Abstract
The precise role of the anterior cingulate cortex in monitoring, evaluating, and correcting behavior remains unclear despite numerous theories and much empirical data implicating it in cognitive control. The present event-related functional magnetic resonance imaging study was able to separate monitoring from error-specific functions by allowing subjects to reject a trial so as to avoid errors. Cingulate and left dorsolateral prefrontal activity was greatest on rejection trials but comparable for correct and error trials, whereas an error-specific response was observed in bilateral insula. A dissociation was also observed between the cingulate and the nucleus accumbens with the latter more active for error than reject trials. These results reveal that the functional role of the cingulate is not particular to errors but instead is related to an evaluative function concerned with on-line behavioral adjustment in the service of avoiding losses.
Keywords: cingulate, dorsolateral, prefrontal cortex, event related, fMRI, accumbens, executive
Introduction
The ability to monitor and dynamically regulate performance is a prerequisite to fluid and responsive behavior. Multiple theories implicate the anterior cingulate cortex (ACC) in these prefrontally dependent processes but have ascribed different functions to this medial frontal region including monitoring conflict between competing responses (Carter et al., 1998; Botvinick et al., 2004), detecting errors in one's behavior (Scheffers et al., 1996; Coles et al., 2001), selecting specific responses in the service of goals (Turken and Swick, 1999), regulating arousal levels (Critchley et al., 2001; Paus, 2001), and predicting the likelihood of committing an error (Brown and Braver, 2005). As a consequence, although ACC activity is one of the most robust neural indices of cognitive control, the precise contribution of the ACC to control operations remains far from clear.
Almost all theories of the function of the ACC in cognitive control have in common the prediction that substantial ACC activity will be observed on error trials: relative to a correct trial, the error trial contains more conflict, is more arousing, clearly will engage more error-specific processes, and also, by definition, has the highest likelihood of being an error; indeed, error trials produce 1 order of magnitude more activity in the ACC than do correct trials with high error-likelihood (Brown and Braver, 2005). To discriminate between the candidate theories of the contribution of the ACC to control, it would be desirable to separate evaluative or monitoring functions from error-specific processes given that the two tend to co-occur in many experimental paradigms. Doing so could reveal whether the function of the ACC is error specific or, alternatively, is specific to the evaluative functions. To this end, subjects performed a task that allowed for within-trial corrective behavior by giving them the option to abort a trial rather than risk failing it.
Sixteen participants performed a visual search task in which they were required, within time limits, to detect the presence of a target in the midst of a varying number of distractors (Fig. 1). Subjects obtained points for accurately reporting the presence or absence of the target but lost points for inaccuracies or for failing to respond within the available time. Subjects also had the option to reject trials to avoid a point loss (rejected trials neither won nor lost points). Exercising the reject option indicates active performance monitoring insofar as the subjects determine that they will not be able to complete the trial within the available time. Conversely, an error or a failure to respond indicates poorer monitoring or a deficient initiation of error-avoidance behavior. In this regard, error trials can be isolated as instances in which within-trial evaluative and corrective functions failed and can be contrasted against rejected trials in which these functions successfully operated, thereby enabling us to disentangle evaluation (monitoring) from errors. Event-related functional magnetic resonance imaging (fMRI) allowed us to identify the brain regions activated during these different trial types.
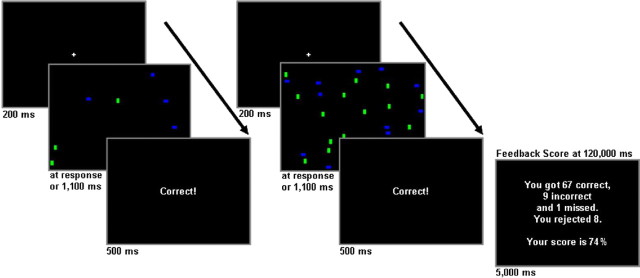
Figure 1.
