (1993). Internucleosomal DNA cleavage and neuronal cell survival/death. J Cell Biol 122:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berra E, Diaz-Meco MT, Moscat J (1998). The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J Biol Chem 273:10792–10797. [DOI] [PubMed] [Google Scholar]
- Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, Courtneidge SA (2000). SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol 20:9018–9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus J, Dressel D, Herold S, Deitmer JW (2004). Purinergic modulation of synaptic input to Purkinje neurons in rat cerebellar brain slices. Eur J Neurosci 19:2221–2230. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Williams M (2000). P2 purinergic receptors: modulation of cell function and therapeutic potential. J Pharmacol Exp Ther 295:862–869. [PubMed] [Google Scholar]
- Chao MV (2003). Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4:299–309. [DOI] [PubMed] [Google Scholar]
- Coutinho-Silva R, Stahl L, Cheung KK, de Campos NE, de Oliveira Souza C, Ojcius DM, Burnstock G (2005). P2X and P2Y purinergic receptors on human intestinal epithelial carcinoma cells: effects of extracellular nucleotides on apoptosis and cell proliferation. Am J Physiol 288:G1024–G1035. [DOI] [PubMed] [Google Scholar]
- Cressman VL, Lazarowski E, Homolya L, Boucher RC, Koller BH, Grubb BR (1999). Effect of loss of P2Y2 receptor gene expression on nucleotide regulation of murine epithelial Cl− transport. J Biol Chem 274:26461–26468. [DOI] [PubMed] [Google Scholar]
- Crowder RJ, Freeman RS (1998). Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J Neurosci 18:2933–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosi N, Murra B, Cavaliere F, Amadio S, Bernardi G, Burnstock G, Volonte C (2001). Interaction between ATP and nerve growth factor signalling in the survival and neuritic outgrowth from PC12 cells. Neuroscience 108:527–534. [DOI] [PubMed] [Google Scholar]
- Davis BM, Malin SA, Koerber HR, Albers KM, Koller BH, Molliver DC (2005). Mice lacking the P2Y2 receptor have deficits in noxious thermal sensation and neuronal responses to capsacin. Soc Neurosci Abstr 31:393.16. [Google Scholar]
- Duque-Parra JE (2005). Note on the origin and history of the term “apoptosis.”. Anat Rec B New Anat 283:2–4. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Litwack G, Alnemri ES (1994). CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem 269:30761–30764. [PubMed] [Google Scholar]
- Franke H, Illes P (2005). Involvement of P2 receptors in the growth and survival of neurons in the CNS. Pharmacol Ther 109:297–324. [DOI] [PubMed] [Google Scholar]
- Franke H, Gunther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D, Illes P (2004). P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol 63:686–699. [DOI] [PubMed] [Google Scholar]
- Gavazzi I, Cowen T (1996). Can the neurotrophic hypothesis explain degeneration and loss of plasticity in mature and ageing autonomic nerves? J Auton Nerv Syst 58:1–10. [DOI] [PubMed] [Google Scholar]
- Gonzalez FA, Weisman GA, Erb L, Seye CI, Sun GY, Velazquez B, Hernandez-Perez M, Chorna NE (2005). Mechanisms for inhibition of P2 receptors signaling in neural cells. Mol Neurobiol 31:65–79. [DOI] [PubMed] [Google Scholar]
- Greene LA (1978). Nerve growth factor prevents the death and stimulates the neuronal differentiation of clonal PC12 pheochromocytoma cells in serum-free medium. J Cell Biol 78:747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson E, Ronnback L (2003). Glial neuronal signaling in the central nervous system. FASEB J 17:341–348. [DOI] [PubMed] [Google Scholar]
- Heuss C, Gerber U (2000). G-protein-independent signaling by G-protein-coupled receptors. Trends Neurosci 23:469–475. [DOI] [PubMed] [Google Scholar]