The visual search monitoring task. Two trial examples, each with the target present, are shown. Each trial was presented after a fixation cross. Immediate feedback was presented after each response, and a final feedback score was shown after each 2 min run. Display durations are reported in milliseconds.
Materials and Methods
Subjects and task.
Nineteen right-handed participants, with normal or corrected to normal vision, free of neurological or psychological disorders, completed a novel visual search task after providing written informed consent. Three subjects were not included in the fMRI analyses because of insufficient numbers of rejected trials. Thus, the fMRI analyses were restricted to 16 of the original subjects (11 females; mean age, 30 years; range, 22–38 years). Stimuli duration varied within a 1.8 s window with the total duration of a trial dictated by a subject's speed of response. After a 200 ms duration fixation cross, stimuli were presented for a maximum duration of 1100 ms, terminating after the subject's response and followed by “error,” “correct,” “missed,” or “rejected” trial feedback that was presented for 500 ms. Subjects were instructed to respond while the stimulus was on screen. The right index and middle fingers were used to respond to target (a blue square) presence or absence in the pattern, respectively. The left index finger was used to reject trials. Eighty percent of the trials contained 8 distracters, and the remaining 20% contained 32 distracters. Equal numbers of vertical green and horizontal blue rectangles were used as distracters. When the target was present (50% of trials), one less distracter of either color was used. Subjects were instructed to maximize their score, shown at the end of each 2 min run, which reflected the difference between the number of correct responses and the sum of incorrect and missed trials. Rejecting did not contribute to the score but, from the subject's perspective, contributed to performance by enabling them to avoid errors on trials in which they could not determine with certainty whether the target was present or absent within the available time.
The design of this experiment allowed the large-set-size trials to be distributed throughout the stimuli stream at a distance of at least two to six small-set-size trials from each other. Because rejection rate could not be predicted in individual participants, we used a version of the task that, based on pilot data, produced approximately comparable numbers of errors and rejects in each subject. Three practice runs were performed outside the magnet. During fMRI scanning, subjects completed, on average, 950 trials divided into 12 runs of 2 min duration (on average, 79 trials each). However, the variability in the subjects' response times dictated the exact number of trials each subject completed. A 3 min rest period was allowed every 4 min (two runs), and a rest period of 30 s separated the different runs. There were, in total, six 5 min scan sessions for a total of 30 min, in addition to ∼15 min that included standard scout images, shimming to reduce the echo-planar imaging artifacts, and magnetization prepared rapid gradient echo (MPRAGE) structural acquisitions.
Imaging and data analysis.
Twenty-two contiguous 5 mm axial slices covering the entire brain were collected using a T2*-weighted echo-planar imaging sequence [echo time, 50 ms; repetition time, 2000 ms; field of view (FOV), 256 mm; 64 × 64 mm matrix size in-plane resolution]. All scanning was conducted on a 1.5 T Siemens (Erlangen, Germany) Vision scanner equipped with a 640 × 480 liquid crystal display (LCD) panel. The LCD panel was mounted on the head coil in the subjects' line of vision. Head movements were restricted using foam padding within the coil. One hundred seventy-two sagittal high-resolution T1-weighted anatomic MPRAGE images (FOV, 307 mm; thickness, 1.2 mm) were acquired at the end of the functional imaging to allow subsequent activation localization and spatial normalization.
All analyses were conducted using AFNI software [http://afni.nimh.nih.gov/afni/ (Cox, 1996)]. After image reconstruction, differences in slice acquisition times were removed using Fourier interpolation, and the time-series data were motion corrected using three-dimensional volume registration (least-squares alignment of three translational and three rotational parameters). Activation outside the brain was removed using edge-detection algorithms. The first five images of each run were excluded from analysis. Separate hemodynamic response functions at a 2 s temporal resolution were identified for rejects and errors using a multiple regression deconvolution analysis. The motion-corrected time-series files were included as additional regressors to accommodate nuisance variance.