- Horn AP, Gerhardt D, Geyer AB, Valentim L, Cimarosti H, Tavares A, Horn F, Lenz G, Salbego C (2005). Cellular death in hippocampus in response to PI3K pathway inhibition and oxygen and glucose deprivation. Neurochem Res 30:355–361. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Rolz W, Dorsch S, Ren S, Pfeilschifter J (2002). Extracellular ATP and UTP activate the protein kinase B/Akt cascade via the P2Y2 purinoceptor in renal mesangial cells. Br J Pharmacol 136:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC (2001). UTP as an extracellular signaling molecule. News Physiol Sci 16:1–5. [DOI] [PubMed] [Google Scholar]
- Lee FS, Chao MV (2001). Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci USA 98:3555–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Rajagopal R, Chao MV (2002). Distinctive features of Trk neurotrophin receptor transactivation by G protein-coupled receptors. Cytokine Growth Factor Rev 13:11–17. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK (2005). Transduction of receptor signals by beta-arrestins. Science 308:512–517. [DOI] [PubMed] [Google Scholar]
- Lindsay RM (1988). Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J Neurosci 8:2394–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipnik-Stangelj M, Carman-Krzan M (2004). Activation of histamine H1-receptor enhances neurotrophic factor secretion from cultured astrocytes. Inflamm Res 53:245–252. [DOI] [PubMed] [Google Scholar]
- Liu J, Liao Z, Camden J, Griffin KD, Garrad RC, Santiago-Perez LI, Gonzalez FA, Seye CI, Weisman GA, Erb L (2004). Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J Biol Chem 279:8212–8218. [DOI] [PubMed] [Google Scholar]
- Luttrell DK, Luttrell LM (2004). Not so strange bedfellows: G-protein-coupled receptors and Src family kinases. Oncogene 23:7969–7978. [DOI] [PubMed] [Google Scholar]
- MacInnis BL, Senger DL, Campenot RB (2003). Spatial requirements for TrkA kinase activity in the support of neuronal survival and axon growth in rat sympathetic neurons. Neuropharmacology 45:995–1010. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR (1995). Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med 182:1545–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdi MZ, Pandey NR, Pandey SK, Srivastava AK (2005). H2O2-induced phosphorylation of ERK1/2 and PKB requires tyrosine kinase activity of insulin receptor and c-Src. Antioxid Redox Signal 7:1014–1020. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Chambers JK, Wahlin JP, Tan KB, Moore GB, Jenkins O, Emson PC, Murdock PR (2001). Expression pattern of human P2Y receptor subtypes: a quantitative reverse transcription-polymerase chain reaction study. Biochim Biophys Acta 1521:107–119. [DOI] [PubMed] [Google Scholar]
- Nagao M, Yamauchi J, Kaziro Y, Itoh H (1998). Involvement of protein kinase C and Src family tyrosine kinase in Galphaq/11-induced activation of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase. J Biol Chem 273:22892–22898. [DOI] [PubMed] [Google Scholar]
- Newman EA (2003). Glial cell inhibition of neurons by release of ATP. J Neurosci 23:1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzveig DR, Heinflink M, Gershengorn MC (1993). Decreased levels of internalized thyrotropin-releasing hormone receptors after uncoupling from guanine nucleotide-binding protein and phospholipase-C. Mol Endocrinol 7:1105–1111. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ (2002). Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3:639–650. [DOI] [PubMed] [Google Scholar]
- Sanada M, Yasuda H, Omatsu-Kanbe M, Sango K, Isono T, Matsuura H, Kikkawa R (2002). Increase in intracellular Ca2+ and calcitonin gene-related peptide release through metabotropic P2Y receptors in rat dorsal root ganglion neurons. Neuroscience 111:413–422. [DOI] [PubMed] [Google Scholar]
- Santer RM, Dering MA, Ranson RN, Waboso HN, Watson AH (2002). Differential susceptibility to ageing of rat preganglionic neurones projecting to the major pelvic ganglion and of their afferent inputs. Auton Neurosci 96:73–81. [DOI] [PubMed] [Google Scholar]
- Saunders JW Jr (1966). Death in embryonic systems. Science 154:604–612. [DOI] [PubMed] [Google Scholar]
- Shaw PJ (2005). Molecular and cellular pathways of neurodegeneration in motor neurone disease. J Neurol Neurosurg Psychiatry 76:1046–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ (2005). Beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta 2 adrenergic receptor. J Biol Chem 281:1261–1273. [DOI] [PubMed] [Google Scholar]
- Shimoke K, Yamagishi S, Yamada M, Ikeuchi T, Hatanaka H (1999). Inhibition of phosphatidylinositol 3-kinase activity elevates c-Jun N-terminal kinase activity in apoptosis of cultured cerebellar granule neurons. Brain Res Dev Brain Res 112:245–253. [DOI] [PubMed] [Google Scholar]
- Soltoff SP, Avraham H, Avraham S, Cantley LC (1998). Activation of P2Y2 receptors by UTP and ATP stimulates mitogen-activated kinase activity through a pathway that involves related adhesion focal tyrosine kinase and protein kinase C. J Biol Chem 273:2653–2660. [DOI] [PubMed] [Google Scholar]
- Taupenot L, Mahata M, Mahata SK, O'Connor DT (1999). Time-dependent effects of the neuropeptide PACAP on catecholamine secretion: stimulation and desensitization. Hypertension 34:1152–1162. [DOI] [PubMed] [Google Scholar]
- Troppmair J, Bruder JT, Munoz H, Lloyd PA, Kyriakis J, Banerjee P, Avruch J, Rapp UR (1994). Mitogen-activated protein kinase/extracellular signal-regulated protein kinase activation by oncogenes, serum, and 12-O-tetradecanoylphorbol-13-acetate requires Raf and is necessary for transformation. J Biol Chem 269:7030–7035. [PubMed] [Google Scholar]
- Twomey C, McCarthy JV (2005). Pathways of apoptosis and importance in development. J Cell Mol Med 9:345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakade AR, Przywara DA, Wakade TD (2001). Intracellular, nonreceptor-mediated signaling by adenosine: induction and prevention of neuronal apoptosis. Mol Neurobiol 23:137–153. [DOI] [PubMed] [Google Scholar]
- Wang L, Olivecrona G, Gotberg M, Olsson ML, Winzell MS, Erlinge D (2005). ADP acting on P2Y13 receptors is a negative feedback pathway for ATP release from human red blood cells. Circ Res 96:189–196. [DOI] [PubMed] [Google Scholar]
- Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, Li P, Xu Q, Liu QS, Goldman SA, Nedergaard M (2004). P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med 10:821–827. [DOI] [PubMed] [Google Scholar]
- Weisman GA, Wang M, Kong Q, Chorna NE, Neary JT, Sun GY, Gonzalez FA, Seye CI, Erb L (2005). Molecular determinants of P2Y2 nucleotide receptor function: implications for proliferative and inflammatory pathways in astrocytes. Mol Neurobiol 31:169–183. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME (1995). Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326–1331. [DOI] [PubMed] [Google Scholar]
- Xie Y, Tisi MA, Yeo TT, Longo FM (2000). Nerve growth factor (NGF) loop 4 dimeric mimetics activate ERK and AKT and promote NGF-like neurotrophic effects. J Biol Chem 275:29868–29874. [DOI] [PubMed] [Google Scholar]
- Zachary I (2003). VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans 31:1171–1177. [DOI] [PubMed] [Google Scholar]
- Zhang FL, Luo L, Gustafson E, Lachowicz J, Smith M, Qiao X, Liu YH, Chen G, Pramanik B, Laz TM, Palmer K, Bayne M, Monsma FJ Jr (2001). ADP is the cognate ligand for the orphan G protein-coupled receptor SP1999. J Biol Chem 276:8608–8615. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Zhao J, Stout JG, Luhm RA, Wiedmer T, Sims PJ (1997). Molecular cloning of human plasma membrane phospholipid scramblase. A protein mediating transbilayer movement of plasma membrane phospholipids. J Biol Chem 272:18240–18244. [DOI] [PubMed] [Google Scholar]