The hemodynamic response functions were fitted to a gamma-variate function using nonlinear regression as described previously (Garavan et al., 1999). Brain activation was operationalized as the area under these event-related response functions expressed as a percentage of the area under the baseline. The baseline in this event-related design consisted of the correct trials. Activation maps were resampled to 1 mm3 (1 μl), warped into a standard Talairach space (Talairach and Tournoux, 1988), and spatially blurred (3 mm isotropic rms Gaussian kernel filter).
Group activation maps for each condition were determined with one-sample t tests against the null hypothesis of zero event-related activation changes. Significant voxels passed a voxelwise statistical threshold (t = 3.3; p < 0.005) and were required to be part of a larger 294 μl cluster of contiguous significant voxels. The cluster size was determined through Monte Carlo simulations and resulted in a 5% probability of a cluster surviving because of chance.
The nucleus accumbens (NAcc) is involved in reward-related processing and is responsive when expected reward is not obtained (Breiter et al., 2001; Knutson et al., 2001). To determine whether its functional role for error and monitoring functions is distinct from that of the ACC, a region of interest (ROI) analysis was conducted, using the left (154 μl; x = −13, y = 9, z = −8) and right (130 μl; x = 11, y = 9, z = −8) NAcc regions defined by the Talairach and Tournoux (1988) atlas of the AFNI toolbox (Cox, 1996) provided by the Research Imaging Center at the University of Texas Health Sciences Center at San Antonio (San Antonio, TX).
Results
Behavioral results (Table 1) show comparable numbers of errors and rejects. An ANOVA on response-time data using the factors of response (correct, reject, and error) and distractor set size (small, large) showed a significant response by size interaction (F(2,30) = 15.82; p < 0.001). Post hoc tests indicated that rejections of large set size were faster than rejections of small set size, errors of both small and large set size, and correct responses of large set size. No main effects were significant.
Table 1.
Behavioral performance across 16 participants on the visual search task
| Response | Correct | Rejection | Error (commission) | Miss (omission) |
|---|---|---|---|---|
| Mean percentage | 0.64 (0.02) | 0.17 (0.01) | 0.15 (0.01) | 0.04 (0.01) |
| 80% size 8 | 0.60 (0.02) | 0.05 (0.01) | 0.12 (0.01) | 0.03 (0.00) |
| 20% size 32 | 0.04 (0.01) | 0.12 (0.01) | 0.03 (0.00) | 0.01 (0.00) |
| Mean RT | 782 (11) | 749 (25) | 813 (15) | |
| RT size 8 | 780 (11) | 826 (24) | 815 (14) | |
| RT size 32 | 829 (17) | 741 (25) | 823 (19) |
Percentage scores and average response times (RTs) are reported for each response and set size. SEs are shown in parentheses.
A number of regions (Fig. 2) were significantly more active for reject trials relative to error trials. Of particular note were the right anterior cingulate [Brodmann's area (BA) 24, 396 μl; x = 5, y = 3, z = 42; t(15) = 5.09; p < 0.001] and the left middle and superior frontal gyrus (BA 9, 370 μl; x = −30, y = 39, z = 33; t(15) = 5.00; p < 0.001). A second right ACC region (BA 32, 369 μl; x = 2, y = 12, z = 38) showed significant activity for the reject trials only (t(15) = 5.31; p < 0.001) but did not significantly differ between rejects and errors. Across subjects, activity in the left dorsolateral prefrontal cortex (DLPFC) on reject trials was correlated positively with reject response times (r = 0.60; p < 0.05) and negatively with both the absolute number of rejections (r = −0.57; p < 0.05) and the number of rejections expressed as a percentage of the total number of trials (r = −0.53; p < 0.05). Activity in the left DLPFC did not correlate with the right ACC (BA 24), but it correlated with the right ACC (BA 32) activity (r = 0.52; p < 0.01). Additional regions more active for reject trials included visual, cerebellar, and subcortical areas (Table 2), but none were correlated with behavioral indices. Only two regions (Fig. 3) were more activated for the error over the reject trials, and these were the left and right insula (left: BA 13/47, 912 μl, x = −40, y = 18, z = 1, t(15) = 4.62, p < 0.001; right: BA 13, 778 μl, x = 36, y = 17, z = 3, t(15) = 3.60, p < 0.003).
Figure 2.
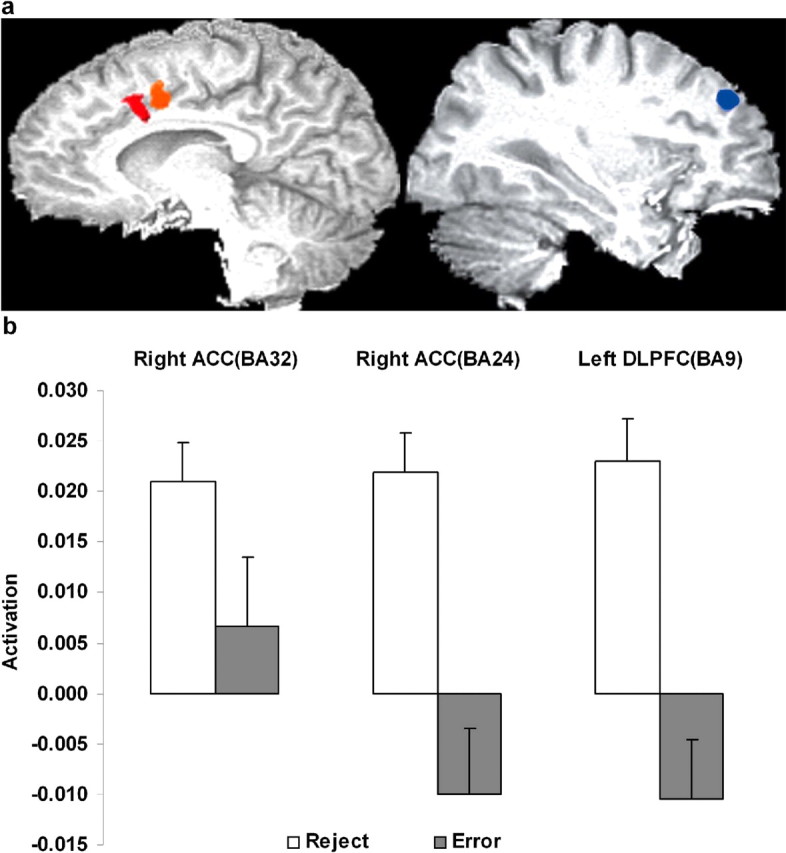
Sagittal images of the greater activations for reject relative to error trials. a, Left, Right ACC (BA 24 and BA 32) activations. Right, Left DLPFC (BA 9) activation. b, Bar charts showing the mean activation for the above areas for rejections and errors.
Table 2.
Clusters of activation and coordinates (x, y, z) in Talairach space for rejections and errors
| Region | Hemisphere | BA | Volume (μl) | x | y | z |
|---|---|---|---|---|---|---|
| Frontal lobes | ||||||
| Cingulate gyrusa | R | 24 | 396 | 5 | 3 | 42 |
| Cingulate gyrus | R | 24, 32 | 369 | 2 | 12 | 38 |
| Cingulate gyrus | R | 32 | 297 | 10 | 22 | 26 |
| Superior frontal gyrus and middle frontal gyrusa | L | 9 | 370 | −30 | 39 | 33 |
| Fronto-parietal lobes | ||||||
| Precentral gyrus and postcentral gyrusa | R | 2, 3, 4, 6 | 7723 | 38 | −17 | 54 |
| Postcentral gyrusa | R | 5 | 553 | 28 | −39 | 64 |
| Parieto-occipital lobes | ||||||
| Cuneus, middle occipital gyrus, superior occipital gyrus, and fusiform gyrusa | R, L | 19, 37 | 15,628 | −2 | −75 | 5 |
| Culmen | L | 19 | 869 | −10 | −54 | −9 |
| Middle occipital gyrus and cuneusa | L | 18 | 553 | −25 | −77 | 18 |
| Middle occipital gyrus and cuneusa | L | 18 | 342 | −31 | −94 | 2 |
| Insula and subcortical | ||||||
| Insula and inferior frontal gyrusa | R | 13, 47 | 778 | 36 | 17 | 3 |
| Insula and inferior frontal gyrusa | L | 13, 47 | 912 | −34 | 18 | 1 |
| Lentiform nucleus and putamena | R | 571 | 23 | 5 | 2 | |
| Thalamusa | R | 469 | 13 | −17 | 9 |
R, Right; L, left. The volume equals the number of voxels in each cluster.
aGreater activity for rejections than errors, except for the insula, which showed the opposite pattern.
Figure 3.
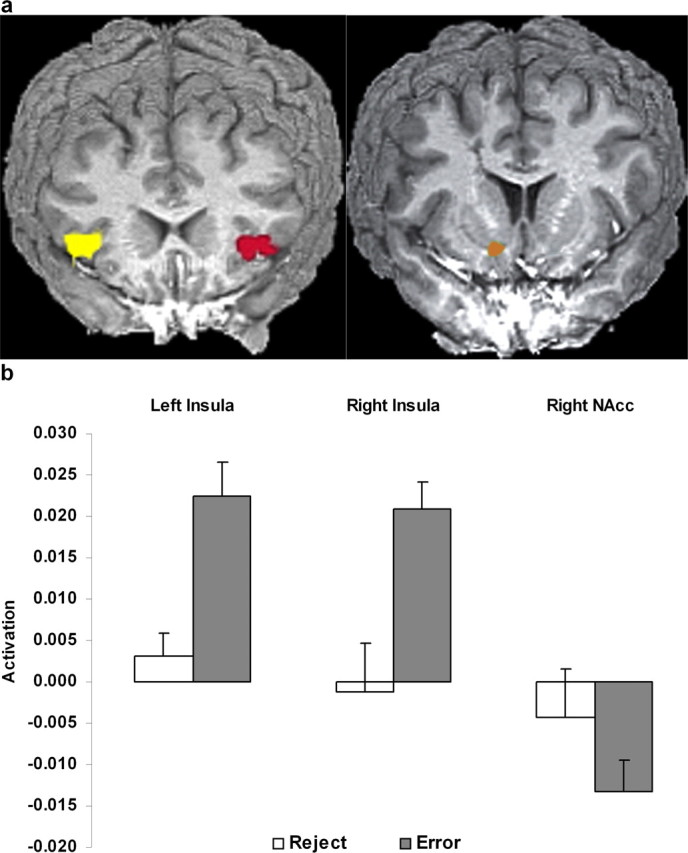
Coronal views of the greater activations for errors relative to reject trials. a, Bilateral insular activations (left) and right NAcc deactivation (right). b, Bar charts show mean activation for these areas for errors and rejections.
The NAcc ROI analysis showed the right NAcc was significantly more deactivated for errors relative to the baseline of correct events (t(15) = −3.48; p < 0.003). A region (right NAcc, ACC BA 24) by response (error, reject) ANOVA revealed a significant interaction (F(1,15) = 7.92; p < 0.025) with post hoc tests confirming ACC activity for reject trials that was significantly greater than NAcc activity for both rejections and errors.
The reject trials tended to be those with larger set sizes (subjects rejected 62% of the large-set-size trials and 5% of the small-set-size trials; t(15) = 7.08; p < 0.001). This leaves open the confound that increased mental effort on larger set sizes may have driven the greater ACC activation (Paus et al., 1998), rather than rejection per se. However, a secondary analysis on a subset of events in which the error and reject trials were equated for the numbers of large and small set sizes (across subject average of 37 events of small size and 22 events of large size in both conditions) identified an ACC region, encompassing both ACC regions (BA 24 and BA 32) identified above, that showed greater activity for rejections than errors (2269 μl; x = 2, y = 11, z = 41; t(12) = 3.14; p < 0.01). Whenever possible, the selected events were also equated for target presence and absence within each size. All other events were included in separate regressors. Only 13 subjects had sufficient numbers of events to be included in this analysis. Although the reaction time difference between conditions no longer remained (802 ms for rejections and 823 ms for errors), significantly greater activation for reject trials over error trials still remained. Also showing greater activity for rejections over errors was the same area of the left DLPFC as observed in the complete analysis [now also including BA 9 (568 μl; x = −28, y = 42, z = 32; t(12) = 3.88; p < 0.005) and left BA 10 (843 μl; x = −37, y = 41, z = 19; t(12) = 2.98; p < 0.01)]. These results indicate that set size differences in the complete analysis were not responsible for ACC activation in the rejections versus errors contrast.
Participants showed appropriate monitoring behavior insofar as they were less likely to be correct on large-set-size trials (subjects were correct on 20% of the large-set-size trials and 75% of the small-set-size trials). To test whether the lower likelihood of accuracy on large-set-size trials was reflected in cingulate error-evaluation activity, we conducted an analysis restricted to correct responses that compared small- and large-set-size trials, in the 12 subjects with a sufficient number of events. The contrast showed greater ACC activity for the larger set sizes (813 μl; x = −3, y = 17, z = 37; t(11) = −4.28; p < 0.001), a finding consistent with Brown and Braver's (2005) results in which correct trials associated with a greater likelihood of errors produced greater ACC activity.
Discussion
In observing greater activity for reject over error trials, the present results are inconsistent with the ACC having a primary role in error detection. Indeed, ACC activity for errors did not differ from the baseline of correct trials and instead showed a nonsignificant deactivation (t(15) = −1.54; p < 0.14) similar to an effect observed in the NAcc (described below), both of which may be driven by a transient depression of midbrain dopaminergic neurons that is observed when an expected reward is not received (Schultz et al., 1997). A conflict monitoring interpretation of the ACC activity on reject trials cannot easily accommodate these results because the present task did not activate competing, antagonistic responses unlike STROOP, flanker or response inhibition tasks for which response conflict and the monitoring of same might be expected. Errors tend to occur on trials in which more than one response representation is activated (Botvinick et al., 2001; Gehring and Fencsik, 2001; Kerns et al., 2004), so it might be suggested that in the present task, conflict was generated between the target-present versus target-absent responses. However, coactivation of these responses would not be expected to be greatest on reject trials because the rejection serves to dispose of these options and, as a result, any conflict between them. In addition, although response times are generally longer on high-conflict trials (Fan et al., 2002), the reject trials were faster than error trials (F(1,15) = 5.20; p < 0.05). In total, these findings suggest that rejecting served to provide escape from conflict rather than to generate it.
An alternative interpretation of the present results notes that the reject trials represent an on-line adjustment of behavior to prevent errors and, in producing greater ACC activity, suggest that the role of this structure in cognitive control is in identifying situations in which errors are likely. Performance was worse on large-set-size trials, consistent with a large body of evidence on conjunction search tasks showing performance decreases as set sizes increase (Carrasco and Yeshurun, 1998), and large-set-size trials were rejected more often. Furthermore, greater ACC activity for the correct trials with larger set sizes relative to those with smaller set sizes supports previous findings of greater ACC activity associated with a greater likelihood of errors (Brown and Braver, 2005). Although the present finding of greater ACC activity on large-set-size correct trials is also consistent with a role for the ACC in effortful processing (Paus et al., 1998), effort alone cannot explain the greater ACC activity on rejection trials because rejecting large-set-size trials was faster than rejecting small-set-size trials. This suggests that rejections served to reduce effort. In this regard, ACC activity for error trials and high-conflict trials might both be considered specific instances of a more general performance evaluation function. By giving subjects the option to reject trials, it was possible to separate these evaluative functions from error trials, in which they would typically be most prevalent, and in doing so, this rendered correct and error trials indistinguishable in terms of ACC activity: error trial activity did not significantly differ from the baseline activity of correct trials.
Both the ACC and left DLPFC were activated on reject trials. Coactivation of these regions is consistent with other imaging studies of performance monitoring (Bush et al., 1998; Carter et al., 1998; Kiehl et al., 2000). Although some studies suggest that the DLPFC detects the need for executive control, whereas the ACC implements such control (Turken and Swick, 1999; Swick and Turken, 2002), the inverse has also been suggested (Carter et al., 1998; MacDonald et al., 2000; Botvinick et al., 2001; Garavan et al., 2002, 2003). The present results suggest that it is the DLPFC that implements behavioral adjustment given the correlations unique to this region and behavioral measures. These correlations were not predicted a priori and may thus allow a number of interpretations; one that we favor is that the DLPFC was more active in the more demanding circumstances (i.e., for those who were slower to reject or who rejected less frequently). We speculate that the left DLPFC is central to maintaining a representation of task-relevant goals that facilitates error avoidance by, for example, keeping active the viability of the reject option. Those who reject less often will require greater phasic activity of these task representations and will require more time to implement the rejection. Active maintenance of the task goals may be necessary to determine that a behavior is inappropriate and may also be involved in post-error correction and error avoidance by phasic strengthening or reactivation of those representations (Garavan et al., 2002). In this regard, bidirectional information flow between the DLPFC and ACC (Gehring and Knight, 2000) may be central to implementing proactive or reactive control and may be reflected in the observed correlation between the DLPFC and ACC, albeit in the ACC BA 32 region that was significantly more activated than baseline for rejections but that did not differ between rejections and errors.
Opposite to the ACC response, an error-specific response was observed in bilateral insula with a region (insula, ACC BA 24) by trial-type (error, reject) ANOVA revealing a significant interaction (F(1,15) = 67.57; p < 0.001) driven by greater insular activity on error trials and greater ACC activity on reject trials. Robust insula activity often accompanies errors (Garavan et al., 2002; Hester et al., 2004; Ullsperger and von Cramon, 2004) and may reflect an autonomic arousal or affective response to the error. Right insula activation correlates with both awareness of emotionally powerful stimuli and with increased autonomic responses (Critchley et al., 2002, 2004), and it is proposed to function as a gateway to the subjective representation of emotion (Critchley, 2004). The present results, which dissociate ACC and insular activity, suggest that a role in arousal regulation related to cognitive control might be ascribed to the insula and not the ACC.
The NAcc and the ACC both receive midbrain dopamine innervations, and both have been implicated in normal reward-related processing and reward-related psychiatric conditions such as drug dependence (Holroyd and Coles, 2002; Garavan and Stout, 2005). The right NAcc (Fig. 3) revealed a different pattern of results to the ACC in that significant activity changes were observed in this region for error trials and not for rejections. The right NAcc activity for error trials was a decrease below the baseline of correct trials, consistent with previous neuroimaging studies in which an anticipated reward is not obtained (Breiter et al., 2001; Knutson et al., 2001). The significant interaction between the NAcc and the ACC for the reject and error trials suggest different functions for each region insofar as the NAcc would seem to respond to primary reward-related information such as an expected reward not being delivered, whereas the ACC uses this information in the service of implementing behavioral change.
In total, these results implicate a number of separate brain structures in the cognitive control functions that are particular to maintaining high levels of performance. Whereas a performance evaluative role might be attributed to the anterior cingulate, the present results are suggestive of a model in which reinforcement information and arousal, both most particular to error trials, are associated with the NAcc and the insula, whereas the change in behavior necessary to avoid errors is implemented by the DLPFC.
Footnotes
This work was supported by the Irish Research Council for the Humanities and Social Sciences (E.M.) and National Institute of Mental Health Grant MH65350 (J.J.F.). The assistance of Raj Sangoi, Beth Higgins, and Marina Shpaner is gratefully acknowledged.
References
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD (2001). Conflict monitoring and cognitive control. Psychol Rev 108:624–652. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8:539–546. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P (2001). Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 30:619–639. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS (2005). Learned predictions of error likelihood in the anterior cingulate cortex. Science 307:1118–1121. [DOI] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL (1998). The counting Stroop: an interference task specialized for functional neuroimaging–validation study with functional MRI. Hum Brain Mapp 6:270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Yeshurun Y (1998). The contribution of covert attention to the set-size and eccentricity effects in visual search. J Exp Psychol Hum Percept Perform 24:673–692. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD (1998). Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280:747–749. [DOI] [PubMed] [Google Scholar]
- Coles MG, Scheffers MK, Holroyd CB (2001). Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and the theory of error-processing. Biol Psychol 56:173–189. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Critchley HD (2004). The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci USA 101:6333–6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ (2001). Neuroanatomical basis for first- and second-order representations of bodily states. Nat Neurosci 4:207–212. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ (2002). Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron 33:653–663. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ (2004). Neural systems supporting interoceptive awareness. Nat Neurosci 7:189–195. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI (2002). Testing the efficiency and independence of attentional networks. J Cogn Neurosci 14:340–347. [DOI] [PubMed] [Google Scholar]
- Garavan H, Stout JC (2005). Neurocognitive insights into substance abuse. Trends Cogn Sci 9:195–201. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA (1999). Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci USA 96:8301–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RAP, Stein EA (2002). Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. NeuroImage 17:1820–1829. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA (2003). A midline dissociation between error-processing and response-conflict monitoring. NeuroImage 20:1132–1139. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Fencsik DE (2001). Functions of the medial frontal cortex in the processing of conflict and errors. J Neurosci 21:9430–9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT (2000). Prefrontal-cingulate interactions in action monitoring. Nat Neurosci 3:516–520. [DOI] [PubMed] [Google Scholar]
- Hester R, Fassbender C, Garavan H (2004). Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cereb Cortex 14:986–994. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG (2002). The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev 109:679–709. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW III, Cho RY, Stenger VA, Carter CS (2004). Anterior cingulate conflict monitoring and adjustments in control. Science 303:1023–1026. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB (2000). Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology 37:216–223. [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D (2001). Dissociation of reward anticipation and outcome with event-related fMRI. NeuroReport 12:3683–3687. [DOI] [PubMed] [Google Scholar]
- MacDonald AW III, Cohen JD, Stenger VA, Carter CS (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288:1835–1838. [DOI] [PubMed] [Google Scholar]
- Paus T (2001). Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2:417–424. [DOI] [PubMed] [Google Scholar]
- Paus T, Koski L, Caramanos Z, Westbury C (1998). Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: a review of 107 PET activation studies. NeuroReport 9:R37–R47. [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Coles MG, Bernstein P, Gehring WJ, Donchin E (1996). Event-related brain potentials and error-related processing: an analysis of incorrect responses to go and no-go stimuli. Psychophysiology 33:42–53. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR (1997). A neural substrate of prediction and reward. Science 275:1593–1599. [DOI] [PubMed] [Google Scholar]
- Swick D, Turken AU (2002). Dissociation between conflict detection and error monitoring in the human anterior cingulate cortex. Proc Natl Acad Sci USA 99:16354–16359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988). In: Co-planar stereotaxic atlas of the human brain New York: Thieme Medical.
- Turken AU, Swick D (1999). Response selection in the human anterior cingulate cortex. Nat Neurosci 2:920–924. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY (2004). Neuroimaging of performance monitoring: error detection and beyond. Cortex 40:593–604. [DOI] [PubMed] [Google Scholar]